Abstract
Schistosomiasis is a parasitic infection prevalent mainly in tropical and subtropical countries. We report a case of urinary infection caused by Schistosoma haematobium in a 16 years-old boy from Mali presented to our Institution with a one-month history of gross hematuria. The physical examination revealed an apparently healthy patient with soft abdomen, treatable, but painful to palpation and otherwise no other significant symptoms. The blood tests were within normal range except for eosinophilia. Urinary ultrasound revealed mild bilateral renal pelvis dilation, and distended bladder with thickened walls and multiple papillomatous growths (up to 31 mm in diameter). Cystoscopy confirmed multiple widespread mucosal lesions, and histology revealed severe eosinophilic cystitis with numerous parasitic eggs, observed, also, in parasitological urine examination, confirming the diagnosis of S. haematobium disease. The patient was diagnosed with schistosomiasis-related cystitis and treated with Praziquantel. At 2 months post-treatment follow-up urine microscope and eosinophil count were normalized and bladder wall irregularities and focal thickenings of variable size (from 3 to 11 mm) were documented. This case highlights the importance of considering hematuria and urinary bladder lesions in patients from areas where Schistosoma spp. is endemic as a strong indicator of parasitosis. Promptly initiating therapy can help prevent potential severe and less manageable consequences.
1 Introduction
Human schistosomiasis (1, 2), a neglected tropical disease, caused by helminth parasites from the Schistosoma genus, affects over 200 million people worldwide, primarily in Africa, Asia, and Latin America representing a major public health issue in tropical and subtropical countries (3–5). Europe is not generally endemic for human schistosomiasis, but it is affected by the disease in multiple ways. Environmental factors, such as water resource development, climate change, intensification of anthropogenic activities, increased migration flows from schistosomiasis-endemic regions, and the prolonged survival of adult parasites within the human host, potentially up to 40 years, may facilitate the emergence of autochthonous schistosomiasis, particularly in the southern regions of Europe (6–9).
Since 2014, numerous cases of acquired urogenital schistosomiasis have been described in Corsica (10, 11) where the parasite was introduced from an endemic region and established in local freshwater snails. In Italy, several studies have been performed on the prevalence of schistosomiasis in African migrants, showing a high incidence in this population. In 2019–2020, a study conducted by the National Institute for Health, found a prevalence of 31% for schistosoma infection, particularly in migrants from West Africa (12). Notably, the chronic urogenital manifestations of S. haematobium infection including hematuria, bladder wall thickening, and mass-like lesions can closely mimic neoplastic conditions, both clinically and radiologically. This diagnostic overlap often leads to delays or misdiagnosis, underscoring the importance of considering parasitic infections in the differential diagnosis of bladder cancers, particularly in individuals from endemic regions (1, 13, 14).
Six species of schistosomes infect humans: S. haematobium, S. mansoni, S. japonicum, S. mekongi, S. intercalatum, and S. guineensis. Among these, S. haematobium causes urogenital schistosomiasis. Infection occurs through contact with water containing schistosome cercariae, often during recreational, occupational, agricultural, or daily activities. Adult S. haematobium worms are localized in the retrovesical and periprostatic venous plexuses, depositing their eggs in the surrounding tissues, primarily in the bladder wall and urinary tract. These eggs cause inflammation and microlesions, resulting in hematuria, chronic cystitis, and, in severe cases, urinary obstructions. Each egg develops miracidium within a week, causing a local tissue reaction that facilitates its movement through the bladder layers into the lumen (13, 14).
Schistosomes have a complex life cycle, characterized of a sexual and asexual reproductive stage, with snails, specifically the Bulinus truncatus snail, as intermediate hosts and humans as the definitive hosts. Infected individuals excrete the parasite’s eggs in their stool and urine, which can contaminate both fresh and warm water, particularly in areas with inadequate sanitation. Once the eggs hatch, they release miracidia that infect the snails, where they reproduce asexually and eventually emerge as cercariae. These cercariae can penetrate human skin and transform into schistosomules. The young schistosomules move into the venous or lymphatic system, passing through the heart and lungs to the portal vein, where they mature within a few weeks. The female is transported by the male into the pelvic veins, completing its life cycle (13).
The most concerning outcome of urogenital schistosomiasis is the development of bladder cancer (14–16). Consequently, S. haematobium is classified as a biological carcinogen by the International Agency for Research on Cancer. Bladder cancer associated with chronic schistosomiasis involves immune dysregulation, where modulation of NK cell receptors plays a dual role in promoting anti-tumor activity while preserving healthy tissue integrity; in this context, tyrosine kinase inhibitors emerge as promising agents capable of targeting oncogenic signaling pathways while potentially influencing NK cell-mediated immune responses (17, 18).
Currently, the epidemiology is still poorly understood and due to gaps in surveillance and reporting.
Moreover, S. haematobium infection is often missed in early diagnoses due to symptom overlap with other urinary diseases, yet timely detection and treatment are crucial to prevent long-term complications. Reducing the risk of late diagnosis is essential, especially as this insidious and neglected disease has recently re-emerged in Europe (9). This issue is particularly relevant among migrant populations, who are affected by structural barriers that compromise timely access to medical care, such as language discordance and the lack of accessible or reliable medical history documentation. Diagnostic delays in such contexts may lead to prolonged morbidity, missed opportunities for early therapeutic intervention, and an elevated risk of adverse outcomes, including irreversible organ damage or malignant transformation. Addressing these gaps is essential to ensure equitable healthcare delivery and to improve clinical outcomes in at-risk and vulnerable populations.
Here, clinical and laboratory approaches were used to investigate a case of S. haematobium infection in a migrant boy from Mali, aiming to confirm diagnosis and assess response to Praziquantel (PZQ) therapy. Case outcomes and implications are discussed.
2 Case report
2.1 Clinical presentation
A 16 years-old patient from Mali was referred to our Institution complaining of gross hematuria since June 2023, without fever, chills, vomiting, diarrhea, and other symptoms. He was apparently healthy, although limited available medical and family history due to language barriers and a lack of cooperation (currently under the care of a family home guardian). Following the appearance of hematuria, a standard urine test and an ultrasound of the upper and lower abdomen were ordered at an outside facility.
On 6 June 2023, the urinalysis showed hemoglobin, numerous red blood cells, and leukocytes. An abdomen ultrasound conducted on 31 June 2023, revealed no evidence of abdominal fluid accumulation, mild bilateral dilation of the renal pelvis, a distended bladder with diffusely thickened walls, and irregular contours caused by multiple papillomatous growths (up to 16 mm in diameter) accompanied by mobile echogenic material in the dependent areas, and a non-uniform prostate structure. No signs of parenchymatous or cholestatic liver injury, or advanced liver pathology were observed.
The patient was referred for urological and cystoscopic evaluation and admitted to our Pediatric Surgery Unit. At the time of admission, the patient’s overall clinical condition was good, except for hematuria episodes over the past month and the presence of a reducible umbilical hernia.
2.2 Differential diagnoses, diagnostic workup and therapeutic interventions
During hospitalization in the Pediatric Surgery Unit, between 28th August and 30th August, the patient underwent laboratory tests (Table 1). In a young patient from a schistosomiasis-endemic area, presenting hematuria and bladder wall lesions, the differential diagnosis is broad and requires a multidisciplinary approach. One of the initial concerns was the possibility of bladder neoplasia. The presence of papillomatous growths on imaging and cystoscopy, along with hematuria, could raise suspicion for this malignancy. Another important differential diagnosis was urinary tract tuberculosis (TB), particularly due to the granulomatous features observed in the bladder biopsies. TB can affect the genitourinary system, and its presentation may mimic neoplastic or inflammatory bladder disease. However, extensive testing including Interferon Gamma Release Assay (LIAISON® QuantiFERON® TB-Gold Plus, Diasorin) with values of 0,012 IU/mL (TB1-CD4+) and 0,082 IU/mL (TB2-CD4+/CD8+), Mycobacterium tuberculosis-polymerase chain reaction of urine and Mantoux tuberculin skin test were performed and were consistently negative, making this diagnosis unlikely. The hypothesis of eosinophilic cystitis also emerged early in the diagnostic workup, especially after histological analysis of bladder biopsies revealed dense eosinophilic infiltration and granulomatous inflammation. Eosinophilic cystitis is a rare inflammatory condition that can clinically and radiologically mimic bladder cancer. It is often associated with allergies, infections, or parasitic infestations and thus served as a valuable diagnostic clue pointing toward a potential helminthic etiology. Chronic bacterial cystitis was also considered, as it can cause hematuria and bladder wall thickening. However, repeated urine cultures did not reveal any pathogenic bacterial growth, and there was no evidence of nitrites in urinalysis or systemic signs of infection. Enterobacteriaceae and enteropathogens (Salmonella, Shigella, Yersinia, Campylobacter, Escherichia coli O157H7) were also negative in the extended stool-culture on Xylose lysine desoxycholate agar (XLD; Difco, Sparks, MD).
TABLE 1
| Microbiological tests | Normal value | Test results |
| Interferon gamma release assay | < 0.35 IU/mL: Negative | 0.012 IU/mL (TB1-CD4+) 0.082 IU/mL (TB2-CD4+/CD8+) |
| Mycobacterium tubercolosis-polimerase chain reaction of urine | Negative | Negative |
| Mantoux tuberculin skin test | Negative | Negative |
| Anti-Strongyloides stercoralis antibodies with ELISA test | < 9 NTU: Negative | 7 NTU |
| Stool extended bacterial pathogens culture (Salmonella, Shigella, Yersinia, Campylobacter, Escherichia coli O157H7) | Negative | Negative |
| Complete blood count | Normal value | Test results |
| White blood cell count | 4.8–10.8 × 103/mL | 9.74 × 103/mL |
| Lymphocytes | 1.0–4.8 × 103/mL | 2.80 × 103/mL |
| Monocytes | 0.1–0.8 × 103/mL | 0.51 × 103/mL |
| Eosinophiles | 0.0–0.45 × 103/mL | 2.78 × 103/mL |
| Basophils | 0.0–0.20 × 103/mL | 0.10 × 103/mL |
| Red blood cell count | 4.2–5.6 × 103/mL | 5.72 × 103/mL |
| Hemoglobin | 12–17.5 g/dL | 15.4 g/dL |
| Platelet count | 130–400 × 103/mL | 207 × 103/mL |
| Inflammatory markers | Normal value | Test results |
| C-reactive protein | 0–5 mg/dL | 3.4 mg/dL |
| Fibrinogen | 160–350 mg/dL | 353 mg/dL |
| Albumin | 3.8–5.4 g/L | 4.3 g/L |
| Total protein | 6–8 g/dL | 8 g/dL |
| Urine examination | Normal value | Test results |
| Erythrocytes | 0–14 cells/μL | 316 cells/μL |
| Leukocytes | 0–18 cells/μL | 382 cells/μL |
| pH | 5–9 | 6.5 |
| Leukocyte esterase | Absent | Revealed in traces |
| Nitrites | Absent | Absent |
| Urine bacteriological test | Negative | Negative |
| Urine parasitological test | Negative | Presence of Schistosoma hematobium eggs |
Results of laboratory workup.
Given the geographical origin of the patient, parasitic infections should be considered predominant in the differential diagnosis. Moreover, no anti-Strongyloides stercoralis antibodies were detected in the patient’s serum using an ELISA method (The NovaLisa® Strongyloides Antibody ELISA, Novatec Immunodiagnostic GmbH) that resulted with a negative value (the diagnostic specificity of this assay is 94.12% (95% confidence interval: 83.76%–98.77%), and the diagnostic sensitivity is 89.47% (95% confidence interval: 75.2%–97.06%).
The complete blood count revealed a white blood cell count of 9.74 × 103/mL (range 4.8–10.8 × 103/mL), hemoglobin 15.4 g/dL (range 12–17.5 g/dL), and platelets 207 × 103/mL (range 130–400 × 103/mL). The differential white blood cell count was normal, except for mild eosinophilia, with 2.78 × 103/mL (range 0.0–0.45 × 103/mL). The blood chemistry levels were all within normal ranges with normal inflammatory markers.
Physical-chemical urine examination showed in the urine sediment the presence of numerous erythrocytes, 316 cells/μl (range 0–14) and leukocytes, 382 cells/μl (range 0–18) associated with traces of leukocyte esterase.
The urinary ultrasound, performed on 28 August 2023, showed normal-sized kidneys with regular margins in their anatomic position. The parenchymal thickness was at the lower limit of normal, and the corticomedullary ratio was appropriate for the patient’s age. Mild hyperechogenicity was noted in the right kidney. No signs of obstructive pathology were observed. The bladder was distended with finely and diffusely thickened walls, showing evidence of diffuse endoluminal sediment. At least six large, nodular, vegetating formations, adherent to the bladder walls, were visible with hyperechoic structures. These were located as follows: - in the left angular basal region (approximately 11 × 15 mm), - two along the left lateral wall (10 × 7 and 15 × 10 mm), - along the right lateral wall (11 × 5 mm), and the two largest on the bladder dome, protruding into the lumen and extending along the right lateral wall, with associated hypoechoic components (26 × 19 mm); the final formation (maximal diameters 31 × 11 mm) wrapped around the right ureter, which appeared ectatic at its distal segment. No free fluid is present in the pelvic cavity.
Further investigation was necessary, including chest X-ray and cystoscopic examination. The chest X-ray of 29th August showed mild reinforcement of the vascular-bronchial markings, particularly in the hiloperibronchial and hilar-basal regions. The diaphragm appeared normal in profile, with clear costophrenic angles. Cardiac volume was within normal limits. Cystoscopic examination revealed multiple papillomatous formations adherent to the bladder walls, with a ubiquitous distribution.
A critical diagnostic pitfall is the visual similarity between schistosomal granulomas and papillomatous tumors. This underlines the importance of systematic histological sampling and urinary cytology in all cases of persistent or atypical bladder lesions. As a result, multiple biopsies of these formations were performed (Figure 1). Histological analysis showed a pattern of eosinophilic cystitis with granulomatous features. This is consistent with an inactive stage of schistosomiasis.
FIGURE 1

Cystoscopic images. (A,B) “Sandy patches” and mucosal nodularity with erythema and hemorrhage, (C,D) multiple and inflamed papillomatous lesions.
In addition to the abnormalities observed in urinalysis and instrumental investigations, a microscopic parasitological examination of urine was also critical. The microscopic observation, as shown in Figure 2, revealed the presence of eggs of approximately above 150 μm in size, embryonated, with elongated or ellipsoid shape and a sub-terminal spine that were morphologically compatible with S. haematobium eggs. Following the detection of S. haematobium in the urine parasitology test, the patient was transferred to the Pediatric Infectious Diseases Department on 01/09. The team there noted that the patient was in good overall clinical condition. The brain CT scan showed no significant abnormalities, and the neurological exam was unremarkable. Blood tests once again revealed eosinophilia, strongly indicative of a parasitic infection.
FIGURE 2
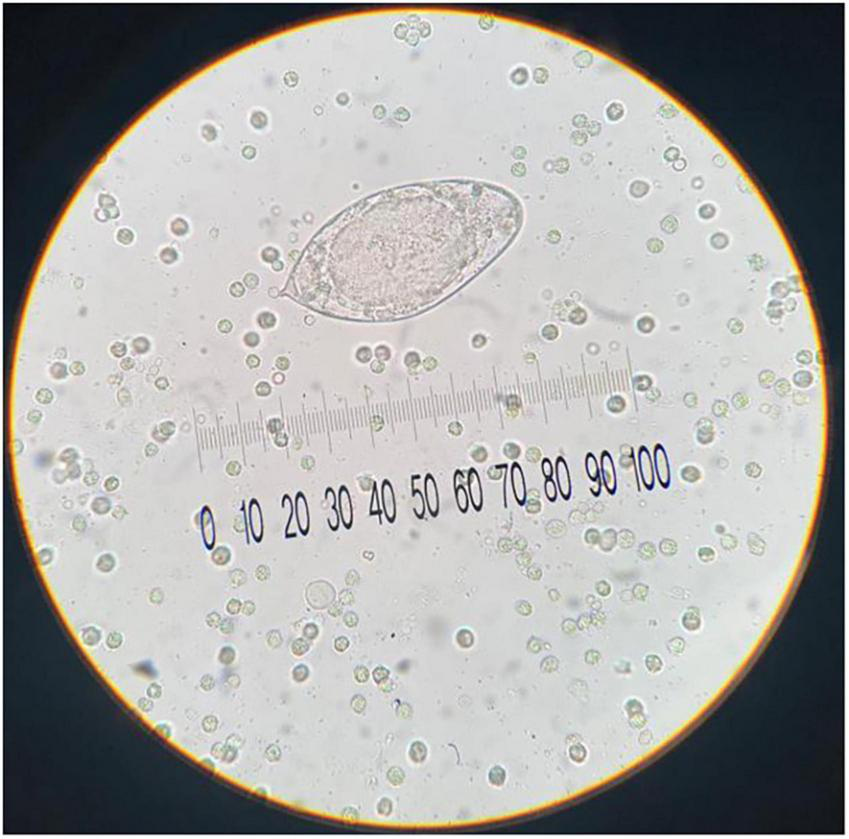
Urine sediment examination shows a representative egg of S. haematobium with diagnostic sub-terminal spine (approximative size: 150 μm; original magnification, 40x; no stain used).
The patient was treated with 600 mg of PZQ (Chandra Bhagat Pharma Ltd) (20 mg/kg in three administrations for 1 day only). After the treatment with PZQ, the patient did not experience any further episodes of hematuria and was discharged once the stability of their general condition was confirmed.
Every month, the patient underwent Day Hospital treatment in the Pediatric Infectious Diseases Department for a complete blood count, PCR, biochemistry tests, and especially for the urine collection to search for Schistosoma. Despite persistent negative urine parasitology and normalized eosinophil counts during follow-up, bladder wall irregularities and focal thickenings of variable size (from 3 to 11 mm) were documented up to date, demonstrating that PZQ was effective. No evidence of malignancy was found in repeated cytological examinations.
3 Discussion
Schistosomiasis is a critical public health concern, especially in its urinary form (14–16, 19). This condition mainly results from the long-term impacts of the infection rather than from the acute phase, which is manageable with treatment. The ongoing presence of the disease in endemic areas is complicated by education gaps and local environmental factors. However, the number of newly diagnosed cases of schistosomiasis is increasing in non-endemic areas, such as the Mediterranean regions of Europe, due to international tourism and immigration from transmission areas (6–9, 20).
Although 40% of infected subjects are asymptomatic, specific signs are present in 24%–66.4% of cases. Clinical outcomes are generally mild, but severe and unusual forms of the disease may occur. Severe infections are often associated with increased clinical severity due to allergic host responses, although significant morbidity may also occur from mild or mild infections. Chronic conditions arise from granulomatous inflammation due to egg deposition in tissues, with dysuria and hematuria being common symptoms at all stages of the disease. Late-stage complications can include proteinuria (often nephrotic), bladder calcifications, chronic bladder ulceration, ureter obstruction, renal colic, hydronephrosis, renal failure, and, ultimately, bladder carcinoma (14–16).
However, Schistosoma spp. infections can be asymptomatic, with no clinical signs or eosinophilia present (1, 2, 21, 22). As a result, schistosomiasis may be misdiagnosed and lead to high severity and chronicity with increased disability.
Therefore, laboratory diagnosis is crucial to accurately identify schistosomiasis. The diagnosis of schistosomiasis requires confirming infection, but the sensitivity of the available tests’ ranges significantly, from 45% to 72%. This includes serological assays for schistosomes antibody and microscopic detection of eggs in urine or stool (2, 21, 22).
Serologic assays for Schistosoma spp. can detect infection earlier than microscopic examination but cannot differentiate between active and prior infections, remaining positive for life. They are most useful in non-immune individuals, such as returning travelers with recent exposure. Identification of the eggs in urine or stools by microscopy technique is the most practical diagnostic method, being the unit of measurement of the number of eggs per gram of urine or stools. For suspected S. haematobium infection, the urine test is preferred, and specimen collection should occur at least two months after the last exposure to allow time for adult worms to mature and produce eggs.
Urine microscopic examination in our patient revealed ova, with specific size, shape, and spine characteristics to support S. haematobium identification.
Moreover, in hospital settings, cystoscopy and endoscopy are utilized to visualize lesions linked to urinary schistosomiasis. A common cystoscopy observation is the presence of “sandy patches” and mucosal nodularity. If the submucosa is involved, fibrosis or calcification may hinder bladder contraction, resulting in hypertonic or atonic bladder conditions (14–16). Radiological methods can reveal pathological lesions associated with S. haematobium infection, with calcifications in the ureter and bladder wall—due to calcified eggs in the submucosa—being the most frequent radiological finding in areas where the disease is common (23). Histopathological examination often reveals eosinophilic infiltration, fibrosis, and squamous metaplasia, with or without identifiable ova, complicating differentiation from malignancy (14–16). A multidisciplinary diagnostic approach, integrating parasitological, radiological, cytological, and histological findings, is essential for accurate diagnosis and appropriate management in patients from endemic regions.
Our patient presented microscopic and macroscopic hematuria associated with moderate eosinophilia, which suggested a helminthic infection. The subsequent investigation by urinary ultrasound and cystoscopy observation revealed the finding of the multiple papillomatous growths, which were resected. Histopathological evaluations consistently demonstrated eosinophilic cystitis with granulomatous inflammation, urothelial metaplasia, and calcified Schistosoma ova. The presence of bladder lesions and urothelial metaplasia raised concern for potential neoplastic transformation. However, repeated negative cytology, absence of dysplasia in biopsies, and stable clinical status argue against malignancy.
Therefore, the key strategies for managing schistosomiasis should include early diagnosis, intensive treatment, and regular follow-up care.
In response to the diagnostic challenges associated with hematuria and bladder lesions, particularly the difficulty in differentiating S. haematobium infection from neoplasia, we recommend a diagnostic algorithm based on the patient’s epidemiological setting. In endemic areas, the initial screening should emphasize non-invasive methods, including clinical assessment focused on urogenital symptoms (gross/microscopic hematuria, dysuria), urine microscopy for detection of schistosome eggs and molecular diagnostics assays for increased sensitivity in low-intensity S. haematobium infections. If results are inconclusive or there is persistent clinical suspicion of neoplasia, further investigation with cystoscopy and histopathological biopsy is appropriate. In non-endemic regions, detailed travel and migration history is crucial. The diagnostic approach should include serological testing for anti-schistosome antibodies used for screening, urine sediment examination and molecular assays before progressing to invasive procedures. Radiological imaging (ultrasound or CT urography) can provide additional information on the morphology and extent of bladder lesions. This stratified, epidemiology-based, approach optimizes diagnostic accuracy while minimizing unnecessary invasive techniques, and facilitates early detection and management of urogenital schistosomiasis, particularly in cases where delayed diagnosis may lead to complications such as bladder fibrosis, calcifications, or squamous cell carcinoma (1).
Praziquantel, a pyrazinoisoquinoline derivative, is currently the only drug available for the treatment of all Schistosoma spp. It is typically administered as a single dose of 40 mg/kg body weight for both school-aged children and adults through mass drug administration programs. However, its effectiveness ranges between 70% and 80% in adults and schoolchildren, with lower efficacy in preschool-aged children. Contributing factors to reduced effectiveness in young children include inadequate dosing, poor adherence due to the drug’s unpleasant taste, and limited understanding of PZQ’s pharmacokinetics and pharmacodynamics in this age group (2, 24).
Extended post-treatment follow-up to 18 months, monitoring of eosinophil level, parasitological urine examination, and clinical/imaging evaluation revealed that PZQ treatment was likely successful. The patient experienced no further episodes of hematuria and was discharged once his general condition stabilized.
4 Conclusion
Schistosomiasis is an emerging health issue in non-endemic areas, largely due to migration. Often misdiagnosed, it can lead to serious complications if not promptly addressed.
This report details a case involving a young boy from Mali with hematuria and a bladder lesion linked to S. haematobium. A combination of parasitological tests, imaging, and tissue analysis enabled early intervention, preventing severe complications like bladder cancer and renal failure.
From a clinical practice perspective in non-endemic regions, this case emphasizes the importance of considering parasitic infections in the differential diagnosis of hematuria and bladder lesions, especially in migrant populations. With rising global mobility, awareness and swift recognition of such infections are crucial to avoid delayed care and long-term damage.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving humans because the patient admitted to the Federico II University Hospital has signed the informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
FC: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – review and editing. ME: Data curation, Methodology, Writing – review and editing. AR: Data curation, Methodology, Writing – review and editing. AS: Data curation, Methodology, Writing – review and editing. GE: Data curation, Methodology, Writing – review and editing. CD: Data curation, Methodology, Writing – review and editing. RC: Conceptualization, Data curation, Investigation, Methodology, Writing – review and editing. PS: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review and editing. CE: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – review and editing. MV: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The funding facilitated specific aspects of the study, including access to specialized diagnostic assays, and supported the follow-up care of the patient. This research was funded by “Next Generation EU” funding within the MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project No. PE00000007, INF-ACT).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Buonfrate D Ferrari T Akim Adegnika A Russell Stothard J Gobbi F . Human schistosomiasis.Lancet. (2025) 405:658–70. 10.1016/S0140-6736(24)02814-9
2.
Chatterji T Khanna N Alghamdi S Bhagat T Gupta N Alkurbi M et al Recent advance in the diagnosis, treatment, and vaccine development for human schistosomiasis. Trop Med Infect Dis. (2024) 9:243. 10.3390/tropicalmed9100243
3.
Aula O McManus D Jones M Gordon C . Schistosomiasis with a focus on Africa.Trop Med Infect Dis. (2021) 6:109. 10.3390/tropicalmed6030109
4.
Li Q Li Y Guo S Li S Wang Q Lin W et al Global trends of schistosomiasis burden from 1990 to 2021 across 204 countries and territories: Findings from GBD 2021 study. Acta Trop. (2025) 261:107504. 10.1016/j.actatropica.2024.107504
5.
Dong B Hou Z Lu K . Schistosomiasis trends and control efforts: A global perspective from 1990 to 2050.Eur J Clin Microbiol Infect Dis. (2025) 44:1219–30. 10.1007/s10096-025-05102-y
6.
Arsuaga M Díaz-Menéndez M Gobbi F . Autochthonous schistosomiasis in Europe: A silent threat.Travel Med Infect Dis. (2022) 45:102244. 10.1016/j.tmaid.2021.102244
7.
Salas-Coronas J Bargues M Lozano-Serrano A Artigas P Martínez-Ortí A MasComa S et al Evidence of autochthonous transmission of urinary schistosomiasis in Almeria (southeast Spain): An outbreak analisis. Trav Med Infect Dis. (2021) 44:102165. 10.1016/j.tmaid.2021.102165
8.
Mulero S Rey O Arancibia N et al Persistent establishment of a tropical disease in Europe: The preadaptation of schistosomes to overwinter. Vector. (2019) 12:379. 10.1186/s13071-019-3635-0
9.
Gabrielli A Garba Djirmay A . Schistosomiasis in Europe.Curr Trop Med Rip. (2023) 10:79–87. 10.1007/s40475-023-00286-9
10.
Berry A Moné H Iriart X Mouahid G Aboo O Boissier J et al Schistosomiasis haematobium, Corsica, France. Emerg Infect Dis. (2014) 20:1595–7. 10.3201/eid2009.140928
11.
Saint F Boissier J Arnaud P Totet A Dinh A Vallee M et al Urinary schistosomiasis: The Corsican file. Fr J Urol. (2025) 35:102799. 10.1016/j.fjurol.2024.102799
12.
Marrone R Mazzi C Ouattara H Cammilli M Pontillo D Perandin F et al Screening for neglected tropical diseases and other infections in African refugees and asylum seekers in Rome and Lazio region, Italy. Travel Med Infect Dis. (2023) 56:102649. 10.1016/j.tmaid.2023.102649
13.
LoVerde PT . Schistosomiasis. In: Rafael T, Bernard F editors. Advances in Experimental Medicine and Biology.Cham: Springer (2019). p. 45–70.
14.
Santos L Santos J Gouveia M Bernardo C Lopes C Rinaldi G et al Urogenital schistosomiasis-history, pathogenesies, and bladder cancer. J Clin Med. (2021) 10:205. 10.3390/jcm10020205
15.
Jalloh M Cassell A Diallo T Gaye O Ndoye M Mbodji M et al Is schistosomiasis a risk factor for bladder cancer? EvidBased Facts. J Trop Med. (2020) 2020:8270810. 10.1155/2020/8270810
16.
Manciulli T Marangoni D Salas-Coronas J Bocanegra C Richter J Gobbi F et al TropNet schisto task force. Diagnosis and management of complicated urogenital schistosomiasis: A systematic review of the literature. Infection. (2023) 51:1185–221. 10.1007/s15010-023-02060-5
17.
Greppi M De Franco F Obino V Rebaudi F Godal R Frumento D et al NK cell receptors in anti-tumor and healthy tissue protection: Mechanisms and therapeutic advances. Immunol Lett. (2024) 270:106932. 10.1016/j.imlet.2024.106932
18.
Frumento D Grossi G Falesiedi M Musumeci F Carbone A Schenone S . Small molecule tyrosine kinase inhibitors (TKIs) for glioblastoma treatment.Int J Mol Sci. (2024) 25:1398. 10.3390/ijms25031398
19.
Onyekwere A Rey O Nwanchor M Alo M Angora E Allienne J et al Prevalence and risk factors associated with urogenital schistosomiasis among primary school pupils in Nigeria. Parasite Epidemial Control. (2022) 18:e00255. 10.1016/j.parepi.2022.e00255
20.
Lingscheid T Kurth F Clerinx J Marocco S Trevino B Schunk M et al Schistosomiasis in european travelers and migrants: Analysis of 14 years Tropnet Surveillance Data. Am J Trop Med Hyg. (2017) 97:567–74. 10.4269/ajtmh.17-0034
21.
González-Sanz M Martín-Rubio I Martín O Muriel A de la Fuente-Hernanz S Crespillo-Andújar C . Description of the serological response after treatment of chronic imported schistosomiasis.Trop Med Infect Dis. (2025) 10:22. 10.3390/tropicalmed10010022
22.
Hinz R Schwarz N Hahn A Frickmann H . Serological approaches for the diagnosis of schistosomiasis - A review.Mol Cell Probes. (2017) 31:2–21. 10.1016/j.mcp.2016.12.003
23.
Cimini A Ricci M Gigliotti P Pugliese L Chiaravalloti A Danieli R et al Medical imaging in the diagnosis of schistosomiasis: A Review. Pathogens. (2021) 10:1058. 10.3390/pathogens10081058
24.
Vale N Gouveia M Rinaldi G Brindley P Gärtner F Correia da Costa JM . Praziquantel for schistosomiasis: Single-drug metabolism revisited, mode of action, and resistance.Antimicrob Agents Chemother. (2017) 61:e02582-16. 10.1128/AAC.02582-16
Summary
Keywords
schistosomiasis, hematuria, urinary bladder lesions, cystoscopic examination, Praziquantel
Citation
Carraturo F, Escolino M, Russo A, Spagnuolo A, Esposito G, Di Mento C, Colicchio R, Salvatore P, Esposito C and Vitiello M (2025) Case Report: Uncommon presentation of Schistosoma haematobium infection in a migrant patient: diagnostic and therapeutic challenges. Front. Med. 12:1583233. doi: 10.3389/fmed.2025.1583233
Received
25 February 2025
Accepted
26 May 2025
Published
17 June 2025
Volume
12 - 2025
Edited by
Amir Sasan Mozaffari Nejad, Jiroft University of Medical Sciences, Iran
Reviewed by
Chiaka Anumudu, University of Ibadan, Nigeria
José Manuel Correia Da Costa, National Health Institute Doutor Ricardo Jorge (INSA), Portugal
Updates
Copyright
© 2025 Carraturo, Escolino, Russo, Spagnuolo, Esposito, Di Mento, Colicchio, Salvatore, Esposito and Vitiello.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paola Salvatore, psalvato@unina.itCiro Esposito, ciro.esposito4@unina.itMariateresa Vitiello, mariateresa.vitiello2@unina.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.