Abstract
Background:
The fourth-generation human immunodeficiency virus (HIV) serology assay, which simultaneously detects the HIV-1 p24 antigen and HIV-1 antibodies, is available either in a combined format or as dual tests that differentiate between the p24 antigen and antibodies. Divergent detection methodologies require distinct confirmatory testing algorithms, which significantly impact the time to HIV infection.
Case presentation:
In this report, we present three cases where the HIV-1 p24 antigen tested reactive, while the HIV-1 antibody remained non-reactive in a dual testing scenario—despite both the combined test and the colloidal gold immunochromatographic assay (GICA) for HIV-1 antibodies yielding reactive results. Upon further analysis of subsequent laboratory procedures, we observed that due to the application of various complementary tests, the assay with high antibody sensitivity such as the GICA paradoxically resulted in a prolonged time to diagnosis, extending the diagnostic window for patients from 5 days to 11 days.
Conclusion:
Our findings underscore the importance of prioritizing HIV-1 RNA testing in cases of discordant results between combined antigen/antibody testing, dual testing, and stand-alone antibody testing, particularly for patients who have not received pre-exposure or post-exposure prophylaxis.
1 Introduction
Human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) are major public health problems worldwide. An HIV screening test is of great importance to identify all HIV-infected people and facilitate their linkage to care (1). The screening and detection of 90% of all HIV-infected people was declared a major goal by the UNAIDS (2). The fourth-generation antigen/antibody (Ag/Ab) immunoassay has become the most commonly used screening test due to its high sensitivity (3). Elecsys® HIV combi PT and Elecsys® HIV Duo are both fourth-generation reagents of electrochemiluminescence immunoassay (ECLIA). A critical distinction between the two assays lies in result interpretation: Elecsys® HIV combi PT yields a single composite result for the simultaneous detection of the p24 antigen and antibodies, whereas the Elecsys® HIV Duo provides discrete differentiation of antigen and antibody reactivity, thereby distinguishing whether a positive result is attributable to the presence of the HIV-1 p24 antigen or specific antibodies against HIV-1/2 (4). For samples reactive to HIV antibodies, the colloidal gold immunochromatographic assay (GICA) is routinely used as a rapid diagnostic test and employed as the retesting method. Concurrent positive results from both the GICA and the initial screening test generally indicate the need for a Western blot (WB) HIV-1 antibody confirmatory test, and a negative GICA result typically warrants supplemental HIV-1 RNA testing (nucleic acid amplification technologies, NAATs) to rule out acute HIV infection. The National Guideline for HIV/AIDS Detection (2020), issued by the Chinese Center for Disease Control and Prevention (5) (hereinafter referred to as the “Guideline”), stipulates distinct supplementary diagnostic algorithms (Figure 1) for these two types of test assays.
Figure 1

Two processing flows for HIV screening positive results. Elecsys® HIV combi PT/ Elecsys® HIV Duo and the GICA antibody test are used as screening procedures for HIV infection. HIV infection can be confirmed in patients with positive NAATs and positive confirmatory tests. The procedure of the Elecsys® HIV combi PT- or Elecsys® HIV Duo-positive supplementary test is shown in the figure. The dotted lines represent the processing flow for our three patients. Case 1 corresponds to the left one, and Cases 2 and 3 correspond to the right one. The HIV-1 antibody confirmatory test uses the Western blot technique to detect antibodies and is regarded as the most important supplementary test for HIV diagnosis in China because it can distinguish the type of HIV proteins targeted by antibodies in patients.
Here, we present three cases demonstrating reactivity in both Elecsys® HIV Duo and Elecsys® HIV combi PT independently. In one case, the diagnostic algorithm prompted GICA testing, which yielded reactive results; however, the time to definitive HIV diagnosis for this patient was nearly twice as long as that for the other two cases.
2 Case presentation
2.1 Case 1
A 43-year-old man was admitted to the emergency department with a fever lasting for 8 days, with the highest recorded temperature reaching 39°C. The inguinal lymph nodes had become enlarged 2 weeks earlier. An adequate, non-leaky qualified serum sample was sent for an HIV screening test. Since Elecsys® HIV combi PT (Roche Diagnostics, Germany, REF 05390095) was reactive (22.01 COI) and the GICA (Lizhu, China, REF20143401976) was positive, the patient was advised to undergo an HIV-1 antibody confirmatory test. Six days later, we obtained another sample from him for WB testing (MP Diagnostics, Singapore, REF 20163401575). Prior to testing, Elecsys® HIV Duo (Roche Diagnostics, REF 07229542190) was used as a conventional additional test according to our workflow.
Notably, Elecsys® HIV Duo demonstrated discordant results compared to the GICA, and HIV antibodies were non-reactive (0.37 COI), whereas the p24 antigen exhibited reactivity (9.94 COI). The WB testing showed gp160 and p24 bands, which were classified as indeterminate. Given this discrepancy, confirmatory HIV-1 RNA testing was performed using the Roche cobas® system (REF 05212294190). Following a 4-day interval, subsequent testing revealed a high HIV-1 viral load of 2.86 × 106 copies/mL, confirming the definitive diagnosis of HIV infection. In this patient, we found low CD4 + T-lymphocyte counts (BD, America, REF 34049) with 55 cells/μL. The diagnostic interval from the initial HIV screening test to the confirmed diagnosis was 11 days (Figure 2).
Figure 2
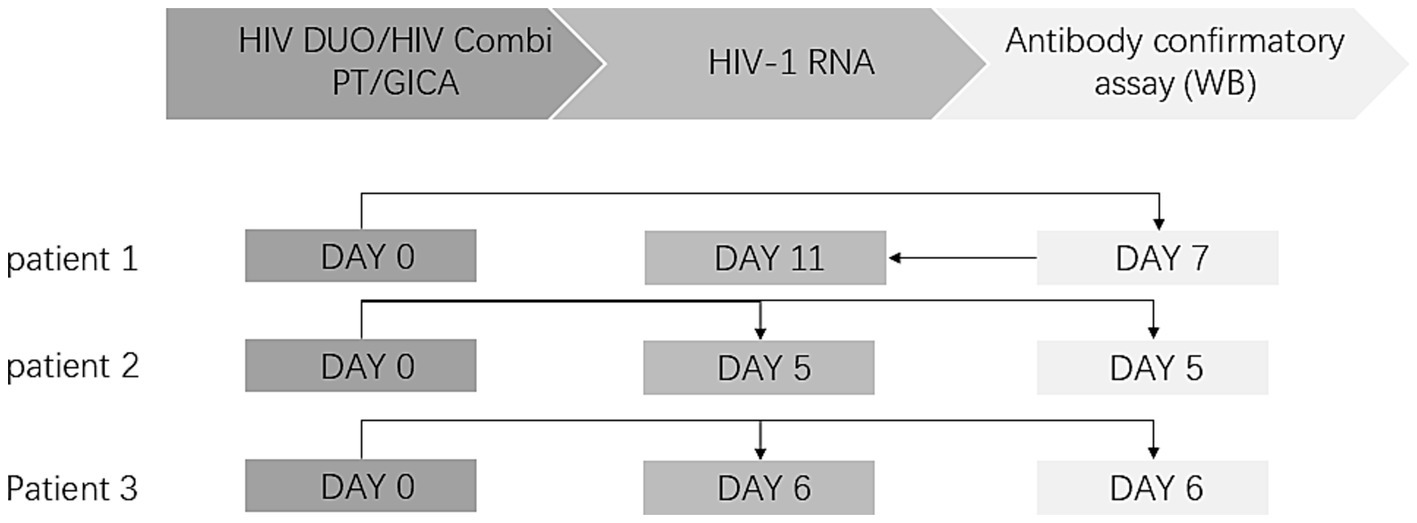
Diagnosis timeline of HIV patients. The laboratory diagnostic test procedure for HIV is divided into three sections: the HIV screening test, HIV RNA testing, and the HIV-1 antibody confirmatory test. The arrow indicates the sequence of the tests. In Cases 2 and 3, both HIV-1 RNA testing and Western blot (WB) antibody confirmation were submitted concurrently.
2.2 Case 2
A 46-year-old male was admitted to the otorhinolaryngology department with upper respiratory tract symptoms and lymphadenopathy. An HIV screening test was immediately conducted. Elecsys® HIV Duo demonstrated reactive p24 antigen (5.52 COI) with concurrent non-reactive HIV antibodies (0.35 COI). To prevent potential false-negative results (as demonstrated in Case 1), antibody retesting was conducted using the GICA, which yielded positive results. To verify this contradictory result, Elecsys® HIV combi PT was added as a third test, and the result was reactive (21.34 COI), prompting us to perform an HIV-1 antibody confirmatory test for this patient. However, in this case, we recommended the concurrent submission of two specimens: one for HIV-1 RNA testing and the other for the HIV-1 antibody confirmatory test. Four days later, the HIV-1 RNA test returned positive with a viral load of more than 1.00 × 107 copies/mL, while the HIV-1 antibody confirmatory test was negative, showing no band. The patient was eventually diagnosed with HIV infection based on a positive HIV-1 RNA result. The diagnostic interval from the initial HIV screening test to the confirmed diagnosis was 5 days (Figure 2), CD4 + T-lymphocyte counts were also low, at a level of 78 cells/μL.
2.3 Case 3
A 46-year-old man with Guillain–Barre syndrome was admitted to the neurology department. Elecsys® HIV Duo showed reactive p24 antigen (8.95 COI) and non-reactive HIV antibodies (0.20COI). Similar to Case 2, retesting with the GICA and Elecsys® HIV combi PT showed positive results. To ensure a comprehensive diagnostic evaluation, we reiterated the recommendation for concurrent HIV-1 RNA testing and confirmatory HIV-1 antibody testing. As a result, the HIV-1 RNA testing was positive, with a viral load above 1.00 × 107 copies/mL, while the WB testing was indeterminate, showing only the gp160 band.CD4 + T-lymphocyte counts were 712 cells/μL. This patient was also diagnosed with HIV infection. The diagnostic interval from the initial HIV screening test to the confirmed diagnosis was 6 days (Figure 2).
The studies involving human participants were reviewed and approved by the Ethics Committee of West China Hospital of Sichuan University. Informed consent was obtained from all patients.
3 Discussion
Serological tests for antigens and antibodies have been the most common method of HIV screening for a long time. Serological testing has evolved through four generations: the first generation used viral lysates for IgG antibody detection, the second generation employed recombinant antigens for IgG antibody detection, the third generation detected immunoglobulin M (IgM) and IgG antibodies, and the fourth generation detected IgM and IgG antibodies alongside the p24 antigen. As a fourth-generation reagent, Elecsys® HIV Duo shows good sensitivity and specificity. A multicenter evaluation of the reagent involving 13,328 blood donor samples showed a specificity of 99.87% (6). Elecsys® HIV Duo is considered to have slightly higher specificity (99.93% vs. 99.84%) and equivalent sensitivity compared to Elecsys® HIV combi PT (7). In a previous study involving 1,505 patients, the BioPlex 4th PLUS assay was assessed and found to have 100% sensitivity and 99.5% specificity (8).
The detection window of fourth-generation reagents is shorter, enabling serological detection within 5–7 days following a positive HIV-1 RNA test, as they can detect the HIV-1 p24 antigen. Approximately 1 week after the appearance of p24, immunoglobulin M (IgM) antibodies are detectable through third-generation immunoassays—several weeks earlier than first- or second-generation immunoassays that only detect immunoglobulin G (IgG) antibodies (9–12). Compared to Elecsys® HIV combi PT, Elecsys® HIV Duo can shorten the window period from 18 days to 14 days after HIV infection (13) because of its slightly different coating of antigens/antibodies, which reduces the proportion of cross-reactions. In addition, Elecsys® HIV Duo can avoid the secondary window period to a certain extent, while the window period for Elecsys® HIV combi PT is more difficult to minimize.
Given reports of HIV misdiagnoses associated with fourth-generation reagents, to avoid false-positive antibody results from Elecsys® HIV Duo and Elecsys® HIV combi PT (3, 14), third-generation reagents such as the GICA with lower detection limits are used as supplementary methods in screening tests. Although only IgG antibodies are detected, due to the high affinity of gp41 and gp120, the GICA reagent has a lower limit for antibody detection than fourth-generation reagents. It usually yields a positive result at the same time as, or earlier than, WB testing (15, 16) (Figure 3). Samples that test positive by ECLIA, the GICA, and WB testing will be used as direct evidence for an antibody-based HIV diagnosis. In contrast, only ECLIA-positive samples will prompt HIV-1 RNA testing (Figure 1). Due to its high specificity and ability to evaluate the stage of infection based on the separation of HIV-1 viral proteins by molecular weight, the WB technique has long been used as a confirmatory assay. However, the HIV-1 Western blot test still relies on first-generation principles, using whole viral lysate as the source of antigens and an enzyme-conjugated anti-IgG to bind to individual HIV proteins. The long detection window period makes it easy to prolong the diagnosis time. HIV-1 RNA is considered the earliest detectable marker of HIV infection and can be detected 7–10 days after infection, even when antigen and antibody tests are still negative (11, 17). It can be detected using PCR or NAATs with high sensitivity. HIV-1 RNA positivity is generally considered direct evidence of HIV infection. However, the risk of false-negative results remains due to viral replication inhibition by antiretroviral therapy and pre-exposure prophylaxis/post-exposure prophylaxis (18–20).
Figure 3
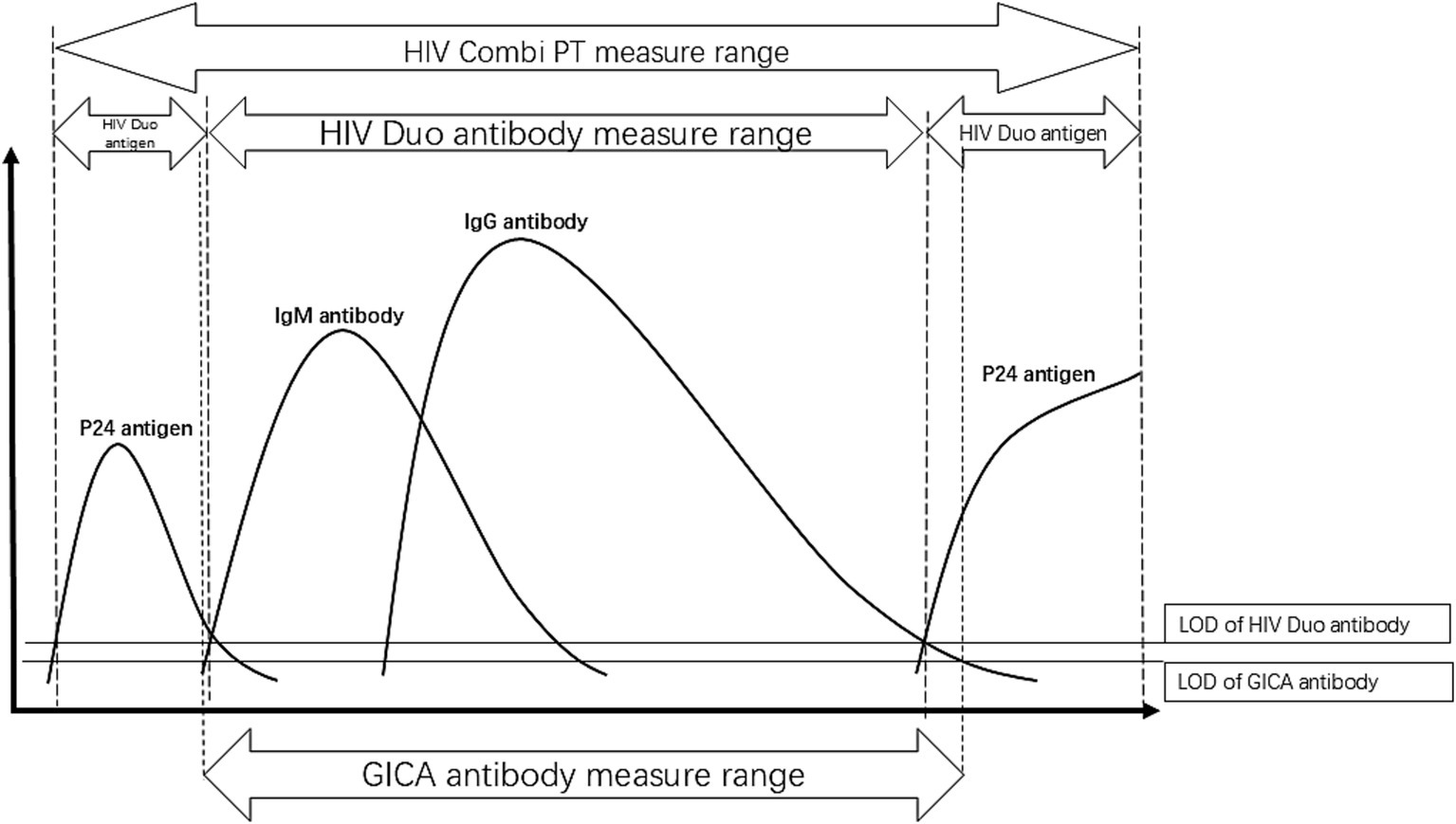
Pattern diagram of serological conversion of HIV infection. The dotted lines represent the detection range of different methods, while the solid lines indicate the detection limits of antigens or antibodies. The limit of detection (LOD) for the GICA antibody test is lower than that of the ROCHE HIV DUO antibody test. When the HIV Duo antibody test is negative, the GICA antibody test may be positive, while the HIV Duo antigen test will be positive, as observed in our cases. In the serological conversion process of HIV infection, this situation occurs during two phases—early infection and late infection. At these stages, different tests provide varying guidance. The HIV Duo antigen test takes precedence for patients whose antibodies are not detected, directing them to HIV RNA testing.
Across all three cases in our series, we observed that, as an antibody supplemental test, the GICA results were inconsistent with the antibody testing results from Elecsys® HIV Duo. Among patients with reactive GICA results, it took much longer for HIV infection to be diagnosed in patients who continued with confirmatory WB testing only (Case 1). We believe that the high sensitivity of the GICA led to reactive results, which subsequently directed patients to WB testing, a method with longer window periods. It is worth noting that in Case 2, the likelihood of missing the diagnosis would have been high if we had not performed additional HIV-1 RNA testing and only performed HIV antibody confirmatory testing based on the supplemental testing procedures of Elecsys® HIV combi PT. Our study demonstrated that incorporating higher-sensitivity antibody detection assays into the initial screening protocol failed to significantly improve diagnostic timeliness. Crucially, HIV-1 RNA testing remained indispensable regardless of the reactivity status in subsequent antibody screening. Furthermore, to preclude the influence of pre-exposure or post-exposure prophylaxis on HIV-1 RNA testing, WB testing is essential and should be conducted concurrently.
In conclusion, our patients presented with unusual cases of discrepant HIV antibody screening results, which led to different recommendations and affected the time to diagnosis and the assessment of infection status. Higher sensitivity antibody screening results lead to HIV-1 antibody confirmatory testing, which, in turn, leads to longer diagnostic times. Therefore, even when the screening procedure confirms HIV antibody reactivity, HIV-1 RNA testing needs to be performed concurrently.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the dataset contains patient information. Requests to access these datasets should be directed to Yuanfang Wang, 180402617@qq.com.
Ethics statement
The studies involving humans were approved by Ethics Committee of West China Hospital of Sichuan University (No. 920). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW: Data curation, Writing – original draft. LL: Data curation, Writing – original draft. JD: Formal analysis, Writing – original draft. XL: Formal analysis, Writing – original draft. DL: Project administration, Writing – review & editing. YX: Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Smith MKRS Powers KA Fidler S Miller WC Eron JJ Jr Cohen MS . The detection and management of early HIV infection: a clinical and public health emergency. J Acquir Immune Defic Syndr. (2013) 63:S187–99. doi: 10.1097/QAI.0b013e31829871e0
2.
UNAIDS . 90–90-90 an ambitious treatment target to help end the AIDS epidemic. Joint United Nations Programme on HIV/AIDS. (2014).
3.
Avidor B Chemtob D Turner D Zeldis I Girshengorn S Matus N . Evaluation of the virtues and pitfalls in an HIV screening algorithm based on two fourth generation assays - a step towards an improved national algorithm. J Clin Virol: Official Pub Pan American Society for Clin Virol. (2018) 106:18–22. doi: 10.1016/j.jcv.2018.06.017
4.
Alexander TS . Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin Vaccine Immunol: CVI. (2016) 23:249–53. doi: 10.1128/CVI.00053-16
5.
Pervention CCFDCA . National Guideline of detection of HIV/AIDS. Joint United Nations Programme on HIV/AIDS. (2020).
6.
Mühlbacher A Sauleda S Piron M Rietz R Permpikul P Klinkicht M et al . A multicentre evaluation of the Elecsys(®) HIV duo assay. J Clin Virol: Official Pub Pan American Society for Clin Virol. (2019) 112:45–50. doi: 10.1016/j.jcv.2018.11.005
7.
Zhang B Ma Q Zhao B Wang L Pu C Han X . Performance evaluation of Elecsys HIV duo on cobas e 801 using clinical samples in China. J Med Virol. (2020) 92:3230–6. doi: 10.1002/jmv.25845
8.
Salmona M Delarue S Delaugerre C Simon F Maylin S . Clinical evaluation of bio Plex 2200 HIV ag-ab, an automated screening method providing discrete detection of HIV-1 p 24 antigen, HIV-1 antibody, and HIV-2 antibody. J Clin Microbiol. (2014) 52:103–7. doi: 10.1128/JCM.02460-13
9.
Delaney KP Hanson DL Masciotra S Ethridge SF Wesolowski L Owen SM . Time until emergence of HIV test reactivity following infection with HIV-1: implications for interpreting test results and retesting after exposure. Clin Infect Dis. (2017) 64:53–9. doi: 10.1093/cid/ciw666
10.
Ma Y Ni C Dzakah EE Wang H Kang K Tang S et al . Development of monoclonal antibodies against HIV-1 p24 protein and its application in colloidal gold Immunochromatographic assay for HIV-1 detection. Biomed Res Int. (2016) 2016:1–6. doi: 10.1155/2016/6743904
11.
Rosenberg NE Pilcher CD Busch MP Cohen MS . How can we better identify early HIV infections?Curr Opin HIV AIDS. (2015) 10:61–8. doi: 10.1097/COH.0000000000000121
12.
White DAE Giordano TP Pasalar S Jacobson KR Glick NR Sha BE et al . Acute HIV discovered during routine HIV screening with HIV antigen-antibody combination tests in 9 US emergency departments. Ann Emerg Med. (2018) 72:29–40.e2. doi: 10.1016/j.annemergmed.2017.11.027
13.
Taylor D Durigon M Davis H Archibald C Konrad B Coombs D et al . Probability of a false-negative HIV antibody test result during the window period: a tool for pre- and post-test counselling. Int J STD AIDS. (2015) 26:215–24. doi: 10.1177/0956462414542987
14.
Lang R Charlton C Beckthold B Kadivar K Lavoie S Caswell D et al . HIV misdiagnosis: a root cause analysis leading to improvements in HIV diagnosis and patient care. Journal of clinical virology: the official publication of the Pan American Society for Clinical. Virology. (2017) 96:84–8. doi: 10.1016/j.jcv.2017.10.005
15.
Masciotra SM Feldman JS Sprinkle J Wesolowski P Owen L . Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. Journal of clinical virology: the official publication of the Pan American Society for Clinical. Virology. (2011) 52:S17–22. doi: 10.1016/j.jcv.2011.09.011
16.
Branson BM . HIV diagnostics: current recommendations and opportunities for improvement. Infect Dis Clin N Am. (2019) 33:611–28. doi: 10.1016/j.idc.2019.04.001
17.
Zhang Y Wang YY Li XF Ma CY Li J Kang W et al . A human immunodeficiency virus-seronegative acquired immunodeficiency syndrome patient with opportunistic infections: a case report. Int J STD AIDS. (2022) 33:515–8. doi: 10.1177/09564624221074507
18.
Iwata K Morishita N Otani S . A case of human immunodeficiency virus (HIV) infection without increase in HIV RNA level: a rare observation during the modern antiretroviral therapy era. J Gen Fam Med. (2022) 23:101–3. doi: 10.1002/jgf2.492
19.
Seed CR Styles CE Hoad VC Yang H Thomas MJ Gosbell IB . Effect of HIV pre-exposure prophylaxis (PrEP) on detection of early infection and its impact on the appropriate post-PrEP deferral period. Vox Sang. (2021) 116:379–87. doi: 10.1111/vox.13011
20.
Elliott T Sanders EJ Doherty M Ndung'u T Cohen M Patel P et al . Challenges of HIV diagnosis and management in the context of pre-exposure prophylaxis (PrEP), post-exposure prophylaxis (PEP), test and start and acute HIV infection: a scoping review. J Int AIDS Soc. (2019) 22:e25419. doi: 10.1002/jia2.25419
Summary
Keywords
HIV serological assay, P24 antigen, antibody HIV, diagnosis period, algorithm, fourth generation assay
Citation
Wang Y, Luo L, Deng J, Li X, Xie Y and Li D (2025) High sensitivity of HIV antibody screening tests may lead to longer time to diagnosis: a Case Report. Front. Med. 12:1562946. doi: 10.3389/fmed.2025.1562946
Received
18 January 2025
Accepted
21 April 2025
Published
15 May 2025
Volume
12 - 2025
Edited by
Alessandro Perrella, Hospital of the Hills, Italy
Reviewed by
SriSowmya Sanisetty, Independent Researcher, Boston, United States
Shilpee Sharma, Université libre de Bruxelles, Belgium
Updates
Copyright
© 2025 Wang, Luo, Deng, Li, Xie and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongdong Li, jiangxili1219@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.