Abstract
Background:
Non-typeable Haemophilus influenzae (NTHi) and Moraxella catarrhalis (Mcat) are major pathogens implicated in bacterial exacerbations of chronic obstructive pulmonary disease (COPD). Their involvement contributes to antibiotic resistance and poses significant immune challenges, underscoring the need for targeted vaccine strategies. This systematic review and meta-analysis assessed the safety and efficacy of NTHi-Mcat/NTHi vaccines in COPD patients.
Research design and methods:
Randomized controlled trials (RCTs) assessing the safety and efficacy of NTHi-Mcat/NTHi vaccines for COPD were systematically searched across four databases (PubMed, CENTRAL, Embase, and Medline) from inception to October 2024. Meta-analyses were conducted using random-effects or fixed-effects models, with subgroup analyses to investigate possible sources of heterogeneity.
Results:
This analysis included eight RCTs involving 1,574 participants, primarily conducted in Europe (n = 3) and Australia (n = 2), with interventions administered orally or intramuscularly at varying frequencies (twice or three times). The Meta-analyses revealed that the NTHi-Mcat/NTHi vaccine did not affect the incidence of acute exacerbations of COPD (relative risk (RR): 1.02, 95% confidence interval (CI): 0.76 to 1.36), all-cause mortality (RR: 0.91, 95% CI: 0.38 to 2.21), and hospitalization rate (RR: 0.50, 95% CI: 0.09 to 2.77). Regarding safety, the NTHi-Mcat/NTHi vaccine did not significantly increase the risk of serious adverse events (RR: 1.00, 95% CI: 0.84 to 1.19) or grade 3 serious events (RR: 1.20, 95% CI: 0.93 to 1.53). However, it was associated with a higher risk of local and systemic reactions, including pain (RR: 5.33, 95% CI: 1.98 to 14.33), swelling (RR: 12.15, 95% CI: 4.67 to 31.67), redness (first dose: RR: 12.74, 95% CI: 3.48 to 46.59; second dose: RR: 11.55, 95% CI: 3.90 to 34.22), headaches (RR: 1.20, 95% CI: 1.00 to 1.43), erythema (RR: 15.38, 95% CI: 5.64 to 41.92), and fever (after the second dose: RR: 2.33, 95% CI: 1.24 to 4.38).
Conclusion:
Although the NTHi-Mcat/NTHi vaccines were well-tolerated in COPD patients, they did not significantly reduce the risk of exacerbations or mortality. These findings suggest that further research is needed to validate these results and identify potential subgroups that may derive clinical benefit.
Systematic review registration:
The study was registered in PROSPERO (ID: CRD42023381488).
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide, and has a significant and growing economic and social burden (1). Among the 55.4 million deaths worldwide in 2019, COPD is the second and third leading cause of death, accounting for 11 and 6% of total deaths, respectively (2, 3). In the United States, the cost of COPD is projected to grow over the next 20 years, with a projected annual cost of $800.9 billion or $40 billion (4, 5). The Global Burden of Disease (GBD) was significantly higher compared to that in 2010 and 2014, especially for moderate to severe cases (5, 6). The prevalence and burden of COPD are expected to increase in the coming decades because of the continued exposure to COPD risk factors and an aging population (7, 8). Furthermore, individuals with COPD are at a higher risk of experiencing acute exacerbation of chronic obstructive pulmonary disease (AECOPD) (9). The pathology of AECOPD is mainly associated with increased airway inflammation, increased mucus production, and pronounced gas trapping (10, 11). These changes lead to increased dyspnea, which in turn leads to a decreased lung function and quality of life, and an increased mortality (9, 12, 13).
GBD report recommended that vaccination against Haemophilus influenza type b should be offered that is largely based on reducing severe illness (e.g., lower respiratory tract infections requiring hospitalization) and death in patients with COPD (14, 15). The US Centers for Disease Control (CDC) has endorsed the pneumococcal vaccine because of its demonstrated ability to lower the occurrence of AECOPD and comorbidities of COPD (16). Notably, Non-typable Haemophilus influenzae (NTHi) and Moraxella catarrhalis (Mcat) have been identified as the predominant bacteria responsible for AECOPD (17–20). Furthermore, NTHi and Mcat often act as co-pathogens in COPD, thereby exacerbating issues related to antibiotic resistance and host immune response when co-infections occur (21, 22). In response to this challenge, an experimental adjuvant multicomponent vaccine has been developed to target NTHi and Mcat, aiming to enhance local airway immunity and pathogen-specific antibody production, thereby potentially reducing the frequency and severity of AECOPD episodes (23).
While several randomized controlled trials (RCTs) have investigated the efficacy and safety of NTHi-Mcat/NTHi vaccines in COPD patients, the findings have been inconclusive. Some RCTs reported positive outcomes, indicating that the vaccine is both safe and effective (24). Conversely, other RCTs have failed to demonstrate the efficacy of the vaccine in reducing AECOPD occurrence in COPD patients (25–28). In a recent Cochrane review, meta-analyses were conducted to evaluate the efficacy of the NTHi vaccine, focusing on adults with chronic bronchitis or COPD (29). This review was an update of a previous publication from 1998, which primarily aimed to assess the protective effects of the NTHi vaccine against recurrent acute episodes in patient with chronic bronchitis (30). Although the 2017 Cochrane review included studies involving adults with chronic bronchitis and COPD, it did not specifically focus on COPD subpopulations or perform robust subgroup analyses, despite incorporating three trials with COPD patients (24, 31, 32). Consequently, there remains a gap in the evidence, as no meta-analysis has specifically evaluated the efficacy of the NTHi vaccine in COPD patients. Our study aims to fill this gap by providing a focused analysis of this population.
Given the evolving landscape of studies on this topic, including recent evidence published in the last few years (23, 26–28), we sought to conduct a comprehensive systematic review and meta-analysis to evaluate the efficacy and safety of NTHi-Mcat/NTHi vaccines in COPD patients. Additionally, we sought to explore potential effect differences by drug delivery methods (e.g., oral vs. intramuscular) and vaccine composition (e.g., NTHi alone vs. NTHi-Mcat). The objective of this study is to offer valuable insight for future studies and guideline development in this area.
Methods
Reporting of the systematic review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (33). The study was registered in PROSPERO (ID: CRD42023381488).
Search strategy
Regarding the search strategy, articles were searched in electronic databases, including PubMed, Cochrane Central Register of Controlled Trials (CENTRAL) databases, Embase (OVID), and Medline (OVID) from inception to October 2024 with “chronic obstructive pulmonary disease (COPD),” “vaccine, “Non-typeable Haemophilus influenzae (NTHi),” and “randomized controlled trial (RCT)” as search terms were retrieved without any restriction of date or language. The reference list of all selected articles was independently screened to identify additional studies. Details of the search strategy are shown in Supplementary Table S1.
Study selection inclusion and exclusion criteria
Studies were included if the following criteria were met: (1) participants diagnosed with COPD or demonstrating immunological characteristics consistent with those of the COPD population, acknowledging the early onset of immune system alterations in smokers prior to COPD manifestation (34–36); (2) adherence to a RCT study design; (3) inclusion of multiple study groups, with at least one group receiving a vaccine containing NTHi or a combination of NTHi and Mcat (NTHi-Mcat); (4) evaluation of the safety and efficacy of the NTHi-Mcat vaccine in COPD patients. Primary outcomes of interest encompassed the incidence of AECOPD, all-cause mortality, hospitalization rates, and a comprehensive array adverse event, spanning serious adverse events, general reactions, and system-specific events affecting the gastrointestinal, respiratory, musculoskeletal, nervous, renal, cardiac, and immune systems. Exclusion criteria were: (1) literature reviews, quasi-randomized or cluster-randomized trials, editorials, conference abstracts, correspondence, and case reports; (2) duplicate publications; (3) studies focused on basic or animal research; (4) investigations focusing solely on reactogenicity or the humoral and cellular immunogenicity of the NTHi-Mcat vaccine; (5) studies lacking adequate data for analysis. If multiple publications from the same trial were identified, we extracted and retained all available data. For outcomes with overlapping populations reported across multiple publications, we prioritized the results with the longest follow-up duration or the largest sample size to ensure the most comprehensive and reliable data were included.
After deleting duplicate studies, two reviewers independently reviewed the title and abstract according to the inclusion criteria. Subsequently, reviewers continued to screen the full text according to the inclusion criteria, and finally the study would be included in the review. Discrepancies in the selection process were resolved through discussion or, if necessary, by consultation with a third reviewer.
Data extraction and quality assessment
Two reviewers independently extracted data and assessed the risk of bias using a standardized data abstraction form. The following information was extracted: (1) study characteristics: first author, publication year, phase, center, blinding, study duration, and follow-up; (2) participant characteristics: mean age, gender, sample size, and region; (3) intervention and comparator information: components of the vaccine, vaccine types, and delivery methods; and (4) outcomes: effect size, and 95% confidence intervals (CI). In case of uncertainty, the reviewers consulted each other. Discrepancies during the data extraction and risk of bias assessment were resolved through discussion or, if necessary, by consultation with a third reviewer.
The risk of bias was assessed using the RCT risk of bias assessment tool (RoB) as recommended in the Cochrane Handbook of Systematic Reviews (37). The evaluation included the following: random sequence generation, allocation concealment, whether to use blinding to participants and personnel, whether to use blinding to the measurers of the study outcome, whether the outcome data were complete, whether to selectively report the study results and other sources of bias. Each domain was rated as “uncertain,” “low risk,” or “high risk.”
Statistical analyses
Meta-analyses were performed using R version 4.2.2. The impact magnitude of the measured data was expressed as relative risks (RRs) and 95% CI. Depending on the I2 and p values, the data were combined using either a random-effects or fixed-effects model. When the I2 statistic was greater than 50% or the p-value was less than 0.05, indicating significant heterogeneity, the random-effects model was used to compute the pooled effect sizes (38). Otherwise, the fixed-effects model was applied. The restricted maximum-likelihood estimator of τ2 was used to assess the between-study heterogeneity (39). The Hartung–Knapp method was used for adjustment of the estimates and confidence intervals (CIs) for random-effects model (40).
To further evaluate heterogeneity, subgroup analyses were conducted to evaluate the results of standardized meta-analysis. Subgroup analyses were conducted based on intervention characteristics (e.g., drug types, methods of administration), region (Europe vs. Australia vs. mixed), sample size (≥50 vs. <50), and study design (multicenter vs. single center). To comprehensively assess the relative impact of each study, sequential removal of each study for sensitivity analysis was performed. Funnel plot, the Egger’s test (41) and Begg’s test (42) were used to assess for publication bias if more than 10 studies were included in the primary outcomes.
Results
A total of 556 records were initially retrieved through the search strategy, and after removing 198 duplicates, 358 articles were included for further screening. These articles were assessed based on title and abstract, resulting in 27 full-text articles being examined for eligibility. After excluding 19 records (details are shown in Supplementary Table S2), eight studies were ultimately included in the analysis (Figure 1).
Figure 1

The flow chart of the study selection.
Characteristics of the studies
Eight eligible RCTs included a total of 1,574 participants, with sample sizes ranging from 33 to 606. The characteristics of the studies are shown in Table 1 and Supplementary Table S3. The majority of the studies were conducted in Europe (n = 3) (23, 27, 28), followed by two studies from Australia (31, 32), one study from the USA and Australia (24), and one study (25) with undisclosed participant origin (Table 1). Drugs were administered orally (24, 25, 31, 32) or via intramuscular injection (23, 26–28). The administration frequency varied, with some studies administering the intervention twice (23, 26–28) and others three times (31, 32); in one study the administration frequency was not specified. One study has two control groups (32), and two studies have two intervention groups (23, 28).
Table 1
| First author | Year | Phase | Multicenter | Blinding | Study duration | Sample size | Country | Follow-up (Months) | Age (Mean) | Men (%) | Intervention | Control | Funding | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | I/C | I/C | I/C | Vaccine | Delivery methods | Drug | Delivery methods | |||||||||
| Andreas S. (26) | 2022 | Phase 2b trial | Yes | Observer-blinded | 2017/11–2020/03 | 606 | 304/302 | Belgium, Canada, France, Germany, Italy, Spain, UK, and USA. | 15 | 65.7/66.3 | 60.5/58.6 | NTHi–Mcat vaccine | Intramuscular injection | Phosphate-buffered saline | Intramuscular injection | GlaxoSmithKline Biologicals SA. |
| Clancy R. (32) | 1985 | NR | No | Double-blind | Winter period in 1983 | 34 | 17/17 | Australia | 3 | 64.7/62.0 | 82.4/94.1 | NTHi vaccine | Oral | 25 mg sodium tauroglycocholate | Oral | GlaxoSmithKline Biologicals SA |
| 33 | 17/16 | Australia | 3 | 64.7/65.5 | 82.4/62.5 | NTHi vaccine | Oral | Enteric-coated glucose tablet | Oral | Ciba-Geigy (Australia) | ||||||
| Clancy R. L. (31) | 2016 | NR | Yes | Double-blind | Winter of 2011 | 320 | 160/160 | Australia | 9 | 71.2/67.9 | 66.9/58.1 | NTHi vaccine | Oral | NR | Oral | Bioxyne Ltd |
| De Smedt P. (28) | 2021 | Phase 1 trial | Yes | Observer-blinded | 2015/8–2017/4 | 55 | 27/28 | Belgium | 48 | 59.7/58.2 | 55.6/67.9 | NTHi–Mcat vaccine | Intramuscular injection | Saline solution | Intramuscular injection | GlaxoSmithKline Biologicals SA |
| 54 | 26/28 | Belgium | 48 | 59.0/58.2 | 53.8/67.9 | NTHi–Mcat vaccine | Intramuscular injection | Saline solution | Intramuscular injection | GlaxoSmithKline Biologicals SA | ||||||
| Tandon M. K. (24) | 2010 | Phase 2 trial | Yes | Double-blind | 2023/10/6 | 38 | 18/20 | USA and AUS | 4 | 69.5/67.3 | 83.3/70.0 | NTHi vaccine | Oral | Enteric-coated placebo tablet | Oral | Hunter Immunology Ltd |
| Philips M. (25) | 2007 | NR | No | Double-blind | NR | 140 | 68/72 | Australia | NR | NR/NR | NR/NR | NTHi vaccine | Oral | NR | NR | NR |
| Van Damme P. (23) | 2019 | Phase 1 trial | Yes | Observer-blinded | 2015/8–2017/3 | 75 | 31/44 | Belgium | 12 | 58.9/47.2 | 58.1/52.3 | NTHi–Mcat vaccine | Intramuscular injection | Saline solution | Intramuscular injection | GlaxoSmithKline Biologicals SA |
| 74 | 30/44 | Belgium | 12 | 58.5/47.2 | 56.7/52.4 | NTHi–Mcat vaccine | Intramuscular injection | Saline solution | Intramuscular injection | GlaxoSmithKline Biologicals SA | ||||||
| Wilkinson T. M. A. (27) | 2019 | Phase 2 trial | Yes | Observer-blinded | 2014/7/8–2017/4/19 | 145 | 73/72 | Sweden and UK | 15 | 67.0/66.8 | 53.4/50.0 | NTHi vaccine | Intramuscular injection | Saline placebo | Intramuscular injection | GlaxoSmithKline Biologicals SA |
Characteristics of randomized trials included in systematic review and meta-analysis.
NTHi–Mcat vaccine, non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine; NTHi vaccine, non-typeable Haemophilus influenzae vaccine; NR, not reported; I, intervention; C, control.
Risk of bias assessment
The RoB assessment (Supplementary Figure S1) showed that high risk of bias was primarily attributed to reporting bias. Specifically, two studies (24, 25) had incomplete reporting of adverse reaction outcomes, possibly due to a different focus of the study as it was not possible to report the important outcome measures consistently to all studies.
The efficacy of non-typeable Haemophilus influenzae vaccine in COPD
This meta-analysis included five studies (24, 26, 27, 31, 32) with a total of 1,176 participants. The results showed that the NTHi-Mcat/NTHi vaccine did not have a significant effect on the risk of AECOPD occurrence (RR: 1.02, 95% CI: 0.76 to 1.36; Figure 2). Sensitivity analysis was conducted and the stability and reliability of the findings were confirmed (Supplementary Figure S2). The research findings presented in Table 2 suggest that the NTHi-Mcat/NTHi vaccine does not increase the risk of AECOPD in multicenter studies (n = 4, RR: 1.03, 95% CI: 0.90 to 1.19). Additionally, subgroup analyses showed no significant differences in AECOPD risk based on region (p = 0.69), sample size (p = 0.46), intervention types (p = 0.67), and drug delivery methods (p = 0.74).
Figure 2
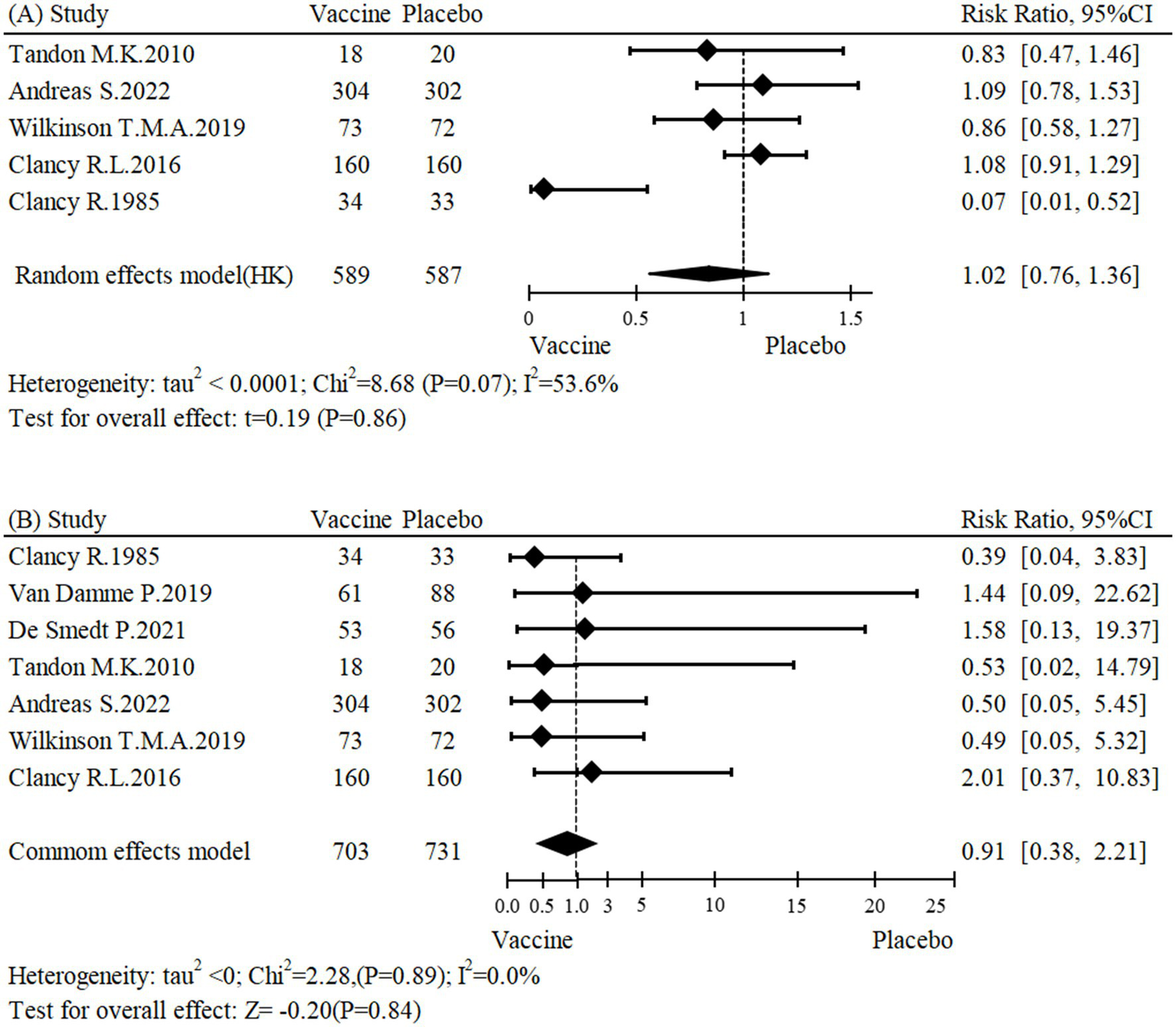
Forest plot for (A) incidence of AECOPD and (B) all-cause mortality.
Table 2
| Subgroups | No. of studies | No. of patients | Effect size | Heterogeneity | p interaction | |
|---|---|---|---|---|---|---|
| RR (95%CI) | I2 (%) | p value | ||||
| AECOPD | ||||||
| Region | ||||||
| Europe | 2 | 751 | 0.98 (0.76,1.27) | 0 | 0.37 | 0.69 |
| Australia | 2 | 387 | 1.06 (0.89,1.26) | 86 | <0.01 | |
| Mixed | 1 | 38 | 0.83 (0.47,1.46) | ─ | ─ | |
| Center | ||||||
| Multicenter | 4 | 1,109 | 1.03 (0.90,1.19) | 0 | 0.62 | <0.01 |
| Single center | 1 | 67 | 0.07 (0.01,0.52) | ─ | ─ | |
| Sample sizes | ||||||
| ≥ 50 | 4 | 1,138 | 1.03 (0.90,1.19) | 63 | 0.04 | 0.46 |
| < 50 | 1 | 38 | 0.83 (0.47,1.46) | ─ | ─ | |
| Intervention types | ||||||
| NTHi vaccine | 4 | 570 | 1.01 (0.86,1.17) | 65 | 0.04 | 0.67 |
| NTHi–Mcat vaccine | 1 | 606 | 1.09 (0.78,1.53) | ─ | ─ | |
| Drug delivery methods | ||||||
| Intramuscular injection | 2 | 751 | 0.98 (0.76,1.27) | 0 | 0.37 | 0.74 |
| Oral | 3 | 425 | 1.04 (0.88,1.22) | 74 | 0.02 | |
| All-cause mortality | ||||||
| Region | ||||||
| Europe | 4 | 1,009 | 0.82 (0.24,2.86) | 0 | 0.86 | 0.89 |
| Australia | 2 | 387 | 1.13 (0.29,4.37) | 22 | 0.26 | |
| Mixed | 1 | 38 | 0.53 (0.02,14.80) | ─ | ─ | |
| Center | ||||||
| Multicenter | 6 | 1,367 | 1.06 (0.41,2.77) | 0 | 0.89 | 0.43 |
| Single center | 1 | 67 | 0.39 (0.04,3.83) | ─ | ─ | |
| Sample size | ||||||
| ≥ 50 | 6 | 1,396 | 0.95 (0.38,2.38) | 0 | 0.83 | 0.74 |
| < 50 | 1 | 38 | 0.53 (0.02,14.80) | ─ | ─ | |
| Drug of interventions | ||||||
| NTHi vaccine | 4 | 570 | 0.87 (0.29,2.63) | 0 | 0.63 | 0.88 |
| NTHi–Mcat vaccine | 3 | 864 | 1.00 (0.23,4.32) | 0 | 0.77 | |
| Drug delivery methods | ||||||
| Intramuscular injection | 4 | 1,009 | 0.82 (0.24,2.86) | 0 | 0.86 | 0.82 |
| Oral | 3 | 425 | 1.01 (0.29,3.56) | 0 | 0.48 | |
| Hospitalization | ||||||
| Region | ||||||
| Europe | 0 | - | ||||
| Australia | 3 | 527 | 1.29 (1.00,1.68) | 91 | <0.01 | 0.06 |
| Mixed | 1 | 38 | 1.23 (0.95,1.58) | - | - | |
| Center | ||||||
| Single-center | 2 | 207 | 0.30 (0.15,0.59) | 7 | 0.3 | <0.01 |
| Multicenter | 2 | 358 | 1.54 (1.17,2.03) | 81 | 0.02 | |
| Sample sizes | ||||||
| <50 | 1 | 38 | 0.42 (0.13,1.33) | - | - | |
| ≥50 | 3 | 527 | 1.29 (1.00,1.68) | 91 | <0.01 | 0.06 |
| Serious adverse events | ||||||
| Region | ||||||
| Europe | 4 | 1,009 | 0.91 (0.73,1.12) | 0 | 0.62 | 0.13 |
| Australia | 1 | 320 | 1.19 (0.90,1.58) | ─ | ─ | |
| Drug of Interventions | ||||||
| NTHi vaccine | 2 | 465 | 1.13 (0.87,1.46) | 0 | 0.37 | 0.23 |
| NTHi–Mcat vaccine | 3 | 864 | 0.91 (0.72,1.15) | 0 | 0.41 | |
| Drug delivery methods | ||||||
| Intramuscular injection | 4 | 1,009 | 0.91 (0.73,1.12) | 0 | 0.62 | 0.13 |
| Oral | 1 | 320 | 1.19 (0.90,1.58) | ─ | ─ | |
| Grade 3 serious adverse events | ||||||
| Region | ||||||
| Europe | 3 | 900 | 1.35 (0.92,1.98) | 0 | 0.39 | 0.43 |
| Australia | 1 | 320 | 1.11 (0.81,1.52) | ─ | ─ | |
| Drug of interventions | ||||||
| NTHi vaccine | 2 | 465 | 1.07 (0.79,1.45) | 0 | 0.41 | 0.20 |
| NTHi–Mcat vaccine | 2 | 755 | 1.49 (0.99,2.23) | 0 | 0.68 | |
| Drug delivery methods | ||||||
| Intramuscular injection | 3 | 900 | 1.35 (0.92,1.98) | 0 | 0.39 | 0.43 |
| Oral | 1 | 320 | 1.11 (0.81,1.52) | ─ | ─ | |
| pIMDs | ||||||
| Drug of interventions | ||||||
| NTHi vaccine | 1 | 145 | 3.95 (0.18,85.99) | ─ | ─ | 0.67 |
| NTHi–Mcat vaccine | 3 | 864 | 1.94 (0.65,5.74) | 0 | 0.98 | |
Subgroup analyses of efficacy and safety of non-typeable Haemophilus influenzae vaccine in chronic obstructive pulmonary disease.
AECOPD, Acute exacerbation of chronic obstructive pulmonary disease; NTHi–Mcat vaccine, non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine; NTHi vaccine, non-typeable Haemophilus influenzae vaccine; pIMDs, potential immune-mediated diseases.
A meta-analysis of seven studies (23, 24, 26–28, 31, 32), encompassing a total of 1,434 participants, assessed the impact of the NTHi-Mcat/NTHi vaccine on all-cause mortality. The findings showed that the vaccine did not have a significant effect on the risk of all-cause mortality (RR: 0.91, 95% CI: 0.38 to 2.21; Figure 2). Furthermore, sensitivity analysis confirmed the stability of the results (Supplementary Figure S3). Subgroup analyses were conducted to investigate potential sources of heterogeneity, including region (p = 0.89), center (p = 0.43), sample size (p = 0.74), intervention types (p = 0.88), and drug delivery methods (p = 0.82). None of these factors were found to be significantly associated with heterogeneity (Table 2).
The safety of non-typeable Haemophilus influenzae vaccine in COPD
The meta-analysis of five studies (23, 26–28, 31) involving 565 participants, showed that the NTHi-Mcat/NTHi vaccine did not affect the incidence of hospitalization (RR: 0.50, 95% CI: 0.09 to 2.77; Figure 3). Sensitivity analyses indicated that the results were stable (Supplementary Figure S4). Moreover, subgroup analysis revealed that the NTHi-Mcat/NTHi vaccine was not associated with a risk of hospitalization in multicenter studies (n = 2, RR: 1.54, 95% CI: 1.17 to 2.03). However, in single-center studies (n = 2), the NTHi-Mcat/NTHi vaccine significantly decreased the risk of hospitalization (RR: 0.30, 95% CI: 0.15 to 0.59). Furthermore, subgroup analyses did not indicate region (p = 0.06) and sample size (p = 0.06) to be significant sources of heterogeneity (Table 2).
Figure 3

Meta-analysis for safety outcomes.
In addition to evaluating the efficacy of NTHi-Mcat/NTHi vaccines in COPD patients, the safety profile was also comprehensively analyzed. This analysis encompassed a wide range of adverse events, including serious adverse events, general events, and events that were specific to different bodily systems, such as gastrointestinal, respiratory, musculoskeletal, nervous, renal, cardiac, and immune system disorders (Supplementary Table S4). To ensure a robust assessment, a meta-analysis of these safety outcomes was conducted when at least three studies reported on a specific event.
The meta-analysis of five studies (25, 27, 31, 32) involving 1,329 participants indicated that administration of the NTHi-Mcat/NTHi vaccine had no significant impact on the incidence of serious adverse events (RR: 1.00, 95% CI: 0.84 to 1.19; Figure 3). Subgroup analyses were performed to explore potential sources of heterogeneity, including region (p = 0.13), intervention types (p = 0.23), and drug delivery methods (p = 0.13), but no significant association was found (Table 2).
Four studies (23, 26, 27, 31), including a total of 1,220 participants, were included in this meta-analysis. The analysis indicated that the NTHi-Mcat/NTHi vaccine did not increase risk of grade 3 serious adverse events (RR: 1.20, 95% CI: 0.93 to 1.53; Figure 3). Furthermore, subgroup analyses were conducted to explore potential sources of heterogeneity, but no significant association was found between region (p = 0.43), intervention types (p = 0.20), drug delivery methods (p = 0.43), and heterogeneity (Table 2).
The findings from the meta-analysis regarding other safety outcomes are presented in Table 3. While there were some differences in general adverse events between the NTHi-Mcat/NTHi vaccine group and the control group, no significant differences in adverse events were found regardless of timing (at any time, after the first dose, or after the second dose). However, compared to the control group, individuals in the NTHi-Mcat/NTHi vaccine group had a higher likelihood of experiencing pain and swelling at any time (pain: n = 3, RR: 5.33, 95% CI: 1.98 to 14.33; swelling: n = 3, RR: 12.15, 95% CI: 4.67 to 31.67), after the first dose (pain: n = 3, RR: 7.72, 95% CI: 5.52 to 10.79; swelling: n = 3, RR: 6.66, 95% CI: 1.88 to 33.76), and after the second dose (pain: n = 3, RR: 7.96, 95% CI: 1.98 to 14.33; swelling: n = 3, RR: 16.10, 95% CI: 3.78 to 68.60). Moreover, individuals in the NTHi-Mcat/NTHi vaccine group were more likely to experience redness after both the first dose (n = 3, RR: 12.74, 95% CI: 3.48 to 46.59) and the second dose (n = 3, RR: 11.55, 95% CI: 3.90 to 34.22). Additionally, individuals in the NTHi-Mcat/NTHi vaccine group had a higher risk of developing headaches (any time: n = 3, RR: 1.20, 95% CI: 1.00 to 1.43; after the first dose: n = 3, RR: 1.30, 95% CI: 1.03 to 1.64), erythema (any time: n = 3, RR: 15.38, 95% CI: 5.64 to 41.92), and fever (after the second dose: n = 3, RR: 2.33, 95% CI: 1.24 to 4.38). Notably, the increased risk of injection-site-related events (pain, swelling, redness) is consistent with many vaccine studies.
Table 3
| General adverse events | Sample size | No. of event | No. of studies | Rate ratio, 95%CI | p value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|
| I2 | τ2 | p value | ||||||
| Any | ||||||||
| Fatigue | 894 | 502 | 3 | 1.28 (0.59, 2.78) | 0.31 | 74.1 | 0.08 | 0.02 |
| Fever | 1,003 | 99 | 3 | 1.38 (0.94, 2.01) | 0.10 | 21.0 | 0.04 | 0.28 |
| Pain | 900 | 386 | 3 | 5.33 (1.98, 14.33) | 0.02 | 74.5 | 0.12 | 0.02 |
| Headache | 894 | 330 | 3 | 1.20 (1.00, 1.43) | 0.05 | 0.0 | 0.0 | 0.64 |
| Swelling | 900 | 55 | 3 | 12.15 (4.67, 31.61) | <0.01 | 0.0 | 0.0 | 0.96 |
| pIMDs | 1,009 | 13 | 4 | 2.10 (0.75, 5.84) | 0.16 | 0.0 | 0.0 | 0.98 |
| Erythema | 900 | 75 | 3 | 15.38 (5.64,41.92) | <0.01 | 0.0 | 0.0 | 0.47 |
| Nasopharyngitis | 900 | 71 | 3 | 0.95 (0.61, 1.49) | 0.84 | 0.0 | 0.0 | 0.51 |
| Oropharyngeal pain | 900 | 16 | 3 | 0.90 (0.35,2.34) | 0.83 | 0.0 | 0.0 | 0.87 |
| Gastroenteritis | 938 | 7 | 4 | 0.84 (0.22,3.22) | 0.79 | 0.0 | 0.0 | 0.77 |
| Gastrointestinal disorders | 900 | 163 | 3 | 0.96 (0.73,1.25) | 0.74 | 0.0 | 0.01 | 0.54 |
| Pneumonia | 900 | 25 | 3 | 0.50 (0.22,1.16) | 0.11 | 18.2 | 0.27 | 0.29 |
| Rib fracture | 900 | 3 | 3 | 2.07 (0.37,11.67) | 0.41 | 0.0 | 0.0 | 1.00 |
| Upper respiratory tract infection | 822 | 23 | 3 | 1.21 (0.54,2.68) | 0.65 | 0.0 | 0.0 | 0.88 |
| Other adverse events* | 900 | 751 | 3 | 1.27 (0.86, 1.87) | 0.12 | 80.5 | 0.02 | 0.01 |
| After dose 1 | ||||||||
| Redness | 877 | 33 | 3 | 12.74 (3.48, 46.59) | <0.01 | 0.0 | 0.0 | 0.80 |
| Fatigue | 877 | 398 | 3 | 0.95 (0.82, 1.09) | 0.44 | 0.0 | 0.0 | 0.86 |
| Fever | 877 | 59 | 3 | 0.92 (0.56, 1.50) | 0.73 | 34.5 | 0.0 | 0.22 |
| Pain | 877 | 280 | 3 | 7.72 (5.52, 10.79) | <0.01 | 22.9 | 0.04 | 0.27 |
| Headache | 877 | 232 | 3 | 1.30 (1.03, 1.64) | 0.03 | 0.0 | 0.0 | 0.91 |
| Swelling | 877 | 23 | 3 | 6.66 (2.16, 20.53) | <0.01 | 0.0 | 0.0 | 0.99 |
| Gastrointestinal symptoms | 877 | 133 | 3 | 0.93 (0.68, 1.27) | 0.63 | 0.0 | 0.0 | 0.75 |
| After dose 2 | ||||||||
| Redness | 823 | 61 | 3 | 11.55 (3.90, 34.22) | <0.01 | 6.8 | 0.0 | 0.34 |
| Fatigue | 823 | 341 | 3 | 1.72 (0.51, 5.83) | 0.20 | 83.3 | 0.21 | <0.01 |
| Fever | 823 | 48 | 3 | 2.33 (1.24, 4.38) | 0.01 | 39.9 | 0.36 | 0.19 |
| Pain | 823 | 294 | 3 | 7.96 (1.88, 33.76) | 0.03 | 78.6 | 0.27 | 0.01 |
| Headache | 823 | 189 | 3 | 1.77 (0.34, 9.07) | 0.27 | 67.2 | 0.26 | 0.05 |
| Swelling | 823 | 44 | 3 | 16.10 (3.78. 68.60) | <0.01 | 0.0 | 0.0 | 0.48 |
| Gastrointestinal symptoms | 823 | 102 | 3 | 1.40 (0.96, 2.03) | 0.08 | 47.4 | 0.19 | 0.15 |
The safety of non-typeable Haemophilus influenzae vaccine in chronic obstructive pulmonary disease.
*Not Including Serious adverse events; pIMDs, potential immune-mediated diseases.
Based on the evidence available, the NTHi-Mcat/NTHi vaccine appears to be safe for use in individuals with COPD. No significant differences were observed in the occurrence of major adverse events between vaccine and placebo groups. However, mild adverse events, such as fatigue, headaches, myalgia, and fever were reported at similar rates in both groups, following both the initial and subsequent doses of the vaccine. These findings were further supported by sensitivity analyses, which enhanced the reliability and consistency of the results obtained (Supplementary Figures S5–S7).
Discussion
This study is a comprehensive systematic review and meta-analysis conducted to assess the efficacy and safety of NTHi-Mcat/NTHi vaccines in COPD patients. Our study findings show no statistically significant differences in adverse events between the NTHi-Mcat/NTHi vaccine group and the control group at any time point, post-first dose, or post-second dose, except for six commonly reported events: pain, swelling, redness, headaches, erythema, and fever. Furthermore, our evidence supports that NTHi-Mcat/NTHi vaccines do not reduce the risk of AECOPD or overall mortality. These findings are significant for clinical practice and future research in this area.
In previous studies (29, 43), it has been reported that vaccination has no significant effect on the number of AECOPD, which is consistent with the findings obtained in our study. However, our study expanded previous results in two critical aspects. First, a larger number of studies was included, totaling 8 articles with a collective sample size of 2,180 participants. The larger sample size encompassed a diverse range of participants, including individuals from different regions, ethnicities, and age groups, thereby enhancing the reliability and generalizability of our results. Consequently, to explore the potential sources of heterogeneity, subgroup analyses were conducted based on various factors, such as regions, centers, intervention types, sample size, and drug delivery methods. Moreover, a comprehensive investigation into the safety of vaccination was conducted, taking into account general adverse events as well as events specific to different bodily systems, including gastrointestinal, respiratory, musculoskeletal, nervous, renal, cardiac, and immune system disorders.
The mechanism of action underlying the efficacy of NTHi-Mcat/NTHi vaccines in patients with COPD likely involves the stimulation of a robust immune response against these pathogens (44). This immune response is characterized by the generation of pathogen-specific antibodies (45) and the activation of cellular immunity (27). Additionally, the vaccines exhibit immunomodulatory effects within the airway microenvironment, thereby contributing to enhanced respiratory function and reduced exacerbations in COPD patients. Nevertheless, our research findings indicate that NTHi-Mcat/NTHi vaccines do not significantly impact the risk of AECOPD or all-cause mortality in COPD patients. This lack of effect may be attributed to the marked genetic heterogeneity of NTHi, including its phase-variable genes, as well as the high genetic variability of NTHi or differences in patients’ baseline immunity. These factors may all potentially reduce the efficacy of the vaccine (46). Further investigations are warranted to elucidate the mechanisms underlying the effects of these vaccines and to explore biomarkers that can predict vaccine responses, thereby helping to identify specific patient subgroups who may benefit from the vaccines.
The overall heterogeneity in this study was low (I2 < 50%), suggesting that heterogeneity had a limited impact on the results. To further explore the sources of heterogeneity and its potential influence, subgroup and sensitivity analyses were conducted. The heterogeneity observed in hospitalization outcomes primarily originated from one single-center study (32). This may be attributed to limitations in sample selection, research methods, or regional differences inherent in single-center studies. Potential explanations include variations in the standard of care, disease severity, or differing definitions of ‘hospitalization’ across study settings.” Nevertheless, this study effectively identified and controlled the main source of heterogeneity through sensitivity analysis, significantly enhancing the robustness of the results. Future research could further validate and optimize the findings by expanding sample sizes and incorporating more high-quality multicenter studies, thereby reducing the potential impact of heterogeneity and improving the generalizability of the conclusions.
Although our study did not show a significant impact of the NTHi-Mcat/NTHi vaccine on the risk of AECOPD and all-cause mortality in COPD patients, it is important to note that this does not negate the potential efficacy of the vaccine in a specific population. Future research should focus on exploring the efficacy of the vaccine in subgroups, such as those with severe COPD or confirmed infections. The overall safety and efficacy of the NTHi-Mcat/NTHi vaccine still remain controversial, and further studies should consider individual differences and personalized vaccine strategies. To improve vaccine efficacy and minimize side effects, future research can delve into patient genotypes, immune status, and pathogen characteristics to develop more targeted vaccines. Personalized vaccine strategies may require the integration of genetic testing, immunological evaluation, and clinical data to provide tailored preventive measures for each patient. It is crucial to ensure the safety of vaccines to implement effective vaccination programs. Although serious adverse events have not been identified in current studies, continuous monitoring of the safety of newly developed vaccines is necessary. Future studies should focus on long-term safety monitoring and establish effective mechanisms for safety management to promptly assess and manage any potential side effects.
Strengths and limitations
This is the first systematic review and meta-analysis to investigate the safety and efficacy of NTHi-Mcat/NTHi vaccines in COPD patients. However, this study has several limitations. First, the participants in our meta-analyses were predominantly from Europe and Australia, which limits the generalizability of our findings to other regions, such as Asia, Africa, or the Americas. Therefore, the results should be interpreted with caution when applied to other populations. Second, subgroup analyses were conducted and more than 5 studies were included to explore the sources of heterogeneity and the impact of factors on the efficacy of NTHi-Mcat/NTHi vaccines in COPD patients based on vaccine composition, administration route, and dosage. However, the number of studies included in our meta-analyses was fewer than ten, and the sample sizes within subgroups were limited. These factors may reduce the statistical power of the subgroup analyses and limit the generalizability of the findings. Third, variations in participant age and gender, definitions of COPD exacerbations or severity, and vaccine dosage may influence the efficacy of NTHi-Mcat/NTHi vaccines. However, due to limited data availability, we were unable to conduct analyses to explore the impact of these factors on vaccine efficacy in COPD patients.
Conclusion
In summary, our findings indicate that the administration of NTHi-Mcat/NTHi vaccines in COPD patients did not result in any significant adverse events. However, no significant reduction in the risk of AECOPD or overall mortality was observed when using these vaccines. To further validate these results, future studies should prioritize larger sample sizes and longer follow-up periods, particularly in the Asian population, to enhance the generalizability of the findings. Additionally, we recommend conducting multinational RCTs focusing on specific subgroups, such as patients with severe COPD, frequent exacerbations, or confirmed NTHi colonization. Furthermore, investigating immunological correlates of protection—such as baseline antibody titers or immune signatures—may help identify subgroups more likely to benefit from vaccination, providing a deeper understanding of vaccine efficacy in this population.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TS: Writing – original draft, Writing – review & editing. JinL: Writing – original draft, Writing – review & editing. MD: Writing – original draft, Writing – review & editing. PW: Writing – original draft, Writing – review & editing. LZ: Writing – original draft, Writing – review & editing. ZF: Writing – original draft, Writing – review & editing. WL: Writing – original draft, Writing – review & editing. JiaL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of Gansu Province (Grant No. 24JRRA1068).
Conflict of interest
PW was employed by KeyMed Biosciences Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1572726/full#supplementary-material
References
1.
GBD 2019 Chronic Respiratory Diseases Collaborators . Global burden of chronic respiratory diseases and risk factors, 1990-2019: an update from the global burden of disease study 2019. EClinicalMedicine. (2023) 59:101936. doi: 10.1016/j.eclinm.2023.101936
2.
Wongsurakiat P Maranetra KN Wasi C Kositanont U Dejsomritrutai W Charoenratanakul S . Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest. (2004) 125:2011–20. doi: 10.1378/chest.125.6.2011
3.
World Health Organization . (2024). The top 10 causes of death. World Health Organization (WHO). Available online at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (Accessed October, 2024)
4.
Guarascio AJ Ray SM Finch CK Self TH . The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. (2013) 5:235–45. doi: 10.2147/CEOR.S34321
5.
Zafari Z Li S Eakin MN Bellanger M Reed RM . Projecting long-term health and economic burden of COPD in the United States. Chest. (2021) 159:1400–10. doi: 10.1016/j.chest.2020.09.255
6.
Collaborators GCRD . Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med. (2020) 8:585–96. doi: 10.1016/s2213-2600(20)30105-3
7.
Hurst JR Han MK Singh B Sharma S Kaur G de Nigris E et al . Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. (2022) 23:213. doi: 10.1186/s12931-022-02123-5
8.
Tamondong-Lachica DR Skolnik N Hurst JR Marchetti N Rabe APJ Montes de Oca M et al . Update: implications for clinical practice. Int J Chron Obstruct Pulmon Dis. (2023) 18:745–54. doi: 10.2147/copd.S404690
9.
Zinellu A Zinellu E Mangoni AA Pau MC Carru C Pirina P et al . Clinical significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute exacerbations of COPD: present and future. Eur Respir Rev. (2022) 31:220095. doi: 10.1183/16000617.0095-2022
10.
Celli BR Fabbri LM Aaron SD Agusti A Brook R Criner GJ et al . An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am J Respir Crit Care Med. (2021) 204:1251–8. doi: 10.1164/rccm.202108-1819PP
11.
Singh B Kampani G Lall B Singh M . Study of inflammatory markers in chronic obstructive pulmonary disease. J Assoc Physicians India. (2022) 70:48–52. doi: 10.5005/japi-11001-0165
12.
Dharmage SC Bui DS Walters EH Lowe AJ Thompson B Bowatte G et al . Lifetime spirometry patterns of obstruction and restriction, and their risk factors and outcomes: a prospective cohort study. Lancet Respir Med. (2023) 11:273–82. doi: 10.1016/s2213-2600(22)00364-2
13.
Peng JC Gong WW Wu Y Yan TY Jiang XY . Development and validation of a prognostic nomogram among patients with acute exacerbation of chronic obstructive pulmonary disease in intensive care unit. BMC Pulm Med. (2022) 22:306. doi: 10.1186/s12890-022-02100-0
14.
Collaborators GL . Age-sex differences in the global burden of lower respiratory infections and risk factors, 1990-2019: results from the global burden of disease study 2019. Lancet Infect Dis. (2022) 22:1626–47. doi: 10.1016/s1473-3099(22)00510-2
15.
Collaborators GLRI . Quantifying risks and interventions that have affected the burden of lower respiratory infections among children younger than 5 years: an analysis for the global burden of disease study 2017. Lancet Infect Dis. (2020) 20:60–79. doi: 10.1016/s1473-3099(19)30410-4
16.
Centers for Disease Control and Prevention . (2023). COPD: Symptoms, diagnosis, and treatment. Centers for Disease Control and Prevention. Available online at: https://www.cdc.gov/copd/features/copd-symptoms-diagnosis-treatment.html (Accessed August, 2023)
17.
Huang YJ Erb-Downward JR Dickson RP Curtis JL Huffnagle GB Han MK . Understanding the role of the microbiome in chronic obstructive pulmonary disease: principles, challenges, and future directions. Transl Res. (2017) 179:71–83. doi: 10.1016/j.trsl.2016.06.007
18.
Mayhew D Devos N Lambert C Brown JR Clarke SC Kim VL et al . Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax. (2018) 73:422–30. doi: 10.1136/thoraxjnl-2017-210408
19.
Wilkinson TMA Aris E Bourne S Clarke SC Peeters M Pascal TG et al . A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax. (2017) 72:919–27. doi: 10.1136/thoraxjnl-2016-209023
20.
Sethi S Evans N Grant BJ Murphy TF . New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. (2002) 347:465–71. doi: 10.1056/NEJMoa012561
21.
Schaar V Nordstrom T Morgelin M Riesbeck K . Moraxella catarrhalis outer membrane vesicles carry beta-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob Agents Chemother. (2011) 55:3845–53. doi: 10.1128/AAC.01772-10
22.
Su YC Jalalvand F Thegerström J Riesbeck K . The interplay between immune response and bacterial infection in COPD: focus upon non-typeable Haemophilus influenzae. Front Immunol. (2018) 9:2530. doi: 10.3389/fimmu.2018.02530
23.
Van Damme P Leroux-Roels G Vandermeulen C De Ryck I Tasciotti A Dozot M et al . Safety and immunogenicity of non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine. Vaccine. (2019) 37:3113–22. doi: 10.1016/j.vaccine.2019.04.041
24.
Tandon MK Phillips M Waterer G Dunkley M Comans P Clancy R . Oral immunotherapy with inactivated nontypeable Haemophilus influenzae reduces severity of acute exacerbations in severe COPD. Chest. (2010) 137:805–11. doi: 10.1378/chest.09-1382
25.
Philips M Tandon M Waterer G Dunkley M Clancy R . HI-164 an oral vaccine to non-typable Haemophilus influenzae reduces antibiotic use respiratory exacerbations and hospitalisations in patients with COPD. Eur Respir J. (2007) 30:E1370:224s.
26.
Andreas S Testa M Boyer L Brusselle G Janssens W Kerwin E et al . Non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine for the prevention of exacerbations in chronic obstructive pulmonary disease: a multicentre, randomised, placebo-controlled, observer-blinded, proof-of-concept, phase 2b trial. Lancet Respir Med. (2022) 10:435–46. doi: 10.1016/s2213-2600(21)00502-6
27.
Wilkinson TMA Schembri S Brightling C Bakerly ND Lewis K Mac Nee W et al . Non-typeable Haemophilus influenzae protein vaccine in adults with COPD: a phase 2 clinical trial. Vaccine. (2019) 37:6102–11. doi: 10.1016/j.vaccine.2019.07.100
28.
De Smedt P Leroux-Roels G Vandermeulen C Tasciotti A Di Maro G Dozot M et al . Long-term immunogenicity and safety of a non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine: 4-year follow-up of a phase 1 multicentre trial. Vaccine X. (2021) 9:100124. doi: 10.1016/j.jvacx.2021.100124
29.
Teo E Lockhart K Purchuri SN Pushparajah J Cripps AW van Driel ML . Haemophilus influenzae oral vaccination for preventing acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2017) 6:CD010010. doi: 10.1002/14651858.CD010010.pub3
30.
Foxwell AR Cripps AW Dear KB . Haemophilus influenzae oral whole cell vaccination for preventing acute exacerbations of chronic bronchitis. Cochrane Database Syst Rev. (2006):Cd001958. doi: 10.1002/14651858.CD001958.pub2
31.
Clancy RL Dunkley ML Sockler J McDonald CF . Multi-site placebo-controlled randomised clinical trial to assess protection following oral immunisation with inactivated non-typeable Haemophilus influenzae in chronic obstructive pulmonary disease. Intern Med J. (2016) 46:684–93. doi: 10.1111/imj.13072
32.
Clancy R Murree-Allen K Cripps A . Oral immunisation with killed Haemophilus influenzae for protection against acute bronchitis in chronic obstructive lung disease. Lancet. (1985) 2:1395–7. doi: 10.1016/s0140-6736(85)92559-0
33.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
34.
Barceló B Pons J Ferrer JM Sauleda J Fuster A Agustí AG . Phenotypic characterisation of T-lymphocytes in COPD: abnormal CD4+CD25+ regulatory T-lymphocyte response to tobacco smoking. Eur Respir J. (2008) 31:555–62. doi: 10.1183/09031936.00010407
35.
Droemann D Goldmann T Tiedje T Zabel P Dalhoff K Schaaf B . Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res. (2005) 6:68. doi: 10.1186/1465-9921-6-68
36.
Takanashi S Hasegawa Y Kanehira Y Yamamoto K Fujimoto K Satoh K et al . Interleukin-10 level in sputum is reduced in bronchial asthma, COPD and in smokers. Eur Respir J. (1999) 14:309–14. doi: 10.1034/j.1399-3003.1999.14b12.x
37.
Higgins JP Altman DG Gøtzsche PC Jüni P Moher D Oxman AD et al . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
38.
Krawczyk A Kurek K Nucera G Pruc M Swieczkowski D Kacprzyk D et al . Effect of COVID-19 on the prevalence of bystanders performing cardiopulmonary resuscitation: a systematic review and meta-analysis. Cardiol J. (2025) 32:9–18. doi: 10.5603/cj.98616
39.
Viechtbauer W . Confidence intervals for the amount of heterogeneity in meta-analysis. Stat Med. (2007) 26:37–52. doi: 10.1002/sim.2514
40.
Rover C Knapp G Friede T . Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol. (2015) 15:99. doi: 10.1186/s12874-015-0091-1
41.
Egger M Davey Smith G Schneider M Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
42.
Begg CB Berlin JA . Publication Bias: a problem in interpreting medical data. J Royal Stat Soc Series A. (1988) 151:419–63. doi: 10.2307/2982993
43.
Teo E House H Lockhart K Purchuri SN Pushparajah J Cripps AW et al . Haemophilus influenzae oral vaccination for preventing acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2014):Cd010010. doi: 10.1002/14651858.CD010010.pub2
44.
Jalalvand F Riesbeck K . Update on non-typeable Haemophilus influenzae-mediated disease and vaccine development. Expert Rev Vaccines. (2018) 17:503–12. doi: 10.1080/14760584.2018.1484286
45.
Bhat TA Panzica L Kalathil SG Thanavala Y . Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. (2015) 12:S169–75. doi: 10.1513/AnnalsATS.201503-126AW
46.
Chatziparasidis G Kantar A Grimwood K . Pathogenesis of nontypeable Haemophilus influenzae infections in chronic suppurative lung disease. Pediatr Pulmonol. (2023) 58:1849–60. doi: 10.1002/ppul.26446
Summary
Keywords
chronic obstructive pulmonary disease, non-typeable Haemophilus influenzae, Moraxella catarrhalis bacteremia, vaccine, efficacy and safety, systematic review, meta-analysis
Citation
Shuai T, Liu J, Dong M, Wu P, Zhang L, Feng Z, Li W and Liu J (2025) The safety and efficacy of non-typeable Haemophilus influenzae and Moraxella catarrhalis vaccine in chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized controlled trials. Front. Med. 12:1572726. doi: 10.3389/fmed.2025.1572726
Received
07 February 2025
Accepted
20 March 2025
Published
04 April 2025
Volume
12 - 2025
Edited by
Xueping Yu, Fujian Medical University, China
Reviewed by
José J. Leija-Martínez, Autonomous University of San Luis Potosí, Mexico
Dóra Paróczai, University of Szeged, Hungary
Dafeng Lu, Capital Medical University, China
Jiefeng Luo, Fudan University, China
Updates
Copyright
© 2025 Shuai, Liu, Dong, Wu, Zhang, Feng, Li and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Liu, medecinliujian@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.