- 1Department of Nephrology, The Second Hospital Affiliated to Kunming Medical University, Kunming, China
- 2Department of Nephrology, Jiujiang City Key Laboratory of Cell Therapy, Jiujiang No. 1 People’s Hospital, Jiujiang, China
- 3The Third Unit of the Department of Hepatology, Beijing Institute of Hepatology, Beijing Youan Hospital, Capital Medical University, Beijing, China
Background: The atherosclerosis index (AIP) in plasma is a novel indicator closely associated with various metabolic abnormalities, and the bidirectional relationship between metabolic dysfunction and chronic kidney disease (CKD) has been extensively documented. However, evidence regarding the association between AIP and mortality in CKD population remains scarce. This study aims to elucidate the association between baseline AIP levels and both all-cause and specific mortality in a diverse cohort of US adults.
Methods: This cohort study utilized data from the National Health and Nutrition Examination Survey (NHANES) spanning from 1999 to 2018. A total of 4,403 participants were included in the analysis. Mortality rates were determined through linkage with the National Death Index (NDI), with follow-up extending through December 31, 2019. The primary outcome variables were all-cause mortality and cause-specific mortality. Multivariable weighted Cox proportional hazards regression models, restricted cubic spline analysis, subgroup stratification, and sensitivity testing were utilized to evaluate the associations between the AIP and both all-cause and cause-specific mortality.
Results: During a median follow-up of 83 months, 1,767 all-cause deaths and 526 CVD deaths occurred. After multivariable adjustment, AIP was independently associated with elevated risks of all-cause mortality (HR: 2.03, 95% CI: 1.62∼2.55, P < 0.001) and cardiovascular mortality (SHR: 1.60, 95% CI: 1.08∼2.39, P = 0.028). Restricted cubic spline analysis confirmed a linear dose-response relationship between AIP and all-cause mortality (P for non-linearity = 0.243). Subgroup analyses confirmed consistent associations across demographics and comorbidities, with significant interactions observed for sex, BMI, and diabetes (P < 0.05). Sensitivity analyses excluding deaths within 2 years showed similar outcomes (all-cause mortality HR: 1.94, 95% CI: 1.53∼2.47, P < 0.001; CVD mortality SHR: 1.69, 95% CI: 1.13∼2.53, P = 0.01).
Conclusion: Data from large cohort studies have revealed a significant positive correlation between AIP levels and the risks of all-cause and cardiovascular mortality in the adult population of the United States. This suggests that AIP is associated with an increased risk of adverse outcomes in CKD and may serve as biomarker.
1 Introduction
Chronic kidney disease (CKD), as a major global public health concern, is receiving increasingly extensive attention. Over the past several decades, its incidence and prevalence have manifested a notable upward trend. According to relevant estimations, approximately 700 million people worldwide are afflicted by CKD, which results in approximately 1.2 million deaths and directly contributes to 35.8 million disability-adjusted life years (DALYs) (1, 2). The prevalence of CKD varies among different regions. In low- and middle-income countries, the prevalence is relatively high, even affecting more than 10% of the local population (3). Taking the United States as an example, the estimated prevalence of CKD is approximately 15%, and in 2017 alone, medical expenditures related to CKD management exceeded 84 billion US dollars (4, 5). Beyond its direct impact on renal function, CKD is intricately linked to cardiovascular complications (6–8). This cardiovascular burden is further exacerbated by metabolic derangements, particularly dyslipidemia, which accelerates glomerular injury and systemic inflammation (9).
In recent years, a considerable number of studies have indicated that lipid metabolism disorders exert a critical role in the pathogenesis and progression of CKD (6, 9–11). Atherosclerosis index (AIP), a novel lipid marker initially proposed by Dobiasova and Frohlich in 2001, is obtained through logarithmic transformation of the ratio of triglycerides (TG) to high-density lipoprotein cholesterol (HDL-C) (12). AIP is capable of reflecting the characteristics and extent of lipid metabolism abnormalities. A growing body of research evidence highlights the significance of AIP in predicting the risk of cardiovascular disease (13, 14), as well as metabolic disorders such as non-alcoholic fatty liver disease (NAFLD) (15). Nonetheless, its role in CKD remains insufficiently explored. Especially, a sufficient and in-depth cognition of the consistency and specificity of this association among different regional populations has not yet been formed. Cross-sectional analyses have associated AIP with albuminuria and reduced glomerular filtration rate (16–18), yet longitudinal data are lacking. Mechanistically, AIP may drive renal damage through endothelial dysfunction, oxidative stress, and proinflammatory pathways (5). Recent trials demonstrate that sodium-glucose cotransporter 2 (SGLT2) inhibitors reduce cardiovascular mortality in CKD patients by modulating lipid metabolism and mitigating inflammation (2, 5). However, these therapies do not address the fundamental dyslipidemia inherent to CKD, underscoring the need for novel biomarkers like AIP. Hence, an in-depth exploration of the specific role of AIP in the CKD population and its impact on prognosis is of paramount significance for optimizing the management strategies for CKD patients.
The present study sought to investigate the longitudinal association between baseline AIP levels and mortality outcomes in a nationally representative CKD cohort.
2 Material and methods
2.1 Study population
NHANES is an ongoing periodic cross-sectional sample survey conducted by the National Center for Health Statistics (NCHS) within the U.S. Centers for Disease Control and Prevention (CDC). This survey aims to collect data from a nationally representative sample of the non-institutionalized civilian population. Mortality data are sourced from the NDI database maintained by the CDC. This study analyzed data from all 11 consecutive survey cycles of NHANES conducted between 1999 and 2018, focusing specifically on non-pregnant adults aged 18 years and older, while excluding individuals with incomplete data. Participants were followed until their death or until December 31, 2019. Ultimately, 4,403 eligible participants were included in the analysis to investigate the relationship between AIP and all-cause mortality within the CKD population (Figure 1).
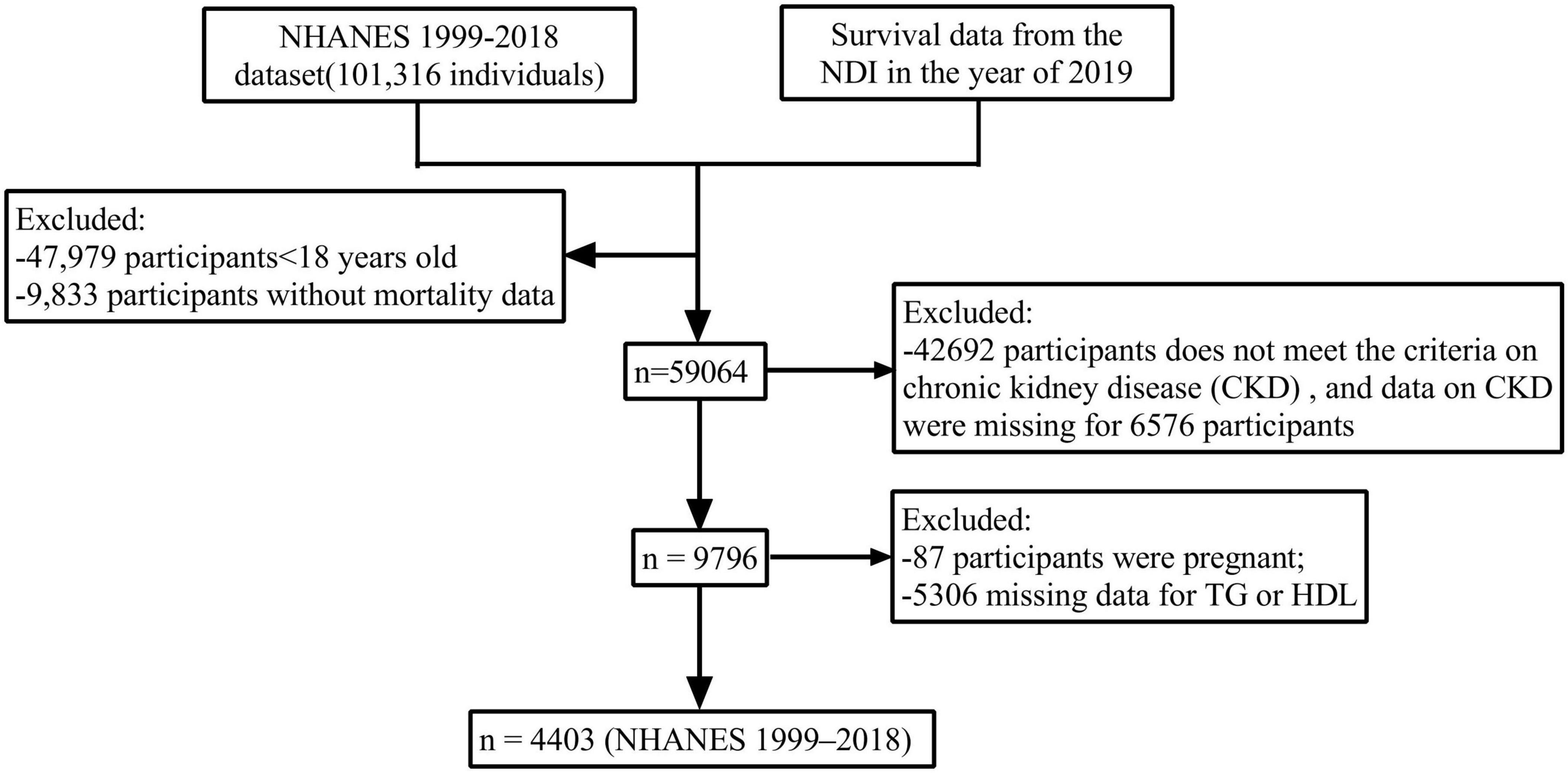
Figure 1. Flow chart of participant selection from the NHANES 1999–2018 dataset. A total of 4,403 adults with chronic kidney disease (CKD) were included in the final analysis. Exclusion criteria included age < 18 years, missing mortality data, incomplete CKD diagnostic criteria, pregnancy, and missing TG/HDL-C measurements.
For details on survey design and data acquisition, please refer to the NHANES website at https://www.cdc.gov/nchs/nhanes/ (accessed January 5, 2025). The NHANES research plan has received approval from the NCHS Ethics Review Board, as outlined at https://www.cdc.gov/nchs/nhanes/about/erb.html?CDC_AA_refVal=https://www.cdc.gov/nchs/nhanes/irba98.htm. Furthermore, All participants provided written informed consent for the use of their data, and all experiments conducted in this study adhered to relevant guidelines and regulations.
CKD can be defined by two primary criteria: firstly, a glomerular filtration rate (GFR) of less than 60 mL/(min⋅1.73 m2); and secondly, the presence of one or more indicators of kidney damage, which include: (a) albuminuria, defined as a urine albumin-to-creatinine ratio (ACR) of ≥ 30 mg/g; (b) abnormal urinary sediment; (c) electrolyte or other abnormalities resulting from renal tubular dysfunction; (d) renal histological abnormalities; and (e) renal structural abnormalities identified through imaging examinations. A diagnosis of CKD can be made if any of these conditions persist for at least 3 months (19). The severity of CKD is categorized into five stages based on the estimated glomerular filtration rate (eGFR): G1 (eGFR ≥ 90 mL/min/1.73 m2), G2 (eGFR 60–89 mL/min/1.73 m2), G3 (eGFR 30–59 mL/min/1.73 m2), G4 (eGFR 15–29 mL/min/1.73 m2), and G5 (eGFR < 15 mL/min/1.73 m2). Each stage reflects varying degrees of renal function impairment (20).
2.2 Study variables
2.2.1 Exposure variable
The calculation formula for the AIP is expressed as follows: AIP = Log [TG (mmol/L)/HDL-C (mmol/L)] (21). This indicator is derived from peripheral blood samples collected after a fasting period of at least 8 hours in the morning, during which TG and HDL-C levels were measured. Following the standardized protocol established by the CDC, serum HDL-C levels were assessed using either a direct immunoassay or a precipitation method, while serum TG levels were measured using enzymatic methods (22). Based on the quartiles of AIP, the study population was categorized into four groups: Q1 group (≤ −0.19), Q2 group (−0.19 to 0.02), Q3 group (0.02–0.24), and Q4 group (> 0.24), for subsequent analysis of the association between AIP and mortality.
2.2.2 Covariates
The included demographic characteristics encompass age, gender, race, family poverty-income ratio (FPIR), marital status, body mass index (BMI), education level (categorized as below high school, high school graduate or equivalent, and above high school), drinking habits (classified as never, former, mild, moderate, and severe), and smoking status (comprising never smokers, former smokers, and current smokers). Laboratory test data consist of white blood cell count (WBC), neutrophils, hemoglobin, creatinine, uric acid, blood urea nitrogen, TG, total cholesterol (TC), HDL-c, low-density lipoprotein cholesterol (LDL-c), C-reactive protein (CRP), estimated glomerular filtration rate (eGFR), and systemic immune inflammatory index (SII). Additionally, chronic comorbidities such as diabetes, hypertension, cardiovascular disease, hyperlipidemia, and the use of lipid-lowering medications were documented. The household poverty-income ratio is determined by comparing total household income to the poverty line. Smoking status is classified into three categories: current smokers (more than 100 cigarettes in lifetime and currently smoking all or some days), former smokers (having smoked more than 100 cigarettes throughout life but having quit currently), and never smokers (having smoked less than 100 cigarettes throughout life).
2.3 Mortality outcome of the study population
The mortality data for this study were obtained from the NDI as of December 31, 2019. The database utilized the 10th revision of the International Classification of Diseases and Related Health Problems (ICD-10) to record the leading causes of death. The aim of this study was to investigate the effect of AIP levels on all-cause and cardiovascular mortality. All-cause mortality refers to death from any cause during the follow-up period. Cardiovascular mortality was identified using the International Classification of Diseases, 10th Revision codes I00–I09, I11, I13, I20-I51, or I60–I69. Deaths due to causes such as infection (ICD-10: J09-J18, or J40-J47) and cancer (CA) (ICD-10: C00-C97) were analyzed as additional competing outcomes (23, 24).
2.4 Statistical analysis
Given that the NHANES database employs a complex, multistage, stratified sampling design, we adhered to the guidelines established by the CDC and utilized appropriate weighting methods to address this complexity. All analyses were weighted to account for issues arising from complex survey designs, ensuring that the estimates were representative of the general population (19). For continuous variables, we report the weighted mean ± standard error (SE) and median (interquartile range), while for categorical variables, we present estimated proportions. We employed analysis of variance (ANOVA) or the rank sum test to evaluate group differences for continuous variables, and the chi-square test for categorical variables. To compare survival estimates and cumulative incidence rates, we employed both the Kaplan-Meier method and competing risk models. After confirming the proportional hazards assumption for the influence of AIP on survival risk through the Schoenfeld residual test, we conducted multivariable Cox proportional hazards regression analyses to assess the association between AIP levels and all-cause mortality. The results were expressed as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). Additionally, we implemented the Fine-Gray competing risk regression model to examine the relationship between AIP levels and CVD mortality, presenting the findings as subdistribution hazard ratios (SHRs) with 95% CIs. Furthermore, a post hoc power analysis, based on observed event rates and effect sizes, confirmed that our sample size was sufficient to detect significant associations (α = 0.05, power = 80%).
Analytical models were refined incrementally to address potential confounding factors. The Crude Model did not adjust for any covariates, while Model 1 included adjustments for gender, age, and race. Model 2 further adjusted for smoking status, drinking status, education levels, marital status, FPIR, hypertension, diabetes, history of cardiovascular disease, history of lipid-lowering drug use, CRP, SII, LDL-c, eGFR, and additional covariates from Model 1. Using patients in the lowest weighted quartile of AIP as the reference group, we calculated the HR, 95% CI, and P for trend regarding the risk of all-cause mortality, as well as the SHR, 95% CI, and P for trend for CVD mortality. Restricted cubic spline plots were employed to illustrate the association between AIP as a continuous variable and all-cause mortality, while likelihood ratio tests were utilized to assess potential non-linear relationships. Subgroup analyses were conducted based on age, gender, BMI, race, FPIR, hypertension, diabetes, and education level. To address potential reverse causality, we conducted sensitivity analyses excluding deaths within the initial two follow-up years. Prognostic performance was assessed through the concordance index (C-index), with incremental value quantified using Integrated Discrimination Improvement (IDI) and Net Reclassification Improvement (NRI) metrics relative to established risk factors. Statistical analyses were performed using R statistical software (version 4.3.2; R Foundation for Statistical Computing, Vienna, Austria), with two-sided P-values < 0.05 considered statistically significant.
3 Results
3.1 Baseline characteristics of study participants
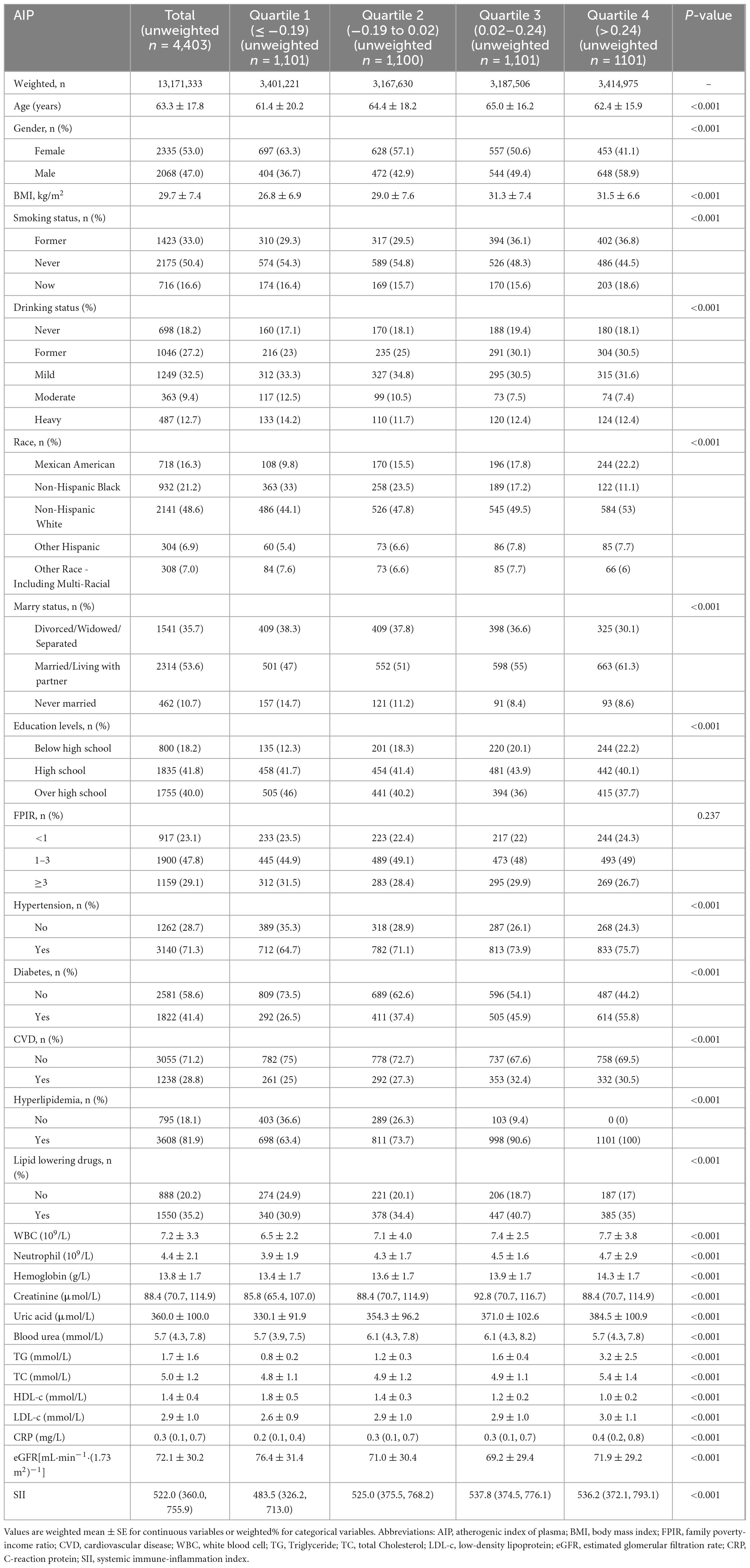
A total of 4,403 patients diagnosed with CKD were encompassed in this study. The weighted median age of the participants was 63.3 years, with 53.0% being female. Table 1 presents the baseline characteristics of the patients in accordance with the weighted quartiles of AIP. The weighted median of AIP was 0.20 (interquartile range: −0.19 to 0.24). The study findings demonstrated that patients with elevated AIP values generally exhibited higher body mass index (BMI), white blood cell count, neutrophil count, C-reactive protein level, systemic inflammation index (SII), uric acid and low-density lipoprotein cholesterol (LDL), while having lower estimated glomerular filtration rate (eGFR). Furthermore, such patients were more likely to have lower educational attainment, be married or cohabiting with a partner, mainly distributed in the Mexican American and non-Hispanic white populations, and had a higher prevalence of hypertension, diabetes, and cardiovascular diseases (including coronary heart disease). In contrast, patients with lower AIP values tended to be younger.
3.2 AIP and mortality in CKD participants
During a median follow-up period of 83 months (interquartile range: 43–137 months), a total of 416,840 person-months of observation data were collected. During this period, 1,767 patients with CKD succumbed to all-cause mortality, including 526 deaths attributable to CVD, 305 due to malignant neoplasms, and 109 resulting from infectious diseases. Results from Kaplan-Meier survival analysis and Fine-Gray competing risk models demonstrated that CKD patients in the highest quartile (Q4) of the AIP exhibited significantly elevated risks of both all-cause mortality and CVD mortality compared to those in lower AIP groups (P < 0.05), as illustrated in Figure 2. Furthermore, Fine-Gray competing risk analysis revealed no significant differences in CA-related or infection-related mortality between CKD patients in the Q4 and those in the lower quartiles (P > 0.05), as detailed in Supplementary Figure 1.
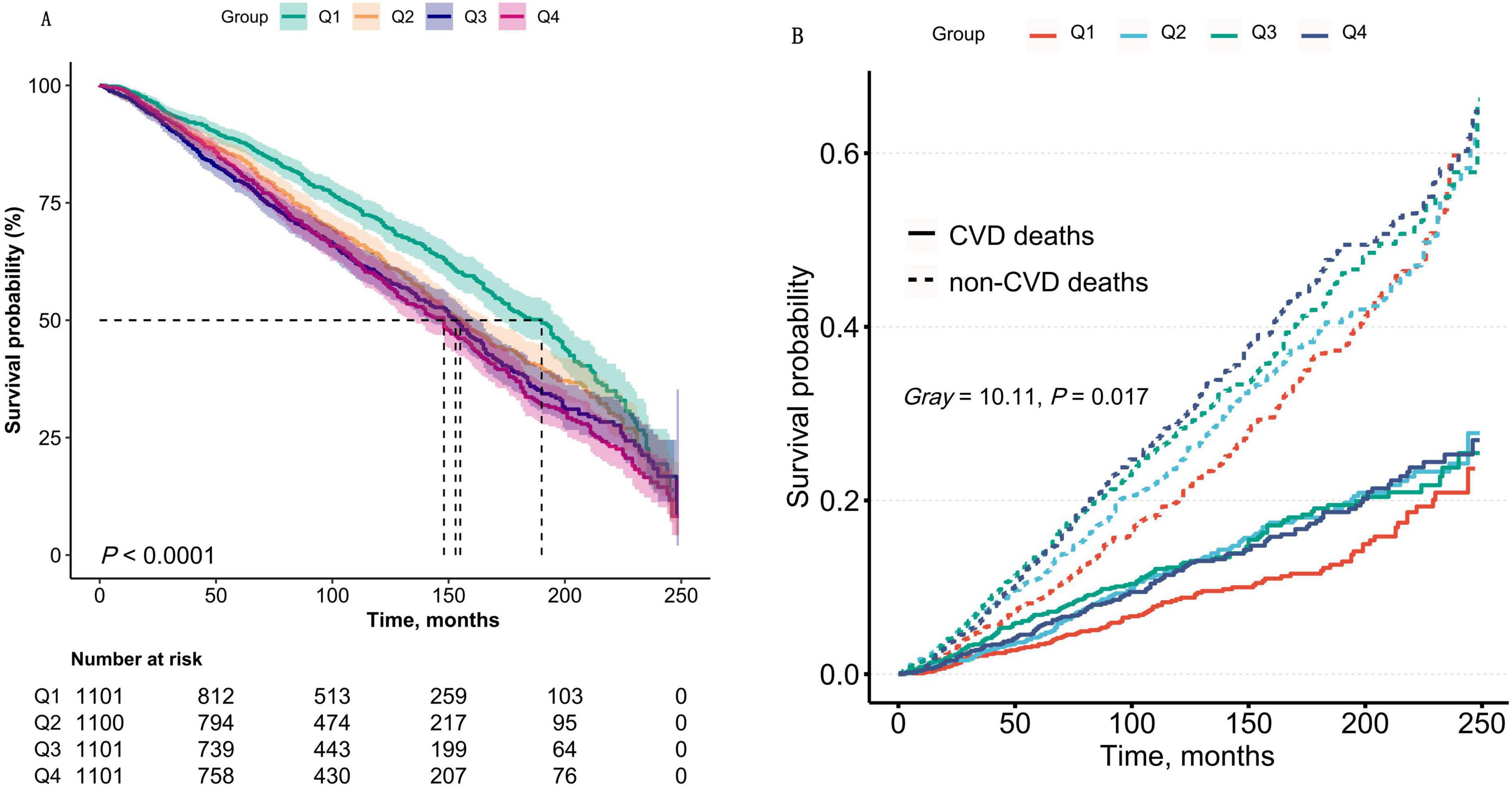
Figure 2. (A) Kaplan–Meier survival curves for all-cause mortality stratified by AIP quartiles (Q1–Q4) in CKD patients. The log-rank test demonstrated a significant difference in survival across quartiles (P < 0.0001). Q1: AIP ≤ -0.19; Q2: - 0.19 to 0.02; Q3: 0.02–0.24; Q4: > 0.24. (B) Cumulative incidence of CVD mortality analyzed using Fine-Gray competing risk models, accounting for non-CVD deaths as competing events. The Gray’s test confirmed significant differences in CVD mortality risk across AIP quartiles (P < 0.0001). Shaded areas represent 95% confidence intervals. Data were weighted to represent the U.S. population.
Table 2 presents detailed hazard ratios (HRs) and 95% CIs for the association between the AIP and all-cause mortality, as well as subdistribution hazard ratios (SHRs) and 95% CIs for cardiovascular mortality, including both unadjusted and multivariable-adjusted analyses. After adjusting for sociodemographic factors, laboratory parameters, health-related lifestyle behaviors, and comorbidities, AIP was found to be significantly associated with an increased risk of all-cause mortality in CKD patients (HR: 2.03, 95% CI: 1.62–2.55, P < 0.001) (Table 2). Additionally, the results indicate a significant positive association between AIP and cardiovascular mortality risk (HR: 1.60, 95% CI: 1.08–2.39, P = 0.02). These findings suggest that each unit increase in AIP corresponds to a 103% higher risk of all-cause mortality and a 60% higher risk of cardiovascular mortality among CKD patients.
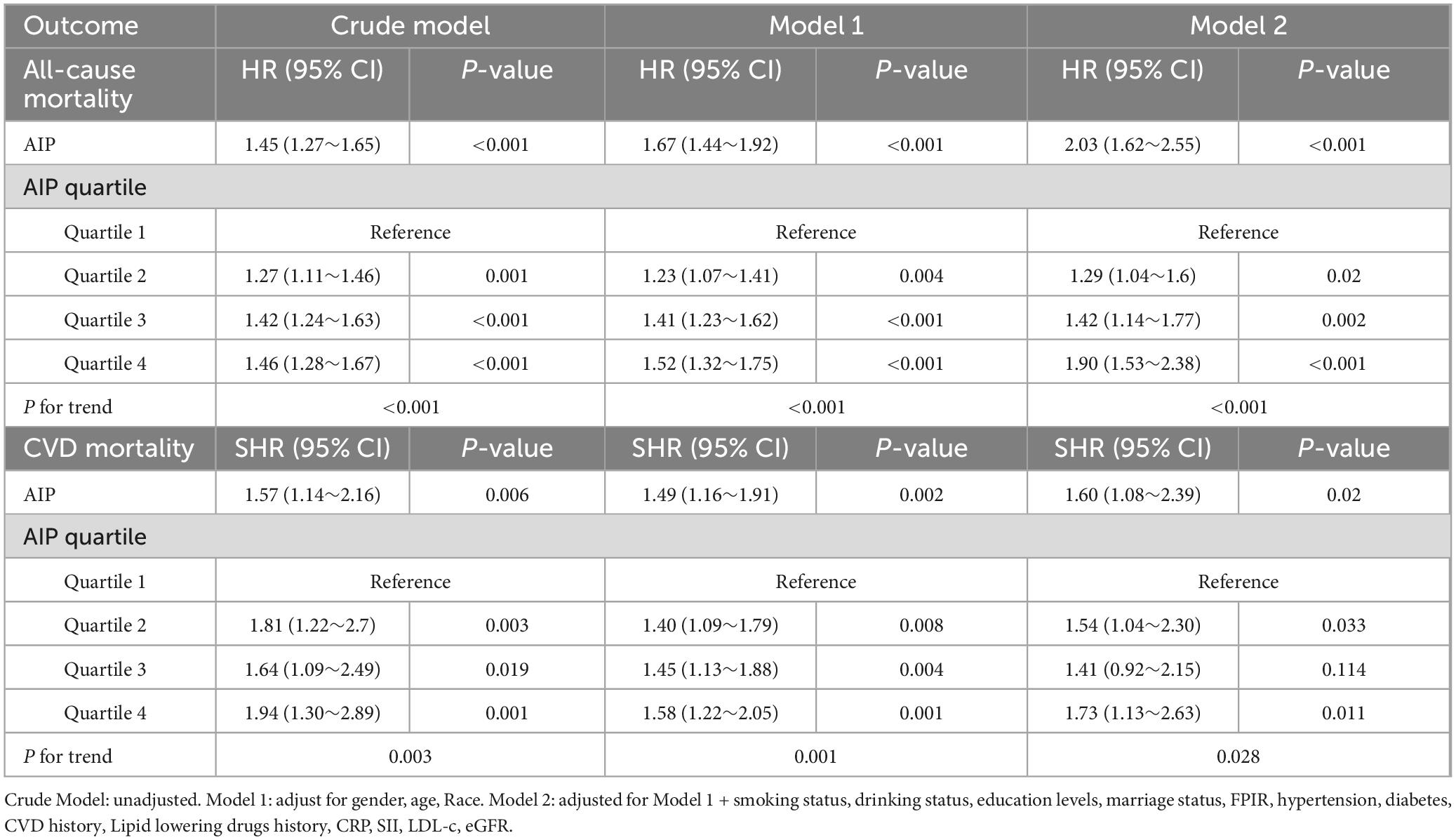
Table 2. Association between AIP and all-cause mortality (Cox regression model), and AIP and CVD mortality (Fine-Gray competing risk model).
When analyzed as a categorical variable, the results are summarized in Table 2. Across the ascending AIP categories (0 to −0.19, >−0.19 to 0.02, > 0.02–0.24, and > 0.24), the weighted estimated hazard ratios (HRs, 95% CIs) for all-cause mortality in CKD patients demonstrated a significant dose-response relationship (P for trend < 0.001). Similarly, the weighted competing risk regression analysis indicated a progressively increasing risk of cardiovascular mortality after multivariable adjustment (P for trend < 0.001). These findings provide robust evidence that elevated AIP is significantly associated with higher mortality risk in CKD patients (P < 0.001).
3.3 The relationship between lead concentration and CKD mortality
A multivariate-adjusted weighted restricted cubic spline analysis revealed a significant linear relationship between AIP and the risk of all-cause mortality (P for non-linearity = 0.243). This finding indicates that higher AIP levels are associated with an increased risk of all-cause mortality, as shown in Figure 3.
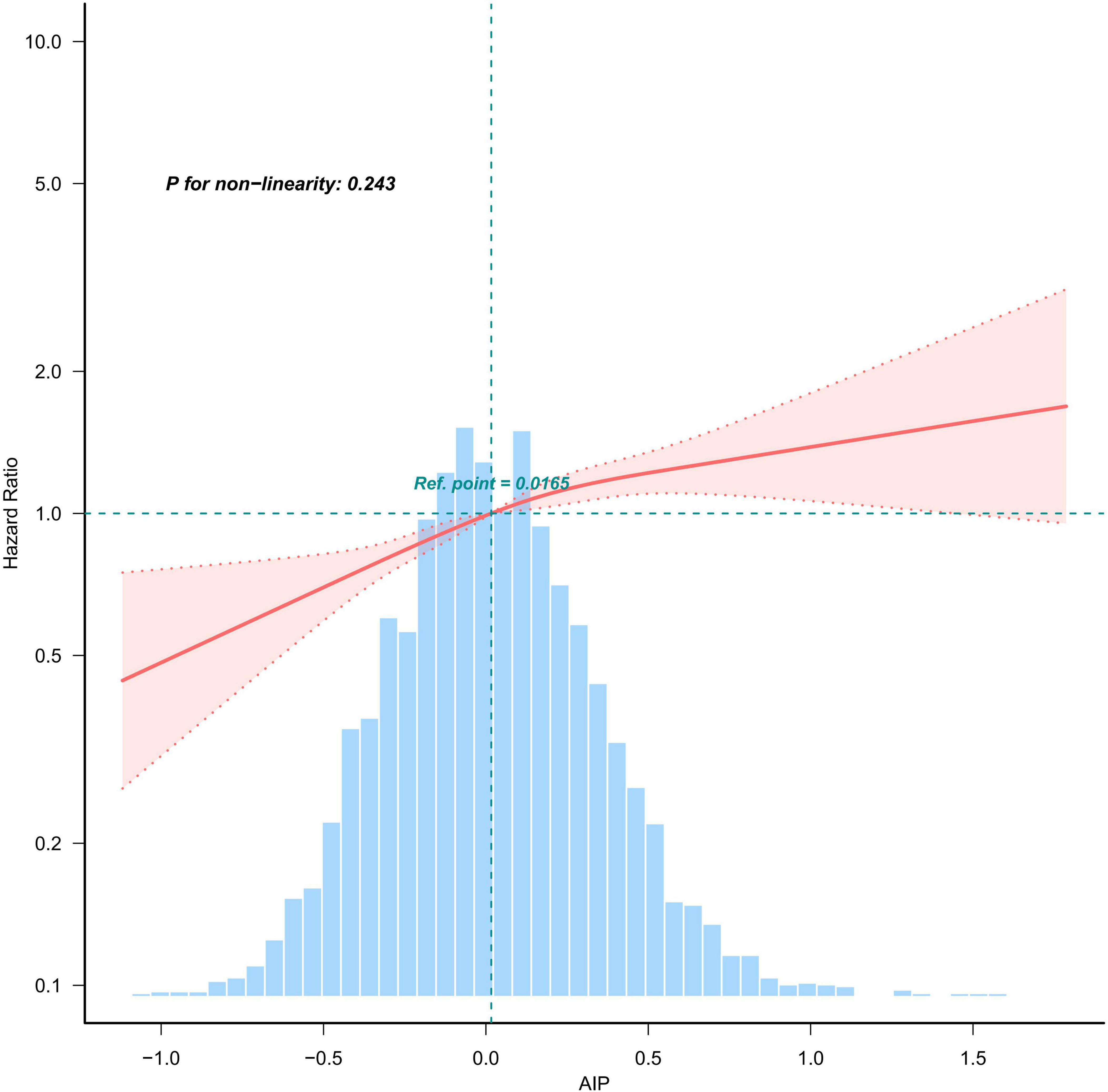
Figure 3. Weighted restricted cubic spline plot showing the linear relationship between AIP (continuous) and all-cause mortality with CKD. The weighted restricted cubic spline model was adjusted for the variables listed in the fully adjusted model in Table 2. The solid red line represents the HR derived from the weighted multivariable Cox model, while shadows represent corresponding 95% CIs of the adjusted HR. The blue histogram illustrates the frequency distribution across various intervals of AIP content.
3.4 Subgroup analysis
This study performed a subgroup analysis to investigate the relationships between AIP values and the risks of all-cause and cardiovascular mortality across various demographic characteristics and comorbid conditions, as detailed in Table 3. Stratifications were conducted by age (< 60 years vs. ≥ 60 years), sex (male vs. female), body mass index (BMI; < 25 kg/m2, 25–30 kg/m2, ≥ 30 kg/m2), FPIR (< 1, 1–3, ≥ 3), race, educational level, hypertension, and diabetes mellitus (DM). The analysis revealed that the association between AIP and cardiovascular mortality was consistently observed across all subgroups (all P for interaction > 0.05). After adjusting for confounding factors, the subgroup findings remained broadly consistent with the primary results. Notably, sex, BMI, and DM were identified as significant interaction factors influencing the relationship between AIP and all-cause mortality (P for interaction < 0.05). Further details are provided in Table 3.
3.5 Sensitivity analysis
Additionally, a sensitivity analysis excluding participants who died within the first 2 years of follow-up yielded results consistent with the primary analysis, further reinforcing the robustness of the study findings (see Table 4). These results indicate a positive association between elevated AIP levels and the risks of both all-cause and cardiovascular mortality.
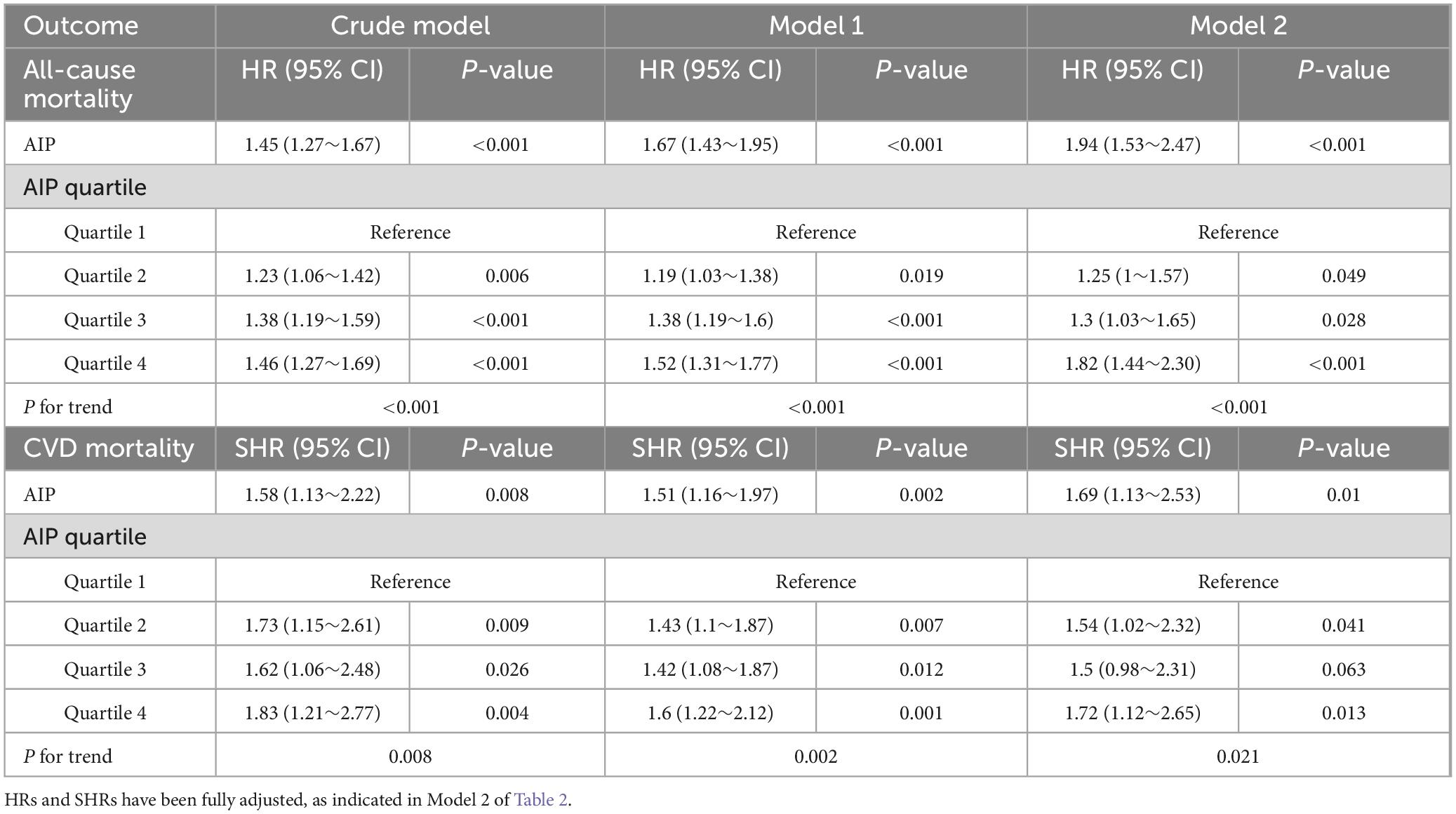
Table 4. Association between AIP and all-cause mortality as well as CVD mortality, excluding participants who died within the first 2 years of follow-up (n = 4,176).
3.6 The forecast value of AIP for mortality
Based on previously identified factors (gender, age, smoking status, drinking status, hypertension, diabetes, CVD history, CRP, SII, LDL-c, eGFR) that influence all-cause mortality prognosis, as well as commonly debated risk factors for adverse CKD prognosis (education levels, marriage status, FPIR, lipid-lowering drugs history), we defined this as the traditional factors model. After incorporating the AIP model, it was renamed the traditional factors + AIP model. The statistical analysis demonstrated that AIP alone produced a C-index of 0.713 (95% CI: 0.571–0.855, P = 0.007) for predicting all-cause mortality. Although AIP exhibited moderate discriminative ability independently, its incremental prognostic value became more apparent when incorporated into the traditional factors model, improving the C-index from 0.811 to 0.823, with an NRI of 0.376 (P < 0.001) and an IDI of 0.025 (P < 0.001) (Supplementary Table 1). These results underscore AIP as a complementary, rather than substitutive, biomarker to existing prognostic models, supporting its role as an independent indicator of residual mortality risk in CKD.
4 Discussion
In this prospective cohort study based on NHANES data, which included 4,403 participants, we identified a significant association between baseline AIP and mortality risk in patients with CKD. This relationship remained robust for both all-cause and cardiovascular mortality, even after extensive adjustment for a wide range of potential confounding factors. The robustness of these findings was further corroborated through restricted cubic spline analysis, subgroup analyses, and sensitivity tests. This discovery broadens the application of AIP as a prognostic marker in patients with CKD. Moreover, we also verified that adding the AIP value to the traditional risk factor model could improve the predictive capacity for all-cause mortality. In brief, this research demonstrates that a reduced AIP value may be a risk factor for poor prognosis in patients with CKD.
The present study demonstrates that higher AIP quartiles are significantly associated with increased mortality risk in patients with CKD compared to those in the lowest quartile. While investigations into the specific relationship between AIP and CKD remain limited, the association between dyslipidemia and CKD has been widely established (25, 26). However, existing evidence is not entirely consistent. For example, You et al. reported a significant correlation between elevated AIP levels and an increased risk of CKD (27). Similarly, other studies have linked higher AIP levels to increased mortality among patients with diabetes, findings that are in agreement with our results (28).
In contrast, a study conducted in dialysis patients revealed a U-shaped relationship between AIP levels and all-cause mortality, with both the lowest quintile (≤ 0.20) and the highest quintile (≥ 0.71) independently associated with increased mortality risk (29). The pathophysiological mechanisms underlying this observation are incompletely understood but may involve the unique pathophysiological characteristics of dialysis patients, who are typically in the late stages of kidney disease and experience profound metabolic and physiological derangements. Patients in the lowest AIP quintile may suffer from more severe malnutrition and inflammatory states, contributing to heightened mortality risk. Moreover, the coexistence of severe cardiovascular disease, anemia, and electrolyte imbalances—common in dialysis patients—may confound the prognostic value of AIP.
By contrast, the participants in this study were non-dialysis CKD patients, predominantly in the early to middle stages of the disease, with relatively fewer pathological and comorbid complications. This distinction highlights the potential modulatory role of CKD stage and comorbid conditions in the relationship between AIP and patient outcomes. These findings underscore the need for future research to elucidate the differential impact of AIP across various CKD stages and clinical contexts.
Although the precise mechanisms underlying the increased risk of CKD-related mortality associated with AIP remain incompletely understood, several potential explanations exist. Firstly, as a key indicator of lipid metabolism dysregulation, AIP reflects the ratio of TG to HDL-C, which plays a pivotal role in the progression of CKD. Elevated TG levels promote the formation of small, dense low-density lipoprotein cholesterol (LDL-C) particles, which are more prone to oxidative modification (30, 31), thereby enhancing the risk of atherosclerosis. In patients with CKD, impaired renal function disrupts normal lipid metabolism, and elevated AIP further exacerbates this dysregulation. Oxidized LDL-C particles are engulfed by macrophages, transforming into foam cells that accumulate in the subendothelial space, ultimately contributing to the formation of atherosclerotic plaques. As these plaques grow and become unstable, vascular lumen narrowing and blood flow obstruction increase, heightening the risk of cardiovascular events such as myocardial infarction and stroke, directly leading to an increased mortality rate from CVD. Secondly, insulin resistance (IR), which is closely associated with elevated AIP and commonly observed in CKD patients (32, 33), further complicates this relationship. Elevated AIP can impair insulin signaling pathways, reducing cellular sensitivity to insulin and triggering IR. In an IR state, the body compensates by secreting more insulin to maintain glucose homeostasis, resulting in hyperinsulinemia. This condition activates the sympathetic nervous system, inducing vasoconstriction and elevated blood pressure, while also stimulating vascular smooth muscle cell proliferation and migration, accelerating the atherosclerotic process, and increasing the risk of CVD (34–36). Moreover, IR disrupts renal hemodynamics, raising intraglomerular pressure and exacerbating renal function decline, thereby creating a vicious cycle (37) that ultimately elevates both all-cause and CVD mortality rates.
Furthermore, inflammatory responses play a crucial role in AIP-mediated adverse outcomes in CKD patients. Table 1 of this study demonstrates that inflammatory markers, including white blood cell count (WBC), C-reactive protein (CRP), and systemic immune-inflammation index (SII), are significantly elevated in the higher AIP quartiles (Q2-Q4) compared to the Q1 group. Chronic inflammation is a well-established feature of CKD (38, 39), and elevated AIP may further activate immune cells such as monocytes and macrophages. These cells release a multitude of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), thereby triggering systemic inflammation. Inflammatory cytokines can directly damage endothelial cells, compromising vascular integrity and function, which promotes thrombus formation. Additionally, these cytokines stimulate the proliferation of smooth muscle cells and extracellular matrix production, leading to thickening and stiffening of the vascular wall, worsening atherosclerosis, and increasing the likelihood of CVD events, thereby raising mortality rates. Locally, inflammation exacerbates damage to the glomeruli and renal tubules, accelerating renal function deterioration (40), thereby contributing to an increased risk of all-cause mortality. Furthermore, endothelial dysfunction represents a critical link in the effect of AIP on mortality in CKD patients. The dyslipidemia and inflammatory responses induced by AIP collaborate to impair endothelial cell function (41). A reduction in vasodilators such as nitric oxide (NO) and an increase in vasoconstrictors like endothelin-1 (ET-1) result in impaired vascular dilation and enhanced constriction, creating hemodynamic abnormalities (42). Endothelial dysfunction also facilitates the deposition of lipids on the vascular wall, promoting the progression of atherosclerosis and elevating the risk of CVD events, leading to increased CVD mortality (43). Moreover, endothelial dysfunction in the renal microvasculature can compromise glomerular filtration and tubular reabsorption, accelerating renal function decline and indirectly increasing the risk of all-cause mortality (44).
Additionally, the AIP-related lipid metabolism dysregulation in CKD involves complex molecular mechanisms that warrant further exploration. Emerging evidence suggests that elevated AIP reflects phospholipid dysregulation in CKD, driven by phospholipase A2(PLA2)-mediated generation of proinflammatory lysophospholipids such as lysophosphatidylcholine (LysoPC) and lysophosphatidic acid (LysoPA) (45). These metabolites not only promote endothelial dysfunction but also accelerate foam cell formation, representing critical steps in atherosclerosis pathogenesis. Furthermore, vitamin D deficiency exacerbates lipid accumulation by impairing mitochondrial fatty acid oxidation (FAO) in renal tubular cells and macrophages, thereby elevating AIP levels and contributing to systemic inflammation (46). Conversely, Klotho, a renal protective protein, inversely correlates with AIP through antioxidative and anti-inflammatory pathways, mitigating fibrosis and oxidative stress in CKD (47). Another key player is adipose triglyceride lipase (ATGL), which maintains FAO and mitochondrial function to reduce lipotoxicity and fibrosis; its deficiency promotes lipid droplet accumulation and inflammatory cytokine release (48). Collectively, these findings highlight PLA2hyperactivity, vitamin D deficiency, Klotho loss, and ATGL dysfunction as pivotal determinants of AIP elevation in CKD. Targeting these pathways may offer novel therapeutic strategies to mitigate cardiovascular complications in this population.
In summary, AIP contributes to an increased risk of both all-cause and cardiovascular mortality in CKD patients through multiple mechanisms, including lipid metabolism disturbances, induction of insulin resistance, activation of inflammatory responses, and endothelial dysfunction. These pathways are interrelated, creating a complex pathological network that drives CKD progression. This further underscores the clinical significance of AIP as a prognostic marker in CKD, offering potential targets for future comprehensive treatment strategies aimed at reducing AIP levels and mitigating its adverse effects, ultimately improving long-term survival outcomes for CKD patients.
The interaction analysis in this study revealed significant interactions between gender, BMI, and DM in their effect on all-cause mortality, particularly among women and individuals with higher BMI, where these factors exhibited a more pronounced influence on all-cause mortality. Surprisingly, the predictive value of AIP for all-cause mortality was not statistically significant in the diabetic group when compared to the non-diabetic group. This may be attributed, in part, to the prevalent insulin resistance observed in diabetic patients, which could alter their lipid profile and thus impair AIP’s ability to adequately reflect lipid abnormalities in this population. As a result, the relationship between AIP and all-cause mortality may be attenuated in diabetic individuals. Furthermore, diabetic patients often undergo more intensive medical interventions, including antidiabetic medications, statins, and other cardiovascular protective measures, which may lower AIP levels, thereby diminishing its prognostic value. Additionally, compared to diabetic individuals, the non-diabetic population is less affected by factors such as insulin resistance and multiple comorbidities. AIP changes in this group are more likely to be driven by traditional lipid metabolism abnormalities (e.g., hypertriglyceridemia, low HDL-C levels), which are more directly and clearly associated with all-cause mortality. Thus, the effect of AIP on all-cause mortality may be more readily apparent in the non-diabetic cohort. Finally, other potential mechanisms warrant further investigation. Notably, a separate study has indicated that although the predictive value of AIP in diabetic patients may be influenced by other factors, it retains incremental prognostic value in individuals with acute coronary syndrome and can serve as a vital tool for risk stratification and prognosis assessment (49), which aligns with the subgroup analysis results of our current study regarding CVD mortality.
The AIP is derived from routine lipid measurements, specifically TG and HDL-C, and requires no additional cost or specialized testing. Its ease of calculation offers excellent clinical applicability, enabling effective implementation even in environments with relatively limited medical resources. Compared to traditional methods that measure TG or HDL-C separately, AIP integrates two key indicators of lipid metabolism. By quantifying the dynamic balance between TG and protective HDL-C, this index provides a more precise assessment of residual cardiovascular risk in patients with CKD. Particularly in the CKD population with LDL-C within the reference range but exhibiting lipid metabolism disorders, AIP demonstrates superior risk prediction capabilities compared to traditional lipid parameters. Furthermore, while traditional markers for predicting adverse outcomes in CKD, such as the urine albumin-to-creatinine ratio (UACR) and eGFR, have been well validated, these parameters primarily reflect renal structural damage and functional decline. In contrast, AIP reflects systemic lipid metabolism disorders, representing a unique pathophysiological pathway associated with cardiovascular complications and inflammation in CKD, which cannot be fully captured by UACR or eGFR alone. Consequently, AIP serves as a complementary assessment tool for the pathological processes not addressed by traditional renal function markers, thereby enhancing the overall prognostic assessment for patients with CKD.
Our study presents several strengths. First, the sample exhibits strong representativeness. The NHANES database employs a complex weighting design, and this study utilized data from 1999 to 2018, encompassing a broad spectrum of adult CKD patients in the United States. This robust sample is well-suited to reflect the overall population characteristics, thereby enhancing the generalizability of the findings. Second, the measurement of variables is precise. AIP and a range of covariates, including demographic information, laboratory markers, and chronic comorbidities, were measured using standardized methodologies. Data collection was performed by trained professionals following standardized protocols, and blood samples were analyzed in the same laboratory, minimizing bias and ensuring high data quality, thereby strengthening the credibility of the findings. Third, the analytical methods employed were comprehensive. Various adjustments for potential confounders, along with stratified analyses, were utilized to ensure the reliability and consistency of the results.
However, there are certain limitations in our study. First, It is crucial to emphasize that this study represents an observational cohort design, and as such, it is not possible to ascertain a causal relationship between AIP and mortality outcomes among CKD patients. Despite our adjustment for an extensive array of potential confounders, residual confounding factors, including unmeasured lifestyle variables or genetic influences, might still affect the observed associations. Further interventional studies are warranted to investigate whether modulating AIP levels can directly enhance clinical outcomes in CKD patients. Second, Our analysis primarily utilized baseline measurements of covariates, including AIP, comorbidities, and medication use. While this approach is common in cohort studies, it does not account for potential dynamic changes during follow-up, such as the onset of new diabetes, decline in eGFR, or initiation of lipid-lowering therapies. These time-varying factors may confound the observed associations, particularly in progressive diseases like CKD, where risk profiles evolve over time. This limitation is inherent to datasets like NHANES, which lack repeated measurements of covariates during follow-up. Future studies with longitudinal biomarker and comorbidity data could refine these findings. Third, while NHANES mortality linkage via the NDI provides robust follow-up data, participants are not actively monitored over time, and certain subgroups (e.g., transient populations) may be underrepresented. However, NHANES’s rigorous sampling methodology and weighting adjustments help ensure generalizability to the U.S. non-institutionalized CKD population. Fourth, while our analysis of CVD mortality accounted for non-CVD deaths using the Fine-Gray model, it is important to note that all-cause mortality inherently accounts for competing risks (e.g., non-CVD deaths). Future studies should explore other competing outcomes (e.g., infection, malignancy) in larger CKD cohorts to comprehensively evaluate the impact of AIP on mortality. Finally, a notable limitation of this study is the absence of external validation in an independent CKD cohort. While NHANES provides robust, population-representative data, replication in diverse cohorts (e.g., Asian or European populations) is essential to confirm the generalizability of our findings. Future studies should prioritize validation in independent datasets to further solidify the prognostic role of AIP in CKD. Notwithstanding this limitation, our study benefits from a large, ethnically diverse sample and long follow-up period, providing robust evidence for the association between AIP and mortality in U.S. CKD patients. The consistency of results across subgroup analyses further supports the reliability of our findings.
5 Conclusion
Based on data from the U.S. NHANES spanning from 1999 to 2018, this study reveals a significant association between AIP and the risk of all-cause mortality as well as CVD mortality in patients with CKD. A significant positive correlation was identified between AIP and mortality risk, which remained robust whether AIP was analyzed as a continuous variable or categorized into quartiles. Incorporating AIP into clinical risk stratification models may enhance the identification of CKD patients at elevated risk for mortality, enabling targeted interventions such as lipid-lowering therapies and lifestyle modifications. However, critical knowledge gaps persist, including the absence of validated AIP-specific therapeutic targets and randomized controlled trials (RCTs) assessing the efficacy of AIP-driven treatment protocols. Prospective validation studies are warranted to determine optimal AIP thresholds and evaluate the impact of AIP-lowering interventions on clinical outcomes. For patients with elevated AIP of Plasma, clinicians should prioritize lifestyle modifications, such as adopting a Mediterranean diet and engaging in aerobic exercise (50). Furthermore, pharmacological interventions, including fibrates and omega-3 fatty acids, should be considered to lower triglyceride levels (51). Intensified monitoring for cardiovascular events and potential decline in renal function is also recommended.
In conclusion, our study demonstrates that AIP is associated with adverse outcomes in CKD and may be considered a potential biomarker for risk assessment. However, the observational nature of our analysis precludes causal inferences. Future research should focus on mechanistic studies elucidating AIP’s pathophysiological role and interventional trials testing AIP-guided therapy. These efforts may ultimately refine prognostic models and inform precision medicine approaches in CKD management.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: For details on survey design and data acquisition, please refer to the NHANES website at https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving human participants were reviewed and approved by NHANES was implemented by the NCHS of the US CDC and approved by the National Center for Health Statistics Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LL: Writing – original draft. JZ: Writing – original draft. MY: Writing – review & editing. HJ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank Zhang Jing (Second Department of Infectious Diseases, Fifth People’s Hospital of Fudan University) for her work on the NHANES database. His excellent work on the nhanesR package and webpage makes it easier for us to explore the NHANES database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1575657/full#supplementary-material
References
1. Hockham C, Bao L, Tiku A, Badve S, Bello A, Jardine M, et al. Sex differences in chronic kidney disease prevalence in Asia: A systematic review and meta-analysis. Clin Kidney J. (2022) 15:1144–51. doi: 10.1093/ckj/sfac030
2. Chen X, Wang J, Lin Y, Yao K, Xie Y, Zhou T. Cardiovascular outcomes and safety of SGLT2 inhibitors in chronic kidney disease patients. Front Endocrinol (Lausanne). (2023) 14:1236404. doi: 10.3389/fendo.2023.1236404
3. Zilli M, Borovac-Pinheiro A, Costa M, Surita F. Perinatal outcomes in women with chronic kidney diseases. Rev Bras Ginecol Obstet. (2022) 44:1094–101. doi: 10.1055/s-0042-1753546
4. Kibria G, Crispen R. Prevalence and trends of chronic kidney disease and its risk factors among US adults: An analysis of NHANES 2003-18. Prev Med Rep. (2020) 20:101193. doi: 10.1016/j.pmedr.2020.101193
5. Zhao B, Hu X, Wang W, Zhou Y. Cardiorenal syndrome: Clinical diagnosis, molecular mechanisms and therapeutic strategies. Acta Pharmacol Sin. (2025): doi: 10.1038/s41401-025-01476-z Online ahead of print.
6. Lecamwasam A, Mansell T, Ekinci E, Saffery R, Dwyer K. Blood plasma metabolites in diabetes-associated chronic kidney disease: A focus on lipid profiles and cardiovascular risk. Front Nutr. (2022) 9:821209. doi: 10.3389/fnut.2022.821209
7. Wang S, Li L, Fu C, Lee Y, Kuo H, Hsu C. Trajectory of low-density lipoprotein cholesterol in patients with chronic kidney disease and its association with cardiovascular disease. Front Cardiovasc Med. (2022) 9:887915. doi: 10.3389/fcvm.2022.887915
8. Padalkar M, Tsivitis A, Gelfman Y, Kasiyanyk M, Kaungumpillil N, Ma D, et al. Paradoxical reduction of plasma lipids and atherosclerosis in mice with adenine-induced chronic kidney disease and hypercholesterolemia. Front Cardiovasc Med. (2023) 10:1088015. doi: 10.3389/fcvm.2023.1088015
9. Wang Y, Liu T, Liu W, Zhao H, Li P. Research hotspots and future trends in lipid metabolism in chronic kidney disease: A bibliometric and visualization analysis from 2004 to 2023. Front Pharmacol. (2024) 15:1401939. doi: 10.3389/fphar.2024.1401939
10. Gui T, Li Y, Zhang S, Alecu I, Chen Q, Zhao Y, et al. Oxidative stress increases 1-deoxysphingolipid levels in chronic kidney disease. Free Radic Biol Med. (2021) 164:139–48. doi: 10.1016/j.freeradbiomed.2021.01.011
11. Rysz-Górzyńska M, Banach M. Subfractions of high-density lipoprotein (HDL) and dysfunctional HDL in chronic kidney disease patients. Arch Med Sci. (2016) 12:844–9. doi: 10.5114/aoms.2016.60971
12. Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. (2001) 34:583–8. doi: 10.1016/s0009-9120(01)00263-6
13. Hang F, Chen J, Wang Z, Zheng K, Wu Y. Association between the atherogenic index of plasma and major adverse cardiovascular events among non-diabetic hypertensive older adults. Lipids Health Dis. (2022) 21:62. doi: 10.1186/s12944-022-01670-6
14. Hamzeh B, Pasdar Y, Mirzaei N, Faramani R, Najafi F, Shakiba E, et al. Visceral adiposity index and atherogenic index of plasma as useful predictors of risk of cardiovascular diseases: Evidence from a cohort study in Iran. Lipids Health Dis. (2021) 20:82. doi: 10.1186/s12944-021-01505-w
15. Ismaiel A, Ciobanu O, Ismaiel M, Leucuta D, Popa S, David L, et al. Atherogenic index of plasma in non-alcoholic fatty liver disease: Systematic review and meta-analysis. Biomedicines. (2022) 10:2101. doi: 10.3390/biomedicines10092101
16. Wang B, Jiang C, Qu Y, Wang J, Yan C, Zhang X. Nonlinear association between atherogenic index of plasma and chronic kidney disease: A nationwide cross-sectional study. Lipids Health Dis. (2024) 23:312. doi: 10.1186/s12944-024-02288-6
17. Zhou Y, Shang X. Usefulness of atherogenic index of plasma for estimating reduced eGFR risk: Insights from the national health and nutrition examination survey. Postgrad Med. (2021) 133:278–85. doi: 10.1080/00325481.2020.1838138
18. Li H, Miao X, Zhong J, Zhu Z. Atherogenic Index of plasma as an early marker of chronic kidney disease and liver injury in type 2 diabetes. Clin Med Insights Endocrinol Diabetes. (2024) 17:11795514241259741. doi: 10.1177/11795514241259741
19. Hsieh M, Everhart J, Byrd-Holt D, Tisdale J, Rodgers G. Prevalence of neutropenia in the U.S. population: Age, sex, smoking status, and ethnic differences. Ann Intern Med. (2007) 146:486–92. doi: 10.7326/0003-4819-146-7-200704030-00004
20. Garasto S, Fusco S, Corica F, Rosignuolo M, Marino A, Montesanto A, et al. Estimating glomerular filtration rate in older people. Biomed Res Int. (2014) 2014:916542. doi: 10.1155/2014/916542
21. Onat A, Can G, Kaya H, Hergenç G. “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. (2010) 4:89–98. doi: 10.1016/j.jacl.2010.02.005
22. Bucholz E, Rodday A, Kolor K, Khoury M, de Ferranti S. Prevalence and predictors of cholesterol screening, awareness, and statin treatment among US Adults with familial hypercholesterolemia or other forms of severe dyslipidemia (1999-2014). Circulation. (2018) 137:2218–30. doi: 10.1161/CIRCULATIONAHA.117.032321
23. Cao Y, Li P, Zhang Y, Qiu M, Li J, Ma S, et al. Association of systemic immune inflammatory index with all-cause and cause-specific mortality in hypertensive individuals: Results from NHANES. Front Immunol. (2023) 14:1087345. doi: 10.3389/fimmu.2023.1087345
24. Li R, Li Y, Fan Z, Liu Z, Lin J, He M. L-shaped association of serum 25-hydroxyvitamin D with all-cause and cardiovascular mortality in older people with chronic kidney disease: Results from the NHANES database prospective cohort study. BMC Public Health. (2023) 23:1260. doi: 10.1186/s12889-023-16165-x
25. Oh D, Lee S, Yang E, Choi H, Park H, Jhee J. Atherogenic indices and risk of chronic kidney disease in metabolic derangements: Gangnam Severance Medical Cohort. Kidney Res Clin Pract. (2023) 44:132–44. doi: 10.23876/j.krcp.23.043
26. Hossein Rouhani M, Mortazavi Najafabadi M, Esmaillzadeh A, Feizi A, Azadbakht L. Direct association between high fat dietary pattern and risk of being in the higher stages of chronic kidney disease. Int J Vitam Nutr Res. (2019) 89:261–70. doi: 10.1024/0300-9831/a000260
27. Smajić J, Hasić S, Rašić S. High-density lipoprotein cholesterol, apolipoprotein E and atherogenic index of plasma are associated with risk of chronic kidney disease. Med Glas (Zenica). (2018) 15:115–21. doi: 10.17392/962-18
28. You F, Gao J, Gao Y, Li Z, Shen D, Zhong W, et al. Association between atherogenic index of plasma and all-cause mortality and specific-mortality: A nationwide population-based cohort study. Cardiovasc Diabetol. (2024) 23:276. doi: 10.1186/s12933-024-02370-4
29. Lee M, Park J, Han S, Kim Y, Kim Y, Yang C, et al. The atherogenic index of plasma and the risk of mortality in incident dialysis patients: Results from a nationwide prospective cohort in Korea. PLoS One. (2017) 12:e0177499. doi: 10.1371/journal.pone.0177499
30. Quispe R, Manalac R, Faridi K, Blaha M, Toth P, Kulkarni K, et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: The Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis. (2015) 242:243–50. doi: 10.1016/j.atherosclerosis.2015.06.057
31. Guérin M, Le Goff W, Lassel T, Van Tol A, Steiner G, Chapman M. Atherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes : Impact of the degree of triglyceridemia. Arterioscler Thromb Vasc Biol. (2001) 21:282–8. doi: 10.1161/01.atv.21.2.282
32. Hermans M, Ahn S, Rousseau M. The atherogenic dyslipidemia ratio [log(TG)/HDL-C] is associated with residual vascular risk, beta-cell function loss and microangiopathy in type 2 diabetes females. Lipids Health Dis. (2012) 11:132. doi: 10.1186/1476-511X-11-132
33. Wu J, Zhou Q, Wei Z, Wei J, Cui M. Corrigendum: Atherogenic index of plasma and coronary artery disease in the adult population: A meta-analysis. Front Cardiovasc Med. (2023) 10:1153914. doi: 10.3389/fcvm.2023.1153914
34. Liu H, Liu K, Pei L, Li S, Zhao J, Zhang K, et al. Atherogenic index of plasma predicts outcomes in acute ischemic stroke. Front Neurol. (2021) 12:741754. doi: 10.3389/fneur.2021.741754
35. Samimi S, Rajabzadeh S, Rabizadeh S, Nakhjavani M, Nakhaei P, Avanaki F, et al. Atherogenic index of plasma is an independent predictor of metabolic-associated fatty liver disease in patients with type 2 diabetes. Eur J Med Res. (2022) 27:112. doi: 10.1186/s40001-022-00731-x
36. Liu Y, Feng X, Yang J, Zhai G, Zhang B, Guo Q, et al. The relation between atherogenic index of plasma and cardiovascular outcomes in prediabetic individuals with unstable angina pectoris. BMC Endocr Disord. (2023) 23:187. doi: 10.1186/s12902-023-01443-x
37. Satirapoj B, Adler S. Comprehensive approach to diabetic nephropathy. Kidney Res Clin Pract. (2014) 33:121–31. doi: 10.1016/j.krcp.2014.08.001
38. Silverstein D. Inflammation in chronic kidney disease: Role in the progression of renal and cardiovascular disease. Pediatr Nephrol. (2009) 24:1445–52. doi: 10.1007/s00467-008-1046-0
39. Marques de Mattos A, Afonso Jordão A, Abrão Cardeal da Costa J, Garcia Chiarello P. Study of protein oxidative stress, antioxidant vitamins and inflammation in patients undergoing either hemodialysis or peritoneal dialysis. Int J Vitam Nutr Res. (2014) 84:261–8. doi: 10.1024/0300-9831/a000212
40. Rutledge J, Ng K, Aung H, Wilson D. Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat Rev Nephrol. (2010) 6:361–70. doi: 10.1038/nrneph.2010.59
41. Lehmann N, Erbel R, Mahabadi A, Rauwolf M, Möhlenkamp S, Moebus S, et al. Value of progression of coronary artery calcification for risk prediction of coronary and cardiovascular events: Result of the HNR study (Heinz Nixdorf Recall). Circulation. (2018) 137:665–79. doi: 10.1161/CIRCULATIONAHA.116.027034
42. Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat Med. (2011) 17:1410–22. doi: 10.1038/nm.2538
43. Si Y, Fan W, Han C, Liu J, Sun L. Atherogenic index of plasma, triglyceride-glucose index and monocyte-to-lymphocyte ratio for predicting subclinical coronary artery disease. Am J Med Sci. (2021) 362:285–90. doi: 10.1016/j.amjms.2021.05.001
44. Wang X, He B. Insight into endothelial cell-derived extracellular vesicles in cardiovascular disease: Molecular mechanisms and clinical implications. Pharmacol Res. (2024) 207:107309. doi: 10.1016/j.phrs.2024.107309
45. Wang Y, Zhang Z, Liu H, Guo Z, Zou L, Zhang Y, et al. Integrative phosphatidylcholine metabolism through phospholipase A(2) in rats with chronic kidney disease. Acta Pharmacol Sin. (2023) 44:393–405. doi: 10.1038/s41401-022-00947-x
46. Gonçalves L, Andrade-Silva M, Basso P, Câmara N. Vitamin D and chronic kidney disease: Insights on lipid metabolism of tubular epithelial cell and macrophages in tubulointerstitial fibrosis. Front Physiol. (2023) 14:1145233. doi: 10.3389/fphys.2023.1145233
47. Liu J, Wang H, Liu Q, Long S, Wu Y, Wang N, et al. Klotho exerts protection in chronic kidney disease associated with regulating inflammatory response and lipid metabolism. Cell Biosci. (2024) 14:46. doi: 10.1186/s13578-024-01226-4
48. Li X, Chen J, Li J, Zhang Y, Xia J, Du H, et al. ATGL regulates renal fibrosis by reprogramming lipid metabolism during the transition from AKI to CKD. Mol Ther. (2025) 33:805–22. doi: 10.1016/j.ymthe.2024.12.053
49. Ma X, Sun Y, Cheng Y, Shen H, Gao F, Qi J, et al. Prognostic impact of the atherogenic index of plasma in type 2 diabetes mellitus patients with acute coronary syndrome undergoing percutaneous coronary intervention. Lipids Health Dis. (2020) 19:240. doi: 10.1186/s12944-020-01418-0
50. Shen S, Lu Y, Dang Y, Qi H, Shen Z, Wu L, et al. Effect of aerobic exercise on the atherogenic index of plasma in middle-aged Chinese men with various body weights. Int J Cardiol. (2017) 230:1–5. doi: 10.1016/j.ijcard.2016.12.132
Keywords: atherogenic index of plasma, mortality, National Health and Nutrition Examination Survey, chronic kidney disease, cardiovascular disease, prognosis
Citation: Li L, Zhao J, Yuan M and Jiang H (2025) Association between the atherogenic index of plasma and mortality in the chronic kidney disease population: evidence from NHANES. Front. Med. 12:1575657. doi: 10.3389/fmed.2025.1575657
Received: 12 February 2025; Accepted: 12 May 2025;
Published: 30 May 2025.
Edited by:
Rodolfo Valtuille, University of Business and Social Sciences, ArgentinaReviewed by:
Hua Miao, Northwest University, ChinaChenyu Li, Universty of Pennsylvania, United States
Copyright © 2025 Li, Zhao, Yuan and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongying Jiang, MTYyNzI0ODk2NUBxcS5jb20=; Mei Yuan, MTUyMjU4MjE4MEBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Mei Yuan, orcid.org/0009-0000-5880-5723; Hongying Jiang, orcid.org/0000-0003-4935-0629
 Luohua Li
Luohua Li Jinhan Zhao
Jinhan Zhao Mei Yuan
Mei Yuan Hongying Jiang
Hongying Jiang