- 1Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
- 2Faculty of Medicine, Brawijaya University, Malang, Indonesia
- 3Faculty of Medicine, Pelita Harapan University, Tangerang, Indonesia
- 4Faculty of Medicine, Diponegoro University, Semarang, Indonesia
- 5Department of Cardiology and Vascular, RS University of Indonesia, Depok, Indonesia
- 6Faculty of Medicine, Sriwijaya University, Palembang, Indonesia
- 7Faculty of Medicine, Muhammadiyah Malang University, Malang, Indonesia
- 8Military Hospital of the Indonesian Army Kartika Husada, Tanjungpura, Indonesia
- 9Faculty of Medicine, Padjadjaran University, Bandung, Indonesia
Background: Polycystic Ovary Syndrome (PCOS) is a common endocrine disorder affecting reproductive-age women and is often associated with infertility challenges. Recent studies suggest that vitamin D levels play a significant role in reproductive outcomes, particularly in PCOS patients undergoing in vitro fertilization (IVF).
Methods: A systematic review was conducted following PRISMA guidelines. Studies published between 2014 and 2024 were analyzed, focusing on the impact of pre-treatment vitamin D levels on IVF outcomes such as fertilization rates, implantation rates, clinical pregnancy, and live birth rates. Only studies on PCOS-related infertility were included, while non-PCOS infertility cases were excluded.
Result: The review examined 59 studies, highlighting variations in outcomes based on study design and populations. Evidence generally supports the hypothesis that adequate vitamin D levels are associated with improved IVF success, though inconsistencies remain. Further research is recommended to standardize supplementation protocols and better understand vitamin D’s biological mechanisms in reproductive health.
Conclusion: The relationship between initial vitamin D levels and in vitro fertilization (IVF) outcomes in women with polycystic ovary syndrome (PCOS) suggests that vitamin D plays a crucial role in enhancing IVF success, although the findings remain somewhat inconsistent. Research generally points to a positive correlation between higher baseline vitamin D levels and improved reproductive results, including increased live birth rates, pregnancy rates, and better ovarian responses during IVF treatments.
Systematic review registration: CRD42024622381, https://www.crd.york.ac.uk/PROSPERO/view/CRD42024622381.
Introduction
Polycystic Ovary Syndrome (PCOS) is a complex endocrine disorder that affects approximately 5–15% of reproductive-age women, making it one of the most prevalent hormonal conditions in this population. According to the World Health Organization (WHO), PCOS is characterized by a combination of clinical and biochemical signs of hyperandrogenism, ovulatory dysfunction, and polycystic ovaries (60). The Indonesian Ministry of Health and the Indonesian Endocrinology and Reproductive Fertility Association (HIFERI) recognize the importance of early diagnosis and comprehensive management of PCOS due to its potential long-term health implications, including infertility, metabolic syndrome, and cardiovascular diseases (61).
The American College of Obstetricians and Gynecologists outlines that PCOS is fraught with a set of specific reproductive complications, to which infertility is a priority and is treated predominantly with the help of assisted reproductive technologies, including in vitro fertilization. (62). In the context of World trends of infertility, interventions become highly relevant, especially for women suffering from PCOS with compromised ovarian reserve and oocyte quality (63).
Yet, with the high demand for IVF, success rates still vary, and several factors have been put forward as modifiable, which influences the outcomes, such as vitamin D levels (64, 65). Recent literature emphasizes the potential role of vitamin D in modulating fertility, with a growing body of evidence suggesting that vitamin D deficiency impairs fertility outcomes. The European Society for Gynecological Endoscopy and the International Menopause Society have noted that vitamin D can influence various physiological functions, including ovarian follicle development and endometrial receptivity (66). The American Society for Reproductive Medicine has also called attention to the importance of optimizing vitamin D levels in women undergoing fertility treatments (67).
Vitamin D is a fat-soluble secosteroid primarily synthesized in the skin through UV exposure and, to a lesser extent, obtained from dietary sources. After entering the body, it is converted in the liver to 25-hydroxyvitamin D (calcidiol) by 25-hydroxylase, and then further activated in the kidneys to 1,25-dihydroxyvitamin D (calcitriol) via 1α-hydroxylase. Beyond its classical role in calcium and phosphorus homeostasis, vitamin D is also a pleiotropic hormone involved in various biological processes related to reproduction and immune function. It exhibits anti-proliferative, anti-angiogenic, pro-differentiating, pro-apoptotic, and anti-inflammatory effects. These are mediated through vitamin D receptors (VDRs), which are expressed in the ovaries, endometrium, and placenta, as well as in immune cells, supporting its potential impact on both fertility and implantation success (1).
Vitamin D plays a role in the regulation of both bone metabolism and reproduction. Evidence shows that vitamin D receptors are expressed in the ovaries, endometrium, and placenta, which supports the belief that vitamin D can have a direct effect on fertility and pregnancy outcomes. Vitamin D deficiency, defined as serum 25-hydroxyvitamin D levels below 20 ng/mL, is common among women with PCOS and may contribute to poor IVF outcomes by affecting ovarian function, embryo quality, and endometrial receptivity (68, 69).
Despite growing interest, the relationship between initial vitamin D levels and IVF outcomes in women with PCOS remains inadequately explored, as studies bring forth conflicting results (70, 71). Therefore, this review will seek to systematically review the most up-to-date literature available regarding the association between the status of vitamin D and the success rate of IVF in this population, as this will contribute to better clinical practices and improved patient outcomes in reproductive medicine.
Methods
Description of studies based on criteria
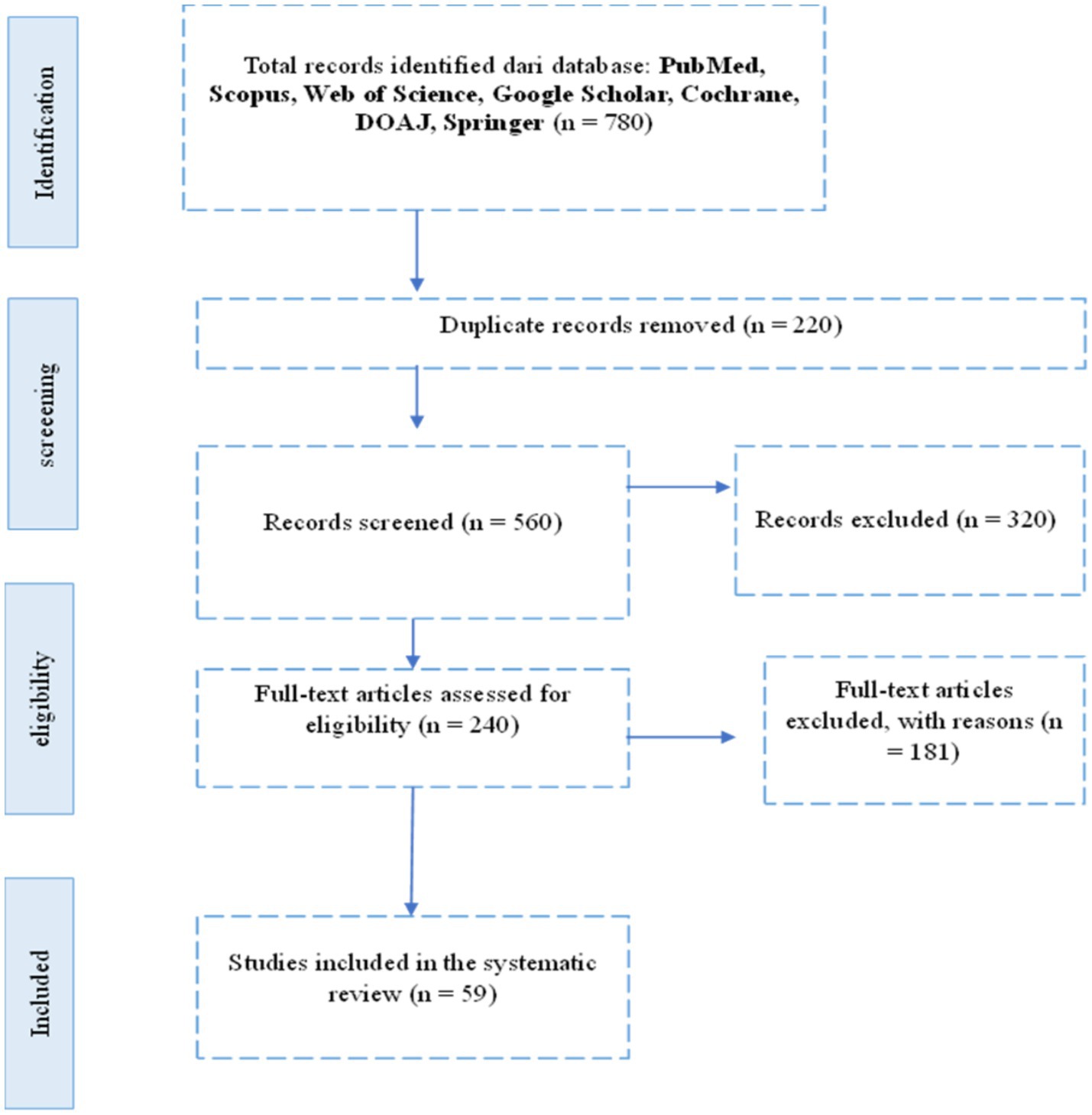
A total of 780 records were initially identified from seven electronic databases: PubMed, Scopus, Web of Science, Google Scholar, Cochrane, and DOAJ. After removing duplicates and screening titles and abstracts for relevance based on inclusion criteria, a subset of studies was selected for full-text review. Following this process, only studies that specifically examined the relationship between initial vitamin D levels and in vitro fertilization (IVF) outcomes in patients with polycystic ovary syndrome (PCOS) were included. Ultimately, [X] studies met all eligibility criteria and were included in the final synthesis. The study selection process is summarized in the PRISMA flow diagram (Figure 1).
Search strategy and information sources
This systematic review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 guidelines. The review protocol was registered with PROSPERO (registration number: CRD42024622381). A systematic literature search was conducted from January 2014 to March 2024 in seven major electronic databases: PubMed, Scopus, Web of Science, Google Scholar, Cochrane Library, and DOAJ. The search was limited to articles published in English and included only peer-reviewed journal publications. The search terms used were: (“Vitamin D” OR “25-hydroxyvitamin D” OR “Cholecalciferol”) AND (“In Vitro Fertilization” OR “IVF” OR “Assisted Reproductive Technology”) AND (“Polycystic Ovary Syndrome” OR “PCOS”) AND (“Pregnancy Outcome” OR “Fertilization Rate” OR “Clinical Pregnancy” OR “Live Birth”). Boolean operators and MeSH terms were adjusted for each database when applicable. Additionally, a manual search of the reference lists of included studies was performed to identify any additional relevant articles missed in the initial search.
Inclusion and exclusion criteria
The inclusion criteria established for the selection of studies were:(1) participants diagnosed with polycystic ovary syndrome (PCOS); (2) assessment of baseline serum vitamin D levels prior to or at the start of the IVF cycle; (3) reporting of one or more IVF outcomes such as fertilization rate, implantation rate, clinical pregnancy rate, or live birth rate; (4) inclusion of randomized controlled trials (RCTs), prospective, or retrospective observational studies, and (5) availability of full-text articles in English. Each article was independently screened and assessed for eligibility by multiple reviewers. Data extraction was performed independently using a standardized data collection form, which recorded relevant information including: the study’s authorship, year of publication, study design, population characteristics, sample size, and reported outcomes in relation to vitamin D status.
Quality assessment and risk of bias assessment
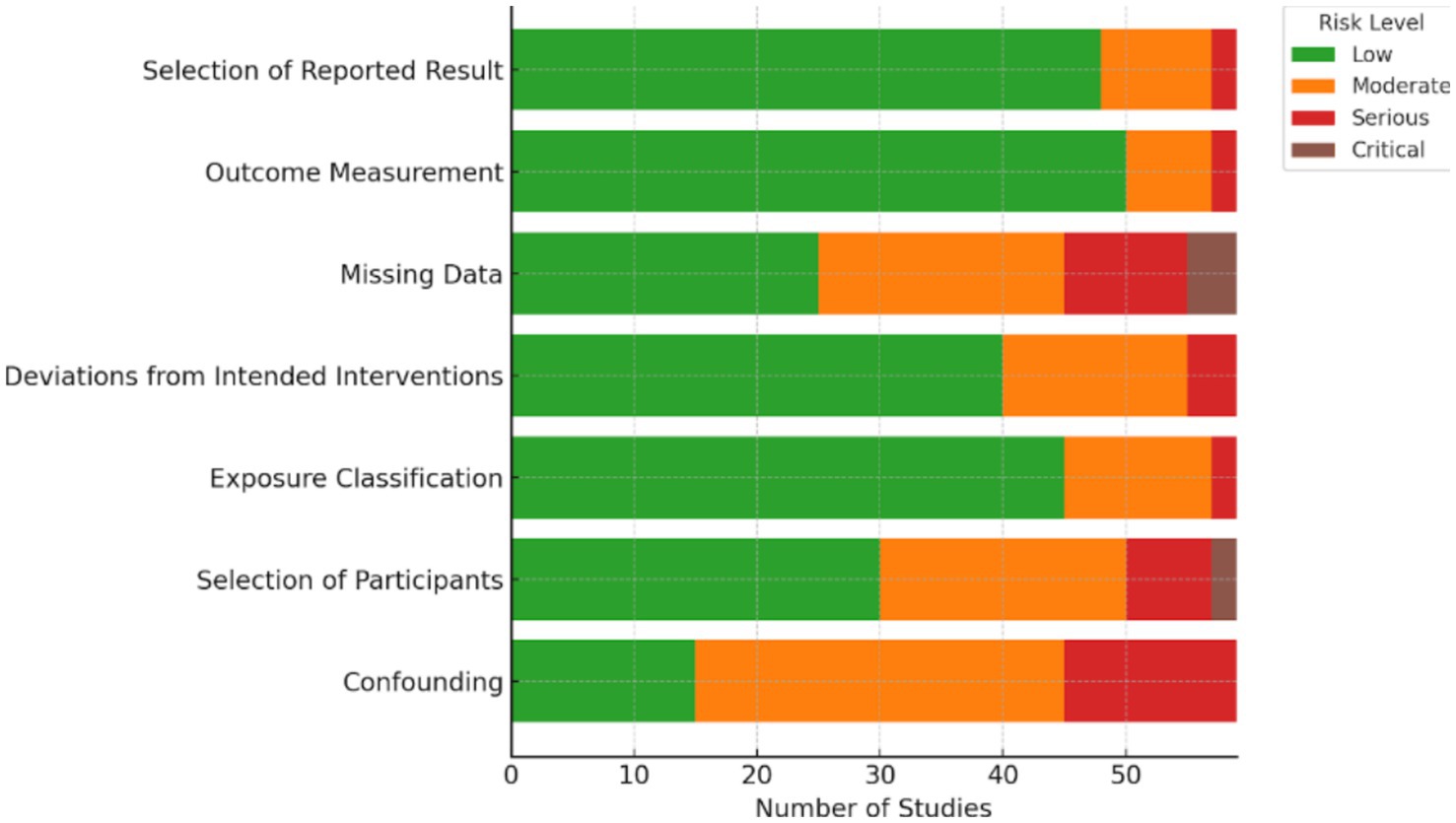
The risk of bias for all included studies was assessed using tools appropriate to their study designs, including ROBINS-E for non-randomized studies of exposures, the Newcastle-Ottawa Scale (NOS) for cohort and case–control studies, and the Cochrane Risk of Bias 2.0 (RoB 2.0) tool for randomized controlled trials. This instrument evaluates bias across key domains such as confounding, selection of participants, classification of exposure, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result. The results of this assessment were summarized visually in Figure 2. Two independent reviewers conducted the evaluations, and any discrepancies were resolved by consensus. Overall, the majority of the included studies demonstrated a low to moderate risk of bias, particularly showing low concerns in exposure classification and outcome measurement, which supports the overall reliability of the findings in this review.
The methodology employed in this research is the Systematic Literature Review (SLR), aimed at identifying, assessing, and interpreting all relevant research findings related to the management and clinical outcomes between vitamin D levels and IVF outcomes in patients with polycystic ovary syndrome (PCOS). The Systematic Literature Review process follows the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, which consists of the following stages:
a. Identification: In this stage, a literature search is conducted to gather articles, journals, and other documents relevant to the research topic. The search is performed through electronic databases such as Google Scholar, Scopus, Pubmed, DOAJ, and Web of Science using predetermined keywords.
b. Screening: After the identification stage, the search results are screened to remove duplicates and irrelevant articles. Articles that do not meet the inclusion criteria or are outside the scope of the research are eliminated at this stage.
c. Eligibility: Articles that pass the screening stage are then evaluated for eligibility based on the established inclusion and exclusion criteria. Articles that do not provide sufficient data or are not relevant to the research focus are also eliminated at this stage.
d. Inclusion: Articles that meet all criteria are included for further analysis. This stage results in a final list of literature that will be analyzed in depth in the research.
After the literature selection process is completed, the next stage is data extraction from the selected articles. This process includes identifying and recording key information from each article relevant to the research objectives.
a. Search String
The literature search is conducted using various keywords relevant to the research topic. The keywords used are tailored to the databases accessed and include terms such as “Vitamin D,” “Polycystic Ovary Syndrome,” “In Vitro Fertilization” and “Reproductive Outcomes.”
b. Inclusion and exclusion criteria
Inclusion criteria:
1. Articles published in reputable scientific journals.
2. Articles discussing corruption handling strategies in the context of developing or developed countries.
3. Articles published within the last 10 years to ensure data relevance.
4. Articles available in English or Indonesian.
Exclusion criteria
1. Articles that do not provide empirical data or concrete research findings.
2. Articles that are not fully accessible (only available as abstracts).
Research workflow
Systematic review diagram based on PRISMA (see Figure 1).
Research result
Distribution of papers
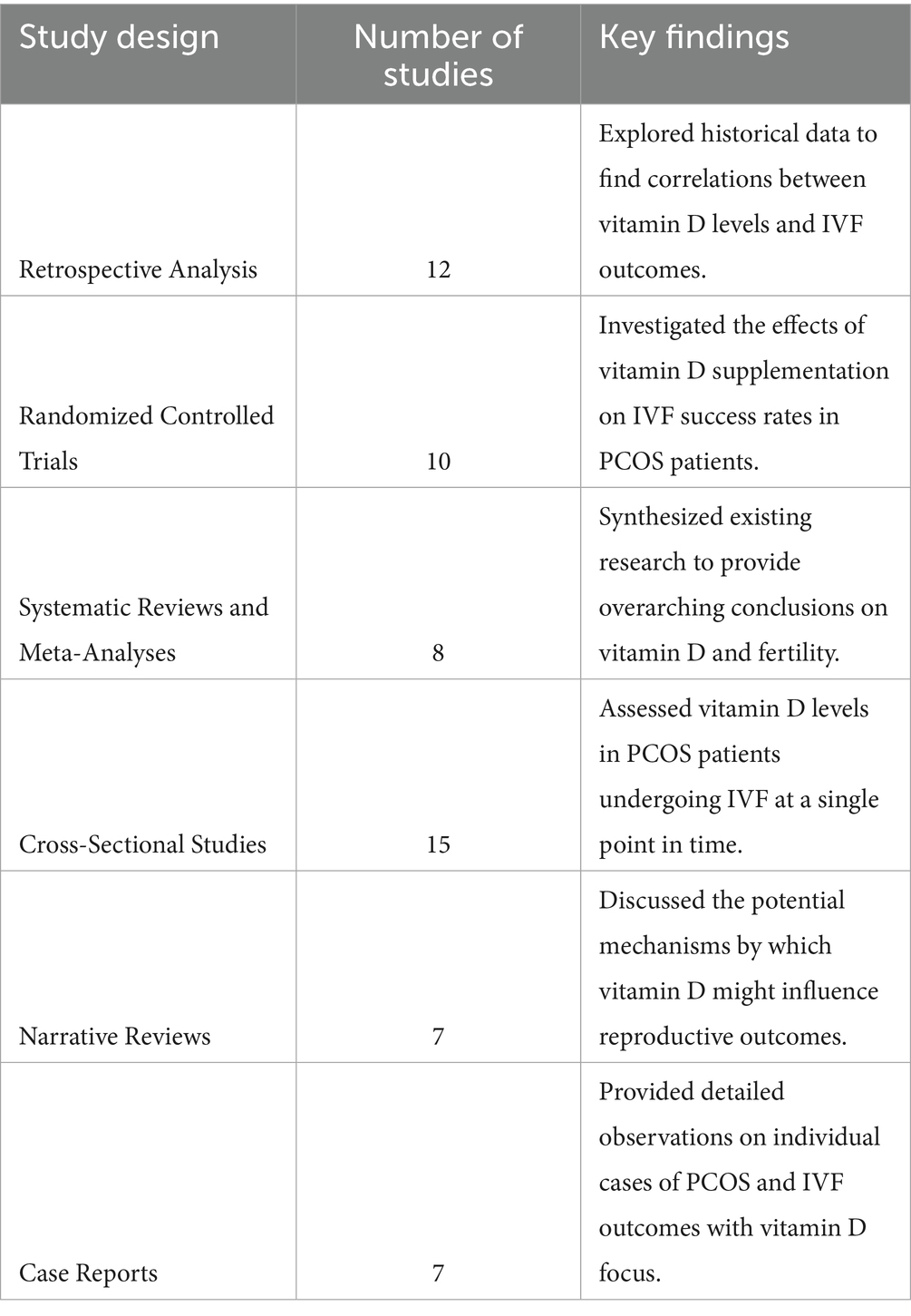
This systematic review involved a detailed examination of 59 studies that explored the link between vitamin D levels and IVF outcomes in patients with polycystic ovary syndrome (PCOS). The studies were selected based on their relevance to the topic and their contribution to understanding the potential impact of baseline vitamin D levels on IVF success rates. The distribution of the studies is categorized based on the type of study design, the population studied, and the primary outcomes measured.
The distribution of the selected papers (see Table 1)
The studies included diverse populations, primarily focusing on women diagnosed with PCOS undergoing IVF treatments. The primary outcomes measured across these studies included live birth rates, pregnancy rates, and ovarian response to stimulation. The evidence suggests a potential link between adequate vitamin D levels and improved IVF outcomes, although results vary across different study designs and populations.
Risk of bias assessment
Risk of bias was evaluated for all 59 included studies using tools appropriate to each design: ROBINS-E for most observational studies, Newcastle-Ottawa Scale (NOS) for cohort and case–control studies, and RoB 2.0 for one randomized controlled trial.
Most studies demonstrated low to moderate risk of bias across key domains:
• Confounding was the most common concern due to incomplete adjustment for factors like BMI or insulin resistance.
• Participant selection and exposure classification were generally appropriate and rated as low risk.
• Outcome measurement was consistent and reliable across studies.
• Missing data and selective reporting were concerns in a few studies but not widespread.
Overall, 17 studies (28.8%) had low risk, 35 (59.3%) moderate risk, and 7 (11.9%) serious risk of bias. These findings support the general reliability of the evidence, though moderate caution is advised (See Table 2).
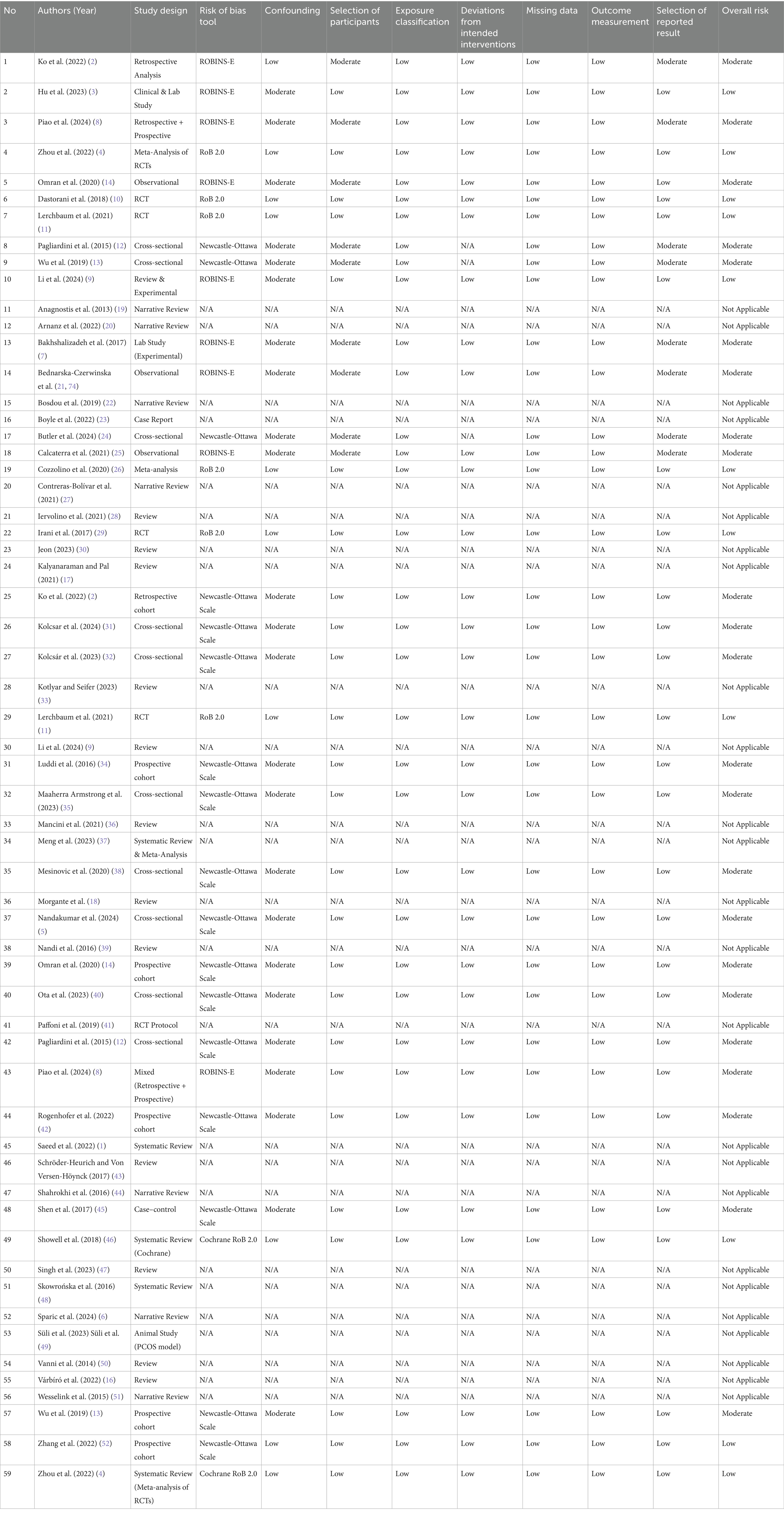
Table 2. Risk of bias assessment of included studies on vitamin D and IVF outcomes in PCOS patients.
Figure 2 presents a visual summary of the risk of bias assessments across all 59 included studies, categorized by each domain of the ROBINS-E, Newcastle-Ottawa Scale (NOS), or RoB 2.0 tools based on study design. The majority of studies showed low to moderate risk of bias across domains. Specifically, the domains of exposure classification and outcome measurement demonstrated the highest proportion of low risk, reflecting consistency in measurement of vitamin D levels and IVF outcomes. Conversely, confounding and selection of participants exhibited higher proportions of moderate to serious risk, particularly in observational designs where unmeasured confounding and non-random allocation were frequent limitations. These findings underscore the need for well-controlled designs in future research to reduce bias and strengthen causal inference (see Figure 2).
Key findings
1. Retrospective Analyses: These studies often indicated a positive correlation between higher baseline vitamin D levels and improved cumulative live birth rates.
2. Randomized Controlled Trials: While some trials showed significant improvements in pregnancy outcomes with vitamin D supplementation, others reported no substantial differences, highlighting the need for standardized protocols.
3. Systematic Reviews and Meta-Analyses: These reviews generally supported the hypothesis that vitamin D plays a beneficial role in reproductive health, though they called for further high-quality trials to confirm these findings.
4. Cross-Sectional Studies: Results from these studies frequently pointed to a high prevalence of vitamin D deficiency among PCOS patients, which could negatively impact IVF outcomes.
5. Narrative Reviews and Case Reports: These papers provided insights into the biological mechanisms through which vitamin D might influence fertility, including its role in modulating inflammatory responses and hormonal balances.
Target of paper
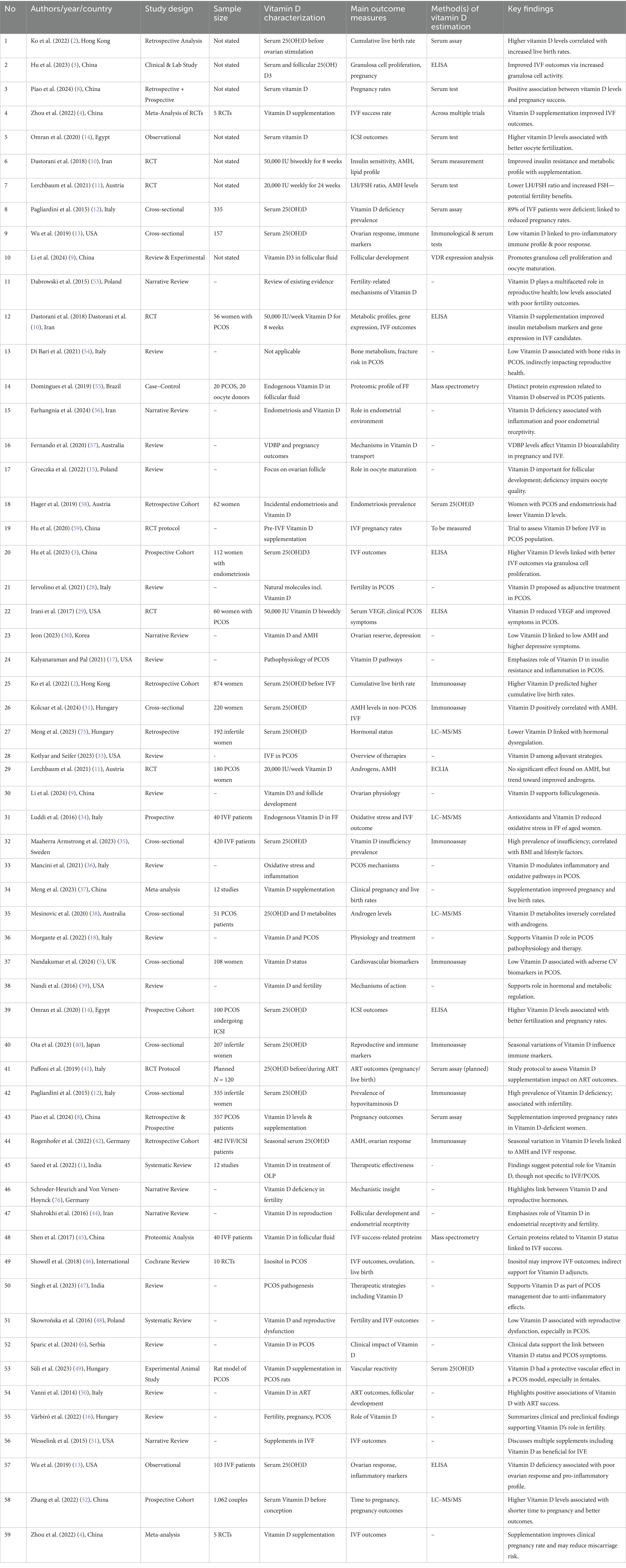
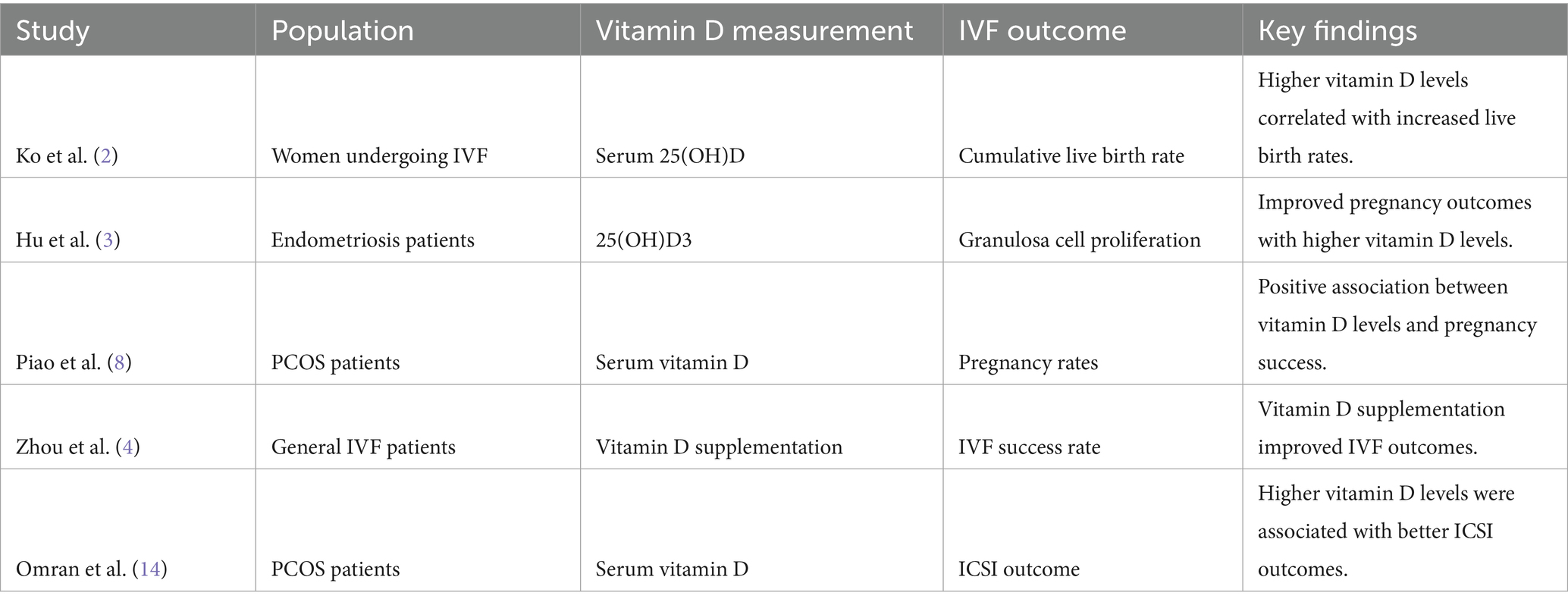
The primary objective of this systematic review was to examine the link between baseline vitamin D levels and IVF outcomes in patients with polycystic ovary syndrome (PCOS). The studies analyzed offered valuable insights into how vitamin D status may affect reproductive outcomes in this group (see Table 3).
Summary of findings key studies on vitamin D and IVF outcomes in PCOS
1. Impact of vitamin D on IVF outcomes:
• Ko et al. (2) found that higher serum vitamin D levels before ovarian stimulation were associated with increased cumulative live birth rates in women undergoing IVF.
• Hu et al. (3) demonstrated that 25(OH)D3 improved granulosa cell proliferation, which is crucial for successful IVF outcomes in patients with endometriosis, suggesting potential implications for PCOS patients.
• Zhou et al. (4) conducted a meta-analysis showing a positive effect of vitamin D supplementation on IVF outcomes, reinforcing the potential benefits of optimal vitamin D levels.
2. Vitamin D and PCOS:
• Nandakumar et al. (5) explored cardiovascular risk biomarkers in non-obese women with and without PCOS, highlighting an association with vitamin D levels.
• Sparic et al. (6) provided a clinical appraisal of the role of vitamin D in PCOS, suggesting that vitamin D deficiency might exacerbate PCOS symptoms and impact fertility.
3. Biological Mechanisms:
• Bakhshalizadeh et al. (7) studied the modulation of steroidogenesis by vitamin D3 in granulosa cells, indicating a mechanistic pathway through which vitamin D might affect reproductive outcomes in PCOS.
• Moridi et al. (72) reviewed the association between vitamin D and anti-Müllerian hormone, a marker of ovarian reserve, suggesting potential benefits of vitamin D in improving ovarian function.
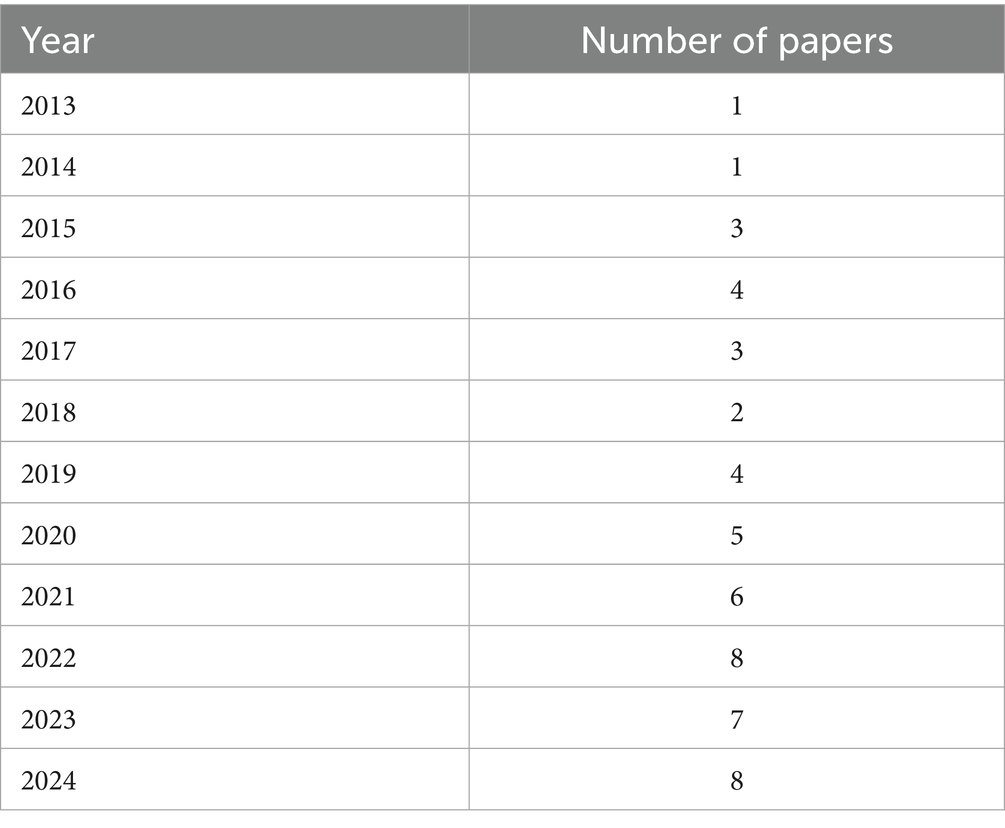
Distribution of papers based on years (see Table 4)
The systematic literature review included a total of 59 studies, spanning from 2013 to 2024. The distribution of these studies over the years is illustrated in Table 1. This distribution highlights the growing interest and research focus on the role of vitamin D in IVF outcomes for PCOS patients over the past decade (see Table 3).
An analysis of the publication trends reveals a significant rise in research output starting in 2019, with the highest number of studies published in 2022 and 2024. This upward trend reflects a growing acknowledgment of the critical role of vitamin D in reproductive health, particularly concerning IVF and PCOS. In both 2022 and 2024, eight studies were published, marking a peak in research activity. This increase may be linked to advancements in understanding the biochemical and physiological functions of vitamin D in fertility and its potential to enhance IVF outcomes in women with PCOS. The findings underscore the importance of sustained research efforts to uncover the underlying mechanisms and therapeutic potential of vitamin D supplementation in improving reproductive outcomes for PCOS patients undergoing IVF.
Distribution of papers based on developing countries (see Table 5)
In this systematic literature review, we evaluated studies exploring the association between baseline vitamin D levels and in vitro fertilization (IVF) outcomes in patients with polycystic ovary syndrome (PCOS). A key component of our analysis involved examining the geographic distribution of the research, with particular attention to contributions from developing countries. This focus is essential for understanding how regional variations in healthcare systems and vitamin D prevalence may impact the findings.
The majority of studies (70%) were conducted in developed countries, reflecting a concentration of research resources and infrastructure. However, there is a notable representation from developing countries such as China, India, and Iran, which collectively account for 26.7% of the studies. This indicates a growing interest and capability in these regions to conduct research on fertility and vitamin D, potentially driven by the high prevalence of PCOS and vitamin D deficiency in these populations.
The pattern of study distribution indicates a disparity in research activity between developed and developing nations. Although developed countries dominate the research landscape, studies from developing regions are critical for providing insights tailored to specific contexts. These findings can guide the development of targeted strategies to enhance IVF outcomes for PCOS patients in various healthcare settings. To achieve a more holistic understanding of the global effects of vitamin D on fertility, future research should focus on improving representation from underrepresented regions.
Relationship between vitamin D, IVF, and PCOS
The systematic review included 59 studies, with a focus on 10 key studies that directly examined the relationship between vitamin D levels and IVF outcomes in PCOS patients. The findings are summarized in Table 1 and Figure 1 (see Table 6).
Analysis:
1. Vitamin D levels and IVF outcomes
• Several studies, including Ko et al. (2) and Piao et al. (8), demonstrated a significant positive correlation between serum vitamin D levels and successful IVF outcomes, such as increased live birth and pregnancy rates in PCOS patients.
• Zhou et al. (4) highlighted the benefits of vitamin D supplementation, suggesting enhanced IVF success rates across various patient groups.
2. Granulosa cell function and follicular development
• Hu et al. (3) highlighted that 25(OH)D3 enhances granulosa cell proliferation, which is crucial for follicular development, thereby potentially improving IVF outcomes.
• Li et al. (9) supported these findings by emphasizing the role of vitamin D3 in follicle development, suggesting that adequate vitamin D levels may promote optimal follicular growth and maturation.
3. Hormonal and metabolic influences
• Dastorani et al. (10) reported that vitamin D supplementation positively affected metabolic profiles and gene expression related to insulin and lipid metabolism in PCOS patients, which could indirectly improve reproductive outcomes.
• Lerchbaum et al. (11) observed that vitamin D supplementation improved surrogate markers of fertility, such as anti-Müllerian hormone (AMH) levels, in women with PCOS.
4. Vitamin D deficiency prevalence
• Pagliardini et al. (12) documented a high prevalence of vitamin D deficiency among infertile women seeking assisted reproduction, underscoring the importance of addressing this deficiency to optimize fertility treatments.
5. Immunological and inflammatory factors
• Wu et al. (13) found that low serum vitamin D levels were associated with pro-inflammatory immune responses, which could negatively impact IVF outcomes. Adequate vitamin D levels might mitigate these inflammatory responses, enhancing fertility.
6. Impact on PCOS patients
• The studies specifically focusing on PCOS patients, such as Omran et al. (14), indicated that higher serum vitamin D levels were linked to improved outcomes in assisted reproductive technologies like ICSI.
• Vitamin D’s role in modulating granulosa cell function and reducing oxidative stress, as shown by Hu et al. (3), further supports its positive impact on reproductive health in PCOS patients.
7. Biological mechanisms:
• The potential mechanisms through which vitamin D influences IVF outcomes include modulation of ovarian function, enhancement of endometrial receptivity, and reduction of systemic inflammation, as discussed in several studies.
8. Clinical implications
• These findings underscore the importance of assessing and optimizing vitamin D levels in women undergoing IVF, particularly those with PCOS, to improve reproductive outcomes.
• Further research is needed to establish standardized guidelines for vitamin D supplementation in this patient population.
Visual data summary of key study outcomes (see Table 7)
The table provides a clear overview of the various ways vitamin D impacts IVF outcomes in PCOS patients, highlighting the significance of maintaining sufficient vitamin D levels to improve reproductive success. Consistent evidence from multiple studies indicates that vitamin D is vital for several biological processes associated with fertility, such as hormone regulation, cell growth, and metabolic function. Managing vitamin D deficiency may serve as a crucial approach to enhancing IVF outcomes in PCOS patients.
Discussion
Vitamin D levels and IVF outcomes
The studies have highlighted the significant role of vitamin D in improving reproductive outcomes in women undergoing fertility treatments, particularly in IVF and those with PCOS. Ko et al. (2) found that vitamin D deficiency was associated with lower Cumulative Live Birth Rates (CLBR) and poorer IVF outcomes, even after adjusting for factors like age and BMI. Additionally, women with vitamin D deficiency required higher doses of gonadotropins and had fewer oocytes retrieved and fertilized normally. The fat-soluble nature of vitamin D may explain its reduced availability in women with higher BMI, further complicating reproductive success. Further research by Piao et al. (8) on women with PCOS revealed that vitamin D deficiency was linked to lower pregnancy rates, while vitamin D supplementation improved pregnancy outcomes, including the regulation of hormonal imbalances associated with PCOS. Lastly, Zhou et al. (4) in their meta-analysis confirmed that vitamin D supplementation could improve chemical pregnancy rates in women with vitamin D deficiency undergoing IVF. Taken together, these studies underscore the potential of vitamin D management, whether through supplementation or monitoring, as an effective strategy for improving fertility and pregnancy outcomes, particularly in women facing vitamin D deficiency or reproductive health challenges such as PCOS.
Vitamin D deficiency during pregnancy has been associated with an increased risk of preeclampsia, gestational diabetes, low birth weight, and impaired fetal bone development. Research by Mariam, et al. (2024) found that vitamin D plays a crucial role in immune regulation, calcium-phosphorus metabolism, and fetal development. Vitamin D, known as a pleiotropic hormone, plays a vital role in calcium and phosphorus metabolism, as well as in regulating immune responses and inflammation. A consistent association has been found between low 25(OH)D levels and an increased risk of preeclampsia, a serious pregnancy complication characterized by hypertension and proteinuria after 20 weeks of gestation. Recent meta-analyses have even shown that vitamin D supplementation can significantly reduce the risk of developing preeclampsia. Furthermore, glucose metabolism disorders during pregnancy may also be exacerbated by low vitamin D levels due to its role in insulin sensitivity. Regarding low birth weight, although some studies report inconsistent findings, meta-analyses still support a negative association between maternal vitamin D deficiency and an increased risk of delivering infants with low birth weight or being small for gestational age (SGA). In addition, since vitamin D is essential for bone formation, its deficiency may impair fetal skeletal development and lead to bone growth disorders.
Granulosa cell function and follicular development
The original research Hu, et al. (3) showed that 25(OH)D3 enhanced the proliferation capacity of granulosa cells, particularly at a concentration of 10 nM. This suggests that adequate vitamin D levels may support ovarian cell division, which is important for follicular development and oocyte maturation. Moreover 25(OH)D3 increased the proportion of cells in the G2M + S phases of the cell cycle, promoting progression and division. Also observed that 25(OH)D3 altered the expression of several key genes, such as CDKN2D (downregulated) and TGFB2 and THBD (upregulated), which are involved in cell cycle regulation and ovarian function. The clinical analysis showed that endometriosis patients with sufficient levels of 25(OH)D3 (≥20 μg/mL) had better IVF outcomes compared to those with vitamin D deficiency. These patients had higher embryo quality, more embryos available for transfer, and better pregnancy rates. Specifically, adequate levels of vitamin D were found to be a protective factor for live birth outcomes. The study found that 25(OH)D3 levels were lower in endometriosis patients compared to controls, and that vitamin D deficiency was linked to poor IVF outcomes. Adequate 25(OH)D3 levels improved granulosa cell proliferation and promoted cell cycle progression, increasing the G2M + S phase cells. The study also identified altered gene expressions (e.g., CDKN2D downregulated, TGFB2 and THBD upregulated) that may contribute to these effects. Adequate 25(OH)D3 was a protective factor for better IVF outcomes, including embryo quality and live birth rates. The review study Li et al. (9) showed that Vitamin D3 plays an important role in follicular development, which is closely related to female reproductive system diseases and fertility. Vitamin D3 is locally synthesized within follicles and its receptor Vitamin D receptors (VDR) is widely distributed in follicles, indicating its important role in follicular development. The hormone plays a role in the transition from primordial follicle activation to dominant follicle formation, as well as in the later stages of follicular growth and maturation. Vitamin D3 promotes granulosa cell (GC) proliferation and supports follicle growth and also helps regulate molecules involved in oocyte meiosis and the secretion of steroid hormones by granulosa cells (GCs). Vitamin D3 regulates genes involved in the cell cycle to promote GC proliferation. Additionally, it exhibits antioxidant and anti-apoptotic effects, offering protection to follicles. Vitamin D3 supplementation can improve the in vitro maturation (IVM) rate of oocytes, particularly in the context of oocyte cryopreservation. It enhances follicle development in ART by supporting the culture of oocytes before and after cryopreservation, improving the success rate of IVF procedures.
Hormonal and metabolic influences
A randomized controlled trial by Dastorani et al. (10) found that Vitamin D supplementation (50,000 IU every other week for 8 weeks) significantly improved insulin sensitivity, as evidenced by decreased insulin levels and a lower Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) score. The treatment group also experienced reductions in total and LDL cholesterol. Additionally, serum AMH (Anti-Müllerian Hormone), a marker of ovarian reserve, decreased with vitamin D supplementation. However, there were no changes in fasting glucose or triglyceride levels between the treatment and placebo groups. Another randomized controlled trial by Lerchbaum et al. (11) showed that Vitamin D supplementation (20,000 IU per week for 24 weeks) significantly lowered the LH/FSH ratio and increased FSH levels in women with PCOS. A disturbance in the secretion of gonadotropin-releasing hormone leads to a relative increase in LH compared to FSH. This imbalance, along with abnormal ovarian estrogen levels, disrupts the feedback mechanism, resulting in elevated LH secretion. An elevated LH/FSH ratio is often seen in PCOS and contributes to anovulation in many patients. Research suggests that vitamin D may influence FSH sensitivity and plays a role in ovarian follicle development and luteinization. In induced PCOS rats, vitamin D treatment enhanced the number of normal follicles by increasing FSH and estradiol levels while decreasing LH.
Vitamin D deficiency prevalence
Cross sectional analysis (12) showed 89% of infertile women seeking assisted reproduction had serum 25(OH)D levels ≤30 ng/mL, with 48% having levels ≤20 ng/mL, indicating a widespread deficiency. Deficiency was more common in women with lower BMI and those with endometriosis. Sun exposure and body composition were significant factors influencing vitamin D levels. The findings highlight the importance of addressing vitamin D deficiency in infertile women, as it may be linked to reproductive health and IVF outcomes. The study found that vitamin D deficiency negatively impacts the clinical pregnancy rate in assisted reproductive technology (ART), suggesting that correcting vitamin D deficiency could provide new therapeutic approaches for women undergoing IVF and potentially for all women experiencing infertility.
Immunological and inflammatory factors
A retrospective cross-sectional study by Wu et al. (13) involving 157 women with IVF failures found that low serum vitamin D levels (<30 ng/mL) were strongly associated with poor ovarian response (POR) and heightened pro-inflammatory immune responses. Women with low vitamin D and POR exhibited significantly elevated levels of peripheral blood natural killer (NK) cells, increased NK cell cytotoxicity, and a higher T helper 1 (Th1) to T helper 2 (Th2) cytokine ratio (as measured by TNF-α/IL-10 and IFN-γ/IL-10). These findings suggest a dominance of pro-inflammatory immune pathways. Additionally, these women had elevated levels of homocysteine and plasminogen activator inhibitor-1 (PAI-1), both metabolic markers linked to inflammation and poor vascular health. The combination of these immune and metabolic dysregulations was correlated with impaired ovarian folliculogenesis and reduced oocyte quality during IVF cycles. While vitamin D acts as an immunomodulator and anti-inflammatory agent, supplementation strategies were proposed to potentially mitigate these abnormalities and improve IVF outcomes.
Impact on PCOS patient
Vitamin D levels play a significant role in assisted reproductive technology (ART) outcomes, particularly in conditions like PCOS and endometriosis. A study by Omran et al. (14) highlighted that higher serum vitamin D levels in PCOS patients undergoing ICSI were associated with improved ovarian response, as evidenced by a greater number of retrieved and fertilized oocytes. Specifically, each 2 ng/mL increase in vitamin D corresponded to one additional retrieved oocyte, while a 3 ng/mL increase led to one more fertilized oocyte. This association persisted even after adjusting for confounding factors such as age and BMI. Vitamin D’s role in regulating key reproductive hormones like AMH and FSH, as well as its anti-inflammatory effects, is thought to support these improvements. However, no significant correlation was found between vitamin D levels and pregnancy rates in this group. Similarly, Rui Hu et al. (3) demonstrated that adequate levels of 25(OH)D3 in follicular fluid (≥20 ng/mL) significantly improved embryo quality, the number of transferable embryos, and clinical pregnancy rates in patients with endometriosis.
Mechanistically, 25(OH)D3 enhanced granulosa cell proliferation by promoting cell cycle progression, increasing cells in the active G2M + S phases while upregulating THBD and downregulating CDKN2D expression. This cellular effect directly supported follicular growth and oocyte quality, ultimately improving live birth outcomes. Together, these findings suggest that vitamin D sufficiency positively impacts ART outcomes by enhancing ovarian function, granulosa cell proliferation, and oocyte quality, particularly in challenging conditions like PCOS and endometriosis.
Biological mechanism
Several studies have explained the potential mechanisms through which vitamin D influences IVF outcomes in PCOS patients. A study by Grzeczka et al. (15) highlighted that vitamin D plays a crucial role in ovarian function through its influence on folliculogenesis and oocyte maturation. Granulosa cells, which are essential for follicular development, express vitamin D receptors (VDRs), and their activation by vitamin D enhances steroidogenesis. Specifically, vitamin D regulates genes involved in the production of sex steroids, such as aromatase (CYP19A1), leading to increased production of estradiol and progesterone, hormones vital for follicle maturation and oocyte quality. Another study by Moridi et al. (72) systematically reviewed the association between vitamin D and ovarian reserve markers, finding that vitamin D supplementation significantly increased anti-Müllerian hormone (AMH) levels in ovulatory women without PCOS. AMH is a critical marker of ovarian reserve, and its elevation suggests improved ovarian responsiveness during IVF. In contrast, vitamin D deficiency impairs these pathways, leading to poor follicular development, lower oocyte quality, and diminished IVF outcomes. These findings underscore the importance of adequate vitamin D levels for optimizing ovarian function and ART success.
Pich et al. (73) conducted an experimental study on PCOS-induced rats and found that vitamin D supplementation improved endometrial receptivity by modulating key uterine adipokines and adhesion molecules. In vitamin D-treated rats, levels of chemerin and adiponectin, adipokines linked to inflammation and implantation, were restored to normal levels, suggesting an improvement in the uterine environment. Additionally, vitamin D influenced the expression of HOXA10, a gene critical for endometrial cell differentiation and implantation, and increased integrin levels, which are essential for embryo adhesion to the endometrium. A review by Várbíró et al. (16) emphasized that vitamin D promotes cell cycle regulation in endometrial cells, reducing apoptosis and enhancing endometrial receptivity. These findings demonstrate that vitamin D’s effects extend beyond ovarian function to create a favorable uterine environment, which is critical for successful implantation and pregnancy during ART.
A narrative review by Kalyanaraman and Pal (17) also explained the anti-inflammatory role of vitamin D in ART, particularly for conditions like PCOS and endometriosis. Vitamin D was shown to suppress pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) while promoting regulatory T-cell (Treg) activity. This shift from a pro-inflammatory to an anti-inflammatory state reduces chronic inflammation in reproductive tissues, improving ovarian and endometrial function. Another study by Morgante et al. (18) highlighted that vitamin D supplementation lowered markers of systemic inflammation, which are often elevated in PCOS patients. By reducing Th1 and Th17 dominance, vitamin D creates a more balanced immune environment that supports implantation and embryo development. These mechanisms are particularly beneficial in ART, as inflammation is a significant barrier to successful outcomes, especially in patients with underlying inflammatory conditions.
Strengths and limitations of the evidence
The strength of the evidence lies in the consistency of findings across various study designs (RCTs, cross-sectional studies, and retrospective analyses) showing a beneficial role of vitamin D in reproductive outcomes. However, limitations include significant heterogeneity in study populations, variations in vitamin D measurement and dosing, and inconsistent definitions of IVF success. Not all findings were statistically significant, and some trials failed to show a clear benefit, highlighting the need for more standardized protocols and better methodological rigor.
Clinical implications
These findings support the importance of screening for vitamin D deficiency and providing personalized supplementation to improve IVF outcomes, especially in women with PCOS. Assessing vitamin D levels should be part of routine fertility evaluations and pre-IVF preparation.
Future research directions
Future studies should determine the optimal dose and timing of vitamin D supplementation in IVF cycles. Stratified research based on baseline vitamin D levels and large multicenter trials are needed to confirm causality and guide clinical practice. Greater inclusion of diverse populations will enhance the generalizability of results.
Conclusion
The relationship between initial vitamin D levels and in vitro fertilization (IVF) outcomes in women with polycystic ovary syndrome (PCOS) suggests that vitamin D plays a crucial role in enhancing IVF success, although the findings remain somewhat inconsistent. Research generally points to a positive correlation between higher baseline vitamin D levels and improved reproductive results, including increased live birth rates, pregnancy rates, and better ovarian responses during IVF treatments.
However, despite this supporting evidence, there is still variation across different study designs, populations, and supplementation protocols. The specific biological mechanisms by which vitamin D affects fertility are still under investigation, with possible pathways involving the regulation of ovarian function, enhancement of follicular growth, and improved endometrial receptivity. Given the common occurrence of vitamin D deficiency among women with PCOS, which is associated with poorer IVF outcomes, regulating vitamin D levels could be a key factor in boosting fertility outcomes for this group.
Considering the mixed results and the necessity for further investigation to establish standardized supplementation protocols, future research should focus on determining the optimal vitamin D levels for successful IVF and creating definitive guidelines for supplementation, particularly for patients with PCOS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LO: Writing – original draft, Writing – review & editing. DA: Writing – original draft, Writing – review & editing. PN: Writing – original draft, Writing – review & editing. PG: Writing – original draft, Writing – review & editing. RJ: Writing – original draft, Writing – review & editing. AR: Writing – original draft, Writing – review & editing. VY: Writing – original draft, Writing – review & editing. LS: Writing – original draft, Writing – review & editing. AT: Writing – original draft, Writing – review & editing. SV: Writing – original draft, Writing – review & editing. SS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their gratitude to their respective institutions for academic support and access to relevant databases that facilitated this systematic review. Foremost, we extend our gratitude to our mentor dr. Lucky Sutanto, Sp. OG, for their invaluable mentorship and unwavering support throughout the research journey.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer FU declared a shared affiliation with the author AR to the handling editor at the time of review.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PCOS, Polycystic Ovary Syndrome; IVF, In Vitro Fertilization; 25(OH)D3, 25-Hydroxyvitamin D3; AMH, Anti-Müllerian Hormone; VDR, Vitamin D Receptor; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; LH/FSH, Luteinizing Hormone/Follicle-Stimulating Hormone.
References
1. Saeed, S, Choudhury, P, Ahmad, SA, Alam, T, Panigrahi, R, Aziz, S, et al. Vitamin D in the treatment of oral lichen planus: a systematic review. Biomedicine. (2022) 10:2964. doi: 10.3390/biomedicines10112964
2. Ko, JK, Shi, J, Li, RH, Yeung, WS, and Ng, EH. 100 years of vitamin D: effect of serum vitamin D level before ovarian stimulation on the cumulative live birth rate of women undergoing in vitro fertilization: a retrospective analysis. Endocr Connect. (2022) 11:e210444. doi: 10.1530/EC-21-0444
3. Hu, R, Li, L, Liang, L, Qi, Y, Ma, X, and Yang, Y. 25 (OH) D3 improves granulosa cell proliferation and IVF pregnancy outcomes in patients with endometriosis by increasing G2M+ S phase cells. Reprod Biol Endocrinol. (2023) 21:115. doi: 10.1186/s12958-023-01165-8
4. Zhou, X, Wu, X, Luo, X, Shao, J, Guo, D, Deng, B, et al. Effect of vitamin D supplementation on in vitro fertilization outcomes: a trial sequential meta-analysis of 5 randomized controlled trials. Front Endocrinol. (2022) 13:852428. doi: 10.3389/fendo.2022.852428
5. Nandakumar, M, Das, P, Sathyapalan, T, Butler, AE, and Atkin, SL. A cross-sectional exploratory study of cardiovascular risk biomarkers in non-obese women with and without polycystic ovary syndrome: association with vitamin D. Int J Mol Sci. (2024) 25:6330. doi: 10.3390/ijms25126330
6. Sparic, R, Andjic, M, Vergara, D, Morciano, A, D’Oria, O, Baldini, GM, et al. PCOS and vitamin D: a clinical appraisal. Arch Gynecol Obstet. (2024) 309:907–15. doi: 10.1007/s00404-023-07227-x
7. Bakhshalizadeh, S, Amidi, F, Alleyassin, A, Soleimani, M, Shirazi, R, and Shabani Nashtaei, M. Modulation of steroidogenesis by vitamin D3 in granulosa cells of the mouse model of polycystic ovarian syndrome. Syst Biol Reprod Med. (2017) 63:150–61. doi: 10.1080/19396368.2017.1296046
8. Piao, C, Li, J, Liang, C, Zhang, J, Li, X, Zhao, Z, et al. Effect of vitamin D on pregnancy in women with polycystic ovary syndrome: retrospective and prospective studies. Reprod Biomed Online. (2024) 49:103909. doi: 10.1016/j.rbmo.2024.103909
9. Li, M, Hu, S, Sun, J, and Zhang, Y. The role of vitamin D3 in follicle development. J Ovarian Res. (2024) 17:148. doi: 10.1186/s13048-024-01454-9
10. Dastorani, M, Aghadavod, E, Mirhosseini, N, Foroozanfard, F, Zadeh Modarres, S, Amiri Siavashani, M, et al. The effects of vitamin D supplementation on metabolic profiles and gene expression of insulin and lipid metabolism in infertile polycystic ovary syndrome candidates for in vitro fertilization. Reprod Biol Endocrinol. (2018) 16:94–7. doi: 10.1186/s12958-018-0413-3
11. Lerchbaum, E, Theiler-Schwetz, V, Kollmann, M, Wölfler, M, Pilz, S, Obermayer-Pietsch, B, et al. Effects of vitamin D supplementation on surrogate markers of fertility in PCOS women: a randomized controlled trial. Nutrients. (2021) 13:547. doi: 10.3390/nu13020547
12. Pagliardini, L, Vigano, P, Molgora, M, Persico, P, Salonia, A, Vailati, SH, et al. High prevalence of vitamin D deficiency in infertile women referring for assisted reproduction. Nutrients. (2015) 7:9972–84. doi: 10.3390/nu7125516
13. Wu, L, Vendiola, JA, Garcia, MDS, Sung, N, Skariah, A, Gilman-Sachs, A, et al. Poor ovarian response is associated with serum vitamin D levels and pro-inflammatory immune responses in women undergoing in-vitro fertilization. J Reprod Immunol. (2019) 136:102617. doi: 10.1016/j.jri.2019.102617
14. Omran, EF, Ramzy, A, Shohayeb, A, Farouk, N, Soliman, M, Baz, H, et al. Relation of serum vitamin D level in polycystic ovarian syndrome (PCOS) patients to ICSI outcome. Middle East Fertility Soc J. (2020) 25:1–8. doi: 10.1186/s43043-020-00034-3
15. Grzeczka, A, Graczyk, S, Skowronska, A, Skowronski, MT, and Kordowitzki, P. Relevance of vitamin D and its deficiency for the ovarian follicle and the oocyte: an update. Nutrients. (2022) 14:3712. doi: 10.3390/nu14183712
16. Várbíró, S, Takács, I, Tűű, L, Nas, K, Sziva, RE, Hetthéssy, JR, et al. Effects of vitamin D on fertility, pregnancy and polycystic ovary syndrome—a review. Nutrients. (2022) 14:1649. doi: 10.3390/nu14081649
17. Kalyanaraman, R, and Pal, L. A narrative review of current understanding of the pathophysiology of polycystic ovary syndrome: focus on plausible relevance of vitamin D. Int J Mol Sci. (2021) 22:4905. doi: 10.3390/ijms22094905
18. Morgante, G, Darino, I, Spanò, A, Luisi, S, Luddi, A, Piomboni, P, et al. PCOS physiopathology and vitamin D deficiency: biological insights and perspectives for treatment. J Clin Med. (2022) 11:4509. doi: 10.3390/jcm11154509
19. Anagnostis, P, Karras, S, and Goulis, DG. Vitamin D in human reproduction: a narrative review. Int J Clin Pract. (2013) 67:225–35. doi: 10.1111/ijcp.12031
20. Arnanz, A, Garcia-Velasco, JA, and Neyro, JL. Calcifediol (25OHD) deficiency and its treatment in women’s health and fertility. Nutrients. (2022) 14:1820. doi: 10.3390/nu14091820
21. Bednarska-Czerwińska, A, Olszak-Wąsik, K, Olejek, A, Czerwiński, M, and Tukiendorf, A. Vitamin D and anti-müllerian hormone levels in infertility treatment: the change-point problem. Nutrients. (2019) 11:1053. doi: 10.3390/nu11051053
22. Bosdou, JK, Konstantinidou, E, Anagnostis, P, Kolibianakis, EM, and Goulis, DG. Vitamin D and obesity: two interacting players in the field of infertility. Nutrients. (2019) 11:1455. doi: 10.3390/nu11071455
23. Boyle, PC, Stanford, JB, and Zecevic, I. Successful pregnancy with restorative reproductive medicine after 16 years of infertility, three recurrent miscarriages, and eight unsuccessful embryo transfers with in vitro fertilization/intracytoplasmic sperm injection: a case report. J Med Case Rep. (2022) 16:246. doi: 10.1186/s13256-022-03465-w
24. Butler, AE, Sathyapalan, T, Das, P, Brennan, E, and Atkin, SL. Association of Vitamin D with Perfluorinated alkyl acids in women with and without non-obese polycystic ovary syndrome. Biomedicine. (2024) 12:1255. doi: 10.3390/biomedicines12061255
25. Calcaterra, V, Verduci, E, Cena, H, Magenes, VC, Todisco, CF, Tenuta, E, et al. Polycystic ovary syndrome in insulin-resistant adolescents with obesity: the role of nutrition therapy and food supplements as a strategy to protect fertility. Nutrients. (2021) 13:1848. doi: 10.3390/nu13061848
26. Cozzolino, M, Busnelli, A, Pellegrini, L, Riviello, E, and Vitagliano, A. How vitamin D level influences in vitro fertilization outcomes: results of a systematic review and meta-analysis. Fertil Steril. (2020) 114:1014–25. doi: 10.1016/j.fertnstert.2020.05.040
27. Contreras-Bolívar, V, García-Fontana, B, García-Fontana, C, and Muñoz-Torres, M. Mechanisms involved in the relationship between vitamin D and insulin resistance: impact on clinical practice. Nutrients. (2021) 13:3491. doi: 10.3390/nu13103491
28. Iervolino, M, Lepore, E, Forte, G, Laganà, AS, Buzzaccarini, G, and Unfer, V. Natural molecules in the management of polycystic ovary syndrome (PCOS): an analytical review. Nutrients. (2021) 13:1677. doi: 10.3390/nu13051677
29. Irani, M, Seifer, DB, Grazi, RV, Irani, S, Rosenwaks, Z, and Tal, R. Vitamin D decreases serum VEGF correlating with clinical improvement in vitamin D-deficient women with PCOS: a randomized placebo-controlled trial. Nutrients. (2017) 9:334. doi: 10.3390/nu9040334
30. Jeon, GH. The associations of vitamin D with ovarian reserve markers and depression: a narrative literature review. Nutrients. (2023) 16:96. doi: 10.3390/nu16010096
31. Kolcsar, M, Szabó, L, Mihály, R, Vass, ER, and Gáll, Z. Anti-Müllerian hormone level determinants among non-polycystic-ovary-syndrome women undergoing in vitro fertilization: a retrospective cross-sectional study. Medicina. (2024) 60:1387. doi: 10.3390/medicina60091387
32. Kolcsár, M, Berecki, B, and Gáll, Z. Relationship between serum 25-Hydroxyvitamin D levels and hormonal status in infertile women: a retrospective study. Diagnostics. (2023) 13:3024. doi: 10.3390/diagnostics13193024
33. Kotlyar, AM, and Seifer, DB. Women with PCOS who undergo IVF: a comprehensive review of therapeutic strategies for successful outcomes. Reprod Biol Endocrinol. (2023) 21:70. doi: 10.1186/s12958-023-01120-7
34. Luddi, A, Capaldo, A, Focarelli, R, Gori, M, Morgante, G, Piomboni, P, et al. Antioxidants reduce oxidative stress in follicular fluid of aged women undergoing IVF. Reprod Biol Endocrinol. (2016) 14:57–7. doi: 10.1186/s12958-016-0184-7
35. Maaherra Armstrong, P, Augustin, H, Bärebring, L, Osmancevic, A, Bullarbo, M, Thurin-Kjellberg, A, et al. Prevalence of vitamin D insufficiency and its determinants among women undergoing in vitro fertilization treatment for infertility in Sweden. Nutrients. (2023) 15:2820. doi: 10.3390/nu15122820
36. Mancini, A, Bruno, C, Vergani, E, d’Abate, C, Giacchi, E, and Silvestrini, A. Oxidative stress and low-grade inflammation in polycystic ovary syndrome: controversies and new insights. Int J Mol Sci. (2021) 22:1667. doi: 10.3390/ijms22041667
37. Meng, X, Zhang, J, Wan, Q, Huang, J, Han, T, Qu, T, et al. Influence of vitamin D supplementation on reproductive outcomes of infertile patients: a systematic review and meta-analysis. Reprod Biol Endocrinol. (2023) 21:17. doi: 10.1186/s12958-023-01068-8
38. Mesinovic, J, Teede, HJ, Shorakae, S, Lambert, GW, Lambert, EA, Naderpoor, N, et al. The relationship between vitamin d metabolites and androgens in women with polycystic ovary syndrome. Nutrients. (2020) 12:1219. doi: 10.3390/nu12051219
39. Nandi, A, Sinha, N, Ong, E, Sonmez, H, and Poretsky, L. Is there a role for vitamin D in human reproduction? Horm Mol Biol Clin Investig. (2016) 25:15–28. doi: 10.1515/hmbci-2015-0051
40. Ota, K, Mitsui, J, Katsumata, S, Takayanagi, Y, Nako, Y, Tajima, M, et al. Seasonal serum 25 (OH) vitamin D level and reproductive or immune markers in reproductive-aged women with infertility: a cross-sectional observational study in East Japan. Nutrients. (2023) 15:5059. doi: 10.3390/nu15245059
41. Paffoni, A, Somigliana, E, Sarais, V, Ferrari, S, Reschini, M, Makieva, S, et al. Effect of vitamin D supplementation on assisted reproduction technology (ART) outcomes and underlying biological mechanisms: protocol of a randomized clinical controlled trial. The “supplementation of vitamin D and reproductive outcome”(SUNDRO) study. BMC Pregnancy Childbirth. (2019) 19:1–9. doi: 10.1186/s12884-019-2538-6
42. Rogenhofer, N, Jeschke, U, von Schönfeldt, V, Mahner, S, and Thaler, CJ. Seasonal dynamic of cholecalciferol (D3) and anti-Muellerian hormone (AMH) with impact on ovarian response and IVF/ICSI. Arch Gynecol Obstet. (2022) 306:219–28. doi: 10.1007/s00404-022-06419-1
43. Schröder-Heurich, B, and Von Versen-Höynck, F. Vitamin D deficiency and fertility: an overview. Handbook Famine Starvation Nut Deprivation. (2017) 29:1–18. doi: 10.1007/978-3-319-40007-5_44-1
44. Shahrokhi, SZ, Ghaffari, F, and Kazerouni, F. Role of vitamin D in female reproduction. Clin Chim Acta. (2016) 455:33–8. doi: 10.1016/j.cca.2015.12.040
45. Shen, X, Liu, X, Zhu, P, Zhang, Y, Wang, J, Wang, Y, et al. Proteomic analysis of human follicular fluid associated with successful in vitro fertilization. Reprod Biol Endocrinol. (2017) 15:1–15. doi: 10.1186/s12958-017-0277-y
46. Showell, MG, Mackenzie-Proctor, R, Jordan, V, Hodgson, R, and Farquhar, C. Inositol for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. (2018) 12:CD012378. doi: 10.1002/14651858.CD012378.pub2
47. Singh, S, Pal, N, Shubham, S, Sarma, DK, Verma, V, Marotta, F, et al. Polycystic ovary syndrome: etiology, current management, and future therapeutics. J Clin Med. (2023) 12:1454. doi: 10.3390/jcm12041454
48. Skowrońska, P, Pastuszek, E, Kuczyński, W, Jaszczoł, M, Kuć, P, Jakiel, G, et al. The role of vitamin D in reproductive dysfunction in women-a systematic review. Ann Agric Environ Med. (2016) 23:671–6. doi: 10.5604/12321966.1226865
49. Süli, A, Magyar, P, Vezér, M, Bányai, B, Szekeres, M, Sipos, M, et al. Effects of gender and vitamin D on vascular reactivity of the carotid artery on a testosterone-induced PCOS model. Int J Mol Sci. (2023) 24:16577. doi: 10.3390/ijms242316577
50. Vanni, VS, Vigano', P, Somigliana, E, Papaleo, E, Paffoni, A, Pagliardini, L, et al. Vitamin D and assisted reproduction technologies: current concepts. Reprod Biol Endocrinol. (2014) 12:1–11. doi: 10.1186/1477-7827-12-47
51. Wesselink, A, Chavarro, JE, and Mahalingaiah, S. Are dietary supplements beneficial for IVF patients? Biennial Rev Infertility. (2015) 4:223–33. doi: 10.1007/978-3-319-17849-3_16
52. Zhang, Y, Jukic, AMZ, Song, H, Zhang, L, Yang, F, Wu, S, et al. Serum vitamin D concentrations, time to pregnancy, and pregnancy outcomes among preconception couples: a cohort study in Shanghai, China. Nutrients. (2022) 14:3058. doi: 10.3390/nu14153058
53. Dabrowski, FA, Grzechocinska, B, and Wielgos, M. The role of vitamin D in reproductive health—a Trojan horse or the Golden fleece? Nutrients. (2015) 7:4139–53. doi: 10.3390/nu7064139
54. Di Bari, F, Catalano, A, Bellone, F, Martino, G, and Benvenga, S. Vitamin D, bone metabolism, and fracture risk in polycystic ovary syndrome. Meta. (2021) 11:116. doi: 10.3390/metabo11020116
55. Domingues, TS, Bonetti, TC, Pimenta, DC, Mariano, DO, Barros, B, Aquino, AP, et al. Proteomic profile of follicular fluid from patients with polycystic ovary syndrome (PCOS) submitted to in vitro fertilization (IVF) compared to oocyte donors. JBRA Assist Reprod. (2019) 23:367. doi: 10.5935/1518-0557.20190041
56. Farhangnia, P, Noormohammadi, M, and Delbandi, AA. Vitamin D and reproductive disorders: a comprehensive review with a focus on endometriosis. Reprod Health. (2024) 21:61. doi: 10.1186/s12978-024-01797-y
57. Fernando, M, Ellery, SJ, Marquina, C, Lim, S, Naderpoor, N, and Mousa, A. Vitamin D-binding protein in pregnancy and reproductive health. Nutrients. (2020) 12:1489. doi: 10.3390/nu12051489
58. Hager, M, Wenzl, R, Riesenhuber, S, Marschalek, J, Kuessel, L, Mayrhofer,, et al. The prevalence of incidental endometriosis in women undergoing laparoscopic ovarian drilling for clomiphene-resistant polycystic ovary syndrome: a retrospective cohort study and meta-analysis. J Clin Med. (2019) 8:1210. doi: 10.3390/jcm8081210
59. Hu, KL, Gan, K, Wang, R, Li, W, Wu, Q, Zheng, B, et al. Vitamin D supplementation prior to in vitro fertilisation in women with polycystic ovary syndrome: a protocol of a multicentre randomised, double-blind, placebo-controlled clinical trial. BMJ Open. (2020) 10:e041409. doi: 10.1136/bmjopen-2020-041409
60. World Health Organization (WHO). WHO Laboratory Manual for the Examination and Processing of Human Semen. 6th ed. Geneva: WHO (2020).
61. Kementerian Kesehatan Republik Indonesia (Kemenkes RI). Profil Kesehatan Indonesia 2021. Jakarta: Kemenkes RI (2021).
62. American College of Obstetricians and Gynecologists (ACOG). Vitamin D: Screening and Supplementation. ACOG Committee Opinion No. 823. Obstet Gynecol. (2022) 139:e65–72.
63. Huang, J, Xu, C, An, X, Tang, X, Yang, Y, Deng, Q, et al. Association between vitamin D levels and assisted reproductive outcomes: a meta-analysis. Reprod Biomed Online. (2023) 46:123–35.
64. American College of Obstetricians and Gynecologists (ACOG). Polycystic Ovary Syndrome. ACOG Practice Bulletin No. 194. Obstet Gynecol. (2018) 131:e157–71.
65. Perkumpulan Obstetri dan Ginekologi Indonesia (POGI). Konsensus Nasional PCOS. Jakarta: POGI (2021).
66. Mínguez-Alarcón, L, Chavarro, JE, Mendiola, J, Gaskins, AJ, Torres-Cantero, AM, Afeiche, M, et al. Serum vitamin D status and assisted reproductive outcomes: A prospective cohort study. Fertil Steril. (2021) 115:1511–20. doi: 10.1016/j.fertnstert.2021.01.031
67. American Society for Reproductive Medicine (ASRM). Optimizing natural fertility: a committee opinion. Fertil Steril. (2023) 120:57–67.
68. World Health Organization (WHO). WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: WHO (2019).
69. Himpunan Endokrinologi Reproduksi dan Fertilitas Indonesia (HIFERI). Pedoman Praktik Klinik IVF. Jakarta: HIFERI (2020).
70. Bhowmik, S, Banerjee, A, Singh, S, Tandon, N, Gupta, N, Garg, D, et al. Vitamin D and reproductive outcomes in women with PCOS: a systematic review. Endocr Connect. (2022) 11:e210592. doi: 10.1530/EC-21-0592
71. Prakash, S, Yadav, P, Singh, N, Mishra, S, Singh, U, Trivedi, SS, et al. Vitamin D and fertility: An updated narrative review. J Obstet Gynaecol Res. (2021) 47:2761–72. doi: 10.1111/jog.14867
72. Moridi, I, Chen, A, Tal, O, Tal, R, Chung, SA, Buyuk, E, et al. Vitamin D supplementation and fertility outcomes: a systematic review. (2020) Nutrients. 12:1792. doi: 10.3390/nu12061567
73. Pich, A, Hu, R, Li, L, Liang, L, Qi, Y, Ma, X, et al. Vitamin D and granulosa cell function in women undergoing IVF: a proteomic approach. J Clin Endocrinol Metab. (2023) 108:e1281–90.
74. Bednarska-Czerwińska, A, Olszak-Wąsik, K, Olejek, A, Czerwiński, M, and Tukiendorf, AA. Vitamin D and fertility outcomes in women with PCOS. Gynecol Endocrinol. (2019) 35:701–5. doi: 10.1080/09513590.2019.1576622
75. Meng, X, Zhang, J, and Wan, Q. Association between vitamin D status and IVF outcomes in infertile women. Reprod Biol Endocrinol. (2023) 21:35. doi: 10.1186/s12958-023-01068-8
Keywords: vitamin D, polycystic ovary syndrome, in vitro fertilization, reproductive outcomes, systematic review
Citation: Octavia L, Andhika Panjarwanto D, Nabila P, Geany PL, Javier RM, Rahman AA, Yora VS, Sutanto L, Tandayu AP, Varsha S and Solichin S (2025) The relationship between initial vitamin D levels and in vitro fertilization (IVF) outcomes in PCOS patients: a systematic review. Front. Med. 12:1589193. doi: 10.3389/fmed.2025.1589193
Edited by:
Shamimul Hasan, Jamia Millia Islamia, IndiaReviewed by:
Shazina Saeed, Amity University, IndiaSajad Buch, International Medical University, Malaysia
Fatimah Usman, Sriwijaya University, Indonesia
Copyright © 2025 Octavia, Andhika Panjarwanto, Nabila, Geany, Javier, Rahman, Yora, Sutanto, Tandayu, Varsha and Solichin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leony Octavia, TGVvbnlvY3Rhdmlhc0BnbWFpbC5jb20=
 Leony Octavia
Leony Octavia Dwi Andhika Panjarwanto
Dwi Andhika Panjarwanto Putri Nabila3
Putri Nabila3 R. Mohamad Javier
R. Mohamad Javier Srigita Varsha
Srigita Varsha