Abstract
Background and aim:
Tegoprazan (TEG) is a novel potassium-competitive acid blocker (P-CAB) that provides long-lasting acid-suppressing effects. The role of TEG-based Helicobacter pylori (H. pylori) eradication regimens in comparison to proton pump inhibitor (PPI)-based regimens requires further investigation.
Methods:
We conducted a comprehensive search across multiple databases. Studies comparing H. pylori eradication rates, adverse events (AEs), and compliance between TEG-based and PPI-based regimens were included. Statistical analyses were performed using RevMan 5.4.
Results:
A total of eight studies involving 4,640 patients were included. Based on intention-to-treat (ITT) analyses, the overall eradication rate (78.6% vs. 76.6%; odds ratio [OR] = 1.08, 95% CI: 0.93–1.24; p = 0.31, I2 = 0%) and compliance (97.8% vs. 97.8%; OR = 1.16, 95% CI: 0.54–2.50; p = 0.33, I2 = 13%) were comparable between the TEG and PPI groups. The AE rate of TEG-based regimens was significantly lower than that of PPI-based regimens (30.8% vs. 34.9%; OR = 0.88; 95% CI: 0.78–1.00; p = 0.04, I2 = 67%), although this difference was not significant in the randomized controlled trials (RCTs). The subgroup analyses showed higher eradication rates in studies conducted in China, those with treatment durations of 10 or 14 days, and those using dual or bismuth quadruple regimens. However, the treatment regimens did not significantly influence eradication rates within any subgroup.
Conclusion:
TEG-based H. pylori eradication treatment demonstrated similar eradication rates, compliance, and safety to PPI-based regimens.
Systematic review registration:
1 Introduction
More than half of the people worldwide have been infected with Helicobacter pylori (H. pylori) (1, 2). H. pylori is a known human carcinogen that is strongly associated with the development of gastric cancer (GC), peptic ulcers, and B-cell mucosa-associated lymphoid tissue lymphoma (3). Many gastrointestinal disorders, including GC, can be prevented and treated through the eradication of H. pylori (2, 4, 5). Therefore, administering H. pylori eradication therapy to infected individuals holds significant clinical importance.
For first-line H. pylori eradication regimens, proton pump inhibitor (PPI)-based triple or quadruple therapies are recommended in different countries (6–8). However, the efficacy of these regimens has declined significantly in recent years, primarily due to antibiotic resistance (9, 10) and insufficient acid suppression (11, 12). Increasing intragastric pH levels may enhance the stability and concentration of antibiotics, such as amoxicillin and clarithromycin (13). Furthermore, sustained acid suppression could render H. pylori more susceptible to antibiotic-induced eradication (12, 14). However, PPIs have limitations, including a short half-life, delayed onset of action, and variability in efficacy influenced by dietary factors and CYP2C19 polymorphisms (15). Therefore, identifying and utilizing more effective acid inhibitors in H. pylori eradication therapy is critical.
The recently developed potassium-competitive acid blocker (P-CAB) class provides potent acid suppression (16). The first developed P-CAB, vonoprazan, has demonstrated similar or superior efficacy in H. pylori eradication treatment, as shown by several meta-analyses (17–20). Tegoprazan (TEG), a subsequent P-CAB, has shown comparable or even superior acid suppression compared to vonoprazan or PPIs (21). In addition to acid suppression, P-CABs may also increase the susceptibility of antibiotic-resistant H. pylori strains (22). While these properties suggest promising eradication outcomes, evidence remains limited and inconsistent. Additionally, the optimal drug combinations and treatment duration for TEG-based regimens remain undefined. A comprehensive comparative analysis of TEG-based versus PPI-based regimens is therefore warranted to establish optimal eradication strategies.
This study aimed to evaluate the relative safety and efficacy of TEG-based versus PPI-based regimens for H. pylori infection through a systematic review and meta-analysis.
2 Methods
The meta-analysis was performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Cochrane Handbook for Systematic Reviews of Interventions, as described in previous meta-analyses (23, 24). We prospectively registered this meta-analysis on PROSPERO (CRD42024629665). The approval from the Ethics Committee was waived due to the study being a systematic review and meta-analysis.
2.1 Search strategy
To identify relevant English-language studies, two independent researchers conducted comprehensive searches of PubMed, Embase, Web of Science, and the Cochrane Library (updated to 20 December 2024). The search strategy included terms related to TEG, eradication, and H. pylori. Detailed search terms for PubMed are provided in Supplementary Table 1.
2.2 Study selection
All articles were assessed by two independent researchers to decide which ones could be included. In case of disagreement, the corresponding author (L.X) was consulted to make the final decision. The inclusion criteria were as follows: (1) patients: diagnosed with H. pylori infection; (2) intervention: TEG-based H. pylori eradication regimens for first-line therapy; (3) comparison: eradication therapy with PPI-based regimens; (4) outcomes: successful eradication rate and adverse event (AE) rate; and (5) study design: randomized controlled trial (RCT) or retrospective case–control study.
2.3 Data extraction
Two researchers independently extracted the following data: first author, publication year, country, patient age, diagnostic method, treatment regimen details, sample size, eradication success rate, and adverse events.
2.4 Risk of bias assessment
The Cochrane risk-of-bias (ROB) tool 2.0 and the Newcastle–Ottawa Scale (NOS) were used to assess bias in RCTs and retrospective studies, respectively. Assessments were performed independently by two researchers (Z.X. and L.J.G.).
2.5 Statistical analysis
Primary endpoints included the eradication rate and AE rate based on intention-to-treat (ITT) analysis. Only drug-related AEs were recorded, with serious AEs analyzed separately. Pooled proportions were calculated using RevMan 5.4, with heterogeneity assessed using the I2 statistic. A random-effects model was applied if I2 > 50%; otherwise, a fixed-effects model was used. Publication bias was evaluated using funnel plot visualization.
Sensitivity analysis was performed by sequentially excluding individual studies to assess their impact on the overall results. Subgroup analyses explored heterogeneity based on treatment regimen (high-dose dual therapy vs. triple therapy vs. bismuth quadruple therapy) and duration (7 vs. 10 vs. 14 days).
The results were reported as odds ratios (ORs) with 95% confidence intervals (CIs). A p-value < 0.05 was considered statistically significant.
3 Results
3.1 Search results and characteristics of the included studies
Initially, 36 studies from different databases were screened. Based on the inclusion and exclusion criteria, eight studies were finally included (25–32). Figure 1 provides a summary of the study selection flow diagram.
Figure 1

Search flow diagram in accordance with the preferred reporting items for systematic reviews and meta-analyses principles.
Table 1 displays the features of the included studies. They were published between 2022 and 2024. A total of six studies were conducted in Korea, and two were conducted in China. In addition, five studies were RCTs, and three were retrospective studies. All patients in these eight studies were treatment-naïve. All doses of TEG were 50 mg twice daily, except for one arm in the study by Lin et al. (31) (50 mg once daily, n = 107). For dual treatment, the antibiotic used was amoxicillin at a dosage of 3 g/day. For triple treatment, the antibiotics used were amoxicillin at a dosage of 1 g and clarithromycin at a dosage of 500 mg twice daily. The bismuth-based triple treatment was combined with the quadruple therapy. Particularly, the study by Jung et al. (29) mentioned concomitant therapies, with one acid inhibition regimen combined with three antibiotics. Another study by Lee et al. (30) described sequential therapies, with a change of antibiotics after 5 days of treatment. The majority of studies (5/8) included a treatment duration of 14 days, two studies had a duration of 10 days, and the other study had a duration of 7 days.
Table 1
| Study | Country | Study design | Treatment experience | Sample size (T/P) | Treatment regimens for TEG group | Treatment regimens for PPI group | Treatment duration | Successful eradication rate (T/P), % | Compliance, (T/P), % | Adverse event rate (T/P), % | Severe adverse event (T/P), n |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Choi et al. (25) | Korea | Multicenter RCT | First | 175/175 | Tegoprazan-based triple therapy | Lansoprazole-based triple therapy | 7 days | 62.86/60.57 | NR | 37.79/33.53 | 0/2 |
| Jung et al. (26) | Korea | Multicenter Retrospective | First | 344/333 | Tegoprazan-based triple therapy | Rabeprazole-based triple therapy | 14 days | 76.7/75.4 | NR | 27.6/25.8 | NR |
| Kim et al. (27) | Korea | Multicenter RCT | First | 93/97 | Tegoprazan-based bismuth quadruple therapy | Lansoprazole-based bismuth quadruple therapy | 14 days | 90.3/84.5 | 99.1/97.0 | 39.1/43.4 | 1/2 |
| Kong et al. (28) | China | Multicenter RCT | First | 184/184 | Tegoprazan-based dual therapy | Esomeprazole-based dual therapy | 14 days | 85.8/84.2 | 98.9/97.2 | 16.3/21.2 | 0/0 |
| Jung et al. (29) | Korea | Multicenter Retrospective | First | 620/854 | Tegoprazan combined with amoxicillin, clarithromycin, and metronidazole | Rabeprazole combined with amoxicillin, clarithromycin, and metronidazole | 10 days | 74.7/72.7 | NR | 39.2/40.6 | 1/3 |
| Lee et al. (30) | Korea | Single-center RCT | First or experienced | 204/202# | Tegoprazan combined with amoxicillin for 5 days, followed by tegoprazan combined with clarithromycin and metronidazole for the next 5 days | Esomeprazole combined with amoxicillin for 5 days, followed by esomeprazole combined with clarithromycin and metronidazole for the next 5 days | 10 days | 87.1/83.8 | 98.0/99.5 | 37.1/30.4 | 21/11 |
| Lin et al. (31) | China | Multicenter RCT | First | 214*/107 | Arm one: tegoprazan-based dual therapy (50 mg twice daily) arm two: tegoprazan-based dual therapy (50 mg once daily) | Esomeprazole-based bismuth quadruple therapy | 14 days | 85.98 and 85.98/85.05 | 96.2/96.2 | 14.56/13.33/27.18 | 1 and 0/ 1 |
| Park et al. (32) | Korea | Single-center Retrospective | First | 435/419 | Tegoprazan-based triple therapy | Esomeprazole/sodium bicarbonate-based triple therapy | 14 days | 78.6/81.4 | NR | 28.0/39.4 | 0/0 |
Characteristics and main results of included studies in this meta-analysis.
#There were 7 and 15 patients in TEG and PPI groups had previous eradication failure, respectively. *107 patients received tegoprazan 50 mg twice daily and 107 patients received tegoprazan 50 mg once daily. RCT: randomized controlled trial; TEG: tegoprazan; PPI: proton pump inhibitor; T/P: tegoprazan-based group/ PPI-based group, NR: not reported.
3.2 Risk of bias for the included studies
All three retrospective studies were of high quality according to the NOS scale (Supplementary Table 2). For the remaining five RCTs, blinding of participants and personnel was the main source of potential bias according to the ROB tool 2.0. The double-blinding method was not used in two studies (Supplementary Figure 1). The study by Lin et al. had a moderate risk of bias, as it did not describe the randomization method in detail, and the blinding method was not used in this study (31).
3.3 Helicobacter pylori eradication rate
3.3.1 Overall eradication rate
A total of 2,269 and 2,371 patients underwent H. pylori eradication treatment in the TEG and PPI groups, respectively. The overall eradication rates were 78.6% (TEG) and 76.6% (PPI), with no statistically significant difference between the two groups (OR = 1.08, 95% CI: 0.93–1.24; p = 0.31; I2 = 0%) (Figure 2). Similarly, no significant differences were observed in RCTs (82.1% vs. 78.8%; OR = 1.20, 95% CI: 0.93–1.54; p = 0.17; I2 = 0%) or retrospective studies (76.4% vs. 75.5%; OR = 1.03, 95% CI: 0.87–1.21; p = 0.77; I2 = 0%) (Figure 2).
Figure 2

Forest plots comparing the overall eradication rate of H. pylori between TEG-based therapy vs. PPI-based therapy.
3.3.2 The impact of regions on the overall eradication rate
Due to regional variations in antibiotic resistance and CYP2C19 polymorphism distribution, the subgroup analyses were stratified by country. The overall eradication rate was significantly higher in studies conducted in China (85.3%) compared to Korea (76.2%) (p < 0.001) (Table 2). However, the TEG-based regimens showed no superiority over the PPI-based regimens in both countries. In Korea, the success rates were 77.0% for TEG-based regimens compared to 75.5% for PPI-based regimens (OR = 1.07, 95% CI: 0.92–1.24; p = 0.58; I2 = 0%). In China, the success rates were 78.6% for TEG-based regimens compared to 76.6% for PPI-based regimens (OR = 1.11, 95% CI: 0.72–1.71; p = 0.91; I2 = 0%) (Figure 3A).
Table 2
| Regions | Total patients, n | Successfully eradicated, n | Eradication rate, % | p value |
|---|---|---|---|---|
| Korea | 3,951 | 3,011 | 76.2 | <0.001 |
| China | 689 | 588 | 85.3 | |
| Treatment length | ||||
| 14 days | 2,410 | 1952 | 81.0 | <0.001a |
| 10 days | 1880 | 1,431 | 76.1 | <0.001b |
| 7 days | 350 | 216 | 61.7 | <0.001c |
| Treatment regimens | ||||
| Dual | 475 | 405 | 85.3 | <0.001d |
| Triple | 1881 | 1,414 | 75.2 | 0.624e |
| Bismuth quadruple | 297 | 257 | 86.5 | <0.001f |
The impact of regions, treatment length, and treatment regimens on eradication rate.
14 days vs. 10 days.
14 days vs. 7 days.
10 days vs. 7 days.
Dual vs. triple.
Dual vs. Bismuth quadruple.
Triple vs. Bismuth quadruple.
Figure 3

Forest plots comparing the H. pylori eradication rate between TEG-based therapy and PPI-based therapy by region (Korea vs. China) (A), length of treatment (7-,10- and 14- days) (B), and treatment regimens (bismuth-containing quadruple therapy, bismuth-containing triple therapy, and non-bismuth-containing dual therapy) (C).
3.3.3 The impact of treatment length on the overall eradication rate
The length of treatment was analyzed. In total, 350 patients received 7-day eradication treatment, 1,880 patients received 10-day eradication treatment, and 2,410 patients received 14-day eradication treatment. The eradication rates for the 10-day and 14-day treatments were significantly higher than those for the 7-day treatment (76.1% vs. 61.7%, p < 0.001, and 81.0% vs. 61.7%, p < 0.001). Moreover, the 14-day treatment showed a superior eradication rate than the 10-day treatment (81.0% vs. 76.1%, p < 0.001) (Table 2).
The TEG-based treatment showed no difference when compared to the PPI-based treatment for 14-day (81.3% vs. 80.7%, OR = 1.01, 95% CI:0.83–1.25; p = 0.59, I2 = 0%), 10-day (77.8% vs. 74.8%, OR = 1.14, 95% CI:0.92–1.41; p = 0.54, I2 = 0%) and 7-day eradication treatments (62.9% vs. 60.6%, OR = 1.10, 95% CI:0.72–1.70; p = 0.66) (Figure 3B).
3.3.4 The impact of treatment regimens on the overall eradication rate
The study by Lee et al. (30) used sequential eradication treatment, while the study by Jung et al. used a three-antibiotic regimen combined with acid suppression, both of which were different from other studies. Therefore, these two studies were excluded from the subgroup analysis. Moreover, one arm in the study by Lin et al. (31) used TEG once daily for eradication treatment, and the arm was excluded from the subgroup analysis.
In total, 475, 1,881, and 297 patients received dual, triple, and bismuth quadruple treatments, with eradication rates of 85.3, 75.2, and 86.5%, respectively. The eradication rates for dual and bismuth quadruple treatments were significantly higher compared to the triple treatment (both p < 0.001) (Table 2).
The TEG-based treatment showed no superiority over the PPI-based treatment among the dual (85.7% vs. 84.2%, OR = 1.14, 95% CI:0.64–2.02; p = 0.66), triple (75.1% vs. 75.3%, OR = 0.98, 95% CI:0.79–1.21; p = 0.86, I2 = 0%), and bismuth quadruple treatments (90.3% vs. 84.5%, OR = 1.71, 95% CI:0.71–4.12; p = 0.23) (Figure 3C).
3.4 Compliance
In total, four studies reported patient compliance. Both groups showed high compliance (97.8% vs. 97.8%, OR = 1.16, 95% CI:0.54–2.50; p = 0.33, I2 = 13%) (Figure 4).
Figure 4

Forest plots comparing TEG-based therapy and PPI-based therapy on compliance.
3.5 Adverse events
The overall AE rate of the TEG-based regimens was significantly lower than that of the PPI-based regimens (30.8% vs. 34.9%; OR = 0.88; 95% CI: 0.78–1.00; p = 0.04, I2 = 67%) (Figure 5). However, the difference was not obvious among the RCTs (27.4% vs. 30.4%, OR = 0.94; 95% CI: 0.75–1.16; p = 0.55, I2 = 67%) (Figure 5). For severe adverse event rates, no significant difference was identified (1.2% vs. 0.9%, OR = 1.27; 95% CI: 0.69–2.34; p = 0.45, I2 = 12%) (Figure 5).
Figure 5
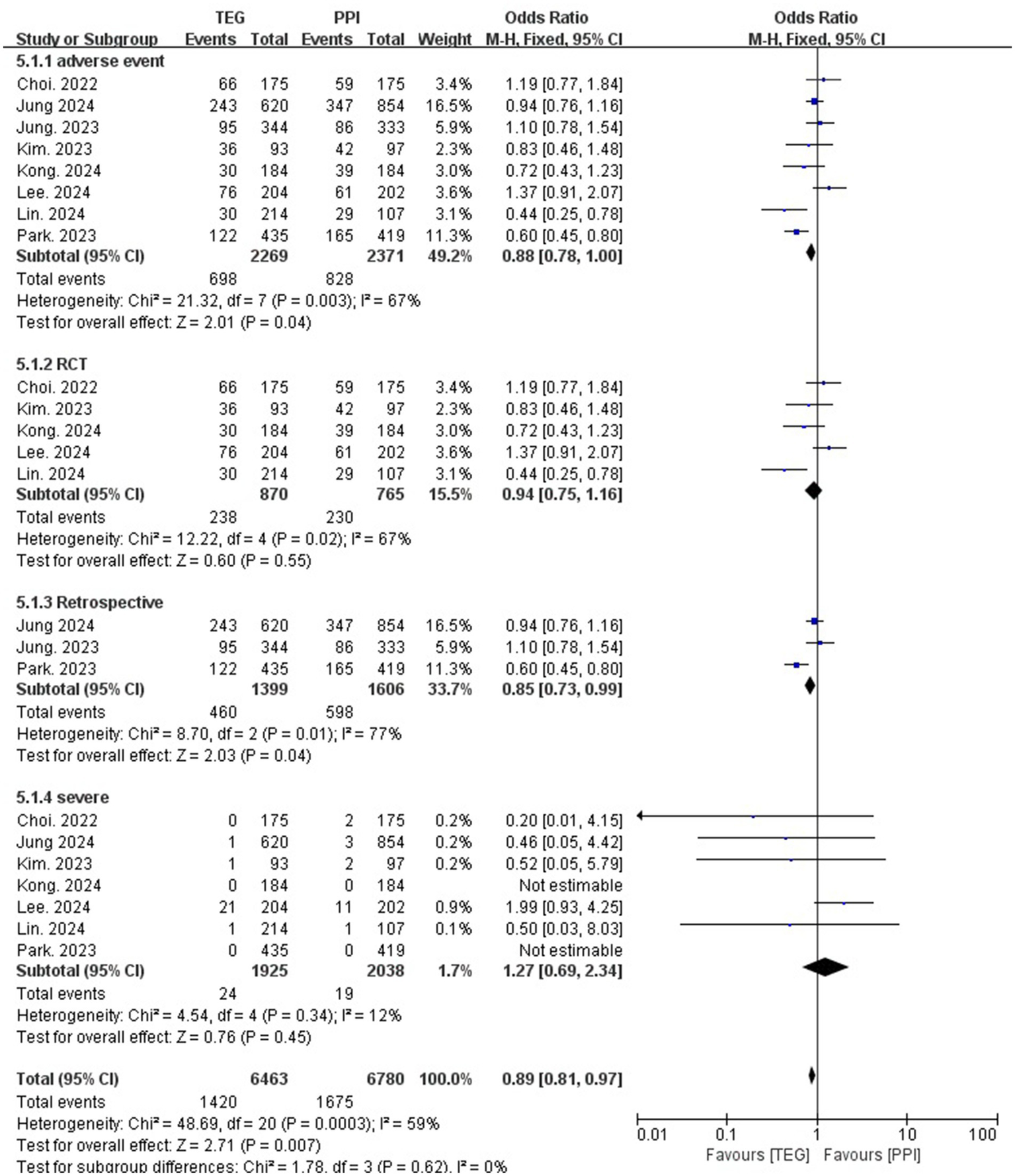
Forest plots comparing TEG-based therapy and PPI-based therapy on overall adverse events and severe adverse events.
3.6 Publication bias and sensitivity analysis
The funnel plot results illustrated that the eradication rate was essentially symmetrical, suggesting that this study did not experience significant publication bias (Supplementary Figure 2). Moreover, the research findings were rather solid since the sensitivity analysis did not significantly alter the results of the overall H. pylori eradication rate.
4 Discussion
In this systematic review and meta-analysis, we comprehensively searched databases to extract the published studies that compared TEG-based regimens with PPI-based regimens in the eradication treatment of H. pylori. The results showed that the TEG-based treatment had a comparable eradication rate and compliance with the PPI-based therapy in the ITT analysis. However, the overall AE rate was significantly lower for the TEG-based treatment, although the difference was not obvious in RCTs.
TEG was first approved in 2018 in South Korea. The drug showed promising advantages in the treatment of acid-associated diseases. When administered at 50 mg once daily, symptom relief rates reached 86.7% at 2 months for the treatment of functional dyspepsia (33). TEG 50 mg administered once daily is comparable to lansoprazole 30 mg taken once daily in Chinese patients suffering from duodenal ulcers (34). Similarly, when compared to esomeprazole once daily, TEG 50 mg once daily showed comparable tolerability and non-inferior effectiveness in healing erosive esophagitis, improving symptoms, and enhancing quality of life (35). Evidence supporting the use of TEG in H. pylori eradication treatment has accumulated in recent years. In 2024 and 2025, two meta-analyses by Kanu et al. (36) and Cho et al. (37) reached the same conclusions as our meta-analysis. However, the lack of subgroup analysis and the smaller number of included studies restricted the reliability of these meta-analyses. Therefore, we conducted a meta-analysis with the most recent evidence to examine how TEG-based regimens may be used to treat H. pylori infection.
Antibiotics, probiotics, and bismuth were the mainstays of previous treatment regimen modifications; nonetheless, the effectiveness of H. pylori eradication has continued to decline. Currently, selecting acid-suppressive medications presents another opportunity for therapeutic innovation. Acid suppressive agents lower the minimum inhibitory concentration (MIC) of medications, which is essential for the sterilization process, while also creating the proper pH environment for antibiotic sterilization (1). Long-term acid suppression, particularly at night, is essential for eliminating H. pylori (37, 38). Compared to patients who tested positive for nocturnal acid breakthrough (NAB), those without NAB had a higher eradication rate (38). P-CABs have been proven to be more effective in inhibiting gastric acid secretion compared to traditional PPIs (39). The first-generation P-CAB, vonoprazan, showed at least non-inferior efficacy and safety compared to conventional PPI-based therapies for both naive patients and patients with previous treatment failure (17, 19). However, the efficacy of TEG-based treatment was not verified in this study. Head-to-head comparisons between vonoprazan and TEG are needed to clarify the results. However, pharmacological studies are limited. The effects of TEG, vonoprazan, and esomeprazole on acid suppression at night in healthy volunteers were compared in a recent study (21). Compared to vonoprazan and esomeprazole, TEG inhibited nocturnal acid production more rapidly when administered at bedtime. However, vonoprazan caused greater and longer-lasting increases in intragastric pH over time. Moreover, P-CABs appear to induce varying degrees of hypergastrinemia, with vonoprazan causing a more significant increase compared to TGE, despite both having nearly identical antisecretory effects (39). Therefore, vonoprazan may have longer-lasting acid suppression effects than TEG, and it may be the reason why TEG-based therapies are not superior to PPI-based therapies. Moreover, as TEG suppresses gastric acid secretion at 50, 100, 200, and 400 mg in a dose-dependent manner (40), higher doses of TPZ may be considered for H. pylori treatment in the future.
The incidence of AEs appeared to be lower in patients who received TEG-based treatment. The most commonly reported adverse events in the two treatment regimens were abdominal pain, abdominal bloating, diarrhea, headache, and dysgeusia. The self-reported nature of these symptoms may explain the high heterogeneity observed in the analysis of adverse events between the two groups. The majority of AEs were well-tolerated, transient, and did not require any medical intervention. As a result, the incidences of severe AEs and compliance in two treatment groups did not differ significantly. Given that the p-value was close to the cutoff (p = 0.04) and the difference was not obvious in the RCTs, either treatment regimen was feasible. The advantage of TEG lies in the convenience of medication, as the efficacy of the drug is not influenced by diet (41).
According to the subgroup analysis, the studies conducted in China with a treatment duration of 10–14 days and using dual or bismuth quadruple treatments showed higher eradication rates compared to the studies conducted in Korea with a 7-day treatment duration and triple treatments. These results are consistent with those of previous studies. In a European RCT, 307 patients were randomized to receive 7-day, 10-day, or 14-day triple therapy. Both the 10-day and 14-day regimens reached eradication rates above the threshold of 80% in the ITT analysis (42). Another meta-analysis by Ding et al. (43) found that 14-day and 10-day bismuth-containing quadruple regimens had similar efficacy and a lower incidence of adverse effects. A network meta-analysis concluded that quadruple and high-dose dual therapies achieved identical eradication rates compared to standard triple therapy (44). In the studies conducted in Korea, triple treatment was the most frequently used regimen, and one study used a 7-day treatment duration. As a result, the overall eradication rate was significantly lower in Korea. More aggressive strategies should be implemented in Korea to ensure the efficacy of eradication treatment.
In our study, only the results from the ITT analysis were analyzed. As mentioned earlier, the treatment regimens were well-tolerated, and the results of the ITT analysis were comparable to those of the pre-protocol analysis. Moreover, it is important for patients to closely follow the instructions of the eradication regimen. PPI should be taken on an empty stomach, while TEG is not affected by dietary intake (45, 46). The difficulty in appropriately using the drugs was not balanced between the two groups. Therefore, the ITT analysis may truly reflect the treatment efficacy for different regimens.
There are several limitations to this study. First, the fact that all of the included studies were from East Asia might have limited the generalizability of the research findings. Second, there was variation in the antibiotics and PPIs used across the studies. This could have introduced bias in the efficacy and safety analyses. Third, the number of studies and sample sizes for the subgroup analysis were limited.
5 Conclusion
The TEG-based H. pylori eradication therapy demonstrated comparable efficacy (eradication rate) and compliance to the PPI-based regimens in the ITT analysis. While the overall AE rate was significantly lower in the TEG-based treatment, this difference was not statistically significant in RCTs. The findings from this meta-analysis suggest that TEG-based regimens represent a viable alternative for H. pylori eradication therapy.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
XZ: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. XL: Data curation, Methodology, Writing – review & editing. JL: Formal analysis, Writing – review & editing. YD: Data curation, Validation, Writing – review & editing. WX: Project administration, Writing – review & editing. DC: Resources, Writing – review & editing. LW: Formal analysis, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1580203/full#supplementary-material
SUPPLEMENTARY FIGURE 1Assessment of bias risk for the included randomized controlled studies based on Cochrane risk of bias tool 2.0.
SUPPLEMENTARY FIGURE 2Funnel plot of the overall eradication rate.
References
1.
MalfertheinerPMegraudFRokkasTGisbertJPLiouJMSchulzCet al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. (2022) 71:1724–62. doi: 10.1136/gutjnl-2022-327745
2.
CheyWDHowdenCWMossSFMorganDRGreerKBGroverSet al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. (2024) 119:1730–53. doi: 10.14309/ajg.0000000000002968
3.
RobinsonKAthertonJC. The Spectrum of Helicobacter-mediated diseases. Annu Rev Pathol. (2021) 16:123–44. doi: 10.1146/annurev-pathol-032520-024949
4.
FordACYuanYMoayyediP. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. (2020) 69:2113–21. doi: 10.1136/gutjnl-2020-320839
5.
LeeYCLinJT. Screening and treating Helicobacter pylori infection for gastric cancer prevention on the population level. J Gastroenterol Hepatol. (2017) 32:1160–9. doi: 10.1111/jgh.13726
6.
DingSZDuYQLuHWangWHChengHChenSYet al. Chinese consensus report on family-based Helicobacter pylori infection control and management. Gut. (2022) 71:238–53. doi: 10.1136/gutjnl-2021-325630
7.
MashikoSIfeanyiSSRoseUAbiodunOJJakaHCharlesOet al. Helicobacter pylori Management in Africa: a survey of diagnostic, treatment, and related resources. Helicobacter. (2024) 29:e13153. doi: 10.1111/hel.13153
8.
KatoMOtaHOkudaMKikuchiSSatohKShimoyamaTet al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. Helicobacter. (2019) 24:e12597. doi: 10.1111/hel.12597
9.
GrahamDYRokkasT. Overcoming the effects of increasing antimicrobial resistance on Helicobacter pylori therapy. Expert Rev Gastroenterol Hepatol. (2024) 18:705–11. doi: 10.1080/17474124.2024.2435520
10.
YuYXueJLinFLiuDZhangWRuSet al. Global primary antibiotic resistance rate of Helicobacter pylori in recent 10 years: a systematic review and Meta-analysis. Helicobacter. (2024) 29:e13103. doi: 10.1111/hel.13103
11.
GrahamDYShiotaniA. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. (2008) 5:321–31. doi: 10.1038/ncpgasthep1138
12.
ScottDRSachsGMarcusEA. The role of acid inhibition in Helicobacter pylori eradication. F1000Res. (2016) 5:1747. doi: 10.12688/f1000research.8598.1
13.
AntequeraCMOrleckKJacobRKenneallyAWrightWL. Potassium-competitive acid blockers: rethinking acid suppression for gastroesophageal reflux disease and Helicobacter pylori. Postgrad Med. (2024) 136:131–40. doi: 10.1080/00325481.2024.2320081
14.
SugimotoMFurutaTShiraiNKodairaCNishinoMIkumaMet al. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. (2007) 12:317–23. doi: 10.1111/j.1523-5378.2007.00508.x
15.
HuntRHArmstrongDJamesCChowdhurySKYuanYFiorentiniPet al. Effect on intragastric pH of a PPI with a prolonged plasma half-life: comparison between tenatoprazole and esomeprazole on the duration of acid suppression in healthy male volunteers. Am J Gastroenterol. (2005) 100:1949–56. doi: 10.1111/j.1572-0241.2005.41956.x
16.
LaineLDeVaultKKatzPMitevSLoweJHuntBet al. Vonoprazan versus lansoprazole for healing and maintenance of healing of erosive esophagitis: a randomized trial. Gastroenterology. (2023) 164:61–71. doi: 10.1053/j.gastro.2022.09.041
17.
YangCLiSHuangTLinHJiangZHeYet al. Effectiveness and safety of vonoprazan-based regimen for Helicobacter pylori eradication: a meta-analysis of randomized clinical trials. J Clin Pharm Ther. (2022) 47:897–904. doi: 10.1111/jcpt.13637
18.
SunYYueLHuW. Effectiveness and safety of vonoprazan-based regimens compared with those of proton pump inhibitor (PPI)-based regimens as first-line agents for Helicobacter pylori: a meta-analysis of randomized clinical trials. Eur J Clin Pharmacol. (2023) 79:279–88. doi: 10.1007/s00228-022-03430-y
19.
LiuLShiHShiYWangAGuoNLiFet al. Vonoprazan-based therapies versus PPI-based therapies in patients with H. pylori infection: systematic review and meta-analyses of randomized controlled trials. Helicobacter. (2024) 29:e13094. doi: 10.1111/hel.13094
20.
HuangSLiBPangXYGaoWW. Efficacy and safety of Vonoprazan-based treatment of Helicobacter pylori infection: a systematic review and network meta-analysis. BMC Infect Dis. (2024) 24:953. doi: 10.1186/s12879-024-09885-x
21.
YangEKimSKimBKimBKimYParkSSet al. Night-time gastric acid suppression by tegoprazan compared to vonoprazan or esomeprazole. Br J Clin Pharmacol. (2022) 88:3288–96. doi: 10.1111/bcp.15268
22.
LeeJWKimNNamRHYuJESonJHLeeSMet al. Efficacy of Tegoprazan for improving the susceptibility of antimicrobial agents against antibiotic-resistant Helicobacter pylori. Gut Liver. (2021) 15:53–60. doi: 10.5009/gnl20247
23.
GuoHZhangRZhangPZhangZLiXLyuSet al. Association of proton pump inhibitors with gastric and colorectal cancer risk: a systematic review and meta-analysis. Front Pharmacol. (2023) 14:1129948. doi: 10.3389/fphar.2023.1129948
24.
YanXShiJZhangYLiuJLinXYuCet al. Effectiveness and safety of tripterygium wilfordii poly-glycosides on glomerulonephritis: a systematic review and meta-analysis. Front Pharmacol. (2024) 15:1339153. doi: 10.3389/fphar.2024.1339153
25.
ChoiYJLeeYCKimJMKimJIMoonJSLimYJet al. Triple therapy-based on Tegoprazan, a new potassium-competitive acid blocker, for first-line treatment of Helicobacter pylori infection: a randomized, double-blind, phase III. Clinical Trial Gut Liver. (2022) 16:535–46. doi: 10.5009/gnl220055
26.
JungYSKimSKimHYNohSJParkJHSohnCIet al. Efficacy and tolerability of 14-day Tegoprazan- versus Rabeprazole-based triple therapy for eradication of Helicobacter pylori: a real-world evidence study. Gut Liver. (2023) 17:711–21. doi: 10.5009/gnl220218
27.
KimJSKoWChungJWKimTH. Efficacy of tegoprazan-based bismuth quadruple therapy compared with bismuth quadruple therapy for Helicobacter pylori infection: a randomized, double-blind, active-controlled study. Helicobacter. (2023) 28:e12977. doi: 10.1111/hel.12977
28.
KongQMirzaIAZhangXSongXLiXZhangQet al. Fourteen-day Tegoprazan-amoxicillin dual therapy as the first-line treatment of Helicobacter pylori infection (SHARE2301): a multicenter, noninferiority, randomized clinical trial. Helicobacter. (2024) 29:e13098. doi: 10.1111/hel.13098
29.
JungBWParkCHJungYS. Efficacy and safety of tegoprazan- and rabeprazole-based concomitant therapies for Helicobacter pylori infection: real-world evidence. J Gastroenterol Hepatol. (2024) 39:2409–16. doi: 10.1111/jgh.16719
30.
LeeJWKimNLeeJJoSYLeeDH. Efficacy of Tegoprazan-containing sequential eradication treatment compared to esomeprazole-containing sequential eradication of Helicobacter pylori in South Korea, a region with high antimicrobial resistance: a prospective, randomized, single tertiary center study. Helicobacter. (2024) 29:e13143. doi: 10.1111/hel.13143
31.
LinXHuangHLiuYZengYLuSXuXet al. Tegoprazan-amoxicillin dual therapy for Helicobacter pylori eradication: a prospective, randomized, multicenter study in Fujian, China. Helicobacter. (2024) 29:e13151. doi: 10.1111/hel.13151
32.
ParkCHParkJHJungYS. Comparative efficacy of Tegoprazan vs esomeprazole/sodium bicarbonate for the treatment of Helicobacter pylori infection. Clin Transl Gastroenterol. (2023) 14:e00632. doi: 10.14309/ctg.0000000000000632
33.
HuhCWYounYHJungDHChaRRKimYJJungKet al. Efficacy of Tegoprazan in patients with functional dyspepsia: a prospective, multicenter single study. Neurogastroenterol Motil. (2024) 30:313–21. doi: 10.5056/jnm23150
34.
ZongYLanCLiXChenWChenHLiaoAet al. Efficacy and safety of tegoprazan for duodenal ulcers in Chinese patients: a multicenter, randomized, double-blind, non-inferiority, phase III study. Curr Med Res Opin. (2024) 40:1855–62. doi: 10.1080/03007995.2024.2414090
35.
ZhuHXueQSongYZhangZLiXLyuSet al. Efficacy and safety of tegoprazan (LXI-15028) vs. esomeprazole in patients with erosive esophagitis: a multicenter, randomized, double-blind, non-inferiority phase III trial. Chin Med J. (2024). doi: 10.1097/CM9.0000000000003276 [Epub ahead of print].
36.
KanuJESolderaJ. Treatment of Helicobacter pylori with potassium competitive acid blockers: a systematic review and meta-analysis. World J Gastroenterol. (2024) 30:1213–23. doi: 10.3748/wjg.v30.i9.1213
37.
ChoJJinSParkS. Comparison of tegoprazan and proton pump inhibitors for first-line Helicobacter pylori eradication: a systematic review with meta-analysis. Expert Rev Anti-Infect Ther. (2025) 23:227–33. doi: 10.1080/14787210.2025.2459722
38.
KimJIParkSHKimJKChungISChungKWSunHS. The effects of nocturnal acid breakthrough on Helicobacter pylori eradication. Helicobacter. (2002) 7:331–6. doi: 10.1046/j.1523-5378.2002.00105.x
39.
EchizenH. The first-in-class potassium-competitive acid blocker, Vonoprazan fumarate: pharmacokinetic and Pharmacodynamic considerations. Clin Pharmacokinet. (2016) 55:409–18. doi: 10.1007/s40262-015-0326-7
40.
HanSChoiHYKimYHNamJYKimBSongGSet al. Randomised clinical trial: safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple oral doses of tegoprazan (CJ-12420), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. (2019) 50:751–9. doi: 10.1111/apt.15438
41.
YoonDYSunwooJShinNKimARKimBSongGSet al. Effect of meal timing on pharmacokinetics and pharmacodynamics of tegoprazan in healthy male volunteers. Clin Transl Sci. (2021) 14:934–41. doi: 10.1111/cts.12958
42.
KaratapanisSGeorgopoulosSDPapastergiouVSkordaLPapantoniouNLisgosPet al. 7, 10 and 14-days rabeprazole-based standard triple therapies for H. pylori eradication: are they still effective? A randomized trial. Acta Gastroenterol Belg. (2011) 74:407–12.
43.
DingYMLiYYLiuJWangJWanMLinMJet al. The cure rate of 10-day bismuth-containing quadruple therapy for Helicobacter pylori eradication is equivalent to 14-day: a systematic review and meta-analysis. Clin Exp Med. (2023) 23:1033–43. doi: 10.1007/s10238-022-00953-7
44.
LiJShiHZhouFXieLLinR. The efficacy and safety of regimens for Helicobacter pylori eradication treatment in China: a systemic review and network Meta-analysis. J Clin Gastroenterol. (2024) 58:12–23. doi: 10.1097/MCG.0000000000001902
45.
ScarpignatoCHuntRH. Potassium-competitive acid blockers: current clinical use and future developments. Curr Gastroenterol Rep. (2024) 26:273–93. doi: 10.1007/s11894-024-00939-3
46.
HwangILeeS. Editorial: deja vu all over again - let the P-CAB wars begin. Aliment Pharmacol Ther. (2023) 57:914–5. doi: 10.1111/apt.17444
Summary
Keywords
Helicobacter pylori, tegoprazan, proton pump inhibitor, eradication, compliance, adverse events
Citation
Zhang X, Li X, Li J, Deng Y, Xu W, Chen D and Wei L (2025) Comparison of tegoprazan-based and proton pump inhibitor-based regimens for Helicobacter pylori eradication: a meta-analysis and systematic review. Front. Med. 12:1580203. doi: 10.3389/fmed.2025.1580203
Received
21 February 2025
Accepted
31 March 2025
Published
18 June 2025
Volume
12 - 2025
Edited by
George Grant, Independent Researcher, Aberdeen, United Kingdom
Reviewed by
Xiao Li, Shandong Provincial Qianfoshan Hospital, China
Jonathan Soldera, University of Caxias do Sul, Brazil
György Miklós Buzás, Ferencváros Health Center, Hungary
Updates
Copyright
© 2025 Zhang, Li, Li, Deng, Xu, Chen and Wei.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Zhang, 13335822216@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.