Abstract
Background:
Abortion usually refers to the loss of pregnancy before viability. Despite a potential link between neutrophil-lymphocyte ratio (NLR) levels and abortion, inconclusive findings remain. This review aimed to comprehensively appraise the predictive utility of NLR levels in abortion, offering a new approach to clarify its potential role as a biomarker.
Methods:
PubMed and Cochrane Library were searched for relevant cohort and case–control studies until September 2024. Odds ratio (OR) or weighted mean difference (WMD) with 95% CI of abortion were computed. Subgroup analyses were implemented to clarify potential sources of heterogeneity.
Results:
Higher NLR levels were linked to an enhanced risk of abortion as a continuous (WMD, 0.58; 95% CI: 0.29, 0.88) and dichotomous variable (OR, 1.33; 95% CI: 1.03, 1.72). In subgroup analyses, pooled results from studies with NLR cut-off>3, Asia populations, missed abortion, spontaneous abortion, and mean age>30 demonstrated an increased risk of abortion. In continuous NLR for predicting abortion, retrospective study, Europe populations, threatened abortion, recurrent pregnancy loss, and abortion had an enhanced risk of abortion for higher NLR levels as a dichotomous variable.
Conclusion:
Pooled analyses demonstrated that higher NLR levels may predict abortion. Further investigations need to determine whether these findings can be generalized to all populations.
Systematic review registration:
1 Introduction
According to the WHO definition, abortion refers to the expulsion or removal of a fetus (embryo) weighing < 500 grams (around 22 weeks of gestation). Abortion is usually defined as the loss of pregnancy before viability. With an estimated 23 million abortions worldwide each year, the overall risk of abortion is 15.3% (95% CI, 12.5–18.7%) of all confirmed pregnancies (1). It significantly raises the risk of complications such as coagulation disorders, endometrial damage, and infections, posing great harm to the physical and mental health of pregnant mothers.
The ability of the maternal immune system to fit in the developmental stages of the embryo is crucial for a successful pregnancy. A balanced inflammatory condition is necessary for healthy implantation and tissue remodeling. During embryo implantation, placenta formation, and the first trimester of pregnancy, abortion may be related to placental dysfunction, leading to systemic inflammation in the mother. An excessive maternal inflammatory response is a significant cause of early abortion (2). Many inflammatory cytokines are elevated in the serum of miscarried women (3). However, these markers are not cheap and are not suitable for routine testing. The neutrophil-lymphocyte ratio (NLR), a new index from the complete blood count, reflects the inflammatory state. Due to its availability and widespread use, more articles have stated that NLR is closely associated with the prognosis and incidence of many diseases (4). In recent years, the link between inflammation and abortion has gained more attention. The association between NLR levels and abortion has been extensively investigated. However, the results have been inconsistent (5–24).
There is a growing demand for potent evidence on the NLR level in abortion and its mechanisms. In a recent meta-analysis included 14 articles that Hantoushzadeh et al. revealed that NLR was higher in abortion patients than in healthy controls (25), but subsequent studies have obtained different findings. Çallıoğlu et al. (26) stated that NLR was not greatly distinct between the early pregnancy loss group and the control group, while Humadi et al. (27) showed that NLR was higher in abortion patients. Consequently, this review intended to illustrate the associations between inflammation, NLR levels, and abortion.
2 Methods
2.1 Literature search
This paper obeyed the PRISMA checklist. The study was prospectively registered in PROSPERO (CRD42023485726). Cochrane Library, PubMed, Embase, and Web of Science were searched until September 2024 for English articles that compared NLR levels between patients with and without abortion. The search terms used were ‘miscarriage’, ‘abortion’, ‘neutrophil’, ‘lymphocyte’, and ‘ratio’. Random combinations of subject terms and free words were utilized to retrieve relevant studies. The specific strategy in Pubmed was ((((“Neutrophils”[Mesh]) OR ((((((((((((((Neutrophil) OR (Leukocytes, Polymorphonuclear)) OR (Leukocyte, Polymorphonuclear)) OR (Polymorphonuclear Leukocyte)) OR (Polymorphonuclear Leukocytes)) OR (Polymorphonuclear Neutrophils)) OR (Neutrophil, Polymorphonuclear)) OR (Polymorphonuclear Neutrophil)) OR (LE Cells)) OR (Cell, LE)) OR (LE Cell)) OR (Neutrophil Band Cells)) OR (Band Cell, Neutrophil)) OR (Neutrophil Band Cell))) AND ((“Lymphocytes”[Mesh]) OR (((((Lymphocyte) OR (Lymphoid Cells)) OR (Cell, Lymphoid)) OR (Cells, Lymphoid)) OR (Lymphoid Cell)))) AND ((abortion) OR (miscarriage))) AND (ratio). Other search strategies are displayed in Supplementary Table S1. Additionally, the reference lists of all eligible studies were manually reviewed. Literature search and evaluation were performed independently by two investigators. Any disagreements were addressed through group discussion with a third investigator to get a final consensus.
2.2 Eligible criteria
The inclusion criteria covered: (a) study subjects: pregnant women without internal or obstetric diseases that would disrupt normal pregnancy, like gestational hypertension, infectious diseases, infertility, and gestational diabetes mellitus. (b) NLR = the neutrophil count/the lymphocyte count. (c) Observation population: Women with abortion. (d) Controls: Healthy women with normal pregnancy/delivery. (e) Study design: Observational study.
Articles were excluded for the following reasons (a) review, meeting report, meta-analysis, case report, editorial, comment, letter, note, trial registry record, or protocol. (b) non-human cases, such as animal research. (c) inadequate data on metal concentration. (d) unavailable full text.
2.3 Data extraction
Two investigators (Mi W Jun X) performed data extraction independently. Any disagreements were addressed by a third investigator (Fang Y) to make a final decision. The extracted information encompassed the first author, publication year, country, study design, period, abortion type, sample size, age, BMI, gestational age, NLR cut-off, NLR levels, odds ratio (OR), and 95% confidence interval (CI). When continuous variables were depicted as median with range or interquartile range, a validated mathematical method was adopted to calculate the mean ± standard deviation.
2.4 Quality assessment
To evaluate the study quality, we employed the Newcastle-Ottawa Scale (NOS), with 7–9 points indicating high quality (28). Two authors (Mi W Jun X) independently appraised each included article, and any disagreements were addressed via discussions with a third author (Fang Y).
2.5 Data analysis
Evidence synthesis was conducted utilizing Review Manager 5.4.1. Weighted mean difference (WMD) and OR were adopted for continuous and dichotomous variables. Forest plots were employed to present 95% CIs. Heterogeneity was appraised via Cochran’s Q-test and I2. A random-effects model was utilized in case of notable heterogeneity (p < 0.05, I2 > 50%). To determine the origin of heterogeneity, a subgroup analysis was conducted. Additionally, five subgroup analyses by different types of abortions, the continent of study populations, study type, age, and thresholds of NLR were performed. Publication bias was appraised utilizing funnel plots via Review Manager 5.4.1 and Egger’s tests via Stata 15.1. A p-value < 0.05 implied notable publication bias.
3 Results
3.1 Literature selection and study traits
The flowchart of literature screening is presented in Figure 1. 148 articles were found in Web of Science (n = 48), PubMed (n = 30), Embase (n = 67), and Cochrane (n = 3). After duplicates were ruled out, the titles and abstracts of 82 documents were scanned. 20 full-text articles with 6,913 patients (3,375 abortion versus 3,538 non-abortion) were finally included. Of these articles, 5 were prospective studies and 15 were retrospective studies. Only studies with quality scores > 6 were considered credible. Table 1 shows the traits and quality scores of the included studies. Quality assessment details are displayed in Supplementary Table S2.
Figure 1

Flowchart of literature screening.
Table 1
| Authors | Study period | Country | Study design | NLR threshold | Types of abortion | NO. | Age | BMI | Gestational age | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| case/control | case/control | case/control | case/control | score | ||||||
| Yavuz | 2018.1–2021.11 | Turkey | Retrospective | 4.65 | Missed abortion | 50/50 | 30.92 ± 5.13/27.85 ± 4.68 | NA | 11.74 ± 1.56/10.18 ± 1.34 | 7 |
| Yakıştıran | 2019.9–2020.1 | Turkey | Retrospective | 3.135 | Spontaneous abortion | 193/164 | 30.6 ± 6.8/27.8 ± 5.6 | NA | 7.5 ± 1.2/7.3 ± 1.3 | 7 |
| Biyik | 2015.1–2018.12 | Turkey | Retrospective | NA | Missed abortion | 40/40 | 29.27 ± 6.84/28.37 ± 5.13 | 25.47 ± 5.34/25.89 ± 5.71 | 54.82 ± 11.54/54.12 ± 12.04(days) | 7 |
| Christoforaki | 2014.1–2016.7 | Greece | Retrospective | NA | Abortion | 64/65 | NA | NA | NA | 6 |
| Wang | 2012.6–2018.5 | China | Retrospective | 2.402 | Missed abortion | 69/53 | 28.35 + 3.79/28.60 ± 5.78 | 22.16 ± 2.25/21.75 ± 2.55 | 7.43 ± 0.43/7.29 ± 0.43 | 7 |
| Cimsir | 2020.9–2020.12 | Turkey | Prospective | 4.27 | Recurrent pregnancy loss. | 44/60 | 30.5 ± 5.6/29.7 ± 6.0 | NA | NA | 7 |
| Uckan | 2020.1–2022.1 | Turkey | Retrospective | 2.99 | Missed abortion | 474/452 | 28.93 ± 3.99/28.55 ± 4.01 | 26.62 ± 1.84/26.70 ± 1.99 | 10.31 ± 0.83/10.49 ± 0.84 | 7 |
| Aydın | 2020.6–2020.11 | Turkey | Prospective | 2.8182 | Threatened abortion | 55/55 | 27.49 ± 5.87/28.25 ± 6.44 | 24.83 ± 5.45/25.67 ± 5.69 | 9.53 ± 3.67/10.66 ± 11.63 | 7 |
| Bas | 2012.1–2017.1 | Turkey | Retrospective | 3.24/3.34 | Spontaneous abortion | 173/152/245 | 31.88 ± 6.43/30.87 ± 6.19/30.15 ± 5.62 | 23.28 ± 1.70/23.56 ± 1.40/23.32 ± 1.65 | NA | 7 |
| Yazdizadeh | 2021.3–2022.3 | Iran | Retrospective | NA | Spontaneous abortion | 120/120 | 30.46 ± 4.44 /30.13 ± 4.18 | 22.52 ± 1.77/22.16 ± 1.73 | 59.98 ± 4.85/59.28 ± 5.82 (days) | 8 |
| Turgut | 2020.7–2021.7 | Turkey | Retrospective | 3.2 | Abortion | 709/676 | 30 ± 6/29 ± 6 | 29 ± 4/28 ± 5 | 7.6 ± 1.5/9.5 ± 3 | 7 |
| Soysal | 2019.1–2020.12 | Turkey | Retrospective | 3.94 | Threatened abortion | 150/150 | 29.0 ± 6.2/28.2 ± 5.9 | 28.0 ± 4.1/28.3 ± 3.5 | 9.1 ± 2.8/9.4 ± 2.5 | 7 |
| Jiang | 2012.1–2018.6 | China | Retrospective | 3.16 | Recurrent pregnancy loss. | 133/140 | 34.1 ± 3.9/33.4 ± 2.9 | 22.3 ± 2.9/21.8 ± 2.8 | NA | 7 |
| GORKEM | 2018.9–2019.8 | Turkey | Prospective | NA | Threatened abortion/ spontaneous abortion | 30/30/30 | 25.5 + 4.2/27.4 + 5.8/25.8 + 3.9 | 25.89 ± 5.99/24.58 ± 4.90/23.23 ± 3.58 | 8 ± 1.56/8.29 ± 1.56/8.18 ± 1.17 | 7 |
| Oğlak | 2019.9–2019.12 | Turkey | Retrospective | NA | Early pregnancy loss | 137/148 | 23.32 ± 3.26/26.09 ± 3.04 | 23.12 ± 3.66/23.78 ± 3.82 | NA | 7 |
| Ata | 2018.1–2019.5 | Turkey | Retrospective | 2.99/2.91 | Early pregnancy loss/threatened abortion | 100/100/100 | 27.7 ± 4.7/28.1 ± 4.0/27.1 ± 5.2 | NA | 10 ± 2.1/11 ± 0.9/10 ± 1.8 | 8 |
| Sert | 2018.1–2021.12 | Turkey | Retrospective | 2.59 | Missed abortion | 142/142 | 28.7 ± 6.9/27.1 ± 5.2 | NA | 7.9 ± 1.7/7.6 ± 1.2 | 7 |
| Uysal | 2014.4–2014.12 | Turkey | Prospective | NA | Missed abortion | 90/143 | 27.2 ± 6.7/26.7 ± 5.7 | 24 ± 3/24 ± 4 | 9.6 ± 1.9/9.3 ± 2.4 | 7 |
| Taskomur | 2020.6–2021.8 | Turkey | Prospective | 2.8083/NA | Threatened abortion/spontaneous abortion | 60/60/60 | 27.38 ± 5.75/29.58 ± 5.52/28.17 ± 6.29 | 24.76 ± 5.30/26.38 ± 5.95/25.76 ± 5.49 | 9.69 ± 3.80/10.11 ± 3.51/10.59 ± 11.16 | 7 |
| Liu | 2018.1–2020.12 | China | Retrospective | NA | Missed abortion | 200/200 | 27.86 ± 2.93/26.93 ± 2.93 | 22.14 ± 2.69/21.96 ± 2.15 | 7.14 ± 1.10/7.14 ± 1.10 | 7 |
Baseline study characteristics and methodological assessment.
3.2 Meta-analysis
3.2.1 Link between abortion and NLR levels
Data were synthesized from 20 studies, containing 6,913 patients (3,375 abortion versus 3,538 non-abortion). Pooled analysis (Figure 2) revealed significantly higher NLR levels in the abortion cohort (WMD: 0.58; 95% CI: 0.29, 0.88; p = 0.0001) with notable heterogeneity (I2 = 94%, p < 0.00001). A funnel plot (Figure 3) noted slight publication bias. But Egger’s test uncovered no publication bias (p = 0.700).
Figure 2
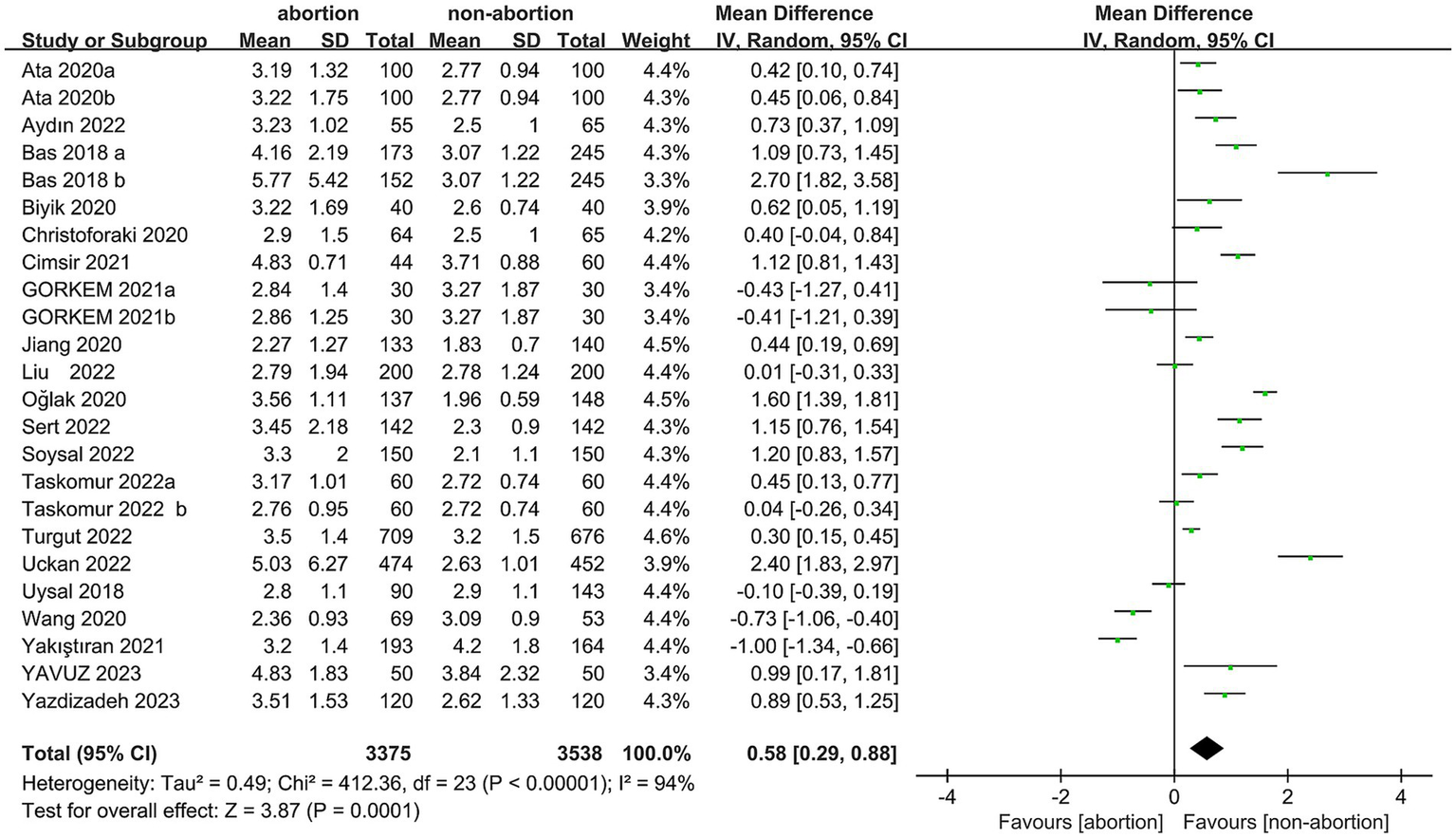
Forest plots of the relationship between abortion and NLR.
Figure 3

Funnel plots of the relationship between abortion and NLR.
3.2.2 The risk of abortion and NLR levels
6 studies with 2,249 patients (1,175 abortion versus 1,074 non-abortion) were included in the analysis for risk of abortion. Pooled analysis (Figure 4) indicated that pregnant women with high NLR were at greatly higher abortion risk (OR: 1.33; 95% CI: 1.03, 1.72; p = 0.03) with marked heterogeneity (I2 = 94%, p < 0.00001). Both the funnel plot (Figure 5) and Egger’s test (p = 0.162) did not showcase publication bias.
Figure 4
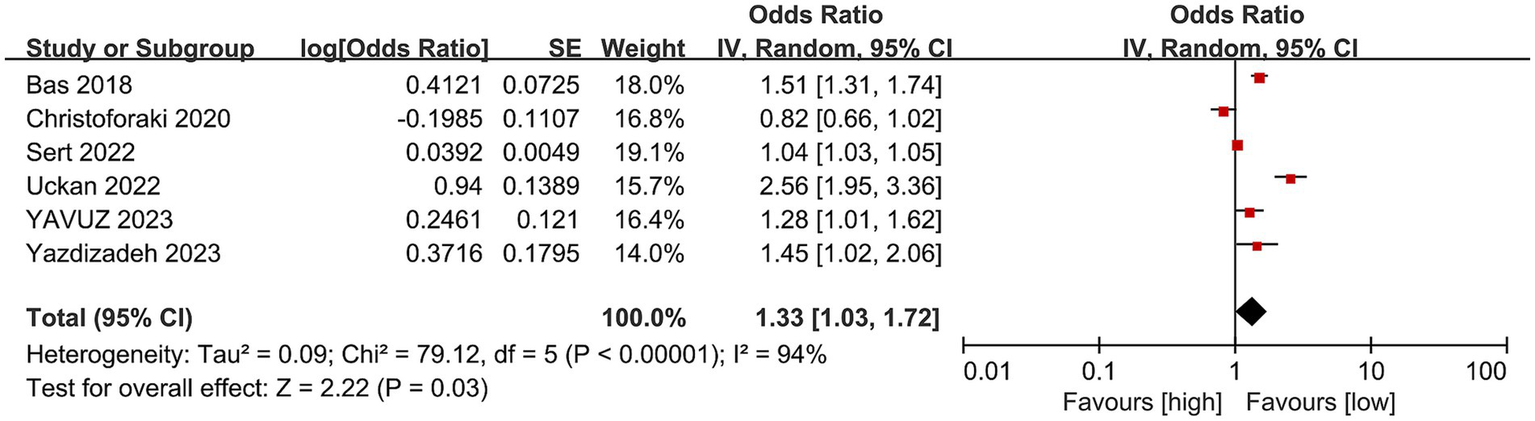
Forest plots of the risk of abortion and NLR.
Figure 5
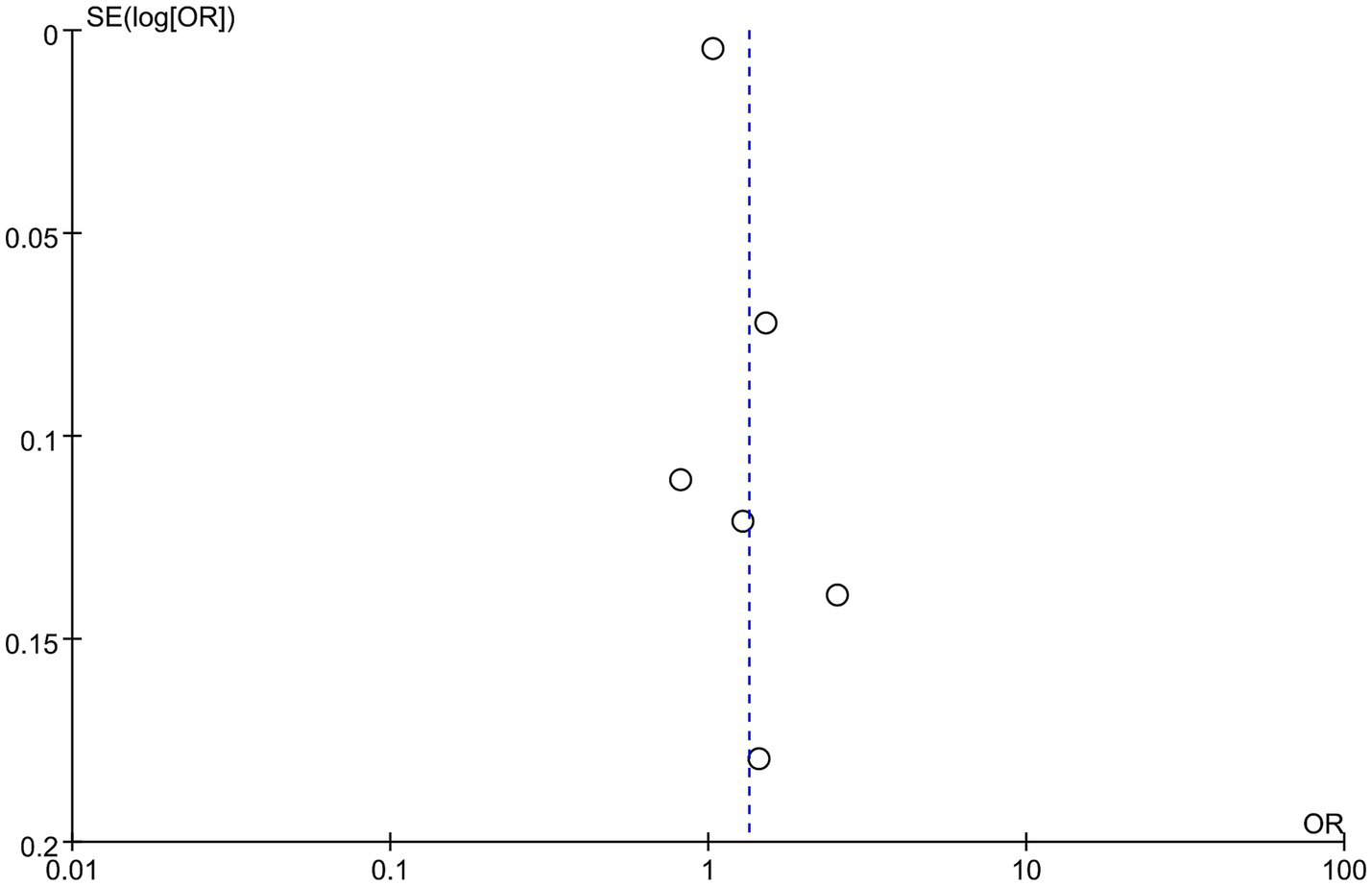
Funnel plots of the risk of abortion and NLR.
3.3 Subgroup
Subgroup analyses were done by study design, the NLR threshold, region, types of abortion, and mean age to compare the prevalence of abortion contraceptive utilization across different studies. Subgroup analysis showed that in retrospective articles, abortion patients had higher NLR levels than controls, whereas this result was not observed in prospective articles. Moreover, NLR levels were markedly higher in European cohorts with abortion than in controls, but no significant difference was noted in Asians. Further subgroup analysis unveiled that NLR levels were greatly higher in threatened abortion patients, recurrent pregnancy loss (RPL) patients, and abortion patients than in controls. However, such differences were not observed in the missed abortion, spontaneous abortion, and early pregnancy loss subgroups (Table 2).
Table 2
| Subgroup | Risk of abortion | NLR level | ||||||
|---|---|---|---|---|---|---|---|---|
| Study | OR [95%CI] | p value | I 2 | Study | MD [95%CI] | p value | I 2 | |
| Total | 6 | 1.33 [1.03, 1.72] | 0.03 | 94% | 24 | 0.58 [0.29–0.88] | 0.0001 | 94% |
| Study design | ||||||||
| Prospective | 0 | NA | NA | NA | 7 | 0.27 [−0.15–0.68] | 0.21 | 88% |
| Retrospective | 6 | 1.33 [1.03, 1.72] | 0.03 | 94% | 17 | 0.72 [0.34–1.10] | 0.0002 | 96% |
| NLR threshold | ||||||||
| >3 | 2 | 1.43 [1.23, 1.67] | <0.00001 | 28% | 8 | 0.79 [0.27–1.31] | 0.003 | 95% |
| ≤3 | 2 | 1.61 [0.67–3.90] | 0.29 | 98% | 7 | 0.67 [0.08–1.27] | 0.03 | 95% |
| Region | ||||||||
| Asia | 1 | 1.45 [1.02–2.06] | 0.04 | NA | 4 | 0.15 [−0.48–0.78] | 0.64 | 94% |
| Europe | 5 | 1.32 [1.00, 1.74] | 0.05 | 95% | 20 | 0.67 [0.34–1.00] | 0.0001 | 94% |
| Types of abortion | ||||||||
| Missed abortion | 3 | 1.48 [0.91–2.43] | 0.12 | 96% | 7 | 0.59 [−0.10–1.29] | 0.1 | 95% |
| Spontaneous abortion | 2 | 1.50 [1.32, 1.71] | <0.00001 | 0% | 6 | 0.52 [−0.32–1.36] | 0.23 | 96% |
| Threatened abortion | 0 | NA | NA | NA | 5 | 0.57 [0.18–0.96] | 0.004 | 78% |
| Recurrent pregnancy loss | 0 | NA | NA | NA | 2 | 0.77 [0.11–1.44] | 0.02 | 91% |
| Early pregnancy loss | 0 | NA | NA | NA | 2 | 1.02 [−0.14–2.17] | 0.08 | 97% |
| Abortion | 1 | 0.82 [0.66–1.28] | 0.07 | NA | 2 | 0.31 [0.17–0.46] | 0.0001 | 0 |
| Mean age | ||||||||
| >30 | 3 | 1.45 [1.29, 1.62] | <0.00001 | 0% | 7 | 0.84 [0.17–1.51] | 0.01 | 95% |
| ≤30 | 2 | 1.61 [0.67–3.90] | 0.29 | 98% | 16 | 0.49 [0.14–0.85] | 0.006 | 95% |
Subgroup analysis.
Subgroup analyses by key study characteristics were also performed. Among different NLR cut-offs, an NLR ≥ 3 linked to a prominently higher incidence of abortion. Among different regions, higher NLR was connected to a visibly higher incidence of abortion in Asian patients. Among different types of abortion, higher NLR was connected to a noticeably higher incidence of missed abortion and spontaneous abortion. Among different mean ages, higher NLR was linked to a sensibly higher incidence of abortion when patients’ ages are above 30 (Table 2).
3.4 Sensitivity analysis
In the prediction of abortion by NLR, sensitivity analyses evinced that the pooled WMD remained unchanged after exclusion of any single study (Figure 6). However, in the link of NLR with abortion risk, sensitivity analysis unraveled that the removal of three studies (6, 18, 23) altered the overall effect (Figure 7).
Figure 6
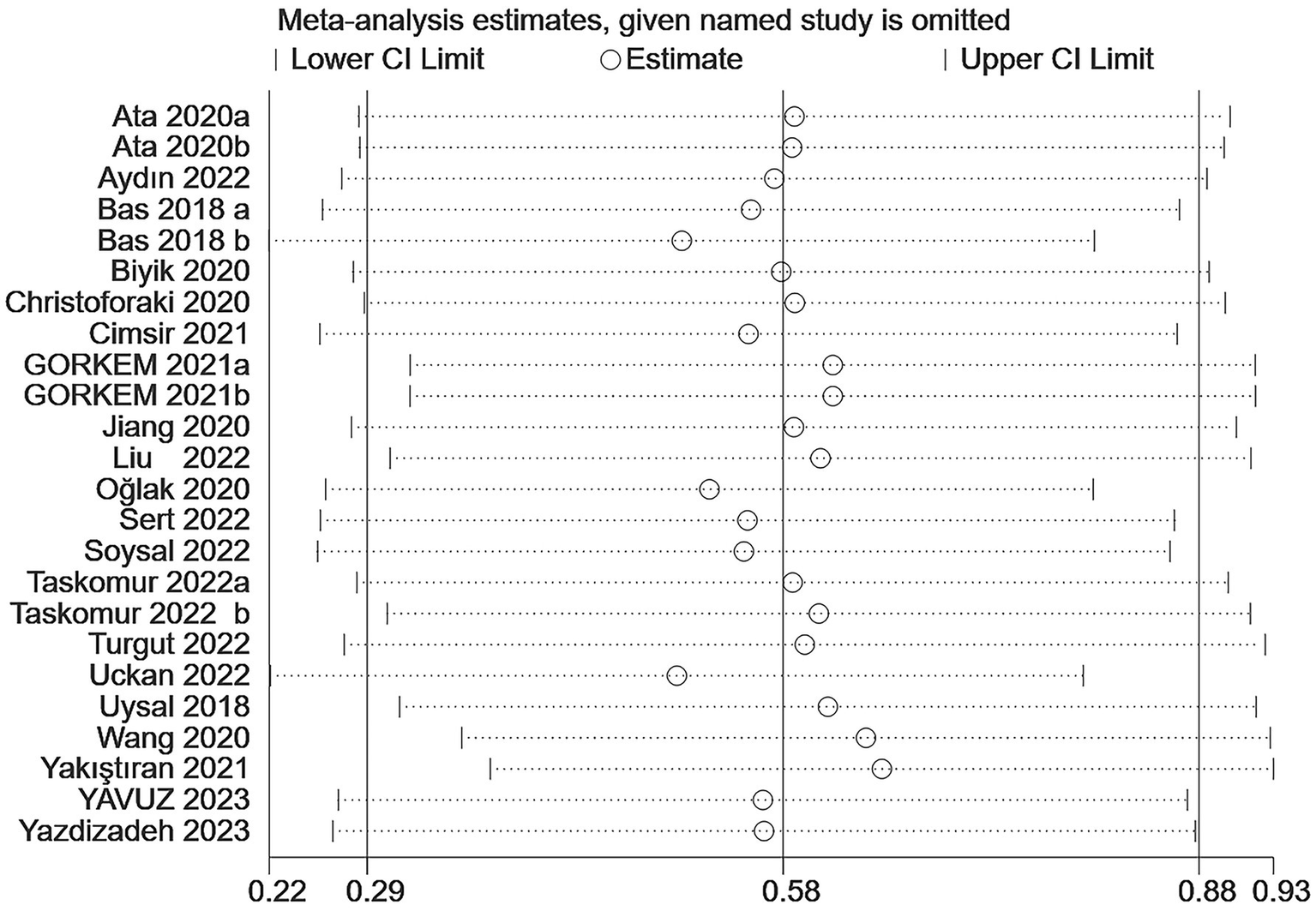
Sensitive analysis of the relationship between abortion and NLR.
Figure 7
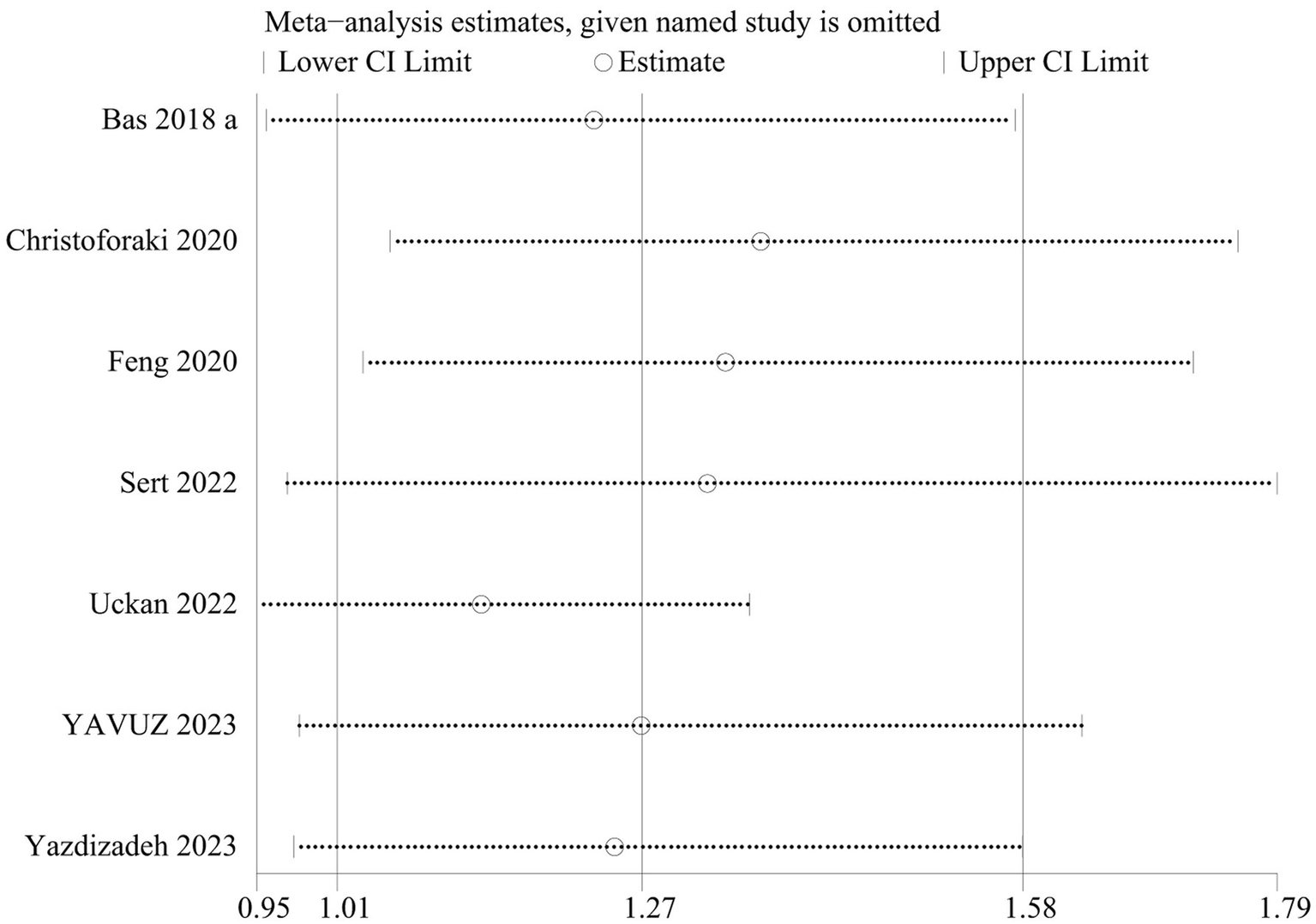
Sensitive analysis of the risk of abortion and NLR.
4 Discussion
This meta-analysis examined the predictive performance of NLR in abortion. In the enrolled 20 articles with 6,913 patients (3,375 abortion versus 3,538 non-abortion), we summarized that NLR could predict abortion.
Subgroup analyses by NLR threshold, regions, types of abortion, and mean age were also performed. Heterogeneity may be due to these factors. Only in NLR cut-off>3, these factors had significant associations with the risk of abortion. A threshold of NLR > 3 is better set to predict abortion. Higher NLR was connected to considerably higher abortion rates in Asia, suggesting that NLR is of greater value in predicting abortion in Asian populations. Further research is needed in other regions. As for different types of abortion, higher NLR was connected to a markedly higher incidence of missed abortion and spontaneous abortion. Neutrophil to lymphocyte ratio may be better at predicting these two types of abortion. Regarding different mean ages, higher NLR was connected to a notably higher incidence of abortion when patients aged above 30. Pregnant women older than 30 are better predicted with NLR. In the relationship between NLR and abortion, only retrospective studies, Europe populations, threatened abortion, RPL, and abortion had significant associations.
Abortion is an extremely distressing incident for couples, leading to diverse psychological consequences. Recent studies have highlighted substantial changes in maternal adaptations and innate immune responses to keep normal pregnancy (29). Under normal pregnancy conditions, there is a marked increase in complete blood count parameters (e.g., white blood cells) and decreases in the proportions of granulocytes, Th-1 lymphocytes, Th-2 lymphocytes, and monocytes (30). Macrophages and monocytes are critical for fetal development since they facilitate extraepithelial trophoblast invasion, spiral dolphin remodeling, and birthing process. In addition, clinical studies have revealed a strong link between inflammation-related parameters and pregnancy complications (31). However, sustained and uncontrolled inflammatory responses have detrimental effects on placental growth, prenatal development, and maternal health (32).
The relationship between inflammation and abortion has garnered more interest in recent years. Experimental research suggests that inflammation is involved in the entire evolution of pregnancy (33, 34). Inflammation and coagulation disorders are crucial in the pathogenesis of abortion, as immunopathological evaluation of abortive material at the site of placenta implantation reveals inflammation and fibrin deposition in the meconium as well as thromboembolism in the meconium vasculature. Normal pregnancies require aseptic inflammation for successful embryo implantation. However, if implantation is uncontrolled and placental growth persists, prenatal development and maternal health may be noted in uterine tissues. Natural Killer cells secrete cytokines that act on the uterus, and activation of vascular endothelial procoagulant initiates ischemia, leading to embryo loss, thrombosis, and inflammation (35). Compared to normal pregnancy, RPL patients have higher levels of cytokines (TNF-a, IFN-γ, IL-12, and IL-2) and increased inflammatory response. Nonetheless, the predicting role of NLR in abortion is elusive (35–38). This study illustrated that higher NLR levels predicted a higher risk of abortion.
There are certain limitations. First, the number of retrospective studies is numerous with low quality. Second, marked heterogeneity was noted. Thus, sensitivity and subgroup analyses were conducted to judge the result stability. There are also strengths. First, this is the meta-analysis with the largest sample size to determine the link between NLR and abortion. Second, the estimates based on WMD and OR data were pooled, which made the results more reliable. Third, data were pooled to evaluate the predictive utility of NLR in abortion. Further prospective studies are needed.
5 Conclusion
Pooled analyses demonstrated that NLR may serve as a potential predictor of abortion. The risk of abortion increases with a higher NLR. The number of retrospective studies and studies conducted in Europe is relatively large. Due to heterogeneity, further studies need to determine whether the findings can be generalized to all populations.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. RY: Data curation, Formal analysis, Investigation, Writing – review & editing. JX: Data curation, Investigation, Writing – review & editing. FY: Conceptualization, Project administration, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study is supported by the General Project of the Xi’an Science and Technology Bureau (NO.24YXYJ0180).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1565979/full#supplementary-material
Abbreviations
OR, Odds ratio; NLR, Neutrophil-lymphocyte ratio; CI, Confidence interval; NOS, Newcastle-Ottawa Scale; RPL, recurrent pregnancy loss.
References
1.
Quenby S Gallos ID Dhillon-Smith RK Podesek M Stephenson MD Fisher J et al . Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. (2021) 397:1658–67. doi: 10.1016/S0140-6736(21)00682-6
2.
Calleja-Agius J Jauniaux E Pizzey AR Muttukrishna S . Investigation of systemic inflammatory response in first trimester pregnancy failure. Hum Reprod. (2012) 27:349–57. doi: 10.1093/humrep/der402
3.
Kalagiri RR Carder T Choudhury S Vora N Ballard AR Govande V et al . Inflammation in complicated pregnancy and its outcome. Am J Perinatol. (2016) 33:1337–56. doi: 10.1055/s-0036-1582397
4.
Yazar FM Bakacak M Emre A Urfalıoglu A Serin S Cengiz E et al . Predictive role of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios for diagnosis of acute appendicitis during pregnancy. Kaohsiung J Med Sci. (2015) 31:591–6. doi: 10.1016/j.kjms.2015.10.005
5.
Soysal C Sarı H Işıkalan MM Özkaya EB Ulaş Ö Taşçı Y et al . Role of the systemic immune-inflammation index in threatened abortion patients and predicting of abortion. J Obstet Gynaecol Res. (2023) 49:1723–8. doi: 10.1111/jog.15655
6.
Bas FY Tola EN Sak S Cankaya BA . The role of complete blood inflammation markers in the prediction of spontaneous abortion. Pak J Med Sci. (2018) 34:1381–5. doi: 10.12669/pjms.346.15939
7.
Yavuz ONUR Özgözen MEHMET Akdöner A Doğan E . Predictivity of platelet to lymphocyte and neutrophil to lymphocyte ratios in the first trimester missed abortion: a retrospective case-control study. J Clin Obstet Gynecol. (2023) 33:66–71. doi: 10.5336/jcog.2023-95319
8.
Liu D Huang X Xu Z Chen M Wu M . Predictive value of NLR and PLR in missed miscarriage. J Clin Lab Anal. (2022) 36:e24250. doi: 10.1002/jcla.24250
9.
Turgut E Yildirim M Sakcak B Ayhan SG Tekin OM Sahin D . Predicting miscarriage using systemic immune-inflammation index. J Obstet Gynaecol Res. (2022) 48:587–92. doi: 10.1111/jog.15156
10.
Biyik I Albayrak M Keskin F . Platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in missed abortion. Rev Bras Ginecol Obstet. (2020) 42:235–9. doi: 10.1055/s-0040-1709693
11.
Jiang S He F Gao R Chen C Zhong X Li X et al . Neutrophil and neutrophil-to-lymphocyte ratio as clinically predictive risk markers for recurrent pregnancy loss. Reprod Sci. (2021) 28:1101–11. doi: 10.1007/s43032-020-00388-z
12.
Gorkem U Kan O Bostanci MO Taskiran D Inal HA . Kisspeptin and hematologic parameters as predictive biomarkers for first-trimester abortions. Medeni Med J. (2021) 36:98–105. doi: 10.5222/MMJ.2021.32549
13.
Christoforaki V Zafeiriou Z Daskalakis G Katasos T Siristatidis C . First trimester neutrophil to lymphocyte ratio (NLR) and pregnancy outcome. J Obstet Gynaecol. (2020) 40:59–64. doi: 10.1080/01443615.2019.1606171
14.
Aydin SM Taskomur AT . Evaluation of serum Procalcitonin, neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in pregnancies with threatened abortion. Aktual Gynekol Porod. (2022) 14:31–7.
15.
Taskomur AT Aydin SM . Evaluation of inflammatory markers in threatened abortions and spontaneous abortions. Ginekol Pol. (2022) 93:721–7. doi: 10.5603/GP.a2022.0069
16.
Cimsir MT Yildiz MS . Could fibrinogen to albumin ratio be a predictive marker for recurrent pregnancy loss. Int J Clin Pract. (2021) 75:e14520. doi: 10.1111/ijcp.14520
17.
Sert ZS Bulbul R . Can the systemic immune-inflammation index be a useful marker for the prediction of a missed abortion in the first trimester of pregnancy?Dubai Med J. (2023) 6:14–9. doi: 10.1159/000527888
18.
Uçkan K Çeleğen İ Baskiran Y Hanligil E . Can platelet mass index be used as a prognostic marker in the diagnosis of missed abortion patients?Eastern J Med. (2022) 27:627–33. doi: 10.5505/ejm.2022.45549
19.
Wang Q Liu F Zhao Y Cui B Ban Y . Can neutrophil-to-lymphocyte and monocyte-to-lymphocyte ratios be useful markers for predicting missed abortion in the first trimester of pregnancy?J Obstet Gynaecol Res. (2020) 46:1702–10. doi: 10.1111/jog.14349
20.
Ata N Kulhan M Kulhan NG Turkler C . Can neutrophil-lymphocyte and platelet-lymphocyte ratios predict threatened abortion and early pregnancy loss?Ginekol Pol. (2020) 91:210–5. doi: 10.5603/GP.2020.0042
21.
Yakıştıran B Tanacan A Altınboğa O Yücel A . Can derived neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and delta neutrophil index predict spontaneous abortion?Z Geburtshilfe Neonatol. (2021) 225:418–22. doi: 10.1055/a-1363-2855
22.
Oğlak SC Aydın MF . Are neutrophil to lymphocyte ratio and platelet to lymphocyte ratio clinically useful for the prediction of early pregnancy loss?Ginekol Pol. (2020) 91:524–7. doi: 10.5603/GP.a2020.0082
23.
Yazdizadeh M Hivehchi N Ghaemi M Azizi S Saeedzarandi M Afrooz N et al . Platelet to lymphocyte and neutrophil to lymphocyte ratio in the first trimester of pregnancy, are they useful for predicting spontaneous miscarriage? A case-control study. Int J Reprod Biomed. (2023) 21:463–70. doi: 10.18502/ijrm.v21i6.13632
24.
Uysal G Çagli F Karakiliç EU Akkaya H Aksoy H Açmaz G . The efficacy of Haematologic parameters in the diagnosis of missed Abortus. Eur J Ther. (2018) 24:17–21. doi: 10.5152/EurJTher.2017.227
25.
Hantoushzadeh S Gargar OK Jafarabady K Rezaei MM Asadi F Eshraghi N et al . Diagnostic value of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio to predict recurrent pregnancy loss and abortion; a systematic review and meta-analysis. Immun Inflamm Dis. (2024) 12:e1210. doi: 10.1002/iid3.1210
26.
Çallıoğlu N Gül DK Arslan İÖ Geyikoğlu İ Demirçivi E . Inflammatory markers in systemic immune-inflammatory index and inflammatory response index to predict early pregnancy loss. Saudi Med J. (2024) 45:808–13. doi: 10.15537/smj.2024.45.8.20240404
27.
Humadi EH Maki H . White blood cell indices to predict first trimester missed miscarriage. J Pak Med Assoc. (2024) 74:S156–s159. doi: 10.47391/JPMA-BAGH-16-34
28.
Shamseer L Moher D Clarke M Ghersi D Liberati A Petticrew M et al . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 349:g7647. doi: 10.1136/bmj.g7647
29.
Mor G Cardenas I . The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. (2010) 63:425–33. doi: 10.1111/j.1600-0897.2010.00836.x
30.
Wegmann TG Lin H Guilbert L Mosmann TR . Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon?Immunol Today. (1993) 14:353–6. doi: 10.1016/0167-5699(93)90235-D
31.
Serin S Avcı F Ercan O Köstü B Bakacak M Kıran H . Is neutrophil/lymphocyte ratio a useful marker to predict the severity of pre-eclampsia?Pregnancy Hypertens. (2016) 6:22–5. doi: 10.1016/j.preghy.2016.01.005
32.
Hustin J Jauniaux E Schaaps JP . Histological study of the materno-embryonic interface in spontaneous abortion. Placenta. (1990) 11:477–86. doi: 10.1016/S0143-4004(05)80193-6
33.
Bae H Lim W Bazer FW Whang KY Song G . Mitigation of ER-stress and inflammation by chemokine (C-C motif) ligand 21 during early pregnancy. Dev Comp Immunol. (2019) 94:73–84. doi: 10.1016/j.dci.2019.01.016
34.
Hedger M Hodyl NA Moss TJ . Inflammation in reproduction, pregnancy and development. J Reprod Immunol. (2018) 130:23–4. doi: 10.1016/j.jri.2018.09.051
35.
Harris LK Benagiano M D’Elios MM Brosens I Benagiano G . Placental bed research: II. Functional and immunological investigations of the placental bed. Am J Obstet Gynecol. (2019) 221:457–69. doi: 10.1016/j.ajog.2019.07.010
36.
Comba C Bastu E Dural O Yasa C Keskin G Ozsurmeli M et al . Role of inflammatory mediators in patients with recurrent pregnancy loss. Fertil Steril. (2015) 104:1467–74.e1. doi: 10.1016/j.fertnstert.2015.08.011
37.
Kasap E Karaarslan S Gene M Gur EB Sahin N Guclu S . The role of cytokines in first trimester pregnancy losses with fetal chromosomal anomaly. Ginekol Pol. (2015) 86:827–32. doi: 10.17772/gp/57827
38.
Challis JR Lockwood CJ Myatt L Norman JE Strauss JF III Petraglia F . Inflammation and pregnancy. Reprod Sci. (2009) 16:206–15. doi: 10.1177/1933719108329095
Summary
Keywords
neutrophil to lymphocyte ratio, abortion, pregnancy, prognosis, NLR
Citation
Wang M, Yue R, Xi J and Yan F (2025) The predictive value of neutrophil to lymphocyte ratio for abortion: a systematic review and meta-analysis. Front. Med. 12:1565979. doi: 10.3389/fmed.2025.1565979
Received
05 June 2025
Accepted
04 September 2025
Published
19 September 2025
Volume
12 - 2025
Edited by
Yin Huang, Sichuan University, China
Reviewed by
Mehmet Nuri Duran, Canakkale Onsekiz Mart Universitesi Tip Fakultesi Hastanesi, Türkiye
Amauche Ngwu, Enugu State University of Science and Technology, Nigeria
Updates
Copyright
© 2025 Wang, Yue, Xi and Yan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Yan, yf980811@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.