Abstract
Background:
Prolonged dialysis can lead patients to multiple complications, with heart failure being the most dangerous and the leading cause of death among dialysis patients. Concurrently, exercise has been shown to improve several indicators of heart function.
Methods:
A comprehensive search was conducted across seven databases. The search was limited to studies published between January 2010 and July 2024.
Results:
(1) Compared to conventional care, exercise significantly increased the left ventricular ejection fraction (LVEF) in dialysis patients (MD = 2.00, 95% CI: 1.15–2.84, p < 0.001). Subgroup analysis revealed that combined aerobic and resistance exercise led to a more substantial increase in LVEF compared to aerobic exercise (MD = 2.78, 95% CI: 1.17–4.38, p < 0.001). Each workout lasting more than 30 min was associated with a significant increase in LVEF compared to sessions lasting 30 min or less (MD = 2.5, 95% CI:0.57–4.43, p = 0.001). An exercise intervention cycle of 10 to 12 weeks resulted in a significant increase in LVEF compared to cycles longer than 12 weeks (MD = 3.36, 95% CI: 2.04–4.68, p < 0.001). A weekly exercise frequency of more than three times per week significantly improved LVEF compared to three times per week or less (MD = 2.73, 95% CI: 1.21–4.25, p < 0.001). (2) In comparison to conventional care, exercise effectively reduced the left ventricular mass index (LVMI) of dialysis patients (MD = −7.93, 95% CI: −14.67--1.19, p = 0.02). However, exercise interventions did not demonstrate statistically significant improvements in pulse wave velocity (PWV), left ventricular end-systolic volume (LVESV), and left ventricular end-diastolic volume (LVEDV).
Conclusion:
Exercise can significantly enhance LVEF and decrease LVMI in dialysis patients. However, no significant improvements were observed in PWV, LVESV, or LVEDV. The subgroup analysis indicated that a combination of aerobic and resistance exercise had a better effect on improving LVEF compared to aerobic exercise, and exercise intervention with each workout lasting for more than 30 min, an exercise intervention cycle of 10 to 12 weeks, and an exercise frequency of more than three times per week were more effective in improving LVEF.
1 Introduction
End-stage renal disease (ESRD) represents the final stage of chronic kidney disease (CKD), and hemodialysis (HD) is a crucial and frequently utilized renal replacement therapy for patients with ESRD. Statistics indicate that the prevalence of ESRD and the utilization rate of renal replacement therapy are increasing annually on a global scale (1). In China, the in-hospital mortality rate for patients with CKD is 2.6%, significantly higher than the 0.8% rate for non-CKD patients. Among CKD patients, those with heart failure experience an even greater in-hospital mortality rate, reaching 7.8% (2). Once heart failure occurs, it can lead to substantial morbidity and mortality.
Heart failure is a common complication among patients undergoing HD. Pathological changes in cardiac structure and function are directly associated with the incidence of cardiovascular events in dialysis patients. These changes include left ventricular hypertrophy (LVH), left ventricular dilation, left ventricular systolic or diastolic dysfunction, and aortic sclerosis, among others (3). When the left ventricular ejection fraction (LVEF) is 40% or lower, the risk of heart failure increases, often accompanied by progressive left ventricular dilation and adverse cardiac remodeling (4). The reduction in cardiac output and coronary artery perfusion results in ischemia of myocardial cells and abnormal regional wall motion, a condition known as myocardial stunning. Repeated episodes of myocardial stunning can exacerbate heart failure. Research has demonstrated that exercise can enhance the quality of life and functional capacity of dialysis patients, as well as alleviate hemodialysis-induced myocardial stunning and reduce its incidence (5, 6). LVH is the most prevalent cardiac abnormality observed in patients with end-stage renal disease (ESRD). Factors associated with LVH include left ventricular mass index (LVMI), arterial stiffness, and volume overload (7). Studies have shown a significant correlation between LVMI and residual renal function, indicating that LVMI is lower when urine output exceeds 250 mL per day (8). Aortic pulse wave velocity (PWV) is a significant predictor of cardiovascular disease and mortality in patients with ESRD. Studies have shown that managing circulating volume can lower blood pressure, promote regression of LVH, and improve survival rates among dialysis patients (9).
According to the Kidney Association’s Clinical Practice Guidelines for Hemodialysis, exercise rehabilitation therapy should be provided for dialysis patients who do not have contraindications to exercise (such as no lower limb fractures, bone or joint lesions, or activity disorders within the past six months) (10). As research progresses, it has been discovered that, in addition to traditional aerobic exercise (such as cycling, recumbent pedaling, stair climbing, and tai chi, etc.), resistance exercise (such as tying sandbags, elastic bands, and dumbbells, etc.) and combined exercise, other innovative forms of exercise (such as virtual reality gaming, yoga for dialysis patients, electrical muscle stimulation, and blood flow restriction training, etc.) can significantly enhance the immune system of individuals undergoing dialysis. These exercises can also reduce the risk of joint stiffness, improve muscle strength, enhance heart function, regulate blood pressure, improve quality of life and sleep, and ultimately promote health (11, 12). Tieming et al. discovered that exercise can significantly enhance the cardiac function of patients undergoing dialysis (13). However, Grigoriou et al. found that exercise had no significant effect on improving the cardiac function of these patients (14).
Therefore, it is essential to collect large samples, comprehensively evaluate eligible studies, and analyze the impact of exercise on improving cardiac function in dialysis patients. This study aims to screen all eligible trials and systematically analyze the effects of exercise rehabilitation on LVEF, PWV, LVMI, left ventricular end-systolic volume (LVESV), and left ventricular end-diastolic volume (LVEDV) in dialysis patients.
2 Methods
2.1 Research registration
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement criteria and the Cochrane Handbook for Systematic Reviews of Interventions were followed in the conduct of this study (15). This systematic review has been registered with PROSPERO, and the registration number is CRD42024574659.
2.2 Search strategy
After discussions among all the members of the research team, a literature search strategy was developed. The primary search terms will included “Renal Dialysis “, “Aerobic Exercise,” “Resistance Exercise,” “Physical Activity “, “Stretching,” “Cycling,” “physical fitness,” “exercise training “, and “Randomized Controlled Trial “, combined with free-text keywords for comprehensive searches. This approach aims to identify randomized controlled trials and assess the impact of exercise intervention programs (aerobic exercise, resistance exercise, or combined exercise) on cardiovascular outcome indicators. The search period was from January 2010 to July 2024 for all published articles. Three English databases (PubMed, Web of Science, and Cochrane Library) and four Chinese databases (China National Knowledge Infrastructure, Wanfang, VIP, and China Biomedical Literature Service System) were searched. The search strategy is detailed in Table 1.
Table 1
| Search strategy in the china national knowledge infrastructure | Search strategy in PubMed |
|---|---|
| #1 (Subject: Dialysis) OR (Subject: Hemodialysis) OR (Subject: Peritoneal Dialysis) | #1 “Renal Dialysis”[Mesh] |
| #2 (Subject: Exercise) OR (Subject: Aerobic Exercise) OR (Subject: Resistance Exercise) OR (Subject: Physical Activity) OR (Subject: Stretching) OR (Subject: Cycling) OR (Subject: Tai Chi) OR (Subject: Physical Fitness) OR (Subject: Baduanjin) | #2 (“Renal Dialysis”[Mesh]) OR (((((((((Dialysis, Renal[Title/Abstract]) OR (Renal Dialyses[Title/Abstract])) OR (Dialyses, Renal[Title/Abstract])) OR (Hemodialysis[Title/Abstract])) OR (Hemodialyses[Title/Abstract])) OR (Dialysis, Extracorporeal[Title/Abstract])) OR (Dialyses, Extracorporeal[Title/Abstract])) OR (Extracorporeal Dialyses[Title/Abstract])) OR (Extracorporeal Dialysis[Title/Abstract])) |
| #3 #1 AND #2 | #3 #1 OR #2 |
| #4 (Abstract: Randomized Controlled Trial) OR (Abstract: Randomized) OR (Abstract: RCT) | #4 ((((((((Exercise[Title/Abstract]) OR (Aerobic Exercise[Title/Abstract])) OR (Resistance Exercise[Title/Abstract])) OR (Physical Activity[Title/Abstract])) OR (Stretching[Title/Abstract])) OR (Cycling[Title/Abstract])) OR (physical fitness[Title/Abstract])) OR (exercise trainin[Title/Abstract])) |
| #5 #3 AND #4 | #5 #3 AND #4 Filters:Randomized Controlled Trial, from 2010/1/1–2024/7/31 |
Literature search strategy.
2.3 Inclusion criteria
(1) Study subjects: patients undergoing hemodialysis or peritoneal dialysis for a minimum of 3 months, exhibiting abnormal cardiac function with LVEF>45%, NYHA score<3, aged between ≥18 and ≤70 years. (2) Intervention measures: dialysis patients who did not engage in any exercise, while the intervention group participated in exercise without restrictions on the form of exercise performed. (3) Control measures: dialysis patients who did not perform any exercise. (4) Outcome indicators: the primary outcome indicator was LVEF, while the secondary outcome indicators included PWV, LVMI, LVESV, and LVEDV. The selected literature must include at least one primary or secondary outcome indicator. (5) Study type: randomized controlled trials.
2.4 Exclusion criteria
(1) Study subjects who are not dialysis patients and do not exhibit abnormal cardiac function. (2) Patients with severe cardiac, vascular, or respiratory diseases, such as unstable angina, valvular heart disease, acute myocardial ischemia, cardiovascular surgery (including valve replacement, coronary artery bypass grafting, and angioplasty), cancer, chronic obstructive pulmonary disease, and other conditions. (3) Intervention measures that do not involve exercise. (4) Literature published before 2010, literature from which valid outcome data cannot be extracted, duplicate publications, or literature with incomplete data. (5) Non-randomized controlled trials.
2.5 Literature screening and data extraction
Two independent researchers (H.Z.L. and M.Q.C.) downloaded the results of the literature search and imported them into the literature management software note express. Using this software, duplicate papers were removed, and the titles and abstracts of all eligible papers were screened. For the papers that passed the initial screening, the full texts were obtained, while those for which the full text could not be retrieved were excluded. A comprehensive assessment was conducted based on the inclusion criteria, and studies that did not meet these criteria were excluded. Any disagreements were resolved by a third researcher (M.Z.). The entire process of selecting research data is presented in the form of a flowchart (Figure 1). The extraction of fundamental data from the included literature was conducted independently by two researchers (H.Z.L. and M.Q.C.) using Excel. Any disagreements were resolved by a third researcher (M.Z.). The extracted data primarily included the first author. For the papers that option, country of study, sample size, patient age, duration of dialysis, and intervention measures, which encompassed types of exercise, duration of exercise, frequency of exercise, intervention period, and exercise intensity.
Figure 1

Schematic diagram of the literature inclusion process.
The primary outcome measured in this study was LVEF, while the secondary outcomes included PWV, LVMI, LVESV, and LVEDV. All outcomes were assessed using echocardiography.
2.6 Literature quality assessment
In this study, two independent researchers (H.Z.L. and M.Q.C.) utilized the risk of bias tool developed by the Cochrane Collaboration to conduct a quality assessment. The assessment criteria included random allocation methods, allocation concealment, blinding of assessors, integrity of outcome data, selective reporting, and other potential biases.
In this study, the GRADE system was employed to assess the quality of evidence. The evaluation was conducted across five dimensions: reference to the risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall strength of the evidence was classified into four categories: high, moderate, low, and very low.
2.7 Statistical analysis
This article utilized RevMan Manager 5.4 to conduct a meta-analysis of more than two randomized controlled trials with identical outcome indicators. The mean difference (MD) was used as the effect measure, and its 95% confidence interval (CI) was calculated simultaneously. The p-value for the combined statistics was determined using the Z test, with P domized controlled trials with identical outcome indigeneity of the research data was assessed using the I2 statistic. If p > 0.1 and I2 < 50%, it indicated no heterogeneity or low heterogeneity, and the fixed-effect model was employed to calculate the combined effect size. Conversely, if p ≤ 0.1 and I2 ≥ 50%, it indicated high heterogeneity, prompting the use of the random-effects model to compute the combined effect size. In instances of significant heterogeneity, subgroup analyses were conducted. A sensitivity analysis was performed on the included literature by systematically removing one study at a time, comparing the changes in I2 values before and after each removal, and assessing the stability of the results. Publication bias was evaluated using a funnel plot. The quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework for pairwise meta-analysis.
3 Results
3.1 Literature retrieval process
We searched seven databases and retrieved a total of 3,969 relevant articles in both Chinese and English. We searched English databases: 212 from PubMed, 429 from Web of Science, 478 from the Cochrane Library. Additionally, we searched Chinese databases: 387 from CNKI, 1,568 from Wanfang, 306 from VIP, and 589 from SinoMed. After excluding duplicate articles, reviews, comments, and animal studies, 92 articles remained. Following the exclusion of those that did not meet the inclusion criteria and those for which the full text could not be obtained, a total of 19 articles were included.
3.2 Methodological quality
Quality evaluation using the Cochrane Risk of Bias tool revealed that only one literature met all evaluation criteria. The remaining literature failed to achieve allocation concealment. Only three literatures explicitly mentioned the implementation of blinding. In exercise trials, it is challenging to meet high-quality evaluation standards, and most studies find it difficult to implement double-blinding. Consequently, the literature is at some risk of bias. The specific literature retrieval process is illustrated in Figure 1.
3.3 Basic characteristics and quality evaluation of the included literature
A total of 19 studies were included in this article, consisting of 16 randomized controlled trials, 3 randomized crossover trials, and 12 publications in English (14, 16–26). Additionally, there were 7 publications in Chinese (13, 27–32). The overall sample size comprised 1,088 individuals, with 615 participants in the intervention group and 610 in the control group. Three studies examined the effects of aerobic exercise combined with resistance training, one study focused solely on resistance training, and the remaining 15 studies investigated aerobic exercise alone. Additionally, one study specifically targeted patients undergoing peritoneal dialysis. Basic information regarding the included literature is presented in Table 2, while detailed quality evaluation tables for the literature are displayed in Figures 2, 3.
Table 2
| Authors and year | Country | Sample size (IG/CG) | Patient Age (years) | Dialysis duration (months) | CG | IG | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| IG | Duration of each exercise (minutes) | Exercise frequency (times per week) | Exercise intervention (weeks) | Exercise intensity (borg rating of perceived exertion scale) | ||||||
| Maufrais, C. 2024 (16) | France | 56 (56/56) | 63 ± 13 | 66 ± 68.4 | HD | AE | 30 | 3 | NR | 11–14 |
| Matthieu Josse 2023 (17) | France | 60 (60/60) | 63 ± 14 | 68.4 ± 72 | HD | AE | 30 | 3 | NR | 11–14 |
| Stefania S. Grigoriou 2022 (14) | Greece | 21 (21/21) | 56 ± 19 | 40 ± 44 | HD | AE + RE | 45 | 3 | NR | 14–16 |
| Matthew P. M. Graham-Brown 2021 (18) | Britain | 101 (51/50) | IG:55.5 ± 15.5 CG:58.9 ± 14.9 |
IG:1.2 ± 2.37 CG:1.3 ± 2.074 |
HD | AE | 30 | 3 | 24 | 12–14 |
| Viviana Rugolo Oliveira e Silva 2019 (19) | Brazil | 30 (15/15) | IG:50 ± 17.2 CG:58 ± 15.0 |
IG:26.0 ± 14.58 CG:21.0 ± 27.1 |
HD | AE | 30 | 3 | 16 | 13 |
| Alexandra B. Cooke 2018 (20) | Canada | 20 (10/10) | IG:58 ± 17 CG:53 ± 15 |
NR | HD | AE | 42.6 | 3 | 16 | 12–16 |
| Gordon McGregor 2018 (21) | Britain | 34 (16/18) | IG:54.3 ± 12.22 CG:52.1 ± 11.63 |
IG:48.1 ± 32.44 CG:49.3 ± 29.19 |
HD | AE | 60 | 3 | 10 | 12–14 |
| Ali Momeni 2014 (22) | Iran | 40 (20/20) | 43.1 ± 10.5 | NR | HD | AE | 30 | 3 | 12 | NR |
| Evangelia Kouidi 2010 (23) | Greece | 44 (24/20) | IG:46.3 ± 11.2 CG:45.8 ± 10.8 |
IG:73.2 ± 55.2 CG:75.6 ± 58.8 |
HD | AE | 60–90 | 3 | 48 | 11–13 |
| Maycon de Moura Reboredo 2010 (24) | Brazil | 22 (11/11) | IG:49.6 ± 10.6 CG:43.5 ± 12.8 |
IG:41.9 ± 42.4 CG:60.1 ± 54.4 |
HD | AE | 60 | 3 | 12 | 4–6 |
| Kirsten P. Koh, BHM 2010 (25) | Australia | 49 (27/22) | IG:52.3 ± 10.9 CG:51.3 ± 14.4 |
30.5 ± 26.6 | HD | AE | 15–45 | 3 | 24 | 12–13 |
| Kenneth R. Wilund 2010 (26) | America | 17 (8/9) | IG:60.8 ± 3.2 CG:59.0 ± 4.9 |
IG:63.3 ± 8.7 CG:44.6 ± 12.2 |
HD | AE | 45 | 3 | 24 | 12–14 |
| Ping Li 2016 (31) | China | 84 (41/43) | IG:49.8 ± 13.5 CG:53.4 ± 14.5 |
IG:37.8 ± 29.4 CG:35.7 ± 30.4 |
HD | AE | 20–30 | 3–5 | 24 | NR |
| Yongyao Wu 2014 (32) | China | 69 (34/35) | IG:45.92 ± 8.64 CG:45.28 ± 8.16 |
IG:55.5 ± 37.3 CG:39.8 ± 29.7 |
HD | AE | 10–15 | 3 | 12 | 12–16 |
| Haiyan Shi 2024 (27) | China | 80 (40/40) | IG;58.28 ± 9.72 CG:59.58 ± 9.12 |
IG:131.57 ± 63.77 CG:121.83 ± 40.56 |
HD | AE | 30 | 3 | 24 | NR |
| Liyang Zhu 2020 (29) | China | 106 (53/53) | IG:46.8 ± 13.72 CG:47.4 ± 12.57 | IG:13.41 ± 5.66 CG:14.87 ± 4.89 |
HD | AE + RE | 10–20 | 4 | 12 | 11–12 |
| Ruihua Li 2019 (30) | China | 50 (25/25) | IG:48.8 ± 12.41 CG:49.1 ± 12.36 |
IG:34.02 ± 15.75 CG:33.79 ± 15.81 |
HD | AE | 30 | 3 | 12 | 11–12 |
| Tieming Niu 2022 (13) | China | 60 (30/30) | IG:57.1 ± 8.7 CG:56.9 ± 9.1 |
IG:27.6 ± 25.2 CG:25.2 ± 26.4 |
PD | AE + RE | 30–60 | 3 | 24 | 11–13 |
| Hong Chen 2022 (28) | China | 125 (63/62) | IG:41.96 ± 5.08 CG:42.75 ± 5.21 |
IG:25.11 ± 4.12 CG:24.33 ± 4.08 |
HD | RE | 40 | 3 | 24 | 11–13 |
Basic information of the included literature.
IG: Intervention Group; CG: Control Group; HD: Hemodialysis; PD: Peritonel Dialysis; AE: Aerobic Exercise; RE: Resistance Exercise; NR: Not Reported.
Figure 2
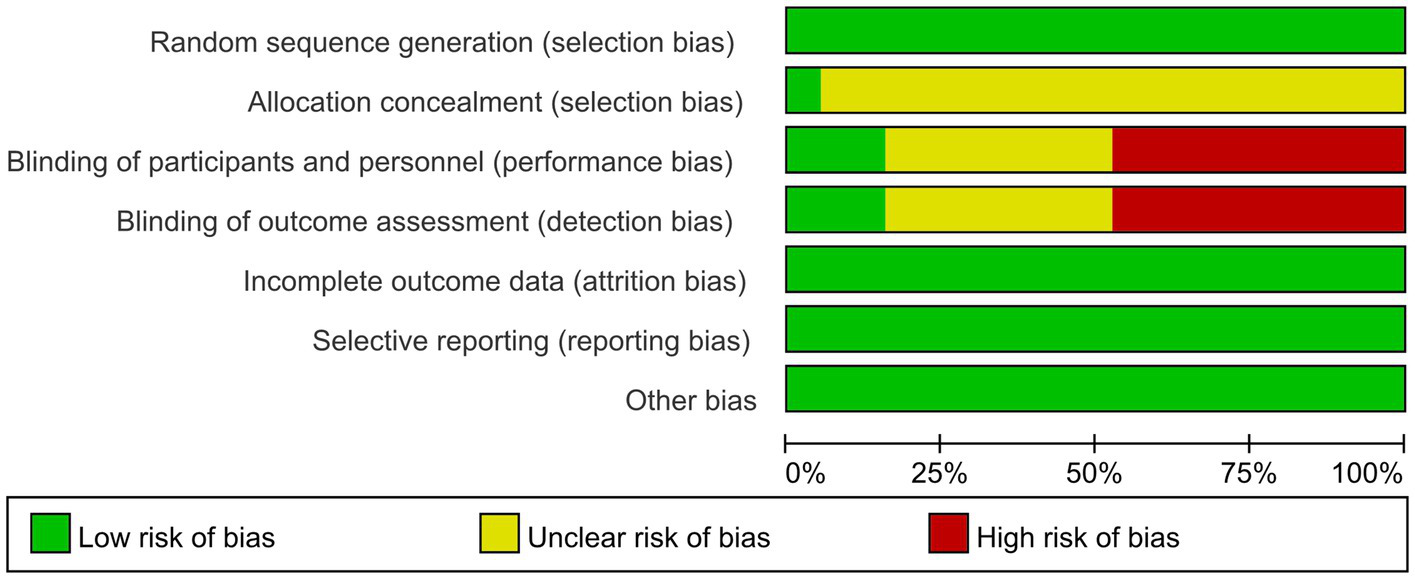
Risk of bias graph.
Figure 3
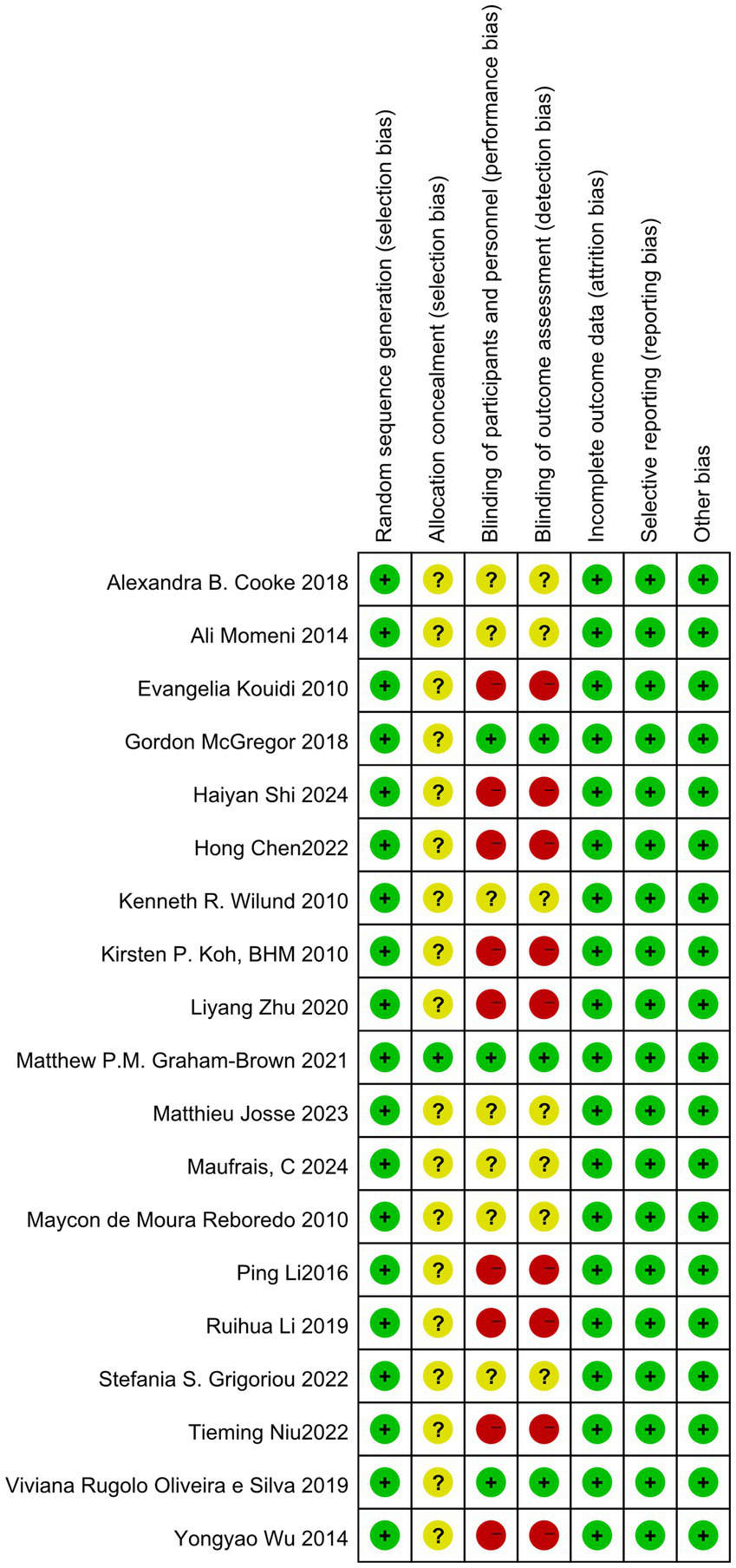
Risk of bias summary.
3.4 Primary outcomes
3.4.1 LVEF
Among the 19 studies included in the analysis, 13 reported the outcome indicator of LVEF. Following a heterogeneity test, the I2 value was found to be 23%, which is less than 50%, and the Q test yielded a p-value of 0.21, indicating low heterogeneity among the studies. A fixed-effect model was employed to combine the effect sizes. Sensitivity analysis was conducted using the one-by-one exclusion method, and the results changed only slightly, suggesting that this study demonstrates good stability. The meta-analysis results indicated that exercise significantly improves LVEF in dialysis patients (MD = 2.00, 95%CI: 1.15–2.84, p < 0.001), as illustrated in Figure 4.
Figure 4
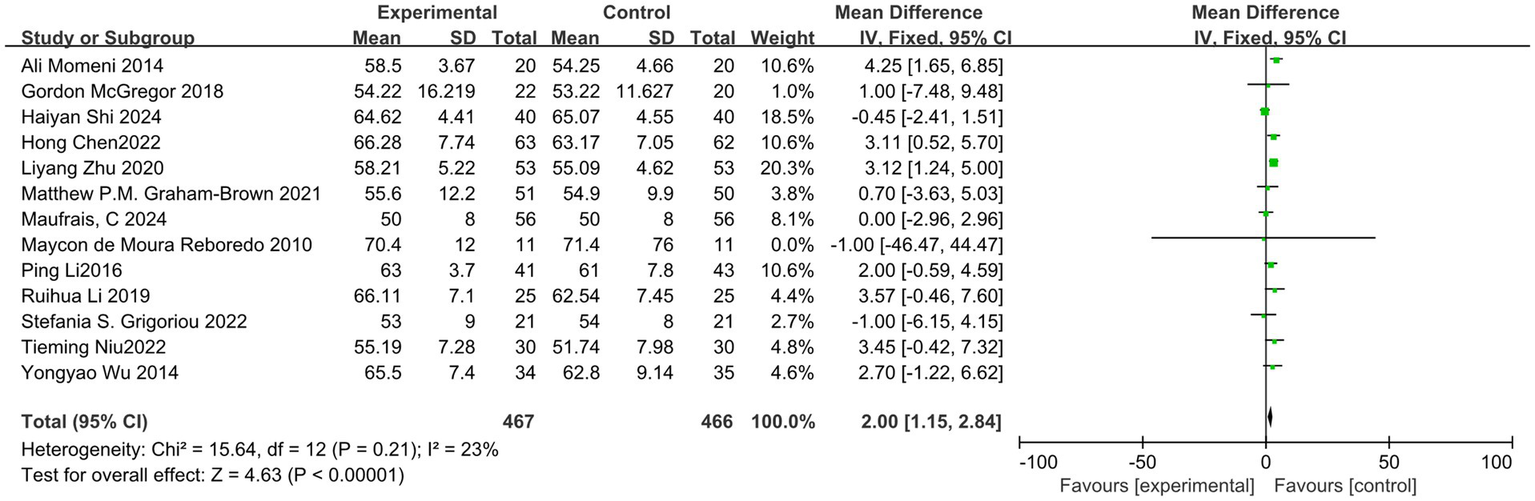
Forest plot of LVEF.
The publication bias of this study was evaluated using a funnel plot. The results indicated that the distribution of points in the funnel plot was largely symmetrical, suggesting a low likelihood of publication bias that would not affect the analysis results, as shown in Figure 5. The overall certainty of this evidence was assessed to be low, as detailed in Table 3.
Figure 5
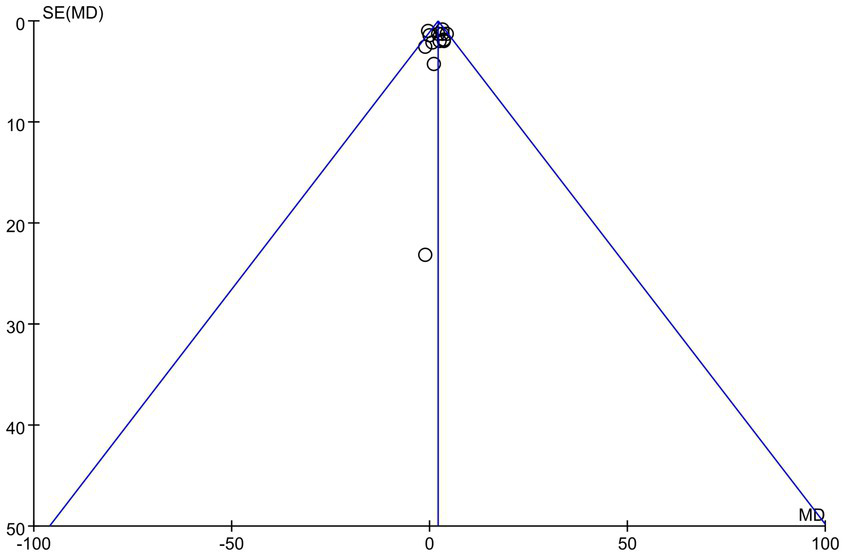
Funnel plot of LVEF.
Table 3
| Outcomes | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | No. of participants | Absolute effect (95% CI) | Quality | |
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | |||||||||
| LVEF | RCTs | Serious | Serious | No | No | No | 467 | 466 | MD 2.00 (1.15 to 2.84) | Low |
| LVMI | RCTs | Serious | Serious | No | Serious | No | 104 | 105 | MD -7.93 (−14.67 to −1.19) | Very low |
| PWV | RCTs | Serious | Serious | No | Serious | No | 74 | 67 | MD -0.48(−1.34 to 0.37) | Very low |
| LVEDV | RCTs | Serious | Serious | No | No | No | 158 | 157 | MD -0.51 (−2.41 to 1.40) | Low |
| LVESV | RCTs | Serious | Serious | No | Serious | No | 51 | 51 | MD -0.96 (−2.94 to 1.02) | Very low |
GRADE assessment.
RCTs, randomized controlled trials. MD, mean difference.
3.4.2 LVMI
Among the included studies, four reported outcome indicators related to LVMI. After conducting a heterogeneity test, we found I2 = 37%, which is less than 50%, and p = 0.19, which is greater than 0.1, indicating low heterogeneity. Therefore, we employed a fixed-effect model to combine the effect sizes. The results of the sensitivity analysis indicated minimal variation, suggesting that this study possesses good stability. The meta-analysis results demonstrated that exercise significantly improves LVMI in dialysis patients (MD = −7.93, 95%CI: −14.67-1.19, p = 0.02), as illustrated in Figure 6.
Figure 6
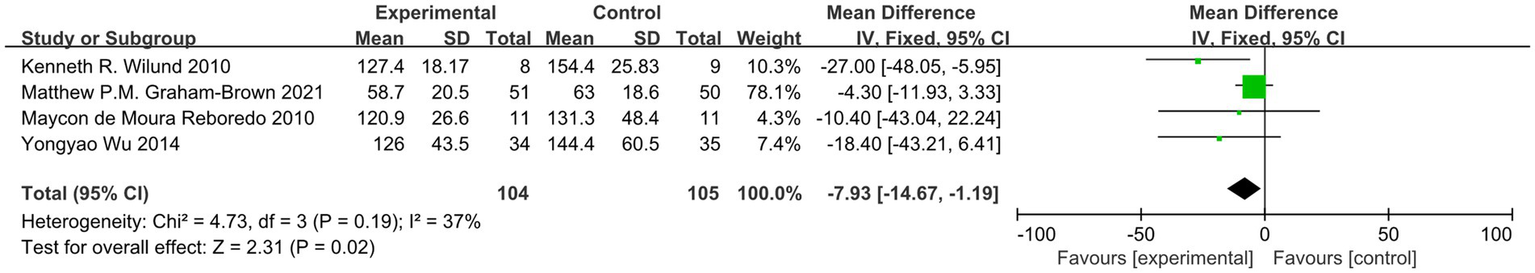
Forest plot of LVMI.
To evaluate potential publication bias in this study, we constructed a funnel plot. The results demonstrated an asymmetric distribution of points within the funnel plot, indicating a possibility of publication bias, which may influence the findings of this study, as illustrated in Figure 7. The overall certainty of this evidence was assessed to be very low, as illustrated in Table 3.
Figure 7
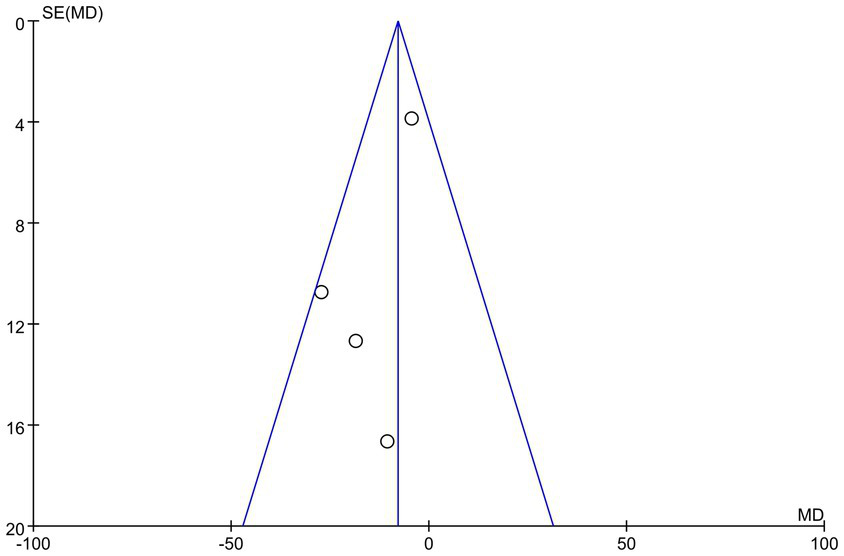
Funnel plot of LVMI.
3.4.3 PWV, LVEDV, and LVESV
Among the studies included in the analysis, four reported the outcome indicator of pulse wave velocity (PWV). After conducting a heterogeneity test, we found I2 = 46%, which is less than 50%, and p = 0.14, which is greater than 0.1, indicating low heterogeneity. Therefore, we employed a fixed-effect model to combine the effect sizes. The results of the meta-analysis indicated a MD of −0.48 (95% CI, −1.34-0.37, p = 0.27), which was not statistically significant. Four studies reported the outcome indicator of LVEDV. After conducting a heterogeneity test, we found I2 = 0%, which is less than 50%, and p = 0.61, which is greater than 0.1, indicating low heterogeneity. Therefore, we employed a fixed-effect model to combine the effect sizes. The meta-analysis results showed an MD of −0.51 (95% CI, −2.41–1.40, p = 0.60), and this difference was not statistically significant. Two studies reported the outcome indicator of LVESV. After conducting a heterogeneity test, we found I2 = 13%, which is less than 50%, and p = 0.28, which is greater than 0.1, indicating low heterogeneity. Therefore, we employed a fixed-effect model to combine the effect sizes. The meta-analysis results indicated an MD of −0.96 (95% CI, −2.94–1.02, p = 0.34), with no statistically significant difference. This study cannot demonstrate that exercise improves the outcome indicators of PWV, LVEDV, and LVESV in dialysis patients, as illustrated in Figures 8–10. The overall quality of the evidence was judged as very low, low, and very low, respectively, as illustrated in Table 3.
Figure 8
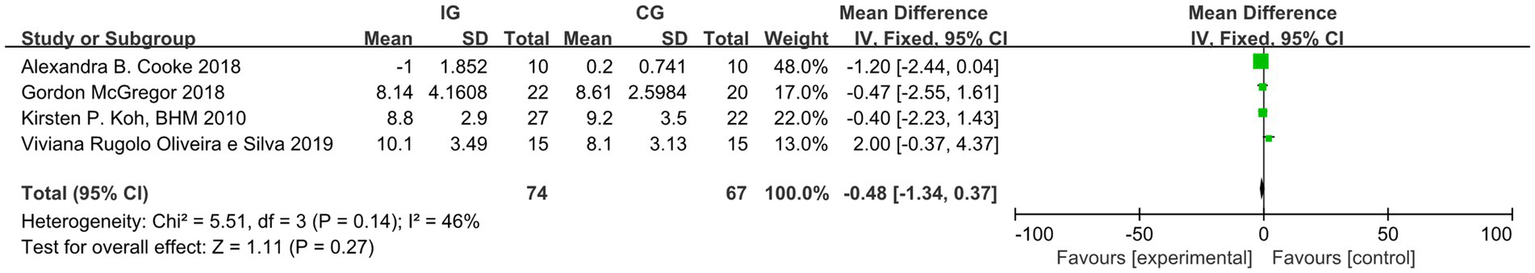
Forest plot of PWV.
Figure 9
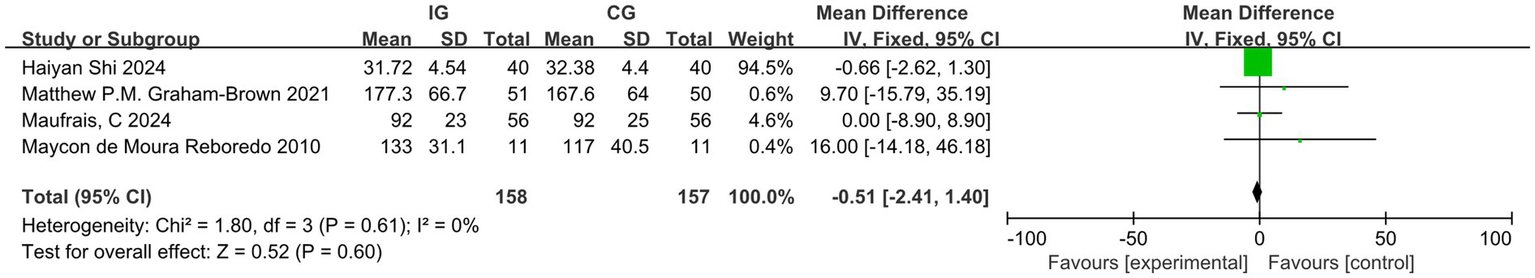
Forest plot of LVEDV.
Figure 10
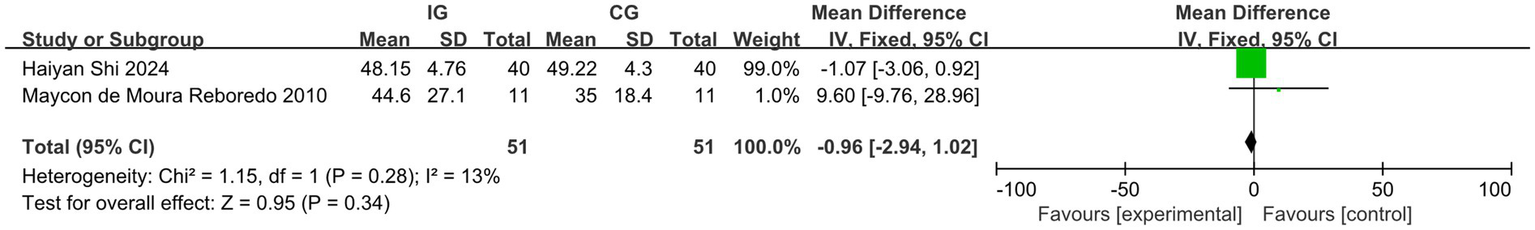
Forest plot of LVESV.
3.4.4 Subgroup analysis of LVEF
Among the included studies, nine utilized pure aerobic exercise, three combined aerobic exercise with resistance training, and only one employed pure resistance exercise (subgroup analysis could not be performed). The results indicated that both aerobic exercise and the combination of aerobic and resistance exercise significantly increased the LVEF in dialysis patients compared to the control group. The groups with workout lasts more than 30 min and less than 30 min, the intervention included groups with cycles longer than 12 weeks and those with cycles of 10 to 12 weeks, as well as groups with a weekly exercise frequency of more than three times per week and those with a frequency of three times per week or less, all demonstrated significant improvements in LVEF among dialysis patients. The differences observed were statistically significant. Please refer to Table 4 for further details.
Table 4
| Subgroup | Number of studies | Research subjects (cases) | Pooled effect size | Effect model | Heterogeneity test | |||
|---|---|---|---|---|---|---|---|---|
| MD [95%CI] | Z-value | p-value | I2-value | p-value | ||||
| Exercise methods | ||||||||
| AE | 9 | 536 | 1.14 [0.07,2.22] | 2.08 | 0.04 | fix | 28% | 0.19 |
| AE + RE | 3 | 187 | 2.78 [1.17,4.38] | 3.39 | <0.001 | fix | 13% | 0.31 |
| Duration of continuous exercise | ||||||||
| >30 min | 5 | 262 | 2.50 [0.57,4.43] | 2.53 | 0.01 | fix | 0% | 0.67 |
| ≤30 min | 8 | 586 | 1.88 [0.94,2.82] | 3.92 | <0.001 | fix | 46% | 0.07 |
| Exercise Intervention period | ||||||||
| >12 weeks | 5 | 450 | 1.35 [0.13,2.56] | 2.17 | 0.03 | fix | 38% | 0.17 |
| 10–12 weeks | 6 | 321 | 3.36 [2.04,4.68] | 4.98 | <0.001 | fix | 0% | 0.97 |
| Exercise frequency per week | ||||||||
| >3 | 2 | 190 | 2.73 [1.21,4.25] | 3.53 | <0.001 | fix | 0% | 0.49 |
| ≤3 | 11 | 658 | 1.67 [0.56,2.68] | 3.21 | 0.001 | fix | 28% | 0.18 |
Subgroup analysis of the effect of exercise intervention on LVEF in dialysis patients.
4 Discussion
The results of this meta-analysis on the effects of exercise interventions on cardiovascular outcomes in dialysis patients indicate that exercise training can significantly enhance several indicators of cardiovascular function, including LVEF and LVMI. However, it does not show statistical significance in improving PWV, LVESV, and LVEDV. The GRADE assessment of the quality of evidence rated the levels of evidence for LVMI, PWV, and LVESV as very low due to the limited quality of the included literature. It is essential to analyze the results of these studies with caution. However, the quality of evidence may be progressively improved in the future through rigorous methodological design, standardized interventions, technological innovations, and multidisciplinary collaboration.
A lifestyle devoid of physical activity is closely linked to the mortality rate among dialysis patients. Studies indicate that nearly 35% of these patients develop cardiovascular disease due to lack of engagement in physical activity (33). Although there are various methods available to treat cardiovascular events in dialysis patients, including pharmacological therapy and surgical interventions, significant financial resources are required, and the outcomes have not substantially improved the survival rates of these patients. On the other hand, exercise is a low-cost intervention that is highly applicable and generally well-accepted by patients. Recent research indicates that exercise has a significant positive impact on cardiovascular health (34, 35).
4.1 LVEF
LVEF is a crucial indicator of left ventricular systolic function, reflecting the heart’s pumping ability. It serves as an important measure for assessing the cardiovascular health of patients undergoing dialysis. Numerous studies have demonstrated that exercise during dialysis can significantly enhance LVEF in these patients (16, 17). The results of this study are consistent and robust. This research conducted subgroup analyses for various types of exercise. The findings indicated that both aerobic plus resistance exercise and simple aerobic exercise can improve the LVEF in dialysis patients, with aerobic plus resistance exercise demonstrating a superior effect compared to simple aerobic exercise. However, a limitation of this study is that the types of exercises examined were relatively limited, and the effects of simple resistance exercise were not compared. Nevertheless, many current studies indicate that personalized exercise regimens can be tailored for dialysis patients in various situations, resulting in different improvement effects across various indicators. For example, AE, respiratory muscle training (PMT), and RE + AE significantly enhance performance on the six-minute walk test (6MWT). AE and RE + AE are associated with a higher maximal oxygen consumption (VO2max) (36). Mind–body training (MBT) and RE + AE significantly improve blood pressure. Moreover, the benefits of MBT in reducing arterial blood pressure are unparalleled compared to other forms of exercise (37). It appears that varying durations of continuous exercise also influence the LVEF in dialysis patients. The study assessed the duration of individual exercise sessions, the length of the exercise intervention period, and the frequency of weekly exercise sessions. It can be concluded that for dialysis patients, the duration of each exercise sessions lasting longer than 30 min, an exercise intervention period of 10 to 12 weeks, and a frequency of more than three sessions per week are associated with greater improvements in LVEF. Due to the limited range of exercise intensities included in this trial, most of the literature employs a rating of perceived exertion between 11 and 16 (medium to hard) on the 6–20 Borg scale. Additionally, a small portion of the literature primarily utilizes 60 to 70% of the maximum heart rate (calculated as 220 minus age) during exercise, a comparison of dialysis intensity was not conducted. One piece of literature involving a study population of patients with Parkinson’s disease was included in this trial but could not be compared statistically. This literature was included in the LVEF study index. We performed a sensitivity analysis of this literature using an exclusion-by-exclusion approach, which resulted in only minor differences, suggesting that this study had a limited impact on the final results. However, due to the different mechanisms of action of HD and peritoneal dialysis, there are advantages and disadvantages to each method. Hemodialysis removes excess water and toxins from the body efficiently and quickly, but may adversely affect the heart, with the most common risk being hypotension. In contrast, peritoneal dialysis enhances water and sodium retention and helps prevent the development of heart failure associated with vascular access. However, patients undergoing peritoneal dialysis are also at increased risk of developing peritoneal dialysis-associated peritonitis. As a result, the choice of dialysis modality varies in its effectiveness in improving heart failure. Comparisons of different dialysis modalities can be made in the future.
4.2 LVMI
Left ventricular hypertrophy (LVH) can result from various factors, including hypertension, diabetes, anemia, hyperparathyroidism, and inflammatory conditions. LVMI is a critical criterion for diagnosing LVH. A study involving 153 participants, with a follow-up period exceeding 54 months, demonstrated that improvements in LVH outcome indicators positively influenced the survival rate of dialysis patients with cardiovascular disease (CVD). Specifically, for every 1 gram reduction in left ventricular mass, the cardiovascular risk decreased by 1% (38). A prospective study revealed that two echocardiographic examinations conducted 18 months apart showed a graded relationship between changes in LVMI and all-cause mortality, which was significantly associated with cardiovascular events (39). This study demonstrates that exercise can significantly improve the LVMI in dialysis patients, thereby reducing the incidence of cardiovascular events and enhancing their survival rates. Interestingly, among the four studies included, two indicated that exercise had no significant effect on improving LVMI, while the other two reported notable improvements. These discrepancies may be attributed to variations in exercise intensity and duration. Due to the limited number of studies included, a subgroup analysis could not be performed.
The outcome indicators of PWV, LVEDV, and LVESV did not show statistical significance. Consequently, it cannot be concluded that exercise improves PWV, LVEDV, and LVESV in dialysis patients. This lack of significance may be attributed to the limited literature included in this study. For LVESV, both studies analyzed indicated that exercise interventions were ineffective, which aligns with the findings of this study. Among the literature concerning PWV and LVEDV, one study showed no significant improvement in PWV, while two studies reported similar results for LVEDV. However, studies have confirmed that for patients with renal failure, every 1 m/s increase in PWV is associated with a 14% increase in the total cardiovascular mortality rate (40). PWV is closely related to hypertension, and an increasing number of studies have established that arteriosclerosis is an independent predictor of hypertension (20). Exercise can improve PWV by dilating blood vessels and enhancing endothelial function. Therefore, regarding the outcomes for PWV, LVEDV, and LVESV, a larger sample size is necessary, and this study should not be considered as evidence-based. In the literature reviewed, both the control and experimental groups reported related adverse events. The incidence of hypotension was relatively high, followed by nausea and vomiting, which may be linked to individual physical conditions or exercise intensity. Although no systematic statistical analysis was conducted in this study, however, future research should include additional statistical analyses regarding the incidence of adverse effects.
The safety of exercise during dialysis remains a significant concern. For example, it is unclear whether exercise may increase the workload on the heart, disrupt cardiac hemodynamics, and consequently elevate the risk of complications in dialysis patients. Additionally, there are questions about whether exercise in patients undergoing peritoneal dialysis could lead to catheter detachment, increased intra-abdominal pressure, or infection. Furthermore, it is important to consider whether medical staff are adequately trained in structured exercise protocols and can provide appropriate exercise recommendations. Although some studies have confirmed that a single session of moderate-intensity aerobic exercise performed during dialysis does not significantly alter hemodynamic parameters, such as blood pressure and cardiac output, further research is needed to fully understand the implications of exercise in this population (16). This confirms that this type of exercise is safe for dialysis patients. However, developing individualized exercise programs requires collaboration among a multidisciplinary team. Several studies have demonstrated that exercise during dialysis, under these conditions, is both safe and effective for patients (41, 42).
5 Conclusion
Our research findings indicate that exercise can significantly enhance LVEF and LVMI in patients undergoing dialysis who experience cardiac function issues. Subgroup analysis suggests that individualized exercise regimens can be tailored for these patients, incorporating both aerobic and resistance training. Effective regimens typically involve exercise sessions lasting more than 30 min, an intervention period of 10 to 12 weeks, and a frequency of more than three times per week. It is important to note that no statistically significant effects were observed for PWV, LVEDV, and LVESV. To enhance the robustness of the research, it may be necessary to increase the sample size and improve the quality of the included literature.
6 Study limitations and future prospects
This study has several significant limitations. Firstly, the quality of the included literature was subpar. Secondly, the types of exercise examined were relatively monotonous, as most studies focused solely on traditional aerobic exercises. The assessment of publication bias was conducted solely through funnel plots, and the limited number of included studies imposed certain constraints on the results. Future research should prioritize higher-quality articles and explore a broader range of exercise modalities, such as virtual reality exercise, yoga during dialysis, electrical muscle stimulation, and more. The outcome measures of the study could be expanded to include inflammatory factors, changes in blood pressure, and other relevant metrics. This would provide dialysis patients with more options to develop appropriate exercise programs. Additionally, the effects of exercise interventions on patients undergoing home peritoneal dialysis should also be considered.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
HL: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. MZ: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. MC: Data curation, Investigation, Software, Writing – review & editing. LC: Data curation, Investigation, Writing – review & editing. TH: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing. ZY: Conceptualization, Formal analysis, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1573498/full#supplementary-material
References
1.
Liyanage T Ninomiya T Jha V Neal B Patrice HM Okpechi I et al . Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. (2015) 385:1975–82. doi: 10.1016/S0140-6736(14)61601-9
2.
Wang F Yang C Long J Zhao X Tang W Zhang D et al . Executive summary for the 2015 annual data report of the China kidney disease network (CK-NET). Kidney Int. (2019) 95:501–5. doi: 10.1016/j.kint.2018.11.011
3.
Parfrey PS Foley RN Harnett JD Kent GM Murray DC Barre PE . Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. (1996) 11:1277–85. doi: 10.1093/ndt/11.7.1277
4.
Murphy SP Ibrahim NE Januzzi JJ . Heart failure with reduced ejection fraction: a review. JAMA. (2020) 324:488–504. doi: 10.1001/jama.2020.10262
5.
Penny JD Salerno FR Brar R Garcia E Rossum K McIntyre CW et al . Intradialytic exercise preconditioning: an exploratory study on the effect on myocardial stunning. Nephrol Dial Transplant. (2019) 34:1917–23. doi: 10.1093/ndt/gfy376
6.
McGuire S Horton EJ Renshaw D Chan K Jimenez D Maddock H et al . Cardiac stunning during haemodialysis: the therapeutic effect of intra-dialytic exercise. Clin Kidney J. (2021) 14:1335–44. doi: 10.1093/ckj/sfz159
7.
Kim ED Sozio SM Estrella MM Jaar BG Shafi T Meoni LA et al . Cross-sectional association of volume, blood pressures, and aortic stiffness with left ventricular mass in incident hemodialysis patients: the predictors of arrhythmic and cardiovascular risk in end-stage renal disease (PACE) study. BMC Nephrol. (2015) 16:131. doi: 10.1186/s12882-015-0131-4
8.
Unver S Kavlak E Gümüsel HK Celikbilek F Esertas K Muftuoglu T et al . Correlation between hypervolemia, left ventricular hypertrophy and fibroblast growth factor 23 in hemodialysis patients. Ren Fail. (2015) 37:951–6. doi: 10.3109/0886022X.2015.1052945
9.
Hur E Usta M Toz H Asci G Wabel P Kahvecioglu S et al . Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. (2013) 61:957–65. doi: 10.1053/j.ajkd.2012.12.017
10.
Ashby D Borman N Burton J Corbett R Davenport A Farrington K et al . Renal association clinical practice guideline on Haemodialysis. BMC Nephrol. (2019) 20:379. doi: 10.1186/s12882-019-1527-3
11.
Wilkinson TJ Mcadams-Demarco M Bennett PN Wilund K . Advances in exercise therapy in predialysis chronic kidney disease, hemodialysis, peritoneal dialysis, and kidney transplantation. Curr Opin Nephrol Hypertens. (2020) 29:471–9. doi: 10.1097/MNH.0000000000000627
12.
Yahui W Guo Q Li X Han P . Research progress on the effects of different exercise modalities on physical function and quality of life in maintenance hemodialysis patients. Nurs Res. (2023) 37:1389–94. doi: 10.12102/j.issn.1009-6493.2023.08.013
13.
Niu T Luan X Dong Q Fu C Yu W Huang T et al . Effects of aerobic exercise combined with resistance training on motor function and factors related to cardiovascular events in peritoneal dialysis patients. Chin J Phys Med Rehabil. (2022) 44:540–2. doi: 10.3760/cma.j.issn.0254-1424.2022.06.014
14.
Grigoriou SS Giannaki CD George K Karatzaferi C Zigoulis P Eleftheriadis T et al . A single bout of hybrid intradialytic exercise did not affect left-ventricular function in exercise-naïve dialysis patients: a randomized, cross-over trial. Int Urol Nephrol. (2022) 54:201–8. doi: 10.1007/s11255-021-02910-x
15.
Moher D Shamseer L Clarke M Ghersi D Liberati A Petticrew M et al . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
16.
Maufrais C Josse M Patrier L Grandperrin A Isnard M Turc-Baron C et al . Cardioprotective effect of intradialytic exercise on left atrial mechanics. Am J Physiol Renal Physiol. (2024) 326:F694–703. doi: 10.1152/ajprenal.00380.2023
17.
Josse M Patrier L Isnard M Turc-Baron C Grandperrin A Nottin S et al . Cardioprotective effect of acute intradialytic exercise: a comprehensive speckle-tracking echocardiography analysis. J Am Soc Nephrol. (2023) 34:1445–55. doi: 10.1681/ASN.0000000000000149
18.
Graham-Brown M March DS Young R Highton BJ Young HML Churchward DR et al . A randomized controlled trial to investigate the effects of intra-dialytic cycling on left ventricular mass. Kidney Int. (2021) 99:1478–86. doi: 10.1016/j.kint.2021.02.027
19.
Silva V Belik FS Hueb JC de Souza Gonçalves R Caramori JCT Vogt BP et al . Aerobic exercise training and nontraditional cardiovascular risk factors in hemodialysis patients: results from a prospective randomized trial. Cardiorenal Med. (2019) 9:391–9. doi: 10.1159/000501589
20.
Cooke AB Ta V Iqbal S Gomez YH Mavrakanas T Barré P et al . The impact of intradialytic pedaling exercise on arterial stiffness: a pilot randomized controlled trial in a hemodialysis population. Am J Hypertens. (2018) 31:458–66. doi: 10.1093/ajh/hpx191
21.
Mcgregor G Ennis S Powell R Hamborg T Raymond NT Owen W et al . Feasibility and effects of intra-dialytic low-frequency electrical muscle stimulation and cycle training: a pilot randomized controlled trial. PLoS One. (2018) 13:e0200354. doi: 10.1371/journal.pone.0200354
22.
Momeni A Nematolahi A Nasr M . Effect of intradialytic exercise on echocardiographic findings in hemodialysis patients. Iran J Kidney Dis. (2014) 8:207–11. PMID:
23.
Kouidi E Karagiannis V Grekas D Iakovides A Kaprinis G Tourkantonis A et al . Depression, heart rate variability, and exercise training in dialysis patients. Eur J Cardiovasc Prev Rehabil. (2010) 17:160–7. doi: 10.1097/HJR.0b013e32833188c4
24.
Reboredo MM Pinheiro BV Neder JA Wermelinger Ávila MP Ribeiro MLBAE Mendonça AF et al . Effects of aerobic training during hemodialysis on heart rate variability and left ventricular function in end-stage renal disease patients. J Bras Nefrol. (2010) 32:367–73. PMID:
25.
Koh KP Fassett RG Sharman JE Coombes JS Williams AD . Effect of intradialytic versus home-based aerobic exercise training on physical function and vascular parameters in hemodialysis patients: a randomized pilot study. Am J Kidney Dis. (2010) 55:88–99. doi: 10.1053/j.ajkd.2009.09.025
26.
Wilund KR Tomayko EJ Wu PT Ryong Chung H Vallurupalli S Lakshminarayanan B et al . Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study (2010) 25:2695–701. doi: 10.1093/ndt/gfq106
27.
Haiyan S Xiujuan Z Peng C Liu H . Effects of quantitative aerobic exercise under the guidance of cardiopulmonary exercise test on cardiopulmonary function and quality of life in hemodialysis patients. Chin J Integ Trad Western Nephrol. (2024) 25:142–5. doi: 10.3969/j.issn.1009-587X.2024.02.017
28.
Hong C Wenlei Z Hong Z . Effects of incremental resistance exercise training during dialysis on dialysis quality and psychological status of middle-aged maintenance hemodialysis patients. Int J Transpl Hemopurif. (2022) 20:46–8. doi: 10.3760/cma.j.cn115399-20220225-02012
29.
Zhu L Lu M Wang H Zhao D Ma R Wang X et al . Effects of planned aerobic-resistance exercise during dialysis intervals on nutritional status and dialysis hypotension of patients. Chin J Mod Nurs. (2020) 26:1894–8. doi: 10.3760/cma.j.cn115682-20191119-04216
30.
Ruihua L Huiling C Xueyu W . Effects of cycling training on laboratory objective indicators of uremic hemodialysis patients. J Guangzhou Med Univ. (2019) 47:116–8. doi: 10.3969/j.issn.2095-9664.2019.02.34
31.
Ping L Donghong W . Effects of aerobic exercise on 6-minute walking distance and omentin-1 in hemodialysis patients. Tianjin Med J. (2016) 44:1014–7. doi: 10.11958/20150248
32.
Wu Y Xia M Cao S Yu F Tong F Liu X et al . Effects and safety of individualized exercise therapy during hemodialysis treatment on cardiac function and exercise capacity of uremic patients. Chin J Blood Purific. (2014) 13:580–4. doi: 10.3969/j.issn.1671-4091.2014.08.08
33.
O'hare AM Tawney K Bacchetti P Johansen KL . Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. (2003) 41:447–54. doi: 10.1053/ajkd.2003.50055
34.
Greenwood SA Koufaki P Mercer TH MacLaughlin HL Rush R Lindup H et al . Effect of exercise training on estimated GFR, vascular health, and cardiorespiratory fitness in patients with CKD: a pilot randomized controlled trial. Am J Kidney Dis. (2015) 65:425–34. doi: 10.1053/j.ajkd.2014.07.015
35.
Zang W Fang M He H Mu L Zheng X Shu H et al . Comparative efficacy of exercise modalities for cardiopulmonary function in hemodialysis patients: a systematic review and network meta-analysis. Front Public Health. (2022) 10:1040704. doi: 10.3389/fpubh.2022.1040704
36.
Ren N Yang H Cai Z Wang R Wang Z Zhao Y et al . Comparative efficacy of nine exercise methods on the prognosis in chronic kidney disease patients with hemodialysis: a systematic review and network meta-analysis. Eur J Med Res. (2023) 28:401. doi: 10.1186/s40001-023-01270-9
37.
Deligiannis A Kouidi E Tassoulas E Gigis P Tourkantonis A Coats A . Cardiac effects of exercise rehabilitation in hemodialysis patients. Int J Cardiol. (1999) 70:253–66. doi: 10.1016/S0167-5273(99)00090-X
38.
London GM Pannier B Guerin AP Blacher J Marchais SJ Darne B et al . Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol. (2001) 12:2759–67. doi: 10.1681/ASN.V12122759
39.
Zoccali C Benedetto FA Mallamaci F Tripepi G Giacone G Stancanelli B et al . Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int. (2004) 65:1492–8. doi: 10.1111/j.1523-1755.2004.00530.x
40.
Blacher J Safar ME Guerin AP Pannier B Marchais SJ London GM . Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. (2003) 63:1852–60. doi: 10.1046/j.1523-1755.2003.00932.x
41.
Li T Lv A Xu N Huang M Su Y Zhang B et al . Barriers and facilitators to exercise in haemodialysis patients: a systematic review of qualitative studies. J Adv Nurs. (2021) 77:4679–92. doi: 10.1111/jan.14960
42.
Liu Y Sun J Ye M Huang L Li H Li M et al . Summary of the best evidence for exercise in peritoneal dialysis patients. J Nurs Sci. (2023) 38:39–43. doi: 10.3870/j.issn.1001-4152.2023.01.039
Summary
Keywords
dialysis, exercise, cardiac function, LVEF, meta-analysis
Citation
Liu H, Zhang M, Chen M, Chen L, Huang T and Ye Z (2025) Meta-analysis of the effects of exercise interventions on dialysis patients with cardiac function disorders. Front. Med. 12:1573498. doi: 10.3389/fmed.2025.1573498
Received
09 February 2025
Accepted
18 April 2025
Published
13 May 2025
Volume
12 - 2025
Edited by
Piergiorgio Messa, University of Milan, Italy
Reviewed by
Maria-Eleni Roumelioti, University of New Mexico, United States
Eleuterio A. Sánchez Romero, Hospital Universitario Puerta de Hierro Majadahonda, Spain
Updates
Copyright
© 2025 Liu, Zhang, Chen, Chen, Huang and Ye.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingrong Huang, 2798612003@qq.comZhao Ye, 381813612@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.