- 1Department of Ophthalmology, Jinan Second People’s Hospital, Jinan, China
- 2Nova Southeastern University College of Optometry, Miami, FL, United States
Purpose: This study aimed to evaluate the surgical treatment of autosomal recessive bestrophinopathy (ARB) combined with angle-closure glaucoma (ACG) through a retrospective case series.
Methods: The treatment of three patients with ACG secondary to ARB was reviewed. The patients were admitted to the Department of Ophthalmology of Jinan Second People’s Hospital from April 2023 to January 2024. Their conditions, treatments, and outcomes were extracted from the medical records and analyzed.
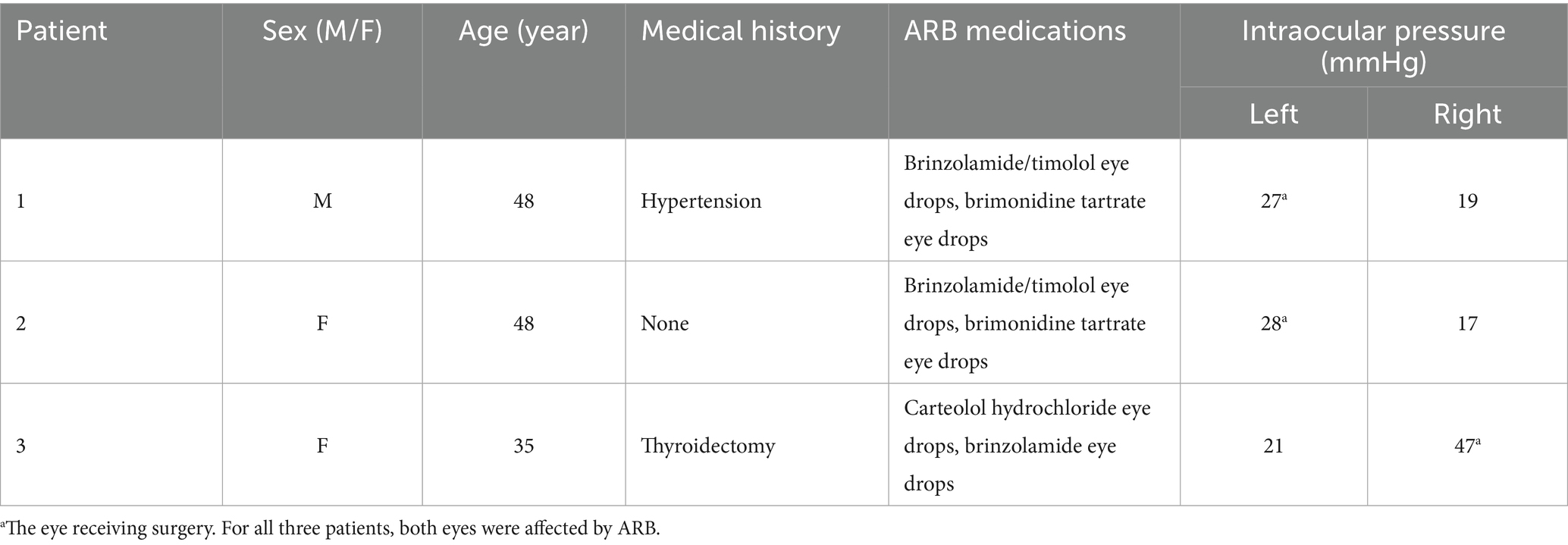
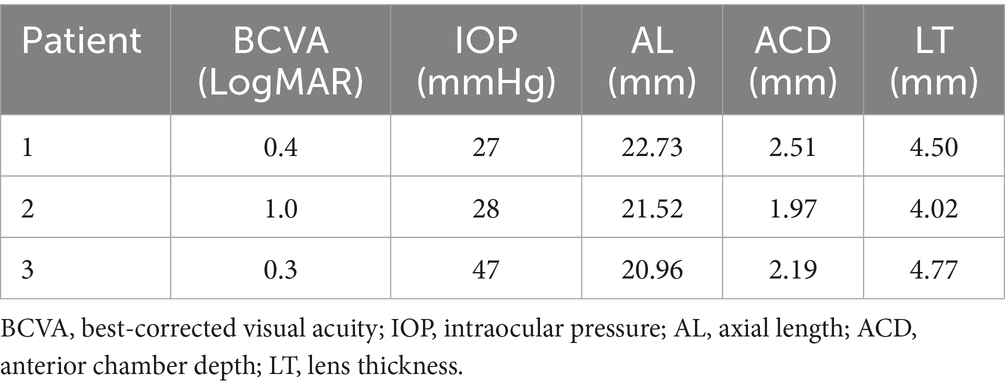
Results: The patients were 48, 48, and 35 years old at the time of surgery. All had bilateral ARB and underwent surgery in the eye more severely affected by ACG. Topical eye drops failed to control the intraocular pressure (IOP), which measured 27, 28, and 47 mmHg before the surgery. The affected eyes also exhibited a shorter axial length (AL) and shallower anterior chamber depth (ACD). The ALs of the surgical eyes measured 22.73 mm, 21.52 mm, and 20.96 mm, while the ACDs were 2.51 mm, 1.97 mm, and 2.19 mm, respectively. After receiving trabeculectomy, they all immediately developed malignant glaucoma, which could not be resolved by conservative treatment. Following a second surgery, which importantly included an anterior vitrectomy and posterior capsulotomy, the IOP was normal, the ACD was satisfactory, and visual function was preserved.
Conclusion: For ACG/ARB patients, the risk of developing malignant glaucoma after glaucoma surgery is very high. Surgical intervention, such as anterior vitrectomy, is needed to increase vitreous fluidity, eliminate vitreous block, assist the formation of the anterior chamber, and stabilize the IOP to save the patient’s vision. Long-term, close follow-up is essential due to the risk of recurrence in the operated eye and occurrence in the non-operated eyes.
Introduction
Autosomal recessive bestrophinopathy (ARB) is a rare retinal degenerative disorder (1). It is caused by biallelic mutations in the BEST1 gene, which are typically characterized by homozygous or compound heterozygous mutations. These result in a greater degree of gene function loss and more extensive lesions. Genetic testing is required to make the diagnosis. The primary lesion of ARB is located in the retinal pigment epithelium (RPE) which might cause the maldevelopment of both the anterior and posterior segments of the eyes characterized by more pronounced crowding of the anterior ocular segment including short axial length (AL), narrow angle, shallow anterior chamber (2), choroidal thickening (3, 4), as well as an increased risk of developing ACG compared to other variants (5, 6).
Current treatment options are primarily symptomatic and supportive, encompassing the correction of macular edema and the monitoring of glaucoma. Surgical interventions are crucial to managing the complications associated with ARB and preventing vision loss. For example, trabeculectomy can be used to reduce the intraocular pressure (IOP) and thus protect the optic nerve (7), and surgeries such as goniosynechialysis and laser peripheral iridotomy (LPI) can prevent further closure of the anterior chamber angle (8). However, the probability of malignant glaucoma and refractory shallow anterior chamber after these surgeries is very high (9). In complex situations, a combination of lens extraction, trabeculectomy, and anterior vitrectomy may be necessary. Experience sharing is particularly important for physicians to be educated and prepared to deal with ARB, as no unified clinical treatment guideline has been established.
Unfortunately, there are few reports on the occurrence of malignant glaucoma or refractory shallow anterior chambers after the surgical treatment of ARB patients, and there are even fewer reports on the treatment of such complications (10–12). In this article, we share our experiences by reviewing the treatment of three ARB patients with angle-closure glaucoma (ACG), who developed malignant glaucoma immediately after the initial surgery. We evaluated the relationship between postoperative complications (malignant glaucoma and refractory shallow anterior chamber) and the clinical characteristics of the patients.
Methods
This study was approved by the Ethics Committee of Jinan Second People’s Hospital (Approval No. JNEYE20240641) and complied with the Declaration of Helsinki. Written informed consent was obtained from all patients or their families.
Routine examinations included best-corrected visual acuity (BCVA), Goldmann applanation tonometry, slit lamp microscopy with goniometry, and fundus examination with autofluorescence imaging. In addition, the ocular biometry profile was assessed using an IOLMaster700 instrument (Carl Zeiss, Germany), and an average value of 10 measurements was taken. The visual field was measured using a Humphrey 750 visual field analyzer (Carl Zeiss, Germany) operating the 30-2 SITA Fast program. During the examination, patients with refractive errors were corrected with glasses, and the mean deviation (MD) was recorded. To determine the angle structure and quantify angle closure, the anterior segment structure was scanned using an SW3200L ultrasound biomicroscope (Tianjin Suowei Electronic Technology Co., Ltd., Tianjin, China) in a room with 60–70 Lux illumination. The retina and choroid were examined under natural pupil conditions by OCT using a Cirrus HD-OCT 4000 instrument (Carl Zeiss, Germany). For color fundus photography, the posterior pole of the fundus was photographed under natural pupil conditions using an Optos Daytona P200T ultra-widefield retinal imaging device (Optos, United Kingdom).
They were diagnosed with ARB based on typical fundus changes in the non-invasive retinal imaging examination, and the diagnosis was verified by their genetic testing results. Specifically, multifocal, subretinal, yellow-white deposits in the posterior pole were detected by color fundus photography. These lesions were mostly around the optic disc, near the retinal vascular arch, or in the macular area. Fundus autofluorescence (FAF) imaging with confocal scanning laser ophthalmoscopy revealed that vitelliform lesions exhibited hyperautofluorescence, distributed predominantly in the macular area, posterior pole, and equatorial regions. However, the fovea typically demonstrated hypoautofluorescence. The OCT showed strongly reflective deposits at the subretinal RPE level, which corresponded to the multifocal yellow-white deposits in the fundus (13).
The diagnosis with ACG is based on the following: (1) history of repeated elevation of IOP (>21 mmHg), with or without symptoms, (2) narrow or closed anterior angle observed in ultrasound biomicroscopy or gonioscope, and (3) glaucomatous optic disc changes and visual field damage.
Malignant glaucoma diagnosis in postoperative cases was confirmed through comprehensive evaluation of key clinical indicators: (1) observed shallowing or complete collapse of the anterior chamber morphology, (2) documented elevation of IOP measurements (>21 mmHg), and (3) definitive exclusion of potential choroidal pathologies, including effusion manifestations and suprachoroidal hemorrhagic events.
In the event of malignant glaucoma, immediate conservative treatment, including mydriasis, intraocular pressure-lowering therapy, and anti-inflammatory therapy, was initiated. If conservative treatment proves ineffective, prompt surgical intervention with anterior vitrectomy combined with posterior capsulotomy is performed. The management procedures were as follows: An infusion cannula was inserted through the limbal side port to maintain anterior chamber stability. A 23-gauge sclerotomy was created 3.5 mm posterior to the corneal limbus, followed by anterior vitrectomy and central posterior capsulotomy. The diameter of the posterior capsule incision was recommended to be approximately 3–4 mm. The cutting rate was set between 500 and 1,000 cuts per minute. Sufficient anterior vitreous was excised until significant anterior chamber deepening was observed and intraocular pressure returned to normal levels.
Results
Patient information
Table 1 summarizes the basic information of the three patients, including one 48-year-old man and two women aged 48 and 35. Patient 3 had bilateral LPI at another hospital 7 years ago. None of the patients had a family history of eye diseases, and using eye drops failed to control the IOP.
Exam findings
Routine exams and biometry
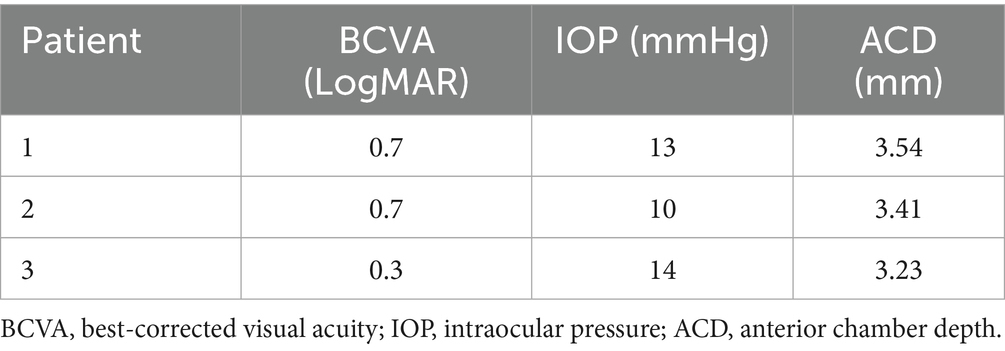
The slit lamp examination of all three patients showed that the cornea was transparent, the anterior chamber was shallow, the iris had a clear texture, the lens was in place, and there was no inflammation or posterior synechiae. For patient 3, the peripheral iridotomy incision was patent (Figure 1A). For all three patients, the eye scheduled for surgery was classified, according to the Scheie system, as grade IV and gonioscopically narrow. None had a visible anterior chamber angle structure, and the angle was always closed (Figure 1B). Their ALs were 22.73, 21.52, and 20.96 mm, and the ACDs were 2.51, 1.97, and 2.19 mm, respectively. The BCVA (LogMAR) before surgery was 0.4, 1.0, and 0.3, respectively, and the preoperative IOP was 27, 28, and 47 mmHg, respectively (Table 2).
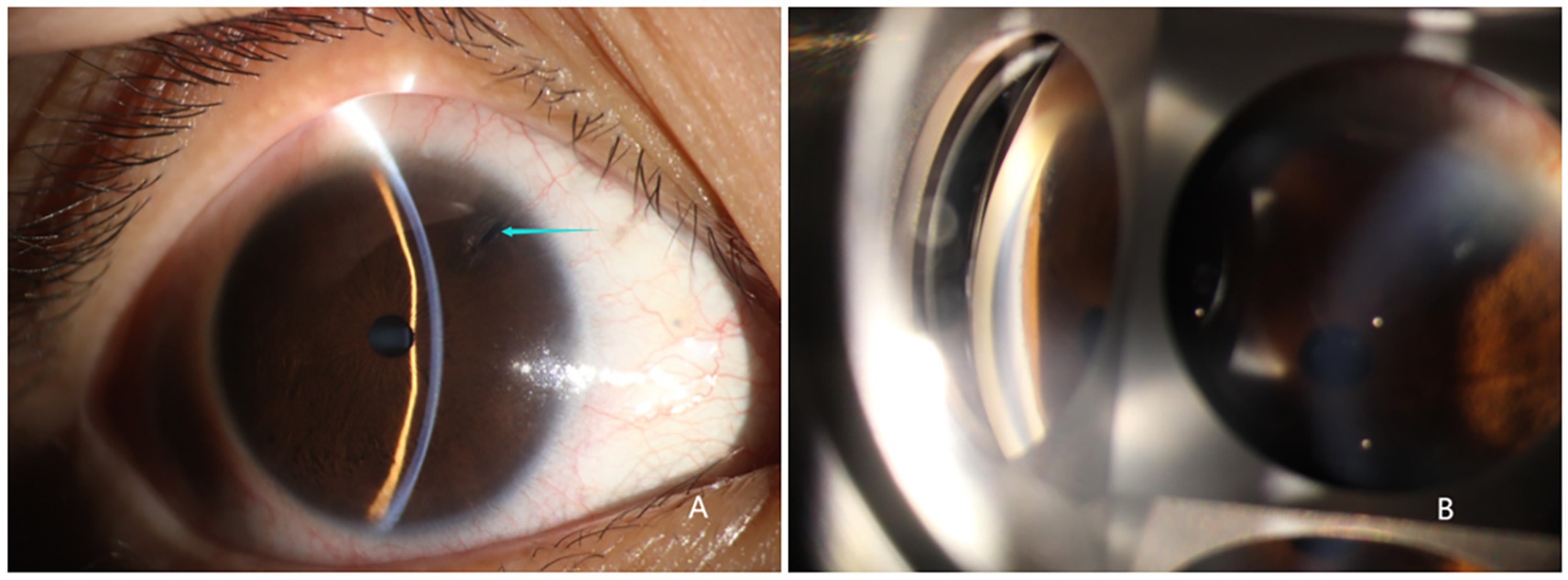
Figure 1. Slit lamp examination of the right eye of patient 3. (A) Transparent cornea, shallow anterior chamber, bulging iris, good patency of the incision from the prior iridotomy, round pupil with ~2 mm diameter post miosis, transparent lens in place. (B) Grade IV and gonioscopically narrow according to the Scheie classification. Angle structures were completely invisible, and the angle was closed.
Ultrasound biomicroscopy
All three patients presented with iris bulging, anterior displacement of the ciliary body, and obstruction of the scleral spur at the iris root. The lens was in a normal position. For patients 1 and 2, one quadrant of the chamber angle was open with an extremely narrow gap, and the rest was all closed. For patient 3, complete angle closure was observed in all four quadrants, and the peripheral iridotomy incision was patent at the 1 o’clock position (Figure 2).
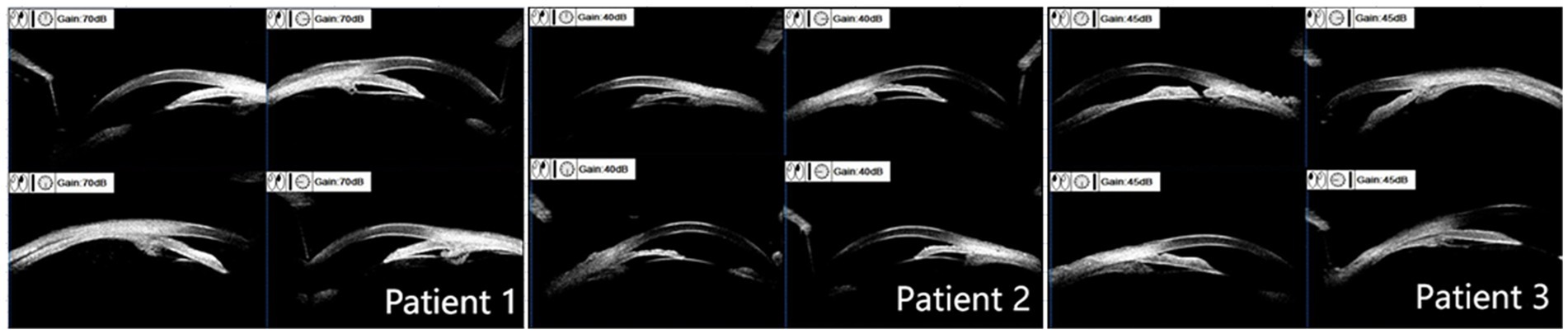
Figure 2. Ultrasound biomicroscope examination results. Patients 1 and 2: mild iris bulging, mild anterior displacement of the ciliary body, open temporal quadrant with an extremely narrow gap, all other quadrants closed, normal lens position. Patient 3: anterior displacement of the ciliary body in all four quadrants, scleral protrusion blocked by the iris root, chamber angle completely closed, patent peripheral iridotomy incision at the 1 o’clock position.
Optical coherence tomography
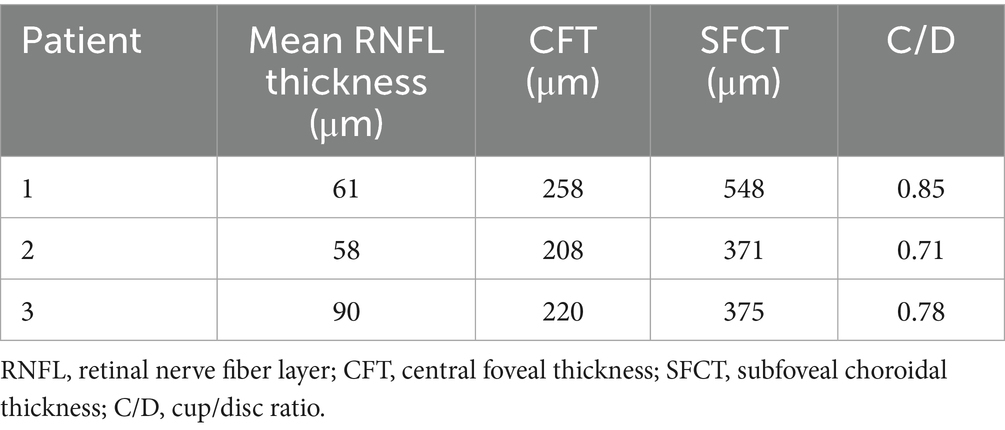
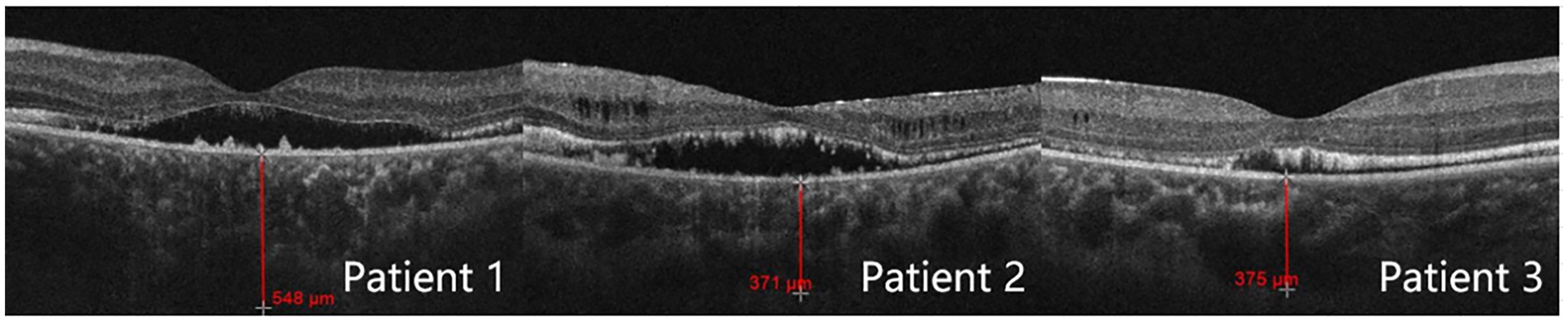
For all three patients, the OCT optic disc examinations revealed an increase in the cup/disc ratio and a decrease in the mean retinal nerve fiber layer (RNFL) thickness. The OCT macula examinations revealed that the central foveal thickness (CFT) increased, and the subfoveal choroidal thickness (SFCT) increased (Table 3). There was serous detachment of the foveal neuroretina, and the choroid thickened (Figure 3).
Color fundus photography
For all patients, multifocal subretinal yellow-white materials, which varied in number and size and appeared in the form of spots or clusters, were deposited in the posterior pole of both eyes. The autofluorescence images showed widespread and uneven enhancement or reduction. The fovea had weak fluorescence. With its strong fluorescence, the posterior pole could be clearly distinguished from the peripheral fundus that had normal fluorescence. On the boundary between the posterior pole and the peripheral fundus, there were multiple hyperfluorescent spots arranged in a nearly circular pattern (Figure 4).
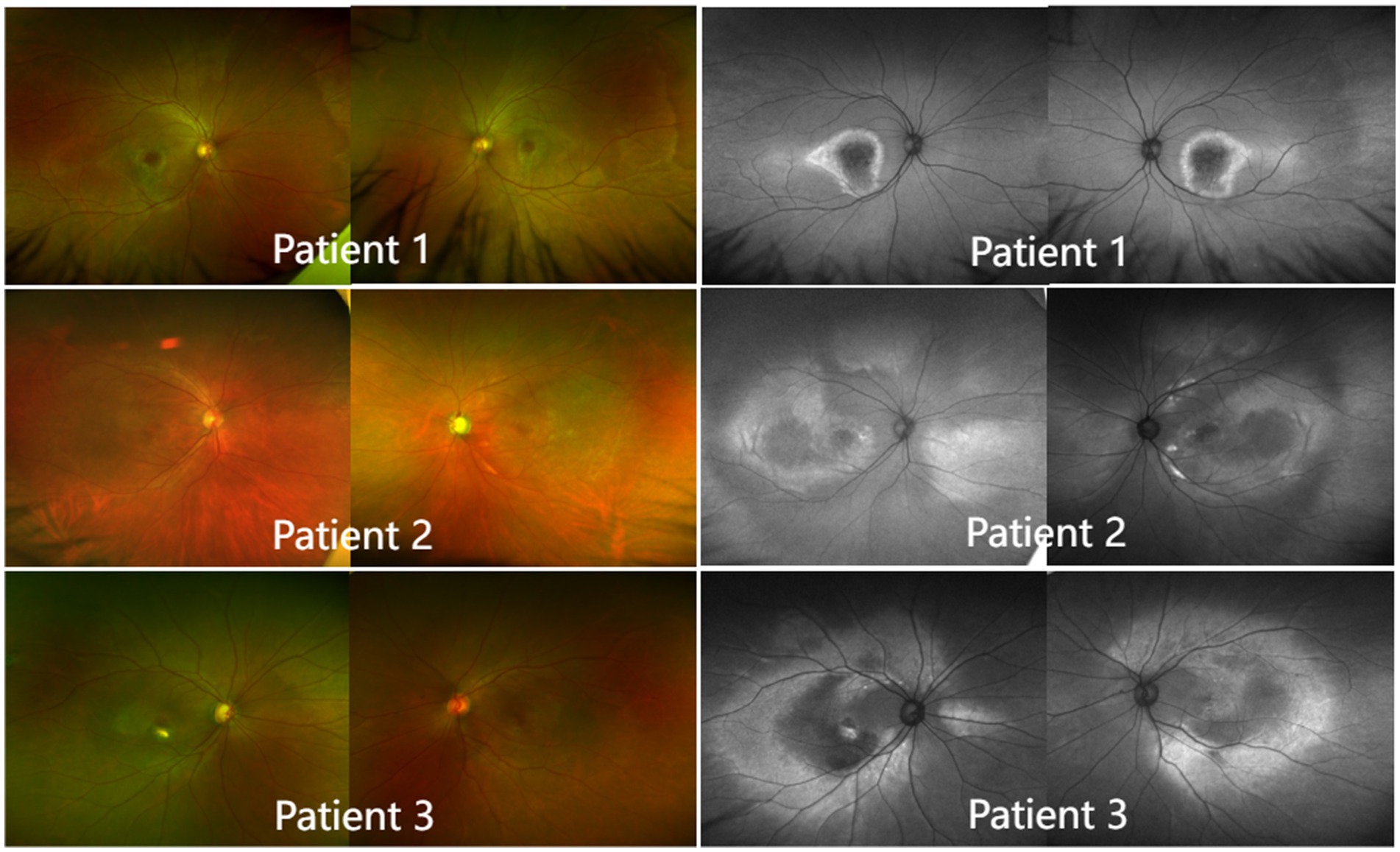
Figure 4. Color fundus photography and autofluorescence imaging (left). In all patients, multifocal subretinal yellow-white materials, which varied in number and size and appeared in the form of spots or clusters, were deposited in the posterior pole of both eyes. Autofluorescence imaging (right). The fovea had weak fluorescence. With its strong fluorescence, the posterior pole could be clearly distinguished from the peripheral fundus, which had normal fluorescence. At the boundary between the posterior pole and the peripheral fundus, there were multiple hyperfluorescent spots arranged in a nearly circular pattern.
Genetic testing
The type of genetic detection was whole exome. All patients had compound heterozygous mutations. Patient 1 detected two heterozygous variations in the BEST1 gene: c.481 + 1G > A/c.256G > T(p.A86S), patient 2 detected two variations in BEST1 gene: c.584C > T(p.A195V)/c.653G > A(p.R218H), and patient 3 detected two compound heterozygous variations in the BEST1 gene: c.584C > T(p.A195V)/c.763C > T(p.R255W).
Case summary
Patient 1 (48-year-old man) presented to our hospital with blurred vision in his left eye for 1 month. His diagnosis was later confirmed by genetic testing. Despite treatment with a triple topical antiglaucoma medication regimen, the IOP remained at 27 mmHg. Consequently, the patient underwent a compound trabeculectomy in the left eye. On the first day after the surgery, malignant glaucoma occurred with shallow ACD (grade II), and the IOP was 23 mmHg. Due to the clear crystalline lens status, the patient refused secondary surgical intervention and was treated conservatively, including reducing posterior chamber pressure, anti-inflammatory steroids, and mydriasis. The shallow ACD persisted (grades I to II). After 4 months of recurring symptoms and worsening medication compliance, the patient finally consented to a combined procedure that included lens extraction, IOL implantation, anterior vitrectomy, and posterior capsulotomy.
Patient 2 (48-year-old woman) presented to our hospital with a 2-year history of blurred vision in her left eye. Comprehensive ophthalmic evaluation and genetic testing established the diagnosis of ARB. Despite treatment with triple topical antiglaucoma medications, the IOP was controlled at 28 mmHg. Due to concurrent lens opacity, the patient initially underwent combined phacoemulsification with intraocular lens implantation and trabeculectomy in the left eye. On the first day after the surgery, malignant glaucoma occurred with shallow ACD (grade II), and the IOP was 25 mmHg. After being treated conservatively, including reducing posterior chamber pressure, anti-inflammatory steroids, and mydriasis, the shallow ACD persisted (grades I to II). On the 18th day after the initial surgery, she received an anterior vitrectomy and posterior capsulotomy.
Patient 3 (35-year-old woman) presented with a 7-year history of bilateral ACG previously diagnosed at an external hospital, having undergone bilateral Laser Peripheral Iridotomy (LPI). Despite treatment with two antiglaucoma medications, the IOP in her right eye remained elevated at 47 mmHg. She was diagnosed with ACG and ARB, and then underwent compound trabeculectomy in the right eye. On the first day after the surgery, malignant glaucoma occurred with shallow ACD (grade III), and the IOP was 35 mmHg. The conservative treatment was ineffective; the anterior chamber did not deepen, and secondary corneal fogging occurred (Figure 5). To avoid further exacerbation, on day 2 after the initial surgery, she received anterior vitrectomy and posterior capsulotomy, along with lens extraction and IOL implantation.
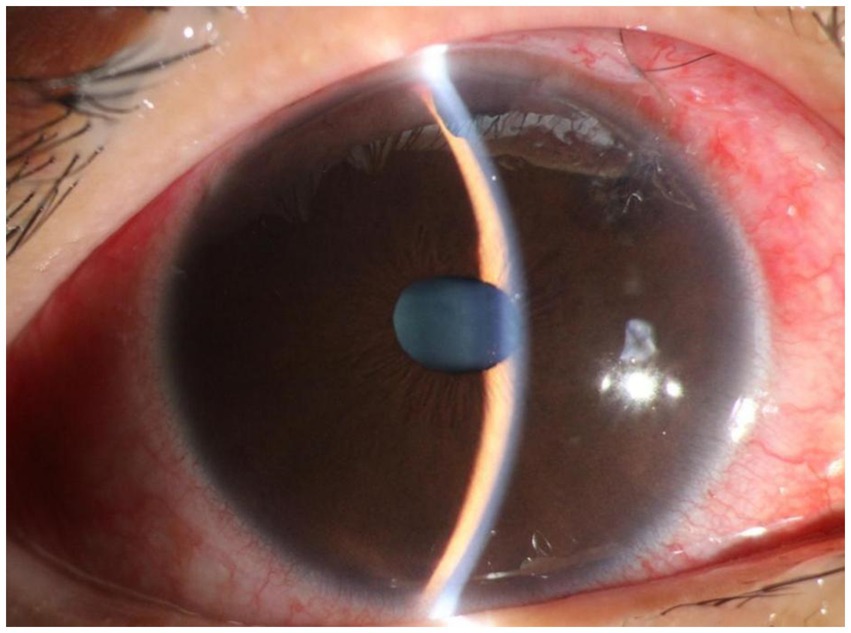
Figure 5. Slit lamp examination of patient 3 on the first day after compound trabeculectomy. The findings included conjunctival hyperemia, diffuse bulging of the filtering bleb above the conjunctiva, good incision apposition, sutures in place, transparent cornea, disappearance of the anterior chamber, iris bulging, patent superior iris incision, and transparent lens.
For all three patients, the anterior chamber was formed on the first day after the second surgery. During the next 3 months, the IOP remained stable, and the anterior chamber was well formed (Figure 6). The follow-up at 3 months after the surgery confirmed the efficacy of their treatment, as the visual function was preserved, the IOP was normal, and the ACD was good (Table 4).
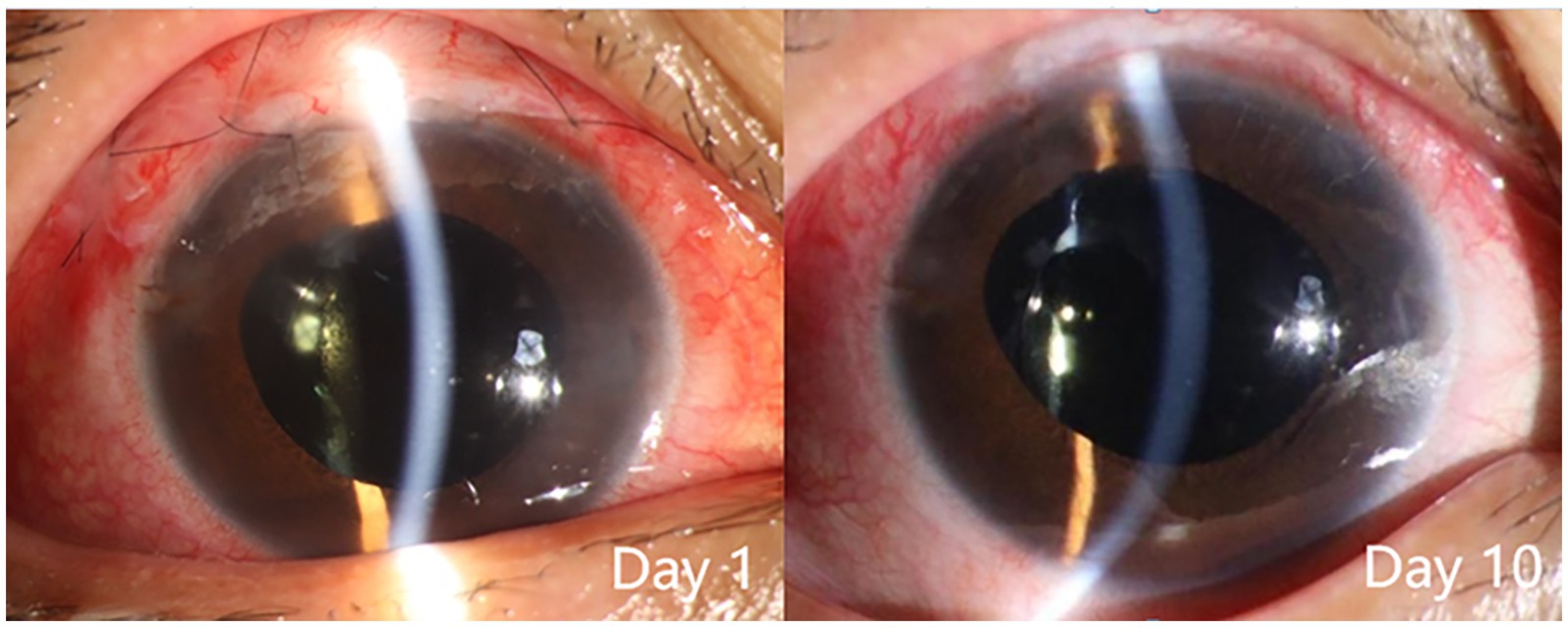
Figure 6. Slit lamp examination of patient 3 after the second surgery. The findings included: day 1, conjunctival hyperemia, good alignment of the conjunctival incision, sutures in place, diffuse elevation of the upper filtering bleb, transparent cornea, normal anterior chamber depth, moderate pupil dilation due to medication, transparent intraocular lens in place, and incision of the central posterior capsule; day 10, reduced conjunctival hyperemia, sutures removed, diffuse elevation of the upper filtering bleb, transparent cornea, normal anterior chamber depth, moderate pupil dilation due to medication, transparent intraocular lens in place, and transparent area of central posterior capsulotomy.
The non-surgical eyes of these three patients were treated with topical anti-glaucoma medications, and their IOP was effectively controlled within the normal range. Considering that their current IOP was well-managed, surgical intervention was not currently warranted. However, close follow-up was required to monitor disease progression.
Discussion
The three patients in this study were aged 48, 48, and 35, respectively. Patient 3 had the shortest AL and the earliest onset. Lin et al. (14) found that 19.3% of ACG patients who are less than 40 years old have ARB, but 44.8% of these patients are misdiagnosed as primary ACG, uveitis, or macular edema. As in the fundus examination without mydriasis, the subretinal vitelliform lesions can be easily overlooked due to the closed angle. We thus recommend that physicians apply a comprehensive evaluation when diagnosing young ACG patients and bear in mind the possibility of ARB, especially when a dilated eye examination is not feasible.
Genetic testing is crucial for a definitive diagnosis. The three patients had compound heterozygous mutations. According to the “ACMG (American College of Medical Genetics and Genomics) Standards and Guidelines for the Classification of Genetic Variants” (15), the variants “c.481 + 1G > A,” “c.584C > T(p.A195V),” and “c.763C > T(p.R255W)” are classified as “pathogenic variants.” The variants “c.256G > T(p.A86S)” and “c.653G > A(p.R218H)” are classified as a “likely pathogenic variant.” Literature reports indicate that these variants have been detected in multiple patients with ARB presenting with similar symptoms. Included are several homozygous cases or compound heterozygous relationships with known (likely) pathogenic variants, such as c.830C > T, c.73C > T, c.598C > T, c.1066C > T, and c.38G > A (2, 16, 17).
With anterior segment abnormal development, it is particularly difficult to treat the ACG associated with ARB. LPI and cataract extraction may not reduce IOP satisfactorily (5, 8, 18). In this study, patient 3 experienced failure in IOP control following bilateral LPI. Trabeculectomy is generally the first-line surgical treatment for ACG in the Chinese population, especially for patients with >180° peripheral anterior synechiae and no significant lens opacities (19–21). However, for ACG/ARB patients, trabeculectomy alone tends to cause postsurgical malignant glaucoma (21, 22). Clear lens extraction is a rational surgical option when malignant glaucoma occurs (23), but most ACG/ARB patients are young and often reluctant to accept clear lens extraction initially. Hence, it is imperative to thoroughly communicate with the patients about their condition and inform them of the likelihood of a second surgery. In this study, both patients 1 and 3 had healthy, clear lenses, and they refused to undergo clear lens extraction along with trabeculectomy. Unfortunately, a refractory shallow anterior chamber occurred in both patients on the first day after the initial surgery.
Moreover, some studies have suggested that even combining lens removal with trabeculectomy may not suffice in preventing postoperative refractory shallow anterior chamber (24). Of the three patients described in this study, the lens thickness was within the normal range in all three cases. Patient 2 underwent lens removal along with trabeculectomy, but still developed malignant glaucoma after the initial surgery. We thus speculate that, for ACG/ARB patients, the angle closure mechanism may not be due to pupillary blockage upon lens enlargement. Instead, the culprit may be the pressure from the posterior segment, which pushes forward the iris diaphragm and thus reduces the ACD (8). Quigley et al. (25) pointed out that the thickening choroid pushes the molded vitreous body forward, creating a high-pressure environment and causing vitreous conduction. The choroidal thickness of patients 1, 2, and 3 was 548, 371, and 375 μm, respectively. The study by Huo et al. (26) revealed that the mean subfoveal choroidal thickness in healthy adults was 288.43 ± 74.60 μm for the 31–40 years group and 278.05 ± 72.87 μm for the 41–50 years group. Notably, the choroidal thickness in our three patients was significantly greater than that of age-matched healthy adults in the corresponding age groups. Since ARB patients are usually young, the vitreous has a high degree of integrity and a low degree of liquefaction, and choroidal thickening passes greater pressure onto the anterior chamber. Once the anterior vitreous contacts the ciliary body and the lens, the effective diffusion area decreases, and the reflux of the aqueous humor occurs. Surgeries such as trabeculectomy create additional outflow channels and can further increase the pressure gradient between the anterior and posterior segments. The lens-iris diaphragm moves further forward and causes angle closure. At this point, the aqueous humor does not have anywhere else to go other than entering the vitreous cavity. Therefore, for patients, especially younger patients with a thicker choroid, glaucoma drainage surgery should be selected with extra caution since the probability of postoperative malignant glaucoma is very high.
For all three patients described in this study, a second surgery to correct the malignant glaucoma may require a combination of anterior vitrectomy, lens extraction, and posterior capsulotomy may be necessary. Following crystalline lens removal, the reduced volume of the intraocular lens and capsular bag increases the ciliary-lenticular space. Posterior capsulotomy disrupts the relative integrity of the lens capsule. Anterior vitrectomy disrupts the integrity of the anterior hyaloid membrane and vitreous block (27). Combined with the communicating effects of peripheral iridotomy and posterior capsulotomy, this approach establishes an antero-posterior communication (28). For most malignant glaucoma patients, this combined surgical approach demonstrates satisfactory therapeutic outcomes (29). Studies have reported a surgical success rate of 64% in such cases (30).
Shi et al. contended that the abnormalities in the ciliary body-zonules-crystalline lens-hyaloid-anterior vitreous (CZLHV) complex play a key role in the development of malignant glaucoma (31). The surgical procedure of irido-zonulo-hyaloid-vitrectomy (IZHV), which directly targets the CZLHV complex, has become a commonly used method for the treatment of malignant glaucoma. However, when only IZHV was performed, the postoperative recurrence rate remained as high as 66%. Some scholars have postulated that this recurrence might be attributed to insufficient anterior vitreous volume reduction, potentially leading to recurrent obstruction of the aqueous outflow pathways by residual vitreous humor (32). Parames et al. (9) reported that all five cases of BEST1 gene-associated glaucoma patients developed malignant glaucoma following filtration surgery, which were effectively managed through combined IZHV along with pars plana vitrectomy (PPV). Therefore, we propose that regardless of the surgical approach adopted, particular attention should be paid to achieving sufficient excision of the anterior vitreous to prevent residual vitreous displacement caused by posterior pressure due to elevated intravitreal pressure and recurrent malignant glaucoma. Tian et al. (33) applied transscleral cyclophotocoagulation (TSCPC) to treat ARB patients who developed malignant glaucoma after the combined surgery of phacoemulsification, IOL implantation, and goniosynechialysis (34). These findings further substantiate the crucial role of improving anterior vitreous liquefaction with higher motility in the therapeutic strategy for malignant glaucoma.
In summary, we reviewed the treatment of three ARB patients with ACG, with a focus on the management of postoperative malignant glaucoma. We recommend that physicians carefully select the suitable treatment for young ACG patients, who are more likely to have secondary ACG, and perform thorough fundus examinations to rule out undiagnosed genetic diseases. For ACG/ARB patients receiving trabeculectomy, with or without concomitant lens removal, there is a very high risk of postoperative malignant glaucoma, and improving the fluidity of the anterior vitreous via combined anterior vitrectomy, diode laser TSCPC, or IZHV may be necessary. We believe that comprehensive evaluations and thorough communication are key for ophthalmologists when treating young ACG patients without cataracts. Simultaneously, long-term, close follow-up is also necessary to monitor the condition of both eyes in patients, to timely detect changes in IOP, and to ensure the visual function of patients over an extended period. The limitation of this study lies in the small sample size, and we anticipate collecting more cases in future studies to provide clinically relevant insights.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Ethics Committee of Jinan Second People’s Hospital (Approval No. JNEYE20240641) and complied with the Declaration of Helsinki. Written informed consent was obtained from all patients or their families. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft. CL: Data curation, Formal analysis, Supervision, Validation, Writing – review & editing. WL: Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. MZ: Formal analysis, Validation, Writing – review & editing. SR: Validation, Writing – review & editing. LG: Validation, Writing – review & editing. ZJ: Conceptualization, Formal analysis, Investigation, Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the Jinan Science and Technology Bureau under the Jinan Scientific and Technological Innovation in Clinical Medicine Program (Grant No. 202328008) and the Young Elite Sponsorship Program of Shandong Provincial Medical Association (2024-JS-0136).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Burgess, R, Millar, ID, Leroy, BP, Urquhart, JE, Fearon, IM, De Baere, E, et al. Biallelic mutation of Best1 causes a distinct retinopathy in humans. Am J Hum Genet. (2008) 82:19–31. doi: 10.1016/j.ajhg.2007.08.004
2. Luo, J, Lin, M, Guo, X, Xiao, X, Li, J, Hu, H, et al. Novel Best1 mutations and special clinical characteristics of autosomal recessive Bestrophinopathy in Chinese patients. Acta Ophthalmol. (2019) 97:247–59. doi: 10.1111/aos.13994
3. Wei, WB, Xu, L, Jonas, JB, Shao, L, Du, KF, Wang, S, et al. Subfoveal choroidal thickness: the Beijing eye study. Ophthalmology. (2013) 120:175–80. doi: 10.1016/j.ophtha.2012.07.048
4. Khojasteh, H, Azarmina, M, Ebrahimiadib, N, Daftarian, N, Riazi-Esfahani, H, Naraghi, H, et al. Autosomal recessive Bestrophinopathy: clinical and genetic characteristics of twenty-four cases. J Ophthalmol. (2021) 2021:1–11. doi: 10.1155/2021/6674290
5. Boon, CJ, van den Born, LI, Visser, L, Keunen, JE, Bergen, AA, Booij, JC, et al. Autosomal recessive bestrophinopathy: differential diagnosis and treatment options. Ophthalmology. (2013) 120:809–20. doi: 10.1016/j.ophtha.2012.09.057
6. Xuan, Y, Zhang, Y, Zong, Y, Wang, M, Li, L, Ye, X, et al. The clinical features and genetic Spectrum of a large cohort of Chinese patients with Vitelliform macular dystrophies. Am J Ophthalmol. (2020) 216:69–79. doi: 10.1016/j.ajo.2020.03.047
7. Fang, Y, Duan, X, Chen, L, Shi, J, Liu, J, Sun, Y, et al. Combination of trabeculectomy and primary pars Plana vitrectomy in the successful treatment of angle-closure Glaucoma with Best1 mutations: self-controlled case series. Ophthalmol Ther. (2022) 11:2271–84. doi: 10.1007/s40123-022-00580-1
8. Shi, Y, Tian, J, Han, Y, Oatts, J, and Wang, N. Pathogenic role of the vitreous in angle-closure Glaucoma with autosomal recessive Bestrophinopathy: a case report. BMC Ophthalmol. (2020) 20:271. doi: 10.1186/s12886-020-01543-5
9. Parameswarappa, DC, Balasubramnian, J, Kumar Padhy, S, Hansraj, S, Natarajan, R, Kannabiran, C, et al. Best1 associated Bestrophinopathies with angle closure and post-surgical malignant Glaucoma. Ophthalmic Genet. (2024) 45:571–82. doi: 10.1080/13816810.2024.2398827
10. Wittström, E, Ponjavic, V, Bondeson, ML, and Andréasson, S. Anterior segment abnormalities and angle-closure Glaucoma in a family with a mutation in the Best1 gene and Best Vitelliform macular dystrophy. Ophthalmic Genet. (2011) 32:217–27. doi: 10.3109/13816810.2011.567884
11. Low, S, Davidson, AE, Holder, GE, Hogg, CR, Bhattacharya, SS, Black, GC, et al. Autosomal dominant best disease with an unusual electrooculographic light rise and risk of angle-closure glaucoma: a clinical and molecular genetic study. Mol Vis. (2011) 17:2272–82. doi: 10.1126/scisignal.2002353
12. Crowley, C, Paterson, R, Lamey, T, McLaren, T, De Roach, J, Chelva, E, et al. Autosomal recessive Bestrophinopathy associated with angle-closure Glaucoma. Doc Ophthalmol. (2014) 129:57–63. doi: 10.1007/s10633-014-9444-z
13. Li, Q, Peng, XY, Wang, XN, Li, Y, and Tian, L. Analysis on the clinical and retinal imaging characteristics of autosomal recessive Bestrophinopathy. Zhonghua Yan Ke Za Zhi [Chin J Ophthalmol]. (2018) 54:263–9. doi: 10.3760/cma.j.issn.0412-4081.2018.04.007
14. Lin, SF, Xiao, H, Chen, LM, Ling, YL, Wei, W, Fang, L, et al. Clinical features of young inpatients with angle-closure Glaucoma. Zhonghua Yan Ke Za Zhi. (2022) 58:28–34. doi: 10.3760/cma.j.cn112142-20210301-00104
15. Richards, S, Aziz, N, Bale, S, Bick, D, Das, S, Gastier-Foster, J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
16. Hufendiek, K, Hufendiek, K, Jägle, H, Stöhr, H, Book, M, Spital, G, et al. Clinical heterogeneity in autosomal recessive Bestrophinopathy with Biallelic mutations in the BEST1 gene. Int J Mol Sci. (2020) 21:9353. doi: 10.3390/ijms21249353
17. Hardin, JS, Schaefer, GB, Sallam, AB, Williams, MK, and Uwaydat, S. A unique case series of autosomal recessive bestrophinopathy exhibiting multigenerational inheritance. Ophthalmic Genet. (2017) 38:570–4. doi: 10.1080/13816810.2017.1318926
18. Raja, V, Manthravadi, SK, and Anjanamurthy, R. Angle-closure Glaucoma associated with autosomal recessive Bestrophinopathy. Indian J Ophthalmol. (2022) 70:2657–8. doi: 10.4103/ijo.IJO_2411_21
19. He, M, Foster, PJ, Johnson, GJ, and Khaw, PT. Angle-closure Glaucoma in east Asian and European people. Different diseases? Eye (Lond). (2006) 20:3–12. doi: 10.1038/sj.eye.6701797
20. Ritch, R, Chang, BM, and Liebmann, JM. Angle closure in younger patients. Ophthalmology. (2003) 110:1880–9. doi: 10.1016/s0161-6420(03)00563-3
21. Liang, YB, Wang, NL, Rong, SS, and Thomas, R. Initial treatment for primary angle-closure Glaucoma in China. J Glaucoma. (2015) 24:469–73. doi: 10.1097/ijg.0000000000000075
22. Zhong, Y, Guo, X, Xiao, H, Luo, J, Zuo, C, Huang, X, et al. Flat anterior chamber after trabeculectomy in secondary angle-closure Glaucoma with Best1 gene mutation: case series. PLoS One. (2017) 12:e0169395. doi: 10.1371/journal.pone.0169395
23. Low, S, Mohamed, R, Ting, M, Webster, AR, and Garway-Heath, DF. The treatment of refractory angle-closure Glaucoma in a patient with X-linked juvenile Retinoschisis. Ophthalmic Genet. (2018) 39:625–7. doi: 10.1080/13816810.2018.1490961
24. Huang, XF, Tu, CS, Xing, DJ, Gan, DK, Xu, GZ, and Jin, ZB. R102w mutation in the Rs1 gene responsible for Retinoschisis and recurrent Glaucoma. Int J Ophthalmol. (2014) 7:169–72. doi: 10.3980/j.issn.2222-3959.2014.01.31
25. Quigley, HA, Friedman, DS, and Congdon, NG. Possible mechanisms of primary angle-closure and malignant Glaucoma. J Glaucoma. (2003) 12:167–80. doi: 10.1097/00061198-200304000-00013
26. Huo, Y, Guo, Y, Wang, H, Li, L, Cao, K, and Wang, N. Change regularity of adult subfoveal choroidal thickness with age and its influencing factors. Chin J Exp Ophthalmol. (2021) 39:29–33. doi: 10.3760/cma.j.cn115989-20190402-00164
27. Hosoda, Y, Akagi, T, and Yoshimura, N. Two cases of malignant Glaucoma unresolved by pars Plana vitrectomy. Clin Ophthalmol. (2014) 8:677–9. doi: 10.2147/opth.s60704
28. Basgil Pasaoglu, I, Altan, C, Bayraktar, S, Satana, B, and Basarır, B. Surgical Management of Pseudophakic Malignant Glaucoma via Anterior Segment-Peripheral Iridectomy Capsulo-Hyaloidectomy and Anterior Vitrectomy. Case Rep Ophthalmol Med. (2012) 2012:794938. doi: 10.1155/2012/794938
29. He, F, Qian, Z, Lu, L, Jiang, J, Fan, X, Wang, Z, et al. Clinical efficacy of modified partial pars Plana vitrectomy combined with phacoemulsification for malignant Glaucoma. Eye (Lond). (2016) 30:1094–100. doi: 10.1038/eye.2016.106
30. Fu, T, and Zhou, X. Clinical analysis of phacoemuisification combined with ant vitrectomy for the treatment of malignant glaucoma. Int Eye Sci. (2017) 17:950–3. doi: 10.3980/j.issn.1672-5123.2017.5.38
31. Shi, Y, Zhong, H, Yu, X, and Fan, Z. Progress in diagnosis and treatment of malignant glaucoma. Ophthalmology. (2024) 33:161–8. doi: 10.13281/j.cnki.issn.1004-4469.2024.03.001
32. Debrouwere, V, Stalmans, P, Van Calster, J, Spileers, W, Zeyen, T, and Stalmans, I. Outcomes of different management options for malignant Glaucoma: a retrospective study. Graefes Arch Clin Exp Ophthalmol. (2012) 250:131–41. doi: 10.1007/s00417-011-1763-0
33. Tian, DD, Li, JM, Song, YM, Fan, XJ, Chen, L, and Qin, L. Clinical observation of high intraocular pressure after the removal of ciliary ring block in the treatment of malignant glaucoma by scleral cyclophotocoagulation. Int Eye Sci. (2021) 21:348–50. doi: 10.3980/j.issn.1672-5123.2021.2.32
Keywords: autosomal recessive bestrophinopathy, angle-closure glaucoma, malignant glaucoma, vitreous humor, compound trabeculectomy
Citation: Sun Z, Liu C, Liu W, Zhang M, Ren S, Gao L and Ji Z (2025) Surgical treatment of autosomal recessive bestrophinopathy with angle-closure glaucoma: vitreous liquefaction as the key to correcting postoperative malignant glaucoma—three case reports. Front. Med. 12:1560475. doi: 10.3389/fmed.2025.1560475
Edited by:
Akio Oishi, Nagasaki University, JapanReviewed by:
Lorenzo Bianco, San Raffaele Scientific Institute (IRCCS), ItalySansar Sharma, New York Medical College, United States
Copyright © 2025 Sun, Liu, Liu, Zhang, Ren, Gao and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Ji, NTc0NzU0ODYwQHFxLmNvbQ==
 Zhonghua Sun1
Zhonghua Sun1 Wei Liu
Wei Liu Lei Gao
Lei Gao Zhen Ji
Zhen Ji