Abstract
Introduction:
KL-6, a pneumocyte derived biomarker, is increased in patients with interstitial lung diseases (ILDs). We aimed to investigate the role of serum KL-6 as a diagnostic and prognostic biomarker in silica-exposed workers.
Material and method:
We studied 108 silica-exposed subjects and 25 healthy controls. Chest radiography (CXR), pulmonary function tests, inflammatory markers were collected. Progressive massive fibrosis (PMF) was defined according to the ILO classification. KL-6 was measured in serum by fully automated CLEIA at first presentation at our institution, intended as baseline visit, with a time point from the initial exposure variable for each patient.
Results:
PMF was present in 23 subjects. Serum KL-6 levels were significantly higher in subjects with PMF than in controls, exposed workers or simple silicosis (p<0.001). In PMF, serum KL-6 positively correlated with C-reactive protein (CRP) and Erythrocyte sedimentation rate (ESR), and negatively with forced vital capacity (FVC) % predicted. At a cut-off of 436 U/mL, serum KL-6 differentiated exposed workers from PMF with a specificity and sensitivity exceeding 90% (p<0.0001), while a cut-off of 445 U/mL differentiated simple silicosis from PMF (p<0.0001). In the multivariate analysis, serum KL-6 levels were independently associated with risk of fibrosis.
Conclusion:
Serum KL-6 appears to be a promising biomarker for the occurrence and progression of PMF in silica-exposed workers.
Introduction
Pneumoconioses represent a group of chronic lung disorders characterized by pulmonary fibrosis, often as a result of occupational exposure to respirable dust or mineral fibers. Respiratory exposure in the workplace environment to pneumoconiogenic minerals, precipitate their accumulation within lung tissues, eliciting tissue responses characterized by inflammation and variable extension of fibrosis (1).
The fibrogenic potential of mineral powders depends on their physical and chemical properties, lung retention, and the individual’s physiological and immunological traits (2). Accumulation of particles in the lungs can trigger a self-sustaining inflammatory process, leading to disease progression even after exposure has ceased, typically following at least 5 years of occupational exposure (2–5). Data on the dust quantities triggering inflammation in humans remains insufficient, though studies in mice indicate that 0.2 mg of retained dust might be enough (5, 6). Thus, exposure limit values and exposure time are only guidelines and do not guarantee protection for all individuals (7).
Despite all the preventive measures implemented in the last decades (8), pneumoconioses still represent a public health concern, both globally and in Romania (8, 9). Studies over the past decade by the Global Burden of Disease (GBD) Center indicate a downward trend in pneumoconioses prevalence, yet approximately 60,000 new cases were confirmed in 2017, with a global prevalence of around 527,500 cases (9).
In accordance with the ILO (International Labor Office) recommendations (10), the diagnosis of pneumoconioses adheres to fundamental criteria, including occupational exposure history, symptomatology, clinical signs, respiratory functional changes, radiological findings compatible with pneumoconiosis, and exclusion of mimicking conditions (10, 11). However, as in other occupational lung diseases, the histopathological onset often precedes early radiological and clinical manifestations (11). The identification of biomarkers for (1) early fibrosis diagnosis, (2) disease severity assessment, (3) progression and survival prognostication is still an unmet need (12).
In light of this, Krebs von den Lungen-6 (KL-6) seems to fulfill the above criteria. KL-6, also known as Mucin-1 (MUC-1), is a circulating high-molecular-weight mucin-like glycoprotein (13, 14), predominantly expressed in the cytoplasm and membrane of type II pneumocytes (15, 16), and in bronchial epithelial cells, particularly Clara cells (non-ciliated bronchiolar secretory cells) (17, 18). Previous research indicates that the elevated level of KL-6 in bronchoalveolar lavage fluid (BALF) in idiopathic pulmonary fibrosis (IPF) reflects the proliferation of type II pneumocytes, which are also stimulated by the exposure to crystalline silica dust (19). Furthermore, serum KL-6 levels correlate with disease severity in different pulmonary conditions (20) and this correlation may also extend to pulmonary fibrosis resulting from exposure to dust and mineral fibers.
The objective of this study was to investigate whether serum KL-6 could have a role as a diagnostic/prognostic marker in exposed workers to crystalline silica dust to predict worse disease outcome.
Patients and methods
Study population and study design
Between 2022 and 2023, 120 subjects with exposure to mineral dust or already diagnosed with pneumoconiosis were admitted in the Occupational Medicine Clinic of Colentina Clinical Hospital, Bucharest, Romania. A detailed anamnesis was performed for all patients and all had a documented occupational exposure of at least 5 years to pneumoconiogenic dusts.
As the current study was dedicated to silicosis, all patients diagnosed with other forms of occupational pulmonary fibrosis (10 patients with asbestosis and 2 with kaolinosis) were excluded and 108 were included in the further analysis. As the prevalence of PMF in the literature ranges from 7.5 to 10% (21, 22), we estimated a sample size between 107 and 139 patients necessary for the purpose of this study.
None of the patients was less than 18 years old and all participants signed an informed consent for inclusion and data processing. Patients with contraindications for pulmonary function tests according to the current international guidelines (23) were also excluded. The study obtained approval from the Ethics Committee of Carol Davila University of Medicine and Pharmacy under protocol No. 8244/28.03.2022.
Clinical and paraclinical investigations were conducted subsequent to consent acquisition.
This retrospective study included 108 subjects who were former or actual cast iron foundry workers, involved in activities such as: abrasive cutting, sand casting, mold dislodgement, cast iron polishing, and furnace maintenance. None of them was miner, as Bucharest in not located in a mining area and there are very few miners with silicosis who are surveilled in this Occupational Medicine Clinic. Given the homogeneity of their professional background, which ensured similar exposure intensity, the primary factors differentiating their exposure were the duration of exposure and retention time. The 108 subjects were monitored for an average duration of 13 years (13.29 ± 5.92), with no statistically significant differences between subgroups. The diagnosis of silicosis was determined based on occupational history, a minimum exposure of 5 years, clinical symptoms, and imaging findings.
As control group, 25 subjects with no occupational exposure to chemical, dust, fibers or biological agents and no medical history of lung disease were selected. In accordance with national regulations, all case group members underwent standard chest radiographs (CXRs) assessed by the Pneumoconiosis Board, aligned with the 2022 revised edition of the ILO Guidelines (10).
Clinical definitions
PMF was defined as complicated silicosis with large opacities (greater than 1 cm) on CXR according to the ILO categories A, B, C [Appendix A]. The following definitions were used: exposure time as effective duration of exposure, retention time as period of time the pneumoconiogenic dusts and fibers were retained in the lungs, and latency time as period from the initial day of exposure to the first diagnosis of the disease. The durations of exposure, retention, and latency were reported in years (3).
Occupational dust exposure: assessment protocol
A standardized questionnaire was applied to all subjects in order to collect data regarding: occupational exposure, occupational history, environmental history, demographic data, and medical background. The questionnaire was administered for both the study and control groups. Its primary objective was to select patients with occupational exposure to crystalline silica dust to be included in the study group and to exclude silica and other occupational respiratory hazards in the control group. The questionnaire was meticulously structured, comprising multiple sections tailored to comprehensively capture occupational and medical histories [Appendix B].
Radiographic evaluation
CXR served as the modality to stratify individuals within the case group (those occupationally exposed to crystalline silica dust) in exposed without silicosis, patients with simple silicosis and patients with complicated silicosis. The classification followed the International Labour Office Guidelines on the International Classification of Radiographs of Pneumoconiosis: simple pneumoconiosis was characterized by small opacities ≤ 1 cm and complicated pneumoconiosis (progressive massive fibrosis) was defined by the presence of larger opacities exceeding 1 cm. Every radiographic image underwent scrutiny by the Pneumoconioses Board, comprising occupational medicine physicians and radiologists with long-term experience in diagnosing occupational pulmonary fibrosis.
Blood tests: laboratory procedures
Venous blood samples were obtained from each patient and stored at −20°C until measurement.
KL-6 serum levels were quantified using Fujirebio Lumiplse G600II through automated chemiluminescent enzyme immunoassay (CLEIA) as previously described (24) employing a two-step immunoassay methodology. The intra-assay coefficient of variation was 2.7% and the inter-assay coefficient of variation was 3.1%, according to the package insert Lumipulse G KL-6 Immunoreaction Cartridges (key-code: FRI87290), Fujirebio Inc. KL-6 serum levels were expressed in U/mL.
Pulmonary function tests
All participants included in the study (N = 133) underwent spirometry to evaluate their lung function with a MasterScreen Pneumo PC spirometer, CareFusion. Spirometric assessments adhered to the guidelines outlined by the American Thoracic Society (ATS) and were performed by experienced practitioners within the Pulmonary Function Lab of the Occupational Medicine Clinic, Colentina Clinical Hospital.
Statistical analysis: methodology
To assess the normality of variables, the Kolmogorov–Smirnov and Shapiro–Wilk tests were employed. Variables demonstrating a normal distribution are presented as mean ± standard error of the mean (SEM), while those with non-normal distribution are depicted as median with interquartile range (IQR). Parametric tests were applied to variables with normal distribution, whereas nonparametric tests were utilized for data exhibiting abnormal distribution. Specifically, ANOVA was employed for quantitative variables with normal distribution, while the Kruskall-Wallis and Mann–Whitney U tests were utilized for data with abnormal distribution. For qualitative variables, Pearson Chi-Square, Likelihood Ratio, and Fisher’s exact test were employed for data comparison. To compare variables between subgroups, we used post hoc tests and made the adjustment for multiple testing using Bonferroni correction.
Pearson’s correlation coefficient was used to analyze the associations between serum KL-6 levels and several covariates, such as pulmonary function parameters and inflammatory markers.
To evaluate the role of serum KL-6 as a progression biomarker in silicosis, ROC curve analysis was conducted. The analysis reported the area under the curve (AUC), 95% confidence interval, and p-value [Significance level P (Area = 0.5)]. Cut-off values were determined with a minimum requirement of a sensitivity and specificity of at least 90%. Univariate and multivariate Cox proportional hazard regression models were employed to assess prognostic factors for silicosis occurrence and progression to PMF. The Kaplan–Meier method with the log-rank test, was utilized to determine the association between KL-6 serum levels and disease outcome. A p-value < 0.05 was considered statistically significant. All statistical analysis was conducted using IBM SPSS Statistics software, version 26.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of study subjects
The demographic characteristics, occupational history, and medical backgrounds of the enrolled subjects are detailed in Table 1. Based on CXR outcomes and occupational history, the case group was stratified into distinct subgroups: 21 exposed workers, without silicosis (EWs), 64 simple silicosis subjects, with opacities <1 cm (SSs), and 23 complicated silicosis subjects, progressive massive fibrosis with opacities >1 cm (CSs).
Table 1
| EWs | SSs | CSs | HCs | p-value | |
|---|---|---|---|---|---|
| Demographics, occupational history, and medical background | |||||
| N | 21 | 64 | 23 | 25 | |
| Age (years) | 53.0 [50.5, 57.5] | 56.0 [54.0, 65.5] | 59.0 [55.0, 65.0] | 57.0 [53.5, 61.5] | 0.502 |
| Female:Male | 3:18 | 27:37 | 5:18 | 9:16 | 0.066 |
| Exposure time (years) | 15.0 [12.0, 22.0] | 19.0 [14.0, 24.75] | 19.0 [14.0, 29.0] | N/A | 0.207 |
| Retention time (years) | 31.0 [23.5, 38.5] | 36.0 [33.25, 40.75] | 37.0 [33.0, 46.0] | N/A | 0.008 |
| Smoker | 10/21 (47.6%) | 33/64 (51.6%) | 6/23 (26.1%) | 3/25 (12%) | 0.003 |
| Alcohol | 7/21 (33.3%) | 15/64 (23.4%) | 4/23 (17.4%) | 8/25 (32%) | 0.312 |
| Other occupational lung diseases, of which: | 13/21 (61.9%) | 50/64 (78.1%) | 17/23 (73.9%) | 0/25 (0%) | <0.001 |
| Occupational lung cancer | 0/21 (0%) | 1/64 (1.6%) | 2/23 (8.7%) | 0/25 (0%) | 0.137 |
| Occupational asthma | 1/21 (4.8%) | 3/64 (4.7%) | 1/23 (4.3%) | 0/25 (0%) | 0.751 |
| Occupational chronic bronchitis | 12/21 (57.1%) | 46/64 (71.9%) | 16/23 (69.6%) | 0/25 (0%) | <0.001 |
| Hypertension | 10/21 (47.6%) | 42/64 (65.6%) | 14/23 (60.9%) | 9/25 (36%) | 0.063 |
| Diabetes | 3/21 (14.3%) | 12/64 (18.8%) | 4/23 (17.4%) | 1/25 (4%) | 0.364 |
| Coronary heart disease | 4/21 (19%) | 23/64 (35.9%) | 8/23 (34.8%) | 4/25 (16%) | 0.180 |
| BMI | 27.4 [23.5, 32.3] | 28.53 [25.54, 32.83] | 26.78 [23.94, 27.41] | 29.4 [26.2, 34.7] | 0.023 |
| Pulmonary function test results | |||||
| VC (L) | 4.42 [3.86, 4.96] | 3.08 [2.66, 3.74] | 3.32 [2.36, 4.28] | 3.82 [3.42, 4.49] | <0.001 |
| VC, % pred | 91.8 [85.4, 106.5] | 91.10 [82.70, 101.0] | 82.90 [70.60, 101.0] | 93.9 [84.7, 99.54] | 0.252 |
| FVC (L) | 4.14 ± 1.104 | 3.08 ± 0.758 | 3.10 ± 1.084 | 3.72 ± 0.707 | <0.001 |
| FVC, %pred | 96.47 ± 24.215 | 89.09 ± 14.729 | 79.33 ± 19.925 | 90.90 ± 13.663 | 0.014 |
| FEV1 (L) | 3.58 [2.87, 3.98] | 2.40 [1.98, 2.88] | 2.20 [1.52, 3.04] | 3.23 [2.77, 3.77] | <0.001 |
| FEV1, %pred | 95.05 [84.65, 110.3] | 87.20 [75.15, 97.55] | 74.90 [52.30, 98.20] | 98.65 [88.55, 104.3] | <0.001 |
| FEV1/FVC (%) | 81.83 [74.90, 84.98] | 81.54 [74.48, 85.62] | 74.36 [61.76, 83.96] | 87.01 [82.10, 91.01] | <0.001 |
| FEF50 (L/s) | 3.94 [2.16, 4.87] | 2.56 [1.99, 3.34] | 1.92 [0.80, 2.88] | 3.98 [3.57, 4.83] | <0.001 |
| FEF50, %pred | 82.3 [52.7, 106.8] | 64.30 [49.60, 80.30] | 46.50 [18.00, 72.50] | 91.6 [85.0, 106.3] | <0.001 |
| Inflammatory markers and KL-6 serum concentrations | |||||
| Fibrinogen (mg/dL) | 298.0 [278.5, 387.5] | 374.5 [310.8, 404.8] | 366.0 [310.0, 394.0] | 305.0 [258.0, 350.0] | 0.001 |
| CRP (mg/L) | 1.98 [1.54, 5.58] | 2.83 [1.39, 5.58] | 2.41 [1.09, 5.48] | 3.28 [2.08, 4.95] | 0.871 |
| ESR (mm/1 h) | 13.00 [7.50, 27.00] | 20.50 [14.00, 29.75] | 20.00 [14.00, 41.00] | 13.00 [9.50, 14.50] | 0.001 |
| LDH (U/L) | 210.0 [200.0, 231.0] | 207.5 [179.3, 242.0] | 211.0 [185.0, 241.5] | 187.0 [145.0, 227.5] | 0.112 |
| KL-6 (U/mL) | 299.0 [271.5, 338.0] | 317.0 [244.5, 506.5] | 532.0 [483.0, 750.0] | 309.0 [235.0, 392.5] | <0.001 |
Clinical, paraclinical and demographic characteristics of the subjects.
Variables with a normal distribution are presented as mean ± standard error of the mean (SEM), while those with non-normal distribution are depicted as median with interquartile range [IQR]. EWs, exposed workers; SSs, simple silicosis subjects; CSs, complicated silicosis (progressive massive fibrosis) subjects; HCs, healthy controls; BMI, body mass index; VC, vital capacity; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; FEV1/FVC, the Tiffeneau-Pinelli index; FEF50%, maximal expiratory flow at 50% of the forced vital capacity; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; LDH, Lactate dehydrogenase; KL-6, Krebs von den Lungen-6.
Differences between subgroups: Retention time: EWs-SSs (p = 0.024), EWs-CSs (p = 0.003); Smoker: SSs-HCs (p = 0.003); Chronic bronchitis: EWs-HCs (p = 0.000), SSs-HCs (p = 0.000), CSs-HCs (p = 0.000); BMI: no significant differences between subgroups; VC(L): SSs-HCs (p = 0.018), EWs-SSs (p = 0.000), EWs-CSs (0.031); FVC(L): EWs-SSs (p = 0.000), EWs-CSs (p = 0.000), SSs-HCs (p = 0.004), CSs-HCs (p = 0.019); FVC, %pred: EWs-CSs (p = 0.002), SSs-CSs (p = 0.025), CSs-HCs (p = 0.029); FEV1 (L): EWs-SSs (p = 0.000), EWs-CSs (p = 0.002), SSs-HCs (p = 0.001), CSs-HCs (p = 0.004); FEV1, %pred: CSs-HCs (p = 0.012); FEV1/FVC (%): EWs-HCs (p = 0.041), SSs-HCs (p = 0.002), CSs-HCs (p = 0.000); FEF50 (L/s): EWs-CSs (p = 0.005), SSs-HCs (p = 0.000), CSs-HCs (p = 0.000); FEF50, %pred: EWs-CSs (p = 0.025), SSs-HCs (p = 0.000), CSs-HCs (p = 0.000); Fibrinogen (mg/dL): SSs-HCs (p = 0.001), CSs-HCs (p = 0.036); ESR (mm/1 h): SSs-HCs (p = 0.002), CSs-HCs (p = 0.013); KL-6 (U/mL): EWs-CSs (p = 0.000), SSs-CSs (p = 0.000), CSs-HCs (p = 0.000). Statistically significant p-values (p < 0.05) are shown in bold.
Pulmonary function test results
Table 1 illustrates the pulmonary function parameters. Significantly differences were observed among the groups in terms of FVC % pred. Between: EWs and CSs (p-value = 0.001), SSs and CSs (p-value = 0.023), CSs and HCs (p-value = 0.027); FEV1% pred. Between: CSs and HCs (p-value = 0.005), CSs and EWs (p-value = 0.031); the FEV1/FVC ratio between: EWs and HCs (p-value = 0.026), SSs and HCs (p-value = 0.001), CSs and HCs (p-value < 0.001); FEF50% pred. Between: EWs and CSs (p-value = 0.011), SSs and HCs (p-value <0.001), CSs and HCs (p-value < 0.001).
Inflammatory markers and KL-6 serum concentrations
Non-specific inflammatory markers assessed across subject groups are shown in Table 1. Significant differences (p-value < 0.05) were noted in Fibrinogen and ESR levels across the SSs, CSs, and HCs groups, with the following findings: (1) Fibrinogen: HCs and SSs, HCs and CSs; (2) ESR: HCs and SSs, HCs and CSs. Conversely, no significant variations were discerned in serum CRP and LDH concentrations among subjects across the four groups. Significant differences (p-value < 0.001) were noted in KL-6 serum concentrations (Figure 1) across the silicosis stages: EWs and CSs, SSs and CSs, and CSs and HCs.
Figure 1

Serum KL-6 levels in the healthy controls (HCs), exposed workers (EWs), simple silicosis (SSs) and complicated silicosis (CSs) groups. White dot: patient; orange square: median; black lines: interquartile range.
Correlations between KL-6 serum concentrations, inflammatory markers and pulmonary function tests among SSs and CSs groups
For the SSs group, there were no correlations between serum KL-6 levels, inflammatory markers, or pulmonary function test parameters, as indicated in Table 2. In the CSs group, KL-6 serum levels were positively correlated with CRP and ESR, and negatively correlated with VC, % pred., and FVC, % pred., as shown in Table 2 and Appendix C.
Table 2
| Fibrinogen | CRP | ESR | LDH | VC, % predicted | FVC, % predicted | FEV1, % predicted | FEV1/FVC | FEF50, % predicted | ||
|---|---|---|---|---|---|---|---|---|---|---|
| KL-6 in SSs group | Pearson Correlation | 0.049 | 0.133 | 0.054 | −0.111 | −0.143 | −0.132 | −0.058 | 0.038 | −0.041 |
| Sig. (2-tailed) | 0.700 | 0.294 | 0.672 | 0.398 | 0.270 | 0.312 | 0.658 | 0.774 | 0.755 | |
| N | 64 | 64 | 64 | 60 | 61 | 61 | 61 | 61 | 61 | |
| KL-6 in CSs group | Pearson Correlation | 0.212 | 0.447* | 0.439* | 0.308 | −0.544** | −0.467* | −0.389 | −0.257 | −0.116 |
| Sig. (2-tailed) | 0.332 | 0.033 | 0.036 | 0.175 | 0.007 | 0.025 | 0.067 | 0.237 | 0.598 | |
| N | 23 | 23 | 23 | 21 | 23 | 23 | 23 | 23 | 23 | |
KL-6 correlations with inflammatory markers and pulmonary function test results.
** Correlation is significant at the 0.01 level (2-tailed); * Correlation is significant at the 0.05 level (2-tailed). KL-6, Krebs von den Lungen-6; SSs, simple silicosis subjects; CSs, complicated silicosis (progressive massive fibrosis) group; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; LDH, Lactate dehydrogenase; VC, vital capacity; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; FEV1/FVC, the Tiffeneau-Pinelli index; FEF50%, maximal expiratory flow at 50% of the forced vital capacity.
Serum KL-6 cut-off values and ROC curve analysis
ROC curve analysis aimed to identify cut-off values for (1) the diagnosis of fibrosis in silica-exposed subjects and (2) progression of pulmonary fibrosis in silicosis patients. As illustrated in Figure 2, the area under the curve (AUC) for serum KL-6 was notably larger in the CSs group compared to EWs (0.971, p < 0.001) and SSs (0.831, p < 0.001) groups. The best positive and negative predictive values (95.45 and 90.90% respectively) were obtained for differentiating exposed workers and silicosis patients with PMF.
Figure 2
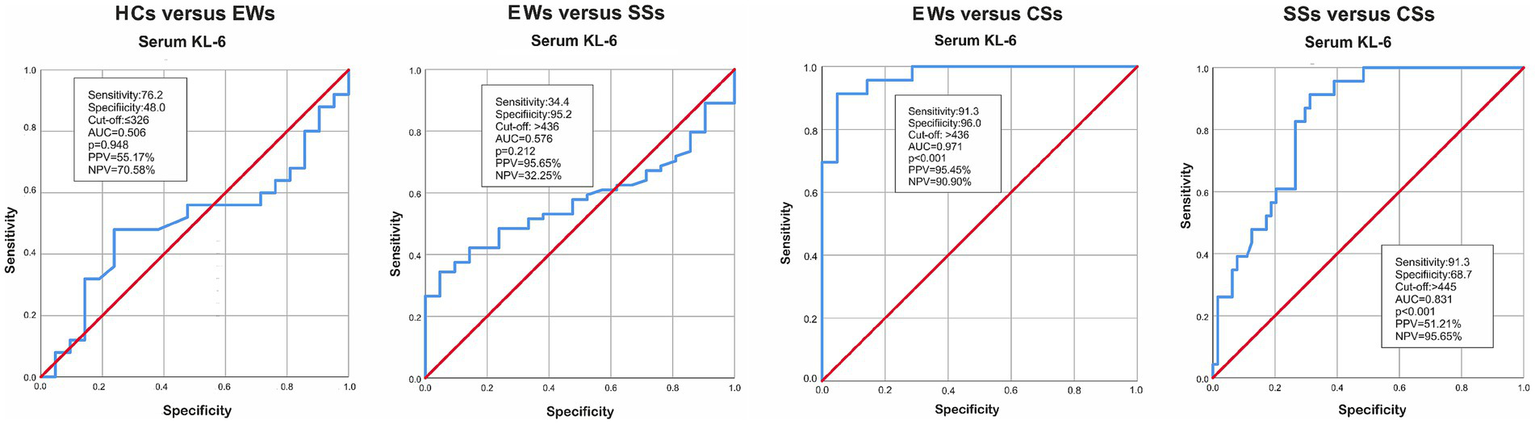
AUC for HCs versus EWs, EWs versus SSs, EWs versus CSs and SSs versus CSs. KL-6, Krebs von den Lungen-6; HCs, healthy controls; EWs, exposed workers; SSs, simple silicosis subjects; CSs, complicated silicosis subjects; AUC, Area under the ROC curve; PPV, positive predictive value; NPV, negative predicted value.
Analysis of predictors of silicosis in silica-exposed workers
Univariate and multivariate analyses were carried out to test whether serum KL-6, in addition to other covariates [age, gender, smoking history, body mass index (BMI), FVC % predicted, serum CRP and LDH, and Neutrophil-to-lymphocyte ratio (NLR)], is a predictor of silicosis onset in silica-exposed workers, as shown in Table 3a. The number of cases available in the analysis was 72, of which 57 events and 15 censored cases. Univariate analysis showed no significant predictors except age (hazard ratio (HR): 0.936; p = 0.001) and body mass index (BMI) (HR: 1.059; p = 0.036). The multivariate analysis showed that female gender was tendentially associated with a 30% increase in the risk of disease occurrence (p = 0.055).
Table 3
| No. of events/total | Hazard ratio | 95% CI | p-value | |
|---|---|---|---|---|
| (a) Silicosis occurrence | ||||
| Univariate analysis | ||||
| Age years (continuous) | 64/82 | 0.936 | 0.900–0.974 | 0.001 |
| Gender (ref. male) | 64/82 | 1.521 | 0.929–2.491 | 0.095 |
| Smoking history (ref. smoker) | 64/82 | 0.885 | 0.540–1.448 | 0.626 |
| BMI kg/m2 (continuous) | 62/80 | 1.059 | 1.003–1.118 | 0.037 |
| FVC % pred (continuous) | 61/78 | 1.001 | 0.988–1.014 | 0.896 |
| CRP mg/L (continuous) | 64/80 | 1.006 | 0.978–1.035 | 0.675 |
| LDH U/L (continuous) | 60/76 | 0.999 | 0.993–1.005 | 0.693 |
| NLR ratio (continuous) | 64/80 | 1.044 | 0.856–1.274 | 0.667 |
| KL-6100 U/mL (continuous) | 64/82 | 1.002 | 0.904–1.111 | 0.966 |
| Multivariate analysis | 57/72 (cumul.) | |||
| Age years (continuous) | 0.912 | 0.867–0.958 | <0.001 | |
| Gender (ref. male) | 1.699 | 0.989–2.919 | 0.055 | |
| BMI kg/m2 (continuous) | 1.092 | 1.030–1.158 | 0.003 | |
| (b) Silicosis progression | ||||
| Univariate analysis | ||||
| Age years (continuous) | 23/84 | 0.912 | 0.842–0.987 | 0.022 |
| Gender (ref. male) | 23/84 | 0.511 | 0.189–1.384 | 0.187 |
| Smoking history (ref. smoker) | 23/84 | 2.358 | 0.926–6.004 | 0.072 |
| BMI kg/m2 (continuous) | 23/82 | 0.906 | 0.821–1.000 | 0.050 |
| FVC % pred (continuous) | 23/81 | 0.986 | 0.964–1.010 | 0.248 |
| CRP mg/L (continuous) | 23/84 | 0.964 | 0.868–1.071 | 0.497 |
| LDH U/L (continuous) | 21/78 | 1.003 | 0.993–1.013 | 0.585 |
| NLR ratio (continuous) | 23/84 | 1.242 | 0.978–1.579 | 0.076 |
| KL-6100 U/mL (continuous) | 23/84 | 1.125 | 1.036–1.221 | 0.005 |
| Multivariate analysis | 21/75 (cumul.) | |||
| Age years (continuous) | 0.907 | 0.836–0.984 | 0.018 | |
| Gender (ref. male) | 0.362 | 0.122–1.076 | 0.067 | |
| Smoking history (ref. smoker) | 0.126 | 0.029–0.539 | 0.005 | |
| KL-6100 U/mL (continuous) | 1.197 | 1.069–1.340 | 0.002 | |
Univariate and multivariate COX proportional analysis for (a) silicosis occurrence and (b) silicosis progression.
Data are presented as hazard ratios, representing the relative risk of developing (a) disease occurrence and (b) disease progression. Backward stepwise (Likelihood Ratio) analysis was performed. BMI, body mass index; FVC, forced vital capacity; CRP, C-reactive protein; LDH, Lactate dehydrogenase; NLR, Neutrophil-to-lymphocyte ratio; KL-6, Krebs von den Lungen-6; 95% CI, 95% Confidence interval.
Analysis of predictors of PMF in silicosis subjects
Kaplan–Meier analysis indicated a predictive value of serum KL-6 for disease progression in silicosis subjects (Figure 3b), but not for the silicosis occurrence (Figure 3a). At a latency time of 30 years, the subjects with KL-6 > 445 U/mL had a worse progression-free survival rate (51%) compared to those with KL-6 levels ≤ 445 U/mL (97%).
Figure 3
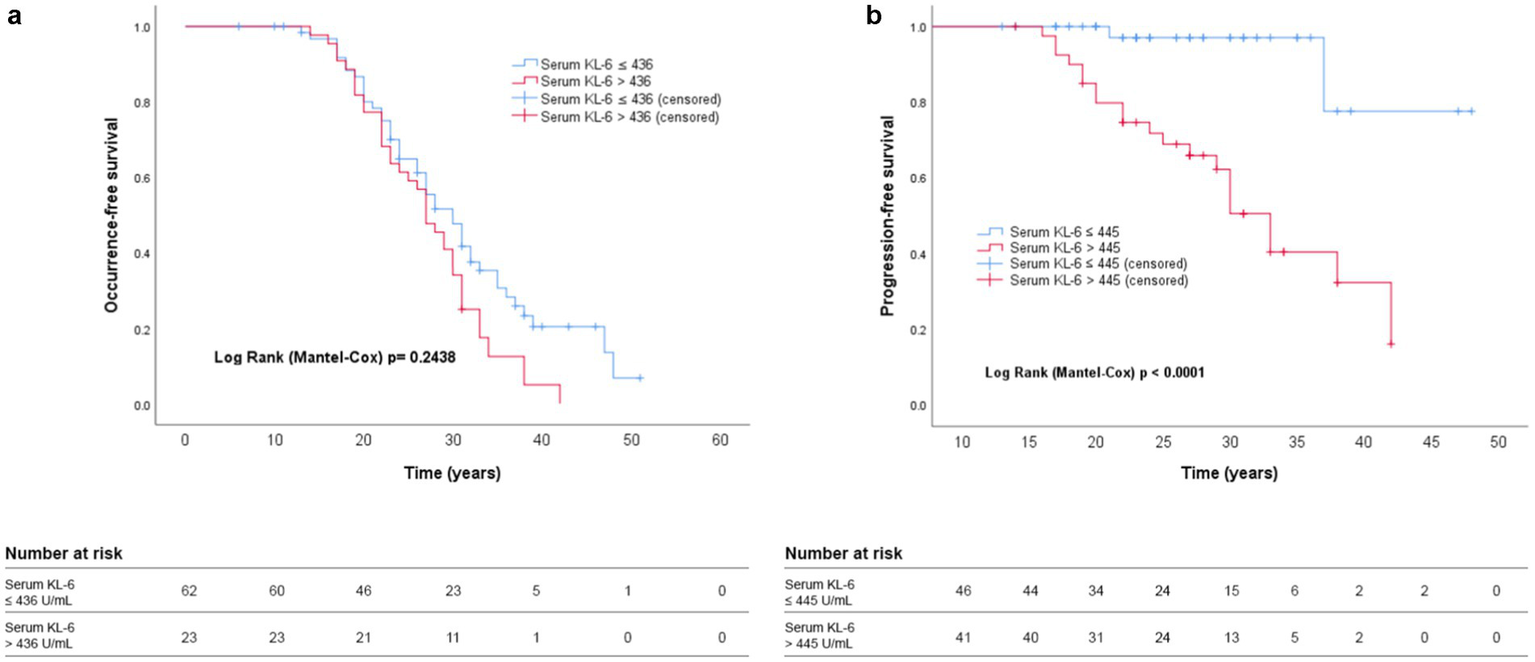
Kaplan–Meier analysis showing (a) silicosis occurrence and (b) silicosis progression according to serum KL-6 levels. The respective cut-off levels and significance (Log rank p) are shown in the graphic.
Table 3b shows the results of the univariate and multivariate analysis to identify PMF predictors. The number of cases available in the analysis was 75, of which 21 events and 54 censored cases. In the multivariate analysis, serum KL-6 levels were independently associated with PMF after adjustment for age, gender, smoking history, BMI, FVC % predicted, serum CRP and LDH, and NLR as covariates (HR 1.197; p = 0.002). Female gender and a negative smoking history were protective against PMF development.
Discussion
Our study found higher serum KL-6 levels in patients with complicated silicosis with progressive massive fibrosis (CSs) compared to exposed workers without radiological signs of silicosis (EWs) and those with simple silicosis (SSs). We identified a cut-off value of serum KL-6 for predicting the progression of pulmonary fibrosis in silicosis patients. Differentiation between simple (opacities ≤ 1 cm) and complicated silicosis (opacities > 1 cm) was achieved at a serum KL-6 cut-off of 445 U/mL, with a specificity and sensitivity exceeding 90%. As with ILDs patients, our data indicated that KL-6 serum levels correlate with the severity of fibrosis assessed by standard radiology (25), supporting the use of KL-6 as biomarker for the lung fibrosis progression. Thus, the increased KL-6 serum levels in complicated and progressive forms could be explained by the alteration of the alveolar-capillary membrane due to extensive pulmonary fibrosis. These findings align with other studies linking KL-6 serum levels to disease progression in ILDs and IPF (13, 26).
Different cut-off values for serum KL-6 levels were proposed in the literature (27). One study suggests a cut-off of 500 U/mL to differentiate ILDs patients from healthy individuals (28). More than 70% of patients with different ILDs (idiopathic interstitial fibrosis, collagenosis, hypersensitivity pneumonitis, radiation pneumonitis, drug-induced pneumonitis, acute respiratory distress syndrome, sarcoidosis, alveolar proteinosis) had KL-6 serum levels exceeding 500 U/mL (26) and therefore, KL-6 cannot be considered a specific marker of silicosis. For patients with known exposure and already diagnosed small opacities in the lungs, the progression toward massive fibrosis has significant clinical implications and serum KL-6 might be of clinical relevance. In our study, a serum KL-6 cut-off of 436 U/mL differentiated EWs from those with PMF with a specificity and sensitivity exceeding 90%. However, KL-6 had an insufficient sensitivity (34.4%) to differentiate between EWs and SSs. Thus, based on these findings, we cannot propose serum KL-6 as a screening marker. However, the high specificity (95.2%) is a strong argument to consider serum KL-6 a marker of fibrosis, potentially serving as a valuable tool for monitoring the progression of pulmonary fibrosis in patients already diagnosed with silicosis. This approach is supported by experimental and clinical research, which demonstrate that exposure to crystalline silica leads to proliferation and hyperplasia of type II pneumocytes (29). Moreover, recent studies suggest that damaged alveolar epithelial cells release KL-6, which may promote the transformation of fibroblasts into myofibroblasts, increase collagen I and III production, and suppress HGF production (30). This highlights KL-6 as a contributor to fibrosis in various ILDs.
In CSs group, the level of KL-6 in serum was positively correlated with several inflammatory biomarkers, namely serum CRP and ESR. While extensive literature supports KL-6 as a marker of alveolar epithelial destruction and increased alveolar-capillary permeability (31), studies to correlate the serum KL-6 to inflammation are much less frequent. The CRP and the ESR were collected in the absence of an acute infection or other clinically manifest inflammatory conditions. In other conditions involving ILDs, such as those related to radiation treatment (32) or associated with connective tissue diseases (33), CRP or ESR levels were correlated with serum KL-6 in predicting disease severity. To the best of our knowledge, this study is the first to demonstrate a correlation between serum KL-6 levels and nonspecific inflammatory markers in patients with occupational progressive massive fibrosis, as expected considering the inflammatory background of fibrosis (34).
Another finding of this study was the negative correlation between KL-6 serum levels and lung function test results (VC % predicted and FVC % predicted), consistent with previous studies on ILDs of different causes (13, 26, 32, 35).
The multivariate analysis showed that serum KL-6 as continuous variable is independently associated with the disease progression in silicosis together with age, gender and smoking status. Higher levels of KL-6 were associated with shorter latency to PMF; subjects with serum KL-6 levels > 445 U/mL had a worse progression-free survival rate (51%) than those with serum KL-6 ≤ 445 U/mL (97%). These findings align with other studies linking serum KL-6 levels to the progression of pulmonary fibrosis in ILDs (36). Male gender has been also identified as an independent risk factor for progression in ILD in several studies (37) and smoking has been associated with a worse evolution in IPF (38). There is evidence that smoking directly is associated with fibrosis in the subpleural and peribronchiolar interstitium (39). In artificial stone dust silicosis, smoking seems to have a protective effect (40), but the mechanism of accelerated silicosis is different compared to the much less aggressive evolution of the chronic silicosis in the foundry workers. In a large cohort of foundry workers, smoking increased the risk of silicosis by 3.79 folds (41). To the best of our knowledge, there are no studies on foundry workers about the impact of smoking on the progression of silicosis. In this respect, the analysis of our patients encourages to prospectively investigate this potential influencer of the silicosis prognosis.
Although the results are promising, this study has potential limitations. First, it was a single-center study with a relatively small number of subjects in each subgroup. We are aware that this number is in the lower range of the estimated necessary numbers, but still remain significant for the long term follow up after the initial exposure (an average retention time exceeding 30 years across all subgroups). The group is rather homogenous in terms of occupational exposure to crystalline silica from cast iron foundries, a type of exposure which is rarely approached in European studies (42). In addition, the average age of our study population corresponds to exposure during their working time, a period in which the foundries had the same technology and protective measures. We therefore consider that the intensity of exposure was homogeneous and unlikely to have significantly influenced the results. Second, although the questionnaire used in this study complies with national regulations and is commonly used in occupational disease department practice, designed to collect basic demographic information, symptoms and data on occupational exposure to pneumoconiogenic dusts and fibers, its validity and reliability were not independently assessed for this study. Moreover, the diagnosis was based on the X-ray and not on CT or HR-CT, to more accurately assess the extension of the pulmonary fibrosis lesions. However, the current classification in clinical practice is based on X-ray, which remains the most extended screening method. Revisions of the X-rays by experienced physicians mitigates this risk, particularly the misclassification between SS and CS. We are aware that several other factors might be related to the level of serum KL-6, such as polymorphisms of MUC1 (43, 44) or the inflammatory/immunological status reflected in other serum biomarkers. To the best of our knowledge there is no information about the interaction between MUC genes expression and the KL-6 serum levels in silicosis; their potential influence might represent a limitation. We have checked on the relation between the complicated silicosis and the CRP, ESR fibrinogen, and LDH levels and found significant association with the progression of silicosis, even though in CS group both CRP and ESR were correlated with KL-6 serum levels. We did not check for other biomarkers such as serum amyloid A or surfactant protein –D, as previous publications found of no relation with the progression of silicosis (45, 46), not even in the stone benchtop industry workers, which have a more aggressive form of silicosis than the silicosis from foundries included in our analysis (47). Translating the lessons learnt from other interstitial lung fibrosis, a panel of biomarkers might also perform better for silicosis. Based on our results we can only support that the selection of biomarkers to characterize the PMF should include serum KL-6. Lastly, we did not monitored KL-6 serum concentrations over time and we cannot draw any conclusion on the prognostic value of KL-6.
Conclusion
In conclusion, there is a scarcity of studies specifically investigating the correlation between serum KL-6 and exposure to pneumoconiogenic mineral dusts in European foundry workers. KL-6 is already approved as a diagnostic and prognostic biomarker for diffuse interstitial lung diseases in some countries, and its pathophysiologic mechanism supports a potential utility in pneumoconioses. From our results, serum KL-6 appears to be a promising biomarker of the progression of occupational fibrosis lesions in silica-exposed patients.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Carol Davila University of Medicine and Pharmacy No. 8244/28.03.2022. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
R-AS: Conceptualization, Data curation, Methodology, Resources, Writing – original draft. M-RO: Conceptualization, Formal analysis, Methodology, Supervision, Writing – review & editing. EP: Data curation, Formal analysis, Resources, Software, Writing – review & editing. AJ: Data curation, Formal analysis, Investigation, Resources, Writing – review & editing. FB: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing. AR: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Acknowledgments
Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fmed.2025.1654729.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1579209/full#supplementary-material
References
1.
DeLight N Sachs H . Pneumoconiosis. Treasure Island, FL: StatPearls Publishing (2024).
2.
Cocarla A . Silicoza In: CocarlaA, editor. Medicina ocupațională. Cluj-Napoca, Romania: Editura Medicala Universitara Iuliu Hatieganu (2008)
3.
Rașcu A Artenie C Naghi E Șerbescu A Gherman D . Silicoza. Cluj-Napoca, Romania: Universitară București (2004).
4.
Barnes H Goh NSL Leong TL Hoy R . Silica-associated lung disease: an old-world exposure in modern industries. Respirology. (2019) 24:1165–75. doi: 10.1111/resp.13695
5.
STEL . (2020). Research report short term exposure limit for respirable crystalline silica SLR ref no: 640.30012-R01-v4.0 (STEL RCS FINAL), Available online at: https://www.safeworkaustralia.gov.au/doc/report-short-term-exposure-limit-respirable-crystalline-silica. (Accessed December 2, 2024).
6.
Adamson IY Prieditis H . Silica deposition in the lung during epithelial injury potentiates fibrosis and increases particle translocation to lymph nodes. Exp Lung Res. (1998) 24:293–306. doi: 10.3109/01902149809041536
7.
Neacșu GRL Rașcu A Bumbăcea D . From stone grinder to lung transplant candidate: A young Worker's Battle with silicosis. Rom J Intern Med. (2023) 74:30–5. doi: 10.2478/rjom-2023-0005
8.
Smărăndescu RA Căluțu IM Rașcu A Bușnatu ȘS . Diagnostic challenges of radiological opacities in silicosis - case reports. Occup Med. (2022) 72:424–7. doi: 10.1093/occmed/kqac044
9.
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/s0140-6736(18)32279-7
10.
International Labour Organization . (2018). Guidelines for the use of the ILO international classification of radiographs of Pneumoconioses, revised edition 2022, Geneva. Available online at: https://www.ilo.org/resource/ilo-international-classification-radiographs-pneumoconioses-1. (Accessed December 2, 2024).
11.
Rascu A Naghi E . Silicoza In: HandraCM, editor. Boli profesionale ale aparatului respirator - Ghid pentru studenti si medici. Bucuresti: Editura Universitara Carol Davila (2019). 76–99.
12.
Căluțu IM Smărăndescu RA Rașcu A . Biomonitoring exposure and early diagnosis in silicosis: A comprehensive review of the current literature. Biomedicines. (2023) 11:100. doi: 10.3390/biomedicines11010100
13.
Jehn LB Costabel U Boerner E Wälscher J Theegarten D Taube C et al . Serum KL-6 as a biomarker of progression at any time in fibrotic interstitial lung disease. J Clin Med. (2023) 12:1173. doi: 10.3390/jcm12031173
14.
Zhou A Tang H Peng W Wang Y Tang X Yang H et al . KL-6 levels in the connective tissue disease population: typical values and potential confounders-a retrospective, real-world study. Front Immunol. (2023) 14:1098602. doi: 10.3389/fimmu.2023.1098602
15.
Kohno N Inoue Y Hamada H Fujioka S Fujino S Yokoyama A et al . Difference in serodiagnostic values among KL-6-associated mucins classified as cluster 9. Int J Cancer. (1994) 57:81–3. doi: 10.1002/ijc.2910570717
16.
Kobayashi J Kitamura S . KL-6: a serum marker for interstitial pneumonia. Chest. (1995) 108:311–5. doi: 10.1378/chest.108.2.311
17.
Ohnishi H Yokoyama A Kondo K Hamada H Abe M Nishimura K et al . Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. (2002) 165:378–81. doi: 10.1164/ajrccm.165.3.2107134
18.
Widdicombe JG Pack RJ . The Clara cell. Eur J Respir Dis. (1982) 63:202–20. PMID:
19.
Lesur O Cantin AM Tanswell AK Melloni B Beaulieu JF Bégin R . Silica exposure induces cytotoxicity and proliferative activity of type II pneumocytes. Exp Lung Res. (1992) 18:173–90. doi: 10.3109/01902149209031679
20.
Ishikawa N Hattori N Yokoyama A Kohno N . Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig. (2012) 50:3–13. doi: 10.1016/j.resinv.2012.02.001
21.
Rosenman KD Reilly MJ Rice C Hertzberg V Tseng CY Anderson HA . Silicosis among foundry workers. Implication for the need to revise the OSHA standard. Am J Epidemiol. (1996) 144:890–900. doi: 10.1093/oxfordjournals.aje.a009023
22.
Nandi SS Dhatrak SV Sarkar K . Silicosis, progressive massive fibrosis and silico-tuberculosis among workers with occupational exposure to silica dusts in sandstone mines of Rajasthan state: an urgent need for initiating national silicosis control programme in India. J Family Med Prim Care. (2021) 10:686–91. doi: 10.4103/jfmpc.jfmpc_1972_20
23.
Graham BL Steenbruggen I Miller MR Barjaktarevic IZ Cooper BG Hall GL et al . Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. (2019) 200:e70–88. doi: 10.1164/rccm.201908-1590ST
24.
d’Alessandro M Bergantini L Cameli P Lanzarone N Antonietta Mazzei M Alonzi V et al . Serum KL-6 levels in pulmonary Langerhans’ cell histiocytosis. Eur J Clin Investig. (2020) 50:e13242. doi: 10.1111/eci.13242
25.
Qin H Xu XP Zou J Zhao XJ Wu HW Zha QF et al . Krebs von den Lungen-6 associated with chest high-resolution CT score in evaluation severity of patients with interstitial lung disease. Pulmonology. (2019) 25:143–8. doi: 10.1016/j.pulmoe.2018.05.008
26.
Jiang Y Luo Q Han Q Huang J Ou Y Chen M et al . Sequential changes of serum KL-6 predict the progression of interstitial lung disease. J Thorac Dis. (2018) 10:4705–14. doi: 10.21037/jtd.2018.07.76
27.
Kattner S Sutharsan S Berger MM Limmer A Jehn LB Herbstreit F et al . Serum KL-6 as a candidate predictor of outcome in patients with SARS-CoV-2 pneumonia. J Clin Med. (2023) 12:6772. doi: 10.3390/jcm12216772
28.
Kitamura S Hiwada K Kobayashi J Kohno N Kawai T Satou A et al . Use of the the ED046 kit to analyze serum KL-6 in patients with pneumonitis. Nihon Kyobu Shikkan Gakkai Zasshi. (1996) 34:639–45. PMID:
29.
Lam M Mansell A Tate MD . Another one fights the dust: targeting the NLRP3 Inflammasome for the treatment of silicosis. Am J Respir Cell Mol Biol. (2022) 66:601–11. doi: 10.1165/rcmb.2021-0545TR
30.
Xu L Yan DR Zhu SL Gu J Bian W Rong ZH et al . KL-6 regulated the expression of HGF, collagen and myofibroblast differentiation. Eur Rev Med Pharmacol Sci. (2013) 17:3073–7. PMID:
31.
Nukiwa T . The role of biomarkers in management of interstitial lung disease: implications of biomarkers derived from type II pneumocytes. Eur Respir Mon. (2009) 46:47–66. doi: 10.1183/1025448x.00046004
32.
Park HK Yoon CS Na YO Lee JK Oh HJ Park HY et al . Serum KL-6 levels predict the occurrence and severity of treatment-related interstitial lung disease in lung cancer. Sci Rep. (2023) 13:18126. doi: 10.1038/s41598-023-45170-8
33.
Lee JS Lee EY Ha YJ Kang EH Lee YJ Song YW . Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res Ther. (2019) 21:58. doi: 10.1186/s13075-019-1835-9
34.
Balci A Düz ME Vurmaz A Çilekar Ş Kaya F . Comprehensive biomarker analysis of patients with idiopathic pulmonary fibrosis and interstitial lung disease with healthy individuals. Eur Rev Med Pharmacol Sci. (2023) 27:5468–79. doi: 10.26355/eurrev_202306_32783
35.
Chung C Kim J Cho HS Kim HC . Baseline serum Krebs von den Lungen-6 as a biomarker for the disease progression in idiopathic pulmonary fibrosis. Sci Rep. (2022) 12:8564. doi: 10.1038/s41598-022-12399-8
36.
Zhang T Shen P Duan C Gao L . KL-6 as an immunological biomarker predicts the severity, progression, acute exacerbation, and poor outcomes of interstitial lung disease: A systematic review and Meta-analysis. Front Immunol. (2021) 12:745233. doi: 10.3389/fimmu.2021.745233
37.
Wong AW Ryerson CJ Guler SA . Progression of fibrosing interstitial lung disease. Respir Res. (2020) 21:32. doi: 10.1186/s12931-020-1296-3
38.
Margaritopoulos GA Vasarmidi E Jacob J Wells AU Antoniou KM . Smoking and interstitial lung diseases. Eur Respir Rev. (2015) 24:428–35. doi: 10.1183/16000617.0050-2015
39.
Sousa C Rodrigues M Carvalho A Viamonte B Cunha R Guimarães S et al . Diffuse smoking-related lung diseases: insights from a radiologic-pathologic correlation. Insights Imaging. (2019) 10:73. doi: 10.1186/s13244-019-0765-z
40.
Ophir N Shai AB Alcalay Y et al . Smoking has a protective effects on functional and inflammatory parameters in workers exposed to artificial stone dust. Eur Respir J. (2016) 48:PA4281. doi: 10.1183/13993003.congress-2016.PA4281
41.
Zhang M Zheng YD Du XY Lu Y Li WJ Qi C et al . Silicosis in automobile foundry workers: a 29-year cohort study. Biomed Environ Sci. (2010) 23:121–9. doi: 10.1016/S0895-3988(10)60041-4
42.
Hoy RF Jeebhay MF Cavalin C Chen W Cohen RA Fireman E et al . Current global perspectives on silicosis-convergence of old and newly emergent hazards. Respirology. (2022) 27:387–98. doi: 10.1111/resp.14242
43.
Bonella F Long X Ohshimo S Horimasu Y Griese M Guzman J et al . MUC1 gene polymorphisms are associated with serum KL-6 levels and pulmonary dysfunction in pulmonary alveolar proteinosis. Orphanet J Rare Dis. (2016) 11:48. doi: 10.1186/s13023-016-0430-2
44.
Horimasu Y Hattori N Ishikawa N Kawase S Tanaka S Yoshioka K et al . Different MUC1 gene polymorphisms in German and Japanese ethnicities affect serum KL-6 levels. Respir Med. (2012) 106:1756–64. doi: 10.1016/j.rmed.2012.09.001
45.
Aloe C Papagianis P Mcqualter J . Systemic ferritin is an early disease biomarker for silicosis patients. Eur Respir J. (2021) 58:PA2255. doi: 10.1183/13993003.congress-2021.PA2255
46.
Xue C Wu N Li X Qiu M du X Ye Q . Serum concentrations of Krebs von den Lungen-6, surfactant protein D, and matrix metalloproteinase-2 as diagnostic biomarkers in patients with asbestosis and silicosis: a case–control study. BMC Pulm Med. (2017) 17:144. doi: 10.1186/s12890-017-0489-0
47.
Aloe CA Leong TL Wimaleswaran H Papagianis PC McQualter JL McDonald CF et al . Excess iron promotes emergence of foamy macrophages that overexpress ferritin in the lungs of silicosis patients. Respirology. (2022) 27:427–36. doi: 10.1111/resp.14230
Summary
Keywords
KL-6, silicosis, occupational fibrosis, crystalline silica, biomarker, disease progression
Citation
Smărăndescu R-A, Oțelea M-R, Panaitescu E, Jitka A, Bonella F and Rașcu A (2025) Serum concentrations of Krebs von den Lungen-6 as prognostic biomarker in patients with silicosis. Front. Med. 12:1579209. doi: 10.3389/fmed.2025.1579209
Received
18 February 2025
Accepted
06 May 2025
Published
06 June 2025
Corrected
28 July 2025
Volume
12 - 2025
Edited by
Annangi Balasubramanyam, Autonomous University of Barcelona, Spain
Reviewed by
Chengliang Yang, University of British Columbia, Canada
Hadiseh Rabiei, Shahid Beheshti University of Medical Sciences, Iran
Updates
Copyright
© 2025 Smărăndescu, Oțelea, Panaitescu, Jitka, Bonella and Rașcu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eugenia Panaitescu, eugenia.panaitescu@umfcd.ro
†These authors share senior authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.