Abstract
Objective:
This study aims to explore the clinical value of the combination of 18F fluorodeoxyglucose (18F-FDG) and N-[2-(dimethylamino) ethyl]-18F-5-fluoropicolinamide (18F-P3BZA) positron emission tomography/computed tomography (PET/CT) in melanoma patients.
Methods:
A retrospective study was conducted on 19 melanoma patients who underwent 18F-FDG and 18F-P3BZA PET/CT scans at the Nuclear Medicine Department/PET Imaging Center of the Second Xiangya Hospital, Central South University, from March 2022 to March 2024. The diagnostic efficacy of 18F-FDG, 18F-P3BZA, and the combination of both for melanoma was compared, and the value of combined imaging for TNM staging and clinical treatment decision-making in melanoma patients was discussed.
Results:
The sensitivity of 18F-P3BZA in diagnosing primary lesions of melanoma, all metastases, sentinel lymph node metastases (SLNM), distant lymph node metastases (DLNM), and bone metastases (BM) was 100% (12/12), 71.4% (40/56), 72.4% (21/29), 66.7% (14/21), and 83.3% (5/6), respectively. The corresponding values for 18F-FDG were 91.7% (11/12), 91.1% (51/56), 86.2% (25/29), 95.2% (20/21), and 100% (6/6), respectively. Combined imaging showed a higher sensitivity in diagnosing SLNM, DLNM, and all metastases than 18F-P3BZA (χ2 = 7.105, p = 0.004; χ2 = 3.860,p = 0.045; χ2 = 15.604; p < 0.001). In addition, the specificity of 18F-FDG in diagnosing all metastases, SLNM, DLNM, and BM, was 50.0, 69.2, 56.3, and 100%, respectively, and the corresponding values for 18F-P3BZA were 81.8, 100, 75.0, and 100%, respectively. Combined imaging improved N and M staging in 31.6% (6/19) of melanoma patients and changed clinical treatment decisions in 26.3% (5/19) of melanoma patients.
Conclusion:
The specificity of 18F-FDG alone in diagnosing melanoma is low, but it can be combined effectively with 18F-P3BZA. The combination of 18F-FDG and 18F-P3BZA PET/CT can further improve the detection efficiency of lesions, TNM staging, and clinical treatment decisions.
Introduction
Melanoma is one of the most aggressive types of cancer, and its incidence continues to rise globally (1). It usually occurs on the skin of the extremities, with its incidence peaking at the age of 65 years; however, it can affect individuals of any age (2). The American Joint Committee on Cancer (AJCC) points out that the 5-year survival rate for early-stage patients after surgery is higher than 95% and that the incidence of distant metastasis is less than 10% (3, 4).
The diagnosis of skin melanoma is performed through physical examination and local biopsy by clinical physicians, whereas for its clinical staging and treatment evaluation, imaging methods are required. However, on the one hand, conventional imaging generally provides morphological information but has low specificity; on the other hand, due to its single-site imaging, its staging value is relatively low (5, 6).
Positron emission tomography/computed tomography (PET/CT) has been widely used for staging, post-treatment restaging, and evaluating the efficacy of various solid malignant tumors in humans (7–10). 18F fluorodeoxyglucose (18F-FDG) PET/CT has changed the diagnostic approach of melanoma and has become the most clinically valuable method for clinical staging and follow-up treatment of melanoma (1, 11, 12). However, 18F-FDG has some limitations, primarily due to its low specificity. In addition, the high uptake of brain tissue during 18F-FDG PET/CT imaging may affect the detection of brain metastases (13, 14).
In recent years, various novel molecular probes for melanoma have been developed, including benzamide (BZA) and its derivatives, antibodies, receptors, very late antigen-4 (VLA-4), and metabolic glutamate receptor-1 (mGluR1) (1, 15–19). Among these, BZA derivatives have been the most extensively investigated. Due to the presence of melanin in more than 90% of melanoma cells, targeting melanoma lesions by targeting melanin is theoretically possible. BZA derivatives are polycyclic aromatic compounds and can specifically bind to melanin in cells (20, 21). Therefore, melanoma lesions can be targeted by labeling BZA derivatives with radioactive isotopes.
In 2013, Liu et al. (22) designed and synthesized several 18F-labeled BZA analogs as positron probes for targeting melanoma lesions in B16F10 tumor-bearing mice. They found that N-[2-(dimethylamino) ethyl]-18F-5-fluoropicolinamide (18F-P3BZA) had a highly specific melanin-binding ability and the highest target-to-background uptake ratio. In 2019, Ma et al. (23) utilized 18F-P3BZA PET/CT for the diagnosis of melanoma and found that its imaging effect was superior to that of 18F-FDG PET/CT. This finding confirms that 18F-P3BZA has the potential for clinical application. However, this study included only five cases of melanoma and did not analyze the detection rate of the lesions, let alone clarify their exact clinical application value.
The present study included 19 patients with melanoma and compared the clinical application value of 18F-P3BZA PET/CT, 18F-FDG PET/CT, and the combination of both. This study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Xiangya Second Hospital, Central South University (ethics approval number: 2021–165). All participants provided their signed informed consent.
Patients and methods
Patients
We conducted a retrospective analysis of melanoma patients who underwent 18F-FDG and 18F-P3BZA PET/CT scans at the Nuclear Medicine Department/PET Imaging Center of the Second Xiangya Hospital, Central South University, from March 2022 to March 2024. Clinical data and PET/CT scan data of the patients were collected, and it was ensured that the interval between two PET/CT scans did not exceed 7 days.
Inclusion criteria were as follows: (1) those diagnosed with melanoma by pathological findings; (2) those with access to clinical and pathological data; (3) those who accepted to undergo 18F-FDG and 18F-P3BZA PET/CT scans with good image quality; and (4) those with complete follow-up information.
Exclusion criteria were as follows: (1) those who had incomplete clinical data or were lost to follow-up; (2) having other malignant tumors or serious systemic diseases; and (3) those with contraindications for radionuclide scanning.
Based on these criteria, 19 patients were included in this study.
Synthesis and quality control of 18F-P3BZA and 18F-FDG
18F-P3BZA: 18F-P3BZA was generated using a cyclotron at the Nuclear Medicine Department/PET Imaging Center of the Second Xiangya Hospital, Central South University (Siemens, Germany). 18F-P3BZA was synthesized using the AllInOne™ synthesizer (TRASIS, Belgium) based on previous research methods (23). Both the radiochemical purity and labeling rate of 18F-P3BZA were higher than 95%.
18F-FDG: 18F-FDG was synthesized using the Explora FDG4 and Explora GN chemical synthesis modules, and its radiochemical purity and labeling rate were both higher than 95%.
For PET/CT, uMI780 PET/CT (Shanghai United Imaging Healthcare Co., Ltd.) was used.
18F-FDG PET/CT image acquisition
The 18F-FDG injection was administered at a dosage of 3.5 MBq/kg. Before administering the injection, patients were asked to fast for at least 6 h, and it was ensured that their blood sugar level did not exceed 11.1 mmol/L. Patients were subjected to scanning 60 min after injection, with the scan area being from the top of the skull to the sole of the plantar. First, CT and PET scans of the body (neck to the plantar) were performed with the following parameters: voltage: 120 kV; radiation dose: 120 mAs; matrix: 600 × 600; and layer thickness: 3.75 mm. PET acquisition was performed in 3D, with a speed of 2 min per bed for a total of 5–9 beds. Then, separate head CT and PET acquisitions were performed, with a head PET acquisition speed of 2 min per bed for one bed. The collected data were reconstructed using iterative methods and transferred to an MMWP image post-processing workstation.
18F-P3BZA PET/CT image acquisition
The 18F-P3BZA injection was administered at a dosage of 2.5 MBq/kg (lower than Ma et al.’s study dose for radiation safety issues), and scanning was performed 60 min after the injection (our team’s previous research confirmed the highest target background uptake ratio at 60 min). Patients were instructed to drink approximately 200–500 mL of water 10 min before scanning to dilute and excrete the radioactive accumulation of 18F-P3BZA in the stomach. The scanning range was from the top of the skull to the plantar, and the patients were asked to remain stationary on the examination bed throughout the entire process. First, a low-dose CT scan was performed with the following parameters: voltage: 120 kV; radiation dose: 120 mAs; matrix: 600 × 600; and layer thickness: 3.75 mm. Then, another PET scan was performed, with 3D PET acquisition at a speed of 2 min per bed for a total of 6–10 beds. The collected data were iteratively reconstructed to obtain whole-body CT images, PET images, and PET/CT fusion images. Then, the reconstructed data were transferred to the MMWP image post-processing workstation.
Image analysis
Two senior nuclear medicine physicians who were involved in PET/CT diagnosis jointly reviewed the PET/CT images. In case of disagreement, the final decision was made through mutual consultation and agreement between the two physicians. The images were primarily analyzed using visual and quantitative methods.
18F-FDG pet/CT
Visual analysis: Except for lesions in the brain and kidneys, any lesions characterized by localized skin thickening, nodules, or masses, along with an increased radioactive uptake or significantly higher radioactive uptake compared with the surrounding normal tissues, were considered primary lesions. For a lymph node (LN) in the drainage pathway of the melanoma, if its volume or radioactive uptake was found to increase, it was considered a metastasis. If there was a bone change on the whole-body scan with increased local radioactive uptake, it was considered a bone metastasis (BM).
Quantitative analysis: For a primary lesion, in addition to monitoring its morphological changes, if its maximum standardized uptake value (SUVmax) was higher than 2.5, it was considered a primary melanoma lesion (24, 25). For an LN in the drainage pathway of the melanoma, if the short diameter was higher than 10 mm or its SUVmax was higher than 2.5, it was considered a metastasis. If there was a change in bone density or the SUVmax was higher than 2.5 in the whole-body scan, it was considered a BM.
18F-P3BZA pet/CT
Visual analysis: In 18F-P3BZA PET/CT images, except for lesions in the kidneys, liver, gallbladder, spleen, and stomach, any lesions characterized by local skin thickening, nodules, or masses, along with an increased radioactive uptake or significantly higher radioactive uptake than the surrounding normal tissues, were considered primary lesions. If the volume or radioactive uptake of an LN in the drainage pathway of the melanoma was found to increase, it was considered a metastasis. If a bone change was observed on the whole-body scan along with increased local radioactive uptake, it was considered a BM.
Quantitative analysis: If the SUVmax of a primary lesion was higher than 2.5, in addition to showing morphological changes, it was considered a primary melanoma lesion. If the short diameter of an LN in the drainage pathway of the melanoma was higher than 10 mm or its SUVmax was higher than 2.5, it was considered a metastasis. If a change in bone density was observed on the whole-body scan and the SUVmax was higher than 2.5, it was considered a BM.
Clinical TNM staging of melanoma patients was recommended according to the latest AJCC guidelines (26).
Follow-up
For each patient, follow-up began at the end of their scan and continued for at least 1 month. Among the 19 melanoma patients, 12 were newly diagnosed, while 7 received postoperative care after their surgery. All primary lesions were validated using pathological results. Metastases were validated through histopathological, clinical, and imaging follow-up (including ultrasound, CT, MRI, and PET/CT) results. If a lesion was found to be significantly enlarged or had a significantly increased metabolism during follow-up, or if a lesion was found to be significantly shrunken or had a significantly decreased metabolism after drug treatment, it was considered a metastasis.
Statistical analysis
The diagnostic consistency between two nuclear medicine physicians was evaluated using the Kappa test. IBS SPSS 26.0 and Origin 2023 were used for statistical analysis and image plotting. Count data were expressed as mean ± standard deviation (x ± s), frequency, and percentage (%). In addition, the t-test was performed to compare the differences in semi-quantitative parameters between 18F-FDG and 18F-P3BZA, and the Chi-squared test was utilized to compare the differences in diagnostic efficacy between 18F-FDG, 18F-P3BZA, and combined imaging. A p-value of less than 0.05 was considered statistically significant.
Results
Study population
Of the 19 melanoma patients, 12 were initially diagnosed, and 7 underwent postoperative follow-up. Partial clinical data and PET/CT scan results of the 19 patients are presented in Table 1.
Table 1
| Patient number | Sex | Age(y) | Height(cm) | Weight(kg) | Primary site | 18F-FDG dose (MBq) | 18F-P3BZA dose (MBq) | Confirmed metastases* | TNM |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 45 | 156 | 61 | Right external genitalia | 248 | 200 | LNM | TxN2M0 |
| 2 | F | 47 | 156 | 65 | Right neck | 274 | 222 | LNM | TxN1M0 |
| 3 | F | 73 | 170 | 59 | Right plantar | 259 | 207 | LNM | TxN0M1a |
| 4 | M | 35 | 168 | 67 | Left plantar | 289 | 204 | - | T3bN0M0 |
| 5 | M | 76 | 170 | 77 | Left plantar | 300 | 248 | LNM | TxN0M1a |
| 6 | F | 36 | 156 | 73 | Right calf | 270 | 204 | LNM | TxN2M0 |
| 7 | F | 74 | 145 | 56 | Right plantar | 241 | 244 | LNM, BM | T3bN3M1c |
| 8 | F | 68 | 153 | 48 | Right thigh | 174 | 222 | - | TxN0M0 |
| 9 | M | 68 | 163 | 65 | Right finger | 259 | 185 | LNM | TxN2M0 |
| 10 | M | 51 | 166 | 57 | Left plantar | 211 | 226 | - | T2aN0M0 |
| 11 | M | 62 | 164 | 65 | Right toe | 259 | 200 | LNM | TxN2M0 |
| 12 | M | 44 | 175 | 97 | Right plantar | 359 | 259 | - | TxN0M0 |
| 13 | M | 72 | 161 | 52 | Left finger | 222 | 211 | LNM | TxN2M0 |
| 14 | F | 39 | 153 | 51 | Right eye | 226 | 189 | - | / |
| 15 | F | 65 | 151 | 69 | Left eye | 344 | 81 | - | / |
| 16 | F | 70 | 150 | 65 | Right plantar | 233 | 130 | - | TxN0M0 |
| 17 | M | 55 | 176 | 80 | Right plantar | 167 | 130 | - | TxN0M0 |
| 18 | M | 33 | 168 | 72 | Left anterior chest | 274 | 215 | - | TxN0M0 |
| 19 | F | 67 | 144 | 48 | Right toe | 178 | 192 | LNM | TxN3M0 |
Clinical and PET/CT scan data of 19 melanoma patients.
Histopathological results and follow-up confirmed 56 metastases in 11 patients (49 lymph node metastases (LNM) in 10 patients and 1 LNM and 6 BM in 1 patient) of the 50 LNM, 29 were sentinel lymph node metastases (SLNM).
Distribution of 18F-P3BZA in melanoma patients
The distribution of 18F-P3BZA in important organs and tissues of 19 patients with melanoma is presented in Table 2.
Table 2
| Sites | Uptake level | SUVmax | SUVmean |
|---|---|---|---|
| Stomach | High, diffuse | 6.4 ± 4.1 | 4.1 ± 2.8 |
| Liver | Medium/high, diffuse | 6.1 ± 2.0 | 4.4 ± 1.7 |
| Gallbladder | High, diffuse | 10.0 ± 5.7 | 7.3 ± 4.0 |
| Spleen | Medium/high, diffuse | 4.0 ± 1.2 | 2.8 ± 0.9 |
| Mediastinal blood pool | Low, diffuse | 1.2 ± 0.3 | 0.7 ± 0.2 |
| Lung | Low, diffuse | 1.2 ± 0.2 | 0.8 ± 0.1 |
| Muscle | Low, diffuse | 1.5 ± 0.3 | 0.9 ± 0.2 |
| Eye (choroid) | Medium/high, diffuse | 1.9 ± 0.6 | 1.3 ± 0.4 |
| Bone | Low/medium, diffuse | 3.6 ± 0.9 | 2.3 ± 0.5 |
Distribution of 18F-P3BZA in important organs and tissues of 19 melanoma patients.
SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value.
18F-P3BZA PET/CT scan results
18F-P3BZA PET/CT detected primary lesions in all newly diagnosed patients (100%, 12/12). The SUVmax of 12 primary tumors was 9.5 ± 9.1, with 10 cases of cutaneous melanoma having an SUVmax of 5.6 ± 3.3 (Figures 1A–D) and 2 cases of ocular melanoma having an SUVmax of 33.4 and 16.8 (Figures 1E–H). No abnormalities were observed at the surgical site in seven postoperative follow-up patients.
Figure 1

(A–D) Findings in a 68-year-old female patient diagnosed with melanoma more than 20 days before the start of the study. 18F-P3BZA PET/CT MIP (maximum intensity projection) (A) showing a radioactive accumulation shadow on the outer side of the right thigh, and CT (B), PET (C), and PET/CT (D) showing local skin thickening on the outer side of the right thigh with increased radioactive uptake (SUVmax: 8.8). (E–H) Findings in a 39-year-old female patient diagnosed with melanoma 1 week before the study. 18F-P3BZA PET/CT MIP (E) showing a radioactive accumulation shadow in the right eye, and CT (F), PET (G), and PET/CT (H) showing high-density nodules on the posterior inner wall of the right eyeball, accompanied by radioactive concentration (SUVmax: 33.4).
As shown in Table 3, 18F-P3BZA PET/CT detected 40 metastases. The SUVmax and SUVmean of all metastases were 12.3 ± 10.0 and 6.8 ± 4.1, respectively. The SUVmax of LNM was 11.7 ± 9.4, and that of BM was 8.4 ± 3.0.
Table 3
| Lesions | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| All metastases | 71.4% (40/56) | 81.8% (18/22) | 90.9% (40/44) | 52.9% (18/34) |
| SLNM | 72.4% (21/29) | 100% (16/16) | 100% (21/21) | 61.9% (13/21) |
| DLNM | 66.7% (14/21) | 75.0% (12/16) | 77.8% (14/18) | 63.2% (12/19) |
| BM | 83.3% (5/6) | 100% (2/2) | 100% (5/5) | 66.7% (2/3) |
Efficiency analysis of 18F-P3BZA PET/CT in the diagnosis of melanoma metastases.
SLNM, sentinel lymph node metastases; DLNM, distant lymph node metastases; BM, bone metastases; PPV, positive predictive value; NPV, negative predictive value.
18F-FDG PET/CT scan results
Of the 12 newly diagnosed patients, 18F-FDG PET/CT detected primary lesions in 11 patients (91.7%, 11/12). No statistically significant difference in sensitivity was observed between 18F-FDG and 18F-P3BZA (100% vs. 91.7%, χ2 = 1.043, p = 1). The SUVmax of 12 primary tumors was 8.1 ± 5.3, with no statistically significant difference between 18F-FDG and 18F-P3BZA (t = 0.501, p = 0.630). In addition, the SUVmax of 10 cases of skin melanoma was 5.6 ± 3.3, and the SUVmax of two cases of ocular melanoma were 3.8 and 8.3. A patient with melanoma of the plantar after surgery presented with false-positive results (mild uptake of 18F-FDG).
As shown in Table 4, 18F-FDG PET/CT detected 51 metastases. The detection rate of 18F-FDG in the diagnosis of DLNM was higher than that of 18F-P3BZA (p = 0.045). The SUVmax of all metastases was 14.2 ± 7.2. In addition, the SUVmax values for LNM and BM were 15.5 ± 6.4 and 9.3 ± 3.3, respectively. No statistically significant difference in SUVmax was observed between 18F-FDG and 18F-P3BZA (t = 0.877, p = 0.410).
Table 4
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| All metastases | 91.2% (51/56) | 50.0% (11/22) | 80.7% (46/57) | 52.3% (11/21) |
| SLNM | 86.2% (25/29) | 69.2% (9/13) | 86.2% (25/29) | 69.2% (9/13) |
| DLNM | 95.2% (20/21) | 56.3% (9/16) | 74.1% (20/27) | 90.0% (9/10) |
| BM | 100% (6/6) | 100% (2/2) | 100% (6/6) | 100% (2/2) |
Efficiency analysis of 18F-FDG PET/CT in the diagnosis of melanoma metastases.
SLNM, sentinel lymph node metastases; DLNM, distant lymph node metastases; BM, bone metastases; PPV, positive predictive value; NPV, negative predictive value.
Combined imaging scan results
Combined imaging detected primary lesions in all newly diagnosed patients (100%, 12/12) (Figures 2, 3 A). No statistically significant difference in the detection rate was observed between combined imaging, 18F-FDG, and 18F-P3BZA (χ2 = 2.057, p = 0.358).
Figure 2
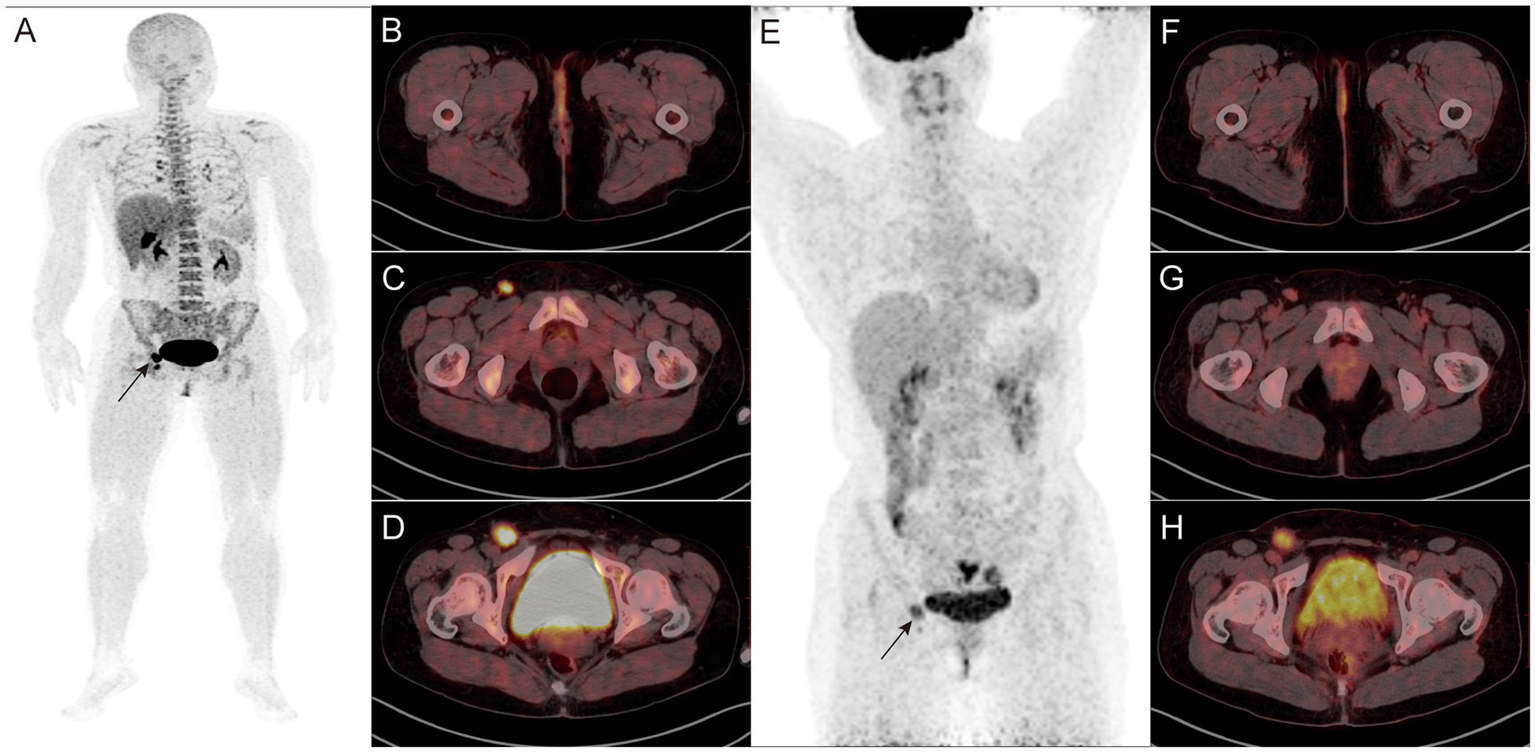
A-H: Findings in a 45-year-old female patient who underwent surgery for external vulvar melanoma 1 year before the start of the study. 18F-P3BZA PET/CT MIP (A) showing a radioactive accumulation shadow in the right inguinal region (black arrow). Axial PET/CT (B–D) showing no significant morphological changes or abnormal uptake in the surgical area, with two enlarged lymph nodes with intense radioactive uptake in the right inguinal region (SUVmax: 7.2 and 14.4, respectively). (E–H)18F-FDG PET/CT images of the patient during the same period. 18F-FDG PET/CT MIP (E) showing a radioactive accumulation shadow in the right inguinal region (black arrow). Axial PET/CT (F–H) showing no abnormalities in the surgical area. The radioactive uptake of the two lymph nodes in the right inguinal region is mild to moderate (SUVmax: 3.6 and 5.8, respectively).
Figure 3
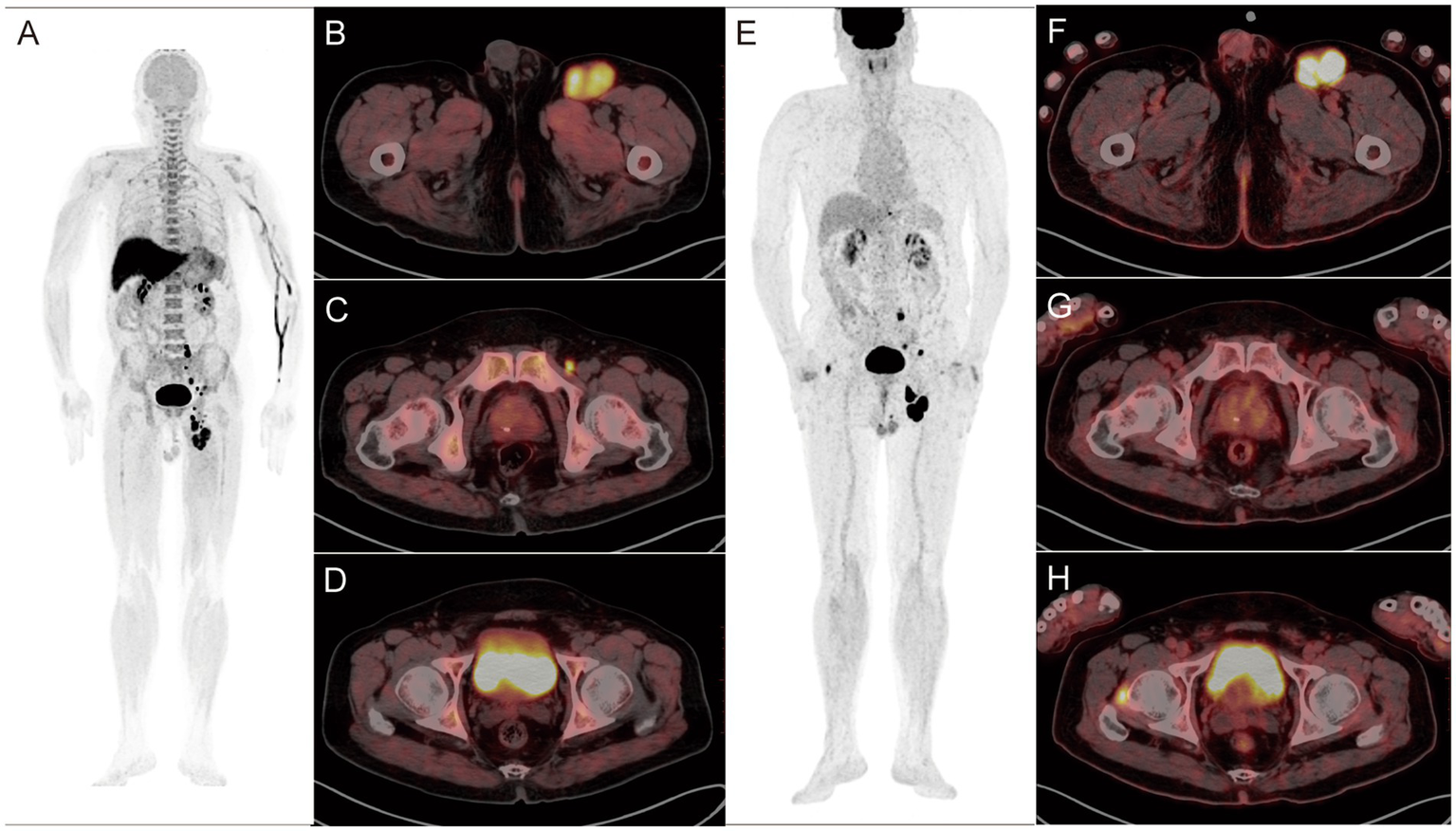
A-H: Findings in a 76-year-old postoperative patient with left plantar melanoma. (A–D)18F-P3BZA PET/CT images of the patient. (E–H)18F-FDG PET/CT images of the patient. 18F-P3BZA PET/CT MIP (A) and axial PET/CT (B,C) showing multiple enlarged lymph nodes with increased radioactive uptake in the left inguinal region. The positive lymph nodes displayed by 18F-FDG PET/CT MIP (E) and axial PET/CT (F,G) are significantly fewer than those displayed by 18F-P3BZA PET/CT. 18F-P3BZA PET/CT (D) showing no abnormal radioactive uptake in the right femoral head muscle, and 18F-FDG PET/CT (H) showing a radioactive accumulation shadow, which was confirmed to be a non-melanoma lesion during follow-up.
As shown in Table 5, combined imaging detected 55 metastases. The sensitivity of combined imaging in the diagnosis of SLNM, DLNM, and all metastases was higher than that of 18F-P3BZA (χ2 = 7.105, p = 0.004; χ2 = 3.860, p = 0.045; χ2 = 15.604, p < 0.001).
Table 5
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| All metastases | 98.2% (55/56) | 100% (22/22) | 100% (55/55) | 95.7% (22/23) |
| SLNM | 100% (29/29) | 100% (13/13) | 100% (29/29) | 100% (13/13) |
| DLNM | 95.2% (20/21) | 100% (16/16) | 100% (20/20) | 94.1% (16/17) |
| BM | 100% (6/6) | 100% (2/2) | 100% (6/6) | 100% (2/2) |
Efficiency analysis of combined imaging in the diagnosis of melanoma metastases.
SLNM, sentinel lymph node metastases; DLNM, distant lymph node metastases; BM, bone metastases; PPV, positive predictive value; NPV, negative predictive value.
For all three imaging methods, the diagnostic consistency between two nuclear medicine physicians was good (18F-P3BZA: Kappa = 0.762, p < 0.001; 18F-FDG: Kappa = 0.877, p < 0.001; combined imaging: Kappa = 0.825, p < 0.001).
Combined imaging improved N and M staging in 31.6% (6/19) of melanoma patients. In addition, it changed the clinical treatment decisions in 26.3% (5/19) of patients (Figure 4; Table 6).
Figure 4
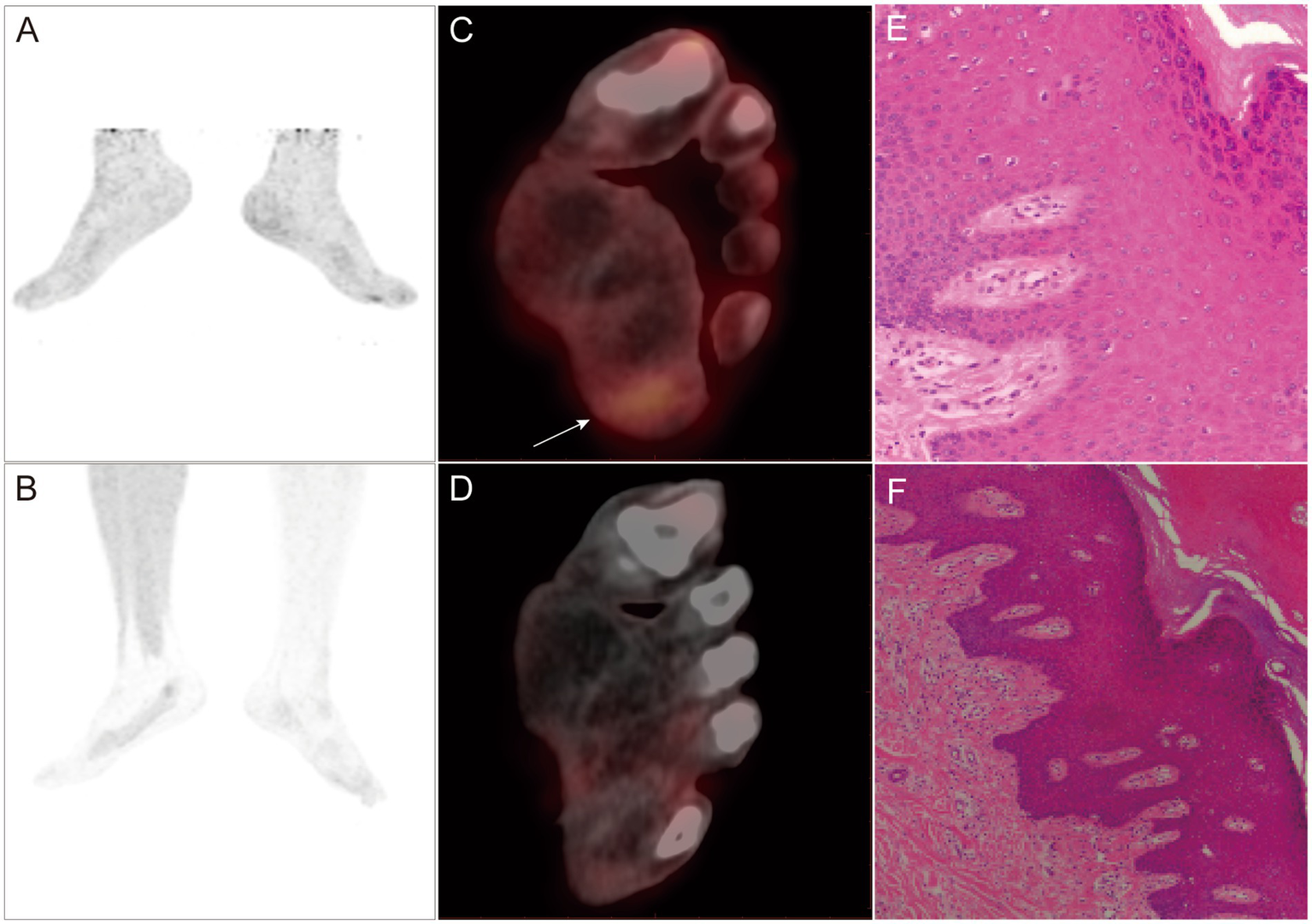
A-F PET/CT and pathological images of a 35-year-old male patient with melanoma who underwent plantar lesion resection surgery 3 weeks before the study took place. 18F-FDG PET/CT MIP (A) and PET/CT (C) showing local radioactive accumulation shadow in the left sole (SUVmax: 2.7). 18F-P3BZA PET/CT MIP (B) and PET/CT (D) without any abnormal radioactive uptake. Five days later, an extended resection of the surgical area was performed, and postoperative pathology confirmed no evidence of tumor recurrence (active local squamous cell proliferation, E,F).
Table 6
| Imaging method | Patient 3 | Patient 6 | Patient 8 | Patient 11 | Patient 12 | Patient 17 |
|---|---|---|---|---|---|---|
| 18F-FDG PET/CT | TxN0M1a | TxN2M0 | TxN1M0 | TxN2M0 | TxN2M0 | TxN2M0 |
| 18F-P3BZA PET/CT | TxN0M0 | TxN0M0 | TxN0M0 | TxN0M0 | TxN0M0 | TxN0M0 |
| Combined imaging | TxN0M1a | TxN2M0 | TxN0M0 | TxN2M0 | TxN0M0 | TxN0M0 |
| Confirmed TNM | TxN0M1a | TxN2M0 | TxN0M0 | TxN2M0 | TxN0M0 | TxN0M0 |
Combined imaging improves N and M staging results in melanoma.
Discussion
The 18F-P3BZA used in this study is a derivative of BZA. One hour after injection, 18F-P3BZA was mainly distributed in the melanoma lesions of the patients, showing a strong melanin-targeting ability. In addition, the probe showed varying degrees of physiological distribution in the stomach, liver, gallbladder, spleen, and eyes of the patients.
Both the primary lesions and metastases showed a high tumor-to-muscle uptake ratio 60 min after 18F-P3BZA injection. The SUVmax of the primary lesion in the eye reached 36.5 ± 19.7. Ma et al. (23) performed 18F-P3BZA PET/CT scans on five melanoma patients, and the results showed that the SUVmax values of their primary lesions and metastases were 19.7 ± 5.3 and 18.2 ± 3.7, respectively. Consistent with the results of this study, our findings indicate that 18F-P3BZA can specifically bind to melanin in vivo, thereby clearly displaying melanoma lesions.
Although 18F-P3BZA PET/CT detected all primary lesions (12/12, 100%), its performance in detecting metastases was unsatisfactory, particularly in detecting LNM (70.0%, 35/50), especially DLNM (66.7%, 14/21). This may not be related to the size of the metastases (this study shows that many small LNM also significantly uptake 18F-P3BZA) but may be related to the level of melanin in the metastases. In a study on Bomirski hamster melanoma cells, Saud et al. (20) showed that tumor cells exhibit a significantly reduced ability to produce melanin after each generation. Therefore, the insufficient uptake of 18F-P3BZA by metastases (especially by DLNM) in the present study may be attributed to a significant decrease in their ability to produce melanin. In addition, we speculate that the synthesis and transport process of melanin requires the participation of multiple enzymes, and the synthesis of these enzymes requires a certain amount of time (27). This leads to a slower increase in melanin levels in metastases (insufficient uptake of 18F-P3BZA).
This study showed that the sensitivity, specificity, PPV, and NPV of 18F-P3BZA combined with 18F-FDG PET/CT in detecting primary lesions were all 100%. Moreover, only one metastasis was missed with the combined imaging, with a sensitivity of 98.2%. It showed significantly lower false-positive and false-negative rates than 18F-FDG and 18F-P3BZA, respectively. In addition, accurate diagnosis of N and M staging is crucial for the clinical management of patients (28). In the present study, combined imaging improved N and M staging in 31.6% (6/19) of patients, and as a result, treatment plans were changed in 26.3% (5/19) of patients. This change is quite helpful for clinical practice as unnecessary or incorrect treatment can be prevented to some extent. Although there was no statistically significant difference in diagnostic efficacy between combined imaging and 18F-FDG alone, the former showed improved final diagnostic results and clinical treatment decisions. However, combined imaging is not suitable for all melanoma patients.
In addition, our PET/CT system is different from the long axial field of view (LAFOV) PET/CT system, which allows for the injection of two tracers on the same day or even simultaneously (29). Although LAFOV PET/CT is currently applied only in a few centers worldwide, this innovative imaging system will soon become available in numerous institutions. Compared to traditional PET/CT systems, LAFOV PET/CT has many advantages in detecting both tumor and non-tumor diseases (30, 31). For instance, using LAFOV, PET/CT PET/CT examinations can be completed on the same day, which can reduce the burden on patients; its higher image quality and optimized lesion quantification can increase lesion detectability; it has low radiation exposure and short collection time; it does not require CT attenuation correction (significantly reducing radiation exposure); and whole-body dynamic imaging can provide more information.
Due to the limitations of using 18F-FDG PET/CT for the diagnosis of melanoma in clinical practice, the addition of 18F-P3BZA PET/CT as a melanin-specific positron tracer can provide more information for diagnosis. Therefore, this study proposes the following indications for using a combination of 18F-FDG and 18F-P3BZA PET/CT scanning: (1) the primary lesion or metastasis is located in the brain; (2) conventional 18F-FDG PET/CT cannot determine the presence of LNM; (3) there is interference from inflammation in the surgical area; and (4) conventional 18F-FDG PET/CT scan is negative although clinical suspicion of melanoma is high.
The findings of this study show that the combination of 18F-FDG and 18F-P3BZA PET/CT imaging can be effective in diagnosing melanoma, guiding TNM staging, and informing clinical treatment decisions. This can enhance physicians’ confidence in managing melanoma and may indirectly improve patients’ prognosis.
This study has some limitations: (1) the number of patients included in this study is relatively small, and further validation of the findings with a large sample size is needed in the future. In addition, melanoma in the skin and other areas was not been analyzed separately; (2) some patients underwent surgery and medication before the PET/CT scanning, which may have affected the semiquantitative parameters of PET; and (3) this study did not quantitatively analyze melanin in melanoma lesions and investigate its correlation with PET metabolic parameters.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Xiangya Hospital, Central South University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
RA: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. TX: Investigation, Methodology, Resources, Visualization, Writing – review & editing. FH: Investigation, Methodology, Validation, Visualization, Writing – review & editing. XM: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing. YW: Conceptualization, Funding acquisition, Methodology, Resources, Software, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Wei W Ehlerding EB Lan X Luo Q Cai W . PET and SPECT imaging of melanoma: the state of the art. Eur J Nucl Med Mol Imaging. (2018) 45:132–50. doi: 10.1007/s00259-017-3839-5
2.
Michielin O van Akkooi ACJ Ascierto PA Dummer R Keilholz U ESMO Guidelines Committee . Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. (2019) 30:1884–901. doi: 10.1093/annonc/mdz411
3.
Trinh VA . Current management of metastatic melanoma. Am J Health Syst Pharm. (2008) 65:S3–9. doi: 10.2146/ajhp080460
4.
Perng P Marcus C Subramaniam RM . (18) F-FDG PET/CT and melanoma: staging, immune modulation and mutation-targeted therapy assessment, and prognosis. AJR Am J Roentgenol. (2015) 205:259–70. doi: 10.2214/AJR.14.13575
5.
Dinnes J Ferrante di Ruffano L Takwoingi Y Cheung ST Nathan P Matin RN et al . Ultrasound, CT, MRI, or PET-CT for staging and re-staging of adults with cutaneous melanoma. Cochrane Database Syst Rev. (2019) 7:CD012806. doi: 10.1002/14651858.CD012806.pub2
6.
Barat M Guegan-Bart S Cottereau AS Guillo E Hoeffel C Barret M et al . CT, MRI and PET/CT features of abdominal manifestations of cutaneous melanoma: a review of current concepts in the era of tumor-specific therapies. Abdom Radiol. (2021) 46:2219–35. doi: 10.1007/s00261-020-02837-4
7.
Tagliabue L Del Sole A . Appropriate use of positron emission tomography with [(18) F] fluorodeoxyglucose for staging of oncology patients. Eur J Intern Med. (2014) 25:6–11. doi: 10.1016/j.ejim.2013.06.012
8.
Dibble EH Karantanis D Mercier G Peller PJ Kachnic LA Subramaniam RM . PET/CT of cancer patients: part 1, pancreatic neoplasms. AJR Am J Roentgenol. (2012) 199:952–67. doi: 10.2214/AJR.11.8182
9.
Davison J Mercier G Russo G Subramaniam RM . PET-based primary tumor volumetric parameters and survival of patients with non-small cell lung carcinoma. AJR Am J Roentgenol. (2013) 200:635–40. doi: 10.2214/AJR.12.9138
10.
Bisschop C de Heer EC Brouwers AH Hospers GAP Jalving M . Rational use of (18) F-FDG PET/CT in patients with advanced cutaneous melanoma: a systematic review. Crit Rev Oncol Hematol. (2020) 153:103044. doi: 10.1016/j.critrevonc.2020.103044
11.
Schwenzer NF Pfannenberg AC . PET/CT, MR, and PET/MR in lymphoma and melanoma. Semin Nucl Med. (2015) 45:322–31. doi: 10.1053/j.semnuclmed.2015.03.006
12.
Ayati N Sadeghi R Kiamanesh Z Lee ST Zakavi SR Scott AM . The value of (18) F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. (2021) 48:428–48. doi: 10.1007/s00259-020-04967-9
13.
Wang L Tang G Hu K Liu X Zhou W Li H et al . Comparison of (68) Ga-FAPI and (18) F-FDG PET/CT in the evaluation of advanced lung Cancer. Radiology. (2022) 303:191–9. doi: 10.1148/radiol.211424
14.
Yi CA Shin KM Lee KS Kim BT Kim H Kwon OJ et al . Non-small cell lung cancer staging: efficacy comparison of integrated PET/CT versus 3.0-T whole-body MR imaging. Radiology. (2008) 248:632–42. doi: 10.1148/radiol.2482071822
15.
Pyo A Kim DY Kim H Lim D Kwon SY Kang SR et al . Ultrasensitive detection of malignant melanoma using PET molecular imaging probes. Proc Natl Acad Sci USA. (2020) 117:12991–9. doi: 10.1073/pnas.1922313117
16.
Wang Y Li M Zhang Y Zhang F Liu C Song Y et al . Detection of melanoma metastases with PET-comparison of (18) F-5-FPN with (18) F-FDG. Nucl Med Biol. (2017) 50:33–8. doi: 10.1016/j.nucmedbio.2017.03.005
17.
Dadachova E Revskaya E Sesay MA Damania H Boucher R Sellers RS et al . Pre-clinical evaluation and efficacy studies of a melanin-binding IgM antibody labeled with 188Re against experimental human metastatic melanoma in nude mice. Cancer Biol Ther. (2008) 7:1116–27. doi: 10.4161/cbt.7.7.6197
18.
Mowlazadeh Haghighi S Zhou Y Dai J Sawyer JR Hruby VJ Cai M . Replacement of Arg with Nle and modified D-Phe in the core sequence of MSHs, ac-his-D-Phe-Arg-Trp-NH (2), leads to hMC1R selectivity and pigmentation. Eur J Med Chem. (2018) 151:815–23. doi: 10.1016/j.ejmech.2018.04.021
19.
Ohtani Y Harada T Funasaka Y Nakao K Takahara C Abdel-Daim M et al . Metabotropic glutamate receptor subtype-1 is essential for in vivo growth of melanoma. Oncogene. (2008) 27:7162–70. doi: 10.1038/onc.2008.329
20.
Saud A Sagineedu SR Ng HS Stanslas J Lim JCW . Melanoma metastasis: what role does melanin play? (review). Oncol Rep. (2022) 48:217–227. doi: 10.3892/or.2022.8432
21.
Beberok A Otreba M Wrzesniok D Buszman E . Cytotoxic effect of lomefloxacin in culture of human epidermal melanocytes. Pharmacol Rep. (2013) 65:689–99. doi: 10.1016/S1734-1140(13)71047-8
22.
Liu H Liu S Miao Z Deng Z Shen B Hong X et al . Development of 18F-labeled picolinamide probes for PET imaging of malignant melanoma. J Med Chem. (2013) 56:895–901. doi: 10.1021/jm301740k
23.
Ma X Wang S Wang S Liu D Zhao X Chen H et al . Biodistribution, radiation dosimetry, and clinical application of a melanin-targeted PET probe, (18) F-P3BZA. Patients J Nucl Med. (2019) 60:16–22. doi: 10.2967/jnumed.118.209643
24.
Byun BH Kong CB Park J Seo Y Lim I Choi CW et al . Initial metabolic tumor volume measured by 18F-FDG PET/CT can predict the outcome of osteosarcoma of the extremities. J Nucl Med. (2013) 54:1725–32. doi: 10.2967/jnumed.112.117697
25.
Jamet B Carlier T Campion L Bompas E Girault S Borrely F et al . Initial FDG-PET/CT predicts survival in adults Ewing sarcoma family of tumors. Oncotarget. (2017) 8:77050–60. doi: 10.18632/oncotarget.20335
26.
Gershenwald JE Scolyer RA Hess KR Sondak VK Long GV Ross MI et al . Melanoma staging: evidence-based changes in the American joint committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. (2017) 67:472–92. doi: 10.3322/caac.21409
27.
Slominski A Zmijewski MA Pawelek J . L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. (2012) 25:14–27. doi: 10.1111/j.1755-148X.2011.00898.x
28.
Carvajal RD Sacco JJ Jager MJ Eschelman DJ Olofsson Bagge R Harbour JW et al . Advances in the clinical management of uveal melanoma. Nat Rev Clin Oncol. (2023) 20:99–115. doi: 10.1038/s41571-022-00714-1
29.
Abgral R Bourhis D Salaun PY . Clinical perspectives for the use of total body PET/CT. Eur J Nucl Med Mol Imaging. (2021) 48:1712–8. doi: 10.1007/s00259-021-05293-4
30.
Vogel WV Steemers-de Boer MM Jansen SM Rijkhorst EJ de Wit-van der Veen BJ van der Hiel B et al . Clinical benefits of LAFOV PET/CT in growing demand for molecular imaging. Eur J Nucl Med Mol Imaging. (2025) 1–4. doi: 10.1007/s00259-025-07159-5
31.
Dimitrakopoulou-Strauss A Pan L Sachpekidis C . Long axial field of view (LAFOV) PET-CT: implementation in static and dynamic oncological studies. Eur J Nucl Med Mol Imaging. (2023) 50:3354–62. doi: 10.1007/s00259-023-06222-3
Summary
Keywords
18F-FDG, 18F-P3BZA, PET/CT, melanoma, melanin
Citation
An R, Xiang T, He F, Ma X and Wang Y (2025) Clinical value of combined 18F-FDG and 18F-P3BZA imaging in the diagnosis of melanoma. Front. Med. 12:1571929. doi: 10.3389/fmed.2025.1571929
Received
06 February 2025
Accepted
01 April 2025
Published
09 May 2025
Volume
12 - 2025
Edited by
Ronan Abgral, Centre Hospitalier Regional Universitaire (CHU) de Brest, France
Reviewed by
Florent L. Besson, Assistance Publique Hopitaux De Paris, France
Kritika Subramanian, Albert Einstein College of Medicine, United States
Updates
Copyright
© 2025 An, Xiang, He, Ma and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaowei Ma, maxiaowei@csu.edu.cnYunhua Wang, 13973186448@139.com
†These authors share senior authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.