- 1Daishan County First People's Hospital, Zhoushan, Zhejiang, China
- 2Jiaxing Second Hospital, Jiaxing, Zhejiang, China
Background: Autoimmune hepatitis (AIH) is a chronic inflammatory disease that can progress to cirrhosis and liver failure. While various biomarkers have been associated with AIH progression and prognosis, their specific roles in diagnosing, predicting, and managing cirrhosis, particularly in compensated cirrhosis, remain unclear. Accurately predicting 2-year survival and clinical outcomes for patients with compensated cirrhosis is a critical challenge.
Methods: This study analyzed clinical data from non-cirrhotic and cirrhotic AIH patients to identify factors influencing cirrhosis development and prognosis. Serum and nutritional indices were compared between groups with differing prognoses. The diagnostic efficacy of TSP-1, Gal-3, Cys-C, AIb, and PA was evaluated in compensated cirrhosis patients, and their prognostic value was validated using linear regression and Cox proportional hazards models.
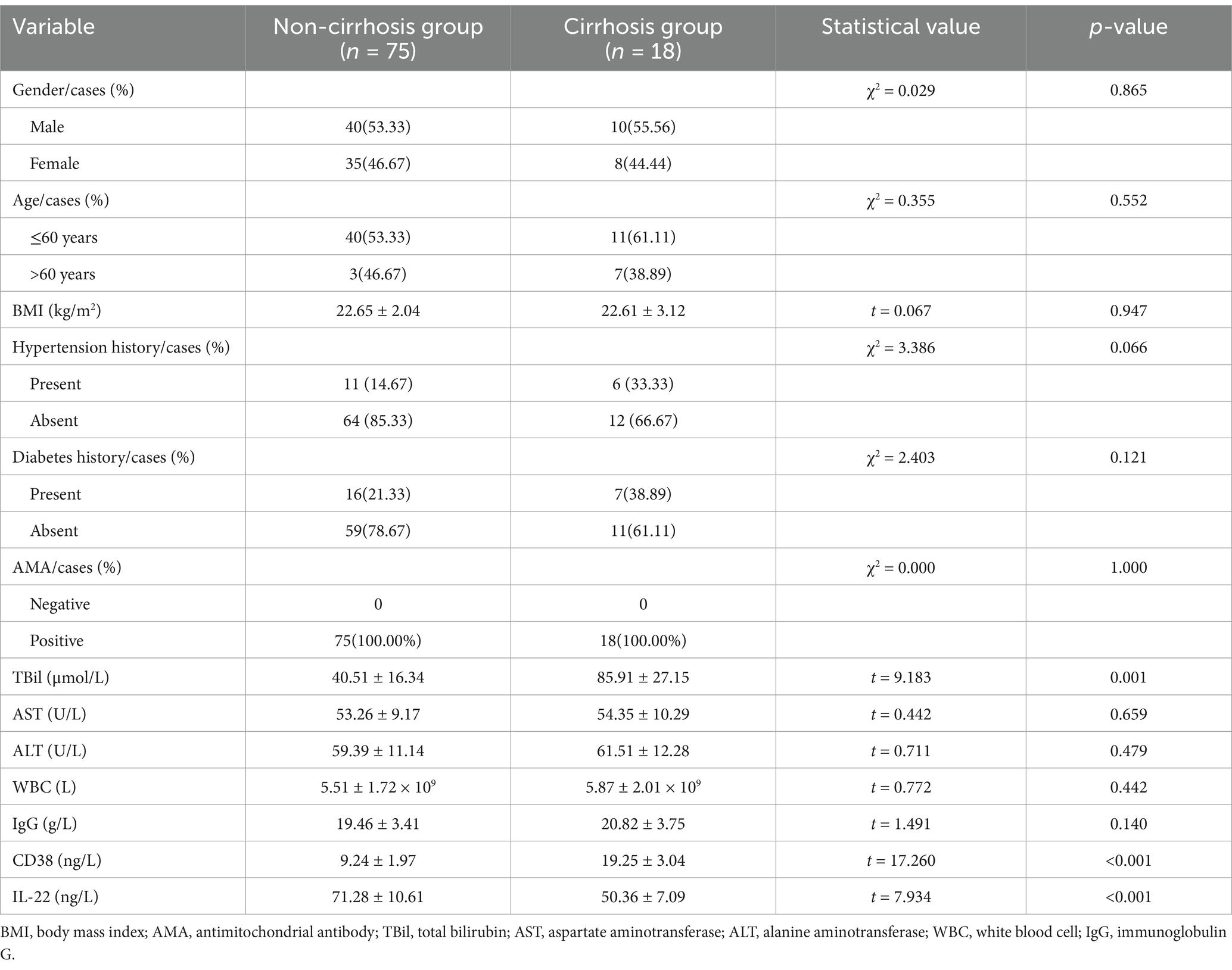
Results: No significant differences in gender, age, BMI, hypertension, diabetes history, or AMA positivity were observed between the non-cirrhotic AIH and cirrhotic AIH groups. However, TBil levels were significantly higher, and CD38 and IL-22 levels were significantly lower in the cirrhosis group. Multivariate logistic regression identified elevated TBil and reduced CD38 and IL-22 levels were associated with increased cirrhosis risk. Prognosis analysis revealed that patients with good outcomes had significantly lower TSP-1, Gal-3, and Cys-C levels and higher AIb and PA levels than those with poor outcomes. Cox proportional hazards modeling identified TSP-1, Gal-3, and Cys-C as independent risk factors and AIb and PA as protective factors influencing 2-year survival.
Conclusion: This study underscores the critical roles of TBil, CD38, and IL-22 in cirrhosis development among AIH patients and validates the diagnostic and prognostic significance of TSP-1, Gal-3, Cys-C, AIb, and PA in compensated cirrhosis. These biomarkers facilitate early risk identification and inform clinical decision-making, contributing to improved management and outcomes for AIH patients.
1 Introduction
Autoimmune hepatitis (AIH) is a chronic liver disease characterized by immune-mediated hepatocellular damage, which can progress to cirrhosis if left untreated (1–3). Cirrhosis, the end-stage of liver fibrosis, is associated with significant morbidity and mortality, making its prevention and early detection critical for improving patient outcomes (4, 5). The pathogenesis of AIH involves a dysregulated immune response, where the liver is attacked by the body’s immune system (6). Although the disease affects individuals across all age groups, it is more commonly diagnosed in women, particularly those in their 50s and 60s.
The diagnosis of AIH is based on clinical, biochemical, immunological, and histological findings (7, 8). The 2022 American Academy of Hepatology Practice Guidelines provide a diagnostic framework for severe forms of liver disease, including decompensated cirrhosis, which can develop in AIH patients (9). These guidelines emphasize the use of imaging, biochemical, and physical assessments for accurate diagnosis. Management strategies for AIH primarily involve immunosuppressive therapy to control the immune response and prevent further liver damage (10). However, the progression to cirrhosis in AIH patients is highly variable, influenced by factors such as age, gender, body mass index (BMI), and comorbidities like hypertension and diabetes mellitus (11, 12). Antimitochondrial antibody (AMA) positivity is also assessed, as it may indicate alternative liver pathologies (13, 14). Despite these known factors, accurately predicting the development of cirrhosis in AIH patients remains a challenge.
In recent years, serum biomarkers have gained attention for their potential in predicting liver disease prognosis, including AIH (15, 16). Biomarkers such as Thrombospondin-1 (TSP-1), Galectin-3 (Gal-3), and Cystatin C (Cys-C) have been studied for their roles in assessing liver fibrosis and predicting cirrhosis progression (17–19). These biomarkers reflect various aspects of liver injury and function, providing valuable insights into disease severity and patient prognosis. This study aimed to investigate the clinical characteristics of AIH patients and identify factors influencing cirrhosis development.
2 Materials and methods
2.1 Research volunteer recruitment
The study adhered to the Declaration of Helsinki and received approval from the hospital’s Ethics Committee. And this study enrolled 75 patients with non-cirrhotic AIH and 18 patients with cirrhosis AIH hospitalized at our institution between June 2020 and June 2023. Clinical data, including gender, age, BMI, hypertension and diabetes history, AMA positivity, total bilirubin (TBil), aspartate aminotransferase (AST), alanine aminotransferase (ALT), white blood cell count (WBC), immunoglobulin G (IgG), CD38, and interleukin-22 (IL-22) levels, were analyzed to compare non-cirrhotic and cirrhotic groups. The non-cirrhotic cohort comprised 40 males and 35 females, aged 45–79 years, with a mean age of 59.31 ± 5.12 years. And the cirrhotic cohort comprised 10 males and 8 females, aged 42 to 80 years, with a mean age of 56.22 ± 6.28 years.
2.1.1 Inclusion criteria
1. Met the diagnostic criteria for decompensated cirrhosis as outlined in the 2022 American Academy of Hepatology Practice Guidelines, confirmed through imaging, biochemical, and physical assessments. 2. No history of metabolic encephalopathy, toxic encephalopathy, or neurological disorders. 3. No active variceal bleeding within 1 month prior to admission. 4. Complete clinical data.
2.1.2 Exclusion criteria
1. Patients with blood or infectious diseases. 2. Patients using immunosuppressants. 3. Patients with abnormal immune function. 4. Patients with other liver tumors. 5. Past recipients of liver transplantation. 6. Patients with severe heart disease.
2.2 Serum protein ELISA assay
A 5 mL fasting blood sample was collected from the cubital vein of each participant, and no repeated measures were taken. Serum was isolated by centrifugation at 2,000 rpm with a 6 cm radius for 3 min and stored at −80°C until analysis. Thrombospondin-1 (TSP-1), Galectin-3 (Gal-3), and Cystatin-C (Cys-C) levels were measured using enzyme-linked immunosorbent assay (ELISA) and a Hitachi Labospec T008AS automatic biochemical analyzer. Blood albumin (Alb) and prealbumin (PA) were analyzed using a Beckman Coulter LH750 total autokinetic blood cell analyzer.
2.3 Statistical analysis
Data analysis was performed using SPSS version 22.0. Variables following a normal distribution were expressed as mean ± standard deviation (SD) and analyzed using an independent t-test. The linear regression model was used to establish regression equations for analyzing correlations. The Cox proportional hazards regression model was employed to identify prognostic factors and develop a predictive model. The model’s accuracy was assessed using the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. Survival rates were analyzed using Kaplan–Meier survival curves. A difference was considered statistically significant when p < 0.05.
3 Results
3.1 Comparison of clinical characteristics between patients in non-cirrhosis and cirrhosis group
To identify factors influencing cirrhosis development in patients with AIH, we analyzed clinical data from non-cirrhotic and cirrhotic patient groups (Table 1). No significant differences were observed between the two groups regarding gender, age, BMI, history of hypertension and diabetes, or AMA positivity. However, significant differences were found in total bilirubin (TBil) levels, which were notably higher in the cirrhotic group compared to the non-cirrhotic group. Levels of CD38 and IL-22 were significantly lower in the cirrhotic group. Multivariate logistic regression analysis identified TBil, CD38, and IL-22 as independent factors influencing cirrhosis development in AIH patients. Combined detection of TBil, CD38, and IL-22 levels demonstrated high sensitivity and specificity for predicting cirrhosis development in AIH patients, highlighting their potential as reliable biomarkers for early identification and risk assessment.
3.2 Comparative of serum and nutritional indexes between the good prognosis group and the poor prognosis group in non-cirrhosis patients
Serum and nutritional indicators were analyzed between the Good Prognosis and Poor Prognosis groups (Table 2). The Good Prognosis Group consisted of 38 subjects, while the Poor Prognosis Group had 37 subjects. The Good Prognosis Group exhibited significantly lower mean levels of Thrombospondin-1 (TSP-1), Galectin-3 (Gal-3), and Cystatin-C (Cys-C) compared to the Poor Prognosis Group. Additionally, the Good Prognosis Group had significantly higher mean levels of albumin (Alb) and prealbumin (PA). These findings demonstrate that the Good Prognosis Group exhibited notably superior serum and nutritional indicators relative to the Poor Prognosis Group.
3.3 Diagnostic performance analysis of biomarkers for predicting prognosis of patients with compensatory cirrhosis
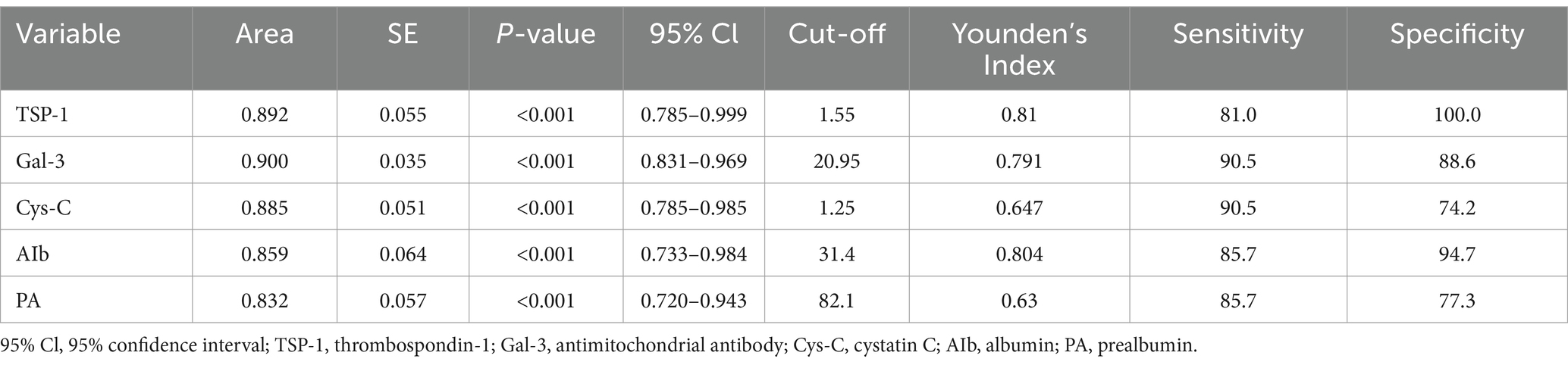
We conducted an in-depth analysis to evaluate the diagnostic capabilities of biomarkers in compensated cirrhosis (Table 3). Our aim was to identify indicators that accurately forecast patient outcomes, thereby guiding clinical decisions and potentially enhancing patient outcomes. Our study assessed the diagnostic efficacy of key biomarkers. TSP-1 and Gal-3 emerged as strong predictors, with TSP-1 showing an area under the curve (AUC) of 0.892, sensitivity of 81.0%, and specificity of 100.0%. Gal-3 exhibited an AUC of 0.900, sensitivity of 90.5%, and specificity of 88.6%. These findings suggest that TSP-1 and Gal-3 have the potential to play a significant role in the early detection and prognosis of compensated cirrhosis. Furthermore, Cys-C, Alb, and PA also demonstrated promising diagnostic performance, with AUCs ranging from 0.832 to 0.885 and varying levels of sensitivity and specificity. Although these biomarkers are less robust than TSP-1 and Gal-3, they still offer valuable insights for clinical decision-making in compensated cirrhosis.
3.4 Relationship between linear regression analysis and prognosis of compensated cirrhosis
To investigate the relationship between patient prognosis in compensated cirrhosis and various biomarkers, we performed linear regression analysis (Table 4). The results showed that TSP-1, Gal-3, Cys-C, Alb, and PA were significantly correlated with prognosis in patients with compensated cirrhosis. TSP-1, Gal-3, and Cys-C were positively correlated with poor prognosis, while Alb and PA demonstrated a negative correlation with poor prognosis. Additionally, the tolerance and variance inflation factor (VIF) of each indicator are listed in the table to evaluate multicollinearity. These findings suggest that this biomarkers may be useful indicators for assessing the prognosis of patients with compensated cirrhosis.
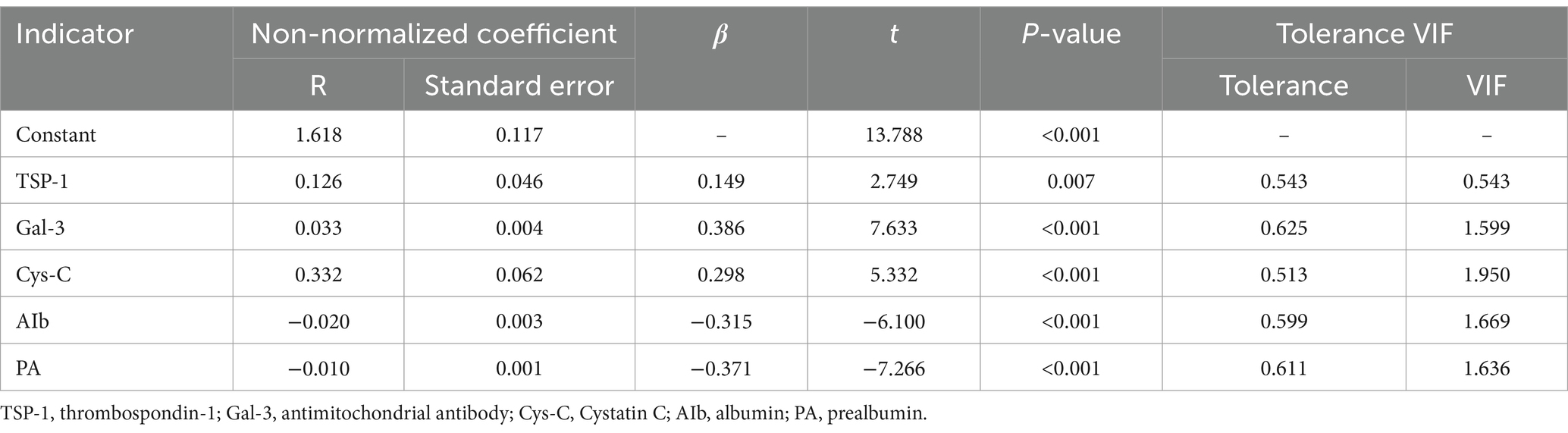
Table 4. Relationship between linear regression analysis indicators and prognosis of compensated cirrhosis.
3.5 Cox proportional hazards analysis of factors affecting 2-year survival rate in compensated cirrhosis
To investigate the impact of various biomarkers on the 2-year survival rate in patients with compensated cirrhosis, we conducted a Cox proportional hazards analysis (Table 5). TSP-1 significantly predicted survival, with a hazard ratio (HR) of 112.562 (95% CI: 31.739–399.203, p < 0.001), suggesting that elevated TSP-1 levels markedly increase mortality risk. Gal-3 (HR = 92.562, 95% CI: 12.392–691.394, p < 0.001) and Cys-C (HR = 43.773, 95% CI: 5.870–326.402, p < 0.001) were also significant risk factors for poor survival outcomes. Conversely, albumin (Alb) and prothrombin activity (PA) were associated with a reduced risk of mortality. The HR for Alb was 0.014 (95% CI: 0.003–0.059, p < 0.001), and for PA, it was 0.020 (95% CI: 0.003–0.147, p < 0.001), indicating that elevated levels of these biomarkers correlate with improved survival outcomes.
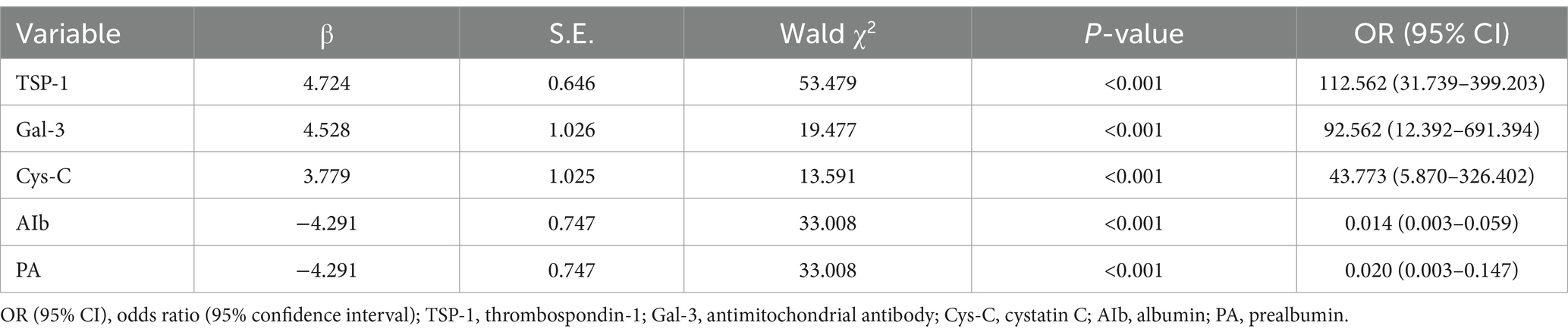
Table 5. Presents the Cox proportional hazards analysis identifying factors influencing the 2-year survival rate in patients with compensated cirrhosis.
4 Discussion
Cirrhosis results from the prolonged destruction of the liver’s normal lobular structure due to various causes, leading to fibrosis, nodular regeneration, and hepatocyte necrosis (20–22). Decompensated cirrhosis represents an advanced stage of liver disease, characterized by portal hypertension and significant liver function impairment, with poor prognosis and high mortality (23). Liver transplantation remains the only curative option but is limited by high risk, cost, and insufficient donor availability. Thus, identifying effective and objective clinical indicators is crucial for treatment planning and prognosis evaluation (24).
Thrombospondin-1 (TSP-1) is a multifunctional trimeric protein involved in apoptosis, angiogenesis, and coagulation, playing a significant role in cirrhosis pathogenesis (25). Decreased liver function during decompensation alters TSP-1 production, making its serum levels a reliable marker for assessing disease severity and prognosis (26). This study identified elevated TSP-1 levels as strongly associated with poor prognosis, suggesting a positive correlation between high TSP-1 expression and adverse outcomes. Activation of TSP-1 precursor complexes can trigger signal transduction, causing extracellular matrix accumulation, reduced stromal degradation, and microenvironment changes, ultimately leading to fibrosis and microcirculatory dysfunction (27, 28). Additionally, TSP-1 acts as a potent activator of transforming growth factor-β1 (TGF-β1), binding to form the TSP1-LAP complex, which regulates cell proliferation and adhesion via signal transduction (29).
Galectin-3 is a β-galactoside-binding lectin extensively involved in inflammatory responses, apoptosis, and fibrotic processes (30–32). In cirrhosis, Galectin-3 is closely associated with the activation of hepatic stellate cells, a key step in liver fibrosis (33). Moreover, Galectin-3 affects the inflammatory response in the liver by regulating the infiltration of inflammatory cells and intercellular signaling (34). High expression of Galectin-3 may reflect the active degree of inflammation and fibrosis in cirrhotic patients, making it an important biomarker for assessing prognosis.
Cystatin-C (Cys-C) is a low-molecular-weight protein that serves as a marker of glomerular filtration rate due to its stable production and rapid metabolic breakdown (35, 36). This study found a positive correlation between elevated Cys-C levels and poor prognosis in decompensated cirrhosis. The proposed mechanism involves portal hypertension and splenomegaly, leading to immune and hematopoietic imbalances that elevate Cys-C levels. These findings align with prior studies linking Cys-C to disease severity. Malnutrition is a common complication in decompensated cirrhosis, driven by impaired liver function and gastrointestinal absorption (37).
Albumin (Alb) and prealbumin (PA) serve as key nutritional indicators, reflecting the liver’s synthetic capacity and the body’s nutritional status (38). This study found lower Alb and PA levels in patients with poor prognosis, indicating a negative correlation with survival outcomes. The mechanisms include increased energy consumption due to cirrhosis, reduced intake of high-quality proteins, and intestinal absorption disorders caused by portal hypertension, leading to malnutrition and decreased Alb and PA levels (39).
In the end, a Cox proportional hazards model identified independent risk factors influencing 2-year survival in decompensated cirrhosis:TSP-1 levels ≥1.55 ng/mL, Gal-3 levels ≥20.95 ng/L, Cys-C levels ≥1.25 mg/L, AIb levels <31.4 g/L, PA levels <82.1 mg/L. Our study demonstrated that TSP-1, Gal-3, and Cys-C independently increased the risk of death in compensated cirrhosis, whereas albumin and plasminogen activity could act as protective factors. These results highlight the potential clinical utility of these biomarkers in predicting survival and guiding therapeutic strategies in patients with compensated cirrhosis.
There are some limitations of our study, firstly, our study is a retrospective study, sample size is small and absence of external validation, future studies expand the sample size through multicenter collaboration to improve the statistical power and future external validation in independent cohorts to confirm the generalizability of our results. Secondly, our study was not externally validated, but we used appropriate models in our statistical analysis and plan to conduct external validation in future studies. Thirdly, our study focused on the assessment of short-term prognosis, the potential value of these biomarkers in long-term prognosis also warrants further exploration. Future studies will aim to validate the performance of these markers in long-term follow-up and explore their potential application in different disease stages.
5 Conclusion
In this study, TSP-1, Galectin-3, and Cystatin-C were found to be independent risk factors for death in patients with cirrhosis, whereas Albumin and Prealbumin were protective. The combined application of these markers provides a new tool for the assessment of short-term prognosis. However, this study has limitations, including a small sample size and the omission of other potential indicators such as vitamin B12, serum iron, and folic acid, which may introduce bias. Future research should address these limitations to enhance the reliability of findings and develop more comprehensive prognostic models.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study protocol was approved by the Jiaxing Second Hospital Ethics Committee (NSQTY12). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JS: Data curation, Writing – original draft, Writing – review & editing. JF: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. CH: Resources, Validation, Writing – original draft, Writing – review & editing. JS: Resources, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Thanks to all those who helped with the study but were not listed as co-authors due to insufficient contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kang, Y, Kuang, X, Yan, H, Ren, P, Yang, X, Liu, H, et al. A novel synbiotic alleviates autoimmune hepatitis by modulating the gut microbiota-liver Axis and inhibiting the hepatic TLR4/NF-κB/NLRP3 signaling pathway. mSystems. (2023) 8:e0112722. doi: 10.1128/msystems.01127-22
2. Pellicano, R, Ferro, A, Cicerchia, F, Mattivi, S, Fagoonee, S, and Durazzo, M. Autoimmune hepatitis and fibrosis. J Clin Med. (2023) 12:1979. doi: 10.3390/jcm12051979
3. Villani, R, Serviddio, G, Avolio, C, Cassano, T, and D'Amico, E. Autoimmune liver disease and multiple sclerosis: state of the art and future perspectives. Clin Exp Med. (2023) 23:3321–38. doi: 10.1007/s10238-023-01128-8
4. Ginès, P, Krag, A, Abraldes, JG, Solà, E, Fabrellas, N, and Kamath, PS. Liver cirrhosis. Lancet. (2021) 398:1359–76. doi: 10.1016/S0140-6736(21)01374-X
5. Parola, M, and Pinzani, M. Liver fibrosis in NAFLD/NASH: from pathophysiology towards diagnostic and therapeutic strategies. Mol Asp Med. (2024) 95:101231. doi: 10.1016/j.mam.2023.101231
6. Sipeki, N, Antal-Szalmas, P, Lakatos, PL, and Papp, M. Immune dysfunction in cirrhosis. World J Gastroenterol. (2017) 20:2564–77. doi: 10.3748/wjg.v20.i10.2564
7. Sucher, E, Sucher, R, Gradistanac, T, Brandacher, G, Schneeberger, S, and Berg, T. Autoimmune hepatitis-immunologically triggered liver pathogenesis-diagnostic and therapeutic strategies. J Immunol Res. (2019) 2019:1–19. doi: 10.1155/2019/9437043
8. Chen, H, Han, Z, Fan, Y, Chen, L, Peng, F, Cheng, X, et al. CD4+ T-cell subsets in autoimmune hepatitis: a review. Hepatol Commun. (2023) 7:e0269. doi: 10.1097/HC9.0000000000000269
9. Long, MT, Noureddin, M, and Lim, JK. AGA clinical practice update: diagnosis and Management of Nonalcoholic Fatty Liver Disease in lean individuals: expert review. Gastroenterology. (2022) 163:764–774.e1. doi: 10.1053/j.gastro.2022.06.023
11. Premkumar, M, and Anand, AC. Overview of complications in cirrhosis. J Clin Exp Hepatol. (2022) 12:1150–74. doi: 10.1016/j.jceh.2022.04.021
12. Reau, NS, Lammert, CS, and Weinberg, EM. Autoimmune hepatitis: current and future therapies. Hepatol Commun. (2024) 8:e0458. doi: 10.1097/HC9.0000000000000458
13. Lammert, C, Chalasani, SN, Green, K, Atkinson, E, McCauley, B, and Lazaridis, KN. Patients with autoimmune hepatitis report lower lifetime coffee consumption. Dig Dis Sci. (2022) 67:2594–9. doi: 10.1007/s10620-021-06989-1
14. Klein, R, Hintz, E, and Staehler, G. Exacerbation of AIH in a patient with an AIH/systemic sclerosis overlap syndrome and pulmonary arterial hypertension treated with the endothelin-1 receptor antagonist sitaxentan. BMJ Case Rep. (2012) 2012:bcr0120125494. doi: 10.1136/bcr-01-2012-5494
15. Tamber, SS, Bansal, P, Sharma, S, Singh, RB, and Sharma, R. Biomarkers of liver diseases. Mol Biol Rep. (2023) 50:7815–23. doi: 10.1007/s11033-023-08666-0
16. Di Sabatino, A, Biagi, F, Lenzi, M, Frulloni, L, Lenti, MV, Giuffrida, P, et al. Clinical usefulness of serum antibodies as biomarkers of gastrointestinal and liver diseases. Dig Liver Dis. (2017) 49:947–56. doi: 10.1016/j.dld.2017.06.010
17. Li, Y, Turpin, CP, and Wang, S. Role of thrombospondin 1 in liver diseases. Hepatol Res. (2017) 47:186–93. doi: 10.1111/hepr.12787
18. Chen, X, Yu, C, Liu, X, Liu, B, Wu, X, Wu, J, et al. Intracellular galectin-3 is a lipopolysaccharide sensor that promotes glycolysis through mTORC1 activation. Nat Commun. (2022) 13:7578. doi: 10.1038/s41467-022-35334-x
19. Assem, NM, Mohammed, AI, Barry, HMA, El Sayed, IET, and Elmadbouh, I. Serum cystatin C is an early renal dysfunction biomarker in patients with hepatitis C virus. Egypt Liver J. (2022) 12:67. doi: 10.1186/s43066-022-00231-x
20. Dezső, K, Paku, S, Kóbori, L, Thorgeirsson, SS, and Nagy, P. What makes cirrhosis irreversible?-consideration on structural changes. Front Med. (2022) 9:876293. doi: 10.3389/fmed.2022.876293
21. Tsochatzis, EA, Bosch, J, and Burroughs, AK. Liver cirrhosis. Lancet. (2014) 383:1749–61. doi: 10.1016/S0140-6736(14)60121-5
22. Çakıroğlu, B. Minimally invasive connective water vapor energy method for benign prostatic hyperplasia. Urologia. (2024) 91:298–305. doi: 10.1177/03915603231216191
23. Tandon, P, Montano-Loza, AJ, Lai, JC, Dasarathy, S, and Merli, M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. (2021) 75:S147–62. doi: 10.1016/j.jhep.2021.01.025
24. Kappe, NN, Stolk, J, and van Hoek, B. Liver transplantation. N Engl J Med. (2024) 390:387. doi: 10.1056/NEJMc2314292
25. Bruel, A, Touhami-Carrier, M, Thomaidis, A, and Legrand, C. Thrombospondin-1 (TSP-1) and TSP-1-derived heparin-binding peptides induce promyelocytic leukemia cell differentiation and apoptosis. Anticancer Res. (2005) 25:757–64.
26. Gwag, T, Reddy Mooli, RG, Li, D, Lee, S, Lee, EY, and Wang, S. Macrophage-derived thrombospondin 1 promotes obesity-associated non-alcoholic fatty liver disease. JHEP Rep. (2020) 3:100193. doi: 10.1016/j.jhepr.2020.100193
27. Liu, X, Jin, J, Liu, Y, Shen, Z, Zhao, R, Ou, L, et al. Targeting TSP-1 decreased periodontitis by attenuating extracellular matrix degradation and alveolar bone destruction. Int Immunopharmacol. (2021) 96:107618. doi: 10.1016/j.intimp.2021.107618
28. Luo, Q, Jiang, Z, Jiang, J, Wan, L, Li, Y, Huang, Y, et al. Tsp-1+ microglia attenuate retinal neovascularization by maintaining the expression of Smad3 in endothelial cells through exosomes with decreased miR-27a-5p. Theranostics. (2023) 13:3689–706. doi: 10.7150/thno.84236
29. Atanasova, VS, Russell, RJ, Webster, TG, Cao, Q, Agarwal, P, Lim, YZ, et al. Thrombospondin-1 is a major activator of TGF-β signaling in recessive dystrophic epidermolysis bullosa fibroblasts. J Invest Dermatol. (2019) 139:1497–1505.e5. doi: 10.1016/j.jid.2019.01.011
30. Dong, R, Zhang, M, Hu, Q, Zheng, S, Soh, A, Zheng, Y, et al. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (review). Int J Mol Med. (2018) 41:599–614. doi: 10.3892/ijmm.2017.3311
31. Soares, LC, Al-Dalahmah, O, Hillis, J, Young, CC, Asbed, I, Sakaguchi, M, et al. Novel Galectin-3 roles in neurogenesis, inflammation and neurological diseases. Cells. (2021) 10:3047. doi: 10.3390/cells10113047
32. Srejovic, I, Selakovic, D, Jovicic, N, Jakovljević, V, Lukic, ML, and Rosic, G. Galectin-3: roles in neurodevelopment, neuroinflammation, and behavior. Biomol Ther. (2020) 10:798. doi: 10.3390/biom10050798
33. Pasmatzi, E, Papadionysiou, C, Monastirli, A, Badavanis, G, and Tsambaos, D. Galectin 3: an extraordinary multifunctional protein in dermatology. Current knowledge and perspectives. An Bras Dermatol. (2019) 94:348–54. doi: 10.1590/abd1806-4841.20198426
34. Nangia-Makker, P, Hogan, V, Balan, V, and Raz, A. Chimeric galectin-3 and collagens: biomarkers and potential therapeutic targets in fibroproliferative diseases. J Biol Chem. (2022) 298:102622. doi: 10.1016/j.jbc.2022.102622
35. Nishimura, F, Oniki, K, Miyamura, S, Ushijima, T, Harada, H, Taki, T, et al. Factors influencing glomerular filtration rate as estimated using preoperative creatinine and cystatin C levels. Int J Clin Pharmacol Ther. (2023) 61:363–70. doi: 10.5414/CP204432
36. Ermetici, F, Filopanti, M, Verga, U, Passeri, E, Dito, G, Malavazos, AE, et al. Estimated glomerular filtration rate by serum cystatin C correlates with cardiometabolic parameters in patients with primary hyperparathyroidism. Eur J Endocrinol. (2015) 173:441–6. doi: 10.1530/EJE-15-0341
37. Dent, E, Wright, ORL, Woo, J, and Hoogendijk, EO. Malnutrition in older adults. Lancet. (2023) 401:951–66. doi: 10.1016/S0140-6736(22)02612-5
38. Warner, BW, James, JH, Hasselgren, PO, Hummel, RP, and Fischer, JE. Effect of sepsis and starvation on amino acid uptake in skeletal muscle. J Surg Res. (1987) 42:377–82. doi: 10.1016/0022-4804(87)90172-7
39. Arroyo, V, Angeli, P, Moreau, R, Jalan, R, Clària, J, Trebicka, J, et al. Grifols chair and European Foundation for the Study of chronic liver failure (EF-Clif). The systemic inflammation hypothesis: towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. (2021) 74:670–85. doi: 10.1016/j.jhep.2020.11.048
Keywords: autoimmune hepatitis (AIH), liver cirrhosis, cytokines, CD38, interleukin-22 (IL-22), biomarkers
Citation: Shi J, Fu J, He C and Shen J (2025) Diagnostic and prognostic biomarkers in autoimmune hepatitis-associated cirrhosis: insights into TBil, CD38, IL-22, TSP-1, GAL-3, and Cyc-C. Front. Med. 12:1564107. doi: 10.3389/fmed.2025.1564107
Edited by:
Talha Bin Emran, Brown University, United StatesReviewed by:
Mohammad Nurul Amin, Atish Dipankar University of Science and Technology, BangladeshMohammad Rashedul Islam, University of Chittagong, Bangladesh
Rashu Barua, New York University, United States
Preethi Jayakumar, Northwell Health, United States
Md Hasif Sinha, University of Louisville, United States
Md Saqline Mostaq, University of Louisiana at Monroe, United States
Copyright © 2025 Shi, Fu, He and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Shen, YWlsYWZ1eW91M2JpQDE2My5jb20=
 Jiani Shi1
Jiani Shi1 Jun Shen
Jun Shen