Abstract
Introduction:
Postural orthostatic tachycardia syndrome (POTS), a type of dysautonomia, has been an enigma to many healthcare providers. As many as 80% of coronavirus disease 2019 (COVID-19) long-hauler patients meet the diagnostic criteria for POTS, highlighting awareness of this debilitating multisystem disorder. The etiology of POTS has not been entirely defined, but researchers have speculated that an immunological stressor such as a viral infection might be an initiating event. Prior to the pandemic, we reported that POTS patients have a bleeding diathesis with platelet dense granule storage pool deficiency (δ-SPD).
Methods:
This report presents a prospective case–control study (n = 252) involving four cohorts, comprising two groups of POTS patients and two groups of healthy controls, to evaluate abnormal bleeding and patient demographics. We compared POTS patients and controls that were naïve to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus with subjects that had been infected and subsequently developed POTS or who recovered healthy. Questionnaires were employed to assess bleeding tendencies and the severity of clinical symptoms commonly reported with POTS. We utilized electron microscopy to assess platelet dense granules and enzyme-linked immunosorbent assay (ELISA) to assess COVID-19 and Epstein–Barr viral titers.
Results:
The most common bleeding symptom was easy bruising in POTS patients naïve to COVID-19 (79.7%) and POTS post-COVID-19 patients (90.5%). Both groups had δ-SPD with means of 2.52 ± 0.9 and 2.44 ± 0.9 DG/PL, respectively, in contrast to a mean of 4.33 ± 0.6 DG/PL for controls naïve to SARS-CoV-2 infection and 4.19 ± 1.0 DG/PL for controls recovered from the virus (p < 0.001).
Discussion:
We found that the results between the two POTS groups have no statistically significant difference. Our results identify an additional comorbidity (δ-SPD) in COVID-19 “long haulers”/post-acute sequela of COVID-19 (PASC) patients, frequently seen in POTS, that could explain several disparate symptoms often a??ecting the severity of the condition.
1 Introduction
Postural orthostatic tachycardia syndrome (POTS) is one of the most common forms of dysautonomia. It is a complex disorder with a myriad of overlapping and sometimes disparate clinical symptoms. The disorder did not have a specific International Classification of Diseases (ICD) diagnostic code assigned by the Centers for Disease Control (CDC) until October 2022 (ICD-10 G90.A) (1).
The definition of postural orthostatic tachycardia syndrome in our clinic is the presence of symptoms of orthostatic intolerance associated with an increased heart rate of 30 beats per minute (BPM) from the basal rate or a rate that exceeds 120 BPM that occurs within the first 10 min of standing or upright tilt (2–4). The disorder is an abnormal physiological state commonly caused by the inability of the peripheral vasculature to maintain adequate resistance in response to orthostatic stress, resulting in excessive pooling of blood in the more dependent areas of the body (5–7). This functional decline in circulatory volume causes a compensatory increase in heart rate and myocardial contractility. In severe cases, the peripheral vasculature resistance is unable to compensate fully, resulting in a reduction in effective circulation and varying degrees of cerebral hypoperfusion. A decrease in arterial blood pressure below the level of cerebral autoregulation due to venous pooling may result in various symptoms, including dizziness, light-headedness, near syncope, and ultimately syncope (2, 8–34). Postural orthostatic tachycardia syndrome has now become more prominently known since the coronavirus disease 2019 (COVID-19) pandemic, as many severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with lingering effects of the viral infection (COVID-19 “long haulers”/post-acute sequela of COVID-19 (PASC) patients) present with dysautonomia and similar multiple clinical morbidities that are seen in POTS patients. This has led to POTS being a recognized complication of PASC. Clinicians with interests in dysautonomia have reported that up to 80% of PASC patients meet POTS diagnostic criteria (19–21). Regardless, a majority of POTS patients have not been documented or incorrectly documented, and awareness has increased identification of the condition. A recent publication reports that the incidence rate of POTS has risen from 1.42 cases per 1,000,000 prior to the pandemic to 22.66 cases per 1,000,000 post-COVID-19 (22). The rise in incidence might also be related to the recently established CDC ICD code, as mentioned above. There are also many reports that vaccination against the SARS-CoV-2 virus may induce the development of POTS (23–25). Long-hauler patients experience the same debilitating symptoms as POTS patients, leading efforts such as the Researching COVID to Enhance Recovery (RECOVER) clinical trial to include an AUTONOMIC arm (26).
A direct link to the development of POTS as a sequela of COVID-19 infection in PASC gives great credence to the idea that the etiology of POTS involves an immunologic stressor such as a preceding viral illness. Viral infections induce the innate immune system to respond, ultimately inducing the adaptive immune system to produce antibodies that attack the infectious agent. The blood platelet appears to be an important mediator of both the innate and adaptive immune system (35–37). The platelet is also the primary storage site of serotonin in the peripheral circulation, with up to 99% stored in platelet dense granules (38, 39). Insufficient peripheral serotonin may create conditions that lead to hypotension and, as stated above, could explain many of the clinical symptoms commonly reported by POTS patients. We have previously published a study establishing that a majority of POTS patients are deficient in the number of platelet dense granules and have lower, but still normal, concentrations of serotonin compared to controls (11). We also reported in that study that POTS patients had significant bleeding symptoms, including histories of easy bruising, epistaxis, and, for women, abnormal menstrual bleeding (11). A deficiency of platelet dense granules is known to result in mucocutaneous bleeding symptoms (40–42).
The purpose of this study was to (1) validate our previous study that a majority of individuals diagnosed with POTS have platelet dense (delta) granule storage pool deficiency (δ-SPD) and (2) to compare clinical symptoms and platelet dense granule numbers in patients diagnosed with POTS prior to the pandemic (naïve POTS) with PASC patients diagnosed with POTS. Our working hypothesis, according to the current understanding of POTS as a post-COVID-19 sequela, was that both naïve POTS and PASC POTS patients would have essentially identical clinical symptoms. This would provide additional evidence that POTS may be preceded by an immunological stressor, such as a viral infection, and that, somehow, platelets may be an important constituent of the disorder.
2 Materials and methods
Our prospective case-control study protocol conforms to the ethical guidelines outlined in the 1975 Declaration of Helsinki, as reflected in a priori approval by the Institutional Review Board (IRB) of the University of Toledo Medical Center. A total of 252 participants were recruited to create four (4) distinct groups; we had two groups of POTS patients, one group diagnosed prior to the COVID-19 pandemic, and a second group who had developed dysautonomia following a COVID-19 infection (PACS) and subsequently diagnosed with POTS. All POTS patients included in this study had been diagnosed based on history, physical examination, and tilt table test performed in the fasting state. All had a 6-month or greater history of orthostatic intolerance manifested by orthostatic tachycardia with a heart rate increase of at least 30 bpm (or a rate exceeding 120 bpm) observed during the first 10 min of upright posture without orthostatic hypotension, weakness, light-headedness, fatigue, and near syncope (6). All subjects were required to sign our IRB-approved informed consent form.
We categorized patients diagnosed with POTS prior to the pandemic as Naïve POTS (n = 70) and those with PACS as POTS post-COVID-19 (n = 67). For each group, we attempted to recruit volunteers who matched both age and sex. To prevent confounding variables, patients were excluded from recruitment if taking a SSRI, which can deplete the dense granule of serotonin (43) for therapeutic management of their condition, or had reported having an autoimmune disorder. We recruited two control groups: one naïve to COVID-19 infections (“naïve” controls, n = 62) and another group comprising patients who had experienced an infection but recovered healthily (“recovered” controls, n = 53). Exclusion criteria for controls were applied after the time of venipuncture. Sixteen individuals were excluded from the naïve control group after ELISA screening for COVID-19 nuclear protein data was obtained, indicating that they had experienced an asymptomatic infection. In addition, exclusion criteria for control groups included a self-reported inflammatory condition (i.e., Hashimoto's disease, inflammatory bowel disease, other autoimmune disorder), taking a SSRI medication, or indications of abnormal bleeding. Naïve controls were not excluded if they had been vaccinated for COVID-19, although several reports have described how the immunologic stressors of both the HPV and COVID-19 vaccinations can induce POTS (25, 44, 45). We also measured antibodies against Epstein–Barr virus infection (EBV), as reactivation of EBV has been reported in PACS (46–49). All volunteers were required to provide peripheral blood samples to determine a complete blood count (CBC), detect SARS-CoV-2 infection, EBV reactivation, and assess their platelet dense granule number.
2.1 POTS diagnosis
Tilt table testing utilized a 70° baseline upright tilt for 30 min, during which time heart rate and blood pressure were continuously monitored (50). The test ended if symptomatic hypotension and bradycardia occurred, reproducing the patient's symptoms. If the patient did not experience symptoms, an intravenous infusion of isoproterenol was initiated in the supine position with a dose sufficient to raise the heart rate to 20%−25% above the resting value. Subsequently, upright tilt was repeated for 15 min. Patients and control participants who were on chronic antihypertensive, diuretic, anti-cholinergic, or antidepressant medications, and those with diabetic neuropathy or multisystem disease of any etiology were excluded from recruitment for this study. Each patient underwent a thorough history and physical examination as well as detailed blood chemistry analysis and thyroid profile analysis.
2.2 Demographic collections
All subjects, following informed consent, completed four different survey questionnaires, including (1) a modified bleeding assessment tool (BAT) designed by the Scientific and Standardization Committee on Platelet Physiology of the International Society on Thrombosis and Haemostasis to obtain an objective bleeding tendency score that can indicate potential platelet dysfunction (51). Our BAT was modified by changing pronouns only (i.e., “I bruise easily” rather than “do you bruise easily?”) so that participants could complete it themselves, in contrast to having a health professional ask questions on the form. We did not validate the modifications, as the questionnaire context remained unchanged, except for the use of personal pronouns or possessive adjectives (i.e., “my”) for self-assessment. A mean BAT score of 5 or more for women and 3 or more for men is indicative of a platelet function disorder (52, 53). (2) To assess the potential for heavy menstrual bleeding, a common clinical symptom seen in women with bleeding of unknown etiology, we used a pictorial chart to discriminate between menorrhagia and normal menstrual blood loss. A score of 185 or more is predictive of abnormal menstrual bleeding (54, 55). (3) A tool to assess an objective POTS severity of symptoms was used [Composite Autonomic Symptom Score-31 (COMPASS-31)] (56). The COMPASS-31 questionnaire enables the calculation of an objective score from 0 to 100, considering six different categories: orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, bladder, and pupillomotor functions. A COMPASS-31 score >20 is considered indicative of moderate to severe autonomic dysfunction (56, 57). (4) Finally, we modified an assessment tool developed at the University of Vanderbilt Heart Institute to measure a relative symptom score for associated clinical co-morbidities in POTS (58). A total of 149 symptoms/conditions were included, such as dizziness, fatigue, and migraine headache, with each symptom assigned a value of 1. This allowed us to determine an objective “clinical symptom score” for each volunteer.
2.3 Platelet preparation
Platelet-rich plasma (PRP) was obtained from whole blood by centrifugation at room temperature for 15 min at 200 g. Electron microscopy-coated copper grids used for platelet support were washed with deionized water after PRP incubation and air-dried as described by White (59). A FEI Tecnai G2 Spirit BioTwin transmission electron microscope (Hillsboro, OR, USA) was used to determine an average number of dense granules per platelet (DG/PL). Figure 1 represents a whole-mounted air-dried platelet as viewed by electron microscopy. Dense granules are readily apparent due to contrast with the platelet cytosol as calcium ions are stored in the same organelle that contains adenine nucleotides (mostly ADP), serotonin, and pyrophosphates. Previous studies from our laboratory have established a mean normal value of 4.60 + 0.47 DG/PL(SE), consistent with the established literature (60).
Figure 1

This image is an entire platelet obtained from platelet-rich plasma that had settled upon an electron microscopy support film, washed, and air-dried without fixation. Dense granules are readily apparent due to the opacity of calcium, one of the constituents stored within delta granules. Bar = 1 μm. Original magnification: 10,000×.
2.4. ELISA assays: viral protein assessments
We assessed both COVID-19 and EBV antibodies to evaluate the exposure to further, or state of activity, of these viruses in participants (2, 9, 15, 61, 62). We purchased ELISA kits from EU-ROIMMUN US, Mountain Lakes, NJ, USA, to measure immunoglobulin concentrations to COVID-19 nuclear (catalog# KTR1035) and spike proteins (catalog # KTR1034). For EBV, ELISA kits were purchased from Serion Diagnostics, Wurzburg, GmbH, Germany, to measure antibody titers for viral capsid antigens immunoglobulins M and G [VCAs; VCA Immunoglobulin M (IgM) (catalog # ESR1361M) and VCA IgG (catalog # ESR1361G)], the early antigens immunoglobulin G [EA IgG (catalog # ESR1363G)], and Epstein–Barr Nuclear Antigen Immunoglobulin G [EBNA IgG (catalog # ESR1362G)]. These immunoglobulins enable the determination of whether an individual has had an Epstein–Barr virus infection in the past or if they may have an active or recently active infection. Table 1 (63) is commonly used to assess EBV antibody positivity/negativity and interpretation; the table can be found easily on numerous web pages when searching the internet.
Table 1
| VCA IgM | VCA IgG | EBNA | EA | Interpretation |
|---|---|---|---|---|
| 44 U/ml | 40 U/ml | 20 U/ml | 11 U/ml | Cut-off values (> is positive) |
| Negative | Negative | Negative | Negative | Seronegative – susceptible |
| Positive | Positive | Negative | Pos/negative | Acute primary infection |
| Negative/positive | Positive | Negative/positive | Positive | Recent primary infection |
| Negative | Positive | Positive | Negative/positive | Past infection |
| Negative | Positive | Positive | Pos/negative | Reactivated infection |
Serologic profiles of the EBV antibody tests.
2.5 Statistical analysis
Unless otherwise stated, data are presented as mean+1 standard error of the mean (SEM). The chi-squared test, Fisher's exact test, one-way analysis of variance (ANOVA), Mann–Whitney rank-sum test, Kruskal–Wallis one-way ANOVA on ranks, and correlation assays were performed using the SAS System (SAS Institute, Inc., Cary, NC, USA) and graphed using SigmaPlot® (Grafiti, LLC, Palo Alto, CA, USA).
3 Results
3.1 Group demographics
Our study recruited both men and women; the naïve controls were 73% women, naïve POTS were 91% women, COVID-19 recovered controls were 79% women, and POTS post-COVID-19 were 96% women. This is consistent with the established literature that POTS is a disorder of young premenopausal Caucasian women (28, 64). There were no statistically significant differences among the four study groups regarding complete blood cell counts. All questionnaires that were utilized demonstrated significant differences between control groups and POTS patients. Data collected using our questionnaires provided objective scores for BAT to assess bleeding severity, the menses assessment to detect abnormal menses, the COMPASS-31 to assess the severity of dysautonomia, and an objective clinical symptom measurement. All questionnaire results correlated significantly with platelet dense granule numbers for each group (Table 2).
Table 2
| Assay | Naïve controls | Naïve POTS | COVID-19 recovered | POTS post-COVID-19 |
|---|---|---|---|---|
| Age | 34.7 ± 15.3 | 35.6 ± 13.3 | 33.7 ± 13.7 | 38.9 ± 17.4 |
| Sex | 73% women | 91% women | 79% women | 96% women |
| COMPASS-31 | 14.4 ± 0.9 | 39.4 ± 1.6** | 12.6 ± 0.2 | 33.3 ± 1.8** |
| Bleeding | 0.8 ± 0.3 | 5.1 ± 0.6** | 0.9 ± 0.3 | 5.8 ± 0.7** |
| Menses | 101.9 ± 15.7 | 296.6 ± 43.5** | 109.7 ± 28.1 | 246.5 ± 53.4** |
| Symptoms score | 10.4 ± 1.2 | 46.9 ± 2.5 ** | 10.5 ± 1.2 | 44.7 ± 2.4 ** |
| Platelet dense granule number | 4.33 ± 0.61 | 2.52 ± 0.93** | 4.19 ± 1.0 | 2.44 ± 0.94** |
Volunteer demographics and questionnaire scores used to assess clinical symptoms in control and POTS groups.
**p < 0.001 for both POTS groups compared to controls.
The COMPASS-31 scores are in line with previously published studies (20, 65). Our naïve and COVID-19 recovered healthy control groups had similar low COMPASS-31 scores (< 20, the normal cut-off score) in contrast with naïve POTS (score = 39.4 ± 1.6, p < 0.001) and POTS post-COVID-19 (score = 33.3 ± 1.8, p < 0.001) patients (Table 2; Figure 2).
Figure 2

Results of COMPASS-31 scores for control groups (n = 99) in comparison to POTS patients (n = 137) are significantly different (**p < 0.001).
Although the BAT was not designed for self-assessment, we found our modified version serviceable. The mean BAT scores for our control groups were normal (scores = 1), whereas our POTS groups had higher bleeding scores (scores = 5+). Regardless of being naïve to COVID-19 infection or having PACS, our POTS patients had BAT scores suggestive of increased bleeding tendencies.
The results of our menstrual bleeding assessment correlated with the BAT results. Both control groups had normal menses scores < 185, whereas both POTS groups had abnormal menses scores, with naïve POTS patients having a mean of 296.6 ± 43.5 and POTS post-COVID-19 patients having a mean score of 246.5 ± 53.4 (Table 2; Figure 3).
Figure 3

POTS patients (n = 137) have increased bleeding tendencies compared to the control groups (n = 99).
3.2 Electron microscopy
Assessments of platelet whole mounts also revealed significant differences between our control and POTS groups. Our naïve control group and our COVID-19 recovered healthy group had means of 4.33 ± 0.6 DG/PL and 4.19 ±1.0 DG/PL, respectively, in contrast to our POTS groups. Naïve to COVID-19, POTS patients, and PACS POTS patients were significantly deficient in platelet dense granules, consistent with δ-SPD. Naïve POTS patients were found to have a mean of 2.52 ± 0.9 DG/PL, and POTS post-COVID-19 patients had a mean of 2.44 ± 0.9 DG/PL (Figure 4). Our cut-off value for diagnosing δ-SPD is 3.68 DG/PL, though other laboratories have reported other ranges (60). The use of both a Mann–Whitney rank-sum test and a Kruskal–Wallis one-way analysis of variance on ranks determined significant differences between control groups and POTS patients (p < 0.001). POTS. These results have validated our earlier publication that most POTS patients have δ-SPD (11). Not only have we validated our previous study, but we now demonstrate that individuals who developed POTS post-COVID-19 also have a near-identical δ-SPD.
Figure 4
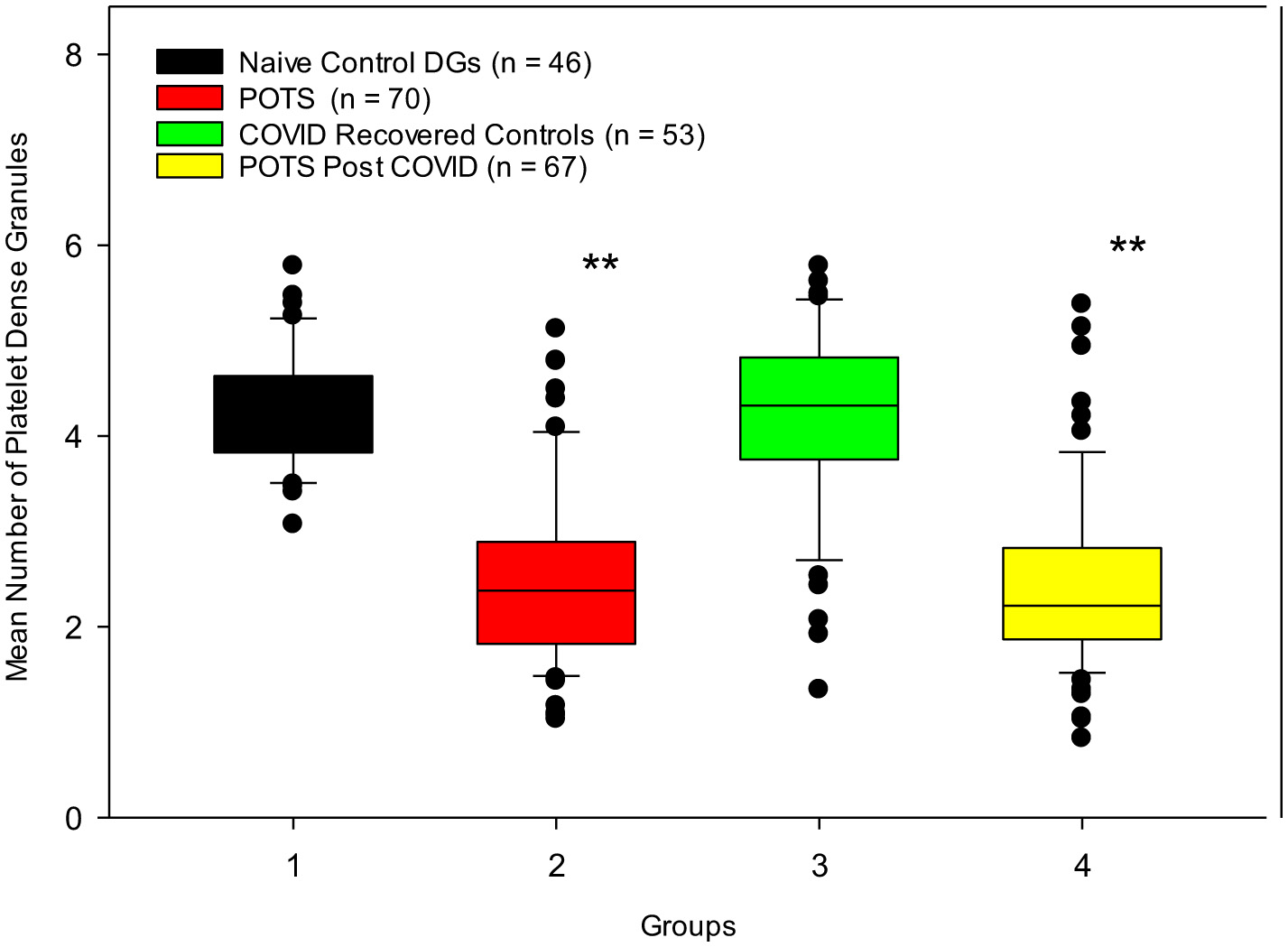
Control subjects who were naïve to COVID-19 infection and those who contracted COVID-19 infection but recovered healthily have normal numbers of dense granules, in contrast to POTS patients diagnosed prior to the pandemic and patients who developed POTS post-COVID-19 infection. **p < 0.001 for both POTS groups vs. control groups.
3.3 SARS-CoV-2 viral protein assessments
We detected nuclear capsid antibodies against the SARS-CoV-2 virus in volunteers who reported having been infected (Table 3). Our cut-off value for a positive COVID-19 infection was 5.0 U/mL. Volunteers who had been infected with SARS-CoV-2 in the study had a mean anti-SARS-CoV-2 nuclear capsid titer ~3-fold higher than both controls and POTS patients naïve to the virus. Almost all volunteers in all four groups had been vaccinated against COVID-19 when they were recruited, indicated by elevated antibody titers against the SARS-CoV-2 viral spike protein (Table 3).
Table 3
| Demographic/ symptom | POTS (201615) | Naïve POTS | POTS post-COVID-19 |
|---|---|---|---|
| Women | 96.1% | 91% | 96% |
| Fatigue | 91.3% | 93.2% | 92.2% |
| Easy bruising | 71.3% | 79.7% | 90.5% |
| Migraine | 64.4% | 61.1% | 81.3% |
| Irritable bowel syndrome | 49.7% | 37.3% | 59.4% |
| Cognitive issues | 37.5% | 69.5% | 62.5% |
| Depression | 33.1% | 38.9% | 34.4% |
| Epistaxis | 33.1% | 16.9% | 23.4% |
| Heavy menstrual bleeding | 32.1% | 38.5% | 42.1% |
| Raynaud's Syndrome | 31.7% | 32.2% | 29.7% |
| Anxiety | 29.8% | 39% | 50% |
| Anemia | 22.7% | 25.4% | 25.1% |
Baseline characteristics and comorbidities in patients with postural orthostatic tachycardia syndrome.
Of unknown significance is the elevation of spike protein antibodies in the COVID-19 recovered healthy group, which is twice as high as that found in POTS PACS patients. Individuals who had been infected with the virus had higher spike protein antibody titers than those who had only received the vaccination. Interestingly, we detected the anti-nucleocapsid IgG in 11 of the control volunteers who claimed that they had never been infected; these individuals were excluded from the naïve control group. We also evaluated EBV antibodies to determine the potential that POTS post-COVID-19 might be due to a reactivation of EBV, as has been suggested in the literature. As defined in Table 1, criteria to determine a recent or reactivated EBV infection can be determined by using four different antibody titer assays, including IgM and IgG antibodies against the viral capsid antigen (VCA), IgG against the nuclear antigen 1 (EBNA1), and IgG against a complex of early antigens (EA). We did not observe evidence that a recent EBV infection or reactivation had occurred in any of our test subjects. Curiously, both POTS groups had slight evaluations of EBV VCA IgM, but these were well below the positive cut-off values (Table 4).
Table 4
| Viral antibody | Naïve control | Naïve POTS | COVID-19-recovered control | POTS post-COVID-19 | Units |
|---|---|---|---|---|---|
| SARS-CoV-2 nuclear capsid IgG | 3.62 | 3.59 | 17.45** | 10.86** | U/ml |
| SARS-CoV-2 Spike Protein IgG | 335.87 | 462.71 | 1,337.75** | 691.39** | U/ml |
| EBV VCA IgG | 117.14 | 119.29 | 85.40 | 105.78 | U/ml |
| EBV VCA IgM | 2.93 | 3.69 | 2.24 | 3.90 | U/ml |
| EBV EBNA1 IgG | 17.80 | 24.86 | 18.50 | 22.38 | U/ml |
| EBV EA IgG | 5.65 | 7.67 | 7.50 | 12.16 | U/ml |
Comparison of viral antibody titers between study groups.
SARS nuclear capsid IgG > 5.0 = COVID-19 infection.
**p < 0.001 for COVID-19 naïve groups vs. infected subjects.
3.4 Other common symptoms
A variety of additional symptoms for both our naïve POTS and our post-COVID-19 POTS groups were also noted in their medical history, similar to what we have previously reported (11). The most frequently reported complaints were fatigue, palpitations, easy bruising, irritable bowel syndrome/gastritis, and cognitive issues; for example, these were determined using a modified questionnaire established at Vanderbilt University (58). There were significant differences in reported clinical symptoms between our control patients and our POTS patients (Table 2). The control groups had clinical symptom scores of ~10, whereas both POTS groups had a symptom score of ~45. Comparisons between the symptoms we reported in 2016 and those in our current study are presented in Table 3. Our results are similar to numerous reports in the current literature (20, 66–68).
4 Discussion
Orthostatic intolerance (OI) has been reported to affect an estimated 500,000 people in the United States in 1999; partial dysautonomia and hyperadrenergic orthostatic intolerance are also referred to as postural orthostatic tachycardia syndrome (POTS) or sympathetic orthostatic intolerance (69). The etiology of POTS has various proposed causes; many studies have reported preceding viral symptoms (8–10), while others have suspected low levels of serotonin (11–13). Additionally, some of the studies describe the presence of autoantibodies in POTS (15–18). Many clinical morbidities are truly related to low levels of serotonin, and descriptions of POTS patients with circulating G-protein coupled receptor autoantibodies have been reported (21, 24, 27–29, 58, 64, 70, 71). The disorder is often misdiagnosed as chronic anxiety or a panic disorder because the autonomic failure in these patients is not severe, and the variety of clinical symptoms, including fatigue, light-headedness, nausea, headache, near syncope, and exercise intolerance, is subtle (72, 73). Furthermore, POTS may be idiopathic and unrelated to other diseases, with most affected patients categorized with a partial dysautonomic condition appearing to be related to mild peripheral autonomic neuropathy (72, 73).
The same symptoms are now common in PACS, and many of these patients are being diagnosed with neurological or psychiatric problems, just as POTS patients were before the pandemic (74–77). Postural orthostatic tachycardia syndrome is now reported to affect one to three million, with various reports suggesting 0.2% of the population to as many as 170 per 100,000, with additional untold millions more. At least 65 million individuals are estimated to have PACS, and of these, up to 79% are reported to meet international criteria for POTS (21, 24, 58, 64, 70, 71). The current post-pandemic literature on PACS, in relation to POTS, recognizes the association between orthostatic intolerance and a preceding immunologic stressor, such as a virus or vaccine.
The majority of POTS patients require numerous visits to several physicians of different disciplines to be accurately diagnosed due to the multitude of conflicting clinical symptoms (78–80). Hypotheses regarding pathophysiology include abnormally increased sympathetic activity, sympathetic denervation leading to hypovolemia and reflex tachycardia, and autoimmunity to G-protein coupled receptors (2, 3, 79, 81, 82). The autoimmunity may occur via a process called molecular mimicry (30, 31). Molecular mimicry has also been postulated as a mechanism by which viruses evade the immune system by mimicking host proteins, but results in the production of cross-reacting autoantibodies (32). Other studies have postulated that COVID-19 causes a number of neuropathology-related symptoms through an indirect means of upregulating the immune system via molecular mimicking proteins in the vagal nuclei and ganglia (33, 34). Observations reported since the pandemic of a high percentage of PACS patients developing dysautonomia and reports that COVID-19 vaccination induces POTS support the hypothesis that the etiology of POTS involves an immunologic stressor (23–25, 83, 84). Before the pandemic, occasional reports had discussed common associations with Ehlers–Danlos syndrome, low serotonin levels, and potential preceding EBV infections.
More than 90% of our patients reported fatigue (Table 3). The most common bleeding symptom for both POTS groups was easy bruising, but frequent nose bleeds and heavy menstrual bleeding were also reported. The results of our electron microscopy assay of platelet dense granules for all four groups correlate well with our BAT and menses scores, as well as the clinical symptoms commonly associated with platelet dysfunction disorders, including easy bruising, epistaxis, and heavy menstrual bleeding, which are seen in our POTS patients. Our observation of mucocutaneous bleeding in these patients is not as severe as seen in other coagulation system anomalies, typically needed to cause significant bleeding. Most publications of PACS that describe coagulopathies report on thrombosis. Still, one recent study reports “that hemorrhage and risk of hemorrhage are not necessarily an infrequent finding in COVID-19, albeit most probably associated with contributing factors” (85, 86). This report provides support to our findings that both COVID-19-induced POTS and POTS naïve to COVID-19 may be associated with platelet dysfunction, specifically platelet δ-SPD. Both of our control groups were found to have normal numbers of dense granules per platelet.
Notably, we observed that study participants who had been infected with COVID-19 exhibited an approximate 2–4-fold increase in viral spike protein IgG antibody titers compared to controls. As stated previously, with few exceptions, all our volunteers had been vaccinated against COVID-19 at the time of enrollment and venipuncture. We did not consider the evaluation of different manufacturers' vaccines. It is most interesting to observe a nearly two-fold increase in both nuclear capsid IgG and spike protein IgG antibodies in the recovered healthy control groups compared to the POTS post-COVID-19 group. Could this imply that some individuals infected with COVID-19 have a reduced innate and/or adaptive immune response to the virus compared to the majority of people, and therefore are more susceptible to lingering effects or continued chronic infections that subsequently lead to PACS? This question should be further evaluated; why do some people develop PACS and dysautonomia, whereas most infected patients recover fully from COVID-19? Evaluating any difference in viral antibody titers, specifically in those infected with SARS-CoV-2, for comparison with clinical symptoms or variances in platelet dense granule numbers, was not within the scope of our project. These assays were utilized to detect asymptomatic COVID-19 infections as an exclusion criterion for our naïve control group.
We report a significant association with platelet δ-SPD, a type of platelet dysfunction disorder, in POTS patients naïve to COVID-19 infection and in PASC patients diagnosed with POTS (p < 0.001 compared to respective control groups). We have validated our previous observation that most POTS patients have platelet δ-SPD. We also demonstrate with statistical significance that COVID-19 long-haulers diagnosed with POTS have essentially identical demographic profiles to COVID-19 naïve patients. The data generated by the use of our questionnaires, including our two bleeding assessment tools, correlate with our platelet dense granule assessment results. Platelet dysfunction disorders such as von Willebrand disease and platelet δ-SPD usually manifest with common symptoms, including easy bruising, frequent nose bleeds, heavy menstrual bleeding for women, excessive bleeding of the gums with brushing, and excessive bleeding with surgery or trauma. Platelet dense granule deficiency is thought to have an autosomal dominant inheritance pattern, but it is also known to be acquired. We postulate that acquired δ-SPD is the result of a chronic inflammatory state induced by an immunological stressor, which could potentially explain the occurrence of δ-SPD in our post-COVID-19 POTS patients. Interestingly, one drug that has been shown to reduce bleeding symptoms related to δ-SPD (Desmopressin) has also been reported to reduce symptoms in POTS (87).
Is our observation that patients diagnosed with POTS have platelet δ-SPD important? It is possible that this may be a factor related to some of the symptoms reported by some POTS patients. Many of their mild bleeding symptoms are suggestive of an underlying platelet dysfunction condition. It is unknown whether or not δ-SPD is a risk factor for the development of POTS. It is possible that δ-SPD is an acquired condition, brought on by an immunological stressor. This is a challenging problem to address, but recent reports of microthrombi found in the blood of PACS patients implicate platelet activation as a result of COVID-19 infection, and may be a factor in some of the symptoms reported by some POTS patients (86, 88, 89).
An additional limitation of our study is that we did not assess serotonin concentrations contained in platelet dense granules. We previously reported that POTS patients have low levels of serotonin, stored in platelet dense granules, when compared to controls (11, 90). The observation of low serotonin in POTS correlates with many symptoms reported by POTS patients. A further limitation of this project is the lack of platelet dense granule data prior to the development of POTS, which hinders consideration of whether δ-SPD is a risk factor for the development of POTS or if it is an acquired condition. We did not utilize PCR to detect EBV for reactivation in this study. For patients with deficient immune systems, in some respects, assessment for EBV infection/reactivation via EBV antibody testing may not be the optimal method for assessing this infection. Finally, we did not assess cohorts for hypermobility spectrum disorders or hypermobile Ehlers–Danlos syndromes that are known to be associated with abnormal bleeding. As these conditions are common comorbidities in POTS, the δ-SPD we report in POTS patients, in contrast to our control cohorts, might be related to these connective tissue disorders rather than POTS.
5 Conclusion
We have validated our previously reported data, which indicates that most POTS patients have a platelet delta granule storage pool deficiency. We do not propose that platelet dense granule deficiency is the etiology of POTS; however, it appears that the condition is somehow associated with POTS. Our observation correlates well with clinical symptoms assessed by questionnaires to determine bleeding tendencies, creating an objective score for comparison with control groups. We have also found that patients diagnosed with POTS post-COVID-19 infection (long haulers/PACS patients) have essentially identical demographics when compared to patients diagnosed with POTS prior to the pandemic. What has not been defined is the association between δ-SPD and POTS. Further research is needed to evaluate whether δ-SPD is a risk factor for the development of POTS or an acquired state, related to an inflammatory response to a viral infection such as COVID-19. Our future research plans include reassessing POTS patients recruited for this study to evaluate any improvement in symptoms and, if present, to investigate potential normalization of their platelet dense granule numbers.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by University of Toledo Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SK: Data curation, Writing – review & editing. JS: Data curation, Investigation, Methodology, Writing – review & editing. BK: Data curation, Investigation, Resources, Writing – review & editing. BG: Conceptualization, Data curation, Investigation, Resources, Writing – review & editing.
Funding
The authors declare that this study received funding from Dysautonomia International, Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
This study could not have been conducted without the help of all the volunteers who were willing to provide a sample of their blood and complete our questionnaires.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor SB declared a past co-authorship with the author BG.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1560120/full#supplementary-material
References
1.
CDC . ICD-10 - International Classification of Diseases, Tenth Revision (2022). Available online at: http://ftpcdcgov-/pub/Health_Statistics/NCHS/Publications/ICD10CM/2023/ (Accessed July 5, 2025).
2.
Fedorowski A . Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. (2018) 285:352–66. 10.1111/joim.12852
3.
Arnold AC Ng J Raj SR . Postural tachycardia syndrome - Diagnosis, physiology, and prognosis. Auton Neurosci. (2018) 215:3–11. 10.1016/j.autneu.2018.02.005
4.
Grubb BP Kosinski DJ Boehm K Kip K . The postural orthostatic tachycardia syndrome: a neurocardiogenic variant identified during head-up tilt table testing. Pacing Clin Electrophysiol. (1997) 20:2205–12. 10.1111/j.1540-8159.1997.tb04238.x
5.
Gazit Y Nahir AM Grahame R Jacob G . Dysautonomia in the joint hypermobility syndrome. Am J Med. (2003) 115:33–40. 10.1016/S0002-9343(03)00235-3
6.
Grubb BP Kanjwal Y Kosinski DJ . The postural tachycardia syndrome: a concise guide to diagnosis and management. J Cardiovasc Electrophysiol. (2006) 17:108–12. 10.1111/j.1540-8167.2005.00318.x
7.
Rowe PC Barron DF Calkins H Maumenee IH Tong PY Geraghty MT . Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers-Danlos syndrome. J Pediatr. (1999) 135:494–9. 10.1016/S0022-3476(99)70173-3
8.
Kimpinski K Figueroa JJ Singer W Sletten DM Iodice V Sandroni P et al . A prospective, 1-year follow-up study of postural tachycardia syndrome. Mayo Clin Proc. (2012) 87:746–52. 10.1016/j.mayocp.2012.02.020
9.
Sandroni P Opfer-Gehrking TL McPhee BR Low PA . Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc. (1999) 74:1106–10. 10.4065/74.11.1106
10.
Schondorf R Low PA . Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia?Neurology. (1993) 43:132–7. 10.1212/WNL.43.1_Part_1.132
11.
Gunning WT Karabin BL Blomquist TM Grubb BP . Postural orthostatic tachycardia syndrome is associated with platelet storage pool deficiency. Medicine. (2016) 95:e4849. 10.1097/MD.0000000000004849
12.
Johnson JN Mack KJ Kuntz NL Brands CK Porter CJ Fischer PR . Postural orthostatic tachycardia syndrome: a clinical review. Pediatr Neurol. (2010) 42:77–85. 10.1016/j.pediatrneurol.2009.07.002
13.
Grubb BP Karas BJ . The potential role of serotonin in the pathogenesis of neurocardiogenic syncope and related autonomic disturbances. J Interv Card Electrophysiol. (1998) 2:325–32. 10.1023/A:1009792000490
14.
Vernino S Hopkins S Bryarly M Hernandez RS Salter A . Randomized controlled trial of intravenous immunoglobulin for autoimmune postural orthostatic tachycardia syndrome (iSTAND). Clin Auton Res. (2024) 34:153–63. 10.1007/s10286-024-01020-9
15.
Gunning WT Kvale H Kramer PM Karabin BL Grubb BP . Postural orthostatic tachycardia syndrome is associated with elevated G-protein coupled receptor autoantibodies. J Am Heart Assoc. (2019) 8:e013602. 10.1161/JAHA.119.013602
16.
Blitshteyn S . Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS). Lupus. (2015) 24:1364–9. 10.1177/0961203315587566
17.
Li H Yu X Liles C Khan M Vanderlinde-Wood M Galloway A et al . Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. (2014) 3:e000755. 10.1161/JAHA.113.000755
18.
Thieben MJ Sandroni P Sletten DM Benrud-Larson LM Fealey RD Vernino S et al . Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. (2007) 82:308–13. 10.4065/82.3.308
19.
Tanking C Lakkananurak C Srisakvarakul C Jitpreeda A Threechod K Sukitpunyaroj D . Postural orthostatic tachycardia syndrome and other autonomic dysfunctions following COVID-19: Incidence, characteristics, and associated factors. J Arrhythm. (2024) 40:230–6. 10.1002/joa3.13001
20.
Cantrell C Reid C Walker CS Stallkamp Tidd SJ Zhang R Wilson R . Post-COVID postural orthostatic tachycardia syndrome (POTS): a new phenomenon. Front Neurol. (2024) 15:1297964. 10.3389/fneur.2024.1297964
21.
Seeley MC Gallagher C Ong E Langdon A Chieng J Bailey D et al . High Incidence of autonomic dysfunction and postural orthostatic tachycardia syndrome in patients with long COVID: implications for management and health care planning. Am J Med. (2025) 138:354–61.e1. 10.1016/j.amjmed.2023.06.010
22.
Dulal D Maraey A Elsharnoby H Chacko P Grubb B . Impact of COVID-19 Pandemic on the incidence and prevalence of postural orthostatic tachycardia syndrome. Eur Heart J Qual Care Clin Outcomes. (2025) 11:698–704. 10.1016/S0735-1097(25)01061-7
23.
Teodorescu DL Kote A Reaso JN Rosenberg C Liu X Kwan AC et al . Postural orthostatic tachycardia syndrome after COVID-19 vaccination. Heart Rhythm. (2024) 21:74–81. 10.1016/j.hrthm.2023.09.012
24.
Tv P Tran TT Hao HT Hau NTH Jain N Reinis A . Postural orthostatic tachycardia syndrome-like symptoms following COVID-19 vaccination: an overview of clinical literature. Hum Antib. (2023) 31:9–17. 10.3233/HAB-220013
25.
Kwan AC Ebinger JE Wei J Le CN Oft JR Zabner R et al . Apparent risks of postural orthostatic tachycardia syndrome diagnoses after COVID-19 vaccination and SARS-CoV-2 infection. Nat Cardiovasc Res. (2022) 1:1187–94. 10.1038/s44161-022-00177-8
26.
RECOVER-AUTONOMIC: A Platform Protocol for Evaluation of Interventions for Autonomic Dysfunction in Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) (2024) . Available online at: http://clinicaltrials.gov/study/NCT06305780 (Accessed July 5, 2025).
27.
Wong AC Devason AS Umana IC Cox TO Dohnalova L Litichevskiy L et al . Serotonin reduction in post-acute sequelae of viral infection. Cell. (2023) 186:4851–67.e20. 10.1016/j.cell.2023.09.013
28.
Geng LN Erlandson KM Hornig M Letts R Selvaggi C Ashktorab H et al . 2024 Update of the RECOVER-Adult long COVID research index. Jama. (2025) 333:694–700. 10.1001/jama.2024.24184
29.
Sunami Y Sugaya K Takahashi K . G protein-coupled receptors related to autoimmunity in postural orthostatic tachycardia syndrome. Immunol Med. (2024) 48:141–8. 10.1080/25785826.2024.2370079
30.
Thaper D Prabha V . Molecular mimicry: an explanation for autoimmune diseases and infertility. Scand J Immunol. (2018):e12697. 10.1111/sji.12697
31.
Bona CA . Molecular mimicry of self-antigens. Immunol Ser. (1991) 55:239–46.
32.
Maguire C Wang C Ramasamy A Fonken C Morse B Lopez N et al . Molecular mimicry as a mechanism of viral immune evasion and autoimmunity. Nat Commun. (2024) 15:9403. 10.1038/s41467-024-53658-8
33.
Proal AD VanElzakker MB . Long COVID or Post-acute Sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. (2021) 12:698169. 10.3389/fmicb.2021.698169
34.
Marino Gammazza A Legare S Lo Bosco G Fucarino A Angileri F Oliveri M et al . Molecular mimicry in the post-COVID-19 signs and symptoms of neurovegetative disorders?Lancet Microbe. (2021) 2:e94. 10.1016/S2666-5247(21)00033-1
35.
Antoniak S Mackman N . Platelets and viruses. Platelets. (2021) 32:325–30. 10.1080/09537104.2021.1887842
36.
Schrottmaier WC Salzmann M Badrnya S Mussbacher M Kral-Pointner JB Morava S et al . Platelets mediate serological memory to neutralize viruses in vitro and in vivo. Blood Adv. (2020) 4:3971–6. 10.1182/bloodadvances.2020001786
37.
Assinger A . Platelets and infection - an emerging role of platelets in viral infection. Front Immunol. (2014) 5:649. 10.3389/fimmu.2014.00649
38.
Dupuis A Bordet JC Eckly A Gachet C . Platelet delta-storage pool disease: an update. J Clin Med. (2020) 9:2508. 10.3390/jcm9082508
39.
Brenner B Harney JT Ahmed BA Jeffus BC Unal R Mehta JL et al . Plasma serotonin levels and the platelet serotonin transporter. J Neurochem. (2007) 102:206–15. 10.1111/j.1471-4159.2007.04542.x
40.
Beirat AF Menakuru SR Kalra M . Platelet Delta (delta)-storage pool deficiency: a case series and review of the literature. Hematol Rep. (2023) 15:405–10. 10.3390/hematolrep15030041
41.
Gunning WT Yoxtheimer L Smith MR . Platelet aggregation assays do not reliably diagnose platelet delta granule storage pool deficiency. J Hematol. (2021) 10:196–201. 10.14740/jh832
42.
Brunet J Badin M Chong M Iyer J Tasneem S Graf L et al . Bleeding risks for uncharacterized platelet function disorders. Res Pract Thromb Haemost. (2020) 4:799–806. 10.1002/rth2.12374
43.
Grech J Chan MV Ochin C Lachapelle A Thibord F Schneider Z et al . Serotonin-affecting antidepressant use in relation to platelet reactivity. Clin Pharmacol Ther. (2022) 111:909–18. 10.1002/cpt.2517
44.
Gómez-Moyano E Rodríguez-Capitán J Gaitán Román D Reyes Bueno JA Villalobos Sánchez A Espíldora Hernández F et al . Postural orthostatic tachycardia syndrome and other related dysautonomic disorders after SARS-CoV-2 infection and after COVID-19 messenger RNA vaccination. Front Neurol. (2023) 14:1221518. 10.3389/fneur.2023.1221518
45.
Blitshteyn S . Postural tachycardia syndrome following human papillomavirus vaccination. Eur J Neurol. (2014) 21:135–9. 10.1111/ene.12272
46.
Bernal KDE Whitehurst CB . Incidence of Epstein-Barr virus reactivation is elevated in COVID-19 patients. Virus Res. (2023) 334:199157. 10.1016/j.virusres.2023.199157
47.
Vojdani A Vojdani E Saidara E Maes M . Persistent SARS-CoV-2 infection, EBV, HHV-6 and other factors may contribute to inflammation and autoimmunity in long COVID. Viruses. (2023) 15:400. 10.3390/v15020400
48.
Gold JE Okyay RA Licht WE Hurley DJ . Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens. (2021) 10:763. 10.3390/pathogens10060763
49.
Chen T Song J Liu H Zheng H Chen C . Positive Epstein-Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci Rep. (2021) 11:10902. 10.1038/s41598-021-90351-y
50.
Low PA Sandroni P Joyner M Shen WK . Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol. (2009) 20:352–8. 10.1111/j.1540-8167.2008.01407.x
51.
Gresele P Falcinelli E Bury L Pecci A Alessi MC Borhany M et al . The ISTH bleeding assessment tool as predictor of bleeding events in inherited platelet disorders: communication from the ISTH SSC subcommittee on platelet physiology. J Thromb Haemost. (2021) 19:1364–71. 10.1111/jth.15263
52.
Jain S Zhang S Acosta M Malone K Kouides P Zia A . Prospective evaluation of ISTH-BAT as a predictor of bleeding disorder in adolescents presenting with heavy menstrual bleeding in a multidisciplinary hematology clinic. J Thromb Haemost. (2020). 10.1111/jth.14997
53.
Gebhart J Hofer S Kaider A Rejtö J Ay C Pabinger I . The discriminatory power of bleeding assessment tools in adult patients with a mild to moderate bleeding tendency. Eur J Intern Med. (2020) 78:34–40. 10.1016/j.ejim.2020.04.023
54.
Magnay JL O'Brien S Gerlinger C Seitz C . Pictorial methods to assess heavy menstrual bleeding in research and clinical practice: a systematic literature review. BMC Womens Health. (2020) 20:24. 10.1186/s12905-020-0887-y
55.
Janssen CA Scholten PC Heintz AP A . simple visual assessment technique to discriminate between menorrhagia and normal menstrual blood loss. Obstet Gynecol. (1995) 85:977–82. 10.1016/0029-7844(95)00062-V
56.
Ruška B Pavičić T Pavlović I Junaković A Adamec I Crnošija L et al . Performance of the COMPASS-31 questionnaire with regard to autonomic nervous system testing results and medication use: a prospective study in a real-life setting. Neurol Sci. (2018) 39:2079–84. 10.1007/s10072-018-3542-8
57.
Larsen NW Stiles LE Shaik R Schneider L Muppidi S Tsui CT et al . Characterization of autonomic symptom burden in long COVID: a global survey of 2,314 adults. Front Neurol. (2022) 13:1012668. 10.3389/fneur.2022.1012668
58.
Shaw BH Stiles LE Bourne K Green EA Shibao CA Okamoto LE et al . The face of postural tachycardia syndrome - insights from a large cross-sectional online community-based survey. J Intern Med. (2019) 286:438–48. 10.1111/joim.12895
59.
White JG . Use of the electron microscope for diagnosis of platelet disorders. Semin Thromb Hemost. (1998) 24:163–8. 10.1055/s-2007-995836
60.
Gunning WT Raghavan M Calomeni EP Turner JN Roysam B et al . A Morphometric analysis of platelet dense granules of patients with unexplained bleeding: a new entity of delta-microgranular storage pool deficiency. J Clin Med. (2020) 9:1734. 10.3390/jcm9061734
61.
Mathias CJ Low DA Iodice V Owens AP Kirbis M Grahame R . Postural tachycardia syndrome–current experience and concepts. Nat Rev Neurol. (2011) 8:22–34. 10.1038/nrneurol.2011.187
62.
Minhas R Bharadwaj AS . COVID-19-induced postural orthostatic tachycardia syndrome and dysautonomia. Cureus. (2023) 15:e40235. 10.7759/cureus.40235
63.
De Paschale M Clerici P . Serological diagnosis of Epstein-Barr virus infection: problems and solutions. World J Virol. (2012) 1:31–43. 10.5501/wjv.v1.i1.31
64.
Harris CI . COVID-19 increases the prevalence of postural orthostatic tachycardia syndrome: what nutrition and dietetics practitioners need to know. J Acad Nutr Diet. (2022) 122:1600–5. 10.1016/j.jand.2022.06.002
65.
Eslami M Mollazadeh R Mirshafiee S Sehat P Alizadeh F Emkanjoo Z et al . Postural orthostatic tachycardia syndrome and orthostatic hypotension post COVID-19. Infect Disord Drug Targets. (2023) 23:e100622205846. 10.2174/1871526522666220610143504
66.
Park JH Park S Kim NH Lee Y Chang Y Song TJ . Postural orthostatic tachycardia syndrome associated with COVID-19: a narrative review. Medicina. (2024) 60:1325. 10.3390/medicina60081325
67.
Meenakshisundaram C Moustafa A Ranabothu M Maraey A Grubb B . Impact of COVID-19 infection on baseline autonomic symptoms in patients with preexisting postural tachycardia syndrome and orthostatic intolerance: a retrospective study. Am J Med Sci. (2024) 367:323–7. 10.1016/j.amjms.2023.12.011
68.
Angeli AM Salonen BR Ganesh R Hurt RT Abdalrhim A Mueller M et al . Symptom presentation by phenotype of postural orthostatic tachycardia syndrome. Sci Rep. (2024) 14:205. 10.1038/s41598-023-50886-8
69.
Robertson D . The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. (1999) 317:75–7. 10.1097/00000441-199902000-00001
70.
Raj SR Bourne KM Stiles LE Miglis MG Cortez MM Miller AJ et al . Postural orthostatic tachycardia syndrome (POTS): Priorities for POTS care and research from a 2019 National Institutes of Health Expert Consensus Meeting - Part 2. Auton Neurosci. (2021) 235:102836. 10.1016/j.autneu.2021.102836
71.
Davis HE McCorkell L Vogel JM Topol EJ . Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. 10.1038/s41579-022-00846-2
72.
Boris JR . The role of the cardiologist in the evaluation of dysautonomia. Cardiol Young. (2010) 3:135–9. 10.1017/S1047951110001198
73.
Kanjwal Y Kosinski D Grubb BP . The postural orthostatic tachycardia syndrome: definitions, diagnosis, and management. Pacing Clin Electrophysiol. (2003) 26:1747–57. 10.1046/j.1460-9592.2003.t01-1-00262.x
74.
Yahya AS Khawaja S . Psychiatric disorder in postural orthostatic tachycardia syndrome and Ehlers-Danlos syndrome-hypermobility type. Prim Care Companion CNS Disord. (2020) 22. 10.4088/PCC.20nr02644
75.
Raj V Haman KL Raj SR Byrne D Blakely RD Biaggioni I et al . Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry. (2009) 80:339–44. 10.1136/jnnp.2008.144360
76.
Taquet M Sillett R Zhu L Mendel J Camplisson I Dercon Q et al . Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. (2022) 9:815–27. 10.1016/S2215-0366(22)00260-7
77.
Taquet M Geddes JR Husain M Luciano S Harrison PJ . 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. (2021) 8:416–27. 10.1016/S2215-0366(21)00084-5
78.
Soroken C Posfay-Barbe KM Caflisch M . Z'Graggen WJ. Postural tachycardia syndrome among adolescents. Arch Pediatr. (2022) 29:398–403. 10.1016/j.arcped.2022.03.006
79.
Sebastian SA Co EL Panthangi V Jain E Ishak A Shah Y et al . Postural orthostatic tachycardia syndrome (POTS): an update for clinical practice. Curr Probl Cardiol. (2022) 47:101384. 10.1016/j.cpcardiol.2022.101384
80.
Vernino S Bourne KM Stiles LE Grubb BP Fedorowski A Stewart JM et al . Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting - Part 1. Auton Neurosci. (2021) 235:102828. 10.1016/j.autneu.2021.102828
81.
Fedorowski A Li H Yu X Koelsch KA Harris VM Liles C et al . Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace. (2017) 19:1211–9. 10.1093/europace/euw154
82.
Robertson D Flattem N Tellioglu T Carson R Garland E Shannon JR et al . Familial orthostatic tachycardia due to norepinephrine transporter deficiency. Ann N Y Acad Sci. (2001) 940:527–43. 10.1111/j.1749-6632.2001.tb03703.x
83.
Rubin R . Large cohort study finds possible association between postural orthostatic tachycardia syndrome and COVID-19 vaccination but far stronger link with SARS-CoV-2 infection. JAMA. (2023) 329:454–6. 10.1001/jama.2023.0050
84.
Raj SR Arnold AC Barboi A Claydon VE Limberg JK Lucci VM et al . Long-COVID postural tachycardia syndrome: an American autonomic society statement. Clin Auton Res. (2021) 31:365–8. 10.1007/s10286-021-00798-2
85.
Dorgalaleh A . Bleeding and bleeding risk in COVID-19. Semin Thromb Hemost. (2020) 46:815–8. 10.1055/s-0040-1713434
86.
Turner S Khan MA Putrino D Woodcock A Kell DB Pretorius E . Long COVID: pathophysiological factors and abnormalities of coagulation. Trends Endocrinol Metab. (2023) 34:321–44. 10.1016/j.tem.2023.03.002
87.
Coffin ST Black BK Biaggioni I Paranjape SY Orozco C Black PW et al . Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm. (2012) 9:1484–90. 10.1016/j.hrthm.2012.05.002
88.
Kell DB Khan MA Kane B Lip GYH Pretorius E . Possible role of fibrinaloid microclots in postural orthostatic tachycardia syndrome (POTS): focus on long COVID. J Pers Med. (2024) 14:170. 10.3390/jpm14020170
89.
Kell DB Pretorius E . Are fibrinaloid microclots a cause of autoimmunity in Long Covid and other post-infection diseases?Biochem J. (2023) 480:1217–40. 10.1042/BCJ20230241
90.
Rinker L Zadzilka N Kanjwal M Karabin B Boehm K Grubb B et al . Platelet delta granule and serotonin concentrations are decreased in patients with postural orthostatic tachycardia syndrome. Blood. (2009) 114:995. 10.1182/blood.V114.22.2419.2419
Summary
Keywords
postural orthostatic tachycardia syndrome (POTS), platelets, storage pool deficiency, COVID-19 long haulers, post-acute sequela of COVID-19 (PASC)
Citation
Gunning WT III, Khan S, Spatafore JW, Karabin BL and Grubb BP (2025) Postural orthostatic tachycardia syndrome in post-COVID-19 long-hauler patients is associated with platelet storage pool deficiency. Front. Med. 12:1560120. doi: 10.3389/fmed.2025.1560120
Received
13 January 2025
Accepted
22 August 2025
Published
11 September 2025
Volume
12 - 2025
Edited by
Svetlana Blitshteyn, University at Buffalo, United States
Reviewed by
Lawrence Afrin, Aim Center for Personalized Medicine, United States
Lesley Kavi, Birmingham City University, United Kingdom
Updates
Copyright
© 2025 Gunning, Khan, Spatafore, Karabin and Grubb.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William T. Gunning III william.gunning@utoledo.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.