Abstract
Background:
Treating severe pneumonia caused by multiple pathogens in immunocompromised hosts (ICHs) presents significant challenges. Isavuconazole (ISA), a next-generation triazole antifungal agent, has shown promise in managing fungal infections. However, clinical evidence regarding its efficacy in cases of complex infections involving multiple pathogens in ICHs remains limited.
Case presentation:
This study describes a case series of three ICHs diagnosed with severe pneumonia, including invasive aspergillosis (IA). All three patients received ISA-based personalized antimicrobial regimens. Alleviation of symptoms was observed in all patients following antimicrobial treatment, with notable absorption of pulmonary lesions and no significant hepatorenal toxic side effects, with no recurrence observed.
Conclusion:
ICHs are highly susceptible to fungal infections, and the severity of their condition can escalate dramatically, with a significant risk of mortality, when severe pneumonia caused by multiple pathogens occurs concurrently. A stepwise treatment strategy, which balances the use between immunosuppressant and effective antimicrobial treatment, is crucial. The selection of appropriate drugs should account for potential adverse drug reactions (ADRs). In this case series, ISA exhibited robust efficacy in treating IA with minimal ADRs. Therefore, ISA represents a valuable option for managing severe pneumonia in ICHs, particularly in the context of IA and co-infections caused by multiple pathogens.
Highlights
-
•
ICHs are susceptible to severe pneumonia caused by IA with high mortality.
-
•
When ICHs are infected with severe pneumonia caused by IA and other pathogens, a stepwise treatment strategy is essential.
-
•
ISA is the next-generation triazole agent with few contraindications, minimal ADRs, proven efficacy, and economic benefits.
-
•
ISA is an effective treatment option for severe pneumonia caused by complex infections including IA in ICHs.
Introduction
Recent epidemiological studies indicate that the use of advanced medical interventions, such as organ transplantation, cytotoxic therapies, and widespread use of immunosuppressive medications, have significantly increased the population of ICHs (1). ICHs are susceptible to opportunistic infections, including Aspergillus, as well as the reactivation of latent pathogens like Pneumocystis jirovecii (PJ) and herpesviruses (2). Such infections in ICHs can lead to severe pneumonia and even acute respiratory distress syndrome (ARDS), necessitating intensive care unit (ICU) admission (3, 4).
Severe pulmonary infections in ICHs, particularly those involving invasive fungal pathogens, are associated with alarmingly high mortality rates (5). Management of these patients poses significant challenges due to the delicate immune balance which complicates the choice of treatment strategies. Therefore, optimizing therapeutic approaches for ICHs with complex infections, including Aspergillus, is crucial for reducing hospital stays, improving survival rates, and enhancing prognosis. ISA, a novel triazole antifungal agent (marketed as Cresemba®), serves as a pivotal therapeutic option for severe pneumonia secondary to invasive fungal infections, particularly when voriconazole (VOR) or posaconazole are contraindicated owing to toxicity, unfavorable pharmacokinetics, or drug interactions (6).
Herein, we report three cases of severe pneumonia involving IA and co-infections with multiple pathogens. All three cases were successfully treated using ISA in combination with other antimicrobial agents.
Materials and method
This report provides the diagnosis and treatment process of three patients with severe pneumonia caused by Aspergillus and other multiple pathogens. These patients were treated from March to August 2023 at the Respiratory and Critical Care Medicine Department of the 900th Hospital of the Joint Logistics Support Force, PLA. The analysis includes their clinical characteristics, treatment plans, prognosis, and outcomes.
Case description
Case 1
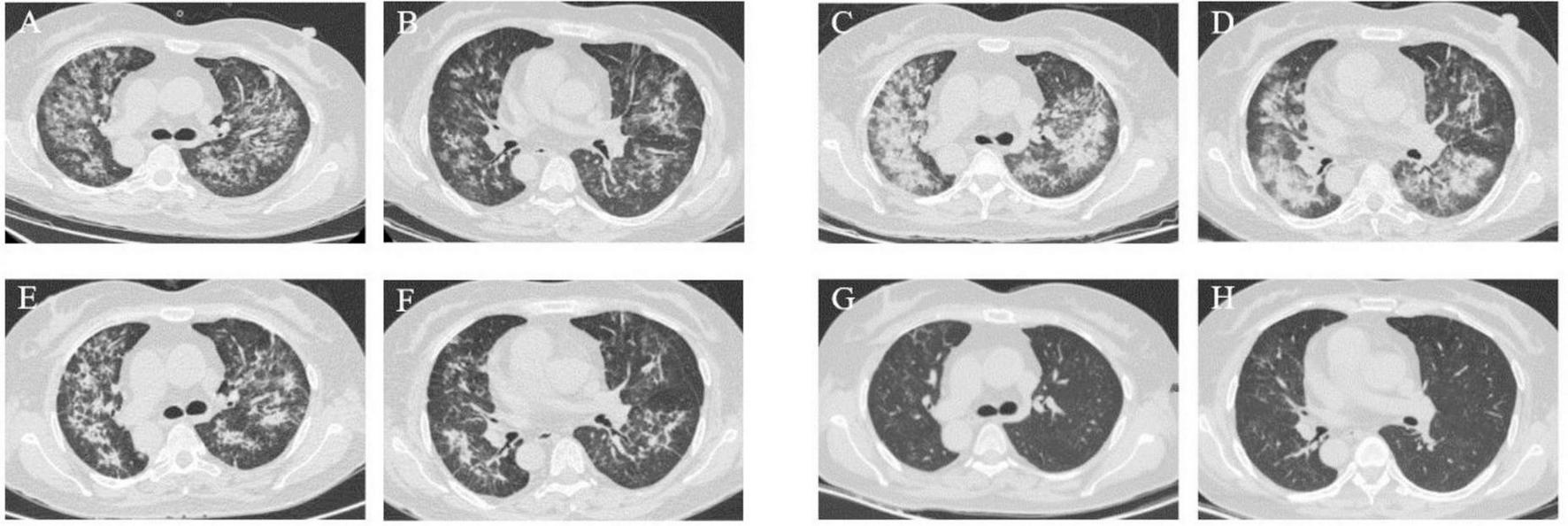
A 73-year-old male was hospitalized on 30 March 2023, with a two-month history of a productive cough and shortness of breath, which had worsened over the past week. A previous chest CT scan from another hospital revealed bronchiectasis and a suspected infection in the upper lobe of the right lung. The patient had experienced a weight loss of 5 Kg in the preceding week. Upon admission, a chest CT scan revealed multiple ill-defined pulmonary nodules in both lungs, with the largest area of consolidation (measuring 3.5 cm × 4.4 cm) located in the left lower lobe, characterized by inhomogeneous density (arrow) (Figures 1A, B).
FIGURE 1

Case 1, Chest CT scans. (A, B) On March 30: multiple ill-defined pulmonary nodules in both lungs. The largest areas of consolidation appearing with inhomogeneous density. In the left lower lobe, diagonal measurement result was 3.5 × 4.4 cm (arrow). (C, D) On April 7: patchy opacities and ground-glass attenuation in both lungs. (E, F) On April 14: partial resolution of the inflammation. The largest consolidated nodules enlarged to 4.8 × 6.2 cm (arrow). (G, H) On April 27: multiple patchy opacities of the left lung were further absorbed than that on April 14, while opacities of the right lung were similar to before. (I, J) On June 26: the pulmonary nodules and patchy opacities were gradually resolving after systemic therapy. The largest consolidation was about 4.0 × 3.3 cm (arrow). (K, L) On August 30: the pulmonary nodules and patchy opacities gradually diminished.
The patient had a 30-year smoking history, a one-month history of hypertension, and a diagnosis of membranous nephropathy two months earlier. He was on a regular regimen of tacrolimus and methylprednisolone (MP) for the membranous nephropathy (specific dosing details unspecified). The results of physical and laboratory tests upon admission were summarized in Table 1, indicated systemic infection, respiratory failure, and hepatic and renal dysfunction. Additionally, arterial blood gas analysis and tests for cytomegalovirus (CMV) DNA and galactomannan (GM) were positive. Cardiac markers, including myoglobin (> 1,000 μg/L), ultra-sensitive troponin I (0.191 μg/L), and creatine kinase MB mass (25.82 μg/L), were elevated.
TABLE 1
| Measure | Patient 1 on admission | Patient 2 on admission | Patient 3 on admission | Reference range |
| Age (year)/sex | 73/M | 44/F | 52/M | |
| Symptoms | Sputum-producing cough; breathlessness | Recurrent fever; breathlessness | Recurring fever; breathlessness; chest pain | |
| Underlying condition | Membranous nephropathy; hypertension | Bilateral uveitis; hypertension | Post-allogeneic kidney transplantation; a left thyroidectomy; hepatitis B; hypertension | |
| Pre-admission immunosuppressant use | Tacrolimus (details unspecified) | MMF 6 pills qd; methotrexate 12.5 mg qw; adalimumab 400 mg q2w | Tacrolimus 1 g bid; sirolimus 1 tablet qd | |
| Pre-admission steroid use | MP (details unspecified) | MP 16 mg qd | Prednisone 2 tablets qd | |
| Body temperature (°C) | 36.5 | 36.2 | 36 | 36.1–37.2 |
| Respiratory rate (breaths/min) | 18 | 18 | 16 | 12–20 |
| Heart rate (beats/min) | 58 | 101 | 102 | 60–100 |
| Blood pressure (mmHg) | 154/85 | 105/77 | 141/95 | 90/60–120/80 |
| pH | 7.43 | 7.45 | 7.33 | 7.35–7.45 |
| HCO3 (mmol/L) | 19 | 16.7 | 12 | 22–27 |
| PO2 (mmHg) | 59 | 59 | 112 | 80–100 |
| PCO2 (mmHg) | 27 | 24 | 23.3 | 35–45 |
| SaO2 (%) | 91.7 | 95.6 | 97 | 95–98 |
| FiO2 (%) | 29 | 50 | 33 | 21–100 |
| OI (mmHg) | 203 | 118 | 339 | > 300 |
| WBC count (109/L) | 6.38 | 5.29 | 7.31 | 3.5–9.5 |
| Neutrophil (%) | 92.9 | 87.1 | 81.7 | 40–75 |
| Granulocyte Count (109/L) | 5.88 | 4.6 | 5.97 | 1.8–6.3 |
| Lymphocyte count (109/L) | 0.24 | 0.45 | 0.8 | 1.1–3.2 |
| CRP (mg/L) | 105 | 83.7 | 148 | 0–10 |
| PCT (ng/mL) | 1.08 | 0.08 | 10.9 | < 0.05 |
| D-Dimer (mg/L) | 0.72 | 11.14 | 2.68 | 0–0.5 |
| ESR (mm/h) | 97 | 98 | 120 | M: 0–15 F: 0–20 |
| Urea (mmol/L) | 19.9 | 6.2 | 20.9 | 2.9–8.2 |
| Creatinine (μmol/L) | 118.4 | 81.8 | 458 | 53–115 |
| Uric acid (μmol/L) | 589.6 | 133.5 | 605.1 | 155–428 |
| LDH (U/L) | 297.1 | 1,180.1 | 780.2 | 109–245 |
| Albumin (g/L) | 21.7 | 33.3 | 33.2 | 40–55 |
| ALT (U/L) | 32 | 168.1 | 17.4 | 7–50 |
| AST (U/L) | 20.7 | 83.7 | 30.6 | 13–40 |
| Cytomegalovirus DNA | Positive | Positive | Negative | Positive: > 5.82 × 104 copies/m |
| GM test | Positive | Negative | Positive | |
| G test | NA | NA | Negative | |
| Tests for respiratory pathogen antibodies | Negative | Negative | Negative | |
| Mycoplasma serological testing | Negative | Negative | NA | |
| Tuberculosis antibody testing | Negative | Negative | Negative | |
| IGRA | Negative | Negative | Negative | |
| TST | Negative | Negative | Negative | |
| Sputum smear | Negative | Negative | Negative | |
| Sputum culture | Negative | Negative | Negative | |
| BALF culture | Aspergillus flavus; Nocardia; Pseudodiphtheritic bacillus | Klebsiella pneumoniae; Penicillium | Aspergillus niger | |
| BALF GM test | Positive | Positive | Negative | |
| BALF tuberculosis DNA | Negative | Negative | Negative |
Patients’ basic conditions and results of physical / laboratory tests.
M, male; F, female; NA, not obtained; PO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide; SaO2, oxygen saturation in arterial blood; FiO2, fraction of inspired oxygen; OI, oxygenation index; WBC, white blood cell; CRP, C-reactive protein; PCT, procalcitonin; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GM test, galactomannan test; G test, (1, 3)-β-D-glucan test; IGRA, interferon-gamma release assay; TST, tuberculin skin test; BALF: bronchoalveolar lavage fluid; MMF, mycophenolate mofetil; MP, methylprednisolone.
On March 31, a fiberoptic bronchoscopy was performed, and bronchoalveolar lavage fluid (BALF) was collected for metagenomic next-generation sequencing (mNGS). The mNGS results were summarized in Table 2. While BALF culture results identified the presence of Aspergillus flavus, Nocardia, and Pseudo-diphtheritic bacillus. Additionally, the BALF GM test returned positive, while the tuberculosis DNA test was negative. Based on clinical symptoms, laboratory tests, and imaging findings, the patient was diagnosed with severe pneumonia and type I respiratory failure, attributed to a complex infection involving at least six pathogens, including PJ, Nocardia, Aspergillus (fumigatus and flavus), nontuberculous mycobacteria (NTM), CMV, Klebsiella aerogenes, yeasts, and bacilli.
TABLE 2
| Microbe | Microbial reads | Relative abundance |
| Pneumocystis jirovecii (PJ) | 38,413 | 15.52% |
| Nocardia arthritidis | 3,768 | 1.53% |
| Nontuberculous mycobacteria (NTM) | 453 | 0.19% |
| Aspergillus fumigatus | 421 | 0.18% |
| Candida albicans | 304 | 0.13% |
| Candida glabrata | 242 | 0.10% |
| Klebsiella oxytoca | 295 | 0.12% |
| Cytomegalovirus (CMV) | 12 | < 0.01% |
| Others | 20,349,980 | 82.22% |
mNGS results of Case 1.
Presented as microbial reads and relative abundance.
Concurrent administration of multiple anti-infective agents (antibacterial, antifungal, antiviral, and anti-NTM therapies) posed a high risk of drug interactions, hepatorenal strain, and adverse effects such as gastrointestinal intolerance. Therefore, treatment was implemented in two phases, prioritizing rapidly progressing pathogens with high mortality risks (PJ, Nocardia, Klebsiella aerogenes, and CMV) in Phase 1 and addressing slow-growing pathogens (e.g., Aspergillus and NTM) in Phase 2.
Phase 1: Initial management
On March 30, the patient continued with 24 mg of MP daily (Figure 2). Starting March 31, sulfamethoxazole-trimethoprim (SMX-TMP) injections were administered every 8 h to target Nocardia and PJ. After six days, due to adequate blood drug levels, the regimen was adjusted to 160 mg every six hours. Meropenem (1 g every 12 h) and ganciclovir (125 mg every 12 h) were prescribed for antibacterial and antiviral purposes, respectively. By April 4, inflammatory markers (CRP: 33.8 mg/L; PCT: 1.26 ng/mL) had decreased, reflecting slight clinical improvement. However, a chest CT on April 7 revealed progression of pneumonia (Figures 1C, D). Despite this, the treatment plan was maintained due to the delayed response in imaging. MP remained the sole immunosuppressive drug.
FIGURE 2
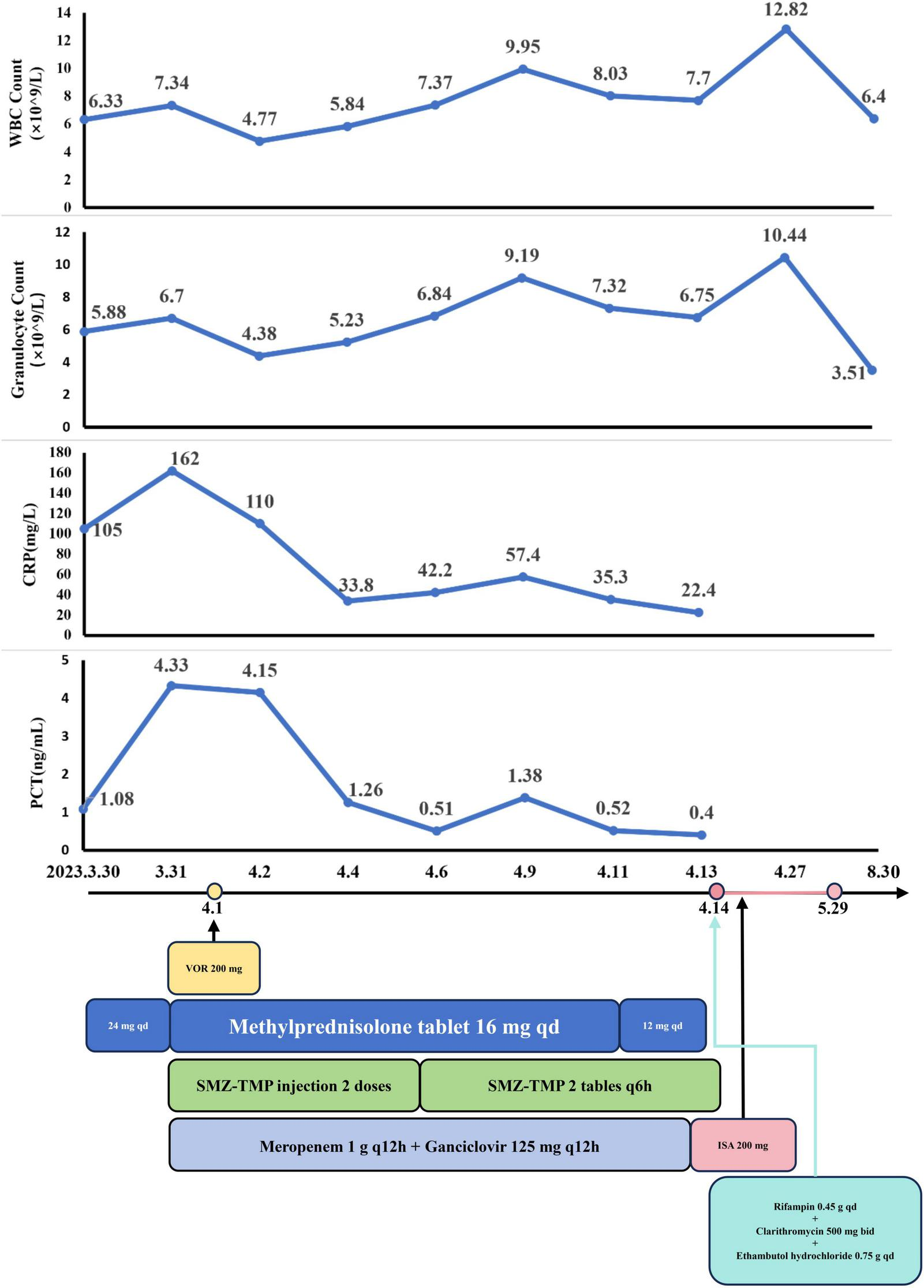
The dynamic of laboratory indicators and treatment timeline for case 1. MP, Methylprednisolone; SMZ-TMP, Sulfamethoxazole and Trimethoprim; VOR, Voriconazole; ISA, Isavuconazole.
Phase 2: Antifungal and anti-NTM therapy
By April 13, there was a significant improvement in the patient’s symptoms, evidenced by the reduction in cough, sputum production, and wheezing. Arterial blood gas analysis showed normalization [FiO2: 43%, pH: 7.54, PO2: 125 mmHg, PCO2: 27 mmHg, : 26 mmol/L, SaO2: 99.4%, oxygenation index (OI): 291 mmHg], and inflammatory markers (CRP: 22.4 mg/L; PCT: 0.40 ng/mL) continued to decline. CMV DNA tests were negative twice consecutively. On April 14, partial resolution of pulmonary inflammation was observed via CT, although the largest consolidated nodule had increased to 4.8 cm × 6.2 cm (arrow) (Figures 1E, F). Meropenem and ganciclovir were discontinued.
VOR (April 1–April 13) was initially used for antifungal treatment but was replaced by ISA on April 14, due to suboptimal blood levels resulting from rapid drug metabolism. ISA was administered as 200 mg every 8 h for the first 48 h, followed by 200 mg daily. Additional treatments included SMX-TMP (21 days), rifampin (0.45 g qd), clarithromycin (500 mg bid), and ethambutol hydrochloride (0.75 g qd) for NTM. On April 17, the BALF fungal culture revealed no growth. By April 27, the left lung opacities showed further resolution, while the right lung opacities remained stable (Figures 1G, H).
Despite financial constraints leading to ISA (April 14–May 29) discontinuation after 45 days, follow-up on June 26 showed further reduction in pulmonary lesions. The largest consolidation had decreased to 4.0 cm × 3.3 cm (arrow) (Figures 1I, J). By August 30, pulmonary nodules and patchy opacities had gradually diminished.
Case 2
A 44-year-old female was admitted to the hospital on 25 June 2023, presenting with a 20-day history of recurrent fever and three days of breathlessness. Her fever peaked at 40°C, accompanied by chills, light-headedness, and exhaustion. After self-medicating without improvement, she sought treatment at a local hospital. A chest-enhanced CT scan revealed diffuse inflammation in both lungs, bronchiectasis in the right middle lobe, lingula of the left upper lobe, and the medial basal segment of the right lower lobe, as well as situs inversus totalis. The patient tested positive for COVID-19.
BALF mNGS results identified multiple pathogens, including PJ, Aspergillus fumigatus, Marnier-Lapostolle blue bacteria, CMV, COVID-19, Klebsiella pneumoniae, and Acinetobacter baumannii. Initial treatment at the local hospital included cephalosporins for bacterial infections, “Simnotrelvir/Ritonavir” for viral infections, “sulfamethoxazole” for PJ, and VOR for fungal infections. Despite these interventions, her symptoms did not improve, and follow-up testing showed abnormal liver function. She was subsequently transferred to our hospital.
On June 26, chest CT scans confirmed the findings of situs inversus totalis, along with bronchial dilation in the upper left and middle right lungs. Multiple patchy opacities and ground-glass attenuation were observed in both lungs (Figures 3A, B). The patient had been diagnosed with bilateral uveitis one year earlier and is on a comprehensive treatment regimen, including MP 16 mg qd, mycophenolate mofetil (MMF) 6 pills qd, methotrexate 12.5 mg qw, and adalimumab 400 mg q2w (Table 1). Besides, the patient had a one-year history of hypertension. Physical examination revealed coarse breathing sounds in both lungs. BALF mNGS results identified several pathogens, including PJ (n = 49,518 reads, 33.20%), Klebsiella pneumoniae (n = 181 reads, 0.13%), Aspergillus fumigatus (n = 82 reads, 0.06%), CMV (n = 8,292 reads, 5.56%), and others (n = 91,050 reads, 61.05%). The GM test was positive, while the tuberculosis DNA test was negative. Cultures from BALF grew Klebsiella pneumoniae and Penicillium. Based on these findings, the patient was diagnosed with severe pneumonia and type I respiratory failure caused by invasive pulmonary aspergillosis (IPA) and concurrent infections, including PJ, CMV, and Klebsiella pneumoniae.
FIGURE 3
Case 2, chest CT scans. This patient had situs inversus totalis. (A,B) On June 26: bronchial dilation in the upper left lung and middle right lung. Multiple patchy opacities and ground-glass attenuation in both lungs. (C,D) On June 30: significant progression of pneumonia. (E,F) On July 6: partial resolution of the patchy opacities and ground-glass attenuation. (G,H) On August 28: significant improvement and absorption of patchy opacities were observed after the systemic antifungal therapy.
Phase 1: Initial management
Given the patient’s abnormal liver function and the risk of hepatotoxicity from VOR, treatment was initiated with liver-protective measures. For PJ, SMX-TMP was administered as two injections q6h for three days, then transitioned to four tablets q6h. Meropenem (50 mL q8h) was prescribed for bacterial infections, ganciclovir (330 mg q12h) for CMV, and MP (16 mg qd) to prevent immunological rejection.
After three days, the patient showed significant clinical improvement, with resolution of fever and wheezing. Blood gas values were as follows: FiO2 50%, pH 7.47, PO2 81 mmHg, PCO2 25 mmHg, 22 mmol/L, SaO2 98.7%, and OI 162 mmHg. CRP levels decreased to 50 mg/L. However, follow-up chest CT scans showed progression of pneumonia (Figures 3C, D). Due to the delayed response in imaging, the initial treatment regimen was maintained.
Phase 2: Antifungal and comprehensive therapy
On June 29, treatment for aspergillosis was initiated with ISA at 200 mg q8h for the first 48 h, followed by 200 mg qd. To better control immunosuppression, MP was replaced with intravenous methylprednisolone sodium succinate (MPS) at 40 mg qd. By July 6, CT scans showed partial resolution of patchy opacities and ground-glass attenuation (Figures 3E, F), and liver function markers (AST, ALT) and CRP levels had normalized.
The patient was discharged on July 10 after completing a 14-day course of ganciclovir and meropenem. SMX-TMP was continued at four tablets q6h for 21 days. GM test results of July 14 and August 2 are negative. Follow-up CT scans on August 28 showed significant improvement, with further absorption of patchy opacities (Figures 3G, H). On September 2, ISA (June 29–September 2) was discontinued due to financial constraints, completing a 66-day treatment course (Figure 4).
FIGURE 4
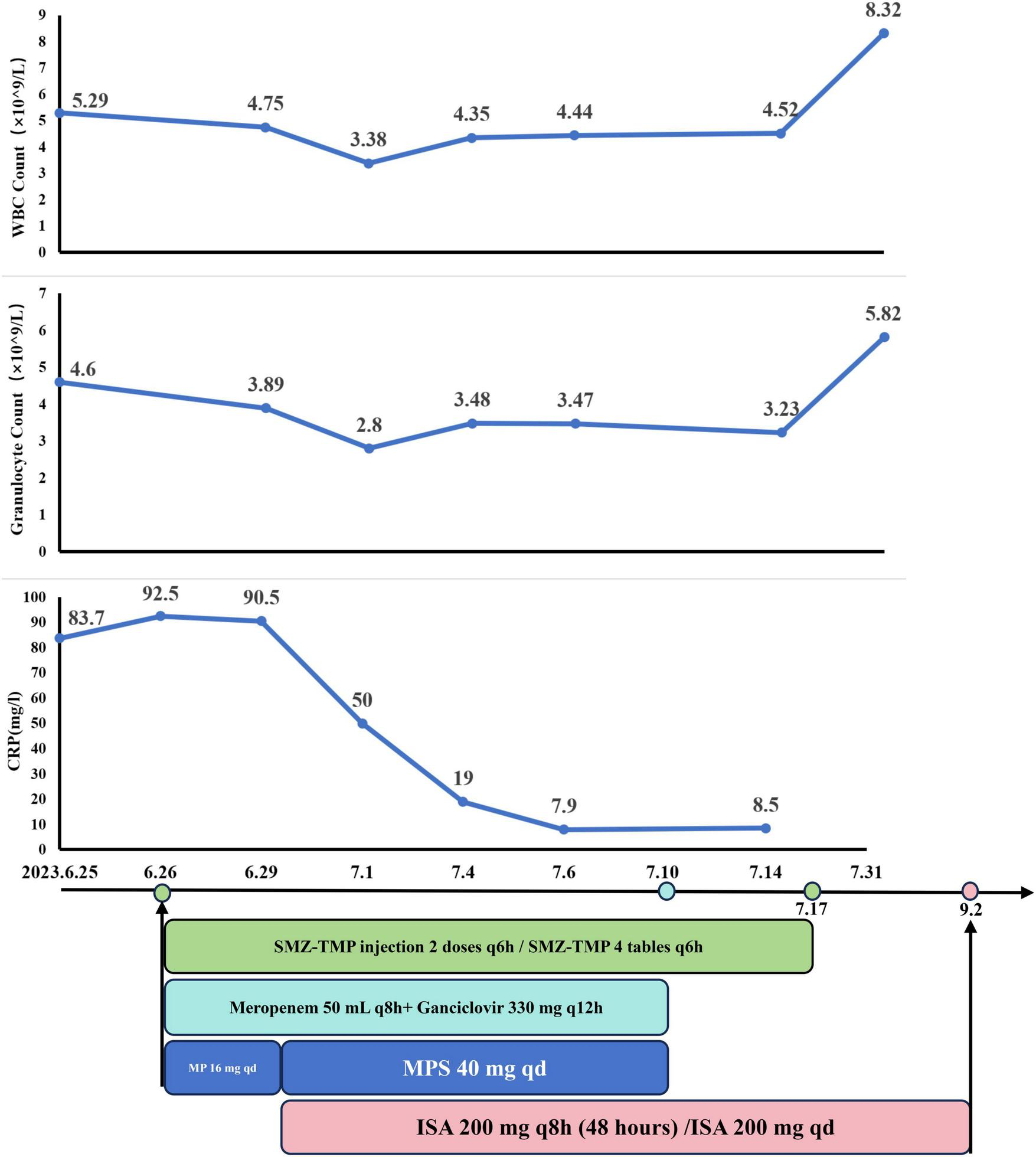
The dynamic of laboratory indicators and treatment timeline for case 2. SMZ-TMP, Sulfamethoxazole and Trimethoprim; MP, Methylprednisolone; MPS, methylprednisolone sodium succinate; ISA, Isavuconazole.
Case 3
A 52-year-old male was admitted to our department on 9 August 2023, with a three-week history of recurrent fever, chest pain, and breathlessness. His temperature had peaked at 39°C, accompanied by chills and fatigue. A chest CT revealed multiple patchy opacities and nodules in both lungs. The largest nodule was 1.8 cm × 1.7 cm located in the basal segment of the right inferior lobe (arrow) (Figures 5A, B).
FIGURE 5
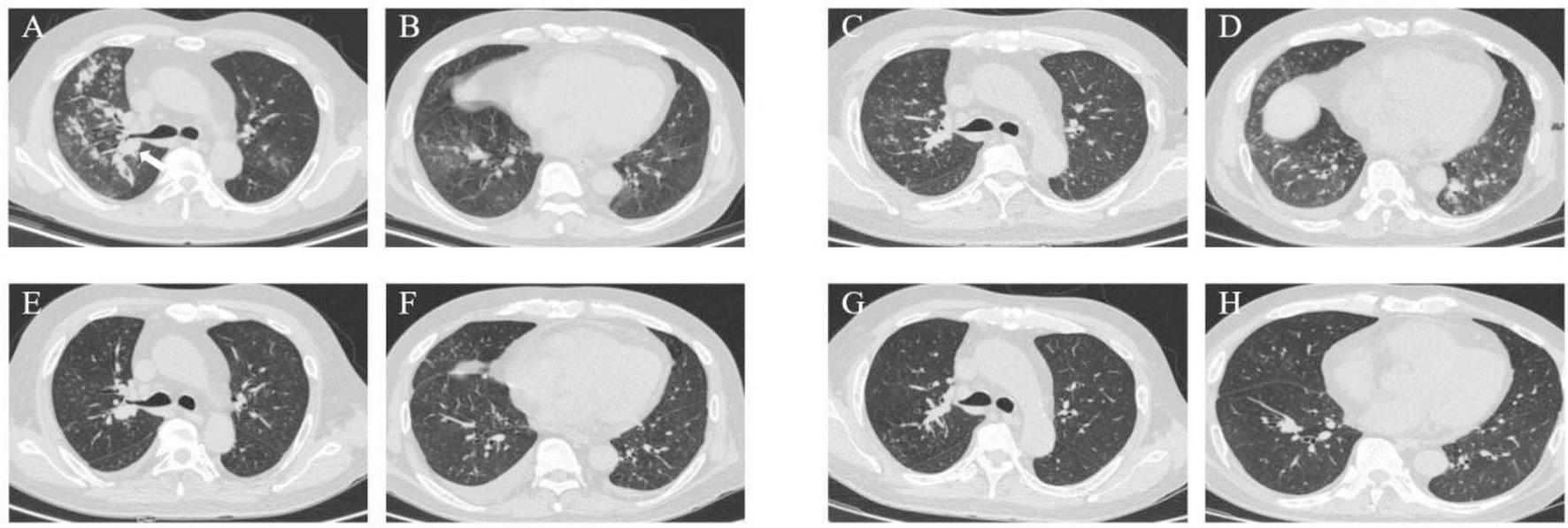
Case 3, chest CT scans. (A, B) On August 9: multiple patchy opacities and nodules in both lungs. The largest nodule was 1.8 × 1.7 cm in the basal segment of the right inferior lobe (arrow). (C,D) On August 17: slight improvement of the right pulmonary patchy opacities while progression of the lower lobe of the left lung. (E,F) On September 11: notable diminishment of multiple patchy opacities in both lungs. (G,H) On November 30: marked absorption of patchy opacities.
The patient received a kidney transplantation 18 years ago and is thus on long-term immunosuppressive medication, including tacrolimus (1 g bid), sirolimus (1 tablet qd), and prednisone (2 tablets qd). He had undergone a left thyroidectomy 11 years ago for thyroid cancer and had a 10-year history of hepatitis B managed with Entecavir, as well as a 4-year history of hypertension managed with Losartan. Following the recurrence and metastasis of thyroid cancer two years ago, the patient had been on regular treatment with Sorafenib. Physical examination upon admission revealed coarse breathing sounds, wet rales, and wheezing in both lungs. BALF mNGS analysis revealed PJ (n = 27,865 reads, 14.40%) and Aspergillus fumigatus (n = 54,451 reads, 28.13%). Laboratory and BALF mNGS findings, along with a positive influenza A RNA test from a throat swab, supported a diagnosis of IPA with concurrent infections from PJ and influenza A virus. Detailed laboratory test results are provided in Table 1.
Phase 1: Initial management
The initial treatment regimen targeted PJ with SMZ-TMP (160 mg q8h) for 21 days. Antiviral therapy included oseltamivir at 30 mg qd for 3 days, and cefoperazone-sulbactam (1.5 g q12h) was administered for antibacterial prophylaxis. Corticosteroids were prescribed to mitigate immunological rejection and inflammation.
A follow-up chest CT on August 17 revealed slight improvement in the right lung but progression in the lower lobe of the left lung (Figures 5C, D). This indicated ongoing pneumonia progression, prompting continuation of the original treatment plan.
Phase 2: Antifungal therapy and liver function management
The patient was discharged on August 28 and initiated antifungal treatment on VOR at 200 mg q12h. A follow-up chest CT on September 11 showed significant resolution of multiple patchy opacities in both lungs (Figures 5E, F). However, subsequent tests revealed a positive blood GM test and elevated liver enzymes (ALT: 212.4 U/L, AST: 202.3 U/L), leading to a diagnosis of drug-induced liver injury.
Consequently, on September 18, after completing a 21-day course of VOR (August 29–September 18), the anti-fungal medication was switched to ISA. By October 3, follow-up laboratory tests showed normalization of liver function, including ALT, AST, and complete blood count. ISA (September 18–November 1) treatment continued for 45 days (Figure 6) but was discontinued by the patient on his own initiative due to financial constraints.
FIGURE 6
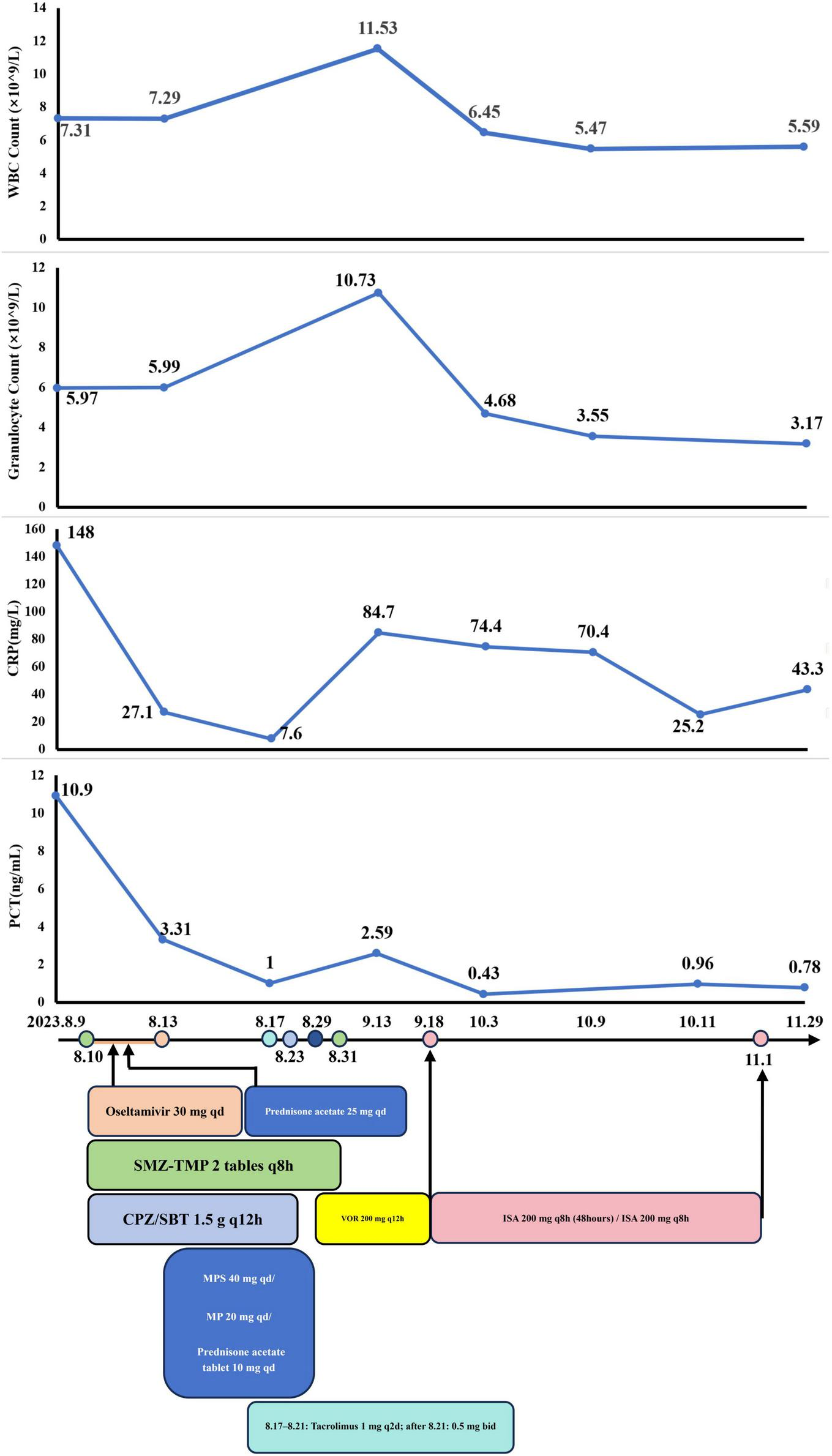
The dynamic of laboratory indicators and treatment timeline for case 3. SMZ-TMP, Sulfamethoxazole and Trimethoprim; CPZ/SBT, Cefoperazone Sulbactam; MP, Methylprednisolone; MPS, methylprednisolone sodium succinate; VOR, Voriconazole; ISA, Isavuconazole.
Aside from the unscheduled review in November for personal reasons, the patient’s monthly routine evaluations of BALF cultures and GM test results from October 2023 to May 2024 were consistently negative. A chest CT on November 30 revealed marked absorption of patchy opacities (Figures 5G, H). The patient achieved substantial clinical and radiological improvement following antifungal therapy, particularly with ISA.
Discussion
The 2022 WHO fungal priority pathogens list identifies Aspergillus as one of the four critical pathogens responsible for invasive fungal infections (7). Clinical presentations of IPA include breathlessness, fever, cough, chest or pleuritic pain, and hemoptysis (8). However, the classic halo sign (a pulmonary nodule surrounded by a region of low attenuation) is often absent in high-risk IPA patients, making radiological findings indistinguishable from other pulmonary infections (9). Due to severe immunodeficiency, ICHs infected with Aspergillus are more likely to develop IPA rather than noninvasive diseases (10).
ICHs include individuals with Human Immunodeficiency Virus (HIV), solid organ transplant recipients, cancer patients, and those undergoing immunomodulatory therapy for autoimmune diseases (11). IPA in ICHs is associated with high mortality and poses significant treatment challenges (12, 13). Recent studies report mortality rate of 40%–50% in Aspergillus infections among patients with acute leukemia and hematopoietic stem cell transplants (14–16). A 2021 review by Paramythiotou et al. (17) evaluated six ICU patients with COVID-19-associated IPA and no prior history of immunosuppression. Five patients were treated with ISA and one with another antifungal medication, resulting in a 67% mortality rate (4/6). Among those treated with ISA, two were transferred to a general ward, while the other three died (17). Nevertheless, reports of successful ISA use in ICHs with IPA and co-infections remain limited. In our study, we present three cases of ICHs with IPA and co-infections causing severe pneumonia and respiratory failure. Combination therapies, including ISA and other medications, effectively managed all three cases, providing insights for clinical practice.
To minimize interactions between antimicrobial drugs and immunosuppressants, corticosteroids were used in the initial phase to reduce immune rejection and inflammation. A 2023 review by Silva et al. (18) highlighted the role of ISA in treating invasive fungal infections in solid organ transplant recipients, emphasizing the importance of adjusting tacrolimus and mTOR inhibitor dosages during treatment to mitigate drug interactions.
In cases of ICHs with severe pneumonia from complex infections, rapid pathogen identification is critical. Traditional diagnostic methods, such as smears, cultures, and serological tests, are inefficient and lack specificity. Advanced molecular techniques like mNGS and PCR enable rapid and sensitive identification of pathogens, even for those undetectable by conventional methods (19). In this case series, immediate bronchoscopy and BALF testing using mNGS clearly identified multiple pathogens and contributed to effective therapy.
Subsequent treatment was tailored based on pathogen virulence, disease progression, treatment duration, patient condition, and medication tolerance. All three patients had multi-system dysfunction and limited tolerance to certain drugs, necessitating a phased treatment approach. Priority was given to rapidly progressing pathogens with high mortality rates, such as PJ, Nocardia, and CMV. Epidemiological data from Germany (2014–2019) show a rise in PJ incidence from 1,857 to 2,172 cases, with related mortality increasing from 516 to 615 cases (20, 21). Early diagnosis and treatment are necessary for PJP patients because of the high risk of respiratory failure. Nocardia pneumonia, which can cause serious complications and life-threatening infections, requires prioritized treatment too (22). CMV pneumonia, which progresses rapidly, can increase the risk of infections from other pathogens like PJP, elevate the risk of disseminated NTM infections, and intensify the severity of COVID-19 infections. Therefore, CMV is also addressed in the initial treatment phase (23–25). In contrast, early Aspergillus infections often show no symptoms and progress relatively slowly in hosts without neutropenia, while symptoms usually appear several weeks later (26). NTM has a long treatment period (usually over 12 months), and its progression is relatively slow (27). Three patients are non-neutropenic hosts. Hence, controlling fast-progressing and highly lethal pathogen infections should be the first priority, followed by long-term comprehensive antibiotic treatment for Aspergillus and NTM.
Furthermore, it’s significant to select antimicrobial drugs that are not only highly effective but also have minimal side effects. Medications for aspergillosis include polyenes, echinocandins, flucytosine, and triazoles. Notably, triazoles consist of itraconazole, posaconazole, VOR, and ISA. ISA, as the latest generation of triazoles, has several advantages, including fewer adverse reactions, straightforward drug interactions, linear and predictable pharmacokinetics, and no QTc interval prolongation (5). Compared to other triazoles, ISA claims to have an oral and intravenous bioavailability of nearly 98%, which is unaffected by food intake, changes in stomach pH, or mucositis (28, 29). Moreover, ISA has the longest half-life and exhibits high tissue penetration. ISA achieves stable drug concentrations in nearly all tissues, including the brain (which has a blood-brain barrier), within two weeks. Importantly, it doesn’t accumulate in physiological fluids or tissues over time (30). However, lipophilic itraconazole and posaconazole may not adequately reach some organs, such as the prostate, brain, and eyes (31). VOR, a primary treatment for invasive fungal diseases since 2002, has a narrow therapeutic window and complex drug interactions. The interactions arise particularly with drugs commonly used in the ICU, due to VOR’s metabolism involving CYP2C19, CYP2C9, and CYP3A4 (32–34). ISA is a recommended treatment for IPA because of the lowest incidence of side effects among triazoles and its efficacy (35). In 2016, Maertens et al. conducted a Phase III, double-blind, global, multicenter, randomized controlled non-inferiority trial that confirmed the efficacy of ISA in treating IPA to be comparable to that of VOR, with fewer adverse reactions (36, 37). Further analysis by Horn et al. (38) highlighted ISA’s ability to significantly reduce hospitalization duration for patients with moderate to severe renal insufficiency. And ISA is the only triazole that doesn’t require routine therapeutic drug monitoring, according to the Guidelines of the Infectious Diseases Society of America and the European Conference on Infections in Leukemia (39, 40). In 2023, a multicenter observational case study in China reported a 75% remission rate for ISA in the treatment of invasive fungal diseases (41). ISA serves as a safe and effective alternative for patients who are resistant or intolerant to other triazoles (42). Throughout the treatment process, our therapeutic decisions were guided by the 2016 IDSA Clinical Practice Guideline, which recommends a minimum treatment duration of 6–12 weeks for IPA (43). In this case series, ISA demonstrated excellent tolerability and efficacy. In Case 1, VOR (13 days) was replaced with ISA (45 days) due to inadequate drug concentrations caused by rapid metabolism. In Cases 2 (ISA: 66 days) and 3 (VOR: 21 days; ISA: 45 days), ISA’s lower toxicity and improved tolerability made it the preferred choice for younger patients with abnormal liver and renal function. Additionally, first-line antibacterial treatment with SMX-TMP effectively managed PJP in all three cases (44). ISA’s benefits include fewer drug interactions, reduced liver and kidney toxicity, and cost-efficiency, as shown by economic models in the UK, US, and Sweden (45–47). According to our follow-up calls or CT scans (Figures 1I–L, 5F, G), none of these patients experienced a recurrence.
The limitations of our paper include patients discontinued ISA treatment due to financial constraints. Consequently, we lack clinical data on comprehensive treatment regimens for ICHs infected with IA and other pathogens. Further studies are warranted to assess ISA’s long-term efficacy. Additionally, because of the extremely low survival rate of ICHs co-infected with IA and other pathogens, we only report three successful cases. ICHs in our study have different underlying diseases taking various immunosuppressants and steroids. Consequently, it is challenging to summarize and formulate a general guideline on how to adjust the doses of specific immunosuppressants and steroids when treating complex infections of IA and other pathogens. The concurrent use of multi-drug therapies is another constraint of the study. Therefore, more controlled studies are needed to evaluate the efficacy of ISA accurately in the future. While our case series reports suggest ISA’s potential clinical utility, further large-scale, multicenter trials are warranted to validate these observations. Nevertheless, our study addresses the existing gaps in the clinical use of ISA for complex infections. Moreover, our study offers a novel insight into the concurrent administration of ISA with immunosuppressants and corticosteroids. Furthermore, our study contributes to improved survival rates in the treatment of ICHs with complex infections. Collectively, this study highlights ISA as a valuable option for treating ICHs with IPA and complex co-infections.
Conclusion
This study highlights the challenges and complexities of managing severe pneumonia with IPA and co-infections in ICHs. Prompt pathogen identification using advanced diagnostic methods, such as mNGS, and a phased treatment approach prioritizing rapidly progressing pathogens, were critical for successful outcomes. ISA demonstrated excellent efficacy and tolerability in all three cases, offering a safe and effective alternative to VOR, particularly in patients with compromised hepatic and renal function. The use of ISA minimized drug interactions and adverse effects, ensuring better compatibility with the patients’ underlying conditions and concurrent therapies. These findings underscore the importance of individualized, stepwise treatment strategies in ICHs and support ISA as a valuable option for the management of IPA and complex co-infections, paving the way for improved clinical outcomes in this high-risk population.
Statements
Data availability statement
The original data presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the 900th Hospital of the Joint Logistics Support Force of the Chinese People’s Liberation Army. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XW: Writing – original draft, Data curation, Formal Analysis, Investigation, Visualization. YY: Writing – original draft, Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review and editing. SC: Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. PW: Data curation, Writing – original draft. SZ: Project administration, Writing – original draft. LZ: Validation, Writing – original draft. MW: Data curation, Writing – original draft. GL: Supervision, Writing – original draft. ZY: Supervision, Writing – original draft. GY: Resources, Writing – review and editing. WW: Funding acquisition, Project administration, Resources, Validation, Writing – original draft, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This research was supported by the High-Quality Medical Specialty Program of the Joint Logistic Support Force (grant no. LQYZ-HX) and the Key Project of the 900th Hospital of the Joint Logistics Support Force (2023SA05 to WW).
Acknowledgments
We would like to express our sincere gratitude to all the physicians involved in the care of the participating patients. We extend our heartfelt thanks to the participating patients and their families for their invaluable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Cheng G Crothers K Aliberti S Bergeron A Boeckh M Chien J et al Immunocompromised host pneumonia: Definitions and diagnostic criteria: An official American thoracic society workshop report. Ann Am Thorac Soc. (2023) 20:341–53. 10.1513/AnnalsATS.202212-1019ST
2.
McGrath B Broadhurst M Roman C . Infectious disease considerations in immunocompromised patients.JAAPA. (2020) 33:16–25. 10.1097/01.JAA.0000694948.01963.f4
3.
Azoulay E Russell L Van de Louw A Metaxa V Bauer P Povoa P et al Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med. (2020) 46:298–314. 10.1007/s00134-019-05906-5
4.
Letourneau A Issa N Baden L . Pneumonia in the immunocompromised host.Curr Opin Pulm Med. (2014) 20:272–9. 10.1097/MCP.0000000000000051
5.
Jenks J Hoenigl M . Treatment of Aspergillosis.J Fungi. (2018) 4:98. 10.3390/jof4030098
6.
Lewis J Wiederhold N Hakki M Thompson G . New perspectives on antimicrobial agents: Isavuconazole.Antimicrob Agents Chemother. (2022) 66:e0017722. 10.1128/aac.00177-22
7.
World Health Organization [WHO]. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. (2022). Available online at: https://www.who.int/publications/i/item/9789240060241(accessed October 25, 2022).
8.
Thompson G Patterson T . Pulmonary aspergillosis: Recent advances.Semin Respir Crit Care Med. (2011) 32:673–81. 10.1055/s-0031-1295715
9.
Denning D . Invasive aspergillosis.Clin Infect Dis. (1998) 26:781–803; quiz 804-5. 10.1086/513943
10.
Giannella M Lanternier F Dellière S Groll A Mueller N Alastruey-Izquierdo A et al Invasive fungal disease in the immunocompromised host: Changing epidemiology, new antifungal therapies, and management challenges. Clin Microbiol Infect. (2025) 31:29–36. 10.1016/j.cmi.2024.08.006
11.
Fung M Babik JM . COVID-19 in immunocompromised hosts: What we know so far.Clin Infect Dis. (2021) 72:340–50. 10.1093/cid/ciaa863
12.
Patterson T . Advances and challenges in management of invasive mycoses.Lancet. (2005) 366:1013–25. 10.1016/S0140-6736(05)67381-3
13.
Ullmann A Aguado J Arikan-Akdagli S Denning D Groll A Lagrou K et al Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. (2018) 24(Suppl 1):e1–38. 10.1016/j.cmi.2018.01.002
14.
Montagna M Lovero G Coretti C Martinelli D Delia M De Giglio O et al SIMIFF study: Italian fungal registry of mold infections in hematological and non-hematological patients. Infection. (2014) 42:141–51. 10.1007/s15010-013-0539-3
15.
Lortholary O Gangneux J Sitbon K Lebeau B de Monbrison F Le Strat Y et al Epidemiological trends in invasive aspergillosis in France: The SAIF network (2005-2007). Clin Microbiol Infect. (2011) 17:1882–9. 10.1111/j.1469-0691.2011.03548.x
16.
Robin C Cordonnier C Sitbon K Raus N Lortholary O Maury S et al Mainly post-transplant factors are associated with invasive aspergillosis after allogeneic stem cell transplantation: A study from the surveillance des aspergilloses invasives en france and société francophone de greffe de moelle et de thérapie cellulaire. Biol Blood Marrow Transplant. (2019) 25:354–61. 10.1016/j.bbmt.2018.09.028
17.
Paramythiotou E Dimopoulos G Koliakos N Siopi M Vourli S Pournaras S et al Epidemiology and incidence of COVID-19-associated pulmonary aspergillosis (CAPA) in a Greek tertiary care academic reference hospital. Infect Dis Ther. (2021) 10:1779–92. 10.1007/s40121-021-00486-8
18.
Silva J Husain S Aguado J . Isavuconazole for treating invasive mould disease in solid organ transplant recipients.Transpl Int. (2023) 36:11845. 10.3389/ti.2023.11845
19.
Li H . Editorial: mNGS for fungal pulmonary infection diagnostics.Front Cell Infect Microbiol. (2022) 12:864163. 10.3389/fcimb.2022.864163
20.
Kolbrink B Scheikholeslami-Sabzewari J Borzikowsky C von Samson-Himmelstjerna F Ullmann A Kunzendorf U et al Evolving epidemiology of pneumocystis pneumonia: Findings from a longitudinal population-based study and a retrospective multi-center study in Germany. Lancet Reg Health Eur. (2022) 18:100400. 10.1016/j.lanepe.2022.100400
21.
Xue T Kong X Ma L . Trends in the Epidemiology of Pneumocystis Pneumonia in Immunocompromised Patients without HIV Infection.J Fungi. (2023) 9:812. 10.3390/jof9080812
22.
Lynch J Reid G Clark N . Nocardia spp.: A rare cause of pneumonia globally.Semin Respir Crit Care Med. (2020) 41:538–54. 10.1055/s-0040-1708816
23.
Alanio C Verma A Mathew D Gouma S Liang G Dunn T et al Cytomegalovirus latent infection is associated with an increased risk of COVID-19-related hospitalization. J Infect Dis. (2022) 226:463–73. 10.1093/infdis/jiac020
24.
Tian B Sun J Bai J Zhang R Liu J Shen Y et al Cytomegalovirus viremia is associated with poor outcomes in AIDS patients with disseminated nontuberculous mycobacterial disease. Biosci Trends. (2022) 15:406–12. 10.5582/bst.2021.01279
25.
Lee S Huh K Joo D Kim M Kim S Lee J et al Risk factors for Pneumocystis jirovecii pneumonia (PJP) in kidney transplantation recipients. Sci Rep. (2017) 7:1571. 10.1038/s41598-017-01818-w
26.
Kosmidis C Denning D . The clinical spectrum of pulmonary aspergillosis.Thorax. (2015) 70:270–7. 10.1136/thoraxjnl-2014-206291
27.
Gopalaswamy R Shanmugam S Mondal R Subbian S . Of tuberculosis and non-tuberculous mycobacterial infections - a comparative analysis of epidemiology, diagnosis and treatment.J Biomed Sci. (2020) 27:74. 10.1186/s12929-020-00667-6
28.
Kovanda L Marty F Maertens J Desai A Lademacher C Engelhardt M et al Impact of mucositis on absorption and systemic drug exposure of isavuconazole. Antimicrob Agents Chemother. (2017) 61:e101–17. 10.1128/AAC.00101-17
29.
Schmitt-Hoffmann A Desai A Kowalski D Pearlman H Yamazaki T Townsend R . Isavuconazole absorption following oral administration in healthy subjects is comparable to intravenous dosing, and is not affected by food, or drugs that alter stomach pH.Int J Clin Pharmacol Ther. (2016) 54:572–80. 10.5414/CP202434
30.
Schmitt-Hoffmann A Kato K Townsend R Potchoiba M Hope W Andes D et al Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob Agents Chemother. (2017) 61:e01292-17. 10.1128/AAC.01292-17
31.
Felton T Troke P Hope W . Tissue penetration of antifungal agents.Clin Microbiol Rev. (2014) 27:68–88. 10.1128/CMR.00046-13
32.
Herbrecht R Denning D Patterson T Bennett J Greene R Oestmann J et al Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. (2002) 347:408–15. 10.1056/NEJMoa020191
33.
Jenks J Mehta S Hoenigl M . Broad spectrum triazoles for invasive mould infections in adults: Which drug and when?Med Mycol. (2019) 57(Suppl_2):S168–78. 10.1093/mmy/myy052
34.
Hoenigl M Duettmann W Raggam R Seeber K Troppan K Fruhwald S et al Potential factors for inadequate voriconazole plasma concentrations in intensive care unit patients and patients with hematological malignancies. Antimicrob Agents Chemother. (2013) 57:3262–7. 10.1128/AAC.00251-13
35.
Bellmann R Smuszkiewicz P . Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients.Infection. (2017) 45:737–79. 10.1007/s15010-017-1042-z
36.
Maertens J Raad I Marr K Patterson T Kontoyiannis D Cornely O et al Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet. (2016) 387:760–9. 10.1016/S0140-6736(15)01159-9
37.
Kontoyiannis D Giladi M Lee M Nucci M Raad I Bow E et al 1211 A Phase 3, Randomized, Double-Blind, Non-Inferiority Trial to Evaluate Efficacy and Safety of Isavuconazole versus Voriconazole in Patients with Invasive Mold Disease (SECURE): Outcomes in Invasive Aspergillosis Patients. Open Forum Infectious Diseases. Oxford: Oxford University Press (2014).
38.
Horn D Goff D Khandelwal N Spalding J Azie N Shi F et al Hospital resource use of patients receiving isavuconazole vs voriconazole for invasive mold infections in the phase III SECURE trial. J Med Econ. (2016) 19:728–34. 10.3111/13696998.2016.1164175
39.
Tissot F Agrawal S Pagano L Petrikkos G Groll A Skiada A et al ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. (2017) 102:433–44. 10.3324/haematol.2016.152900
40.
Patterson T Thompson G Denning D Fishman J Hadley S Herbrecht R et al Executive summary: Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. (2016) 63:433–42. 10.1093/cid/ciw444
41.
Wu L Li S Gao W Zhu X Luo P Xu D et al Real-world, multicenter case series of patients treated with isavuconazole for invasive fungal disease in China. Microorganisms. (2023) 11:2229. 10.3390/microorganisms11092229
42.
Bongomin F Maguire N Moore C Felton T Rautemaa-Richardson R . Isavuconazole and voriconazole for the treatment of chronic pulmonary aspergillosis: A retrospective comparison of rates of adverse events.Mycoses. (2019) 62:217–22. 10.1111/myc.12885
43.
Patterson T Thompson G Denning D Fishman J Hadley S Herbrecht R et al Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. (2016) 63:e1–60. 10.1093/cid/ciw326
44.
Li H Lu Y Tian G Wu Y Chen T Zhang J et al A regimen based on the combination of trimethoprim/sulfamethoxazole with caspofungin and corticosteroids as a first-line therapy for patients with severe non-HIV-related pneumocystis jirovecii pneumonia: A retrospective study in a tertiary hospital. BMC Infect Dis. (2024) 24:152. 10.1186/s12879-024-09031-7
45.
Floros L Kuessner D Posthumus J Bagshaw E Sjölin J . Cost-effectiveness analysis of isavuconazole versus voriconazole for the treatment of patients with possible invasive aspergillosis in Sweden.BMC Infect Dis. (2019) 19:134. 10.1186/s12879-019-3683-2
46.
Floros L Pagliuca A Taie A Weidlich D Rita Capparella M Georgallis M et al The cost-effectiveness of isavuconazole compared to the standard of care in the treatment of patients with invasive fungal infection prior to differential pathogen diagnosis in the United Kingdom. J Med Econ. (2020) 23:86–97. 10.1080/13696998.2019.1638789
47.
Harrington R Lee E Yang H Wei J Messali A Azie N et al Cost-effectiveness analysis of isavuconazole vs. Voriconazole as first-line treatment for invasive aspergillosis. Adv Ther. (2017) 34:207–20. 10.1007/s12325-016-0443-1
Summary
Keywords
severe pneumonia, immunocompromised hosts, invasive aspergillosis, isavuconazole, antifungal therapy
Citation
Wang X, You Y, Chen S, Wang P, Zeng S, Zhuang L, Wang M, Lai G, Yu Z, Yu G and Wen W (2025) Isavuconazole as an optimal treatment option for multiple pathogens induced severe pneumonia in immunocompromised hosts: a case series report. Front. Med. 12:1565071. doi: 10.3389/fmed.2025.1565071
Received
22 January 2025
Accepted
03 April 2025
Published
28 April 2025
Volume
12 - 2025
Edited by
James John, Sathyabama Institute of Science and Technology, India
Reviewed by
Sara Mina, Beirut Arab University, Lebanon
Nurul Asyikin Abdul Rahman, Universiti Teknologi Mara, Malaysia
Updates
Copyright
© 2025 Wang, You, Chen, Wang, Zeng, Zhuang, Wang, Lai, Yu, Yu and Wen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Wen, fjfzfisher@163.comGuoqing Yu, fjfzfisher@sina.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.