- Department of Pathology, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
Background: Extraskeletal osteosarcoma (ESOS), a malignant soft tissue neoplasm characterized by the presence of osteoid or cartilage matrix, arises without bone involvement. ESOS within the esophagus is particularly rare. This study reported a case of esophageal ESOS with PPM1D-BCAS3 fusion, offering an analysis of its clinicopathological features and a comprehensive literature review aimed at advancing the diagnostic understanding of clinical pathologists.
Case demonstration: A 76-year-old male presented with a 2-month history of subxiphoid pain and dysphagia. Chest computed tomography CT revealed irregular mid-esophageal wall thickening and luminal stenosis, raising suspicion for mid-esophageal carcinoma. Painless endoscopic ultrasonography detected a hypoechoic intraluminal esophageal mass. Endoscopic submucosal dissection (ESD) and histopathological analysis confirmed primary ESOS of the mid-esophagus. No adjuvant therapy was administered postoperatively. However, the patient experienced a recurrence with multiple metastases 9-months later.
Conclusion: The diagnosis of esophageal ESOS, a condition of exceptional rarity, necessitates an integrative evaluation that encompasses clinical history, imaging modalities, and histopathological analysis, with a critical distinction from metastatic osteosarcoma and carcinosarcoma. This study presented the first case of esophageal ESOS with PPM1D-BCAS3 fusion treated via ESD, providing a significant point of reference for clinicians and pathologists. The management of ESOS demands vigilant monitoring and precise follow-up care.
Background
Extraskeletal osteosarcoma (ESOS), a malignant neoplasm of mesenchymal origin, was first identified by Wilson in 1941 (1). Representing merely 1–2% of all soft tissue sarcomas and 2–4% of osteosarcomas (2, 3), ESOS predominantly affects elderly males, manifesting primarily as soft tissue masses in the limbs, particularly in the thighs (lower limbs, 46.6%; upper limbs, 20.5%) and retroperitoneum (17%) (4). While less common, ESOS can also arise in the orbits (5), liver (6), kidneys (7), penis (8), breast (9), colon, skin (10), and other anatomical regions. The occurrence of ESOS in the esophagus is exceedingly uncommon, with the first esophageal osteosarcoma case reported by McIntyre et al. (11) in 1982; only three such cases have been documented thus far (11–13). This report detailed the inaugural case of esophageal ESOS with PPM1D-BCAS3 fusion identified post-ESD.
Case demonstration
A 76-year-old male patient presented with a 2-month history of subxiphoid discomfort and dysphagia. Two months ago, the patient developed subxiphoid pain without obvious inducement, swelling pain and reflux after eating, obstruction feeling when eating, mild cough, expectoration, and white serous sputum. He had a history of hypertension for 3 years. He had a history of smoking and drinking for 50 + years. There was no history of genetic disease or cancer in the family. Physical examination showed T 36.2°C, R 20 beats/min, P 108 beats/min, Bp 160/86 MMHG. The body was thin and the superficial lymph nodes were not palpable. The neck was soft and the trachea was centered. There was no bilateral chest symmetry deformity, bilateral lung breath sounds were clear, and no dry or wet rales were heard.
Endoscopy
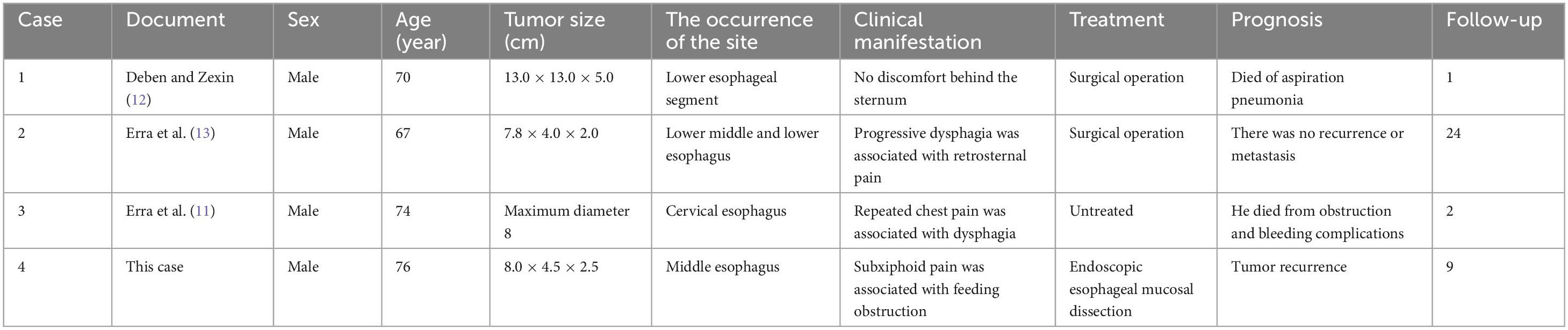
Endoscopic findings: A pedunculated mass was seen protruding into the lumen of the esophagus about 24–30 cm from the incisor tooth, with erosion and bleeding on the surface (Figure 1).
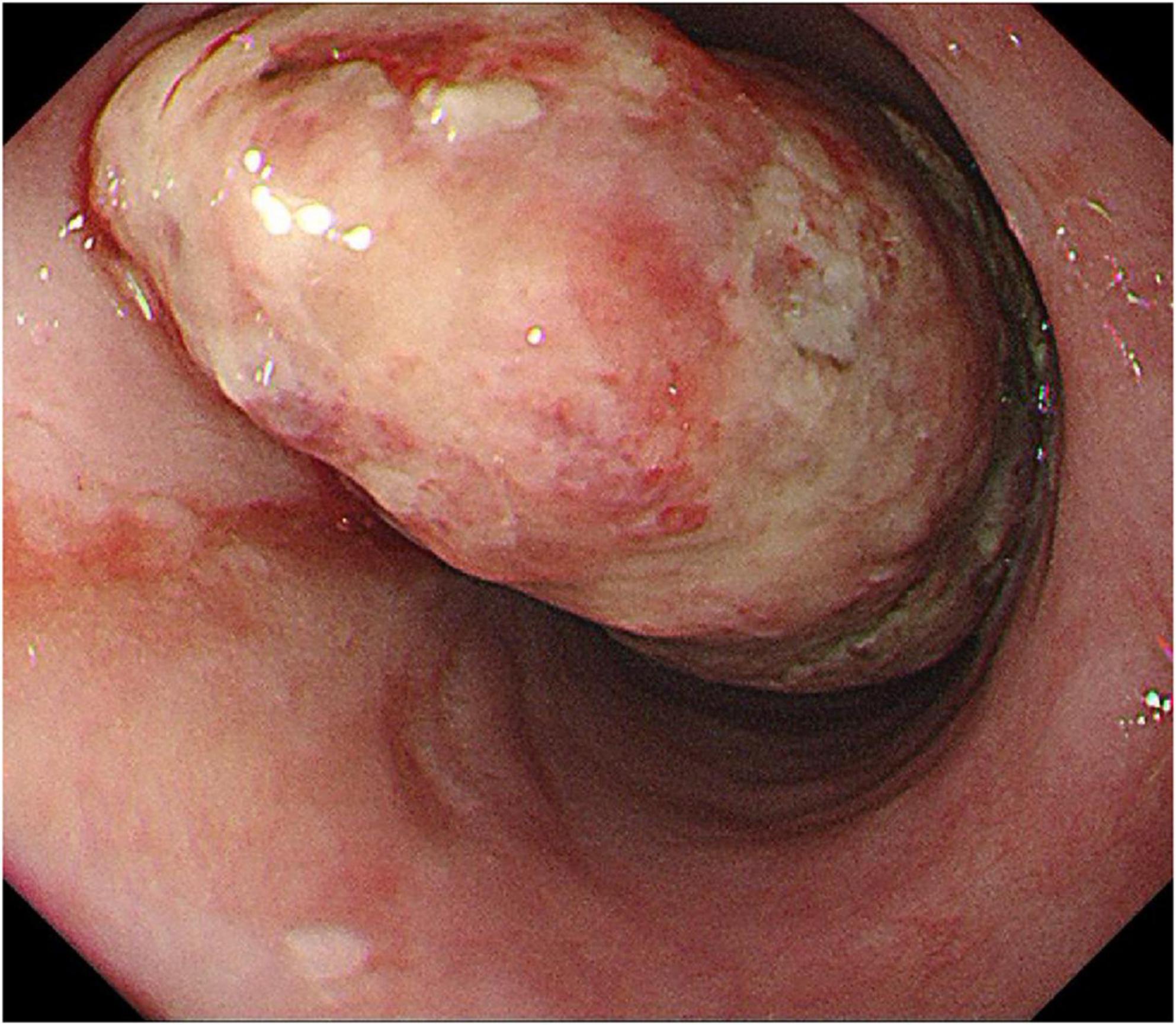
Figure 1. Esophageal endoscopy revealed an intraluminal growing mass in the esophagus 24 to 29 cm from the incisor.
EUS (endoscopic ultrasonography)
Ultrasound imaging (Figure 2) revealed pronounced esophageal wall thickening characterized by hypoechoic regions, interspersed hyper- and anechoic fluid areas, and poorly demarcated boundaries between specific segments and the outer membrane. The imaging features were indicative of an esophageal tumor, accompanied by chronic atrophic gastritis with erosive lesions.
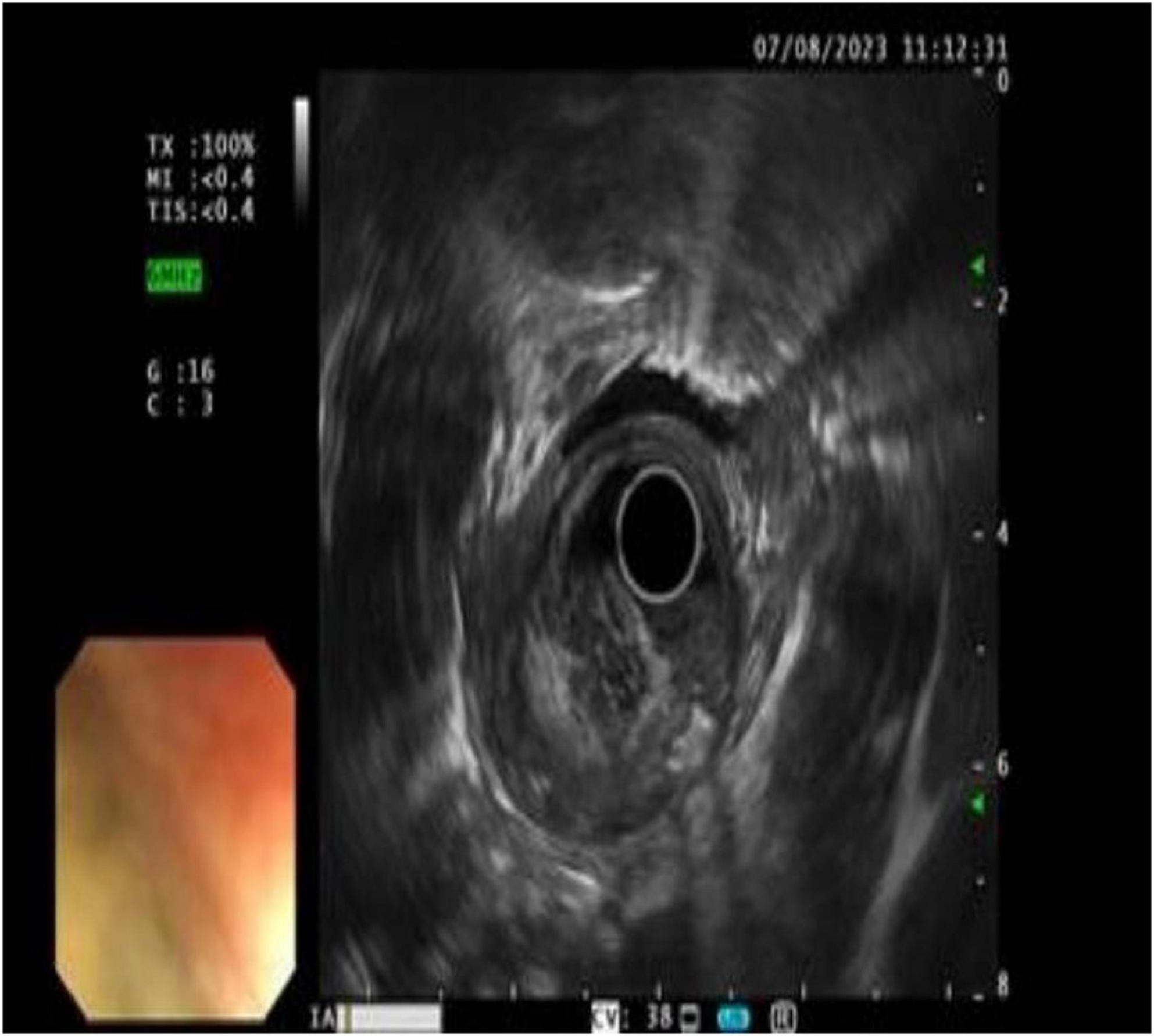
Figure 2. Ultrasound findings: the wall of the esophageal lesions was significantly thickened and hypoechoic, with hyperechoic and fluid anechoic areas in the wall, and some areas had blurred boundaries with the outer membrane.
Chest computed tomography (CT)
Chest computed tomography (CT) identified heterogeneous thickening of the mid-esophageal wall, correlating with luminal stenosis indicative of mid-esophageal carcinoma (Figures 3a,b).
Figure 3. CT showed that the wall of the middle esophagus was uneven and significantly thickened, corresponding to the stenosis of the lumen. The middle esophagus cancer was considered (A: coronal view, B: transverse view).
Initial endoscopic biopsy revealed extensive necrosis and exudate on pathology, necessitating further investigation due to the suspicion of malignancy. Consequently, endoscopic submucosal dissection (ESD) was performed to facilitate comprehensive pathological evaluation.
Gross macroscopic view
The pathological examination revealed a well-demarcated gray-white to gray-brown mass with a smooth surface, measuring 8.0 × 4.5 × 2.5 cm. The cut surface exhibited consistent gray-white to gray-brown coloration, a medium texture, and areas of localized induration.
Histomorphology
Microscopic analysis (Figures 4A–D) identified a tumor with well-defined borders, encapsulated by a thin esophageal squamous epithelium. The tumor featured densely stained regions comprised of polygonal or fusiform cells organized in nests or interwoven patterns. Cellular atypia was prominent, characterized by vacuolated nuclei, inconspicuous nucleoli, and visible mitotic figures. Focal chondroid differentiation was observed, with markedly atypical nuclei present within the cartilage lacunae. The tumor’s central region exhibited abundant eosinophilic osteoid tissue, forming irregular lace-like and grid-like structures. The vascular network within the tumor was extensive, with large vascular cavities infiltrated by tumor cells, containing numerous erythrocytes and eosinophilic homogeneous red-stained material.
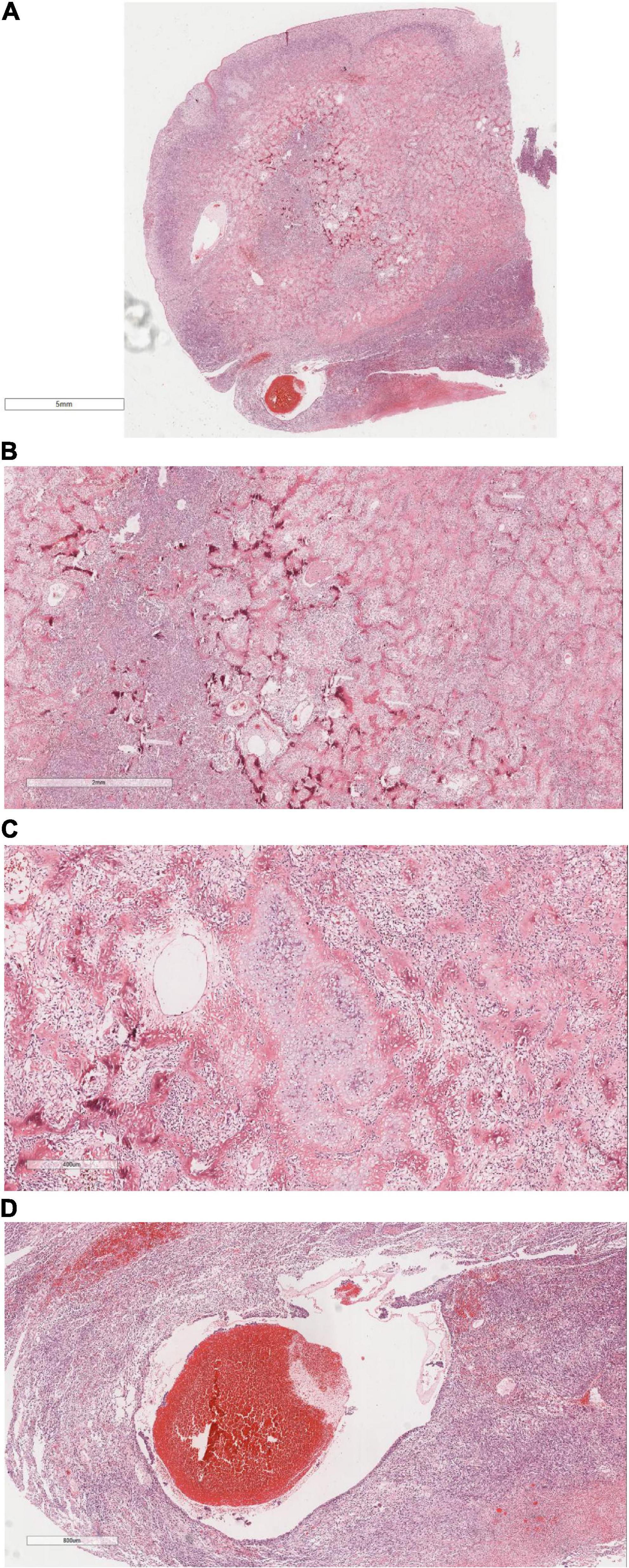
Figure 4. It shows under the microscope, (A) The tumor was well circumscribed and covered with a thin layer of esophageal squamous epithelium. H&E × 4. (B) Tumor cells were composed of polygonal or rapid-hammer cells, which were nested or braid shaped. H&E × 16. (C) The tumor showed focal chondroid differentiation with mild nuclear atypia in the cartilage lacunae. H&E × 50. (D) The tumor was hypervascularized. There were large vascular lumens with tumor cells at the edge of the lumens, a large number of red blood cells and eosinophilic homogeneous red staining in the lumens. H&E × 26.
IHC
Immunohistochemical profiling demonstrated the presence of Vimentin (+), SATB2 (+) (Figure 5A), CDK4 (+) (Figure 5B), with an absence of CK, CK5/6, P40, Desmin, MDM2, and P16, while SMA showed focal positivity in tumor cells.
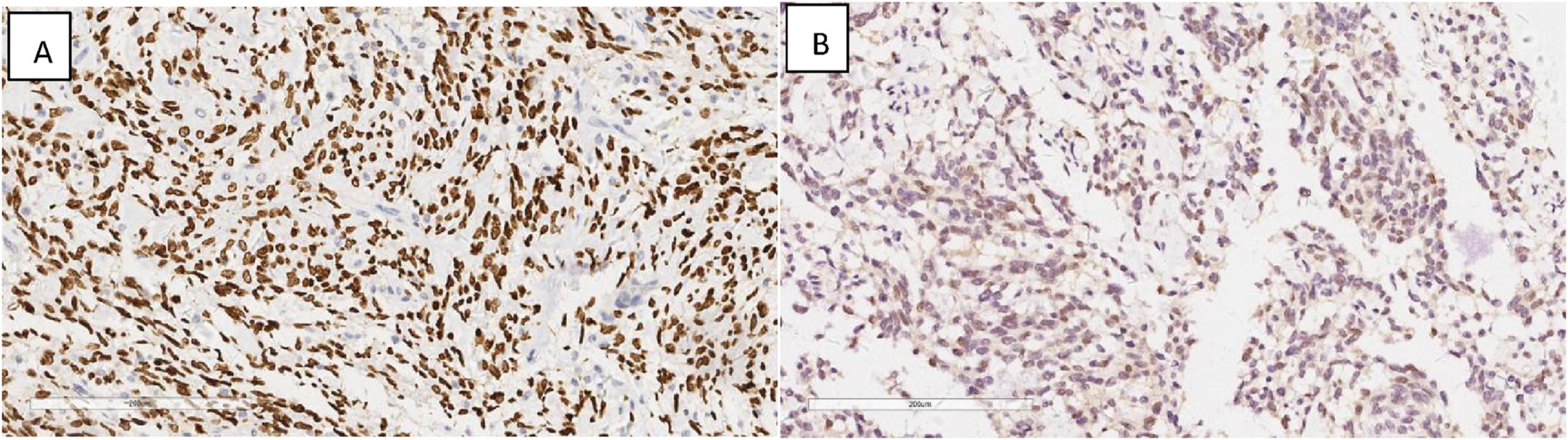
Figure 5. Immunohistochemical results showing tumor cells SATB2 (+) (A), CDK4 (+) (B). EnVision, × 200.
Molecular genetics
Targeted next-generation sequencing (Figure 6) identified 1166 gene mutations, notably including a PPM1D-BCAS3 fusion (PPM1D-BCAS3 Fusion P4: B23; the fusion involved exon 4 of PPM1D and exon 23 of BCAS3, with 12 reads confirming the mutation within the sample).
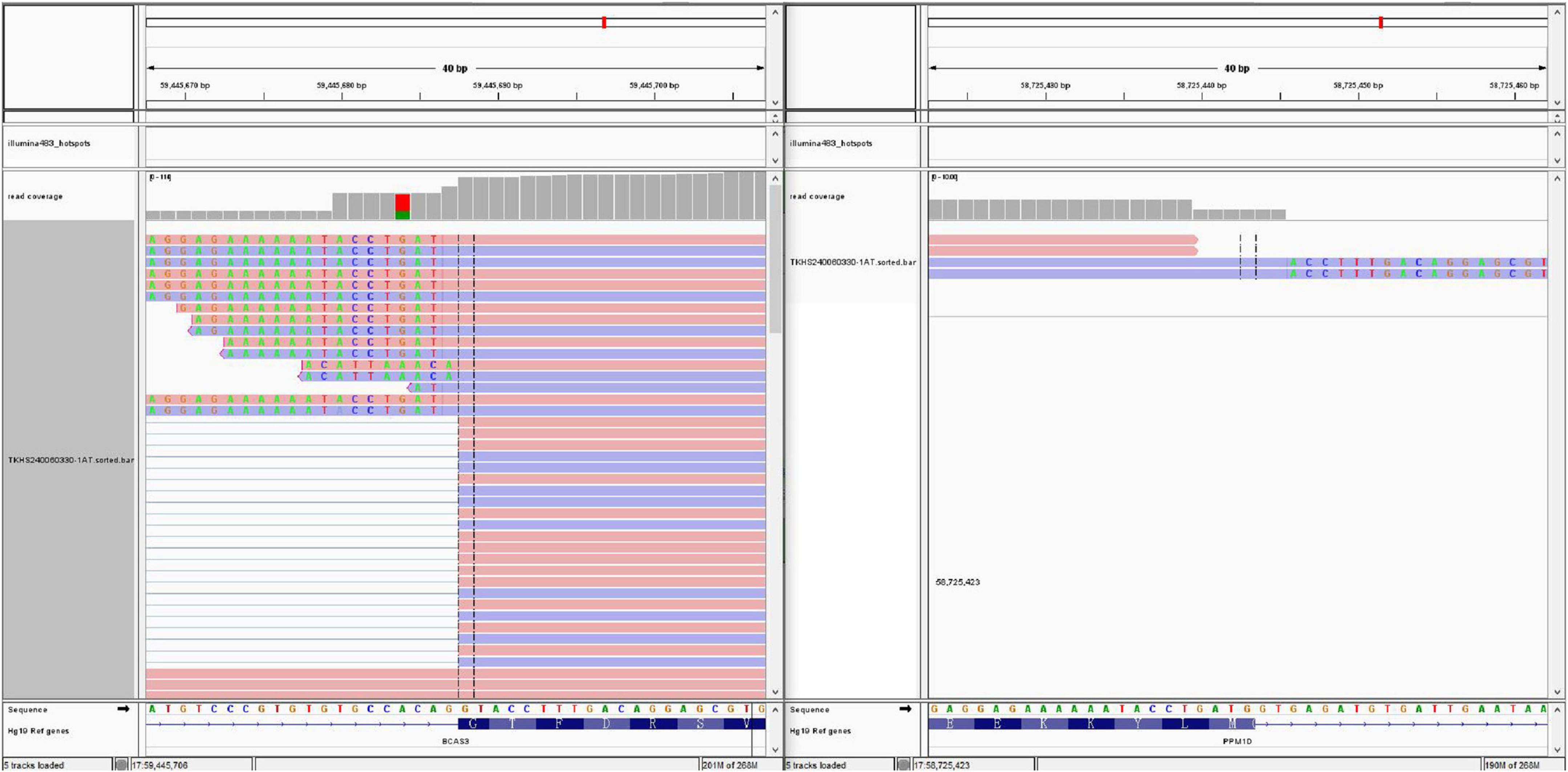
Figure 6. Next-generation sequencing revealed a PPM1D-BCAS3 Fusion P4:B23, with a fusion between exon 4 of the PPM1D gene and exon 23 of the BCAS3 gene.
Taking into account the patient’s clinical history, morphological characteristics, immunohistochemical profile, and sequencing data, a pathological diagnosis of mid-esophageal osteosarcoma was rendered. Given the extreme rarity of primary esophageal osteosarcoma, we considered the need to exclude the possibility of carcinosarcoma, so all the samples of the mass were taken and made into HE, but there was no epithelial cancer component in the tumor component, further clinical evaluation is warranted to definitively determine the primary origin and exclude potential metastasis.
Follow-up
Nine months after surgery, the patient was readmitted due to postprandial esophageal obstruction. Thin-layer chest CT with 3D reconstruction identified the following: (1) Esophageal carcinoma affecting the middle and upper segments, infiltrating adjacent structures, necessitating further diagnostic assessment; (2) Mediastinal lymphadenopathy indicative of possible metastatic spread. Whole-body skeletal planar imaging combined with fusion CT revealed: (1) Symmetrical hypermetabolic foci in the distal femurs bilaterally, indicative of the “hot patella sign,” with no evidence of osseous lesions or abnormal metabolic activity elsewhere; (2) Subpleural nodules in the right upper lobe and a mediastinal mass demonstrating uptake of 99mTc-MDP. Painless electronic esophagoscopy with NBI detected a large mass within the esophagus, located 18–30 cm from the incisors (Figures 7a,b), consistent with tumor recurrence.
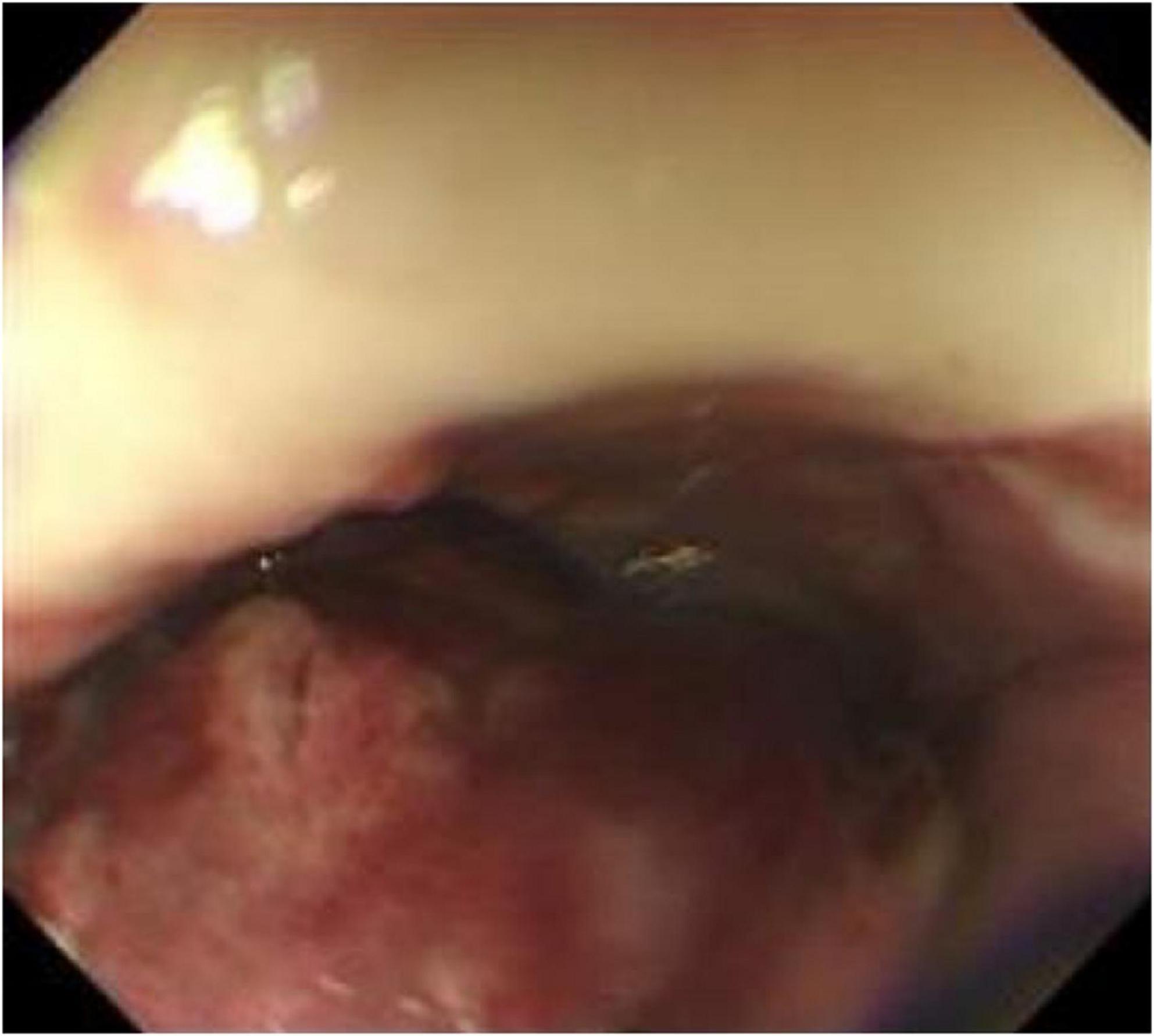
Figure 7. Reexamination of gastroscopy 9 months after surgery showed a large mass in the esophagus 18–30 cm from the incisor, suggesting tumor recurrence.
Diagnosis
The diagnostic findings excluded the possibility of metastatic disease originating from a primary non-esophageal source, thereby confirming recurrent esophageal ESOS with multiple metastases characterized by PPM1D-BCAS3 fusion.
Discussion
ESOS, a rare mesenchymal malignancy, manifests as an osteosarcoma within extraosseous organs and soft tissues. According to the criteria established by Lee et al. 15, early-stage diagnosis of ESOS necessitates: (1) origin within soft tissues, independent of bone or periosteum; (2) uniform sarcomatous histology, ruling out mixed malignant mesenchymal tumors; (3) osteoid and/or cartilage matrix production. This case aligns with the aforementioned diagnostic parameters.
Clinical characteristics
In contrast to primary osteosarcoma, which originates in the bones and predominantly affects adolescents, ESOS is more commonly identified in adults over 50 years of age, with a marked male predominance. Prior to this study, only three cases had been reported, making this the fourth documented case. The clinical presentation of these four ESOS cases primarily features dysphagia (Table 1). Onset occurred between ages 67 and 74, with a median age of 72 years. ESOS can manifest in any part of the esophagus. Follow-up periods varied from 1 to 24 months, with three patients succumbing to the disease and one remaining tumor-free. Due to the non-specificity of clinical symptoms, rapid progression, and significant tumor size, ESOS is frequently misdiagnosed as a tumor of esophageal epithelial origin. The etiology remains uncertain, although prior literature suggests potential links to trauma and X-ray exposure (1, 4, 14).
Imaging manifestations
Large soft tissue masses can be identified through X-rays and CT scans, although imaging alone does not provide definitive differentiation between primary and metastatic lesions. Endoscopic evaluation reveals a solitary, sizable mass with a polypoid morphology. The endoscopic characteristics in this case closely align with those described in cases 1 and 2 in Table 1, suggesting a potentially distinctive presentation of esophageal osteosarcoma. Due to the rarity of reported cases, additional studies involving larger patient cohorts are necessary to validate these findings.
Pathological characteristics
ESOS typically manifests as a soft tissue mass, predominantly occurring in the thigh, upper limbs, and retroperitoneum, with esophageal involvement being an extreme rarity, documented in only three cases. Tumor sizes vary from 2.5 to 30 cm (15), with this case measuring between 7.8 and 13 cm. ESOS is categorized into six pathological subtypes: osteoblastic, fibroblastic, chondroblastic, giant cell, capillary dilation, and small cell types. Morphologically, it bears a strong resemblance to osteosarcoma, with the osteoblastic subtype being the most common. In this case, extensive osteoid production is observed, characteristic of the osteoblastic subtype, along with the presence of chondroblasts, fibroblasts/pleomorphic malignant fibrous histiocytoma, and capillary dilation. Tumor cells are also noted within the margins of dilated vascular lumina.
The diagnosis of ESOS necessitates ruling out metastatic lesions from other origins before attributing it to a primary tumor. Immunohistochemistry lacks specificity, as it does not express epithelial markers like CK and CK5/6; however, it shows expression of SATB2 and vimentin, with occasional MDM2 positivity. SATB2, a marker highly sensitive to osteoblastic differentiation, is primarily observed in both benign and malignant bone tumors with osteoblastic features, as well as in soft tissue tumors exhibiting heterogeneous bone differentiation (16). In this case, SATB2 and CDK4 expression, along with focal chondrocyte differentiation, were identified. Masson trichrome staining, which highlighted the bone matrix in blue, contributed to the diagnosis (17). Markers such as CK, P40, and P16 effectively excluded common epithelial cell-derived esophageal tumors, while negative staining for SMA, desmin, and MDM2 further supported the differential diagnosis.
Molecular genetics
Primary osteosarcoma is linked to a spectrum of inherited germline mutations, with TP53 being the most frequently altered gene. Mutations in other significant driver genes, including RB, c-Myc, NOTCH1, FOS, NF2, WIF1, BRCA2, APC, PTCH1, and PRKAR1A, are also prevalent (18). In this case, targeted next-generation sequencing approach identified 1,166 gene mutations and uncovered a PPM1D-BCAS3 fusion.
PPM1D encodes a serine/threonine phosphatase involved in regulating cell cycle arrest. Its overexpression, mutation, and amplification are frequently observed across a spectrum of cancers. PPM1D exerts negative regulation on several tumor suppressor pathways, including ATM, CHK2, p38 MAPK, and p53, contributing to enhanced osteosarcoma proliferation and migration through the upregulation of PKP2, which has potential as an early diagnostic biomarker for the disease (19). Furthermore, TRPM2-AS may exacerbate osteosarcoma malignancy by acting as a molecular sponge for the miR-15b-5p/PPM1D axis (20).
BCAS3 represents a protein coding gene that acts as an acetyltransferase activator, binding to both β-tubulin and histone acetyltransferase. It contributes to cellular responses to estrogen, augments catalytic activity, and promotes RNA polymerase II-driven transcription, while also playing a role in ectoderm differentiation. Evidence suggests BCAS3 mediates the coupling of microtubules with intermediate filaments, thereby supporting cell migration essential for angiogenic remodeling (21). Furthermore, BCAS3, in conjunction with PPM1D, may be implicated in the initiation, progression, and metastasis of osteosarcoma.
Differential diagnosis
The differential diagnosis for this case included:
1. Metastatic osteosarcoma: This possibility was excluded based on the patient’s medical history and comprehensive imaging, including bone scans, X-rays, and CT, which revealed no primary lesions outside the esophagus.
2. Carcinosarcoma: Consideration was given to carcinosarcoma, a rare biphasic malignancy comprising both squamous cell carcinoma and sarcoma components. The carcinomatous element typically manifests as squamous cell carcinoma, although adenocarcinoma has been occasionally reported. The sarcomatous component usually consists of spindle cells mimicking malignant fibrous histiocytoma, with potential differentiation into muscle, cartilage, or bone. However, the absence of a squamous cell carcinoma component in this case, along with negative epithelial markers CK and CK5/6, ruled out carcinosarcoma.
Treatment and prognosis
ESOS remains an exceptionally rare malignancy with no established treatment protocol. Current therapeutic strategies focus predominantly on surgical intervention, with the approach determined by the tumor’s anatomical location, size, and extent. Surgical options include local, wide, and radical resections. Although radical resection is preferred for achieving local control, it has no effect on distant metastasis (22). Wide resection offers limited survival advantage. Adjuvant chemotherapy or preoperative radiotherapy may provide additional benefit, particularly in cases of large lesions or positive margins, with chemotherapy typically reserved for palliative purposes. The effectiveness and optimal chemotherapy regimens for ESOS continue to be a subject of debate. Ahmad et al. (23) identified that among 60 ESOS patients (24), received doxorubicin-based chemotherapy, with a response rate of 19%. Wang et al. (25) documented that most patients were administered chemotherapy regimens including methotrexate, doxorubicin, and cisplatin, while a smaller subset received either doxorubicin or ifosfamide. A retrospective analysis by Tsukamoto et al. (26) involving 760 ESOS patients compared outcomes between adjuvant chemotherapy post-local resection and post-surgical resection. The 5-year disease-free survival rate was 47.9% (187/390) in the surgery plus (neo)adjuvant chemotherapy cohort, versus 40.4% (150/371) in the surgery-only cohort. The addition of (neo)adjuvant chemotherapy did not significantly enhance the 5-year disease-free survival rate, indicating its limited efficacy for localized ESOS. Chemotherapy regimens for advanced and metastatic ESOS have consistently resulted in poor outcomes. Although some studies suggest radiotherapy or systemic chemotherapy as treatment options, an established standard of care remains elusive. In Torigoe et al. (24), a partial response was observed in 5 out of 11 patients treated with doxorubicin and cisplatin, yielding a 45% response rate. Despite the utilization of various chemotherapy regimens for advanced and metastatic disease, therapeutic outcomes remain inadequate. Although radiotherapy and systemic chemotherapy have been applied in certain instances, an established standard of care for ESOS remains elusive. ESOS is characterized by aggressive behavior, with a high propensity for local recurrence and metastasis.
In this case, the tumor recurred 9 months postoperatively, followed by metastasis to the lungs and mediastinal lymph nodes. Tumor size may play a critical role in determining prognosis. Further research is necessary to clarify the relationship between histological subtype, clinicopathological features, and patient outcomes. This patient did not undergo lymph node dissection, the absence of pre-ESD EUS staging limits the rationale for ESD, as initial tumor depth and lymph node status were not evaluated, so there is a certain limitation in the evaluation of tumor staging and a certain reference basis for guiding the subsequent treatment.
Conclusion
ESOS, an aggressive malignancy, typically originates in the deep soft tissues of the lower and upper extremities, as well as the retroperitoneum, with esophageal involvement being exceptionally uncommon. Accurate diagnosis demands a thorough approach, incorporating clinical history, imaging, and histopathology to differentiate it from metastatic osteosarcoma and carcinosarcoma. Due to the poor prognosis associated with ESOS, early detection and surgical intervention are imperative. This report details the first documented case of esophageal ESOS with PPM1D-BCAS3 fusion treated via ESD, contributing valuable insights to clinical and pathological practice. The management of ESOS necessitates vigilant monitoring and diligent follow-up care.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SL: Writing – original draft. XT: Writing – review and editing. TX: Writing – review and editing. QJ: Writing – review and editing. YL: Writing – review and editing. JW: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Zunyi Science and Technology Program [grant No. ZunShiKeHe HZ Zi(2024)194 Hao, ZunShiKeHe HZ Zi(2023)268 Hao], Guizhou Population Health Foundation (grant No. 2024GZYXKYJJXM0029), and Science and technology Support Program of Guizhou Province (Qiankehezhicheng [2022]Yiban182). The immunohistochemistry in this study was supported by the above foundation.
Acknowledgments
The authors would like to thank all the reviewers who participated in the review and thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ESOS, Extraskeletal osteosarcoma; CT, computed tomography; ESD, Endoscopic submucosal dissection.
References
1. Wilson H. Extraskeletal ossifying tumors. Ann Surg. (1941) 113:95–112. doi: 10.1097/00000658-194101000-00013
2. Nystrom L, Reimer N, Reith J, Scarborough M, Gibbs C. The treatment and outcomes of extraskeletal osteosarcoma: Institutional experience and review of the literature. Iowa Orthop J. (2016) 36:98–103.
3. Tamura T, Suzuki K, Yasuda T, Nogami S, Watanabe K, Kanamori M, et al. Extraskeletal osteosarcoma arising in the subcutaneous tissue of the lower leg: A case report and literature review. Mol Clin Oncol. (2018) 9:287–92. doi: 10.3892/mco.2018.1662
4. Chung E, Enzinger F. Extraskeletal osteosarcoma. Cancer. (1987) 60:1132–42. doi: 10.1002/1097-0142(19870901)60:5<1132::aid-cncr2820600536>3.0.co;2-l
5. Fan J, Lamont D, Greenbaum A, Ng S. Primary orbital extraskeletal osteosarcoma. Orbit. (2011) 30:297–99. doi: 10.3109/01676830.2011.602170
6. Qian Y, Yu H, Lu T. Liver of primary osteosarcoma in 1 case and the literature review. J Clin Exp Pathol. (2023) 33:592–5. doi: 10.13315/j.cnki.cjcep.2023.05.016
7. Lan-feng Z, Xue-wen X, Qin-he F. A case report of primary renal osteosarcoma. Chin J Clin Exp Pathol. (2016) 302:471–2. doi: 10.13315/j.cnki.cjcep.2016.04.032
8. Wu C, Li C, Han S, Wu S. Extraskeletal osteosarcoma of penis: A case report. Chin J Cancer Res. (2012) 24:164–6. doi: 10.1007/s11670-012-0164-1
9. Zhang QY, Peng F, Sun Y. Primary breast osteosarcoma case and the literature review. J Clin Med Res Pract. (2023) 8:1–20. doi: 10.19347/j.cnki.2096-1413.202308001
10. Habeeb O, Weigelt M, Goldblum J, Ko J, Habermehl G, Rubin B, et al. Primary cutaneous extraskeletal osteosarcoma: A series of 16 cases. Pathology. (2023) 55:315–23. doi: 10.1016/j.pathol.2022.10.002
11. McIntyre M, Webb J, Browning G. Osteosarcoma of the esophagus. Hum Pathol. (1982) 13:680–2. doi: 10.1016/s0046-8177(82)80016-6
12. De-ben L, Ze-xin W. A case report of primary polypoid osteosarcoma of esophagus. Chin J Pathol. (2002) 31:377. doi: 10.3760/j.issn:0529-5807.2002.04.033
13. Erra S, Costamagna D, Durando R. A rare case of extraskeletal osteosarcoma of the esophagus: An example of difficult diagnosis. G Chir. (2010) 31:24–7.
14. Lee J, Fetsch J, Wasdhal D, Lee B, Pritchard D, Nascimento A. A review of 40 patients with extraskeletal osteosarcoma. Cancer. (1995) 76:2253–9. doi: 10.1002/1097-0142(19951201)76:11<2253::aid-cncr2820761112>3.0.co;2-8
15. Bane B, Evans H, Ro J, Carrasco C, Grignon D, Benjamin R, et al. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. (1990) 65:2762–70. doi: 10.1002/1097-0142(19900615)65:12<2762::aid-cncr2820651226>3.0.co;2-k
16. Li S, Xue Q, Shi W. Primary osteosarcoma of the breast: A rare case report and literature review. Front Oncol. (2022) 12:875793. doi: 10.3389/fonc.2022.875793
17. Li Q, Gong X, Ma F, Zhao Y, Zhu X. [Application of histochemical staining in diagnosis of osteosarcomas]. Zhonghua Zhong Liu Za Zhi. (2005) 27:489–91. doi: 10.3760/j.issn:0253-3766.2005.08.012
18. Czarnecka A, Synoradzki K, Firlej W, Bartnik E, Sobczuk P, Fiedorowicz M, et al. Molecular biology of osteosarcoma. Cancers (2020) 12:2130. doi: 10.3390/cancers12082130
19. Cai Y, Yang Y, Zhang X, Ma Q, Li M. TRPM2-AS promotes the malignancy of osteosarcoma cells by targeting miR-15b-5p/PPM1D axis. Cell Cycle. (2022) 21:835–50. doi: 10.1080/15384101.2022.2033414
20. Valle G, Lemberg L. An acute cardiovascular event in an endurance-trained athlete. Heart Lung. (1988) 17:216–22
21. Joshi D, Inamdar M. Rudhira/BCAS3 couples microtubules and intermediate filaments to promote cell migration for angiogenic remodeling. Mol Biol Cell. (2019) 30:1437–50. doi: 10.1091/mbc.E18-08-0484
22. Schneider J, Sener S, Barrera E. Combined replacement of infrarenal aorta and inferior vena cava after en bloc resection of retroperitoneal extraosseous osteosarcoma. J Vasc Surg. (2008) 48:478–9. doi: 10.1016/j.jvs.2007.12.052
23. Ahmad S, Patel S, Ballo M, Baker T, Yasko A, Wang X, et al. Extraosseous osteosarcoma: Response to treatment and long-term outcome. J Clin Oncol. (2002) 20:521–7. doi: 10.1200/JCO.2002.20.2.521
24. Torigoe T, Yazawa Y, Takagi T, Terakado A, Kurosawa H. Extraskeletal osteosarcoma in Japan: Multiinstitutional study of 20 patients from the Japanese Musculoskeletal Oncology Group. J Orthop Sci. (2007) 12:424–9. doi: 10.1007/s00776-007-1164-8
25. Wang H, Miao R, Jacobson A, Harmon D, Choy E, Hornicek F, et al. Extraskeletal osteosarcoma: A large series treated at a single institution. Rare Tumors. (2018) 10:2036361317749651. doi: 10.1177/2036361317749651
Keywords: extraskeletal osteosarcoma, esophageal, pathological diagnosis, differential diagnosis, gene fusion
Citation: Luo S, Tian X, Xu T, Ji Q, Li Y and Wang J (2025) Primary extraskeletal osteosarcoma of the esophagus with PPM1D-BCAS3 fusion: a case report and literature review. Front. Med. 12:1558831. doi: 10.3389/fmed.2025.1558831
Received: 15 January 2025; Accepted: 26 May 2025;
Published: 25 June 2025.
Edited by:
Matiullah Khan, AIMST University, MalaysiaReviewed by:
Sheng Wang, Gezhouba Central Hospital of Sinopharm, ChinaAnahita Mirzasadeghi, Tehran University of Medical Sciences, Iran
Copyright © 2025 Luo, Tian, Xu, Ji, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinjing Wang, amluamluZ3dhbmdsc0AxNjMuY29t
 Shuai Luo
Shuai Luo Xiaoxue Tian
Xiaoxue Tian Ting Xu
Ting Xu