- 1Department of Critical Care Medicine, The First Hospital of Jilin University, Changchun, China
- 2Department of Emergency Medicine, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, China
Background: A recent large multicenter randomized controlled trial (RCT) found that continuous infusion (CI) of meropenem did not improve clinical outcomes in critically ill patients, contradicting previous meta-analysis results.
Methods: We conducted a search of the PubMed, EMBASE, and Cochrane databases up to March 19, 2024.
Results: Our study included a total of 1,075 critically ill patients with sepsis from five RCTs. The primary outcome indicated that CI of meropenem did not reduce all-cause mortality in patients (RR = 0.89; 95% CI, 0.75–1.04; P = 0.15; Chi2= 5.75; I2 = 30%). The secondary outcomes revealed that compared to II of meropenem, patients receiving CI had shorter ICU length of stay (MD = –2.39; 95% CI, –2.98 to –1.81; P < 0.00001; Chi2= 6.63; I2 = 40%), higher clinical cure rates (RR = 1.88; 95% CI, 1.23–2.87; P = 0.004; Chi2 = 1.87; I2 = 0%), and shorter duration of meropenem therapy (MD = –0.86; 95% CI, –1.36 to –0.36; P = 0.0008; Chi2 = 3.65; I2 = 45%).
Conclusion: In critically ill patients with sepsis, CI of meropenem did not reduce mortality but was associated with shorter ICU length of stays, higher clinical cure rates, and shorter duration of meropenem therapy. Further large-scale RCTs are needed to validate these findings.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024528380, identifier CRD42024528380.
1 Background
Sepsis is a severe inflammatory syndrome characterized by a dysregulated host response to infection (1–3), serving as a significant contributor to both intensive care unit (ICU) admission and mortality among patients. Beta-lactam antibiotics represent the most commonly used class of antimicrobial agents in the current armamentarium against infectious diseases, constituting 65% of all injectable antibiotic prescriptions in the United States (4).
Beta-lactam antibiotics have a short half-life, and if administered over a brief period, the peak blood concentration typically rapidly decreases below the minimum inhibitory concentration. Prolonged exposure below the minimum inhibitory concentration may diminish therapeutic efficacy, potentially allowing residual bacterial populations to resume growth and facilitate the emergence of resistant strains (5). Therefore, guidelines recommend prolonging the administration time of beta-lactam antibiotics to extend the duration above the minimum inhibitory concentration and achieve enhanced antimicrobial effects (6, 7). The findings of multiple pharmacokinetic studies also support the notion of extending the administration time to improve the efficacy of beta-lactams (8, 9).
Recent meta-analyses have indicated that continuous infusion (CI) of beta-lactams can reduce mortality and improve clinical cure rates in septic patients (10–15). There was a recent BLING III RCT (N = 7031) did not find that continuous infusion of beta-lactams improved 90-day mortality in critically ill patients (16). In addition, a meta-analysis did not find a difference in mortality between the two types of infusion (17). However, these meta-analyses are subject to certain limitations: (1) Variations in the types of antibiotics used across the included studies may introduce some bias, as the diverse pharmacological characteristics, spectrum of activity, and pharmacokinetics of different classes of beta-lactam antibiotics could potentially impact clinical outcomes in patients. (2) The inclusion of retrospective cohort studies and observational studies in some of the meta-analyses may also lower the quality of evidence for the outcomes.(3) These meta-analyses did not incorporate trial sequential analysis (TSA), which could increase the potential for type I or type II errors, thereby reducing the credibility of the study conclusions. Moreover, TSA provides a means to calculate the necessary sample sizes for clinically significant results and offers valuable perspectives on the potential lack of benefit from future trials, thus informing the feasibility and selection of outcome measures (18–20).
Meropenem is one of the most commonly used types of beta-lactam antibiotics, with its post-antibiotic effect demonstrated across various pathogens (21). CI of meropenem has been shown to potentially increase bacterial clearance rates and improve clinical outcomes in septic patients (11, 22). However, a recent high-quality clinical study did not find any benefit in terms of clinical outcomes for patients receiving CI of meropenem (23). Although a recent meta-analysis did not find a difference in mortality between the two meropenem infusions (24), three of the studies included in this meta-analysis did not use meropenem as their primary antimicrobial agent, leading to this highly misleading conclusion (9, 25, 26).
Therefore, it is necessary to conduct this systematic review of RCTs with meta-analysis and TSA to compare the effects of CI and intermittent infusion (II) of meropenem in critically ill patients with sepsis. This will help clarify whether meropenem can improve the clinical outcomes of critically ill septic patients.
2 Methods
2.1 Protocol and registration
The present systematic review and meta-analysis was conducted in strict adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines (27) (Supplementary Table S1). The study protocol for this meta-analysis has been registered in PROSPERO (CRD42024528380) on April 6, 2024, and is publicly accessible.
2.2 Eligibility criteria
This review exclusively incorporated randomized controlled trials (RCTs) involving patients with severe sepsis who received meropenem antimicrobial therapy. The specific inclusion and exclusion criteria are detailed in Table 1.
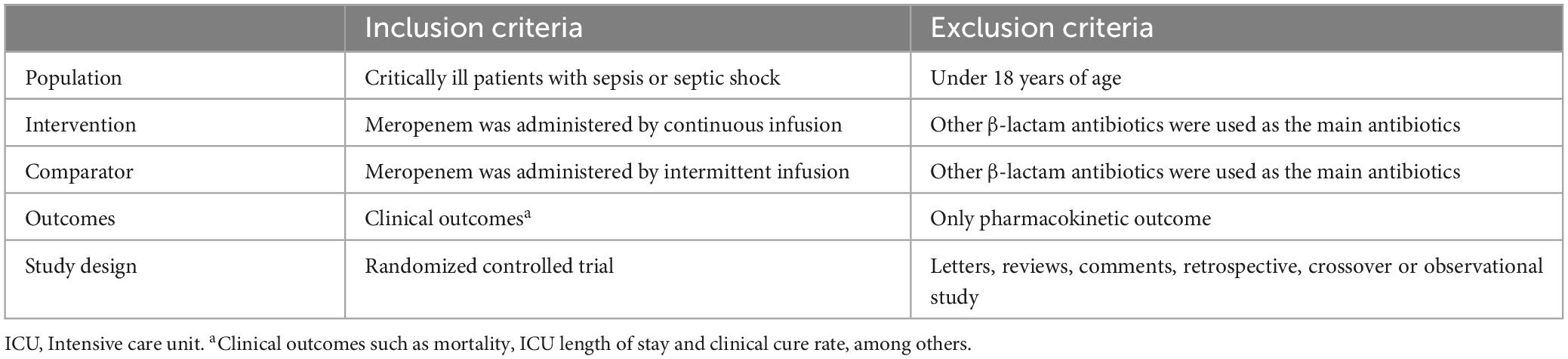
Table 1. Criteria to choose studies for the review based on the population, intervention, comparator, outcomes and study designs (PICOS) structure.
2.3 Data sources and search strategies
A comprehensive literature search was conducted across three electronic databases (PubMed, EMBASE, and Cochrane Library) to identify original studies assessing the effectiveness and safety profile of continuous vs. intermittent meropenem administration in adult sepsis patients, with the search period extending from the inception of the databases through March 2024. Database-specific modifications were applied to the search strategy, which was developed in collaboration with our institutional librarians (detailed search strategy available in Supplementary Table S2). Following initial screening based on titles and abstracts, two independent investigators (YW and YL) performed full-text evaluations of potentially relevant studies. The research team additionally conducted a manual review of the reference lists in the included articles to identify further relevant studies. Any discrepancies between reviewers were adjudicated by a third researcher (HL). The study selection process was managed using EndNote 20.0 software.
2.4 Types of outcome measures
The primary outcome was all-cause mortality (ICU mortality, hospital mortality, 28 or 90-day mortality). When multiple mortality outcomes were available, we preferred ICU mortality, which is the most critical for critically ill patients. We gave preference to intention-to-treat (ITT) dataset in both ITT and clinically evaluable datasets, because the ITT dataset includes all patients who were randomly assigned to treatment and control groups, it reflects real-world treatment effects and is more representative and general.
The secondary outcomes were Length of ICU length of stay, clinical cure rate and duration of meropenem therapy. Weighted means were calculated based on the number of patients in each study.
2.5 Quality assessment
Two independent reviewers (YL and YW) evaluated the methodological quality of the included trials using the Cochrane risk of bias assessment tool. The assessment focused on the following domains to evaluate potential biases: (1) randomization sequence generation (selection bias), (2) allocation concealment (selection bias), (3) blinding of study personnel and participants (performance bias), (4) blinding of outcome assessors (performance bias), (5) completeness of data reporting, including avoidance of arbitrary patient exclusions and minimal loss to follow-up (attrition bias), (6) selective reporting bias, and (7) other potential sources of bias. Each domain was rated using a color-coded system: green for satisfactory performance, yellow for unclear performance, and red for unsatisfactory performance. Any discrepancies between the reviewers were resolved through consultation with a third reviewer (HL). The results of the risk of bias assessment are summarized in a graph and table, which are provided in Supplementary Figure S1.
2.6 Statistical analysis
Studies meeting the inclusion criteria and not violating the exclusion criteria were imported into Review Manager Version 5.3 (RevMan, The Cochrane Collaboration, Oxford, UK) for meta-analysis. For dichotomous outcomes, relative risk (RR) with 95% confidence intervals (CI) was calculated. For continuous outcomes, the mean difference (MD) with 95% CI was calculated as the effect measure. In cases where significant heterogeneity was detected, as indicated by a chi-squared test (P < 0.10) and an inconsistency index (I2 ≥ 50%) (28), a random-effects model was employed to pool the data. For continuous variables reported as median (interquartile range), the mean and standard deviation were estimated using a validated calculator (29) based on sample size, enabling their inclusion in the meta-analysis. Sensitivity analysis was conducted using Stata 16 to assess the stability and reliability of the results. Publication bias was evaluated through funnel plots and Egger’s weighted regression tests, with a P-value of < 0.05 considered statistically significant for determining the overall intervention effect.
2.7 Trial sequential analysis
In our meta-analysis, we utilized TSA to manage random errors and determine the conclusiveness of our findings. Employing a random effects model, we plotted the cumulative Z curve. The TSA was conducted to uphold a 5% risk of type I error overall. Based on previous high-quality RCTs in the field (22, 23), we assumed a 15.0% anticipated relative risk reduction (RRR) with 90% power to calculate the necessary information size for detecting or refuting an intervention effect. By adjusting the control event rate based on the relevant data from the II group in our meta-analysis, we monitored the cumulative Z curve’s trajectory. If the curve intersected the trial sequential monitoring boundary or entered the futility zone, it suggested sufficient evidence to either accept or reject the expected intervention effect, eliminating the need for further studies. Conversely, if the Z curve did not breach any boundaries and the required information size had not been attained, the evidence would be deemed insufficient for drawing a conclusion, indicating the necessity for additional research (30).
3 Results
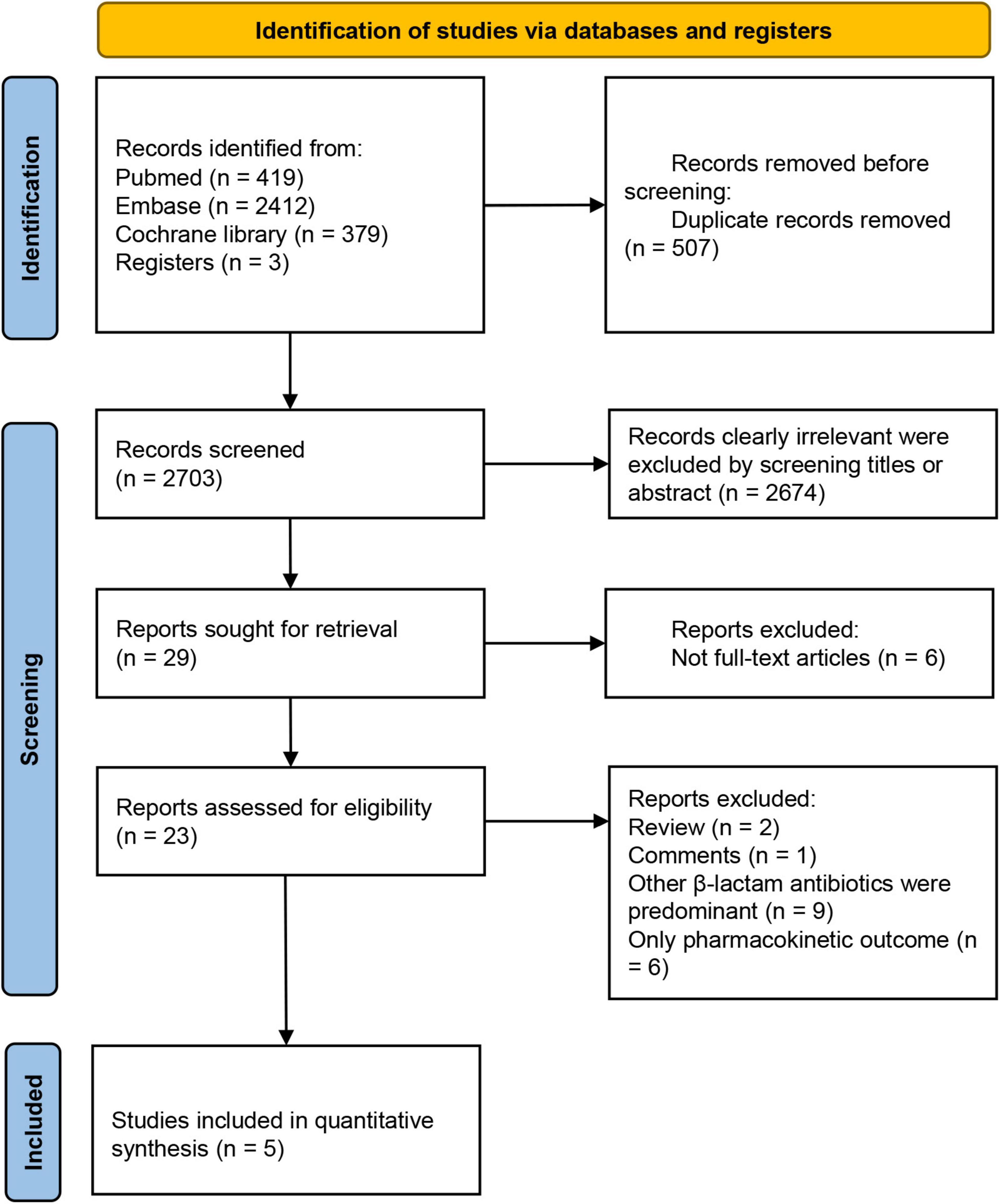
3.1 Study selection process
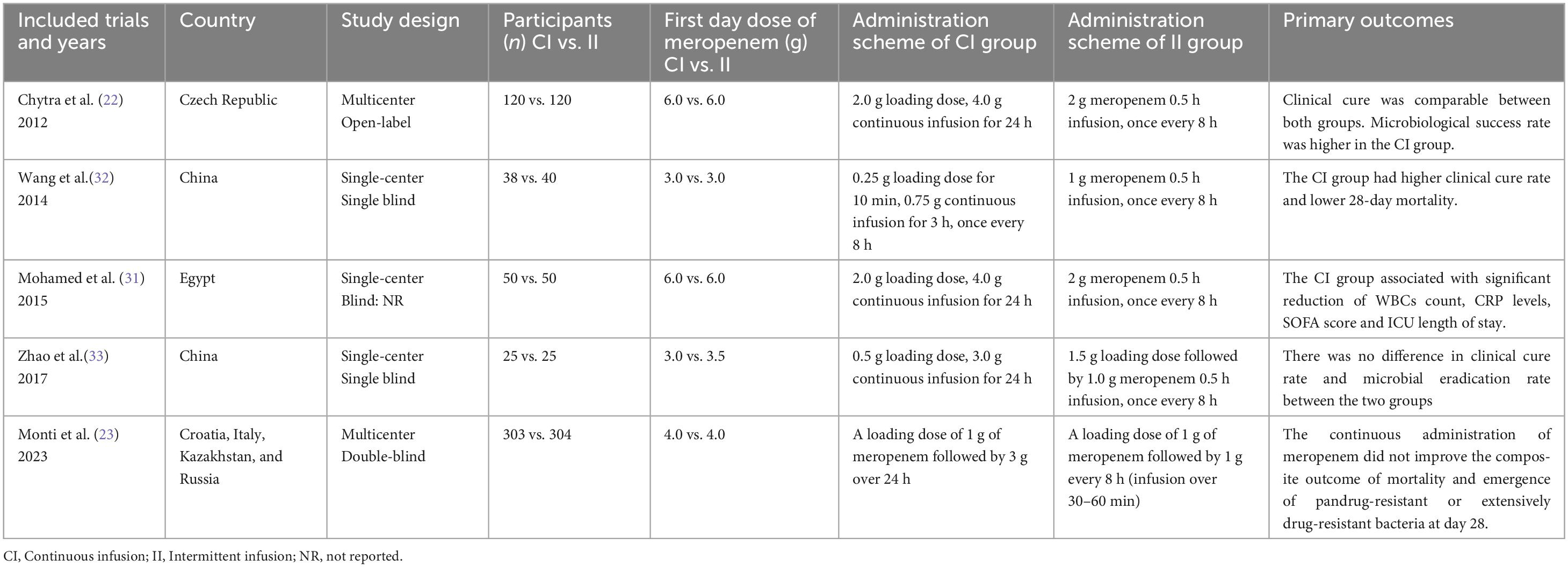
The initial search yielded 3,210 potential studies, with three additional studies identified through cross-referencing and the authors’ personal reference collections. Following the removal of 507 duplicates, 2,703 manuscripts were screened based on titles and abstracts, and 29 trials underwent full-text review. The study selection process is illustrated in Figure 1. The studies that met the inclusion criteria but not the exclusion criteria, and the reasons for exclusion in each study are presented in Supplementary Table S3. Five RCTs met the inclusion criteria for this review and were included for data extraction. These studies included one conducted in the Czech Republic, two in China, one in Egypt, and one conducted across Croatia, Italy, Kazakhstan, and Russia. Four of the studies were single-center trials, while one was a multicenter study. Detailed characteristics of the trials and their participants are provided in Table 2 and Supplementary Table S4, respectively (22, 23, 31–33). In addition, we counted the type of infection and the proportion of patients in these studies (Supplementary Table S5).
3.2 Primary outcome
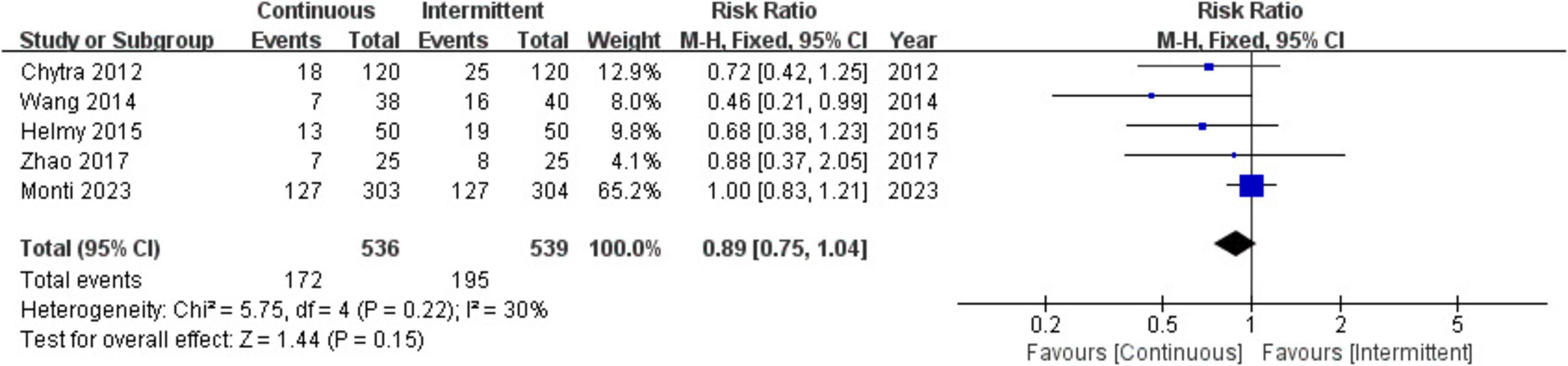
Five trials involving 1,075 patients reported all-cause mortality in both the continuous infusion (CI) and intermittent infusion (II) groups and were included in the meta-analysis. The results indicated no significant difference in all-cause mortality between the CI and II groups (RR = 0.89; 95% CI, 0.75–1.04; P = 0.15; Chi2 = 5.75; I2 = 30%) (Figure 2).
3.3 Secondary outcomes
3.3.1 ICU length of stay
Five trials involving 1,075 patients reported ICU length of stay for both the CI and II groups and were included in the meta-analysis. The results demonstrated that the ICU length of stay was significantly shorter in the CI group compared to the II group (MD = –2.39; 95% CI, –2.98 to –1.81; P < 0.00001; Chi2 = 6.63; I2 = 40%) (Figure 3A).
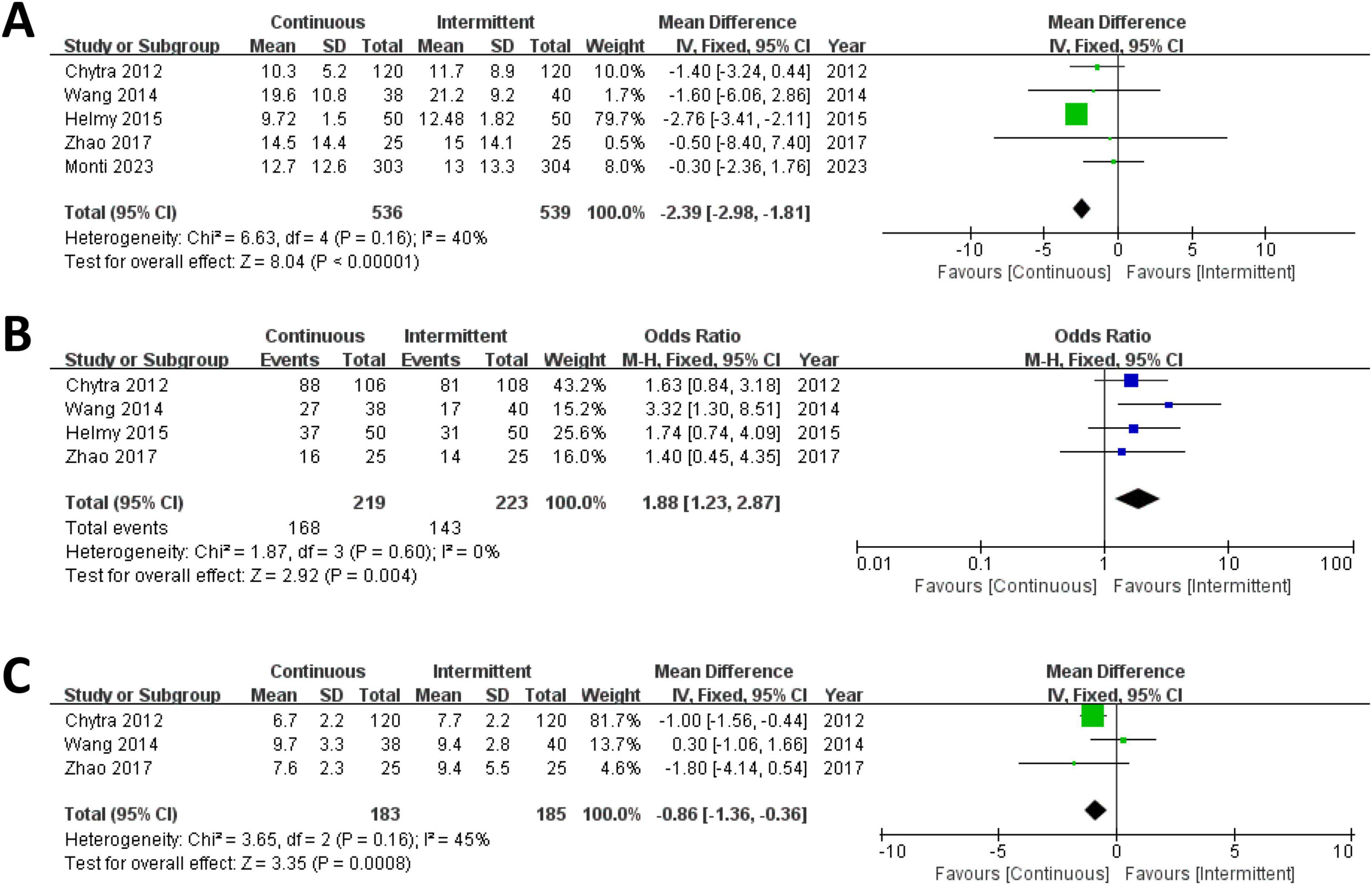
Figure 3. Forest plot for ICU length of stay (A), clinical cure rate (B) and duration of meropenem therapy (C).
3.3.2 Clinical cure rate
Four trials involving 442 patients reported clinical cure rates for both the CI and II groups and were included in the meta-analysis. The results indicated that the clinical cure rate was significantly higher in the CI group compared to the II group (RR = 1.88; 95% CI, 1.23–2.87; P = 0.004; Chi2 = 1.87; I2 = 0%) (Figure 3B).
3.3.3 Duration of meropenem therapy
Three trials involving 368 patients reported the duration of meropenem therapy for both the CI and II groups and were included in the meta-analysis. The results revealed that the duration of meropenem therapy was significantly shorter in the CI group compared to the II group (MD = –0.86; 95% CI, –1.36 to –0.36; P = 0.0008; Chi2 = 3.65; I2 = 45%) (Figure 3C).
3.4 Risk of bias and sensitivity analyses within outcomes
Funnel plots were utilized to evaluate publication bias across all outcomes, with no significant bias detected for any outcome. This conclusion was supported by the funnel plot results (I2 < 50%) and Egger’s test (P > 0.05) (Supplementary Figure S2). To assess the robustness of the findings, a sensitivity analysis was conducted by sequentially excluding one study at a time and recalculating the combined effect sizes for the remaining studies. The direction and magnitude of the pooled estimates remained consistent regardless of the exclusion of any single study. Sensitivity analyses confirmed the stability of the results for both the primary and secondary outcomes (Supplementary Figure S3).
3.5 Trial sequential analysis
The findings of the TSA are detailed in Supplementary Table S6 and illustrated in Figure 4, indicating that the current systematic review did not reach the necessary information sizes to identify the pre-specified effect sizes for all-cause mortality, ICU length of stay, clinical cure rate, and duration of meropenem therapy. In the TSA of all-cause mortality, a transient significance is observed in the Z-curve (Figure 4A), where the initially detected effect may appear significant but gradually diminishes and eventually disappears as the data volume increases or other factors come into play. Researchers should exercise caution in interpreting the results, taking into consideration the stability and persistence of the effect, indicating the potential necessity for further research to validate these outcomes.
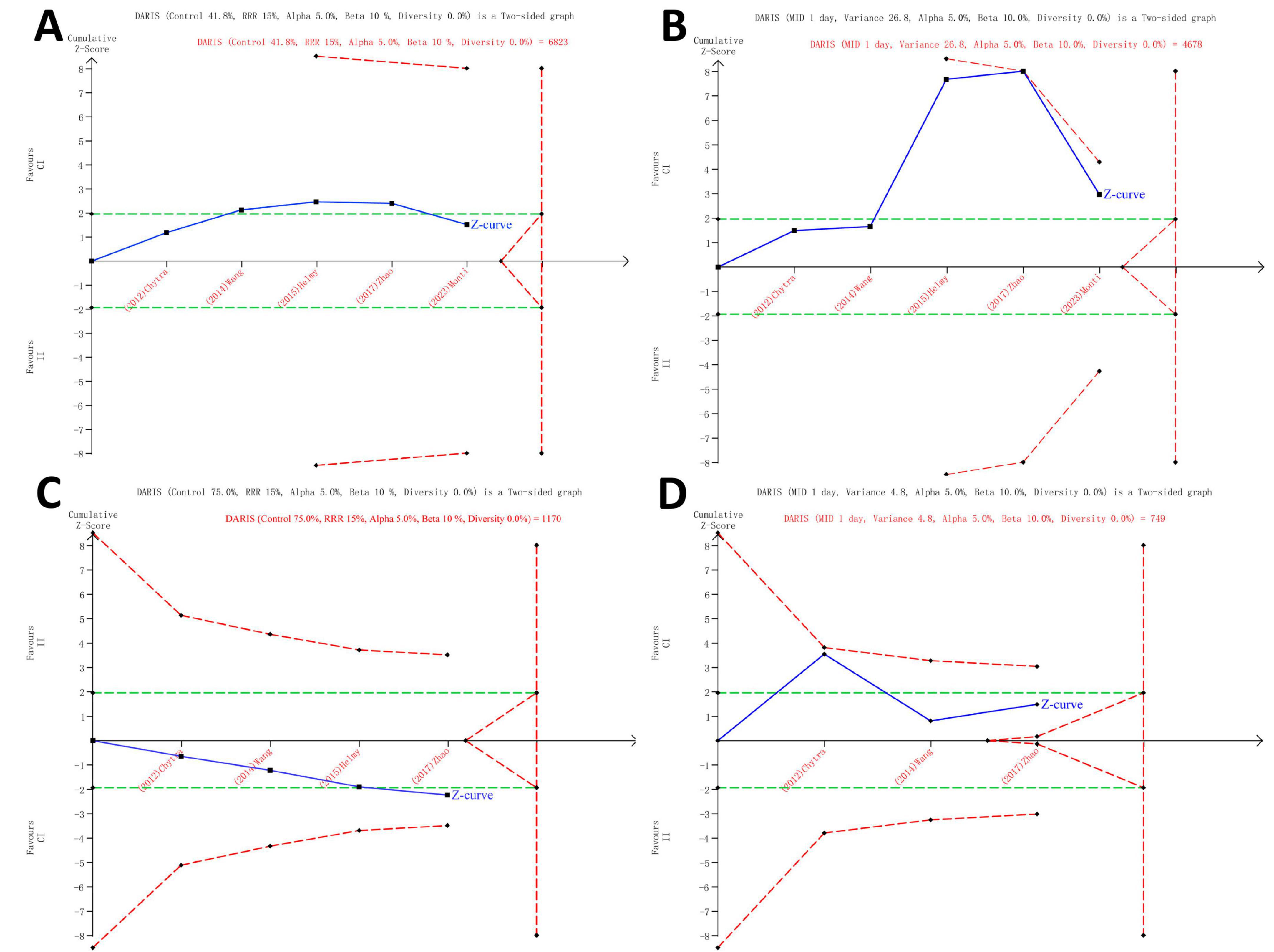
Figure 4. Trial Sequential Analysis of Clinical Outcomes. All-cause mortality in all patients (5 studies, n = 1075) (A), ICU length of stay (5 studies, n = 1075) (B), clinical cure rate (4 studies, n = 442) (C) and duration of meropenem therapy (3 studies, n = 368) (D). TSA was analyzed using Der Simonian and Laird random-effects model. The Z curve in blue measures the treatment effect (pooled relative risk). The parallel lines in green are the boundaries of conventional meta-analysis (alpha 5%), and the boundaries of benefit and harm are boundaries of conventional meta-analysis adjusted for between-trial heterogeneity and multiple statistical testing (TSA boundaries). A treatment effect outside the TSA boundaries of benefit/harm indicates reliable evidence for a treatment effect, and a treatment effect within the futility zone (the triangle between the parallel lines) indicates that there is reliable evidence of no treatment effect. DARIS: diversity adjusted required information size is the calculated optimum sample size for statistical inference, MID: minimally important difference, RRR: relative risk reduction, TSA: trial sequential analysis.
4 Discussion
This systematic review and meta-analysis of 5 RCTs, including 1,075 patients, the effects of CI and II of meropenem on the clinical outcomes of critically ill patients with sepsis were compared. This meta-analysis focused on the primary outcome (all-cause mortality) in critically ill patients with sepsis. The study findings did not reveal a significant difference in all-cause mortality between the CI group and the II group. Interestingly, this result contradicts the meta-analysis results reported by Roberts et al. (12), Zhao et al. (10) and other meta-analyses (11, 13). Our study also found that the CI group had a shorter ICU length of stay, a higher clinical cure rate, and a shorter duration of meropenem therapy compared to the II group. The three secondary outcomes of this study, which share some similarities with previous meta-analytical findings. However, TSA indicated that more trials are needed to further confirm these findings.
Prior to this meta-analysis, there have been multiple meta-analyses specifically comparing the clinical outcomes of septic patients CI vs. II of beta-lactam antibiotics or meropenem (10–14). Although they had differences in their inclusion and exclusion criteria, they all arrived at a consistent clinical outcome: compared to intermittent infusion, CI beta-lactam antibiotics or meropenem can reduce mortality in septic patients. The different choices of beta-lactam antibiotics may indicate the heterogeneity of the condition, and focusing on the use of a single antibiotic may reduce the impact of this heterogeneity. Currently, there is a lack of systematic review and meta-analysis of RCTs comparing CI vs. II of meropenem in critically ill patients with sepsis. Therefore, we sought to address this gap through our meta-analysis. Interestingly, we arrived at an unexpected conclusion: CI of meropenem did not reduce mortality in critically ill septic patients. This finding is consistent with the results of a recent high-quality RCTs (23), as well as a study by Dulhunty et al. (25), which included 25 ICUs and 432 critically ill patients and found that CI or II beta-lactam antibiotics had no impact on mortality in critically ill patients. Several similar RCTs also failed to find a significant difference in mortality between the two infusion methods (22, 34, 35). While the meta-analysis conducted by Falagas et al. (14) indicated that CI beta-lactam antibiotics can reduce mortality in septic patients, it should be noted that this analysis included some non-severe cases. The pharmacokinetic and pharmacodynamic advantages of CI of meropenem are indisputable. Moreover, prolonged or continuous administration of meropenem, with higher plasma and tissue concentrations, particularly against resistant pathogens such as Acinetobacter spp. and Pseudomonas aeruginosa, can provide greater exposure to achieve optimal antimicrobial efficacy (8, 36, 37). However, the management of severe sepsis is a complex endeavor: factors such as the source and type of infection, the severity of the patient’s illness, and even differences in diagnostic and treatment practices all play crucial roles in determining clinical outcomes. Therefore, based on the results of our study’s meta-analysis of RCTs, the conclusion that CI of meropenem can reduce mortality in patients cannot be definitively drawn. In addition, TSA indicated that there may be a type-II error, and the effect of the two infusion modes on mortality in patients with severe sepsis needs to be validated by further studies.
CI of meropenem, at equivalent dosages, can enhance its antimicrobial efficacy. The secondary outcomes of this study indicate a higher clinical cure rate in the CI group, consistent with previous meta-analysis outcomes (11, 13). The improved antimicrobial efficacy expedites the antimicrobial treatment process, leading to a shorter duration of meropenem therapy in the CI group, in line with the results reported by Chytra et al. (22). Additionally, the meta-analysis results revealed a shorter ICU stay in the CI group, akin to the findings of Nicasio et al. (38) and other studies (31, 39), despite the absence of this conclusion in larger RCTs (23, 25, 40). ICU length of stay and mortality, being indirect clinical outcomes influenced by meropenem antimicrobial therapy for infections, may be affected by multiple covariates. Therefore, the variability of these outcomes across different studies is understandable. Although there is minimal heterogeneity (I2 < 50% and Egger’s test P > 0.05) between the studies reporting these secondary outcomes, caution is warranted in interpreting the results due to the limited number of studies and patients included, as well as a type-1 error demonstrated by TSA in all secondary outcomes. Further high-quality RCTs are necessary to validate these two conclusions.
Strengths and limitations
This study has several strengths: (1) Different from other meta-analyses, we exclusively included studies utilizing meropenem as the primary antimicrobial agent, significantly reducing the influence of different antibiotics on the clinical outcomes in the meta-analysis; (2) This study only included RCTs, excluding low-quality studies such as retrospective and observational studies; (3) We incorporated the latest RCT with a total sample size of 607 patients, representing the highest quality study to date (23); (4) The use of TSA enabled us to identify the risk of type-I or type-II errors in our findings. The diversity adjusted required information size (DARIS) estimated from TSA will also inform the sample size needed for adequately powered future trials. These strengths will enhance the stability and value of the conclusions.
This study also has several limitations: (1) Although we constructed funnel plots to assess heterogeneity and both the I2 value and Egger’s test supported minimal heterogeneity among the studies, it is noteworthy that out of the 5 included RCTs, 3 were single-center studies and not all studies employed a double-blind experimental design, which may introduce bias to the conclusions; (2) For the primary outcome of all-cause mortality rate, despite establishing prioritized selection, we combined different mortality rate outcomes, inevitably introducing bias; (3) While all included studies primarily utilized meropenem as the main antimicrobial agent, there were variations in the distribution of infection types, disease severity (APACHE II, SOFA, and SAPS II scores), and other baseline data among the studies, which are important factors influencing clinical outcomes; (4) Despite conducting a comprehensive search for studies utilizing meropenem as the main antimicrobial agent, only 5 RCTs were included in this study, with a relatively small overall sample size. Additionally, some studies had sample sizes of only a few dozen cases, further contributing to outcome uncertainty; (5) Due to the small number of included studies and sample size, and because all studies were not focused on a specific type of infection, no subgroup analysis was conducted. However, the impact of factors such as different infection types, sites, and disease categories on outcomes cannot be ignored. This limitation should be addressed in future high-quality studies. In summary, cautious interpretation of the results of this study is warranted, and further research is needed to validate the conclusions drawn.
Given the current research and its limitations, we attempt to propose some future directions: (1) Conduct larger, multicenter, double-blind RCTs with standardized protocols for mortality definitions, PK/PD monitoring, and subgroup analyses. (2) Consider economic effects to quantify cost-effectiveness and clinical outcomes. (3) CI theoretically offers advantages; it is necessary to explore patient populations (subgroup analyses) that would benefit from CI, rather than continuing clinical studies targeting all patient groups. The importance of personalized treatment should be emphasized.
5 Conclusion
In critically ill patients with sepsis, compared to II administration, CI of meropenem did not reduce mortality. However, CI of meropenem was associated with a reduction in ICU length of stay, an increase in clinical cure rates, and a shorter duration of meropenem therapy. Further large-scale randomized controlled trials evaluating the effects of CI and II on clinical outcomes in critically ill patients with sepsis are needed to confirm these results.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: not applicable.
Author contributions
YW: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft. YHL: Conceptualization, Investigation, Writing – review & editing. MG: Data curation, Software, Writing – original draft. MZ: Project administration, Supervision, Writing – review & editing. YL: Methodology, Writing – review & editing. DW: Data curation, Writing – review & editing. XL: Software, Writing – review & editing. CZ: Data curation, Writing – review & editing. YF: Supervision, Writing – review & editing. HL: Conceptualization, Supervision, Writing – review & editing. DZ: Conceptualization, Investigation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1580116/full#supplementary-material
Abbreviations
ICU, Intensive care unit; CI, Continuous infusion; RCTs, Randomized controlled trials; II, Intermittent infusion; TSA, Trial sequential analysis; RRR, Relative risk reduction; RR, Relative risk; CI, Confidence interval; ITT, Intention-to-treat; DARIS, diversity adjusted required information size.
References
1. Seymour C, Liu V, Iwashyna T, Brunkhorst F, Rea T, Scherag A, et al. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:762–74. doi: 10.1001/jama.2016.0288
2. Chen J, Huang M. Intensive care unit-acquired weakness: Recent insights. J Intensive Med. (2024) 4:73–80. doi: 10.1016/j.jointm.2023.07.002
3. Li H, Li Y, Fu Y, Zhang X, Zhang D. The intensity of organ support: Restrictive or aggressive therapy for critically ill patients. J Intensive Med. (2023) 3:298–302. doi: 10.1016/j.jointm.2023.04.002
4. Bush K, Bradford P. β-Lactams and β-Lactamase inhibitors: An overview. Cold Spring Harb Perspect Med. (2016) 6:a025247. doi: 10.1101/cshperspect.a025247
5. Drusano G. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat Rev Microbiol. (2004) 2:289–300. doi: 10.1038/nrmicro862
6. Rhodes A, Evans L, Alhazzani W, Levy M, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. (2017) 43:304–77. doi: 10.1007/s00134-017-4683-6
7. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith C, French C, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47:1181–247. doi: 10.1007/s00134-021-06506-y
8. Roberts J, Kirkpatrick C, Roberts M, Robertson T, Dalley A, Lipman J. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: Intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother. (2009) 64:142–50. doi: 10.1093/jac/dkp139
9. Abdul-Aziz M, Sulaiman H, Mat-Nor M, Rai V, Wong K, Hasan M, et al. Beta-lactam infusion in severe sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. (2016) 42:1535–45. doi: 10.1007/s00134-015-4188-0
10. Zhao Y, Zang B, Wang Q. Prolonged versus intermittent β-lactam infusion in sepsis: A systematic review and meta-analysis of randomized controlled trials. Ann Intensive Care. (2024) 14:30. doi: 10.1186/s13613-024-01263-9
11. Chen P, Chen F, Lei J, Zhou B. Clinical outcomes of continuous vs intermittent meropenem infusion for the treatment of sepsis: A systematic review and meta-analysis. Adv Clin Exp Med. (2020) 29:993–1000. doi: 10.17219/acem/121934
12. Roberts J, Abdul-Aziz M, Davis J, Dulhunty J, Cotta M, Myburgh J, et al. Continuous versus intermittent β-Lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med. (2016) 194:681–91. doi: 10.1164/rccm.201601-0024OC
13. Yu Z, Pang X, Wu X, Shan C, Jiang S. Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: A meta-analysis. PLoS One. (2018) 13:e0201667. doi: 10.1371/journal.pone.0201667
14. Falagas M, Tansarli G, Ikawa K, Vardakas K. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: A systematic review and meta-analysis. Clin Infect Dis. (2013) 56:272–82. doi: 10.1093/cid/cis857
15. Abdul-Aziz M, Hammond N, Brett S, Cotta M, De Waele J, Devaux A, et al. Prolonged vs intermittent infusions of β-Lactam antibiotics in adults with sepsis or septic shock: A systematic review and meta-analysis. JAMA. (2024) 332:638–48. doi: 10.1001/jama.2024.9803
16. Dulhunty J, Brett S, De Waele J, Rajbhandari D, Billot L, Cotta M, et al. Continuous vs intermittent β-Lactam antibiotic infusions in critically Ill patients with sepsis: The BLING III randomized clinical trial. JAMA. (2024) 332:629–37. doi: 10.1001/jama.2024.9779
17. Li X, Long Y, Wu G, Li R, Zhou M, He A, et al. Prolonged vs intermittent intravenous infusion of β-lactam antibiotics for patients with sepsis: A systematic review of randomized clinical trials with meta-analysis and trial sequential analysis. Ann Intensive Care. (2023) 13:121. doi: 10.1186/s13613-023-01222-w
18. Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive–Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. (2009) 38:287–98. doi: 10.1093/ije/dyn188
19. Thorlund K, Devereaux P, Wetterslev J, Guyatt G, Ioannidis J, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. (2009) 38:276–86. doi: 10.1093/ije/dyn179
20. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. (2008) 61:64–75. doi: 10.1016/j.jclinepi.2007.03.013
21. Hurst M, Lamb H. Meropenem: A review of its use in patients in intensive care. Drugs. (2000) 59:653–80. doi: 10.2165/00003495-200059030-00016
22. Chytra I, Stepan M, Benes J, Pelnar P, Zidkova A, Bergerova T, et al. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: A randomized open-label controlled trial. Crit Care. (2012) 16:R113. doi: 10.1186/cc11405
23. Monti G, Bradic N, Marzaroli M, Konkayev A, Fominskiy E, Kotani Y, et al. Continuous vs intermittent meropenem administration in critically Ill patients with sepsis: The MERCY randomized clinical trial. JAMA. (2023) 330:141–51. doi: 10.1001/jama.2023.10598
24. Ai M, Chang W, Liu C. Mortality of continuous infusion versus intermittent bolus of meropenem: A systematic review and meta-analysis of randomized controlled trials. Front Microbiol. (2024) 15:1337570. doi: 10.3389/fmicb.2024.1337570
25. Dulhunty J, Roberts J, Davis J, Webb S, Bellomo R, Gomersall C, et al. A multicenter randomized trial of continuous versus intermittent β-Lactam Infusion in severe sepsis. Am J Respir Crit Care Med. (2015) 192:1298–305. doi: 10.1164/rccm.201505-0857OC
26. Dulhunty J, Roberts J, Davis J, Webb S, Bellomo R, Gomersall C, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: A multicenter double-blind, randomized controlled trial. Clin Infect Dis. (2013) 56:236–44. doi: 10.1093/cid/cis856
27. Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
28. Biggerstaff B, Jackson D. The exact distribution of Cochran’s heterogeneity statistic in one-way random effects meta-analysis. Stat Med. (2008) 27:6093–110. doi: 10.1002/sim.3428
29. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
30. Wetterslev J, Jakobsen J, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. (2017) 17:39. doi: 10.1186/s12874-017-0315-7
31. Helmy TA, Abdelghaffar AA, Fathy EM. Continuous versus intermittent intravenous meropenem in severe sepsis. IJPBS. (2015) 5:4457.
32. Wang Z, Shan T, Liu Y, Ding S, Li C, Zhai Q, et al. [Comparison of 3-hour and 30-minute infusion regimens for meropenem in patients with hospital acquired pneumonia in intensive care unit: A randomized controlled clinical trial]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2014) 26:644–9. doi: 10.3760/cma.j.issn.2095-4352.2014.09.008
33. Zhao H, Gu J, Lyu J, Liu D, Wang Y, Liu F, et al. Pharmacokinetic and pharmacodynamic efficacies of continuous versus intermittent administration of meropenem in patients with severe sepsis and septic shock: A prospective randomized pilot study. Chin Med J (Engl). (2017) 130:1139–45. doi: 10.4103/0366-6999.205859
34. Roberts J, Kirkpatrick C, Roberts M, Dalley A, Lipman J. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int J Antimicrob Agents. (2010) 35:156–63. doi: 10.1016/j.ijantimicag.2009.10.008
35. Roberts J, Roberts M, Robertson T, Dalley A, Lipman J. Piperacillin penetration into tissue of critically ill patients with sepsis–bolus versus continuous administration? Crit Care Med. (2009) 37:926–33. doi: 10.1097/CCM.0b013e3181968e44
36. Zeitlinger M. Extended infusion-putting the benefit into context. Lancet Infect Dis. (2018) 18:380–1. doi: 10.1016/S1473-3099(18)30115-4
37. Paul M, Theuretzbacher U. β-lactam prolonged infusion: It’s time to implement! Lancet Infect Dis. (2018) 18:13–4. doi: 10.1016/S1473-3099(17)30614-X
38. Nicasio A, Eagye K, Nicolau D, Shore E, Palter M, Pepe J, et al. Pharmacodynamic-based clinical pathway for empiric antibiotic choice in patients with ventilator-associated pneumonia. J Crit Care. (2010) 25:69–77. doi: 10.1016/j.jcrc.2009.02.014
39. Lodise T, Lomaestro B, Drusano G. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: Clinical implications of an extended-infusion dosing strategy. Clin Infect Dis. (2007) 44:357–63. doi: 10.1086/510590
40. Mirjalili M, Zand F, Karimzadeh I, Masjedi M, Sabetian G, Mirzaei E, et al. The clinical and paraclinical effectiveness of four-hour infusion vs. half-hour infusion of high-dose ampicillin-sulbactam in treatment of critically ill patients with sepsis or septic shock: An assessor-blinded randomized clinical trial. J Crit Care. (2023) 73:154170. doi: 10.1016/j.jcrc.2022.154170
Keywords: meropenem, continuous infusion, intermittent infusion, sepsis, critical illness
Citation: Wang Y, Li Y, Gao M, Zhang M, Li Y, Wang D, Li X, Zhang C, Fu Y, Li H and Zhang D (2025) Continuous vs. intermittent meropenem infusion in critically ill patients with sepsis: a systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Front. Med. 12:1580116. doi: 10.3389/fmed.2025.1580116
Received: 20 February 2025; Accepted: 05 May 2025;
Published: 10 June 2025.
Edited by:
Jihad Mallat, Cleveland Clinic Abu Dhabi, United Arab EmiratesReviewed by:
Aidos Konkayev, Astana Medical University, KazakhstanFeng Shen, Affiliated Hospital of Guizhou Medical University, China
Copyright © 2025 Wang, Li, Gao, Zhang, Li, Wang, Li, Zhang, Fu, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Zhang, emhhbmdkb25nQGpsdS5lZHUuY24=
 Youquan Wang
Youquan Wang Yanhua Li
Yanhua Li Meng Gao1
Meng Gao1 Yuting Li
Yuting Li Xinyu Li
Xinyu Li Dong Zhang
Dong Zhang