Abstract
Background:
Invasive mucinous adenocarcinoma (IMA) is a rare malignant tumor of the lung, characterized as a distinct subtype of lung adenocarcinoma, with unique histological features and clinical behavior. Because the diagnosis is often delayed due to its imaging characteristics resembling pneumonia, this neoplasm is associated with a poor prognosis.
Case presentation:
This case report describes a 69 years-old man who underwent an asymptomatic health examination, during which small ground-glass opacities were found in the periphery of both lungs. There was no significant change in the lesions during the subsequent 2 years follow-up. However, at the third year of follow-up, the lesions had significantly enlarged. A CT-guided puncture biopsy was performed, and the pathological results indicated IMA of the lung. Subsequently, the patient underwent surgical treatment, and the postoperative pathological findings were consistent with those of the biopsy. In this case, the patient believed that he had been following the doctor’s orders for asymptomatic health checks and follow-up reexamination, yet his cancer diagnosis was still significantly delayed. Therefore, the patient demanded that the doctor take medical responsibility for the alleged negligence. After the doctor carefully described the imaging features of lung IMA, the patient ultimately decided to forgo pursuing medical responsibility and expressed satisfaction with the doctor’s diagnosis.
Conclusion:
This case illustrates the evolving imaging signs of lung IMA. Medical professionals should avoid diagnosing lung cancer as pneumonia, with the aim of enhancing the accuracy of early diagnosis and assisting in clinical evaluation. Additionally, it serves as a reference for patients to better understand this disease.
Introduction
Invasive mucinous adenocarcinoma (IMA) is a rare malignant tumor of the lung, characterized by its unique histological and imaging features (1). This subtype of lung adenocarcinoma often presents challenges in diagnosis due to its imaging characteristics, such as airspace opacities and ground-glass opacities (2). It is frequently misdiagnosed as pneumonia due to its pneumonic-type appearance on imaging studies. Early recognition and accurate diagnosis are crucial for improving patient outcomes, as delayed treatment can lead to worse prognoses. The use of advanced imaging techniques, including thin-slice computed tomography (CT) and 18F-FDG PET/CT, has been associated with a high misdiagnosis rate in the differentiation of IMA from pneumonia also (3). Given the rarity of IMA, there is a pressing need for increased awareness among clinicians regarding its potential imaging manifestations and the importance of distinguishing it from other pulmonary conditions. Since there is no previous literature on the persistent evolution of invasive mucinous adenocarcinoma from small lesions, we report such a case here.
Case report
A 69 years-old male patient was hospitalized with lung nodules that had been present for the past 3 years. The patient was found to have small ground-glass opacities in both lungs on chest CT during a screening process for COVID-19 3 years ago (see Figure 1). One lesion in the lower lobe of the left lung was slightly larger, measuring about 0.5 × 0.4 cm, and had an irregular shape. The patient had a temperature of 36.6°C, a respiratory rate of 14 breaths per minute, and a heart rate of 62 beats per minute. The patient and his companion denied that he had asthma, chronic obstructive pulmonary disease, or gastroesophageal reflux disease. He recently denied having a fever, low-grade fever, obvious night sweats, diarrhea, skin-related abnormalities, or edema in his lower extremities. The patient has been smoking for more than 50 years and has not quit, with a daily average of 15 cigarettes. His professional occupation was not related to any special exposure or radiation exposure, and he reported no alcohol consumption, illicit drug use, or recent travel. The patient has no history of pet ownership or unclean sexual contact.
FIGURE 1

A total of 16-slice computerized tomography images of the chest at initial diagnosis period and in the subsequent 2 years of annual chest computed tomography (CT) re-examination of the patient.
Physical examination showed that the patient had good oral hygiene and no swelling of the tonsils. The patient did not have significantly enlarged lymph nodes, and had a clear respiratory sound in both lungs, and no dry or wet rales heard in either lung field, as well as no pathological murmurs detected during cardiac auscultation. The patient has a history of cerebral infarction for more than 10 years and has been treated with aspirin 100 mg orally once a day. There are currently no special clinical symptoms. The patient denies a history of hypertension, coronary heart disease, diabetes, chronic obstructive pulmonary disease, chronic interstitial pneumonia, and other chronic diseases. The patient also denies allergies to food, drugs, dust, and other substances.
Further examination of the peripheral blood cells counts, liver function, kidney function, electrolytes, myocardial enzymes, blood gas analysis, blood D-dimer, blood glucose, and blood tumor markers all indicated normal results. The patient also underwent tests for erythrocyte sedimentation rate, C-reactive protein, HIV antibody, syphilis antibody, rheumatoid factor, anti-streptococcal hemolysin “O” test, anti-nuclear antibody, anti-double-stranded DNA antibody, and anti-neutrophil cytoplasmic antibody, all of which were normal (see Table 1).
TABLE 1
| Laboratory tests | Normal range | Results |
| WBC (× 109/L) | 4∼10 | 8.22 |
| C-reactive protein (mg/L) | < 10 | 6 |
| Neutrophile granulocyte percentage (%) | 50∼70 | 62.1 |
| Eosinophils percentage (%) | 0∼5 | 4.6 |
| Monocytes percentage (%) | 3∼8 | 5.3 |
| Albumin(g/L) | 35∼55 | 38.7 |
| Alanine aminotransferase (U/L) | 7∼40 | 21 |
| Aspartate aminotransferase (U/L) | 13∼35 | 15 |
| Direct bilirubin (μmol/L) | 0∼6.8 μmol/L | 5.5 |
| Creatinine (μmol/L) | 41∼81 | 54.7 |
| Urea nitrogen (μmol/L) | 3.6∼9.5 | 3.12 |
| Erythrocyte sedimentation rate(mm/h) | 0∼20 | 12 |
| Procalcitonin (ng/mL) | < 0.05 | 0.02 |
| Interleukin-6 (pg/mL) | 0∼40 | 11 |
| Mycoplasma pneumoniae antibody | Negative | Negative |
| Chlamydia pneumoniae antibody | Negative | Negative |
| T-SPOT-TB | Negative | Negative |
| Protein (urinalysis routine) | Negative | Negative |
| Urobilinogen (urinalysis routine) | Negative | Negative |
| Occult blood (urinalysis routine) | Negative | Negative |
| Anti-nRNP/Sm antibody | Negative | Negative |
| Anti-Sm antibody | Negative | Negative |
| Anti-SSA antibody | Negative | Negative |
| Anti-Ro52 antibody | Negative | Negative |
| Anti-Scl-70 antibody | Negative | Negative |
| Anti-Jo-1 antibody | Negative | Negative |
| Anti-CENP B antibody | Negative | Negative |
| Anti-PCNA antibody | Negative | Negative |
| Anti-ds DNA antibody | Negative | Negative |
| Anti- nucleosome antibody | Negative | Negative |
| Anti-histone antibody | Negative | Negative |
| Anti-rRNP antibody | Negative | Negative |
| Anti-AMA M2 antibody | Negative | Negative |
| Anti-proteinase 3 antibody | Negative | Negative |
| Anti-myeloperoxidase antibody | Negative | Negative |
| Anti-neutrophil cytoplasmic antibody-pANCA | Negative | Negative |
| Anti-neutrophil cytoplasmic antibody-cANCA | Negative | Negative |
| Serology (colloidal gold-labeled technique) | Negative | Negative |
Patient laboratory test results.
Pulmonary interstitial changes were noted by both respiratory doctors and radiologists. So, the doctor gave the diagnosis of interstitial pulmonary pneumonia. The patient was advised to take oral moxifloxacin 400 mg once daily for 2 weeks. At the same time, the doctor asked the patient to undergo a review 3 months later. At the same time, the doctor asked the patient to undergo a review 3 months later. A chest CT reexamination conducted 3 months later found that the features of both lung lesions were similar to those in the previous health examination images, and the lesions were neither significantly larger nor significantly smaller (see Figure 1).
The doctors suggested that the patient undergo percutaneous lung biopsy, but the patient refused. So, the patient was recommended a CT reexamination within 1 year. In the subsequent 2 years of annual chest CT reexamination, the imaging changes of both lung lesions were still not significant (see Figure 1). These imaging features did not reveal air bronchogram, and interlobular fissure bulging. However, in the third year of re-examination, chest CT showed that the lesion in the patient’s left lung was significantly larger than before, measuring approximately 3.2 × 3.1 cm. It appears that there are blood vessels running through it, and there is a burr sign (see Figure 2). 18F-FDG PET/CT was performed to identify the nature of lung lesions (see Figure 3). From the images, we can observe that the ground-glass opacities were diffusely distributed in both lungs, especially in the lower lobes of the left lung. The FDG uptake values varied, with a standard uptake value (SUVmax) range of 6.4–24.3, while the SUVmax was 3.7. Which was greater than the SUV value of 2.5 that could be diagnosed as lung cancer. There were no obvious enlarged lymph nodes in the lung hilum or mediastinum.
FIGURE 2
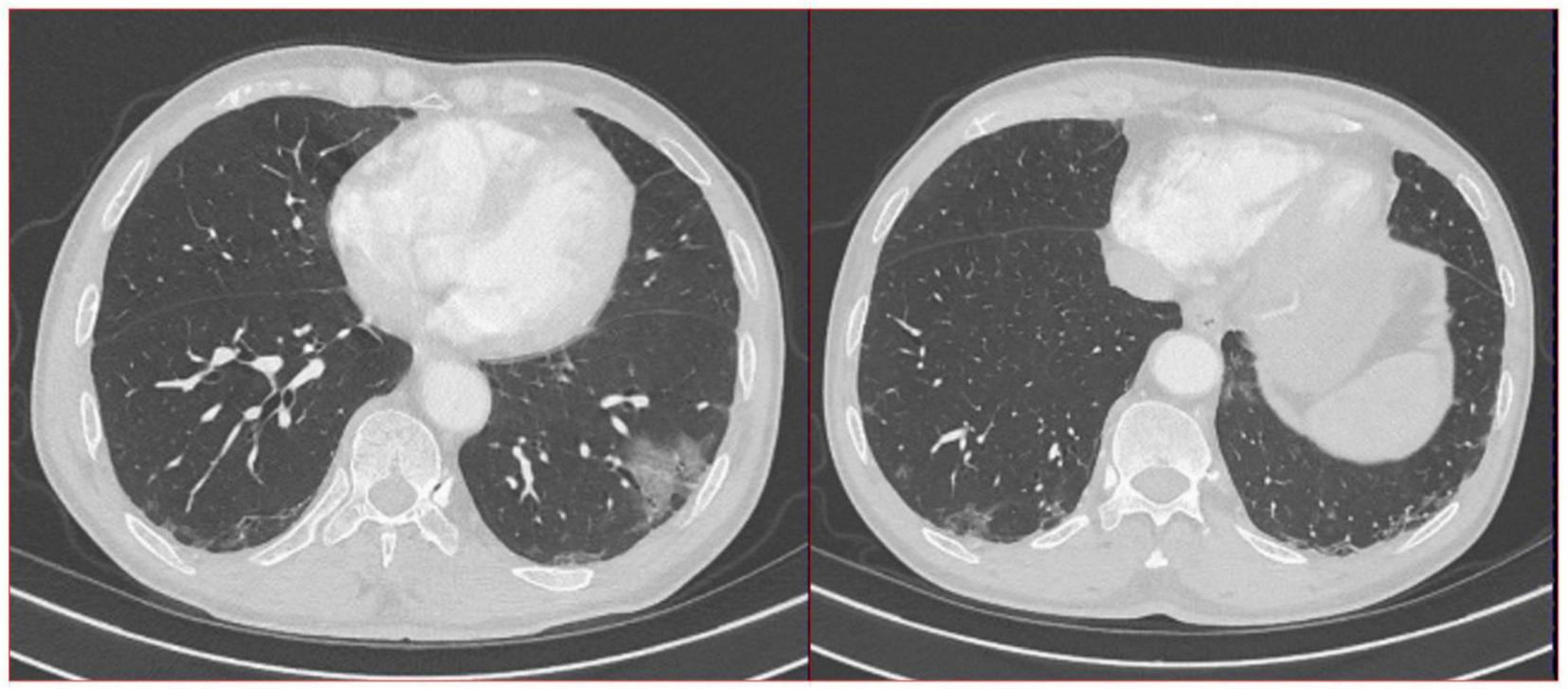
A total of 16-slice computerized tomography images of the chest in the third year (12/27/2023) of re-examination of the patient.
FIGURE 3
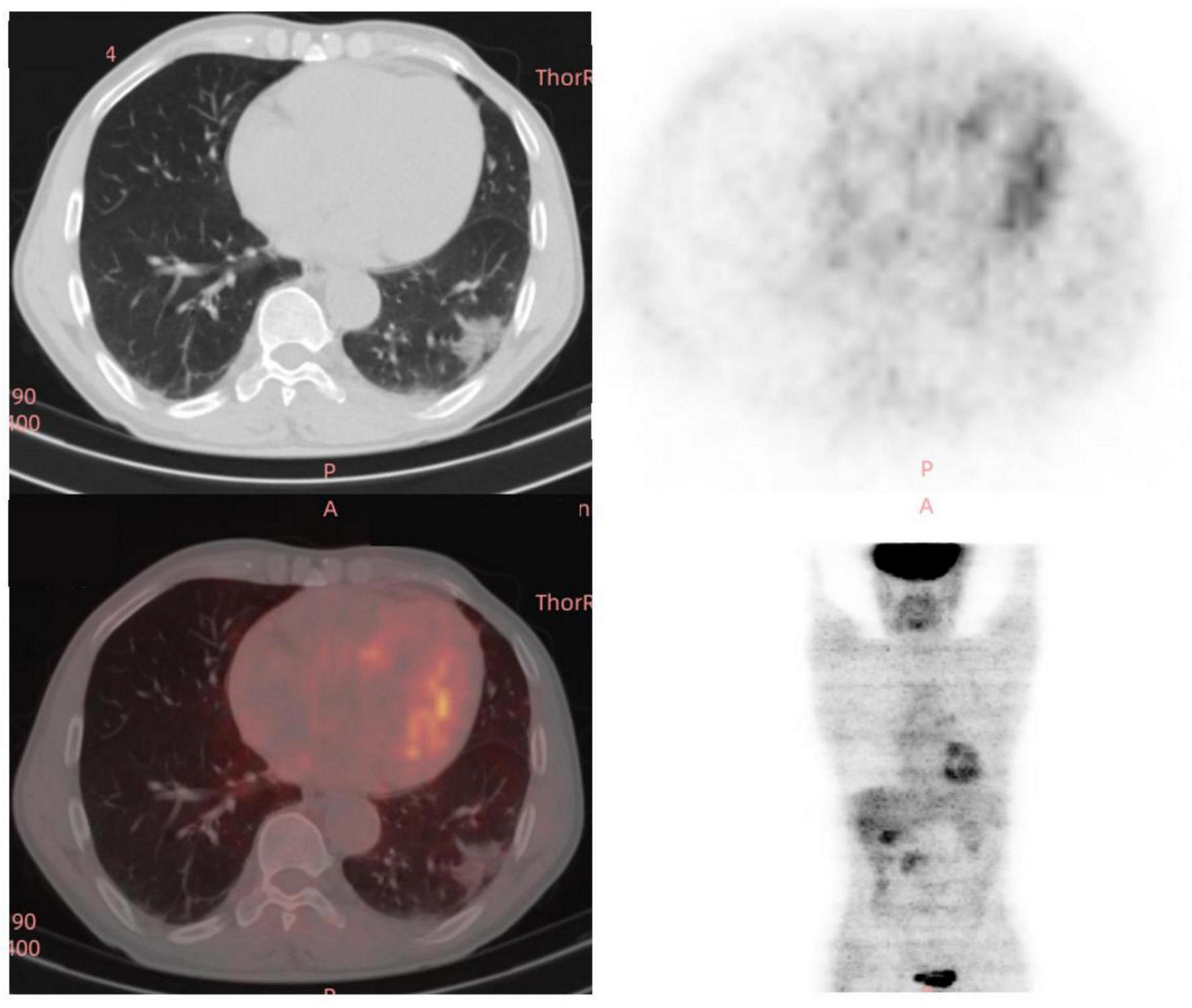
A total of 18 fluoro-D-glucose positron emission tomography/computed tomography (18F-FDG-PET/CT) images of the chest in the third year (12/29/2023) of re-examination of the patient.
Therefore, the radiologist and the respiratory doctor believe that cancer cannot be excluded as the cause of the patient’s lesions. A CT-guided percutaneous lung biopsy was performed, and the lesion was identified microscopically as an IMA of the lung. The tumor cells are in the shape of goblet cells, with abundant mucus in the cytoplasm and nuclei located at the base and perpendicular to the base. Nuclear atypical is not obvious. The surrounding alveolar cavities are filled with mucus. Immunohistochemical examination showed the following results, Cytokeratin (+), Epithelial Membrane Antigen (+), and Thyroid Transcription Factor - 1 (−), with Ki-67 at 10% (see Figure 4). The patient subsequently underwent a left inferior lobectomy, and the postoperative pathological findings were consistent with those of the biopsy tissue.
FIGURE 4
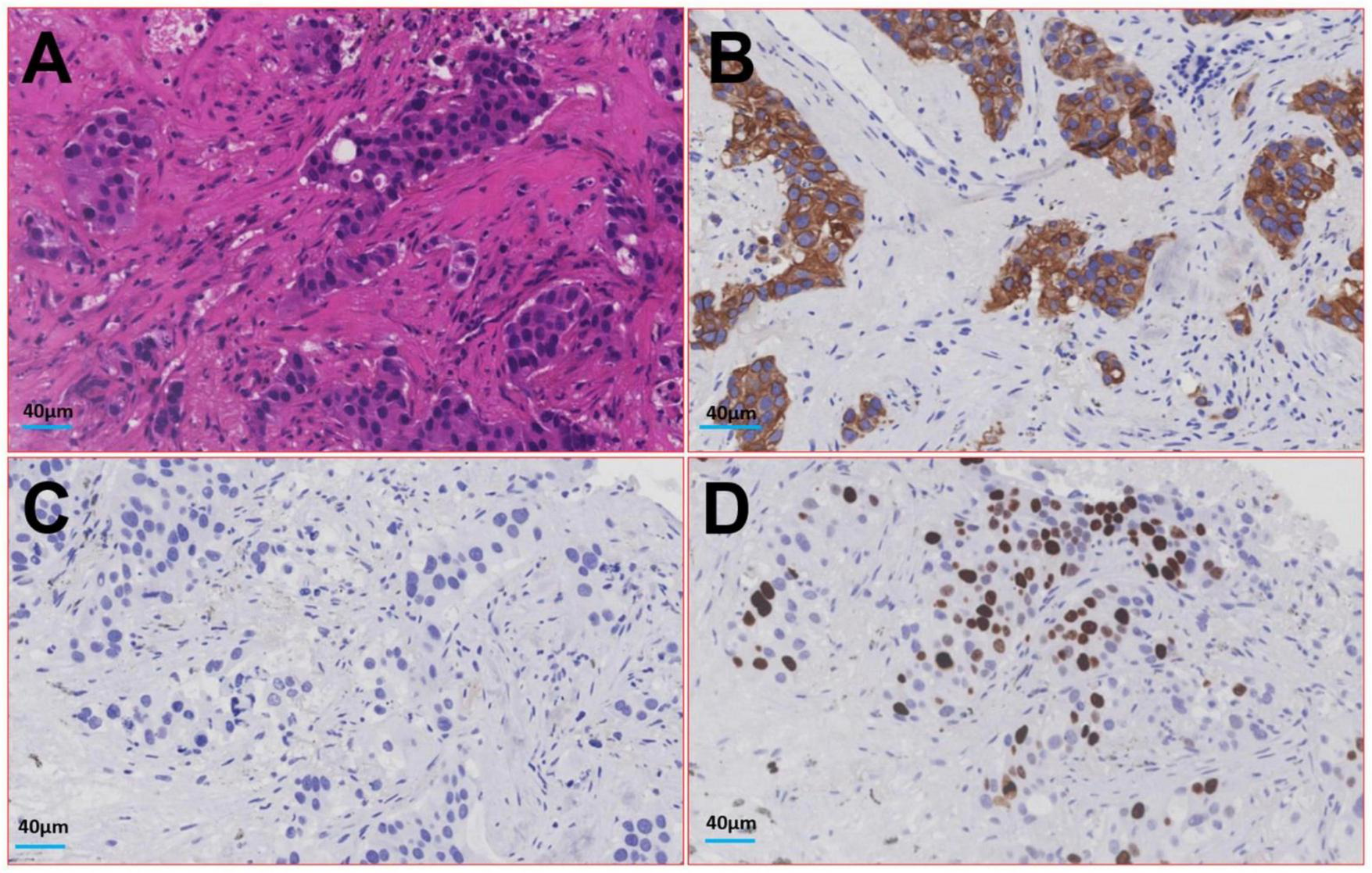
Image of histologic diagnosis from the lesion in the patient’s lower lobe of the left lung. (A) Hematoxylin-eosin staining; (B) Cytokeratin; (C) Thyroid Transcription Factor - 1; (D) Ki-67.
However, the patient believed that he had been following the doctor’s orders for asymptomatic health checks and follow-up reexamination, yet his cancer diagnosis was still significantly delayed. Therefore, the patient demanded that the doctor take medical responsibility for the alleged negligence. After the doctor carefully described the imaging features of lung IMA, the patient ultimately decided to forgo pursuing medical responsibility and expressed satisfaction with the doctor’s diagnosis and treatment.
Discussion
Mucinous adenocarcinoma may originate from goblet cells and columnar cells with secretory functions (4). According to the WHO classification of lung adenocarcinoma, carcinoma in situ and invasive adenocarcinoma may be accompanied by mucous secretion (5). However, the term “mucinous adenocarcinoma” is specifically used for invasive cases. Mucinous adenocarcinoma may also exhibit various growth patterns, including adherent, acinar, papillary, and micropapillary forms, similar to those seen in common adenocarcinomas (6).
Lung IMA can occur at any age, but it is more common in middle-aged and older individuals. There is no significant difference in the incidence of the disease between men and women, regardless of smoking history (7, 8). Most patients with this disease have no obvious clinical symptoms in the early stage. The main symptoms, when the lesions are significant, include repeated cough and sputum production, primarily white mucus sputum, along with fever. These symptoms are similar to pneumonia but do not respond to anti-inflammatory treatment (9, 10). Laboratory tests may show elevated white blood cell counts and C-reactive protein levels in patients with this disease, though not as significantly as in pneumonia (11, 12).
Imaging findings of lung IMA are critical for diagnosis and management. IMA is a rare subtype of lung adenocarcinoma characterized by distinct radiological features that could aid in differentiating it from other types of lung cancer. Common findings include solid nodules or masses, often with lobulated or spiculated margins. Lung IMA with ground-glass opacities lesion presents challenges in diagnosis (3, 6, 7). Pleural traction is also frequently observed. The imaging findings of it were mainly inferior lobe and dorsal side (gravity distribution characteristics), which easily metastasized along the spread through air spaces (STAS) and led to intrapulmonary dissemination (3). Lesions with unclear boundary of the solid or ground glass shadow, the lesions of the bronchus irregular narrow, rigid, distorted, prone to false cavity sign or vacuole sign (2). In the later stage, the lesions were often mixed in various forms, including consolidation, ground glass, multiple nodules and cystic cavity. Due to the high mucous content in the tumor, the lesion appeared as a lower density shadow than the muscle on CT, which was slightly enhanced on enhanced scan. The median maximum standardized uptake value (SUVmax) of IMA lesions is typically around 3.0, with higher values correlating with advanced disease stages and larger tumor sizes (3). In addition to CT, fluorine-18-fluorodeoxyglucose positron emission tomography (PET)/CT can provide valuable metabolic information (3). The morphologic-metabolic dissociation sign, where a malignant-appearing nodule on CT shows a low SUVmax, has been identified as a potential indicator for distinguishing IMA from invasive non-mucinous adenocarcinomas. Furthermore, thin-slice computed tomography (TSCT) has been shown to effectively classify pulmonary subsolid nodules, helping to differentiate between pre-invasive lesions, minimally invasive adenocarcinomas, and invasive adenocarcinomas (13). The morphologic-metabolic dissociation sign, based on CT and PET/CT, has been evaluated for its efficacy in distinguishing IMA from invasive non-mucinous adenocarcinomas of the lung (3).
Misdiagnosis of lung IMA can lead to significant clinical implications, as this rare subtype of lung cancer often presents with symptoms that mimic other conditions, such as pneumonia. Both of the two kind diseases have flaky, ground-glass shadows on the image features and can even be diagnosed as viral pneumonia if the pneumonia virus is prevalent (14–16). Moreover, in this case, the patient had radiographic findings of lesions in both lungs. In order to obtain a more accurate diagnosis for these lesions, invasive procedures such as thoracoscopic or open lung biopsy, CT-guided percutaneous lung biopsy, and transbronchial lung biopsy are required to obtain lung tissue. According to a case report, both surgical lung biopsy and CT-guided percutaneous lung biopsy had a diagnostic rate of 100%, whereas transbronchial lung biopsy with bronchoalveolar lavage had a rate of 80.9% (14, 17). So, the patient believed that he had been following the doctor’s orders for asymptomatic health checks and follow-up reexamination, yet his cancer diagnosis was still significantly delayed. After we carefully informed and popularized the diagnostic difficulty and current situation of IMA, the patient expressed satisfaction with the doctor’s diagnosis and treatment.
Of course, other technologies, including but not limited to artificial intelligence, microbial genetic testing, and virological antigen detection, all help in the two kind diseases identification (18–20). One of the most common genetic mutations found in IMA is the KRAS mutation, which is present in a significant proportion of cases. This mutation is often accompanied by other genetic alterations, such as those in the STK11 gene, which can further influence the tumor’s behavior and response to treatment (21). The genetic landscape of IMA is further characterized by a low overall mutation burden, which is atypical compared to other lung adenocarcinomas. This low mutation burden is associated with a distinct mutational signature, predominantly linked to endogenous mutational processes rather than external factors like smoking. This suggests that the pathogenesis of IMA may involve different mechanisms compared to other lung cancers (22).
Limitations
This was a case report study, which was not highlight the long follow-up period, and lack of molecular diagnostic methods.
Conclusion
In conclusion, this case illustrates the evolving imaging signs of lung IMA. Medical professionals should avoid diagnosing lung cancer as pneumonia, with the aim of enhancing the accuracy of early diagnosis and assisting in clinical evaluation. Additionally, it serves as a reference for patients to better understand this disease.
Statements
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Y-NZ: Data curation, Formal Analysis, Writing – original draft. KC: Investigation, Methodology, Project administration, Software, Writing – original draft. YY: Data curation, Formal Analysis, Resources, Validation, Visualization, Writing – original draft. TW: Data curation, Visualization, Writing – review and editing. M-JL: Methodology, Writing – review and editing. J-XW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review and editing. Q-LL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Ou G Luo W Zhang W Wang S Zhao J Zhao H et al Gefitinib improves severe bronchorrhea and prolongs the survival of a patient with lung invasive mucinous adenocarcinoma: A case report. World J Clin Cases. (2023) 11:441–8. 10.12998/wjcc.v11.i2.441
2.
Zhang S Yu X Huang Y Nie P Deng Y Mao N et al Pneumonic-type invasive mucinous adenocarcinoma and infectious pneumonia: Clinical and CT imaging analysis from multiple centers. BMC Pulm Med. (2022) 22:460. 10.1186/s12890-022-02268-5
3.
Sun X Zeng B Tan X Chen Z Pan X Jiang L . Invasive mucinous adenocarcinoma of the lung: Clinicopathological features, 18F-FDG PET/CT findings, and survival outcomes.Ann Nucl Med. (2023) 37:198–207. 10.1007/s12149-022-01816-7
4.
Chae Y Oh Y Kim L Park J Djunadi T Shah Z et al Bilateral orthotopic lung transplantation for the patient with lung-limited invasive mucinous adenocarcinoma: A case-based literature review. Oncologist. (2024): 10.1093/oncolo/oyae263Online ahead of print.
5.
Travis W Brambilla E Nicholson A Yatabe Y Austin J Beasley M et al The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. (2015) 10:1243–60. 10.1097/JTO.0000000000000630
6.
Chang W Zhang Y Nicholson A . Pulmonary invasive mucinous adenocarcinoma.Histopathology. (2024) 84:18–31. 10.1111/his.15064
7.
Xu L Li C Lu H . Invasive mucinous adenocarcinoma of the lung.Transl Cancer Res. (2019) 8:2924–32. 10.21037/tcr.2019.11.02
8.
Cha Y Shim H . Biology of invasive mucinous adenocarcinoma of the lung.Transl Lung Cancer Res. (2017) 6:508–12. 10.21037/tlcr.2017.06.10
9.
Higashiyama M Kobayashi Y Kashima J Muraoka Y Watanabe H Kusumoto M et al Invasive mucinous adenocarcinoma of the lung with a mural nodule-like lesion. Am J Surg Pathol. (2022) 46:1524–32. 10.1097/PAS.0000000000001938
10.
Zhao T Yi J Luo D Liu J Fan X Wu Q et al Prognostic factors for invasive mucinous adenocarcinoma of the lung: Systematic review and meta-analysis. World J Surg Oncol. (2024) 22:41. 10.1186/s12957-024-03326-4
11.
Aoki A Hara Y Okudela K Ishikawa Y Doshita K Hashimoto H et al Unusual lung involvements of invasive mucinous adenocarcinoma with chylothorax. Thorac Cancer. (2020) 11:3407–8. 10.1111/1759-7714.13665
12.
Nakamura S Sugimoto H Negoro K Tanaka R . Invasive mucinous adenocarcinoma of the lung presenting with multiple cavities.Cureus. (2021) 13:e13795. 10.7759/cureus.13795
13.
Li M Zhu L Lv Y Shen L Han Y Ye B . Thin-slice computed tomography enables to classify pulmonary subsolid nodules into pre-invasive lesion/minimally invasive adenocarcinoma and invasive adenocarcinoma: A retrospective study.Sci Rep. (2023) 13:6999. 10.1038/s41598-023-33803-x
14.
Zhu D Zhang Q Rui Z Xu S . Pulmonary invasive mucinous adenocarcinoma mimicking pulmonary actinomycosis.BMC Pulm Med. (2022) 22:181. 10.1186/s12890-022-01971-7
15.
Morichika D Miyahara N Hotta K Okamoto Y Minami D Irie M et al Invasive mucinous adenocarcinoma mimicking organizing pneumonia associated with Mycobacterium fortuitum infection. Intern Med. (2014) 53:2795–9. 10.2169/internalmedicine.53.2783
16.
Beom J Lee J . Case of invasive mucinous adenocarcinoma mimicking chronic eosinophilic pneumonia.Thorac Cancer. (2014) 5:179–83. 10.1111/1759-7714.12056
17.
Liu Y Li J Wang S Chen M Zhao J Jiang D et al [Advanced pneumonic-type lung carcinoma: A retrospective study of clinical-radiological-pathological characteristics with survival analysis in a single chinese hospital]. Zhongguo Fei Ai Za Zhi. (2019) 22:329–35. 10.3779/j.issn.1009-3419.2019.06.01
18.
Wu X Ding F Li K Huang W Zhang Y Zhu J . Analysis of the causes of solitary pulmonary nodule misdiagnosed as lung cancer by using artificial intelligence: A retrospective study at a single center.Diagnostics (Basel). (2022) 12:2218. 10.3390/diagnostics12092218
19.
Zhu J Huang W Huang B Zhu Y Jiang X Zou J et al Clinical characteristics and prognosis of COVID-19 patients with initial presentation of lung lesions confined to a single pulmonary lobe. Am J Transl Res. (2020) 12:7501–9.
20.
Li M Wang B Liu P Wang H Zhu J . Prostatitis as initial manifestation of Chlamydia psittaci pneumonia diagnosed by metagenome next-generation sequencing: A case report.Open Life Sci. (2023) 18:20220596. 10.1515/biol-2022-0596
21.
Boland J Maleszewski J Wampfler J Voss J Kipp B Yang P et al Pulmonary invasive mucinous adenocarcinoma and mixed invasive mucinous/nonmucinous adenocarcinoma-a clinicopathological and molecular genetic study with survival analysis. Hum Pathol. (2018) 71:8–19. 10.1016/j.humpath.2017.08.002
22.
Kim M Hwang J Kim K Hwang S Lee H Jung J et al Genomic characteristics of invasive mucinous adenocarcinoma of the lung with multiple pulmonary sites of involvement. Mod Pathol. (2022) 35:202–9. 10.1038/s41379-021-00872-0
Summary
Keywords
invasive mucinous adenocarcinoma, lung cancer, computed tomography imaging, diagnosis, ground-glass opacities lesion, case report
Citation
Zhang Y-N, Cao K, Yang Y, Wei T, Li M-J, Wei J-X and Liu Q-L (2025) Case Report: Do not diagnose lung cancer as pneumonia: continue to monitor a case of invasive mucinous adenocarcinoma as it progresses from small to large. Front. Med. 12:1578874. doi: 10.3389/fmed.2025.1578874
Received
18 February 2025
Accepted
12 May 2025
Published
12 June 2025
Volume
12 - 2025
Edited by
Karolina Henryka Czarnecka-Chrebelska, Medical University of Lodz, Poland
Reviewed by
Jiuyu Gong, Armed Police Corps Hospital of Hubei Province, China
Sevilay Özmen, Atatürk University, Türkiye
Updates
Copyright
© 2025 Zhang, Cao, Yang, Wei, Li, Wei and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiu-Lian Liu, 13607023266@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.