Abstract
The potential and positioning of controlled human infection models (CHIMs) and human challenge trials (HCTs) in the investigation of infectious pathogens and efficacy of new anti-infectives or vaccines are under evaluation. CHIMs and HCTs can provide supporting data for decision-making in the development of new medicines (“fast failure”). However, it is important to consider that, like in any phase 1 trial, CHIM volunteers have no direct health benefit. Approval by an ethics or regulatory board implies cautious evaluation of risk and potential safety issues. In this study, we chose a syndromic approach to summarize CHIM and HCT adverse events (AEs). AEs were grouped by disease entities, e.g., enteric, respiratory, vector-borne, and parasitic infections. The analysis concludes that severe AEs are rare. It confirms that AEs reflect symptoms of CHIM infections and are less prevalent in CHIM intended for the induction of carriage. Furthermore, the number of subjects affected reflects the attack rate and individual predisposition. Rarely, AEs affect the study participants’ daily activities, ranging from impairing and preventing routine tasks to requiring emergency room visits or hospitalizations. Nevertheless, while AEs guide ethical and regulatory considerations, symptoms are needed as endpoints for evaluation of the efficacy of drugs or vaccines. Finally, we observe a lack of harmonization in the reporting and grading of AEs. This reveals an eminent need for a reporting structure that allows accessibility and comparability of data sets.
Highlights
-
Grouping of CHIM studies by disease entities identified disease group-specific symptoms, adverse events, and required medical interventions.
-
Distinction of adverse events from vaccines or drugs from infection symptoms provoked by infectious challenge can be difficult.
-
Medical interventions reduce disease-specific risks but mitigate specific symptoms and severity of infection in CHIM studies.
-
Standardization of AEs reporting in CHIM studies should be sought to allow better comparison of study data and provide a better understanding of the risks.
-
Communication on potential scientific and social value and risks is key to societal acceptance of CHIM studies.
Introduction
In humanity’s fight against infections, infection control and sanitation measures have dramatically reduced transmission rates for many infectious pathogens over the last centuries. Concomitantly, the availability of vaccines and anti-infectives has made many infections treatable and preventable. Nevertheless, infections remain a frequent cause of death worldwide. This surges the clinical need for the development of new vaccines and anti-infectives. Contemporary challenges include the rise in antimicrobial resistance and the spread of zoonotic emerging viruses to humans.
In the past decades, controlled human infection models (CHIMs) have been established and evaluated for many pathogens. However, their value and positioning in drug and vaccine development have remained an ethical matter of debate fueled by historical misconduct (1, 2). Currently available CHIMs include the full range of bacterial, viral, and parasitic infections. These studies have been used to describe immune correlates of protection and for testing new medicines and vaccines for efficacy in so-called human challenge trials (HCTs). This can facilitate early decision-making in product development (“fast failure”) or the testing of medicines in specific populations, such as travelers at risk for infections.
Recently, significant efforts have been made to establish ethical guidelines for CHIM development and HCTs (1–4). Notably, the most relevant basis for decision-making on going forward with a challenge model is the assessment of risk versus benefit of potential results. Since these studies imply that healthy human subjects are intensively exposed to infection, it is necessary to carefully predict and evaluate the safety risks for these individuals. The most important principle is to avoid harm, “primum non nocere.” This implies that in order to minimize adverse events (AEs) in the study population, it is necessary to balance the severity of disease manifestation that serves as a clinical endpoint with safety considerations and to ensure the stability of the genotype and robustness of the phenotypical and functional characteristics of the challenge strains across trials (5–7). Thus, selection and specifications of challenge agents are key to an understanding of the present and future potential of CHIMs. Furthermore, when treatment is established, CHIM studies are considered feasible, but this safety measure is not always available, especially in diseases where high clinical need drives the search for new therapies and vaccines.
Despite multiple reports on CHIM and HCT outcomes and their positioning in decision-making (8, 9), only few, usually disease-specific reports specifically address safety issues in HCTs and CHIMs and attempt to define an acceptable residual risk for volunteers that could be used to generate disease- and pathogen-specific CHIM recommendations. This could arise from uncertainties in regard to the requirement for differentiation of infection symptoms and AEs, as well as procedure-related needs in CHIM strain selection, e.g., safety and acceptability versus infection requirements. A clearer and more specific AE definition in CHIMs and HCTs could, therefore, be beneficial. The objective of the present study was, thus, to provide a basis for an understanding of the attributable risks and acceptability thresholds as well as an improved informed consent. This could increase acceptance from both regulators and subjects. In this study, we provide an analysis and summary of AEs using a syndromic approach by grouping challenge agents by disease entity.
Methods
Literature search and selection
Studies were preselected based on PubMed searches for either ‘CHIM (or HCT) AND safety OR adverse events (AE)’. A second search retrieved articles from clinical databases and Google Scholar. The reports selected were peer-reviewed articles, published in English language, free full texts, and screened for duplicates. Due to language barriers, only studies in English could be included in the review. White papers were not added because they do not provide study data and are not peer-reviewed.
All reports were independently screened by two reviewers to ensure the consistency of the selection process. To ensure methodological rigor and credibility of our findings, gray (non-peer-reviewed) literature and unpublished or preprint data were excluded from this report. Studies were included based on coherent reporting of symptoms and AEs in predetermined disease entities, e.g., enteric, respiratory, vector-borne, and water- or soil-transmitted parasitic infections. Of note, this approach resulted in a limited but representative number of reports for analysis. Nevertheless, in view of the high number of publications in an emerging field, the authors cannot exclude that individual publications might not have been assessed. The present analysis summarizes the results obtained in 41 reports on CHIMs and HCTs published or re-analyzed after the year 2000 to provide a clear picture of the current practice. Notably, in some cases, reference is made to earlier studies to highlight the evolvement of the specific trials in regard to standardization and safety reporting. Symptoms and AEs documented in the studies were grouped by disease entity to provide a more general picture of the burden for participating volunteers during infection type-specific CHIMs. In the tables with summarized data, we included only studies that reported absolute numbers or percentages of subjects experiencing a defined AE; studies limited to “AEs recorded” without quantification were excluded. For HCTs, it was often more precise to refer to the placebo group instead of the total population. When applicable, this is denoted with (*) in all tables.
Distinction of clinical symptoms and adverse events
In CHIMs and, in particular, in HCTs, there is an uncertainty and potentially an inherent overlap of AE and CHIM-inherent symptoms of infection that often remain unaddressed. The available non-binding recommendations and guidelines are neither suitable for distinguishing these nor do they provide guidance for precise and comparable pathogen or disease-specific grading. In many studies, it remained unclear whether AEs during CHIMs were potentially underreported or neglected by rating them as clinical endpoints (disease manifestation defined by a predetermined combination of symptoms) and according to which criteria AEs were graded from mild to potentially severe, life-threatening AEs. Severity grading was often based on study-specific rating scales such as symptom scorecards and pro-flu questionnaires, especially when other disease-relevant, evidence-based scales were not available or deemed inappropriate. Inconsistent severity grading prevents comparability and can lead to inaccurate interpretation. We, therefore, decided to summarize and report AEs after challenge without differentiating according to the diverging definitions and severity grading.
Results
Enteric infections with fecal-oral transmission
CHIMs have frequently been employed in the context of vaccine development against enteric pathogens such as typhoid and paratyphoid fever (10–13), cholera (14–16), enterotoxigenic E. coli (17–19), Shigella (20–22), Campylobacter jejuni (23, 24), and norovirus infections (25). Thus, we evaluated 15 reports on CHIMs describing symptoms (e.g., AEs) caused by bacterial diarrheal disease manifestation and n = 1 on viral (norovirus) infection. A detailed summary of AEs categorized by CHIMs is shown in Tables 1–6. with references and summarized in Figure 1.
Table 1

Adverse events in CHIMs for enteric infections—(para)typhoid.
Table 2

Adverse events in CHIMs for enteric infections—cholera.
Table 3

Adverse events in CHIMs for enteric infections—ETEC.
Table 4

Adverse events in CHIMs for enteric infections—Shigellosis.
Table 5
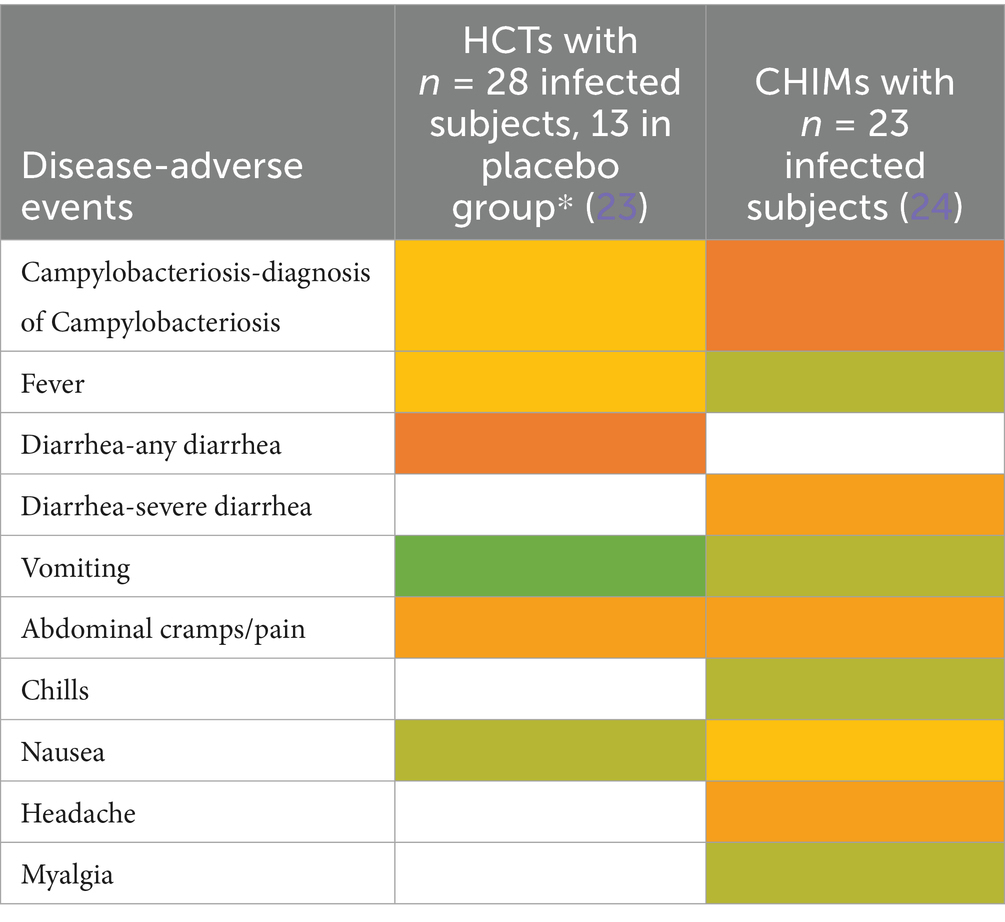
Adverse events in CHIMs for enteric infections—Campylobacteriosis.
Table 6

Adverse events in CHIMs for enteric infections—Norovirus.
Figure 1

Graphical summary of symptom frequencies (AEs) in CHIMs for enteric infections. Reported AEs are given in percentages (%). (A) (Para)typhoid fever. (B) Cholera. (C) ETEC (enterotoxigenic Escherichia coli). (D) Shigellosis. (E) Campylobacteriosis. (F) Norovirus.
Prediction of attack rates is essential for study design and estimation of power. However, the definition of the primary clinical endpoint varied strongly among trials. Attack rates varied, ranging from 49 to 56% in (para)typhoid CHIM, 42 to 92% for cholera, 54% in ETEC CHIM (19), 25 to 100% for shigellosis CHIM, 50 and 96% in campylobacteriosis studies, and 92% in the norovirus study. The differences in obtaining infection manifestation reflect the virulence of the challenge strain and individual predisposition, which are hard to entangle. For cholera, one study enriched for blood group O participants to assess risk and vaccine protection in the more susceptible blood group O individuals (14). Moderate-to-severe diarrhea in the unprotected control subjects was observed in 59% of the control population and in 69% of the blood group O controls. Reference is made to similar results in studies performed before the year 2000 (26–29).
The most important clinical endpoints and AEs were fever, diarrhea, and vomiting in this disease category. These three parameters were inconsistently subcategorized for severity grading. For exemplification, different fever definitions are provided in Tables 1–6 in the section on typhoid fever. Notably, fever > 40°C, which is characteristic of typhoid, was only reported in one study and one patient (13). This might be due to prophylactic medication or the choice of the challenge agent. Notably, in some studies, clinical symptoms were accompanied by laboratory abnormalities, which include imbalances and elevated liver transaminases. Further AEs such as “reduced daily activity” or “requirement for early antibiotic (or intravenous fluids)” listed in (19) are not commonly reported. However, they are indicators of the clinical burden of study participants.
Respiratory diseases
Ten studies were summarized to extract the most frequent AEs described in respiratory CHIMs. These studies include infection with viruses SARS-CoV-2 (30, 31), influenza (32, 33), RSV (34, 35), bacterial colonization studies (Bordetella pertussis (36), Streptococcus pneumoniae (37, 38)), and the Mycobacterium bovis BCG vaccination strain for mimicking tuberculosis (39). AEs are summarized in Tables 7–12 and are shown in Figure 2.
Table 7

AEs documented in CHIMs and HCTs for respiratory pathogens —COVID-19.
Table 8

AEs documented in CHIMs and HCTs for respiratory pathogens —Influenza.
Table 9

AEs documented in CHIM and HCT for respiratory pathogens—RSV.
Table 10

AEs documented in CHIMs and HCTs for respiratory pathogens —B. pertussis carriage.
Table 11

AEs documented in CHIMs and HCTs for respiratory pathogens—Streptococcus pneumoniae carriage.
Table 12

AEs documented in CHIMs and HCTs for respiratory pathogens —tuberculosis with BCG strain.
Figure 2

Adverse events in CHIMs and HCTs for respiratory pathogens. Reported values are given in percentages (%). A detailed list for each AEs categorized by CHIMs and references is presented in Tables 7–12. (A–D) Infection models for (A) COVID-19 (coronavirus disease of 2019); (B) Influenza; (C) RSV (respiratory syncytial virus infection); (D) tuberculosis with BCG strain; (E,F) colonization models for (E)B. pertussis carriage and (F)S. pneumoniae.
In this category, diagnosis of infection was usually defined clinically as moderate-to-severe infection and confirmed by laboratory diagnosis. The latter includes asymptomatic infections with low severity. For example, in CHIMs for influenza (32, 33), 45 or 69% of subjects were clinically diagnosed and, as expected, more (e.g., 55 and 88%, respectively) were diagnosed positive for influenza by laboratory testing. However, in CHIMs developed for COVID-19, 61% of participants were symptomatic and only 50% were positive for SARS-CoV-2 (30). In individuals with a confirmed history of infection, only 14% were transiently infected after challenge and reported symptoms, which were not specific to the challenged group (31). Notably, community-acquired SARS-CoV-2 infections were observed in 39% of volunteers (31). RSV infection was determined by viral load (53 versus 65%) (34, 35). Colonization with B. pertussis or S. pneumoniae was dependent on the inoculum size (36–38).
A detailed list of AEs observed in respiratory CHIMs can be found in Tables 7–12. Fever was detected in 19% of SARS-CoV-2-inoculated subjects and in 17% of those inoculated with influenza. Disease-typical symptoms (AEs) included smell disturbances in COVID-19-CHIM. The HCTs for RSV vaccines exemplify the difficulty of distinguishing AEs related to immunization from those induced by pathogen challenge. Despite the time interval between immunization and challenge, the data provided do not sufficiently differentiate the events, albeit a trend for more AEs in the vaccinated group is seen in (35). However, the placebo group can be used to identify challenge-related AEs. AEs in bacterial colonization studies were rare, which fits well with the absence of infection.
Vector-borne diseases, including malaria
We next followed up 11 reports on CHIMs developed for vector-borne diseases, i.e., two on dengue fever (40–43), eight on malaria (44–51), and one study on Leishmania major (52), regarding documented AEs. In the CHIM studies for dengue, viremia was found in 85–100% (40). The most frequent AEs were rash (67–90%), headache (41–98%), and postorbital pain (35–93%), which was found only in Dengue-CHIM. Laboratory anomalies varied. For example, leucopenia reached 100% in one study and 83% in another, but was not reported in (43).
Clinical diagnosis of malaria and parasitemia was found in 100% with the exception of one study with 95% (49). The manifestation of malaria-typical fever in CHIM volunteers ranged from 48 to 88% (Tables 13–15). Unspecific symptoms were frequently reported but varied strongly: headache (7–100%), malaise (38–94%), fatigue (3–100%), nausea (4–64%), myalgia (3–81%), the wide range possibly reflecting differences in inoculation and the volunteer population. Among laboratory abnormalities, elevated liver transaminases were the most frequent finding, with alanine transaminase (ALT) increased in 11–40%. Recrudescence after treatment in HCTs is described in (46); a second recrudescence occurred in five subjects, but parasitemia was cleared after treatment. For more specific findings in CHIMs, see Tables 13–15 and shown in Figure 3. These include scarring and wound infections typical for the Leishmania model (52).
Table 13

AE in CHIM for vector-borne diseases—dengue.
Table 14

AEs in CHIMs for vector-borne diseases—malaria.
Table 15

AE in CHIM for vector-borne diseases—Leishmania.
Figure 3

AEs in CHIMs for vector-borne diseases. The graphs summarize the reported AEs for (A) dengue; (B) malaria; (C) Leishmania. Frequencies are provided in percentages (%).
Water- and soil-borne parasitic infections
CHIMs for two parasite infections were analyzed. For hookworm, two HCT studies were included (53, 54) that described abdominal pain as the main symptom in all subjects. Of note, blistering (6/10 (60%)) and exudation (4/10 (40%)) were observed after vaccination and are, therefore, an AE attributable to the tested vaccine, which was confirmed in 5/15 vaccinated and infected subjects (Table 16). Eosinophilia was also observed upon exposure to larvae in (55). In Schistosomiasis-CHIM (56, 57), the predominant symptoms were fever and headaches, both of which resulted in the study participants being unable to carry out their daily activities. Pruritus and cercarial dermatitis developed upon successful infection in approximately 80–94% of subjects; accompanying eosinophilia was higher in vaccinated individuals (57) (Figure 4).
Table 16

AE in parasitosis acquired in the environmental habitat—Hookworm.
Table 17

AE in parasitosis acquired in the environmental habitat—Schistosomiasis.
Figure 4

Adverse events in CHIMs of parasitosis acquired in environmental habitat. The frequencies are provided in percent (%) for hookworm (A) and schistosomiasis (B) infections. A detailed list for each AEs categorized and references is presented in Tables 16, 17.
Reporting of delayed adverse events
Only few studies among the screened studies reported on delayed AE CHIMs. This was due to factors such as short study duration, lack of long-term follow-up, limited sample size, and confounding factors. The reported delayed AEs have been stratified by diseases categories and are summarized presented in the Tables 18–21 below according to the diseases category and for the vaccine group, and placebo group accordingly.
Table 18
| Disease | Vaccine/placebo group | |
|---|---|---|
| Delayed AEs | Outcome/Follow-up (source) | |
| Enteric infections | ||
| Cholera (16) * | Pyelonephritis (Grade 3) P* | 8 Weeks after discharge (D68 after enrollment), unrelated to study treatment |
| ETEC (17) | Post-infectious irritable bowel syndromeV/P* | Workshop discussions |
| Shigellosis (20) | One participant P, 4 SAEs: Deep vein thrombosis, pelvic venous thrombosis at D107*, hematoma at D121, and carotid artery aneurysm at D130 from challenge | All events unrelated to any study treatment and resolved |
| Campylobacteriosis (23) | Asymptomatic recrudescence (n = 2) receiving rifaximin V* and (n = 3) placebo | D21–D56 |
| A second recrudescence (77 days after challenge) P | ATB + Probiotic for 7 days, lost to follow-up | |
Delayed AEs by enteric infections.
V, vaccine group; P, placebo group; V/P, vaccine & placebo group; D107, Day 107.
Table 19
| Respiratory pathogens | Delayed AE | Outcome/Follow-up |
|---|---|---|
| COVID-19 (30) | At Day 180:
|
6 months and after smell training advice (n = 6) short courses of oral and intranasal steroids (n = 2) |
| RSV (34, 35) | Mild myocarditis (n = 1) (↑troponin level), normal electrocardiogram (ECG) and a cardiac scan interpreted as mild myocarditis (34)P | no time point provided: The event resolved spontaneously |
| Right ovarian cyst (n = 1) at 8 weeks post-challenge (35) V | unrelated to study IP or challenge virus |
Delayed AEs by respiratory pathogens.
IP, investigational product.
Table 20
| Vector-borne diseases | Delayed AE | Outcome/Follow-up |
|---|---|---|
| Malaria (44–47, 50) | Bleeding or thrombogenic complications are not reported, but in trials where longer parasitemia is expected, platelet count monitoring should be considered (44)V | no time point provided: platelet count monitoring |
| Thrombocytopenia, Grade 3 (n = 4 participants) (45)V | no time point provided: resolved after malaria treatment | |
| Ventricular extrasystoles (n = 1 ART-S and n = 1 ART-R infected participants) on D9 (46)V | ongoing by the EOS* | |
| Transient prolongation in QT interval (n = 4 ART-R and n = 1 ART-S infected participants) (46)V | Resolved by the EOS (pilot study, D90; comparative study, D55) | |
| ↑Transaminases (n = 2) at 4 weeks PDOC * and in (n = 1) at 8 weeks PDOC (47)V | Normal transaminases upon completion of treatment | |
| Increase in QTcF (corrected QT interval) inconsistently (50)V | Up to D42 post-CHIMs, clinical relevance could not be established |
Delayed AEs by vector-borne diseases.
EOS, end of the study; PDOC, post-day of challenge.
Table 21
| Parasitosis | Delayed AE | Outcome/Follow-up |
|---|---|---|
| Hookworm (53, 54) | ↑ in eosinophil count occurred in all participants (among vaccine group > placebo group) (53)V | D161 |
| ↑ Total IgE only in the vaccinated group (53)V | D1—D112 | |
| Gastrointestinal symptoms: |
Week 8 and after | |
| Schistosomiasis (56) | Persistent infection (n = 4/13, all after exposure to 20 cercariae) despite multiple treatment with PZQ or artemether did not result in cure (56)V | At the 1-year follow-up over time 3 participants self-cured. |
Delayed AEs by parasitosis.
Ig E, immunoglobulin E; PZQ, praziquantel.
Discussion
Development of new vaccines and anti-infectives can benefit from an established CHIM and the possibility to perform HCTs (8, 9). This became a driver for COVID-19 CHIM development in the SARS-CoV-2 pandemic (30, 31, 58, 59) and is pursued in infectious diseases where the efficacy of vaccines is difficult to assess in classical clinical trials, such as tuberculosis (39, 60). However, the relevance of data obtained in CHIMs and HCTs strongly depends on the reproducibility and the challenge agents´ mimicking of natural disease (5–7, 61), which comes at a cost for participants, which are subject to symptoms potentially interfering with daily life activities. There is currently no definition of the grading of severity of disease that is needed to provide reliable data on vaccine or drug efficacy in HCTs, and, in addition, no definition of acceptable and unacceptable risks. Thus, decision-making on the feasibility of CHIMs and HCTs is strongly dependent on a study-specific ethics approval, which is primarily based on “doing no harm,” e.g., assessing the potential safety risks for participants, thus favoring low risk and low AE profiles (2–4, 62). The inherent contradiction arising from the requirement to obtain a disease course with predictive value for natural infections remains an unresolved issue and leads to potentially inconsistent trial-specific decisions of the relevant ethics boards and regulatory bodies (2, 59, 62, 63). Moreover, most of the studies included in our analysis were conducted in upper-middle-income countries, which might have resulted in differences in reported AEs when compared to low- and middle-income countries (LMIC) where some of the infections are endemic. In addition, the higher disease burden in LMIC results in a greater need for vaccine development. Thus, there might be a requirement to conduct more CHIM studies in LMIC or countries with comparable epidemiology and socioeconomic conditions (64).
Here, we provide an overview of symptoms and AEs described in the evaluated studies pooled by disease entity, to provide a more general overview and pave the way for more general guidelines on evaluating CHIMs and HCTs. Overall, the conclusions drawn from our review indicate that symptoms and AEs correspond to those expected upon loco-typical manifestation of infection. Vector-borne and environmental uptake of parasites is also associated with typical symptoms for the pathogen and the infection route, such as scarring in Leishmaniosis, fever and chills in malaria, or eosinophilia in hookworm and schistosomiasis infections. In some CHIMs, symptoms are mitigated due to protective measures taken, such as continuous intravenous fluid and antibiotics administration in CHIMs for cholera (14–16) as well as other enteric pathogens, which serves to secure study participant safety.
Importantly, HCTs were not designed as safety studies. In addition, we cannot exclude that the occurrence of infection symptoms is masking AEs related to vaccination or a drug as long as AEs are unspecific and compatible with the infection. It is further difficult to discriminate whether ALT and AST elevations are caused by infection or treatment in malarial studies with artemisinin (45). By contrast, the blistering described in the hookworm vaccinated group in (52) is specific and noticeable. Nevertheless, the current analysis does not include sufficient data to evaluate whether AEs originating from drugs or vaccines tested in HCTs are sufficiently detected. In some cases, inadequate or incomplete data on adverse events following immunization (AEFI) can be deemed either ineligible for causality assessment or unclassifiable (65).
Notably, fever is a measurable parameter and, in many cases, reflects systemic disease manifestation as well as severity of infection. Independent of the infection, fever is the most frequently and probably most sensitive indicator described in all models. It is therefore a key parameter evaluated in all studies. When comparing malaria and dengue fever models, CHIMs for malaria report rates of nearly 48–88% while the fever rate is lower (25%) in Dengue-CHIM. Despite the low number of subjects per study, the latter most likely reflects the variability of disease manifestation in a genetically diverse population rather than the suitability of the challenge agent, and it is, of course, influenced by trial-specific criteria for medical intervention such as early-onset treatment based on positive qPCR (66).
Moreover, the manifestation of specific symptoms such as cough or hives in respiratory models is only documented in a minority of subjects. This could again be related to the reduced virulence of challenge agents and the mild course of infection in healthy volunteers. From a safety perspective, attenuated virulence of the infectious agent is advisable, but marked variation in disease manifestation can also limit the conclusions that can be drawn from the study results.
A recent report by Adams-Phipps et al. (67) performed a systematic review and meta-analysis of trial design and safety reporting in CHIMs over several decades. Despite a possible bias based on the study selection criteria in this report, our analysis confirms the observation that side effects are inadequately documented and discussed in many publications on CHIMs and HCTs. Nevertheless, Adams-Phipps et al. conclude that the overall risk profiles of HCTs and CHIMs are low. Here, we conclude that it lies in the nature of the induced infections that symptoms such as fever and diarrhea, or vomiting can impede daily activities in study subjects. The data reviewed in this study identified potentially severe AEs such as reactive arthritis in typhoid-CHIM (13), elevated AST and ALT levels in CHIMs for cholera (16), influenza (24), RSV (35), dengue (40), malaria (45, 46, 48, 50, 51), or excessive diarrhea in enteric infection models, which required medical intervention related to the infection with the challenge agent such as administration of intravenous fluids and antibiotics. In Shigella-CHIM, i.v. fluid administration was reported in 13/29 (45%) (22) and 36/60 (60%) (20), respectively, emergency room visits for hypotensive shock in 16/60 (27%) in (20), and early need for antibiotics in 18/29 (62%) in (22). Similarly, in ETEC CHIM, the authors reported requirements for i.v. fluid in 18/56 (32%) and for early antibiotics in 28/56 (50%) along with reduced daily activity in 32/56 (57%). Intravenous fluid substitution and antibiotics were also needed in 8/23 study participants (35%) in C. jejuni-CHIM and in 20/23 (87%), respectively (24), and administration of both fluid and antibiotics was reported in all Cholera-CHIM subjects (14–16). Notably, i.v. fluid administration was only reported in 2% (3/175) in a Malaria CHIM study (44). In view of the specific medical intervention needed, i.v. fluid and antibiotic administration is, thus, more frequent in enteric models.
These experiences further denote that the symptoms and AEs resulting from CHIMs can be medically managed and are not considered life-threatening, but can interfere with daily activities and result in significant stress. This is important because it reflects morbidity and disease burden that need to be evaluated for informed consent and ethical considerations. Notably, no deaths were reported in the evaluated studies nor mentioned by Adams-Phipps et al. (67). To improve tracking of delayed AEs in CHIM studies, extended follow-up periods, post-study surveillance studies, and real-world data integration should be considered.
Diarrhea and vomiting are characteristic of CHIMs with most enteric pathogens. Acknowledging that AEs and AE severity are disease- and in some cases pathogen-specific, recommendations for categorizing and grading AEs could alternatively be based on a syndromic approach by organ or disease type rather than with sole reference to a single challenge agent. We further observed that available guidance on severity scoring and grading was frequently adapted to serve the individual study’s purpose. For example, a retrospective reevaluation on the influence of the challenge strain on diarrheal disease severity resulted in a modified scale rather than an assessment of residual risk for volunteers (17).
Despite existing legal frameworks (such as in the EU (68, 69)) and guidance on performance of CHIMs and HCTs (70) as well as on toxicity grading and AE classification (71–73), in the current settings, comparability of data regarding the severity of AEs can therefore not be assumed and was therefore not systematically analyzed in this study. However, improved standardization of trials could provide a means to categorize AEs and define the residual risk associated with a certain type of infection. AE-informed risk–benefit assessments in CHIM design could further be considered as a basis for informed consent of subjects and support ethics committee decisions. Structured benefit/risk evaluation as provided by the European Medicines Agency (EMA) represents an important prerequisite in this research area (74).
Well-defined standards further permit the comparison of studies and thus facilitate the evaluation of a larger study population. This implies that study sites implement high standards in training and effective measures in quality management and risk mitigation strategies to secure the safety of subjects, patients, and the environment as proposed in Ref. (75). As recently proposed, specialized ethics boards and/or CHIM observers or auditors could pave the path for implementation of appropriate ethical frameworks and standards and thereby drive the development of guidance and criteria for the performance of CHIMs and HCTs (76). This comes along with the requirement to build public trust through transparent communication on potential scientific and social value and risks with the public, patients, and the medical communities (77).
Statements
Author’s note
This report summarizes data retrieved from the publications selected for analysis. The analysis and interpretation of data reflect the conclusions drawn by the authors, but do not necessarily reflect the view of Paul-Ehrlich-Institut.
Author contributions
KG: Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. PL: Writing – original draft, Validation, Methodology, Investigation, Writing – review & editing. JR: Conceptualization, Investigation, Writing – review & editing, Methodology, Writing – original draft, Data curation. MM: Data curation, Methodology, Investigation, Writing – review & editing. MJ-H: Investigation, Conceptualization, Writing – review & editing, Data curation, Methodology. IB-D: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study received support from the Innovative Medicines Initiative/European Union/European Federation of Pharmaceutical Industries and Associations (IMI2/EU/EFPIA) Joint Undertaking under grant agreement no. 101007799 (Inno4Vac). This communication reflects the authors’ views and that neither IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained therein.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Metzger WG Ehni HJ Kremsner PG Mordmüller BG . Experimental infections in humans-historical and ethical reflections. Trop Med Int Health. (2019) 24:1384–90. doi: 10.1111/tmi.13320
2.
Battisti D Capulli E Picozzi M . The first- and second-order ethical reasons approach: the case of human challenge trials. Ethics Hum Res. (2024) 46:26–36. doi: 10.1002/eahr.500223
3.
Jamrozik E Littler K Bull S Emerson C Kang G Kapulu M et al . Key criteria for the ethical acceptability of COVID-19 human challenge studies: report of a WHO working group. Vaccine. (2021) 39:633–40. doi: 10.1016/j.vaccine.2020.10.075
4.
Jamrozik E Selgelid MJ . Ethical issues surrounding controlled human infection challenge studies in endemic low-and middle-income countries. Bioethics. (2020) 34:797–808. doi: 10.1111/bioe.12802
5.
Balasingam S Meillon S Chui C Mann A la C Weller CL et al . Human infection studies: key considerations for challenge agent development and production. Wellcome Open Res. (2022) 7:140. doi: 10.12688/wellcomeopenres.17869.1
6.
Bekeredjian-Ding I Trouvin JH Depraetere H la C Suvarnapunya AE Bell A et al . Controlled human infection studies: proposals for guidance on how to design, develop and produce a challenge strain. Biologicals. (2021) 74:16–23. doi: 10.1016/j.biologicals.2021.09.002
7.
Bekeredjian-Ding I van Molle W Baay M Neels P Berthels N Conrad C et al . Human challenge trial workshop: focus on quality requirements for challenge agents, Langen, Germany, October 22, 2019. Biologicals. (2020) 66:53–61. doi: 10.1016/j.biologicals.2020.04.005
8.
Roestenberg M Hoogerwerf MA Ferreira DM Mordmüller B Yazdanbakhsh M . Experimental infection of human volunteers. Lancet Infect Dis. (2018) 18:e312–22. doi: 10.1016/S1473-3099(18)30177-4
9.
Abo YN Jamrozik E McCarthy JS Roestenberg M Steer AC Osowicki J . Strategic and scientific contributions of human challenge trials for vaccine development: facts versus fantasy. Lancet Infect Dis. (2023) 23:e533–46. doi: 10.1016/S1473-3099(23)00294-3
10.
Darton TC Jones C Blohmke CJ Waddington CS Zhou L Peters A et al . Using a human challenge model of infection to measure vaccine efficacy: a randomised, controlled trial comparing the typhoid vaccines M01ZH09 with placebo and Ty21a. PLoS Negl Trop Dis. (2016) 10:e0004926. doi: 10.1371/journal.pntd.0004926
11.
Dobinson HC Gibani MM Jones C Thomaides-Brears HB Voysey M Darton TC et al . Evaluation of the clinical and microbiological response to Salmonella Paratyphi a infection in the first paratyphoid human challenge model. Clin Infect Dis. (2017) 64:1066–73. doi: 10.1093/cid/cix042
12.
Gibani MM Jin C Shrestha S Moore M Norman L Voysey M et al . Homologous and heterologous re-challenge with Salmonella typhi and Salmonella paratyphi a in a randomised controlled human infection model. PLoS Negl Trop Dis. (2020) 14:e0008783. doi: 10.1371/journal.pntd.0008783
13.
Jin C Gibani MM Moore M Juel HB Jones E Meiring J et al . Efficacy and immunogenicity of a vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella typhi: a randomised controlled, phase 2b trial. Lancet. (2017) 390:2472–80. doi: 10.1016/S0140-6736(17)32149-9
14.
Chen WH Cohen MB Kirkpatrick BD Brady RC Galloway D Gurwith M et al . Single-dose live Oral cholera vaccine CVD 103-HgR protects against human experimental infection with Vibrio cholerae O1 El Tor. Clin Infect Dis. (2016) 62:1329–35. doi: 10.1093/cid/ciw145
15.
Cohen MB Giannella RA Bean J Taylor DN Parker S Hoeper A et al . Randomized, controlled human challenge study of the safety, immunogenicity, and protective efficacy of a single dose of Peru-15, a live attenuated oral cholera vaccine. Infect Immun. (2002) 70:1965–70. doi: 10.1128/IAI.70.4.1965-1970.2002
16.
Erdem R Ambler G al-Ibrahim M Fraczek K Dong SD Gast C et al . A phase 2a randomized, single-center, double-blind, placebo-controlled study to evaluate the safety and preliminary efficacy of oral iOWH032 against cholera diarrhea in a controlled human infection model. PLoS Negl Trop Dis. (2021) 15:e0009969. doi: 10.1371/journal.pntd.0009969
17.
Porter CK Gutierrez RL Kotloff KL . Clinical endpoints for efficacy studies. Vaccine. (2019) 37:4814–22. doi: 10.1016/j.vaccine.2019.03.051
18.
Porter CK Riddle MS Alcala AN Sack DA Harro C Chakraborty S et al . An evidenced-based scale of disease severity following human challenge with Enteroxigenic Escherichia coli. PLoS One. (2016) 11:e0149358. doi: 10.1371/journal.pone.0149358
19.
Darsley MJ Chakraborty S DeNearing B Sack DA Feller A Buchwaldt C et al . The oral, live attenuated enterotoxigenic Escherichia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease. Clin Vaccine Immunol. (2012) 19:1921–31. doi: 10.1128/CVI.00364-12
20.
Frenck RW Jr Conti V Ferruzzi P Ndiaye AGW Parker S McNeal MM et al . Efficacy, safety, and immunogenicity of the Shigella sonnei 1790GAHB GMMA candidate vaccine: results from a phase 2b randomized, placebo-controlled challenge study in adults. EClinicalMedicine. (2021) 39:101076. doi: 10.1016/j.eclinm.2021.101076
21.
Porter CK Lynen A Riddle MS Talaat K Sack D Gutiérrez RL et al . Clinical endpoints in the controlled human challenge model for Shigella: a call for standardization and the development of a disease severity score. PLoS One. (2018) 13:e0194325. doi: 10.1371/journal.pone.0194325
22.
Talaat KR Alaimo C Martin P Bourgeois AL Dreyer AM Kaminski RW et al . Human challenge study with a Shigella bioconjugate vaccine: analyses of clinical efficacy and correlate of protection. EBioMedicine. (2021) 66:103310. doi: 10.1016/j.ebiom.2021.103310
23.
Rimmer JE Harro C Sack DA Talaat KR Gutierrez RL DeNearing B et al . Rifaximin fails to prevent Campylobacteriosis in the human challenge model: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. (2018) 66:1435–41. doi: 10.1093/cid/cix1014
24.
Tribble DR Baqar S Carmolli MP Porter C Pierce KK Sadigh K et al . Campylobacter jejuni strain CG8421: a refined model for the study of Campylobacteriosis and evaluation of Campylobacter vaccines in human subjects. Clin Infect Dis. (2009) 49:1512–9. doi: 10.1086/644622
25.
Kirby AE Streby A Moe CL . Vomiting as a symptom and transmission risk in norovirus illness: evidence from human challenge studies. PLoS One. (2016) 11:e0143759. doi: 10.1371/journal.pone.0143759
26.
Levine MM Kaper JB Herrington D Ketley J Losonsky G Tacket CO et al . Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. (1988) 2:467–70. doi: 10.1016/s0140-6736(88)90120-1
27.
Tacket CO Cohen MB Wasserman SS Losonsky G Livio S Kotloff K et al . Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El tor inaba three months after vaccination. Infect Immun. (1999) 67:6341–5. doi: 10.1128/IAI.67.12.6341-6345.1999
28.
Tacket CO Losonsky G Nataro JP Cryz SJ Edelman R Kaper JB et al . Onset and duration of protective immunity in challenged volunteers after vaccination with live oral cholera vaccine CVD 103-HgR. J Infect Dis. (1992) 166:837–41. doi: 10.1093/infdis/166.4.837
29.
Levine MK . Live oral cholera vaccine: from principle to product. Bull Inst Pasteur. (1995) 93:243–53.
30.
Killingley B Mann AJ Kalinova M Boyers A Goonawardane N Zhou J et al . Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med. (2022) 28:1031–41. doi: 10.1038/s41591-022-01780-9
31.
Jackson S Marshall JL Mawer A Lopez-Ramon R Harris SA Satti I et al . Safety, tolerability, viral kinetics, and immune correlates of protection in healthy, seropositive UK adults inoculated with SARS-CoV-2: a single-Centre, open-label, phase 1 controlled human infection study. Lancet Microbe. (2024) 5:655–68. doi: 10.1016/S2666-5247(24)00025-9
32.
Han A Czajkowski L Rosas LA Cervantes-Medina A Xiao Y Gouzoulis M et al . Safety and efficacy of CR6261 in an influenza a H1N1 healthy human challenge model. Clin Infect Dis. (2021) 73:e4260–8. doi: 10.1093/cid/ciaa1725
33.
Watson JM Francis JN Mesens S Faiman GA Makin J Patriarca P et al . Characterisation of a wild-type influenza (a/H1N1) virus strain as an experimental challenge agent in humans. Virol J. (2015) 12:13. doi: 10.1186/s12985-015-0240-5
34.
DeVincenzo J Tait D Efthimiou J Mori J Kim YI Thomas E et al . A randomized, placebo-controlled, respiratory syncytial virus human challenge study of the antiviral efficacy, safety, and pharmacokinetics of RV521, an inhibitor of the RSV-F protein. Antimicrob Agents Chemother. (2020) 64:e01884–19. doi: 10.1128/AAC.01884-19
35.
Sadoff J de Paepe E DeVincenzo J Gymnopoulou E Menten J Murray B et al . Prevention of respiratory syncytial virus infection in healthy adults by a single immunization of Ad26.RSV.preF in a human challenge study. J Infect Dis. (2022) 226:396–406. doi: 10.1093/infdis/jiab003
36.
de Graaf H Ibrahim M Hill AR Gbesemete D Vaughan AT Gorringe A et al . Controlled human infection with Bordetella pertussis induces asymptomatic, immunizing colonization. Clin Infect Dis. (2020) 71:403–11. doi: 10.1093/cid/ciz840
37.
Adler H German EL Mitsi E Nikolaou E Pojar S Hales C et al . Experimental human pneumococcal colonization in older adults is feasible and safe, not immunogenic. Am J Respir Crit Care Med. (2021) 203:604–13. doi: 10.1164/rccm.202004-1483OC
38.
Morton B Burr S Chikaonda T Nsomba E Manda-Taylor L Henrion MYR et al . A feasibility study of controlled human infection with Streptococcus pneumoniae in Malawi. EBioMedicine. (2021) 72:103579. doi: 10.1016/j.ebiom.2021.103579
39.
Davids M Pooran A Hermann C Mottay L Thompson F Cardenas J et al . A human lung challenge model to evaluate the safety and immunogenicity of PPD and live Bacillus Calmette-Guerin. Am J Respir Crit Care Med. (2020) 201:1277–91. doi: 10.1164/rccm.201908-1580OC
40.
Endy TP Wang D Polhemus ME Jarman RG Jasper LE Gromowski G et al . A phase 1, open-label assessment of a dengue Virus-1 live virus human challenge strain. J Infect Dis. (2021) 223:258–67. doi: 10.1093/infdis/jiaa351
41.
Simmons JS FHK R . Experimental studies of dengue. Philipp J Sci. (1931) 44:1–247.
42.
Nishiura H Halstead SB . Natural history of dengue virus (DENV)-1 and DENV-4 infections: reanalysis of classic studies. J Infect Dis. (2007) 195:1007–13. doi: 10.1086/511825
43.
Pierce KK Durbin AP Walsh MCR Carmolli M Sabundayo BP Dickson DM et al . TV005 dengue vaccine protects against dengue serotypes 2 and 3 in two controlled human infection studies. J Clin Invest. (2024) 134:3328. doi: 10.1172/JCI173328
44.
Roestenberg M O'Hara GA Duncan CJA Epstein JE Edwards NJ Scholzen A et al . Comparison of clinical and parasitological data from controlled human malaria infection trials. PLoS One. (2012) 7:e38434. doi: 10.1371/journal.pone.0038434
45.
Laurens MB Billingsley P Richman A Eappen AG Adams M Li T et al . Successful human infection with P. falciparum using three aseptic Anopheles stephensi mosquitoes: a new model for controlled human malaria infection. PLoS One. (2013) 8:e68969. doi: 10.1371/journal.pone.0068969
46.
Watts RE Odedra A Marquart L Webb L Abd-Rahman AN Cascales L et al . Safety and parasite clearance of artemisinin-resistant Plasmodium falciparum infection: a pilot and a randomised volunteer infection study in Australia. PLoS Med. (2020) 17:e1003203. doi: 10.1371/journal.pmed.1003203
47.
Kamau E Bennett JW Yadava A . Safety and tolerability of mosquito bite-induced controlled human infection with Plasmodium vivax in malaria-naive study participants-clinical profile and utility of molecular diagnostic methods. J Infect Dis. (2022) 225:146–56. doi: 10.1093/infdis/jiab332
48.
Collins KA Wang CYT Adams M Mitchell H Robinson GJ Rampton M et al . A plasmodium vivax experimental human infection model for evaluating efficacy of interventions. J Clin Invest. (2020) 130:2920–7. doi: 10.1172/JCI134923
49.
Lyke KE Laurens M Adams M Billingsley PF Richman A Loyevsky M et al . Plasmodium falciparum malaria challenge by the bite of aseptic Anopheles stephensi mosquitoes: results of a randomized infectivity trial. PLoS One. (2010) 5:e13490. doi: 10.1371/journal.pone.0013490
50.
Kublin JG Murphy SC Maenza J Seilie AM Jain JP Berger D et al . Safety, pharmacokinetics, and causal prophylactic efficacy of KAF156 in a Plasmodium falciparum human infection study. Clin Infect Dis. (2021) 73:e2407–14. doi: 10.1093/cid/ciaa952
51.
Epstein JE Rao S Williams F Freilich D Luke T Sedegah M et al . Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: an update. J Infect Dis. (2007) 196:145–54. doi: 10.1086/518510
52.
Parkash V Ashwin H Dey S Sadlova J Vojtkova B van Bocxlaer K et al . Safety and reactogenicity of a controlled human infection model of sand fly-transmitted cutaneous leishmaniasis. Nat Med. (2024) 30:3150–62. doi: 10.1038/s41591-024-03146-9
53.
Chapman PR Webster R Giacomin P Llewellyn S Becker L Pearson MS et al . Vaccination of human participants with attenuated Necator americanus hookworm larvae and human challenge in Australia: a dose-finding study and randomised, placebo-controlled, phase 1 trial. Lancet Infect Dis. (2021) 21:1725–36. doi: 10.1016/S1473-3099(21)00153-5
54.
Hoogerwerf MA Koopman JPR Janse JJ Langenberg MCC van Schuijlenburg R Kruize YCM et al . A Randomized controlled trial to investigate safety and variability of egg excretion after repeated controlled human hookworm infection. J Infect Dis. (2021) 223:905–13. doi: 10.1093/infdis/jiaa414
55.
Hoogerwerf MA Janse JJ Kuiper VP van Schuijlenburg R Kruize YCM Sijtsma JC et al . Protective efficacy of short-term infection with Necator americanus hookworm larvae in healthy volunteers in the Netherlands: a single-centre, placebo-controlled, randomised, controlled, phase 1 trial. Lancet Microbe. (2023) 4:e1024–34. doi: 10.1016/S2666-5247(23)00218-5
56.
Koopman JPR Houlder EL Janse JJ Casacuberta-Partal M Lamers OAC Sijtsma JC et al . Safety and infectivity of female cercariae in Schistosoma-naive, healthy participants: a controlled human Schistosoma mansoni infection study. EBioMedicine. (2023) 97:104832. doi: 10.1016/j.ebiom.2023.104832
57.
Langenberg MCC Hoogerwerf MA Koopman JPR Janse JJ Kos-van Oosterhoud J Feijt C et al . A controlled human Schistosoma mansoni infection model to advance novel drugs, vaccines and diagnostics. Nat Med. (2020) 26:326–32. doi: 10.1038/s41591-020-0759-x
58.
Deming ME Michael NL Robb M Cohen MS Neuzil KM . Accelerating development of SARS-CoV-2 vaccines - the role for controlled human infection models. N Engl J Med. (2020) 383:e63. doi: 10.1056/NEJMp2020076
59.
Levine MM Abdullah S Arabi YM Darko DM Durbin AP Estrada V et al . Viewpoint of a WHO advisory group tasked to consider establishing a closely-monitored challenge model of coronavirus disease 2019 (COVID-19) in healthy volunteers. Clin Infect Dis. (2021) 72:2035–41. doi: 10.1093/cid/ciaa1290
60.
Carter E Morton B ElSafadi D Jambo K Kenny-Nyazika T Hyder-Wright A et al . A feasibility study of controlled human infection with intradermal Bacillus Calmette-Guerin (BCG) injection: pilot BCG controlled human infection model. Wellcome Open Res. (2023) 8:424. doi: 10.12688/wellcomeopenres.19811.1
61.
Corti N Chiu C Cox RJ Demont C Devaster JM Engelhardt OG et al . Regulatory workshop on challenge strain development and GMP manufacture - a stakeholder meeting report. Biologicals. (2024) 85:101746. doi: 10.1016/j.biologicals.2024.101746
62.
Shah SK Miller FG Darton TC Duenas D Emerson C Lynch HF et al . Ethics of controlled human infection to address COVID-19. Science. (2020) 368:832–4. doi: 10.1126/science.abc1076
63.
Cavaleri M Kaslow D Boateng E Chen WH Chiu C Choy RKM et al . Fourth controlled human infection model (CHIM) meeting, CHIM regulatory issues, may 24, 2023. Biologicals. (2024) 85:101745. doi: 10.1016/j.biologicals.2024.101745
64.
Gordon SB Rylance J Luck A Jambo KC Ferreira DM Manda-Taylor L et al . A framework for controlled human infection model (CHIM) studies in Malawi: report of a Wellcome Trust workshop on CHIM in low income countries held in Blantyre, Malawi. Wellcome Open Res. (2017) 2:70. doi: 10.12688/wellcomeopenres.12256.1
65.
Organization, W.H . (2018). Causality assessment of an adverse event following immunization (AEFI), User manual for the revised WHO classification.
66.
Walk J Schats R Langenberg MCC Reuling IJ Teelen K Roestenberg M et al . Diagnosis and treatment based on quantitative PCR after controlled human malaria infection. Malar J. (2016) 15:398. doi: 10.1186/s12936-016-1434-z
67.
Adams-Phipps J Toomey D Więcek W Schmit V Wilkinson J Scholl K et al . A systematic review of human challenge trials, designs, and safety. Clin Infect Dis. (2023) 76:609–19. doi: 10.1093/cid/ciac820
68.
Council, T.E.P.a.t (2014). REGULATION (EU) on clinical trials on medicinal products for human use, in REGULATION (EU) No 536/2014, T.E.P.a.t. Council, Editor. Official Journal of the European Union. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0536 (Accessed May 25, 2025).
69.
Commission, E . (2011). Communication from the Commission — Detailed guidance on the collection, verification and presentation of adverse event/reaction reports arising from clinical trials on medicinal products for human use (‘CT-3’), in 2011/C 172/01, Commission, E., Editor. European Commission. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52011XC0611(01) (Accessed May 25, 2025).
70.
WHO . WHO guidance on the ethical conduct of controlled human infection studies. Geneva: World Health Organization (2021).
71.
Division of AIDS, N.I.o.A.a.I.D . (2017). Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.1.
72.
National Institutes of Health, N.C.I . (2009). Common Terminology Criteria for Adverse Events (CTCAE), Version 4.
73.
Research, F.a.D.A.C.f.B.E.a . (2007). Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. U.S. Department of Health and Human Service.: Available online at: https://www.fda.gov/media/73679/download (Accessed May 25, 2025).
74.
(EMA), E.M.A., Benefit-risk methodology . (2009), European Medicines Agency (EMA) Available online at: https://www.ema.europa.eu/en/about-us/what-we-do/regulatory-science-research/benefit-risk-methodology (Accessed May 25, 2025).
75.
Higham HE Morgan L Cooper C Marshall J Mawer A Jackson S et al . Adopting human factors in early phase and experimental medicine research: a nested pilot study observing controlled human infection with SARS-CoV-2. Br J Clin Pharmacol. (2024) 90:1586–99. doi: 10.1111/bcp.15949
76.
Sharma A Apte A Rajappa M Vaz M Vaswani V Goenka S et al . Perceptions about controlled human infection model (CHIM) studies among members of ethics committees of Indian medical institutions: a qualitative exploration. Wellcome Open Res. (2022) 7:209. doi: 10.12688/wellcomeopenres.17968.1
77.
Jamrozik E Littler K Meln I van Molle W Morel S Olesen OF et al . Ethical approval for controlled human infectious model clinical trial protocols - a workshop report. Biologicals. (2024) 85:101748. doi: 10.1016/j.biologicals.2024.101748
Summary
Keywords
human challenge trials, CHIM, vaccine, anti-infective, adverse effects, safety
Citation
Götz K, Luga P, Rengel J, Masur M, Juárez-Hernández M and Bekeredjian-Ding I (2025) Symptoms and adverse events in controlled human infection models. Front. Med. 12:1578560. doi: 10.3389/fmed.2025.1578560
Received
17 February 2025
Accepted
14 July 2025
Published
14 August 2025
Volume
12 - 2025
Edited by
Beatriz S. Lima, Research Institute for Medicines (iMed.ULisboa), Portugal
Reviewed by
Bian Yuan, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, China
Tang Yongsheng, Third Affiliated Hospital of Sun Yat-sen University, China
Updates
Copyright
© 2025 Götz, Luga, Rengel, Masur, Juárez-Hernández and Bekeredjian-Ding.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabelle Bekeredjian-Ding, Isabelle.bekeredjian-ding@uni-marburg.de
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.