Abstract
Background:
Overactive bladder (OAB) is a common condition in women, affecting quality of life with symptoms like urgency, frequency, and nocturia. Current treatments, such as antimuscarinic drugs, have side effects that limit their effectiveness. Electroacupuncture (EA) shows promise as an alternative, but its mechanisms and effectiveness for OAB are not fully understood.
Objective:
This blinded, randomized controlled trial aimed to evaluate the efficacy and safety of electroacupuncture as a therapeutic intervention for female patients with OAB and to explore potential mechanisms involving the sacral and posterior tibial nerves.
Methods:
Sixty-eight female OAB patients were stratified and randomized into two groups. One group received EA treatment at BL33 and SP6 acupoints three times weekly for 4 weeks, while the control group received tolterodine, a standard antimuscarinic medication. Outcome measures included urgency symptoms, Overactive Bladder Symptom Score (OABSS), and quality of life at 2 and 4 weeks post-treatment, as well as at a 3-month follow-up. Safety and acceptance of EA were also assessed. Additionally, urinary cytokine levels were analyzed to investigate the neurobiological impact of the treatments.
Results:
No significant baseline differences were observed between the groups. At 2 weeks, EA significantly improved quality of life scores (p = 0.002), and by 4 weeks, both groups showed improvements in urgency symptoms and quality of life (p < 0.05), with no significant difference in OABSS (p = 0.081). The EA group demonstrated a significantly higher overall effective rate (88.6%) compared to the medication group (48.5%) (p = 0.002). Safety assessments indicated high acceptance and minimal discomfort with EA, while post-treatment urinary cytokine analysis revealed significant changes in BDNF levels, suggesting a neurobiological effect of EA.
Conclusion:
Electroacupuncture at BL33 and SP6 is a promising, well-tolerated, and effective intervention for OAB, supporting its integration into treatment paradigms. Further research is needed to optimize its clinical application.
Clinical trial registration:
clinicaltrials.gov, identifier ChiCTR-1900021372.
1 Introduction
Overactive bladder (OAB) is a common functional urological disorder characterized by urinary urgency, often accompanied by increased frequency and nocturia, with or without urgency incontinence. According to the International Continence Society (ICS), OAB is defined as a symptom-based syndrome in the absence of urinary tract infection or other obvious pathology (1, 2). Globally, the condition affects approximately 12–21% of adults, with a higher prevalence among women (22.1%) than men (19.5%), and incidence increases substantially with age (3, 4). Despite its high prevalence and significant impact on health-related quality of life (HRQoL), underdiagnosis and limited healthcare-seeking behavior remain common (5). Current pharmacotherapies, particularly muscarinic receptor antagonists, are associated with adverse effects such as dry mouth, constipation, and cognitive decline, leading to poor long-term adherence (6–9). These limitations underscore the need for safer and more tolerable therapeutic alternatives.
Neuromodulation has emerged as a key non-pharmacological approach in the management of refractory OAB. Sacral neuromodulation (SNM) delivers electrical impulses to the sacral nerve roots to modulate abnormal reflex pathways involved in bladder function and has shown proven efficacy (10–12). However, the high cost, invasiveness, and potential for diminishing efficacy over time limit its widespread use (13–15). Posterior tibial nerve stimulation (PTNS) offers a less invasive alternative, stimulating afferent fibers of the tibial nerve via surface or needle electrodes, but often requires multiple sessions with uncertain long-term outcomes (16, 17).
Electroacupuncture (EA), a therapy rooted in traditional Chinese medicine and increasingly recognized in modern neuromodulation frameworks, may offer a promising complementary strategy. The BL33 (Zhongliao) and SP6 (Sanyinjiao) acupoints are anatomically aligned with sacral and tibial nerve trajectories, allowing EA to mimic dual-site neuromodulation through minimally invasive means (18–21). This study employed a randomized, evaluator-blinded, positive-controlled design to evaluate the clinical efficacy and safety of EA in female patients with OAB. The EA group received stimulation at bilateral BL33 and SP6, simulating sacral and tibial nerve pathways, while the control group received tolterodine tartrate extended-release tablets. The primary objective was to assess whether EA can effectively alleviate OAB symptoms, improve patient-reported outcomes, and provide mechanistic insight into its neuromodulatory action through urinary cytokine analysis.
2 Methods
2.1 Design
This study was a single-center, assessor-blinded, parallel-group randomized controlled trial (RCT). Participants were randomized into two groups: an EA group and a medication control group. Outcome measurements were assessed at baseline, 2 weeks after the start of treatment, 4 weeks after the start of treatment, and at a 3 months follow-up. The trial adhered to the Consolidated Standards of Reporting Trials (CONSORT) (22) and the Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) guidelines (23).
The trial was conducted at the Xiyuan Hospital, China Academy of Chinese Medical Sciences, with approval from the hospital’s ethics committee (Approval number: 2018XLA070-3). A qualified clinical trial monitor was involved to ensure the quality of the trial, providing oversight and recommendations for any issues during the trial process. The trial was registered in the Chinese Clinical Trial Registry (ChiCTR-1900021372).
2.2 Participants
Participants were recruited from Xiyuan Hospital, China Academy of Traditional Chinese Medicine, between January 2019 and February 2022. Recruitment included outpatient clinics and online advertisements. All participants provided written informed consent before enrollment.
Participants were included if they met the following criteria: (1) female patients aged between 18 and 60 years; (2) met the diagnostic criteria for overactive bladder (OAB), as defined by the International Continence Society; (3) had not used any Chinese or Western medications for OAB within the past week; and (4) voluntarily signed the informed consent form and agreed to participate in the trial.
Participants were excluded if they met any of the following conditions: (1) presence of urinary tract infection or lower urinary tract obstruction; (2) diagnosis of neurogenic bladder; (3) urethral dysfunction; (4) urinary retention or post-void residual volume >50 mL; (5) severe myasthenia gravis; (6) history of ulcerative colitis or narrow-angle glaucoma; (7) known allergy to tolterodine tartrate tablets; (8) deemed by the investigators to be unable to comply with the study procedures; (9) non-compliance with medication, incomplete data, or other conditions that affect efficacy evaluation; (10) pregnant or breastfeeding; and (11) use of a cardiac pacemaker, metal allergy, or severe needle phobia.
Subjects were excluded from efficacy analysis under the following circumstances: (1) severe protocol violations of the inclusion or exclusion criteria; that is, participants who should not have been randomized; (2) failure to receive any treatment after enrollment; and (3) premature discontinuation due to adverse drug reactions (these cases were excluded from efficacy analysis but included in safety evaluation).
Participants were withdrawn from the trial under the following conditions: (1) withdrawal as decided by the investigators; (2) worsening of the condition during the trial, necessitating termination of participation as judged by the physician; and (3) poor medication adherence (compliance <80%), or self-initiated changes in medication regimen, including addition of medications prohibited by the protocol.
Participants who deviated from the inclusion or exclusion criteria, failed to receive treatment, or discontinued treatment due to adverse drug reactions were excluded from the efficacy evaluation but included in adverse event reporting.
2.3 Sample size and randomization
Based on the anticipated efficacy rates derived from preliminary studies and literature reviews (24–26), with effectiveness rates estimated between 58 and 68% for one group and 81 and 90% for the other, a sample size calculation was performed. Assuming a significance level (α) of 0.05 and a statistical power (1-β) of 0.90, and accounting for an expected dropout rate of 20%, the minimum required sample size for each group was calculated to be 34 participants. Thus, the total sample size for both groups combined was 68 participants, ensuring the study was adequately powered to detect clinically significant differences in outcomes. Stratified blocked randomization was employed to allocate participants into two groups (EA group and medication group) based on the severity of baseline OAB symptoms (mild–moderate and severe). A computer-generated random number table was used to assign participants in a 1:1 ratio. The randomization process was concealed, and the allocation sequence was held by a single administrator. This administrator was the only person aware of group assignments until the intervention began. The allocation was concealed from participants, healthcare providers, and other researchers involved in the study to ensure blinding.
While the treatment group assignment was disclosed to the acupuncturists at the time of treatment initiation, outcome assessors and statisticians remained blinded throughout the study to ensure unbiased evaluation of outcomes.
2.4 Electroacupuncture group
All treatments were performed by trained acupuncturists with at least 5 years of clinical acupuncture experience. Participants in the EA group received real acupuncture with electrostimulation at the BL33 (Baliao) and SP6 (Sanyinjiao) acupoints. Acupuncturists performed needle manipulation, including lifting, thrusting, and rotating the needles to achieve the de qi sensation (a characteristic needle sensation indicating proper stimulation). A 3-inch (0.30 × 75 mm) filiform needle was used for BL33, inserted at a depth of 50–60 mm, while a 2-inch (0.30 × 50 mm) needle was used for SP6, inserted to a depth of 30–40 mm. BL33 Acupoint: Located approximately 1 cm lateral to the third sacral foramen. The needle was inserted at a 30–45° angle downward. SP6 Acupoint: Located on the medial aspect of the lower leg. The needle was inserted perpendicularly (Figure 1). Once the de qi sensation was achieved, electrodes were connected to the needles at BL33 and SP6, and electroacupuncture stimulation was applied using a sparse-dense wave with a frequency of 50 Hz (SDZ-V Huatuo Electronic Acupuncture Instrument, Suzhou Medical Supplies Factory Co., Ltd.). The electrical current intensity was adjusted from 1 mA to 5 mA, based on the participant’s tolerance. Each EA session lasted for 30 min (27, 28).
Figure 1

Electroacupuncture stimulation of the sacral nerve S3 via BL33 and the posterior tibial nerve via SP6.
2.5 Control group
Participants in the control group received Tolterodine tartrate extended-release tablets (4 mg/day) manufactured by Nanjing Meirui Pharmaceutical Co., Ltd. (National Drug Approval H20000602). The treatment lasted for 4 weeks, and participants were instructed not to take any additional medications for overactive bladder during this period.
2.6 Outcome measures
Baseline and clinical data collection: At the start of the study, baseline demographic and clinical data were gathered to ensure consistency among participants. The primary outcome, assessed with the Overactive Bladder Symptom Score (OABSS) (29, 30), focused on urgency symptoms and urination frequency. Secondary outcomes included Quality of Life (QOL) scores (31) and subjective efficacy, evaluated at 2 weeks after the start of treatment, 4 weeks after the start of treatment, and at a 3 months follow-up. This methodical approach allowed for detailed analysis of treatment efficacy over time, with expansive details chronicled in Annex 1, 2, and 3.
Urine cytokine analysis: Urine samples were collected at the start and end of the four-week treatment to assess the biological effects of EA on OAB (BDNF, EGF, NGF, PGE2, MIP1β, and MCP1). Strict protocols minimized diurnal variations, and samples were analyzed via enzyme-linked immunosorbent assay (ELISA), correlating biochemical data with clinical results.
Safety and acceptance evaluation: Before each EA session, safety checks were performed to monitor for adverse events such as needle issues, dizziness, severe pain (VAS ≥ 8), and prolonged post-treatment discomfort (VAS ≥ 4). Additional metrics included monitoring for local hematoma, infections, or abscesses and documenting any secondary discomforts like fatigue and headache where VAS scores were ≥4 (32). Participant acceptance was rated on a five-point scale from “very difficult to accept” to “very easy to accept,” assessed after the first and sixth treatment sessions to gauge ongoing patient tolerance and satisfaction.
2.7 Statistical analysis
Intention-to-treat (ITT) analysis will be employed to account for all randomized participants, with missing data handled by carrying forward the last observation from the previous examination. Statistical analyses will be performed using SPSS version 25.0. For normally distributed or approximately normally distributed continuous data, results will be presented as mean ± standard deviation (SD). Comparisons between groups will be conducted using independent-samples t-tests. For skewed distribution data, results will be presented as median (interquartile range, IQR), and comparisons between groups will be performed using the Mann–Whitney U-test.
For repeated measurements from the same subjects, repeated-measures analysis of variance (ANOVA) will be used to evaluate differences across time points, between groups, and for interaction effects between time and group. Additionally, randomized block ANOVA with Dunnett’s method will be used to compare differences at multiple time points after treatment with baseline values.
3 Results
3.1 Patient demographics and baseline characteristics
This study enrolled 68 female OAB patients using a stratified randomization protocol, allocating 35 to the EA group and 33 to the medication group after excluding two severe cases to meet the sample cap (Figure 2). Despite slight discrepancies in severe case distribution, statistical blinding was maintained, identifying groups as “Group 1” and “Group 2” Statistical tests, including t-tests and chi-square tests, showed no significant differences between groups in age, disease duration, menopausal status, or disease severity, confirming comparable baseline characteristics. The EA group had an average age of 37.51 ± 12.61 years with a disease duration of 6.80 ± 2.06 months, while the medication group had an average age of 38.76 ± 10.19 years with a disease duration of 6.67 ± 2.41 months. Initial symptom scores and cytokine levels were also matched across groups, demonstrating balanced distribution at the outset. Dropout occurred with three in the medication group due to adverse reactions and two in the EA group due to work constraints shown in Table 1.
Figure 2
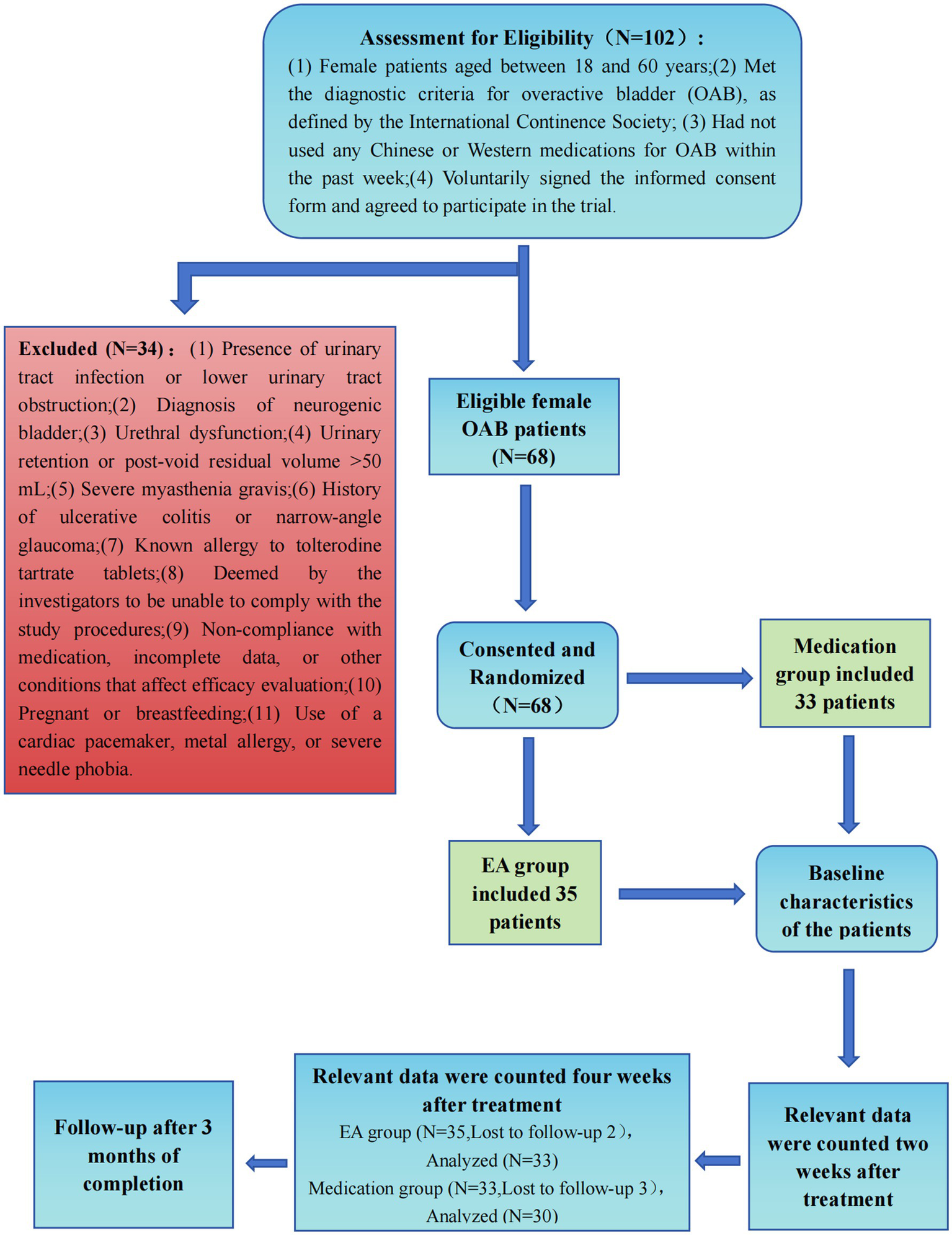
Consolidated standards of reporting trials profile. EA, Electroacupuncture.
Table 1
| Pre-treatment | EA group (N = 35) | Medication group (N = 33) | P | |
|---|---|---|---|---|
| Age (years) | 37.51 ± 12.61 | 38.76 ± 10.19 | 0.657 | |
| Disease duration (months) | 6.80 ± 2.06 | 6.67 ± 2.41 | 0.806 | |
| Menopausal status (%) | Postmenopausal | 7(20) | 5(15.2) | 0.600 |
| Not postmenopausal | 28(80) | 28(84.8) | ||
| Disease severity (%) | Mild | 17(48.6) | 17(51.5) | 0.872 |
| Moderate | 11(31.4) | 11(33.3) | ||
| Severe | 7(20) | 5(15.2) | ||
| Urgency symptoms score | 3(0) | 3(1) | 0.937 | |
| OABSS score | 6(4) | 5.5(3) | 0.964 | |
| Quality of life score | 5(0) | 5(1) | 0.592 | |
| BDNF(ng/mL) | 2.97 ± 1.47 | 2.95 ± 1.61 | 0.950 | |
| EGF(ng/mL) | 0.97 ± 0.21 | 0.96 ± 0.25 | 0.913 | |
| NGF(ng/mL) | 11.14 ± 5.17 | 12.75 ± 6.43 | 0.258 | |
| PGE2(ng/mL) | 3.81 ± 1.31 | 3.81 ± 0.93 | 0.999 | |
| MIP1β(pg/mL) | 104.74 ± 33.43 | 112.18 ± 28.50 | 0.328 | |
| MCP 1(pg/mL) | 94.43 ± 37.92 | 93.3 ± 40.03 | 0.905 | |
Baseline characteristics.
3.2 Outcome measures analysis results
3.2.1 Changes in scores following treatment
Two weeks post-treatment, the EA group exhibited significant QOL improvements compared to the medication group, as shown by a statistically significant Mann–Whitney U-test result (p = 0.002). No significant differences were observed in urgency symptoms or OABSS between the groups at this time (p > 0.05), emphasizing EA’s impact on QOL without affecting core symptoms. By 4 weeks, both treatments significantly improved urgency symptoms and QOL scores (p < 0.05), though OABSS changes remained statistically insignificant (p = 0.081), suggesting gradual therapeutic effects.
Detailed analysis within the EA group demonstrated marked improvements across all evaluated measures at both 2 and 4 weeks (p < 0.001), affirming EA’s efficacy in managing OAB and enhancing patient-reported outcomes. The medication group displayed similar improvements, especially at 4 weeks across most parameters, except for marginal significance in OABSS scores at 2 weeks (p = 0.052), indicating a delayed symptom reduction.
Three months after treatment, follow-up showed the EA group outperforming the medication group in urgency symptoms, OABSS, and QOL scores, substantiating EA’s long-term efficacy in OAB management and its potential as a sustainable treatment option (Table 2).
Table 2
| Post-treatment | EA group (N = 35) | Medication group (N = 33) | P | |
|---|---|---|---|---|
| Urgency symptoms score | 2 weeks | 1 (1)* | 2 (2)* | 0.173 |
| 4 weeks | 2 (1)* | 2 (1)* | 0.043 | |
| OABSS score | 2 weeks | 3 (3)* | 4 (5) | 0.291 |
| 4 weeks | 3 (3)* | 4 (2)* | 0.081 | |
| Quality of life score | 2 weeks | 2 (1)* | 4 (2)* | 0.002 |
| 4 weeks | 2 (1)* | 3 (1)* | 0.001 | |
| OABSS efficacy evaluation (4 weeks) | Marked effective | 8 (22.9) | 4 (12.1) | 0.002 |
| Effective | 23 (65.7) | 12 (36.4) | ||
| Ineffective | 4 (11.4) | 17 (51.5) | ||
| Calculation of efficacy rate | 31 (88.6) | 16 (48.5) | ||
| Subjective efficacy evaluation (2 weeks) | Very helpful | 8 (24.2) | 0 (0) | <0.001 |
| Moderately helpful | 21 (63.6) | 17 (56.7) | ||
| Slightly helpful | 4 (12.1) | 12 (40.0) | ||
| Not helpful at all | 0 (0) | 1 (3.3) | ||
| Subjective efficacy evaluation (4 weeks) | Very helpful | 23 (69.7) | 10 (33.3) | 0.002 |
| Moderately helpful | 9 (27.3) | 10 (33.3) | ||
| Slightly helpful | 1 (3.0) | 10 (33.3) | ||
| Not helpful at all | 0 (0) | 0 (0) | ||
| Three months follow-up after treatment completion | Urgency symptom score | 2.30 ± 0.68 | 2.86 ± 0.81 | 0.004 |
| OABSS score | 3.75 ± 2.16 | 5.06 ± 2.09 | 0.018 | |
| Quality of life score | 2.45 ± 0.79 | 2.96 ± 1.06 | 0.034 | |
Comparison of immediate OABSS, and subjective efficacy evaluation between two groups and their scores at 3 months follow-up.
* represents p < 0.001 compared with before treatment. The description of efficacy evaluation is presented as the number of cases (%).
3.2.2 Evaluation of EA efficacy
Our study demonstrated a clear statistical advantage of EA over medication in treating OAB. A chi-square test revealed a significant difference in treatment efficacy between groups (p = 0.002), with 88.6% of patients in the EA group showing marked improvement compared to 48.5% in the medication group. Subjective efficacy assessments from 63 participants revealed significant enhancements in patient-reported outcomes at both 2-week and 4-week intervals post-treatment (p < 0.05), as detailed in Table 2, confirming EA’s superior effectiveness in symptom relief and enhancing overall well-being, advocating for its broader application in OAB treatment.
3.2.3 Urinary cytokines analysis
Our analysis of urinary cytokines in 63 participants using independent t-tests found no significant differences in post-treatment concentrations of BDNF, EGF, NGF, PGE2, MIP1β, and MCP1 between the EA and medication groups, suggesting similar biochemical impacts. Both treatments led to significant reductions in BDNF levels (EA: p < 0.001, medication: p = 0.038), pointing to a common physiological effect. No other cytokines showed significant changes, underscoring BDNF’s specific modulation. A further comparison of pre- and post-treatment cytokine levels confirmed these findings, reinforcing the comparable efficacy of both treatments (Table 3). Notably, in patients with severe OAB, significant changes in all measured cytokines post-treatment highlighted both therapies’ potential to affect inflammatory and neurotrophic pathways in more advanced cases (Table 4).
Table 3
| Urinary cytokine | Difference (x ± s) | Pair t-values | P | |
|---|---|---|---|---|
| EA group(N = 33) (ng/mL) | BDNF | −2.25 ± 2.62 | −4.928 | <0.001 |
| EGF | −0.01 ± 0.36 | −0.102 | 0.919 | |
| NGF | −1.86 ± 7.64 | −1.397 | 0.172 | |
| PGE2 | −0.15 ± 1.61 | −0.556 | 0.582 | |
| MIP1β | −1.51 ± 35.95 | −0.242 | 0.811 | |
| MCP1 | −9.85 ± 67.06 | −0.844 | 0.405 | |
| Medication group(N = 30) (ng/mL) | BDNF | −1.08 ± 2.73 | −2.171 | 0.038 |
| EGF | 0.096 ± 0.39 | 1.338 | 0.191 | |
| NGF | 1.49 ± 7.73 | 1.059 | 0.298 | |
| PGE2 | 0.10 ± 1.29 | 0.432 | 0.669 | |
| MIP1β | 12.65 ± 38.89 | 1.782 | 0.085 | |
| MCP1 | −20.99 ± 64.75 | −1.775 | 0.086 | |
Comparison of urinary cytokine levels before and after treatment.
Table 4
| Urinary cytokine | Pre-treatment (N = 12) | Post-treatment (N = 11) | P | |
|---|---|---|---|---|
| Severe female OAB patients (EA group and medication group, N = 12) (ng/mL) | BDNF | 4.46 ± 2.40 | 2.11 ± 0.59 | 0.013 |
| EGF | 1.58 ± 0.25 | 1.00 ± 0.19 | <0.001 | |
| NGF | 8.92 ± 2.42 | 4.26 ± 1.08 | <0.001 | |
| PGE2 | 4.59 ± 0.98 | 2.10 ± 0.72 | <0.001 | |
| MIP1β | 126.75 ± 25.79 | 89.53 ± 13.82 | 0.007 | |
| MCP1 | 71.03 ± 16.17 | 63.39 ± 26.72 | 0.468 | |
Comparison of urinary cytokine levels before and after treatment in severe female OAB patients.
3.2.4 Safety and acceptance evaluation
Safety and acceptance of EA were thoroughly evaluated among 35 participants, with minimal discomfort reported; 73.5% had a VAS pain score of 1, and 26.5% scored 2. Additionally, acceptance was high, with 82.4% finding EA very easy to accept. The absence of serious complications or significant discomfort during or after sessions underscores EA’s safety and tolerability, supporting its use as an effective treatment for overactive bladder. These results are further detailed in subsequent sections of the study.
4 Discussion
This randomized controlled trial revealed that EA significantly enhances quality of life and mitigates urgency symptoms in female patients with OAB, demonstrating comparable efficacy to conventional medication. Notably, our findings highlight the potential of EA as a safe, well-tolerated, and effective treatment modality, with a higher overall effective rate (88.6%) compared to medication (48.5%). The analysis of urinary cytokines post-treatment, particularly the significant changes in BDNF levels within the EA group, supports the therapeutic role of EA in modulating neural pathways involved in OAB.
The theoretical underpinnings of our research, supported by existing literature, suggest that normal micturition relies on the integrity of neural pathways (33), which can be modulated by therapies like EA. EA stimulation of the bilateral BL33 (S3, posterior sacral foramen) effectively targets the S3 nerve, known for its robust innervation of detrusor muscles. The sacral three nerve roots emerge as pivotal neural pathways for stimulating the vesicourethral muscle, making S3 the primary acupoint choice. Another selected acupoint in the study is SP6, positioned directly above the tip of the medial malleolus and posterior to the tibia according to international standards for localization. The cutaneous distribution of the iliohypogastric nerve of the fourth lumbar spinal segment encompasses this acupoint, with the tibial nerve traversing beneath the SP6 site. From a neuroanatomical perspective, the application of stainless steel needle electrodes above the medial malleolus for electrical stimulation of the tibial nerve aids in achieving direct sensory and motor control of the bladder and pelvic floor. This indirect approach contributes to the overall therapeutic objectives. However, the challenge lies in accurate localization, impeding widespread clinical application. Electroacupuncture sensation alone is insufficient for judging BL33 localization and entry accuracy due to the deep location of BL33 and the physiological curvature of the caudal vertebrae. Addressing this, the study combines ancient localization methods with modern medical sacral nerve stimulators, enhancing the precision of BL33 acupuncture targeting the S3 nerve. Considering the distinct responses of different sacral nerves to EA stimulation further enhances the objective determination of needle accuracy, ensuring precise needle placement. Our findings provide empirical support for this, with EA’s targeted stimulation of BL33 and SP6 potentially modulating the neural pathways involved in OAB.
Currently, the diagnosis of OAB is based primarily on patient-reported symptoms, with confirmatory tests used to exclude other conditions, reflecting a lack of objective, measurable diagnostic markers (34). Recent studies suggest that specific urinary cytokines are closely associated with urgency and frequency symptoms, and may serve as potential biomarkers for OAB. Key cytokines include NGF, BDNF, MCP-1, PGE2, EGF, and MIP-1β (35–38). Elevated urinary levels of NGF and BDNF have been linked to involuntary detrusor contractions via receptor pathways, contributing to urgency, frequency, and incontinence. Clinical trials have reported a significant increase in NGF in OAB patients, positively correlated with symptom severity, while treatment with anticholinergics reduces NGF levels, supporting its relevance to disease activity (39). BDNF, essential for neuronal remodeling, has also been implicated in OAB through its effects on sensory nerve hyperexcitability and dysfunctional signaling (40, 41), underscore the biological impact of EA treatment and suggest avenues for further exploration. PGE2 is involved in non-cholinergic detrusor contraction and is elevated in idiopathic OAB patients, with levels correlating with symptom severity (42). EGF, recognized for its urothelial regulatory role, is also increased in the urine of patients with chronic interstitial cystitis (IC) (43). Notably, patients with mild to moderate IC often present with only urgency and frequency (44–46), suggesting possible overlap with OAB. Furthermore, EGF, MIP-1β, and MCP-1 may contribute to voiding symptoms in IC and could also be relevant to OAB pathogenesis (36, 47, 48). Despite no significant differences in cytokine levels between treatment groups post-treatment, the within-group changes in BDNF levels and the clinical improvement in severe OAB patients post-treatment highlight the potential of these markers in understanding OAB’s pathophysiology and treatment response. This points to the necessity for further research into the mechanisms of EA in OAB management and its role in neural modulation.
The strengths of our investigation lie in its rigorous randomized controlled design, the innovative application of EA based on acupuncture principles and neural modulation, and the comprehensive evaluation of clinical outcomes and urinary cytokines as biological markers. Our methodological rigor, including randomization and statistical blinding, enhances the credibility of the findings, providing a robust framework for comparing the efficacy, safety, and patient acceptance of EA versus medication. However, the study acknowledges certain limitations. The focus on a female patient cohort limits the generalizability of our results to broader populations, including male patients. Additionally, despite efforts to maintain statistical blinding, the inherent differences in treatment modalities could introduce biases. Future research should aim to overcome these limitations, exploring larger and more diverse patient populations to validate and broaden our findings. Further investigation is needed to delineate specific patient profiles that may derive the most benefit from EA versus medication, enhancing the precision and personalization of OAB treatment strategies.
5 Conclusion
The study substantiates electroacupuncture’s efficacy and safety in managing female OAB, offering a valuable addition to the existing therapeutic modalities. Future research should aim to expand on these findings, exploring the long-term effects of EA, its applicability in diverse patient populations, and further elucidation of its underlying mechanisms through urinary cytokine analysis. The exploration of EA’s role in neural modulation opens new avenues for non-pharmacological OAB treatment, advocating for its integration into clinical practice.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Xiyuan Hospital, China Academy of Chinese Medical Sciences (Grant No.: 2018XLA070-3) and registered in the China Clinical Trials Registry (Reg. No.: ChiCTR1900021372). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing, Visualization. MD: Data curation, Formal analysis, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing, Investigation, Supervision. JL: Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RL: Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, Data curation, Project administration. JS: Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Investigation, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Beijing Municipal Health Commission, Capital Health Development Scientific Research Special Project (No. Capital Health Development 2022-3-4176), China Academy of Traditional Chinese Medicine, Science and Technology Innovation Project Major Research Project (No. C12021A02206), Research on the application of clinical characteristics in the capital (No. Z181100001718046).
Acknowledgments
The data and safety monitoring committee members of this trial include members of the ethics committee of Xiyuan Hospital, China Academy of Chinese Medical Sciences. In addition, we would like to thank Professor Zheng Weijun of Zhejiang University of Traditional Chinese Medicine for his data analysis and technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1579276/full#supplementary-material
References
1.
Gormley EA Lightner DJ Faraday M Vasavada SP American Urological Association Society of Urodynamics et al . Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. (2015) 193:1572–80. doi: 10.1016/j.juro.2015.01.087
2.
Nambiar AK Arlandis S Bo K Cobussen-Boekhorst H Costantini E de Heide M et al . European Association of Urology guidelines on the diagnosis and Management of Female non-neurogenic Lower Urinary Tract Symptoms. Part 1: diagnostics, overactive bladder, stress urinary incontinence, and mixed urinary incontinence. Eur Urol. (2022) 82:49–59. doi: 10.1016/j.eururo.2022.01.045
3.
Bartley JM Blum ES Sirls LT Peters KM . Understanding clinic options for overactive bladder. Curr Urol Rep. (2013) 14:541–8. doi: 10.1007/s11934-013-0353-6
4.
Chuang YC Liu SP Lee KS Liao L Wang J Yoo TK et al . Prevalence of overactive bladder in China, Taiwan and South Korea: results from a cross-sectional, population-based study. Low Urin Tract Symptoms. (2019) 11:48–55. doi: 10.1111/luts.12193
5.
Peyronnet B Mironska E Chapple C Cardozo L Oelke M Dmochowski R et al . A comprehensive review of overactive bladder pathophysiology: on the way to tailored treatment. Eur Urol. (2019) 75:988–1000. doi: 10.1016/j.eururo.2019.02.038
6.
Araklitis G Robinson D Cardozo L . Cognitive effects of anticholinergic load in women with overactive bladder. Clin Interv Aging. (2020) 15:1493–503. doi: 10.2147/CIA.S252852
7.
Suehs BT Caplan EO Hayden J Ng DB Gaddy RR . The relationship between anticholinergic exposure and falls, fractures, and mortality in patients with overactive bladder. Drugs Aging. (2019) 36:957–67. doi: 10.1007/s40266-019-00694-5
8.
Welk B Richardson K Panicker JN . The cognitive effect of anticholinergics for patients with overactive bladder. Nat Rev Urol. (2021) 18:686–700. doi: 10.1038/s41585-021-00504-x
9.
Yamada S Ito Y Nishijima S Kadekawa K Sugaya K . Basic and clinical aspects of antimuscarinic agents used to treat overactive bladder. Pharmacol Ther. (2018) 189:130–48. doi: 10.1016/j.pharmthera.2018.04.010
10.
Chen LC Kuo HC . Current management of refractory overactive bladder. Low Urin Tract Symptoms. (2020) 12:109–16. doi: 10.1111/luts.12304
11.
De Wachter S Knowles CH Elterman DS Kennelly MJ Lehur PA Matzel KE et al . New technologies and applications in sacral neuromodulation: An update. Adv Ther. (2020) 37:637–43. doi: 10.1007/s12325-019-01205-z
12.
De Wachter S Vaganee D Kessler TM . Sacral neuromodulation: mechanism of action. Eur Urol Focus. (2020) 6:823–5. doi: 10.1016/j.euf.2019.11.018
13.
Liao L Zhou Z Chen G Xu Z Huang B Chong T et al . Sacral neuromodulation using a novel device with a six-contact-point electrode for the treatment of patients with refractory overactive bladder: a multicenter, randomized, single-blind, parallel-control clinical trial. Eur Urol Focus. (2022) 8:1823–30. doi: 10.1016/j.euf.2022.04.006
14.
Marcelissen TA Leong RK Serroyen J van Kerrebroeck PE De Wachter SG . The use of bilateral sacral nerve stimulation in patients with loss of unilateral treatment efficacy. J Urol. (2011) 185:976–80. doi: 10.1016/j.juro.2010.10.065
15.
Xiang H Zhang T Al-Danakh A Yang D Wang L . Neuromodulation in chronic pelvic pain: a narrative review. Pain Ther. (2022) 11:789–816. doi: 10.1007/s40122-022-00405-w
16.
Booth J Connelly L Dickson S Duncan F Lawrence M . The effectiveness of transcutaneous tibial nerve stimulation (TTNS) for adults with overactive bladder syndrome: a systematic review. Neurourol Urodyn. (2018) 37:528–41. doi: 10.1002/nau.23351
17.
Sayner AM Rogers F Tran J Jovanovic E Henningham L Nahon I . Transcutaneous Tibial nerve stimulation in the Management of Overactive Bladder: a scoping review. Neuromodulation. (2022) 25:1086–96. doi: 10.1016/j.neurom.2022.04.034
18.
Liu S Wang Z Su Y Qi L Yang W Fu M et al . A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. (2021) 598:641–5. doi: 10.1038/s41586-021-04001-4
19.
Liu S Wang ZF Su YS Ray RS Jing XH Wang YQ et al . Somatotopic organization and intensity dependence in driving distinct NPY-expressing sympathetic pathways by Electroacupuncture. Neuron. (2020) 108:436–450.e7. doi: 10.1016/j.neuron.2020.07.015
20.
Molsberger AF Manickavasagan J Abholz HH Maixner WB Endres HG . Acupuncture points are large fields: the fuzziness of acupuncture point localization by doctors in practice. Eur J Pain. (2012) 16:1264–70. doi: 10.1002/j.1532-2149.2012.00145.x
21.
Pan WX Fan AY Chen S Alemi SF . Acupuncture modulates immunity in sepsis: toward a science-based protocol. Auton Neurosci. (2021) 232:102793. doi: 10.1016/j.autneu.2021.102793
22.
Schulz KF Altman DG Moher D Group C . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg. (2011) 9:672–7. doi: 10.1016/j.ijsu.2011.09.004
23.
MacPherson H Altman DG Hammerschlag R Youping L Taixiang W White A et al . Revised STandards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. J Evid Based Med. (2010) 3:140–55. doi: 10.1111/j.1756-5391.2010.01086.x
24.
Halme AS Fritel X Benedetti A Eng K Tannenbaum C . Implications of the minimal clinically important difference for health-related quality-of-life outcomes: a comparison of sample size requirements for an incontinence treatment trial. Value Health. (2015) 18:292–8. doi: 10.1016/j.jval.2014.11.004
25.
Happ M Bathke AC Brunner E . Optimal sample size planning for the Wilcoxon-Mann-Whitney test. Stat Med. (2019) 38:363–75. doi: 10.1002/sim.7983
26.
Weaver CS Leonardi-Bee J Bath-Hextall FJ Bath PM . Sample size calculations in acute stroke trials: a systematic review of their reporting, characteristics, and relationship with outcome. Stroke. (2004) 35:1216–24. doi: 10.1161/01.STR.0000125010.70652.93
27.
Leung AY Park J Schulteis G Duann JR Yaksh T . The electrophysiology of de qi sensations. J Altern Complement Med. (2006) 12:743–50. doi: 10.1089/acm.2006.12.743
28.
Meng L Tian Z Diao T Wang M Liu X Zhang W et al . Variable- versus constant-frequency sacral neuromodulation in black-zone overactive bladder patients: a study protocol for a multicenter, prospective, randomized, blind, self-controlled trial. Transl Androl Urol. (2021) 10:504–11. doi: 10.21037/tau-20-1257
29.
Blaivas JG Panagopoulos G Weiss JP Somaroo C . Validation of the overactive bladder symptom score. J Urol. (2007) 178:543–7. doi: 10.1016/j.juro.2007.03.133
30.
Liu M Wang J Yang Y An R Wen J Guan Z et al . Overactive bladder symptom score to evaluate efficacy of solifenacin for the treatment of overactive bladder symptoms. Chin Med J. (2014) 127:261–5. doi: 10.3760/cma.j.issn.0366-6999.20131349
31.
Xu ZH Zhang PF Wang YF Ma A Bano Y Ibrohimov A et al . A multi-center, randomized, blind, controlled clinical trial of the safety and efficacy of Micro radio frequency therapy system for the treatment of overactive bladder. Front Med. (2022) 9:746064. doi: 10.3389/fmed.2022.746064
32.
He S Renne A Argandykov D Convissar D Lee J . Comparison of an emoji-based visual analog scale with a numeric rating scale for pain assessment. JAMA. (2022) 328:208–9. doi: 10.1001/jama.2022.7489
33.
Fowler CJ Griffiths D de Groat WC . The neural control of micturition. Nat Rev Neurosci. (2008) 9:453–66. doi: 10.1038/nrn2401
34.
Lightner DJ Gomelsky A Souter L Vasavada SP . Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment 2019. J Urol. (2019) 202:558–63. doi: 10.1097/JU.0000000000000309
35.
Antunes-Lopes T Cruz F . Urinary biomarkers in overactive bladder: revisiting the evidence in 2019. Eur Urol Focus. (2019) 5:329–36. doi: 10.1016/j.euf.2019.06.006
36.
Ma E Vetter J Bliss L Lai HH Mysorekar IU Jain S . A multiplexed analysis approach identifies new association of inflammatory proteins in patients with overactive bladder. Am J Physiol Renal Physiol. (2016) 311:F28–34. doi: 10.1152/ajprenal.00580.2015
37.
Steers WD Tuttle JB . Mechanisms of disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol. (2006) 3:101–10. doi: 10.1038/ncpuro0408
38.
Yu WR Jiang YH Jhang JF Kuo HC . Use of urinary cytokine and chemokine levels for identifying bladder conditions and predicting treatment outcomes in patients with interstitial cystitis/bladder pain syndrome. Biomedicine. (2022) 10:1149. doi: 10.3390/biomedicines10051149
39.
Liu HT Chancellor MB Kuo HC . Decrease of urinary nerve growth factor levels after antimuscarinic therapy in patients with overactive bladder. BJU Int. (2009) 103:1668–72. doi: 10.1111/j.1464-410X.2009.08380.x
40.
Pinto R Frias B Allen S Dawbarn D McMahon SB Cruz F et al . Sequestration of brain derived nerve factor by intravenous delivery of TrkB-Ig2 reduces bladder overactivity and noxious input in animals with chronic cystitis. Neuroscience. (2010) 166:907–16. doi: 10.1016/j.neuroscience.2010.01.015
41.
Song QX Chermansky CJ Birder LA Li L Damaser MS . Brain-derived neurotrophic factor in urinary continence and incontinence. Nat Rev Urol. (2014) 11:579–88. doi: 10.1038/nrurol.2014.244
42.
Yokoyama O Miwa Y Oyama N Aoki Y Ito H Akino H . Antimuscarinic drug inhibits detrusor overactivity induced by topical application of prostaglandin E2 to the urethra with a decrease in urethral pressure. J Urol. (2007) 178:2208–12. doi: 10.1016/j.juro.2007.06.044
43.
Tyagi P Barclay D Zamora R Yoshimura N Peters K Vodovotz Y et al . Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. (2010) 42:629–35. doi: 10.1007/s11255-009-9647-5
44.
Castro-Diaz D Cardozo L Chapple CR Espuna M Kelleher C Kirby M et al . Urgency and pain in patients with overactive bladder and bladder pain syndrome. What are the differences?Int J Clin Pract. (2014) 68:356–62. doi: 10.1111/ijcp.12317
45.
Chung MK Butrick CW Chung CW . The overlap of interstitial cystitis/painful bladder syndrome and overactive bladder. JSLS. (2010) 14:83–90. doi: 10.4293/108680810X12674612014743
46.
Grundy L Caldwell A Brierley SM . Mechanisms underlying overactive bladder and interstitial cystitis/painful bladder syndrome. Front Neurosci. (2018) 12:931. doi: 10.3389/fnins.2018.00931
47.
Apostolidis A Jacques TS Freeman A Kalsi V Popat R Gonzales G et al . Histological changes in the urothelium and suburothelium of human overactive bladder following intradetrusor injections of botulinum neurotoxin type a for the treatment of neurogenic or idiopathic detrusor overactivity. Eur Urol. (2008) 53:1245–53. doi: 10.1016/j.eururo.2008.02.037
48.
Jiang YH Jhang JF Hsu YH Kuo HC . Usefulness of urinary biomarkers for assessing bladder condition and histopathology in patients with interstitial cystitis/bladder pain syndrome. Int J Mol Sci. (2022) 23:12044. doi: 10.3390/ijms231912044
Summary
Keywords
electroacupuncture, overactive bladder, sacral neuromodulation, tibial neuromodulation, urinary cytokines
Citation
Tan Z, Ding M, Li J, Luo R and Shen J (2025) Effectiveness and safety of electroacupuncture in female overactive bladder: a randomized controlled trial investigating sacral and tibial nerve modulation. Front. Med. 12:1579276. doi: 10.3389/fmed.2025.1579276
Received
19 February 2025
Accepted
01 September 2025
Published
16 September 2025
Volume
12 - 2025
Edited by
Alice Chen, Consultant, Potomac, MD, United States
Reviewed by
Jens Wöllner, Swiss Paraplegic Center, Switzerland
Rui Viana, Fernando Pessoa Foundation, Portugal
Updates
Copyright
© 2025 Tan, Ding, Li, Luo and Shen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianwu Shen, 0907shenjianwu@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.