- 1Department of Clinical Medicine, The Third Clinical School of Guangzhou Medical University, Guangzhou, China
- 2Department of Respiratory and Critical Care Medicine, Key Laboratory for Major Obstetric Diseases of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 3Department of Respiratory and Critical Care Medicine, Key Laboratory for Major Obstetric Diseases of Guangdong Province, Guangdong Provincial Clinical Medical Research Center for Obstetrics and Gynecology, Guangdong-Hong Kong-Macao Greater Bay Area Higher Education Joint Laboratory of Maternal-Fetal Medicine, The Third Affiliated Hospital of Guangzhou Medical University Guangdong Provincial, Guangzhou, China
Introduction: Acute Fatty Liver of Pregnancy (AFLP) is a rare, life-threatening complication during pregnancy, characterized by acute liver failure, endangering both mother and fetus. Albumin (ALB), synthesized by the liver, is vital for maintaining plasma oncotic pressure and transporting substances, acting as a liver function indicator. Given the scarce research on the link between serum albumin levels and adverse outcomes in AFLP, our study aimed to explore the association between serum albumin levels and 42-day postpartum mortality in women with AFLP.
Methods: The study included 139 women with AFLP from the Third Affiliated Hospital of Guangzhou Medical University, from 2010 to 2022. Severe hypoalbuminemia is albumin <25 g/L; patients categorized as ≥25 or <25 g/L. Multivariable Cox proportional hazards regression analyses examined the relationship between serum albumin levels and 42-day postpartum mortality. The main outcome was mortality through 42 days postpartum, with secondary outcomes including maternal complications and fetal outcomes.
Results: Of the participants (average age 30.1 ± 5.4, 60.4% primiparous, the median gestational age was 36.1 ± 3.1 weeks), there were 15 deaths within 42 days postpartum, 10.8% mortality rate. After adjustment, multivariable Cox proportional hazards regression showed that patients with severe hypoalbuminemia faced a markedly higher risk of 42-day postpartum mortality (HR = 5.55, 95% CI 1.5 ~ 20.5). Multiple organ dysfunction and hepatic encephalopathy were more common in the ALB < 25 g/L group. Fetal death and low birth weight were associated with low serum albumin, though not significantly.
Conclusion: Hypoalbuminemia serves as a critical and alterable risk factor for postpartum adverse complications related to AFLP.
1 Introduction
Acute fatty liver of pregnancy (AFLP) is a rare, potentially life-threatening condition occurring in the early postpartum phase or the third trimester of pregnancy, which can result in serious maternal and fetal complications including multiorgan failure or even death of the mother and fetus (1). It is currently estimated that there are one to three instances of AFLP for every 10,000 deliveries, indicating a low prevalence among pregnant women (2).
Pathologically, AFLP is characterized by fatty infiltration in the liver (3). During gestation, physiological challenges in the oxidation of long- and medium-chain fatty acids may cause an increase in maternal serum fatty acids, which can be toxic to the liver (4–6). Additionally, enzyme deficits can induce decreased mitochondrial β-oxidation of fatty acids, contributing to the development of acute fatty liver during pregnancy. Insufficient levels of this enzyme lead to the buildup of harmful long-chain fatty acid metabolites in the fetus, which can migrate into the mother’s bloodstream, causing maternal liver toxicity, mitochondrial dysfunction, and hepatic failure (7, 8).
Plasma colloid osmotic pressure is regulated in large part by albumin, the most prevalent plasma protein. Other physiological roles of albumin include endothelium stability, hemostatic, anti-inflammatory, and antioxidant actions, solubilization, dissolving, binding, and transporting both endogenous and exogenous chemicals, and capillary permeability modification (9, 10).
Throughout the course of pregnancy, numerous physiological changes may affect the levels of albumin. In the third trimester, plasma volume in pregnant women was increased by 42 to 48% (11). This may lead to hemodilution, known as physiological hemodilution. In this case, although the total amount of albumin may remain unchanged or even increase, the concentration of albumin may decrease. Research has indicated that in healthy pregnancies, serum albumin concentrations decline from a mean of 42 g/L in nonpregnant women to 31 g/L near the end of pregnancy due to an increase in plasma volume (12). Albumin commonly has a lengthy half-life (15–19 days), however, in critically ill individuals, plasma albumin levels can decline by 10–15 g/L in 3 to 5 days (13). According to a prior study, serum albumin experiences structural and functional anomalies in liver damage women, which jeopardizes the protein’s non-oncotic roles as an antioxidant, scavenger, immunological modulator, and endothelial protector. This can lead to a significant decrease in the amount of circulating “effective” albumin (14).
There have been studies quantifying the relationship between other liver and renal function indicators (such as INR, creatine, ALT, etc.) and the poor prognosis of AFLP (15–18); however, no research has yet explored the impact of albumin on mortality and complications in AFLP patients. Currently, whether a decreased albumin can indicate a poor prognosis for women with acute fatty liver of pregnancy (AFLP) remains unknown. According to WHO’s conventional definitions, maternal and pregnancy-related deaths only include those that take place within 42 days after delivery, termination, or abortion (19). Therefore, our study aimed to analyze a large sample of 139 women with AFLP to explore the relationship between serum albumin levels and mortality through 42 days postpartum.
2 Materials and methods
2.1 Study design and participants
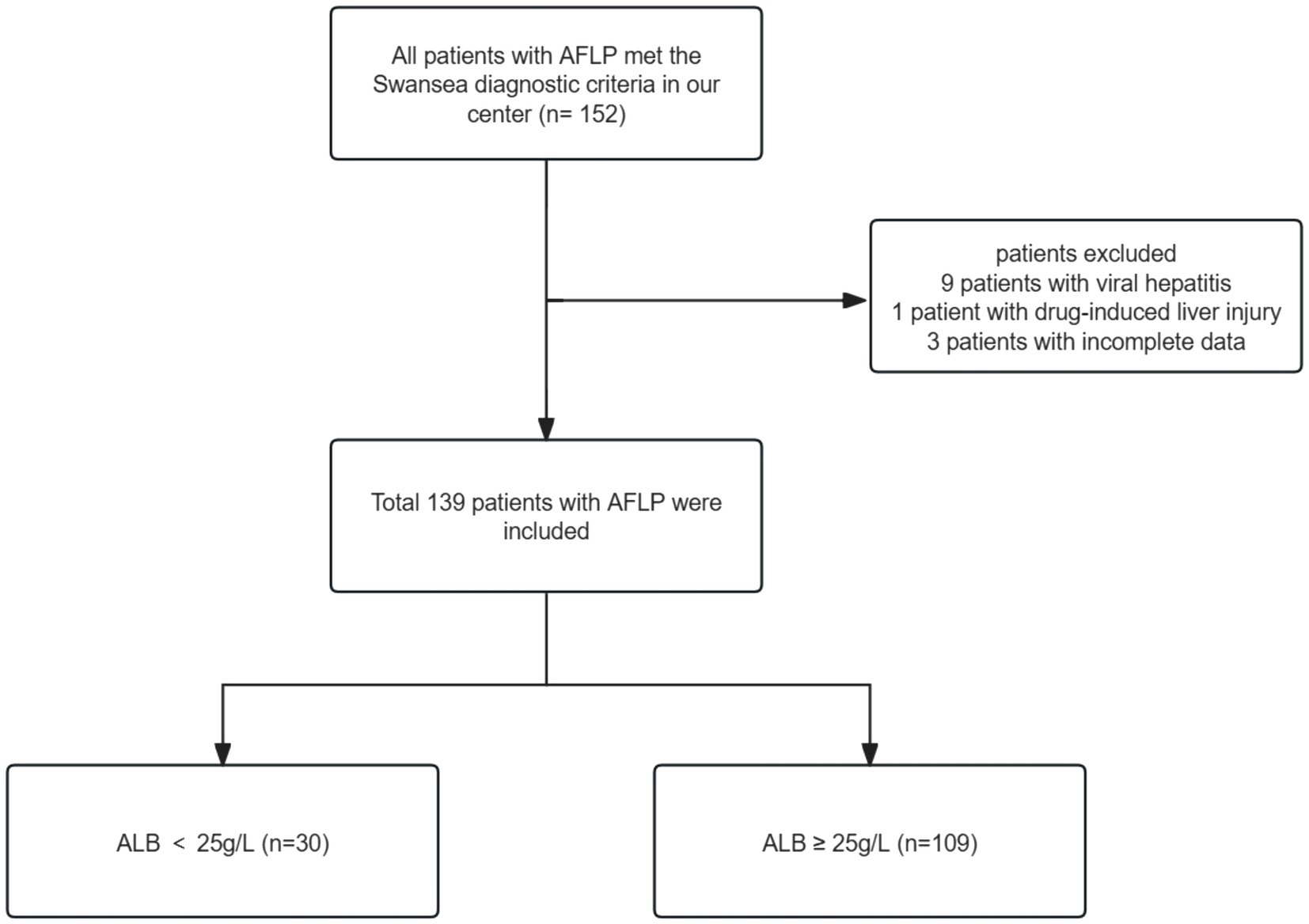
We retrospectively analyzed the data of 139 women who were admitted to the Third Affiliated Hospital of Guangzhou Medical University and diagnosed with AFLP from January 2010 and August 2022. We retrieved AFLP from the medical records system and identified 152 patients who met the criteria. The diagnosis of AFLP was based on the Swansea criteria (20). All women exhibited 6 or more of the Swansea criteria to confirm the diagnosis of AFLP. Upon excluding patients with incomplete data (n = 3), viral hepatitis (n = 9), and drug-induced liver injury (n = 1), we arrived at a final cohort of 139 participants. The patient enrollment workflow is depicted in Figure 1. Given that severe hypoalbuminemia is defined as having an albumin level that is less than 25 g/L (21, 22), we divided patients into two groups based on serum albumin levels: albumin ≥25 g/L (n = 109) and albumin <25 g/L (n = 30).
The study was approved by the Ethics Committee of the Guangzhou Medical University Third Affiliated Hospital (2,020,087, approved in 2021), following the Declaration of Helsinki (2000) of the World Medical Association. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. The severe circumstances, prolonged prothrombin time (PT), and low platelets prevented any of the women from undergoing liver biopsy or liver transplantation. Research has demonstrated that even in the absence of a liver biopsy, no patient would have been overlooked if the Swansea criteria were met (23). Acute kidney injury (AKI) was characterized by serum creatinine (Scr) levels over 90 μmol/L. The diagnosis of disseminated intravascular coagulation (DIC) during pregnancy was established using the DIC score (24). A blood loss of more than 500 mL following vaginal delivery or 1,000 mL following cesarean section delivery within 24 h was referred to as postpartum hemorrhage (PPH) (25). The diagnosis of multiple organ dysfunction syndrome (MODS) and hepatic failure (HE) relies on current guidelines and expert consensus (26, 27).
2.2 Collection of clinical data
In this single-center study, a total of 139 women with AFLP were categorized into two groups, according to their serum albumin levels. Given that the level of serum albumin in women may rise after the treatment, all analyses were stratified by serum albumin before treatment. We collected the laboratory data from the first monitoring conducted after the patient’s admission. The laboratory examination results included regular blood tests, hepatic function, kidney functioning, and blood coagulation tests. Serum albumin and other laboratory indicators were determined using a Roche Large Biochemistry Analyzer (cobas c8000).
The primary outcome was mortality through 42 days postpartum, with secondary outcomes including maternal complications and fetal outcomes. All women were followed up for up to 42 days postpartum. Demographic data, such as maternal age, parity, birth mode, anesthetic techniques, gestational age at diagnosis, and interval between symptom start and delivery, were gathered from medical records. Major complications included PPH, MODS, liver failure, HE, and AKI. Fetal outcomes included fetal death, premature delivery, low birth weight, fetal distress, and fetal asphyxia.
The therapies involved plasma exchange, plasma transfusion, antibiotic therapy, and supportive care. In addition, individuals with AFLP commonly face complications such as MODS and hepatorenal syndrome, necessitating artificial liver support therapy and blood purification therapy. Since the long-term effects of liver transplantation are uncertain and acute liver failure from AFLP can resolve on its own within 7 to 10 days following delivery (28), we did not carry out a liver transplant for the patient.
2.3 Statistical analysis
Firstly, the data were initially categorized into continuous and categorical variables. Continuous variables were summarized as mean ± standard deviation and compared using Student’s t-test if normally distributed, or as median ± interquartile range (IQR) and compared using the Wilcoxon rank-sum test if not normally distributed. Categorical variables were expressed as percentages and compared using the chi-square test. Baseline and clinical characteristics were compared between participants with albumin levels ≥25 g/L and those with levels <25 g/L. Univariate analysis of risk factors for death was performed using the Cox proportional hazard model, and covariates with p ≤ 0.05 were considered significant (to avoid eliminating significant variables). Variables found to be significant in univariate analysis were included in a multivariate Cox regression analysis, performed using a likelihood ratio (LR) approach, p ≤ 0.05 (two-tailed) was considered statistically significant.
The Kaplan–Meier method was used for survival analyses across admission ALB levels. We calculated hazard ratios (HRs) and 95% confidence intervals (CIs) through multivariate Cox proportional hazards regression to assess the 42-day postpartum mortality associated with serum albumin. After adjusting for predefined covariates known to be associated with hypoproteinemia, including maternal age, gestational age, parity, red blood cells (RBC), hemoglobin (Hb), activated partial thromboplastin time (APTT), alanine transaminase (ALT), lactic acid (LACT), creatinine (Cr), the mode of delivery, time from onset to termination of pregnancy, plasma exchange, and ICU admission, the results remained robust. We also conducted a smooth curve fitting to explore whether there exists a non-linear relationship between serum albumin and 42-day postpartum mortality. To evaluate the relationship between albumin levels and various secondary outcomes separately, we employed univariate logistic regression analysis.
Every analysis was conducted using the statistical software packages R version 3.3.21 (The R Foundation) and Free Statistics version 1.7 (29).
3 Results
3.1 Patient selection
152 patients with AFLP met the Swansea diagnostic criteria in our center. Following the exclusion of patients with viral hepatitis (n = 9), drug-induced liver injury (n = 1), and incomplete data (n = 3), the final study cohort consisted of 139 participants. Figure 1 outlines the enrollment process.
3.2 Baseline characteristics of the study participants
A total of 139 AFLP women with available data were included in the analysis, and 15 women (10.8%) suffered from death within 42 days postpartum. In all, 109 women (78.42%) had serum albumin levels above 25 g/L while 30 women (21.58%) had values below 25 g/L. There were 15 total deaths (10.8%), with 8 (7.3%) occurring in patients with ALB ≥25 g/L and 7 (23.3%) in patients with ALB < 25 g/L. The mean admission ALB levels were 29.4 ± 5.7 g/L.
Table 1 presents the clinical and biochemical features of the study population, categorized by ALB level (≥ 25 g/L or < 25 g/L). Participants’ mean age ± SD was 30.1 ± 5.4 years, 60.4% were primiparous women, and the median gestational age was 36.1 ± 3.1 weeks. The maternal complications were AKI (n = 72, 51.8%), PPH (n = 47, 33.8%), MODS (n = 26, 18.7%) and HE (n = 8, 5.8%). Cesarean section was used in 108 (77.7%) patients. A total of 20 patients (14.4%) underwent plasma exchange (PE). Fetal outcomes include fetal death (n = 8, 5.8%), low birth weight (n = 64, 47.4%), fetal distress (n = 54, 40.6%), and fetal asphyxia (n = 48, 36.1%). At baseline, women with severe hypoalbuminemia ALB levels had lower hemoglobin (p = 0.001) and red blood cell (p = 0.008), and higher lactic acid (p = 0.006).
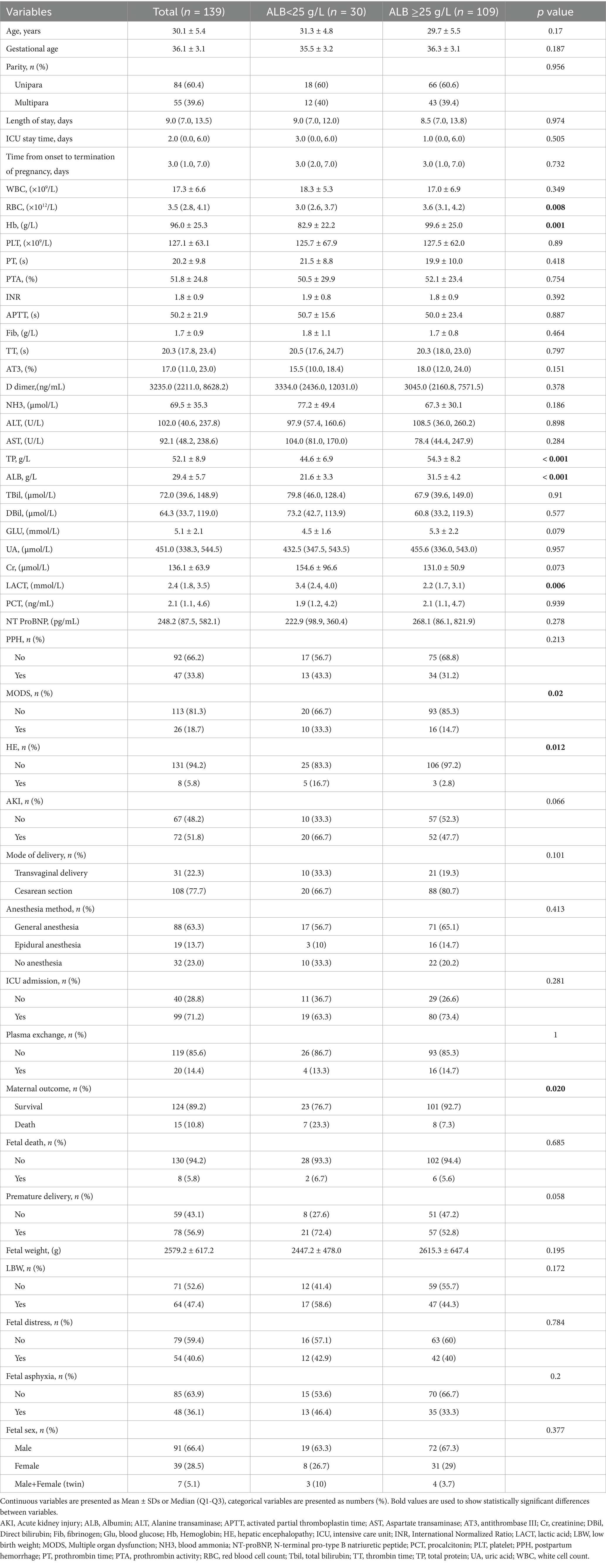
Table 1. Clinical characteristics of women with AFLP of the study population according to serum albumin.
3.3 Kaplan–Meier survival curve
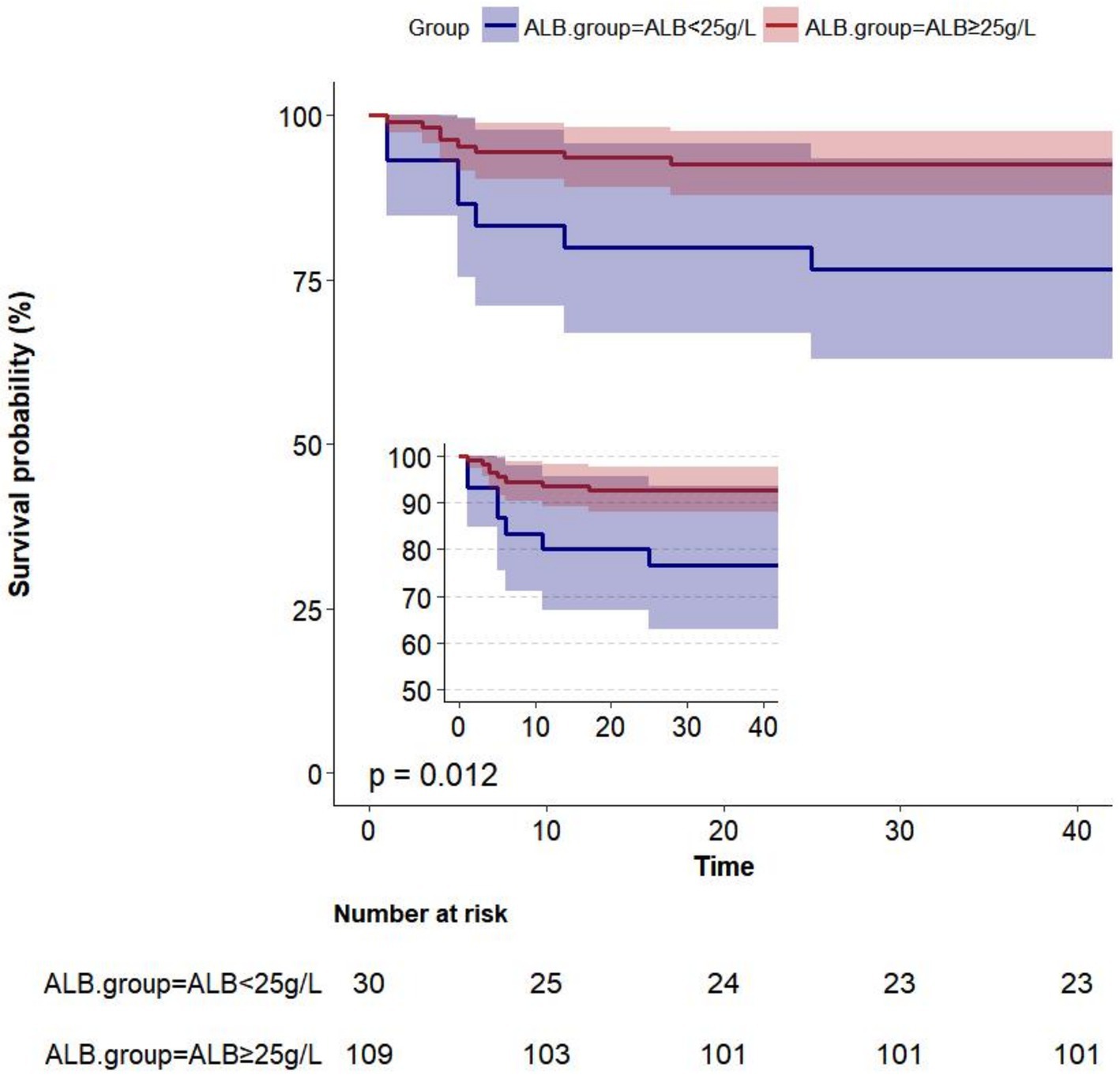
Albumin level at admission affected the Kaplan–Meier survival predictions (p = 0.012). Within 42 days, 139 women had been followed for postpartum death. At 42 days postpartum, 15 out of 139 women (10.8%) passed away. The Kaplan–Meier predictions of cumulative mortality are shown in Figure 2. The cumulative mortality rate was higher in women with ALB < 25 g/L than in those with ALB ≥ 25 g/L (p < 0.05).
3.4 Maternal and fetal outcomes
Univariate logistic regression analysis was used to evaluate the relationship between serum albumin (ALB) levels and secondary outcomes (LBW, HE, MODS, fetal death, and premature delivery), as illustrated in Figure 3. MODS was more common in the ALB <25 g/L group than in the ALB ≥25 g/L group (p = 0.02). Additionally, compared to women with ALB<25 g/L, individuals in the ALB < 25 g/L group had a greater level of HE (p = 0.012). Fetal mortality and low birth weight were associated to low serum albumin, though not significantly.
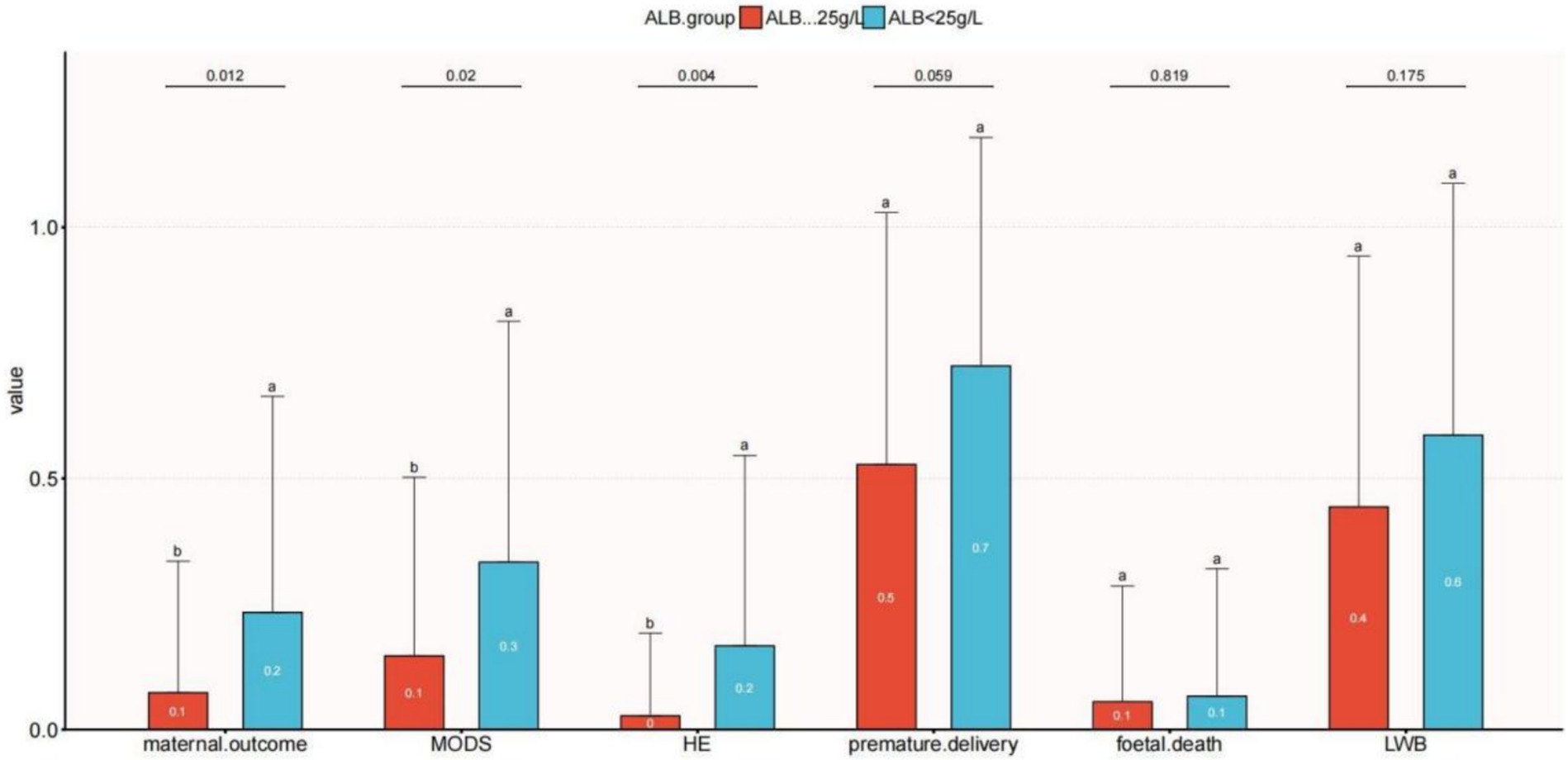
Figure 3. Relationship between serum albumin and clinical outcomes with acute fatty liver of pregnancy. The letters a and b are used to show statistically significant differences between variables. For all variables with the same letter, the difference between the means is not statistically significant. If two variables have different letters, they are significantly different. HE, hepatic encephalopathy; LBW, low-birth-weight infant; MODS, multiple organ dysfunction.
3.5 Association between albumin levels and postpartum mortality
Findings from the multivariable Cox proportional hazards regression for the association between serum albumin levels and 42-day postpartum mortality are shown in Table 2. When albumin was analyzed as a continuous variable, a significant independent negative association was discovered between serum albumin and 42-day postpartum mortality in the non-adjusted crude model (HR: 0.88, 95% CI: 0.81–0.96; p = 0.005); meanwhile, further adjustment did not significantly affect the results. Model I was adjusted for sociodemographic variables (age, gestational age, parity). Model II was further adjusted for red blood cells (RBC), hemoglobin (Hb), activated partial thromboplastin time (APTT), alanine transaminase (ALT), lactic acid (LACT), and creatinine (Cr). Model III was further adjusted for the mode of delivery, time from onset to termination of pregnancy, plasma exchange, and ICU admission. After adjusting for all covariates that are potentially connected to low serum albumin, the results remained robust. We can see from Table 2, above 25 g/L, for every1 g/L increase in serum albumin, there was a 19% decrease in 42-day postpartum mortality (adjusted HR = 0.81; 95% CI 0.71 ~ 0.92, Model III, Table 2).
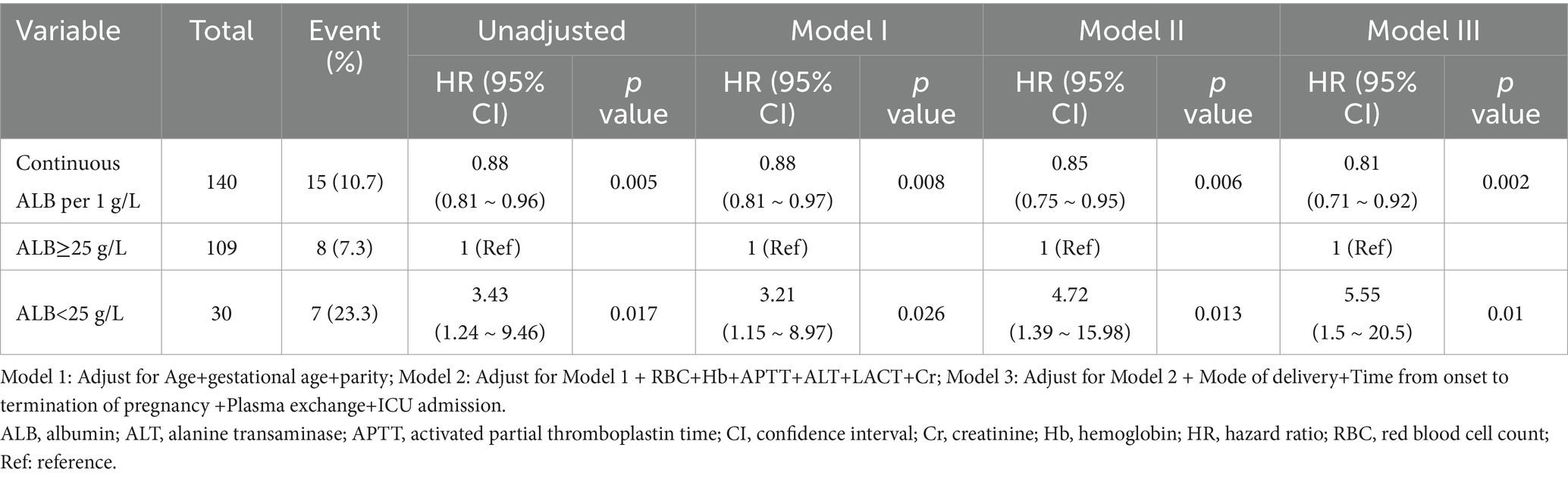
Table 2. Association between serum albumin levels and mortality through 42 days postpartum in multivariable Cox proportional hazards regression analyses.
After adjusting for all variables, the multivariable Cox proportional hazards regression indicated that women with an ALB < 25 g/L had a 4.55-fold higher risk of 42-day postpartum mortality (HR = 5.55, 95% CI 1.5 ~ 20.5, Model III, Table 2) than women with an ALB ≥ 25 g/L.
3.6 Curve fitting for linear association
Finally, non-linear relationships between serum albumin and 42-day maternal postpartum mortality were examined using a smooth curve fitting technique (Supplementary Figure S1). Serum albumin levels and 42-day postpartum mortality had a negative association after all potential confounders were taken into account (nonlinearity: p = 0.856).
4 Discussion
To our knowledge, this study provided the first assessment of 42-day postpartum mortality associations with serum albumin based on a large sample of 139 women between 2010 and 2022. The Swansea standards serve as a recognized international benchmark for the diagnosis of acute fatty liver of pregnancy (AFLP). While the guidelines do not address albumin in the Swansea standards, we found that women with serum albumin levels below 25 g/L had a significantly higher risk of 42-day postpartum mortality compared to those with albumin levels of 25 g/L or higher, thus highlighting the critical need for careful monitoring of albumin pregnant women with AFLP. Additionally, MODS and HE were more commonly observed in the group with severe hypoalbuminemia. These results emphasize the importance of monitoring serum albumin as a predictor of postpartum complications.
Up to now, research on AFLP has primarily concentrated on exploring risk factors. Nulliparity, male fetal sex, and multifetal gestation are potential risk factors of AFLP (4, 8, 30, 31). These factors, however, are unchangeable and static. Peng et al. reported that INR, total bilirubin, fibrinogen, and low platelet were confirmed as risk factors for AFLP-related three-month mortality (15). The days between the onset of symptoms and hospitalization and parturition were the high-risk factors for AFLP fatality, according to a prior study that Li reported (18). According to Gao et al. (32), serum creatinine, total bilirubin, and abortion history are all separate risk factors for maternal death. Chen et al.’s (33) study revealed a correlation between the postpartum recovery duration of AFLP and the levels of platelets, total protein, and total bilirubin. While liver and kidney function indicators and coagulation indicators have received considerable attention in AFLP risk factor studies, albumin, which is a vital liver function indicator, remains overlooked.
Although serum albumin has not been considered an independent risk factor for the prognosis of women with AFLP (15–17, 34), hypoalbuminemia was frequently observed in patients suffering from AFLP. A recent study found that albumin levels in 88% of the women with AFLP had dropped to less than 30 g/L (35). In another study, abnormal liver, renal, and coagulation tests, as well as extremely low serum albumin, were seen in the laboratory results of 91–100% of women with AFLP (36).
Decreased hepatic synthesis causes a progressive decrease in serum albumin, which affects colloid osmotic pressure (37). In the early stages of MODS, fluid retention, and systemic tissue edema are caused by increased permeability of systemic capillaries to water and protein, and decreased plasma colloidal osmotic pressure (38). A reduction in osmotic pressure linked to hypoalbuminemia can lead to a deficiency in intravascular fluid, thereby impairing organ perfusion and potentially causing multiple organ dysfunction syndrome (39). This helps to clarify why MODS are more prevalent in the group with severe hypoalbuminemia (ALB < 25 g/L).
According to a cohort study of 298 individuals, the most serious and potentially fatal maternal consequences are hepatic encephalopathy and postpartum hemorrhage (18). Hepatic encephalopathy can span from mild cases to severe ones, frequently accompanied by elevated intracranial pressure (40, 41). And mild hepatic encephalopathy can sometimes go unnoticed if ammonia (NH3) is not monitored effectively. Our study found that individuals with ALB < 25 g/L were more susceptible to hepatic encephalopathy. Hence, clinicians should be vigilant regarding the risk of hepatic encephalopathy in those with lower albumin levels. Monitoring in an intensive care unit is necessary for patients with lower albumin, along with careful assessment of their neurological condition and encephalopathy severity.
Despite considering various important factors, we cannot establish a causal relationship, and serum albumin might be a predictive factor rather than a direct cause of health. However, it remains crucial to prioritize primary prevention of albumin levels falling beyond the physiological range during pregnancy due to its significant effect on various maternal and offspring outcomes. Furthermore, the goal of treatment is to assist the liver in returning to normal function, which includes avoiding potentially hepatotoxic chemicals and providing adequate nutritional support. In this process, a gradual rise of serum albumin levels can be seen as a positive sign of restored liver function and improved prognosis.
Plasma exchange has become a focal point for treating AFLP. Endotoxin elimination, coagulation factor support, intravascular volume and albumin, electrolyte regulation, and acid–base balance are the suggested advantageous mechanisms (42). Combining plasma exchange and plasma perfusion may result in better survival outcomes than conventional treatment by itself (2).
Multidisciplinary collaboration is required for the management of women with AFLP. Early identification and intervention, including close collaboration between hepatologists, obstetricians, dietitians, and other professionals, provides comprehensive care for people with AFLP. Serum albumin levels should be closely monitored as part of this collaborative effort. A comprehensive treatment strategy, including nutritional support and close monitoring of serum albumin levels, can optimize maternal and fetal health, reduce mortality, and improve maternal and fetal prognosis.
There are various limitations on this study. First, there was only a limited number of women included in this study because of the low incidence of AFLP. Even yet, our sample size was greater than that of other comparable research (15–17, 33, 34, 43, 44). Future multicenter collaborations are needed to validate these findings in larger sample sizes of prospectively enrolled populations. Furthermore, because this was a single-center trial, bias was unavoidable. Additionally, the lack of follow-up information post-discharge restricted our focus to short-term postpartum outcomes, rather than examining the lasting effects of hypoalbuminemia. We acknowledge that the 12-year study period may introduce temporal bias, though the core management strategy—timely pregnancy termination for this condition—remained unchanged in institutional guidelines during the entire 12-year period.
Taken together, we show that severe hypoalbuminemia has procedure-specific effects and calls for targeted management strategies to enhance maternal outcomes. In the absence of definitive guidelines, our findings encourage obstetricians to measure albumin levels and treat hypoalbuminemia appropriately.
Data availability statement
The datasets presented in this article are not readily available because the datasets analyzed in this study are not publicly available due to their inclusion in an ongoing research project, but can be obtained from the corresponding author upon reasonable request. Requests to access the datasets should be directed to YG eXVhbm1laWdhb0AxMjYuY29t.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Guangzhou Medical University Third Affiliated Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YuF: Writing – original draft, Writing – review & editing. GL: Data curation, Visualization, Writing – review & editing. XL: Validation, Writing – review & editing. YiF: Writing – review & editing. YG: Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Guangzhou Medical University (Grant number S202310570022) and Plan on enhancing scientific research in GMU (Grant number GMUCR2025-02004). YG has received research support from Guangzhou Medical University and the Third Affiliated Hospital of Guangzhou Medical University.
Acknowledgments
We express our gratitude to the attending personnel and the women who took part in this retrospective study for their dedication and assistance. The Guangzhou Medical University Third Affiliated Hospital and Guangzhou Medical University provided funding for this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1574686/full#supplementary-material
Footnotes
References
1. Nelson, DB, Byrne, JJ, and Cunningham, FG. Acute fatty liver of pregnancy. Obstet Gynecol. (2021) 137:535–46. doi: 10.1097/AOG.0000000000004289
2. Naoum, EE, Leffert, LR, Chitilian, HV, Gray, KJ, and Bateman, BT. Acute fatty liver of pregnancy: pathophysiology, anesthetic implications, and obstetrical management. Anesthesiology. (2019) 130:446–61. doi: 10.1097/ALN.0000000000002597
3. Ramanathan, R, and Ibdah, JA. Mitochondrial dysfunction and acute fatty liver of pregnancy. Int J Mol Sci. (2022) 23:3595. doi: 10.3390/ijms23073595
4. Castro, MA, Fassett, MJ, Reynolds, TB, Shaw, KJ, and Goodwin, TM. Reversible peripartum liver failure: a new perspective on the diagnosis, treatment, and cause of acute fatty liver of pregnancy, based on 28 consecutive cases. Am J Obstet Gynecol. (1999) 181:389–95. doi: 10.1016/S0002-9378(99)70567-3
5. Grimbert, S, Fisch, C, Deschamps, D, Berson, A, Fromenty, B, Feldmann, G, et al. Effects of female sex hormones on mitochondria: possible role in acute fatty liver of pregnancy. Am J Phys. (1995) 268:G107–15. doi: 10.1152/ajpgi.1995.268.1.G107
6. Grimbert, S, Fromenty, B, Fisch, C, Letteron, P, Berson, A, Durand-Schneider, AM, et al. Decreased mitochondrial oxidation of fatty acids in pregnant mice: possible relevance to development of acute fatty liver of pregnancy. Hepatology. (1993) 17:628–37. doi: 10.1002/hep.1840170417
7. Natarajan, SK, Thangaraj, KR, Eapen, CE, Ramachandran, A, Mukhopadhya, A, Mathai, M, et al. Liver injury in acute fatty liver of pregnancy: possible link to placental mitochondrial dysfunction and oxidative stress. Hepatology. (2010) 51:191–200. doi: 10.1002/hep.23245
8. Bacq, Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. (2011) 35:182–93. doi: 10.1016/j.clinre.2010.11.011
9. Jagdish, RK, Maras, JS, and Sarin, SK. Albumin in advanced liver diseases: the good and bad of a drug! Hepatology. (2021) 74:2848–62. doi: 10.1002/hep.31836
10. Bernardi, M, Angeli, P, Claria, J, Moreau, R, Gines, P, Jalan, R, et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut. (2020) 69:1127–38. doi: 10.1136/gutjnl-2019-318843
11. Aguree, S, and Gernand, AD. Plasma volume expansion across healthy pregnancy: a systematic review and meta-analysis of longitudinal studies. BMC Pregnancy Childbirth. (2019) 19:508. doi: 10.1186/s12884-019-2619-6
12. Elliott, JR, and O’Kell, RT. Normal clinical chemical values for pregnant women at term. Clin Chem. (1971) 17:156–7. doi: 10.1093/clinchem/17.3.156
13. Marik, PE. The treatment of hypoalbuminemia in the critically ill patient. Heart Lung. (1993) 22:166–70.
14. Takegawa, R, Kabata, D, Shimizu, K, Hisano, S, Ogura, H, Shintani, A, et al. Serum albumin as a risk factor for death in patients with prolonged sepsis: an observational study. J Crit Care. (2019) 51:139–44. doi: 10.1016/j.jcrc.2019.02.004
15. Peng, Q, Zhu, T, Huang, J, Liu, Y, Huang, J, and Zhang, W. Factors and a model to predict three-month mortality in patients with acute fatty liver of pregnancy from two medical centers. BMC Pregnancy Childbirth. (2024) 24:27. doi: 10.1186/s12884-023-06233-w
16. Gao, Q, Qu, X, Chen, X, Zhang, J, Liu, F, Tian, S, et al. Outcomes and risk factors of patients with acute fatty liver of pregnancy: a multicentre retrospective study. Singapore Med J. (2018) 59:425–30. doi: 10.11622/smedj.2018001
17. Li, P, Lin, S, Li, L, Cui, J, Wang, Q, Zhou, S, et al. Utility of MELD scoring system for assessing the prognosis of acute fatty liver of pregnancy. Eur J Obstet Gynecol Reprod Biol. (2019) 240:161–6. doi: 10.1016/j.ejogrb.2019.06.030
18. Li, L, Huang, D, Xu, J, Li, M, Zhao, J, Shi, Q, et al. The assessment in patients with acute fatty liver of pregnancy (AFLP) treated with plasma exchange: a cohort study of 298 patients. BMC Pregnancy Childbirth. (2023) 23:171. doi: 10.1186/s12884-023-05503-x
19. Gazeley, U, Reniers, G, Eilerts-Spinelli, H, Prieto, JR, Jasseh, M, Khagayi, S, et al. Women’s risk of death beyond 42 days post partum: a pooled analysis of longitudinal health and demographic surveillance system data in sub-saharan africa. Lancet Glob Health. (2022) 10:e1582–9. doi: 10.1016/S2214-109X(22)00339-4
20. Gao, Y, Wang, X, Li, X, Fang, Y, Lv, C, and Chen, D. Association between platelet counts and clinical outcomes in acute fatty liver of pregnancy: a retrospective cohort study. Int J Gynaecol Obstet. (2024) 164:173–83. doi: 10.1002/ijgo.14955
21. Fieber, JH, Sharoky, CE, Wirtalla, C, Williams, NN, Dempsey, DT, and Kelz, RR. The malnourished patient with obesity: a unique paradox in bariatric surgery. J Surg Res. (2018) 232:456–63. doi: 10.1016/j.jss.2018.06.056
22. Camino-Willhuber, G, Tani, S, Schonnagel, L, Caffard, T, Haffer, H, Chiapparelli, E, et al. Association of frailty and preoperative hypoalbuminemia with the risk of complications, readmission, and mortality after spine surgery. World Neurosurg. (2023) 174:e152–8. doi: 10.1016/j.wneu.2023.03.095
23. Goel, A, Ramakrishna, B, Zachariah, U, Ramachandran, J, Eapen, CE, Kurian, G, et al. How accurate are the Swansea criteria to diagnose acute fatty liver of pregnancy in predicting hepatic microvesicular steatosis? Gut. (2011) 60:138–9. doi: 10.1136/gut.2009.198465
24. Erez, O, Novack, L, Beer-Weisel, R, Dukler, D, Press, F, Zlotnik, A, et al. DIC score in pregnant women--a population based modification of the international society on thrombosis and hemostasis score. PLoS One. (2014) 9:e93240. doi: 10.1371/journal.pone.0093240
25. Giouleka, S, Tsakiridis, I, Kalogiannidis, I, Mamopoulos, A, Tentas, I, Athanasiadis, A, et al. Postpartum hemorrhage: a comprehensive review of guidelines. Obstet Gynecol Surv. (2022) 77:665–82. doi: 10.1097/OGX.0000000000001061
26. Patidar, KR, and Bajaj, JS. Covert and overt hepatic encephalopathy: diagnosis and management. Clin Gastroenterol Hepatol. (2015) 13:2048–61. doi: 10.1016/j.cgh.2015.06.039
27. Nanchal, R, Subramanian, R, Karvellas, CJ, Hollenberg, SM, Peppard, WJ, Singbartl, K, et al. Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. (2020) 48:e173–91. doi: 10.1097/CCM.0000000000004192
28. Ch’ng, CL, Morgan, M, Hainsworth, I, and JGC, K. Prospective study of liver dysfunction in pregnancy in Southwest Wales. Gut. (2002) 51:876–80. doi: 10.1136/gut.51.6.876
29. Wu, B, Guo, Y, Yang, HH, Gao, QG, and Tian, Y. Predicting bone metastasis risk based on Skull Base invasion in locally advanced nasopharyngeal carcinoma. Front Oncol. (2022) 12:812358. doi: 10.3389/fonc.2022.812358
30. Knight, M, Nelson-Piercy, C, Kurinczuk, JJ, Spark, P, and Brocklehurst, PUK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. (2008) 57:951–6. doi: 10.1136/gut.2008.148676
31. Davidson, KM, Simpson, LL, Knox, TA, and D’Alton, ME. Acute fatty liver of pregnancy in triplet gestation. Obstet Gynecol. (1998) 91:806–8.
32. Gao, Q, Ma, Y, Qu, X, and Zheng, X. Risk factors in patients with acute fatty liver of pregnancy: the role of abortion, total bilirubin and serum creatinine. Arch Gynecol Obstet. (2023) 310:153–9. doi: 10.1007/s00404-023-07234-y
33. Chen, G, Huang, K, Ji, B, Chen, C, Liu, C, Wang, X, et al. Acute fatty liver of pregnancy in a Chinese tertiary care center: a retrospective study. Arch Gynecol Obstet. (2019) 300:897–901. doi: 10.1007/s00404-019-05259-w
34. Meng, Z, Fang, W, Meng, M, Zhang, J, Wang, Q, Qie, G, et al. Risk factors for maternal and fetal mortality in acute fatty liver of pregnancy and new predictive models. Front Med. (2021) 8:719906. doi: 10.3389/fmed.2021.719906
35. Meng, J, Wang, S, Gu, Y, Lv, H, Jiang, J, and Wang, X. Prenatal predictors in postpartum recovery for acute fatty liver of pregnancy: experiences at a tertiary referral center. Arch Gynecol Obstet. (2016) 293:1185–91. doi: 10.1007/s00404-015-3941-5
36. Martin, JN, and Tucker, JM. Missing or making the timely diagnosis of acute fatty liver of pregnancy (AFLP): lessons learned. J Matern Fetal Neonatal Med. (2022) 35:3595–601. doi: 10.1080/14767058.2020.1832075
37. Aman, J, van der Heijden, M, van Lingen, A, Girbes, ARJ, van Nieuw Amerongen, GP, van Hinsbergh, VWM, et al. Plasma protein levels are markers of pulmonary vascular permeability and degree of lung injury in critically ill patients with or at risk for acute lung injury/acute respiratory distress syndrome. Crit Care Med. (2011) 39:89–97. doi: 10.1097/CCM.0b013e3181feb46a
38. Fullerton, JN, and Singer, M. Organ failure in the ICU: cellular alterations. Semin Respir Crit Care Med. (2011) 32:581–6. doi: 10.1055/s-0031-1287866
39. Eljaiek, R, and Dubois, MJ. Hypoalbuminemia in the first 24h of admission is associated with organ dysfunction in burned patients. Burns J Int Soc Burn Inj. (2013) 39:113–8. doi: 10.1016/j.burns.2012.05.008
40. Polson, J, and Lee, WM. American Association for the Study of liver disease. AASLD position paper: the management of acute liver failure. Hepatology. (2005) 41:1179–97. doi: 10.1002/hep.20703
41. European Association for the Study of the Liver. EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J Hepatol. (2017) 66:1047–81. doi: 10.1016/j.jhep.2016.12.003
42. Yu, CB, Chen, JJ, Du, WB, Chen, P, Huang, JR, Chen, YM, et al. Effects of plasma exchange combined with continuous renal replacement therapy on acute fatty liver of pregnancy. Hepatobiliary Pancreat Dis Int. (2014) 13:179–83. doi: 10.1016/S1499-3872(14)60028-X
43. Cheng, N, Xiang, T, Wu, X, Li, M, Xie, Y, and Zhang, L. Acute fatty liver of pregnancy: a retrospective study of 32 cases in South China. J Matern Fetal Neonatal Med. (2014) 27:1693–7. doi: 10.3109/14767058.2013.871704
Keywords: serum albumin, acute fatty liver of pregnancy, maternal mortality, maternal complications, fetal outcomes
Citation: Fang Y, Liao G, Li X, Fang Y and Gao Y (2025) Association between serum albumin and 42-day postpartum mortality in women with acute fatty liver of pregnancy: a retrospective study. Front. Med. 12:1574686. doi: 10.3389/fmed.2025.1574686
Edited by:
Wentao Ni, Peking University People’s Hospital, ChinaReviewed by:
Xiaoyan Wang, First Affiliated Hospital of Chengdu Medical College, ChinaHuanxian Liu, Chinese PLA General Hospital, China
Copyright © 2025 Fang, Liao, Li, Fang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanmei Gao, eXVhbm1laWdhb0AxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Yuxin Fang1,2†
Yuxin Fang1,2† Yi Fang
Yi Fang Yuanmei Gao
Yuanmei Gao