- 1Department of Rheumatology and Immunology, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, China
- 2First Veterans Hospital of Sichuan Province, Chengdu, China
Objectives: This study aimed to evaluate the efficacy of iguratimod (IGU) and hydroxychloroquine (HCQ) in the treatment of primary Sjögren’s syndrome (pSS).
Methods: This was a randomised controlled study. A total of 60 patients with pSS in Mianyang Central Hospital were recruited between December 2020 and December 2022. They were randomly divided into two groups: the IGU group and the HCQ group. Treatment in the IGU group was as follows: ≤10 mg of prednisone per day, 25 mg of IGU twice a day; treatment in the HCQ group was as follows: ≤10 mg of prednisone per day, 0.2 g of HCQ twice a day. The EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) and the European League against Desiccation Sjögren’s Syndrome Patient Reported Index (ESSPRI) were used to assess disease activity.
Results: After 6 months of treatment, the levels of immunoglobulin G (IgG) and ESSPRI in the IGU group were significantly lower than those in the HCQ group and the levels of CD19+CD5+CD1d+ B cells were significantly higher than those in the HCQ group (p < 0.05). Compared with baseline, the serum IgG level, erythrocyte sedimentation rate (ESR), B lymphocytes, ESSDAI, ESSPRI and Functional Assessment of Chronic Illness Therapy (FACIT) were significantly decreased and CD19+CD5+CD1d+ B cells were significantly increased in the IGU group after 6 months of treatment. In the HCQ group, C-reactive protein, ESR, ESSDAI, ESSPRI and FACIT were significantly decreased; there was no significant difference in regulatory B cells before and after treatment.
Conclusion: Both IGU and HCQ can reduce the disease activity and fatigue score of patients with pSS. However, IGU was superior to HCQ in reducing IgG levels. Furthermore, IGU can affect the levels of peripheral blood B lymphocytes and CD19+CD5+CD1d+ B cells.
Introduction
Primary Sjögren’s syndrome (pSS) is an autoimmune disease characterised by a large number of lymphocytes and plasma cells invading the lacrimal gland, salivary gland and other exocrine glands. In China, the prevalence of pSS is 0.33–0.77%, with a predominance in women, and the peak onset age is 40–50 years. The male-to-female ratio is 1:9–1:20 (1). Its pathogenesis is complex and involves the combined effects of genetic susceptibility, environmental factors and immune disorders (2, 3). In terms of genetic inheritance, studies have shown that certain specific human leukocyte antigen (HLA) genes are associated with the onset of pSS; genes in the non-HLA region are also involved. Moreover, the susceptibility genes vary among different populations (4). Among environmental factors, viral infections, especially Epstein–Barr virus infection, are considered major risk factors. The Epstein–Barr virus may trigger pSS through mechanisms such as B-cell activation, molecular mimicry and the protective role of tertiary lymphoid structures (TLS) in maintaining abnormal viral infections (5). Sex hormones also play a role in the pathogenesis. Oestrogens may increase the risk of developing the disease by enhancing inflammatory responses and elevating the levels of autoantibodies. Therefore, the incidence rate in women is much higher than in men (6). In terms of immune response, the activation of both innate and adaptive immunity is thought to play a major role in the pathogenesis of pSS. The dysfunction or abnormal activation of these immune cells can lead to lymphocyte infiltration and the production of autoantibodies, ultimately promoting the development of pSS. Specifically, the imbalance of the cytokine network is a key factor in the pathogenesis of pSS. For example, the overproduction of pro-inflammatory cytokines, such as interleukin (IL)-6, IL-17 and tumour necrosis factor alpha (TNF-α), as well as the insufficient secretion of anti-inflammatory cytokines, such as IL-10 and transforming growth factor beta (TGF-β), can exacerbate inflammatory responses and lead to tissue damage (7). In addition, the abnormal activation of signalling pathways, such as the interferon gamma (IFN-γ) signalling pathway, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signalling pathway and Toll-like receptor (TLR) signalling pathway, also plays a key role in the pathogenesis of pSS. These signalling pathways are involved in the regulation of immune cell functions and the production of inflammatory mediators. For example, the IFN-γ signalling pathway can stimulate salivary gland epithelial cells to secrete various inflammatory factors and chemokines and activate the NF-κB and JAK/STAT signalling pathways, mediating the inflammatory process in the salivary glands of patients with pSS (8). The formation of TLS in the salivary glands of patients with pSS is an important pathological feature of the disease. These structures are formed and maintained by the continuous crosstalk between immune cells and non-immune cells. The lack of IL-17 signalling indirectly promotes the expansion and abnormal activity of TLS, leading to a strong IFN-γ response (9), which in turn causes abnormal activation of the interferon pathway and leads to immune system dysregulation, making disease progression more severe. The pathological changes in pSS are mainly characterised by lymphocyte infiltration in the minor salivary glands, with T and B lymphocytes being the predominant cell types involved, and only about 10% infiltration of macrophages and dendritic cells (10). Among these cells, B cells play a central role in the pathogenesis of pSS. The over-activation of B cells is one of the typical features of pSS, which is clinically manifested as hypergammaglobulinemia, the production of various autoantibodies, high levels of B-cell-activating factor, B-cell subset disorders and a significant increase in the risk of lymphoma, with approximately 5% of patients with pSS eventually progressing to lymphoma (11).
Human peripheral blood B cells are derived from haematopoietic stem cells in the bone marrow. Studies have shown that they can be divided into different subgroups based on five different surface molecules: CD19, CD45, CD24, CD38 and CD27. These subgroups include regulatory B cells (Bregs, CD19+CD24hiCD38hi), naive B cells (CD19+CD24intCD38int), two types of memory B cells (CD19+CD38−CD24+ and CD19+CD38−CD27+) and two types of plasmablasts (CD19+CD24−CD38hi and CD19+CD27hiCD38hi) (12). Among them, Bregs are a group of B cells that negatively impact immune responses. They can exert anti-inflammatory effects by inhibiting T- and B-cell responses and producing cytokines, such as IL, TGF-β and granzyme B, and may play a role in suppressing excessive autoimmunity in pSS (13). Based on different surface markers, Bregs in the human body are mainly divided into different phenotypes, such as CD19+CD25hiCD71hiCD73-B cells, CD19+CD24hiCD38hi B cells, CD19+CD24+CD27+B cells, CD19+CD5+CD1dhi B cells and CD19+CD38+CD1d+IgM+CD147+ B cells (14). All play a role in negatively regulating immunity. Current research on Breg cells mainly focuses on three phenotypes: CD19+CD24hiCD38hi B cells, CD19+CD24+CD27+ B cells and CD19+CD5+CD1dhi B cells. One study (15) found that Bregs are more elevated in patients with pSS with clinical activity and extraglandular manifestations.
Primary Sjögren’s syndrome treatment includes local and systemic treatment. Local treatment mainly uses drugs that promote the secretion of glands or artificial saliva or tears to improve dry mouth and dry eye symptoms. Immunomodulatory drugs are often used according to the situation of system damage, and the recommended drug is hydroxychloroquine (HCQ) (16, 17). Previous literature has reported that HCQ can reduce dry mouth symptoms, improve fatigue and reduce C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels in patients with pSS (18–21). However, pSS is a highly heterogeneous disease; different immunosuppressant treatments are needed according to the different organ involvement of patients. The molecular structure of iguratimod (IGU) is n-[7-[(methanesulfonyl) amino]-4-oxo-6-phenoxy-4 h-1-benzopyran-3-yl]-formamide. It can selectively inhibit cyclooxygenase-2 (Cox-2) and inhibit the production of cytokines, such as IFN-γ, IL-1, IL-6, TNF-α, IL-17, immunoglobulin (Ig) M and IgG, substantially reducing the inflammatory response (22, 23). It was approved in China and Japan in 2012 for the treatment of rheumatoid arthritis (RA). Iguratimod has been subsequently used for the treatment of other autoimmune diseases, such as pSS, IgG4-related diseases and lupus nephritis (24, 25). Multiple randomised controlled trials and meta-analyses have shown that IGU achieves significant effects in pSS; in particular, IGU was better than HCQ at reducing the level of Ig (18, 26, 27). Therefore, the 2020 diagnosis and treatment norms of pSS in China recommended IGU as an immunosuppressant (28).
Several studies have shown that IGU has achieved certain efficacy in the treatment of pSS, which can effectively inhibit the activation of B cells, reduce Ig levels, avoid multiple organ damage caused by hypergammaglobulinemia and reduce the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) and the European League Against Desiccation Sjögren’s Syndrome Patient Reported Index (ESSPRI) (22, 27, 29, 30). However, there is no study examining whether IGU affects Bregs in patients with pSS. The purpose of this study is to evaluate the effect of IGU and HCQ in the treatment of pSS and to analyse the influence of these two drugs on Bregs in peripheral blood.
Materials and methods
Patients
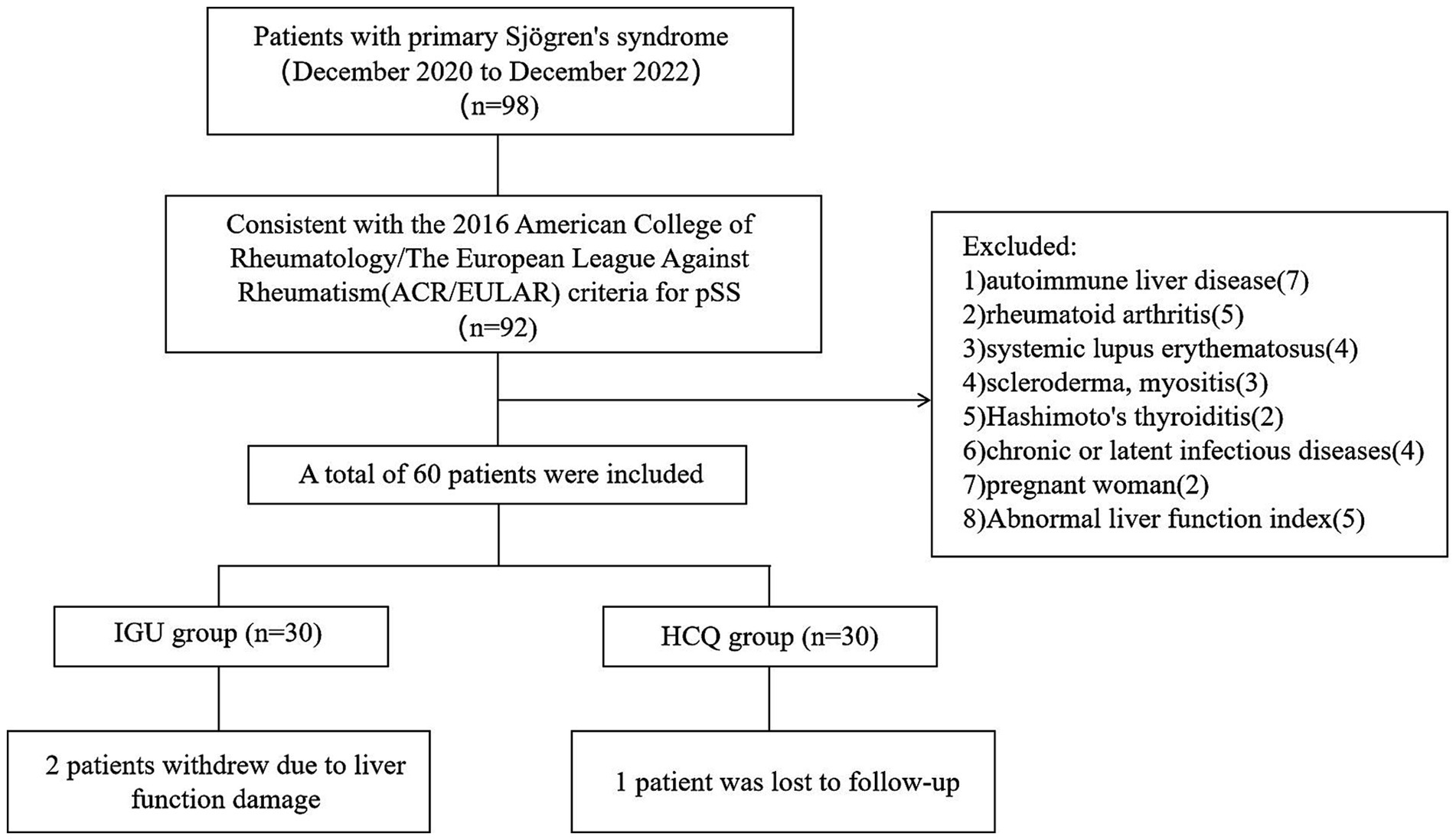
This was a prospective, single-centre, randomised controlled study. Patients from the Mianyang Central Hospital were recruited between December 2020 and December 2022. The protocol was approved by the Ethics Committee of the Mianyang Central Hospital (S-2020-048), and written informed consent was obtained from all patients before they participated in the study. This study has been registered at the registry https://www.isrctn.com with the number: ISRCTN15824224. According to the G*Power software tool (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany), the efficacy of statistical analysis is required to be within the acceptable range of 70–80%, and the samples of the two groups should be >23 cases. A total of 60 patients who fulfilled the 2016 American College of Rheumatology/The European League Against Rheumatism criteria for pSS were recruited for this study (Figure 1). The eligibility criteria were as follows: aged 18–65 years; no glucocorticoids, immunosuppressants or biological agents within 3 months before baseline; consent to contraception during the trial and within 3 months after the end of the trial. The exclusion criteria were as follows: patients with other immune system diseases, such as autoimmune liver disease, RA, systemic lupus erythematosus, scleroderma, myositis or Hashimoto’s thyroiditis; patients with serious organ involvement, such as severe pericardial effusion (echocardiography showing pericardial effusion thickness >10 mm), pulmonary interstitial lesions (high-resolution computed tomography showing ground-glass opacity or honeycomb lung), renal tubular acidosis (serum bicarbonate level >30 mmol/L and a urine pH value persistently >6.0) or atrophic gastritis (endoscopy showing gastric mucosal atrophy); patients with underlying cardiac, pulmonary, renal, gastrointestinal or metabolic conditions; patients with chronic or latent infectious diseases or a history of malignancy, mental diseases or alcohol abuse; pregnant and lactating women; patients with the following abnormal indicators – haemoglobin ≤90 g/L, platelet count <100 × 109/L, white blood cell count <3.0 × 109/L or >14 × 109/L, estimated glomerular filtration rate ≤45 mL/min/1.73 m2, total bilirubin >1.5 × upper limit of normal (ULN), aspartate aminotransferase and alanine aminotransferase both >1.5 × ULN.
Reagents and materials
The flow cytometry tubes were purchased from Zhejiang Gongdong Medical Instruments Co., Ltd., China. The PK-31 diluent for blood cell analysis was obtained from Sysmex. Foetal bovine serum (1%) was sourced from GIBCO life, and paraformaldehyde (1%) was acquired from Chengdu Kelong Chemicals Co., Ltd., China. The pipette tips were purchased from Axygen, Mexico (0.5–10 μL), Haimen Guolong Laboratory Instruments, China (10–200 μL), and Jiangsu Kangjian Medical, China (100–1,000 μL). The antibodies used for flow cytometry include CD5 FITC (Catalogue Number: 555352, BD Biosciences, USA), CD24 PE (Catalogue Number: 983602, BioLegend, USA), CD19 ECD (Catalogue Number: 6604551, Beckman Coulter, USA), CD1d PC7 (Catalogue Number: 350308, BioLegend), CD38 APC-A750 (Catalogue Number: A86049, Beckman Coulter), CD27 PacBlue (Catalogue Number: B30635, Beckman Coulter) and CD45 BV510 (Catalogue Number: 563204, BD Biosciences). The red blood cell lysing solution OptiLyse C was obtained from Beckman Coulter (Catalogue Number: A11895).
Study design
The patients were randomly assigned to an IGU group (n = 30) or an HCQ group (n = 30) at a ratio of 1:1. All the patients were allowed to receive <0 mg of prednisone per day and vitamin D and calcium for 24 weeks to prevent osteoporosis; 25 mg of IGU was administered orally twice a day in the IGU group, and 0.2 g of HCQ was administered orally twice a day in the HCQ group. However, other immunosuppressants, such as methotrexate, leflunomide, mycophenolate mofetil and cyclosporine, and biological agents, such as belimumab and rituximab, were not allowed during the treatment period.
Disease activity was evaluated by ESSDAI and ESSPRI. A 10-cm visual analogue scale was used for rating the patient global assessment. The Functional Assessment of Chronic Illness Therapy (FACIT) was used to assess the fatigue degree. Clinical and laboratory data were collected at the same time. All variables were assessed at baseline and week 24.
Flow cytometric analysis
The B lymphocytes and Bregs (CD19+CD24hiCD38hi, CD19+CD24+CD27+and CD19+ CD5+CD1d+ B cells) were detected by flow cytometry, and the values were expressed by the percentage of Breg cells in B lymphocytes. Peripheral blood samples (5-ml EDTA anticoagulant tube) from all the patients were collected at baseline and week 24. Peripheral blood mononuclear cells were prepared and stained with the following antibodies: CD5 FITC, PE anti-human CD24, CD19-ECD, PE/Cyanine7 anti-human CD1d, CD38-APC-ALexarFluor 750, CD27-Pacific Blue, BV510 Mouse Anti-Human CD45 (CD5FITC 20ul/test, CD24PE 5 μL/test, CD19ECD 10 μL/test, CD1dPC7 5 μL/test, CD38APC-A750 10 μL/test, CD27PacBlue 5 μL/test, CD45BV510 5 μL/test) (Beckman Coulter). The samples were analysed on a DXflex 13 colour flow cytometer (Beckman Coulter). The gating strategy for Breg cells can be found in the Figure 2.
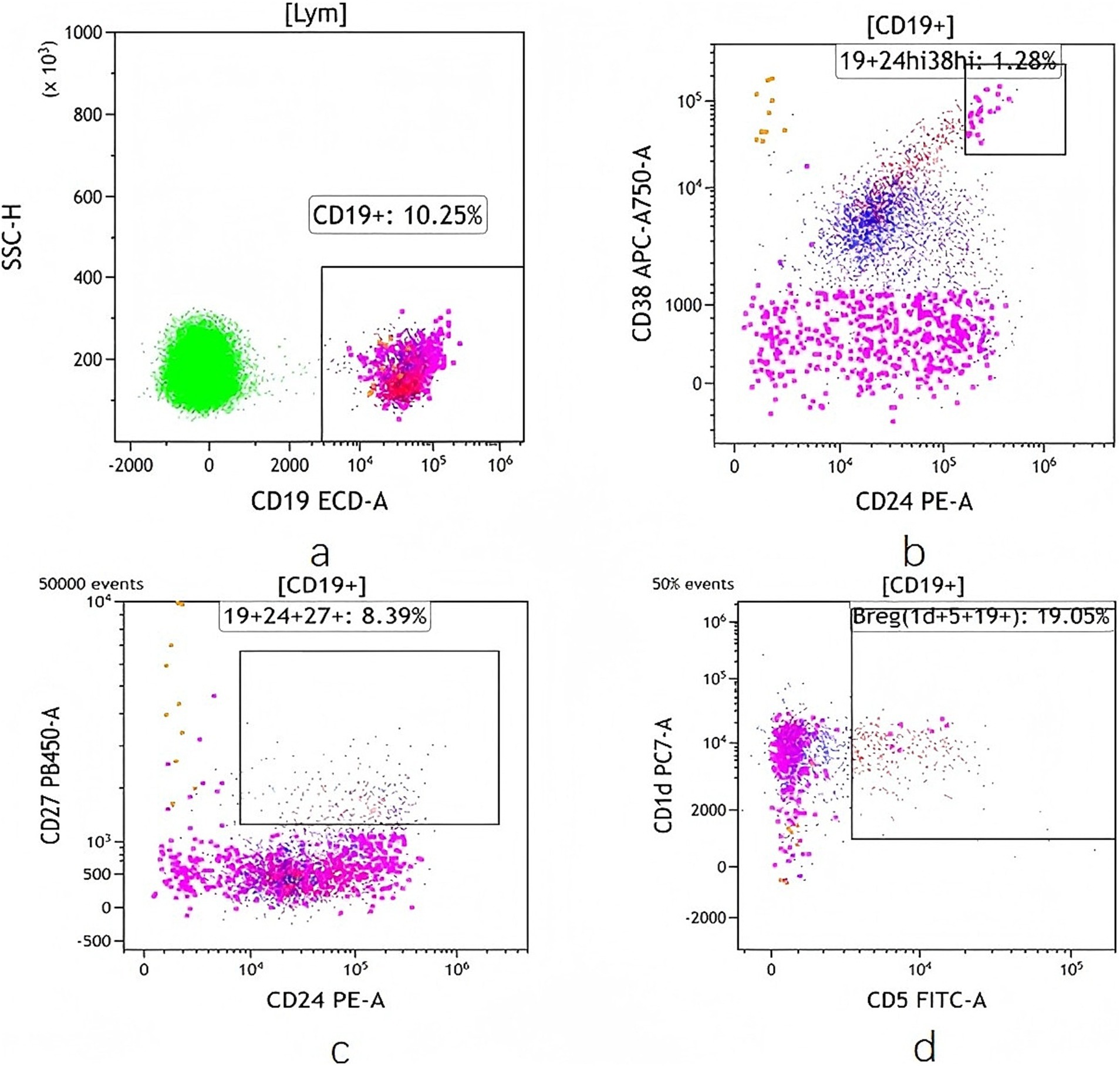
Figure 2. Frequency of Breg cells detected by flow cytometry. (a) CD19 + Bcell. (b) CD19 + CD24hi CD38hi Bcell. (c) CD19 + CD24 + CD27 + Bcell. (d) CD19 + CD5 + CD1d + Bcell.
Statistical methods
All data were analysed using SPSS 26.0 software (IBM Corporation, Armonk, NY, USA). When the measurement data obeyed the normal distribution, they were described by the mean ± standard deviation and the paired t-test was used. If the data did not obey the normal distribution, the median (interquartile interval) was used to describe them and the nonparametric test was used to compare the differences. The χ2 test was used for counting data, and a p-value of <0.05 was considered statistically significant.
Results
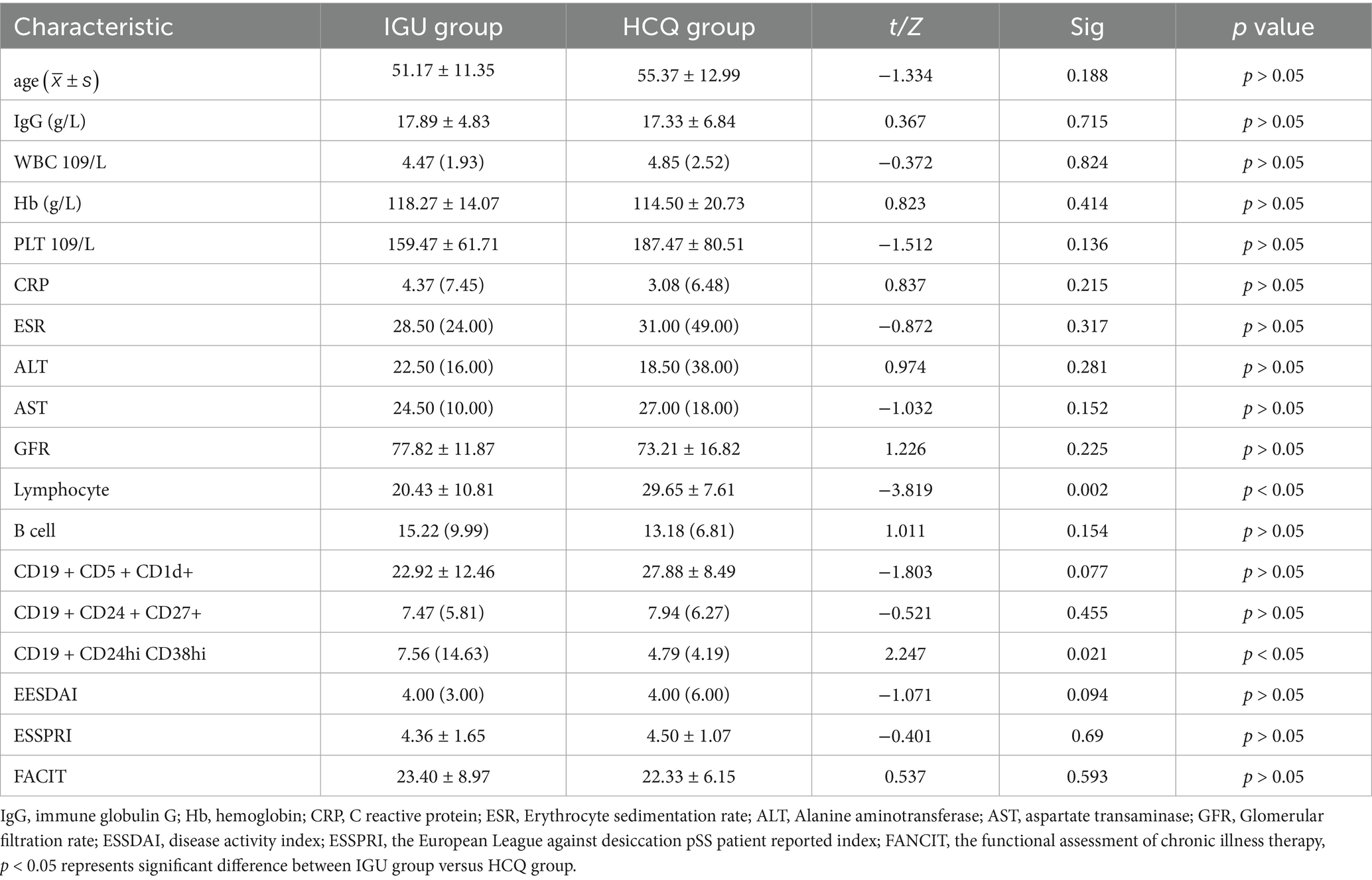
All the patients were female. Two patients in the IGU group withdrew due to liver function damage, one patient in the HCQ group was lost to follow-up and 28 and 29 patients in these two groups completed the 24-week treatment, respectively (Figure 1). Before treatment, there was no statistical difference between the IGU group and the HCQ group in the baseline data. The clinical and demographic characteristics of the study participants are shown in Table 1.
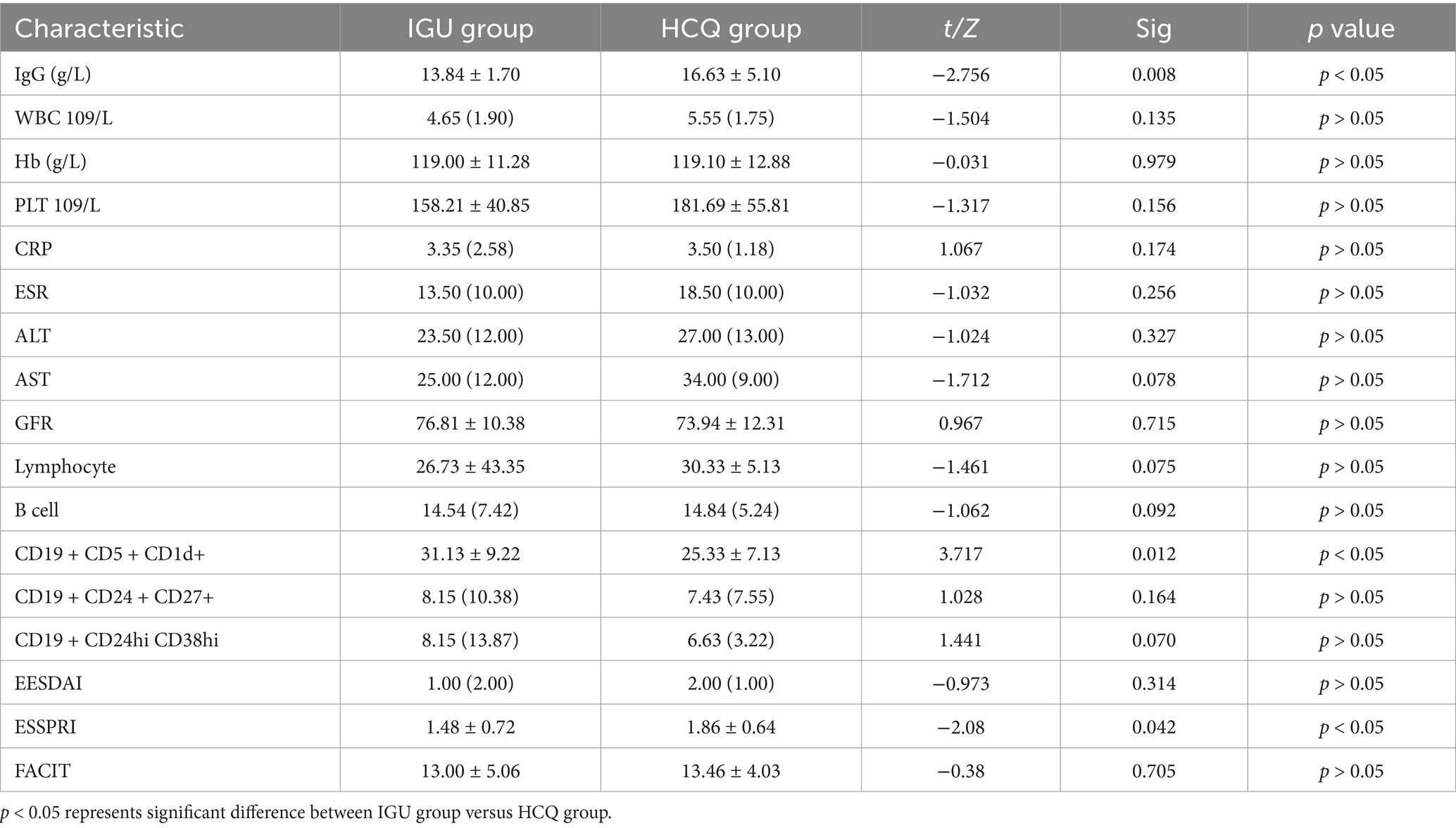
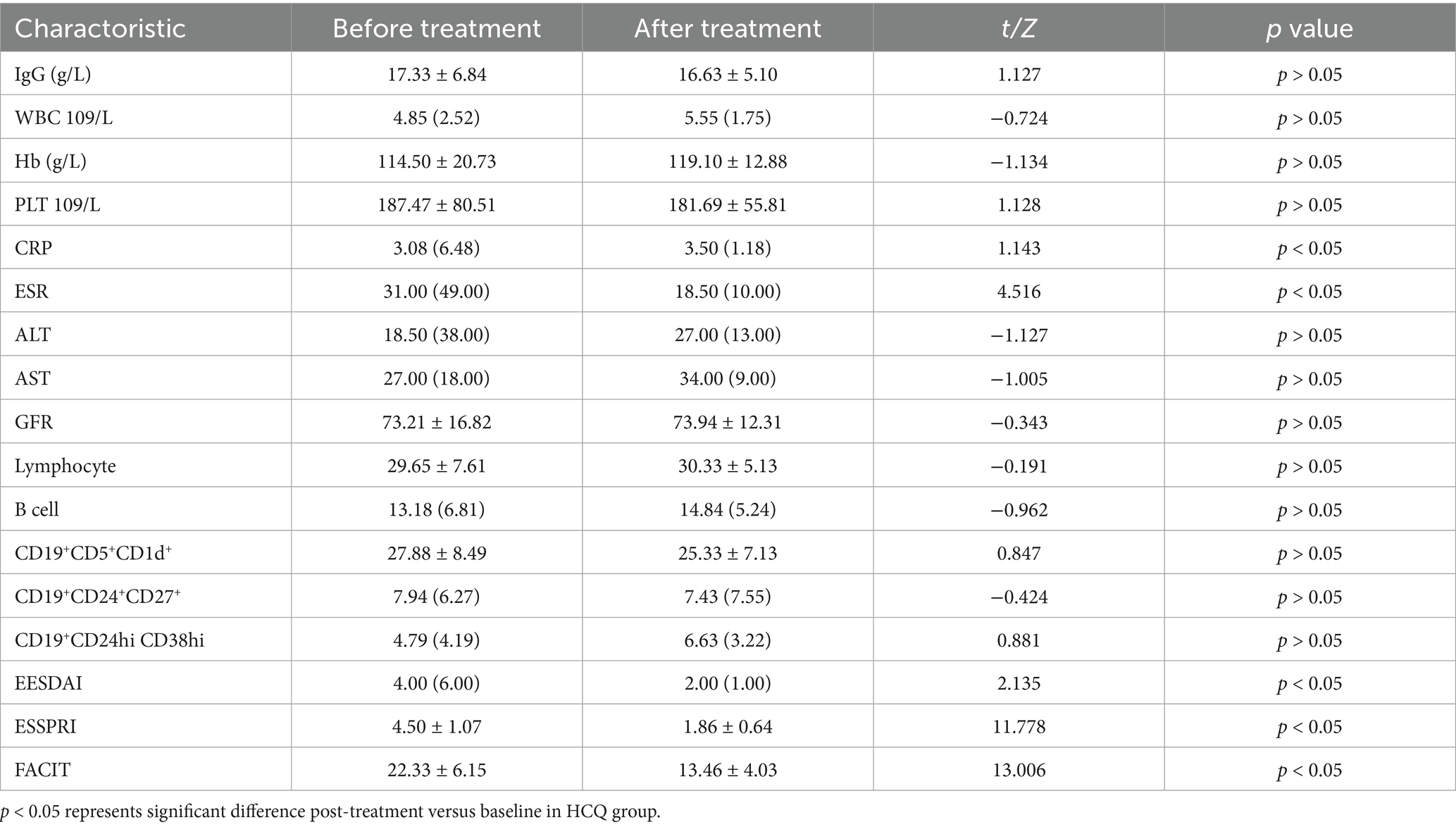
After 6 months of treatment, the IgG levels and ESSPRI scores in the IGU group were significantly lower than those in the HCQ group; the number of CD19+CD5+CD1d+ B cells was higher in the IGU group than in the HCQ group (Table 2).
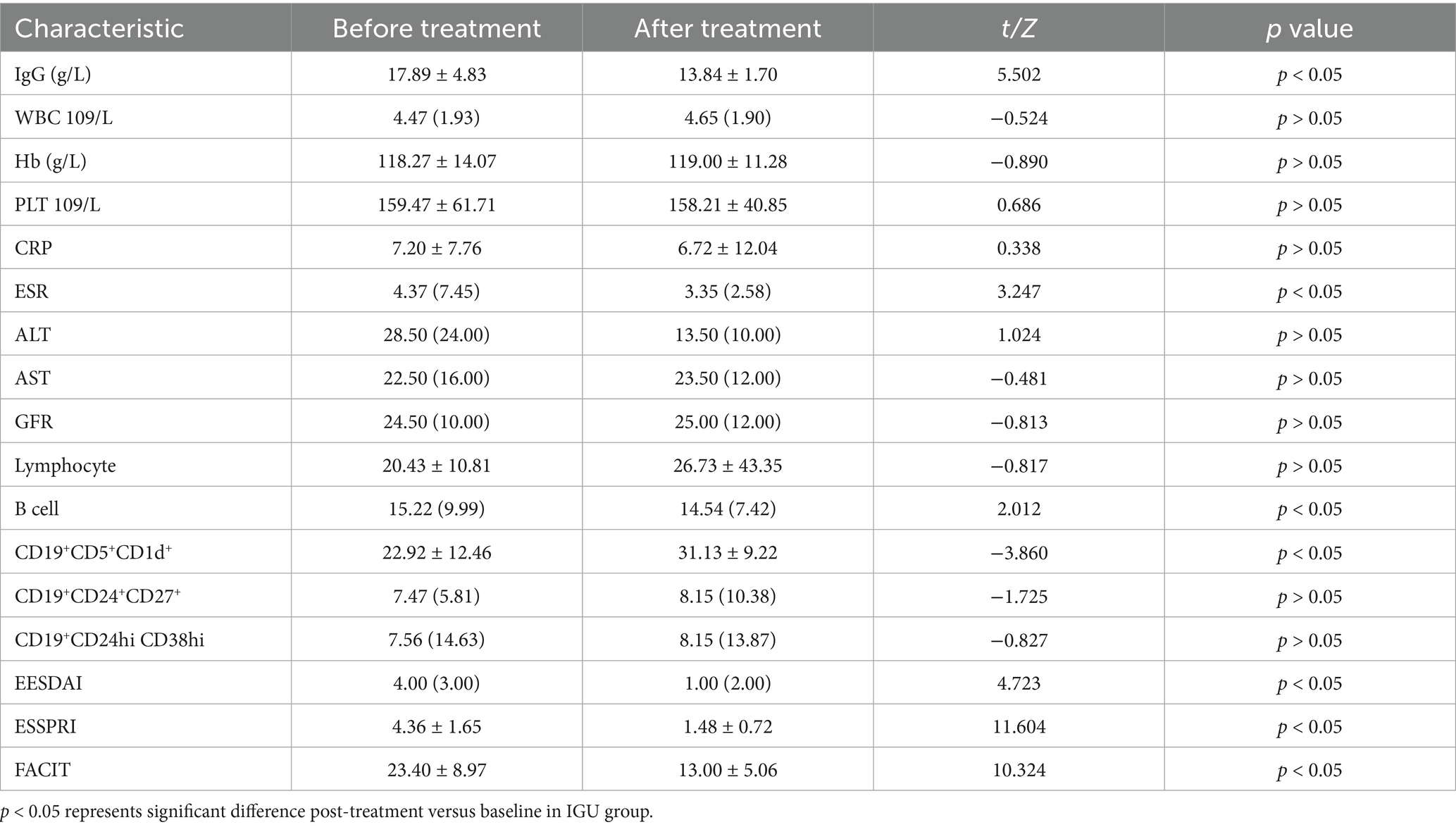
In the IGU group, compared with baseline, serum IgG, ESR, B lymphocytes, ESSDAI, ESSPRI and FACIT were significantly decreased, whereas the number of CD19+CD5+CD1d+ B cells was significantly increased after 6 months of treatment (Table 3; Figure 3).
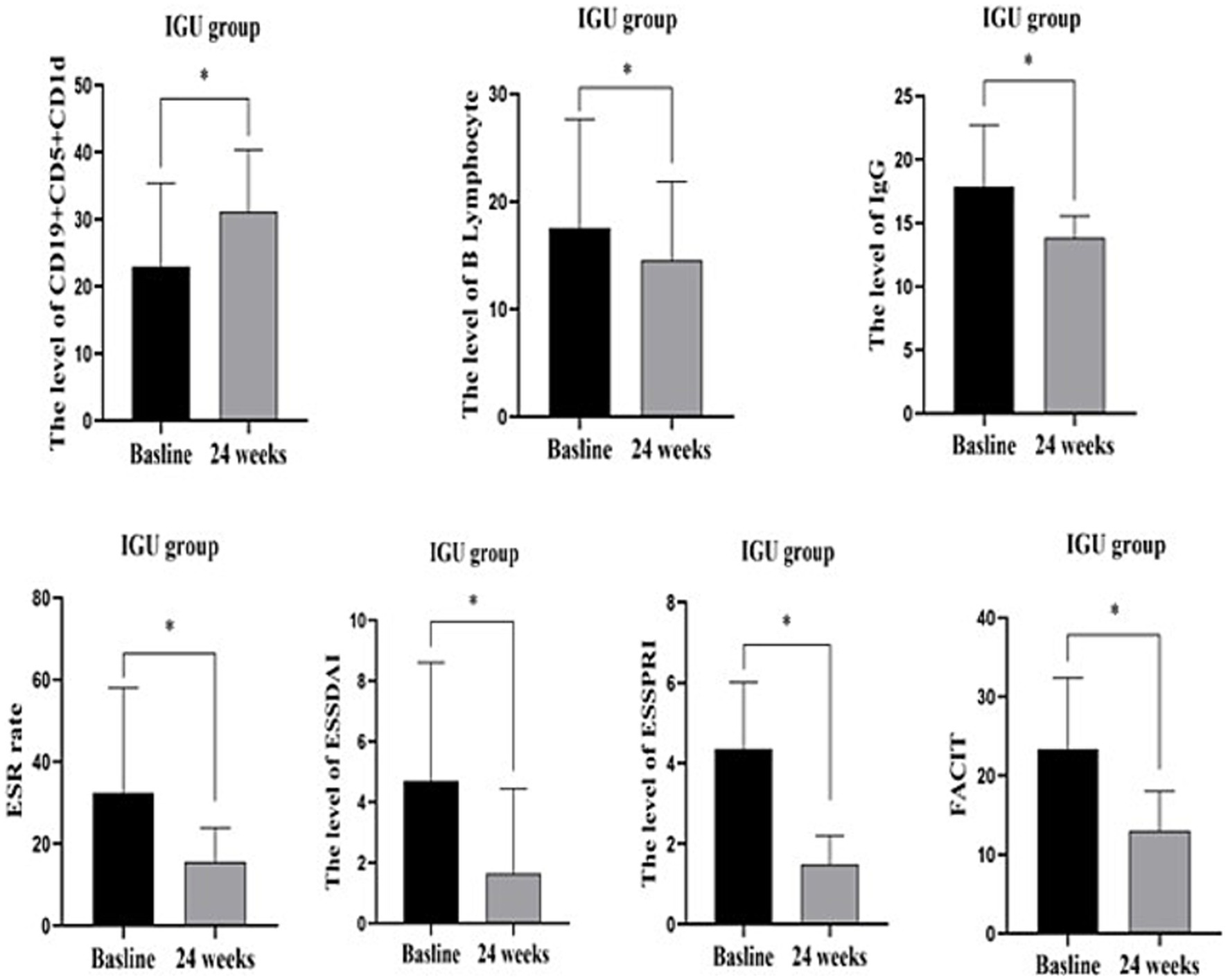
Figure 3. IGU group before and after 24 weeks treatment: CD19+CD5+CD1d+ was significantly increased, while B lymphocytes, serum IgG, ESR, ESSDAIL, ESSPRI, FACIT were significantly decreased compared with the baseline. *: p < 0.05 represents significant difference post-treatment versus baseline in IGU group. IgG, immune globulin G; CRP, C reactive protein; ESR, Erythrocyte sedimentation rate; ESSDAIL, disease activity index; ESSPRI, the European League against desiccation pSS patient reported index; FANCIT, the functional assessment of chronic illness therapy.
In the HCQ group, compared with baseline, CRP, ESR, ESSDAI, ESSPRI and FACIT were significantly decreased after 6 months of treatment. There was no significant difference in the rest of the characteristics, including B lymphocytes and Bregs (Table 4; Figure 4).
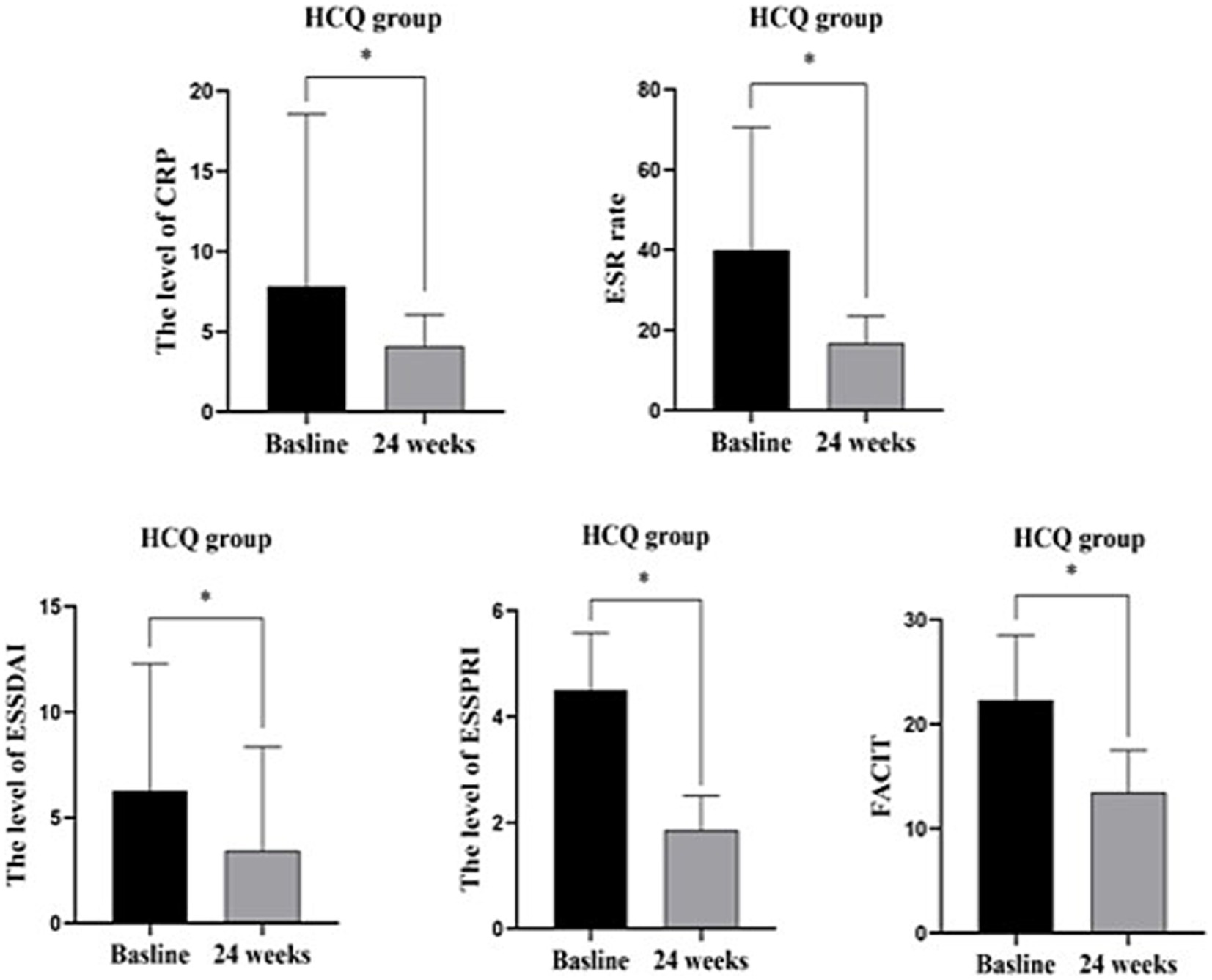
Figure 4. HCQ group before and 24 weeks treatment: CRP, ESR, ESSDAIL, ESSPRI, FACIT were significantly decreased compared with the baseline. There was no significant difference in other characteristics including B lymphocytes and Bregs. *: p < 0.05 represents significant difference post-treatment versus baseline in IGU group.
The adverse reactions in these two groups were mainly liver function abnormalities, gastrointestinal symptoms, rash, leukopenia, skin itching and headache; there was no significant difference between these two groups. Skin pigmentation occurred in two cases in the HCQ group, although no special treatment was provided (Table 5).
Discussion
This study compared the therapeutic effects of IGU and HCQ in pSS and analysed their impacts on Bregs. The results demonstrated that after 24 weeks of treatment, IGU outperformed HCQ in reducing IgG levels and ESSPRI scores and significantly increased the levels of peripheral blood B lymphocytes and CD19+CD5+CD1d+ B cells. In contrast, the influence of HCQ on Bregs was not significant. After treatment, serum IgG levels, ESR, B lymphocytes, ESSDAI, ESSPRI and FACIT were significantly decreased compared with those before treatment in the IGU group. The CRP, ESR, ESSDAI, ESSPRI and FACIT values were significantly decreased, although the serum IgG level had no significant difference compared with those before treatment in the HCQ group.
The differences in therapeutic effects between IGU and HCQ may be attributed to their distinct mechanisms of action. Iguratimod, as a novel immunomodulatory drug, can selectively inhibit Cox-2, thereby reducing the production of prostaglandins in inflamed tissues and inhibiting the release of bradykinin from inflamed tissues, which further alleviates inflammatory responses. Moreover, IGU can selectively act on B cells, inhibiting their excessive proliferation and activation and modulating B cell functions; this leads to a reduction in autoantibody production and a decrease in Ig levels, thus mitigating autoimmune damage to the exocrine glands (31). In contrast, HCQ, a common immunomodulatory drug, exerts anti-inflammatory, immunomodulatory and immunosuppressive effects through multiple pathways, including the inhibition of TLR signalling, the blockade of antigen presentation, the inhibition of autophagy and the suppression of calcium mobilisation. Its impact on Ig production is relatively limited (32). This may explain why IGU is more effective than HCQ in reducing IgG levels.
In addition, this study focused on the levels of B lymphocytes and Bregs in the two groups. The study results showed that before treatment, the proportion of the CD19+CD24hiCD38hi B cell subpopulation in the IGU group was significantly lower than that in the HCQ group, whereas the proportion of the CD19+CD5+CD1d+ B cell subpopulation reached the highest level in the IGU group. This unique phenomenon suggests that in patients with pSS, there may be considerable distribution differences among different Breg subpopulations, which may be closely related to the disease state or individual characteristics of the patients. Current studies have shown that there may be a certain degree of functional overlap and synergistic effects among different Breg subpopulations; however, the specific degree of overlap and interrelationships still need to be further explored in depth (33). After treatment, the level of B lymphocytes in the peripheral blood of the IGU group decreased, indicating that the level of B lymphocytes may be affected by IGU treatment. Moreover, the proportion of CD19+CD5+CD1d+ B cells in the IGU group increased significantly, suggesting that this subpopulation may be the key cell type for IGU to exert its immune regulatory effects. Compared with the HCQ group, the proportion of CD19+CD5+CD1d+ B cells in the two groups after treatment showed a significant difference, with the level in the IGU group being significantly higher than that in the HCQ group. In contrast, there was no significant difference in the proportions of the CD19+CD24hiCD38hi and CD19+CD24+CD27+ B cell subpopulations. This may be because HCQ mainly exerts its effects by inhibiting TLR signalling and reducing the production of pro-inflammatory cytokines, without directly affecting the levels of Bregs. In comparison, IGU can interact with PDK1 to inhibit the Akt–mTOR-STAT3 signalling pathway, leading to the downregulation of the 5CZ6 gene expression and the upregulation of another gene expression downstream. This ultimately results in reduced T follicular helper cell differentiation and the decreased expression of helper function molecules, thereby enhancing the inhibitory effects on B cells. This mechanism may enhance the regulatory functions of Bregs to achieve a more balanced immune response (34). Previous studies have shown that HCQ can effectively relieve dry mouth symptoms in patients with pSS, improve fatigue status and reduce the levels of CRP and ESR; however, its impact on Bregs levels has not been fully investigated (18). Furthermore, multiple studies have demonstrated that IGU has notable therapeutic effects on improving IgG levels, ESSPRI scores and ESR in patients with pSS (26, 27, 31). This study further supports these findings and provides new insights into the different impacts of IGU and HCQ on Bregs, offering important references for future precision treatment strategies targeting pSS.
In summary, this study presents a detailed analysis of the differences in the impact of IGU and HCQ on Bregs in patients with pSS. Previous research has primarily focused on the clinical efficacy and general immune regulatory effects of these drugs; however, the effects on specific B cell subsets, especially Bregs, have not been fully investigated. Our study results highlight the potential role of Bregs in the therapeutic effects of IGU and suggest that targeting Bregs may be a promising strategy for treating pSS. This study also emphasises the importance of further exploring the mechanisms by which IGU and HCQ regulate B cells, which may help to develop more effective treatments for pSS.
Limitations of this study
This study has several limitations. The sample size is relatively small, and further research is needed to expand the sample size, and the role of CD19+CD5+CD1d+ B cells in the occurrence and development of Sjogren’s syndrome needs further study. Furthermore, due to the limited funding of this study and the high cost of Bregs marker detection, healthy individuals were not included as controls in the study design. This limitation prevents us from comparing the proportion of Bregs in patients with pSS with that in healthy individuals. Future studies should include a healthy control group to provide a baseline reference for the proportion of Breg cells, thereby better elucidating the changes in Bregs before and after treatment.
Conclusion
Both IGU and HCQ have beneficial effects in the treatment of pSS and can reduce the disease activity and fatigue score. However, IGU can reduce the level of IgG better than HCQ, and IGU also affects the levels of peripheral blood B lymphocytes and Bregs in patients with pSS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethic Committee of the MianYang Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
J-MZ: Conceptualization, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. LY: Conceptualization, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. J-AL: Data curation, Investigation, Writing – review & editing. YR: Data curation, Investigation, Writing – review & editing. S-YL: Data curation, Investigation, Writing – review & editing. YZ: Data curation, Investigation, Writing – review & editing. J-LD: Data curation, Investigation, Writing – review & editing. D-HD: Data curation, Investigation, Writing – review & editing. Y-PN: Data curation, Investigation, Writing – review & editing. ML: Data curation, Investigation, Writing – review & editing. X-SY: Data curation, Investigation, Writing – review & editing. JY: Data curation, Formal analysis, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Guangzhou pukang charitable foundation (PK-CF-2020-Z-1231) and The Incubation Project of Mianyang Central Hospital (2022FH006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cheng, F, Zhao, FT, Shen, XM, Li, J, Chen, XJ, Wang, GF, et al. Expert consensus on multidisciplinary diagnosis and treatment of primary Sjogren's syndrome (2024 edition). J Int Med Conc Prac. (2024) 19:357–62. doi: 10.16138/j.1673-6087.2024.06.01
2. Sandhya, P, Kurien, BT, Danda, D, and Scofield, RH. Update on pathogenesis of Sjogren's syndrome. Curr Rheumatol Rev. (2017) 13:5–22. doi: 10.2174/1573397112666160714164149
3. Zhan, Q, Zhang, J, Lin, Y, Chen, W, Fan, X, and Zhang, D. Pathogenesis and treatment of Sjogren's syndrome: review and update. Front Immunol. (2023) 14:1127417. doi: 10.3389/fimmu.2023.1127417
4. Lessard, CJ, Li, H, Adrianto, I, Ice, JA, Rasmussen, A, Grundahl, KM, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren's syndrome. Nat Genet. (2013) 45:1284–92. doi: 10.1038/ng.2792
5. Wang, XQ, Liu, DZ, Liu, CL, Hu, Q, Chen, WH, and Xu, WY. Research progress on the pathogenesis of primary Sjogren's syndrome. Clin Res Pract. (2017) 2:189–91. doi: 10.19347/j.cnki.2096-1413.201703103
6. Bruno, KA, Morales-Lara, AC, Bittencourt, EB, Siddiqui, H, Bommarito, G, Patel, J, et al. Sex differences in comorbidities associated with Sjögren's disease. Front Med (Lausanne). (2022) 9:958670. doi: 10.3389/fmed.2022.958670
7. Hong, X, Meng, S, Tang, D, Wang, T, Ding, L, Yu, H, et al. Single-cell RNA sequencing reveals the expansion of cytotoxic CD4+ T lymphocytes and a landscape of immune cells in primary Sjögren's syndrome. Front Immunol. (2021) 11:594658. doi: 10.3389/fimmu.2020.594658
8. Imgenberg-Kreuz, J, Sandling, JK, Almlöf, JC, Nordlund, J, Signér, L, Norheim, KB, et al. Genome-wide DNA methylation analysis in multiple tissues in primary Sjögren's syndrome reveals regulatory effects at interferon-induced genes. Ann Rheum Dis. (2016) 75:2029–36. doi: 10.1136/annrheumdis-2015-208659
9. Luo, S, Zhu, R, Yu, T, Fan, H, Hu, Y, Mohanta, SK, et al. Chronic inflammation: a common promoter in tertiary lymphoid organ Neogenesis. Front Immunol. (2019) 10:2938. doi: 10.3389/fimmu.2019.02938
10. Christodoulou, MI, Kapsogeorgou, EK, and Moutsopoulos, HM. Characteristics of the minor salivary gland infiltrates in Sjögren's syndrome. J Autoimmun. (2010) 34:400–7. doi: 10.1016/j.jaut.2009.10.004
11. Bodewes, ILA, Björk, A, Versnel, MA, and Wahren-Herlenius, M. Innate immunity and interferons in the pathogenesis of Sjögren's syndrome. Rheumatology (Oxford). (2021) 60:2561–73. doi: 10.1093/rheumatology/key360
12. Dong, X, Zhou, J, Guo, X, Li, Y, Xu, Y, Fu, Q, et al. A retrospective analysis of distinguishing features of chest HRCT and clinical manifestation in primary Sjögren's syndrome-related interstitial lung disease in a Chinese population. Clin Rheumatol. (2018) 37:2981–8. doi: 10.1007/s10067-018-4289-6
13. Du, W, Han, M, Zhu, X, Xiao, F, Huang, E, Che, N, et al. The multiple roles of B cells in the pathogenesis of Sjögren's syndrome. Front Immunol. (2021) 12:684999. doi: 10.3389/fimmu.2021.684999
14. Wang, L, Fu, Y, and Chu, Y. Regulatory B cells. Adv Exp Med Biol. (2020) 1254:87–103. doi: 10.1007/978-981-15-3532-1_8
15. Shi, L, Fu, Q, Chen, N, Liu, R, and Zheng, Y. Angiopoietin-like protein 2 as a novel marker for patients with primary Sjogren's syndrome-related interstitial lung disease. Clin Exp Med. (2020) 20:393–9. doi: 10.1007/s10238-020-00623-6
16. Sumida, T, Azuma, N, Moriyama, M, Takahashi, H, Asashima, H, Honda, F, et al. Clinical practice guideline for Sjögren's syndrome 2017. Mod Rheumatol. (2018) 28:383–408. doi: 10.1080/14397595.2018.1438093
17. Price, E, Allen, A, Rauz, S, Tappuni, A, Sutcliffe, N, Bombardieri, M, et al. The management of Sjögren's syndrome: British Society for Rheumatology guideline scope. Rheumatology (Oxford). (2021) 60:2122–7. doi: 10.1093/rheumatology/keaa870
18. Wang, X, Zhang, T, Guo, Z, Pu, J, Riaz, F, Feng, R, et al. The efficiency of hydroxychloroquine for the treatment of primary Sjögren's syndrome: a systematic review and Meta-analysis. Front Pharmacol. (2021) 12:693796. doi: 10.3389/fphar.2021.693796
19. Wang, SQ, Zhang, LW, Wei, P, and Hua, H. Is hydroxychloroquine effective in treating primary Sjogren's syndrome: a systematic review and meta-analysis. BMC Musculoskelet Disord. (2017) 18:186. doi: 10.1186/s12891-017-1543-z
20. Fang, W, Z, Q, L, Q, Z, B, H, X, and X, J. Safety and efficacy of oral hydroxychloroquine in the treatment of ophthalmic disease associated with Sjögren's syndrome. Altern Ther Health Med. (2023) 29:656–62.
21. Tishler, M, Yaron, I, Shirazi, I, and Yaron, M. Hydroxychloroquine treatment for primary Sjögren's syndrome: its effect on salivary and serum inflammatory markers. Ann Rheum Dis. (1999) 58:253–6. doi: 10.1136/ard.58.4.253
22. Ye, Y, Liu, M, Tang, L, Du, F, Liu, Y, Hao, P, et al. Iguratimod represses B cell terminal differentiation linked with the inhibition of PKC/EGR1 axis. Arthritis Res Ther. (2019) 21:92. doi: 10.1186/s13075-019-1874-2
23. Jiang, H, Gao, H, Wang, Q, Wang, M, and Wu, B. Molecular mechanisms and clinical application of Iguratimod: a review. Biomed Pharmacother. (2020) 122:109704. doi: 10.1016/j.biopha.2019.109704
24. Zhang, P, Gong, Y, Liu, Z, Liu, Y, Lin, W, Li, J, et al. Efficacy and safety of iguratimod plus corticosteroid as bridge therapy in treating mild IgG4-related diseases: a prospective clinical trial. Int J Rheum Dis. (2019) 22:1479–88. doi: 10.1111/1756-185X.13633
25. Kang, Y, Yan, Q, Fu, Q, Wang, R, Dai, M, Du, F, et al. Iguratimod as an alternative induction therapy for refractory lupus nephritis: a preliminary investigational study. Arthritis Res Ther. (2020) 22:65. doi: 10.1186/s13075-020-02154-7
26. Chen, H, Qi, X, Li, Y, Wu, Q, Shi, Q, Wang, L, et al. Iguratimod treatment reduces disease activity in early primary Sjögren's syndrome: an open-label pilot study. Mod Rheumatol. (2021) 31:394–8. doi: 10.1080/14397595.2020.1789335
27. Shao, Q, Wang, S, Jiang, H, and Liu, L. Efficacy and safety of iguratimod on patients with primary Sjögren's syndrome: a randomized, placebo-controlled clinical trial. Scand J Rheumatol. (2021) 50:143–52. doi: 10.1080/03009742.2020.1809701
28. Zhang, W, Li, XM, Xu, D, Liu, DZ, Xu, J, Zhao, FT, et al. Recommendations of diagnosis and treatment of primary Sjögren's syndrome in China. Zhonghua Nei Ke Za Zhi. (2020) 59:269–76. doi: 10.3760/cma.j.cn112138-20200113-00021
29. Zeng, L, He, Q, Yang, K, Hao, W, Yu, G, and Chen, H. A systematic review and Meta-analysis of 19 randomized controlled trials of Iguratimod combined with other therapies for Sjogren's syndrome. Front Immunol. (2022) 13:924730. doi: 10.3389/fimmu.2022.924730
30. Pu, J, Wang, X, Riaz, F, Zhang, T, Gao, R, Pan, S, et al. Effectiveness and safety of Iguratimod in treating primary Sjögren's syndrome: a systematic review and Meta-analysis. Front Pharmacol. (2021) 12:621208. doi: 10.3389/fphar.2021.621208
31. Jiang, W, Zhang, L, Zhao, Y, He, X, Hu, C, and Liu, Y. The efficacy and mechanism for action of iguratimod in primary Sjögren's syndrome patients. Int Ophthalmol. (2020) 40:3059–65. doi: 10.1007/s10792-020-01490-6
32. Biswas, M, and Sukasem, C. Pharmacogenomics of chloroquine and hydroxychloroquine: current evidence and future implications. Pharmacogenomics. (2023) 24:831–40. doi: 10.2217/pgs-2023-0124
33. Catalán, D, Mansilla, MA, Ferrier, A, Soto, L, Oleinika, K, Aguillón, JC, et al. Immunosuppressive mechanisms of regulatory B cells. Front Immunol. (2021) 12:611795. doi: 10.3389/fimmu.2021.611795
Keywords: primary Sjögren’s syndrome, regulatory B cells, iguratimod, hydroxychloroquine, treatment
Citation: Zou J-M, Yang L, Luo J-A, Ren Y, Li S-Y, Zhang Y, Dong J-L, Deng D-H, Ni Y-P, Li M, Yin X-S and Yang J (2025) Comparison of the effect of iguratimod and hydroxychloroquine on regulatory B cells in the treatment of primary Sjögren’s syndrome. Front. Med. 12:1573973. doi: 10.3389/fmed.2025.1573973
Edited by:
Cong-Qiu Chu, Oregon Health and Science University, United StatesReviewed by:
Xiaofei Shi, The First Affiliated Hospital of Henan University of Science and Technology, ChinaXiao-Mei Li, The First Affiliated Hospital of University of Science and Technology of China (USTC), China
Copyright © 2025 Zou, Yang, Luo, Ren, Li, Zhang, Dong, Deng, Ni, Li, Yin and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Yang, eWFuZ2ppbmdfMjAyNXlqQDEyNi5jb20=; Jin-Mei Zou, em91X2ppbm1laTAwOUAyMWNuLmNvbQ==
†These authors have contributed equally to this work
 Jin-Mei Zou1*†
Jin-Mei Zou1*† Jing Yang
Jing Yang