- 1College of Clinical Medicine for Obstetrics and Gynecology and Pediatrics, Fujian Medical University, Fujian Maternity and Child Health Hospital, Fuzhou, Fujian, China
- 2Fujian Clinical Research Center for Maternal-Fetal Medicine, Fuzhou, Fujian, China
- 3National Key Obstetric Clinical Specialty Construction Institution of China, Fuzhou, Fujian, China
- 4Department of Obstetrics and Gynecology, Quanzhou Women & Children's Hospital, Quanzhou, Fujian, China
- 5Department of Epidemiology and Health Statistics, School of Public Health, Fujian Medical University, Fuzhou, Fujian, China
- 6Department of Healthcare, Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, Fujian, China
- 7Medical Genetic Diagnosis and Therapy Center, Fujian Key Laboratory for Prenatal Diagnosis and Birth Defect, Fujian Maternity and Child Hospital College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, Fujian, China
Introduction: Fetal macrosomia is related to adverse neonatal and maternal health outcomes. Therefore, we aimed to evaluate the risk factors for macrosomia and establish multivariable prediction models to enable early identification, prevention, and mitigation of its adverse outcomes.
Methods: This retrospective case-control study included 800 singleton pregnant women who delivered in 2022 at Fujian Maternity and Child Health Hospital and Quanzhou Women and Children's Hospital. They were categorized into the macrosomia [birth weight (BW) ≥ 4,000 g, n = 400] and non-macrosomia (BW = 2,500–3,999 g, n = 400) groups according to the BW of the newborns. Prediction models in singleton fetuses during mid-to-late pregnancy and before delivery were constructed.
Results: Maternal height ≥ 165 cm [odds ratio (OR) = 2.303, 95% confidence interval (CI): 1.232–4.305], pre-pregnancy overweight (OR = 2.166, 95% CI: 1.119–4.195), pre-pregnancy obesity (OR = 3.189, 95% CI: 1.020–9.968), excessive gestational weight gain in the second trimester (OR = 2.083, 95% CI: 1.250–3.470), and at least two abnormal blood glucose values in the oral glucose tolerance test (OR = 5.267, 95% CI: 1.814–15.29) were identified as risk factors for macrosomia. Additionally, maternal abdominal circumference (AC) plus fundal length ≥ 140 cm (OR = 6.283, 95% CI: 3.976–9.927), fetal biparietal diameter ≥ 10 cm (OR = 3.373, 95% CI: 1.103–10.31), fetal head circumference ≥ 35 cm (OR = 3.473, 95% CI: 1.334–9.041), and fetal AC ≥ 36 cm at pre-delivery (OR = 23.46, 95% CI: 14.81–37.16) were risk factors for macrosomia.
Discussion: The construction of the macrosomia prediction model in singleton fetuses during mid-to-late pregnancy and before delivery showed a strong predictive value. This study identified key high-risk factors for macrosomia during the perinatal period. The macrosomia prediction model developed here is expected to enable early identification of macrosomia, allowing for timely interventions aimed at reducing the risk of adverse perinatal outcomes.
1 Introduction
Fetal macrosomia is a major concern for neonatal and maternal health that potentially leads to severe perinatal complications (1). The American College of Obstetrics and Gynecology defines fetal macrosomia as an estimated fetal weight or birth weight (BW) exceeding 4,000 g, regardless of gestational age (2). In developed countries, the incidence rate of macrosomia has recently risen from 5%−20% to 15%−25% (3). Moreover, the rapid socioeconomic development in China has significantly altered the dietary habits and lifestyles of residents, contributing to an increasing prevalence of macrosomia (4). The overall prevalence of macrosomia in China increased from 6.6% in 1996 to 8.7% in 2014 (5–7).
Numerous studies have shown a significant relationship between macrosomia and an increased risk of maternal and neonatal complications in the short and long term. Maternal complications primarily include emergency cesarean section, postpartum hemorrhage (PPH), and anal sphincter injury (8, 9). In contrast, neonatal complications involve fractures, perinatal asphyxia, cerebral hemorrhage, and brachial plexus injuries (1, 10). Macrosomia also increases the risk of metabolic diseases, including obesity, diabetes, and hypertension in adulthood (11–13). Beyond physical impacts, it may elevate psychiatric risk during adolescence, particularly among males (14). Therefore, early identification and prediction of macrosomia, followed by the implementation of preventive measures, are crucial for reducing its incidence and mitigating its adverse outcomes, which have significant scientific and practical implications.
Studies have demonstrated that macrosomia is associated with various factors, including environmental influences (15), maternal blood lipid levels (16), gestational weight gain (GWG) (17), gestational diabetes mellitus (GDM) (4), delivery history of macrosomic infants (6), pre-pregnancy body mass index (BMI) (18), parity (19), and other reproductive factors (20). Currently, fetal weight is estimated during prenatal care through maternal physical examinations or ultrasonographic measurements. However, the accuracy of macrosomia prediction using a single clinical indicator remains low. Therefore, developing a practical and accurate predictive model for macrosomia is essential. Multivariable prediction models, which are increasingly used in healthcare, estimate an individual's risk of developing a condition by incorporating multiple characteristics (21, 22). These models have been employed in obstetrics to predict preterm birth (23), small-for-gestational-age infants (24), and preeclampsia (25).
Therefore, this study aimed to evaluate the risk factors for macrosomia and establish multivariable prediction models to enable early identification, prevention, and mitigation of its adverse outcomes.
2 Materials and methods
2.1 Study design and population
This retrospective case-control study included 800 singleton pregnant women who delivered in 2022 at Fujian Maternity and Child Health Hospital and Quanzhou Women and Children's Hospital. Participants were categorized into two groups based on the BW of newborns exceeding 4,000 g—the macrosomia (case group) and non-macrosomia (control group) groups. This study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics Review Committee of Fujian Provincial Maternity and Child Hospital, Fuzhou, China (approval number: 2023KY046). Written informed consent was obtained from the guardians of all participants after a detailed description of the study's purpose was provided.
2.2 Data collection
At pregnancy registration, data on maternal demographic characteristics (e.g., age, education, and occupation), anthropometric measurements (e.g., body weight, height, and blood pressure), and clinical history (e.g., parity and disease history) were recorded. Anthropometric measurements were also collected during mid and late pregnancy. Fasting blood samples and laboratory tests (e.g., routine blood checkup and blood biochemistry examination, including lipids and glucose levels) were performed at 24 weeks of gestation. Subsequently, results from the 75 g oral glucose tolerance test (OGTT) for diagnosing GDM were recorded. Data on maternal blood lipid levels, including total triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), were collected during late pregnancy. The most cost-effective method for detecting fetal macrosomia is selective ultrasound scanning for all suspected cases during late pregnancy (26). Ultrasound results, including biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), and femur length (FL), were recorded. Clinical records related to delivery (e.g., delivery mode, BW, sex, and Apgar score) were retrieved.
Macrosomia was defined as a BW of ≥ 4,000 g (27). Maternal pre-pregnancy BMI was calculated based on self-reported height and weight values before conception, as provided by the participants in the questionnaire. This was categorized as low (BMI < 18.5 kg/m2), normal (BMI: 18.5–23.9 kg/m2), overweight (BMI: 24.0–27.9 kg/m2), and obese (BMI ≥ 28.0 kg/m2) according to the Chinese Nutrition Society Group standard “weight monitoring and evaluation during the pregnancy period of Chinese women” (https://www.cnsoc.org/otherNotice/392100200.html/T/CNSS-009-2021). GDM was diagnosed following the International Association of Diabetes and Pregnancy Study Group criteria, using 75 g 2-h OGTT: fasting glucose ≥ 5.1 mmol/L, 1-h glucose ≥ 10.0 mmol/L, or 2-h glucose ≥ 8.5 mmol/L (28).
GWG was calculated as the difference between the weight at delivery and the pre-pregnancy self-reported weight. Excessive GWG was considered when the total weight gain recommendations of the Chinese Nutrition Society Group standard were exceeded. These recommendations are as follows: (1) underweight: 11.0–16.0 kg, (2) normal weight: 8.0–14.0 kg, (3) overweight: 7.0–11.0 kg, and (4) obese: 5.0–9.0 kg. The recommended GWG in early pregnancy is 2 kg. Furthermore, the normal rate of GWG during mid and late pregnancy for underweight, normal weight, overweight, and obese women is 0.37–0.56, 0.26–0.48, 0.22–0.37, and 0.15–0.30 kg/week, respectively (https://www.cnsoc.org/otherNotice/392100200.html/T/CNSS-009-2021). High maternal TG, TC, and LDL-C, as well as fetal BPD, HC, AC, and FL, were defined as values ≥ 95th percentile. Low maternal HDL-C was defined as values ≤ 5th percentile. High maternal height were defined as ≥ 165 cm (29, 30).
2.3 Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, N.Y., USA). Normally distributed continuous variables were expressed as mean ± standard deviation, with independent samples used to compare both groups and one-way analysis of variance for multiple groups. Non-normally distributed data were presented as median and interquartile range, with non-parametric tests (Mann–Whitney U test) employed for data with unequal variances. Categorical data were presented as frequency (n) and percentage (%), with chi-square or Fisher's exact test used for intergroup comparisons. Univariate logistic regression was performed to assess the relationship between macrosomia and individual predictor variables. Multivariate logistic regression analysis was performed to build multivariate prediction models in singleton fetuses during mid-to-late pregnancy and before delivery using all relevant predictors. Final predictors for the model were selected using bidirectional elimination with stepwise backward regression, starting with a P-value threshold of < 0.15. The final predictor selection for the models involved evaluating all combinations of candidate predictors with entry and retention criteria set at P-values of 0.40 and 0.20, respectively. Multivariable prediction models were evaluated using the Hosmer–Lemeshow goodness-of-fit tests. Subsequently, the models' predictive performance was assessed using receiver operating characteristic (ROC) curves. The predictive accuracy was determined at the model probability cut-off corresponding to the maximum Youden's index. Two-tailed tests were used, with P < 0.05 considered statistically significant.
3 Results
3.1 Maternal and neonatal characteristics of the 800 participants included in the case-control study
Overall, 800 women with singleton pregnancies were included in this study. Table 1 shows the maternal and neonatal characteristics. No significant differences in the mean maternal age, proportion of pregnant women aged >35 years, proportion of assisted reproductive technology conception, or mean gestational age at delivery were observed between the macrosomia and non-macrosomia groups (P > 0.05). The mean maternal height, pre-pregnancy BMI, GWG, and maternal fundal length at pre-delivery were higher in the macrosomia group than in the non-macrosomia group (P < 0.05). Additionally, the proportions of pre-pregnancy overweight and obese women, with gravidity of ≥2 times and parity of ≥1 times in the macrosomia group, were higher than those in the non-macrosomia group. Although no significant difference in the mean GWG rates during the first, second, and third trimesters was observed between the macrosomia and non-macrosomia groups, the proportions of patients with excessive GWG rates in each trimester were higher in the macrosomia group than in the non-macrosomia group. The mean BW of newborns and proportions of male infants were significantly higher in the macrosomia group than in the non-macrosomia group (P < 0.001). Conversely, both groups showed no significant differences in newborn birth height.
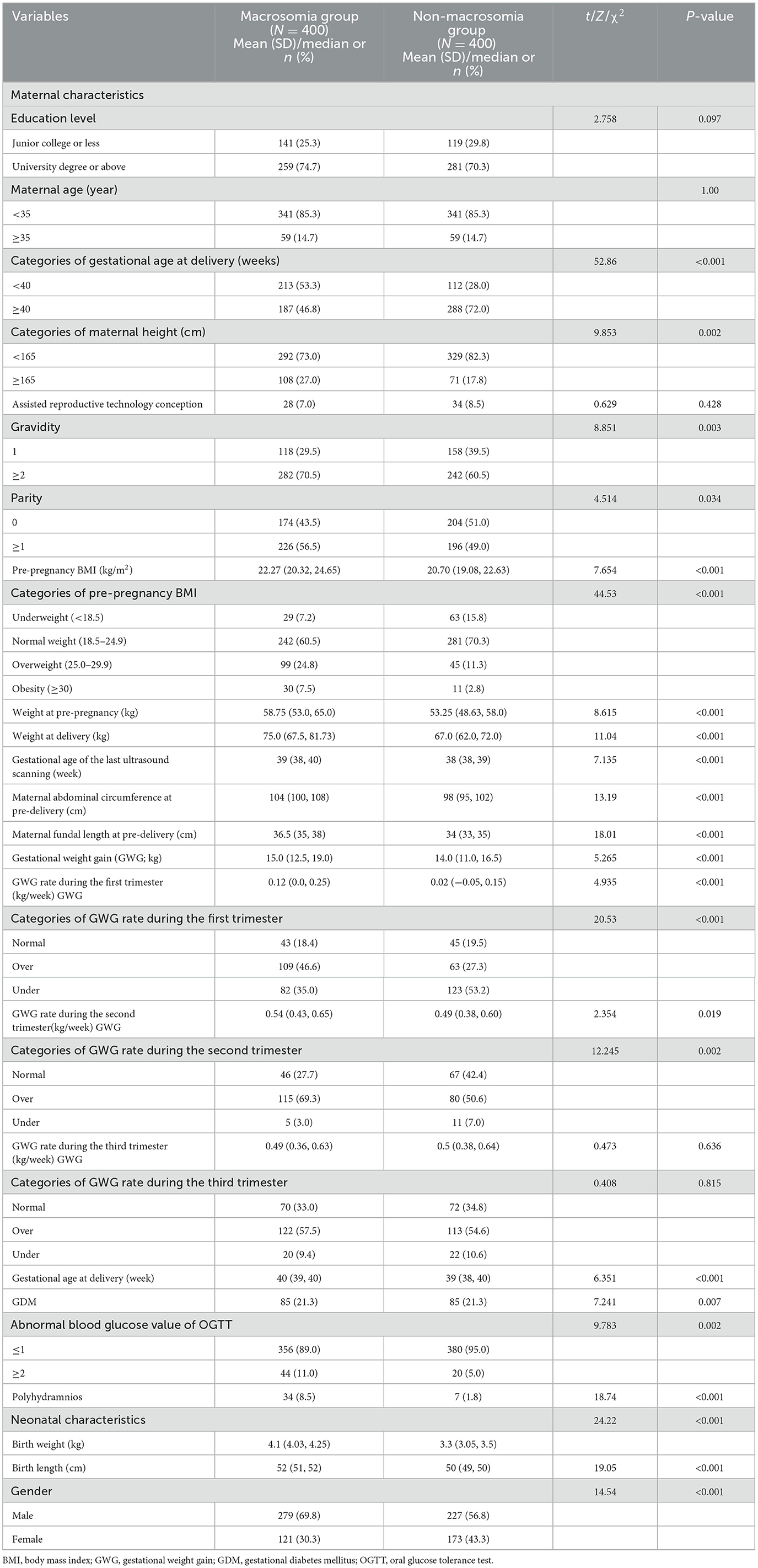
Table 1. Maternal and neonatal characteristics of the 800 cases from Fujian Maternity and Child Health Hospital and Quanzhou Women and Children's Hospital.
3.2 Comparisons of blood glucose and lipid levels between the macrosomia and non-macrosomia groups in late pregnancy
To assess the effect of maternal glucose and lipid levels on fetal weight during pregnancy, blood glucose levels in the OGTT and blood lipids in late pregnancy were compared between the macrosomia and non-macrosomia groups.
Pregnant women in the macrosomia group exhibited a higher GDM incidence, a greater proportion of at least two abnormal OGTT values, and higher mean blood glucose levels at OGTT0 and OGTT2 than those in the non-macrosomia group (P < 0.05). In late pregnancy, the mean HDL-C levels and proportions of pregnant women with HDL-C ≤ 1.27 mmol/L were significantly lower in the macrosomia group than in the non-macrosomia group. However, no significant differences were observed in TG, TC, and LDL-C levels between the two groups (P > 0.05; Table 2).
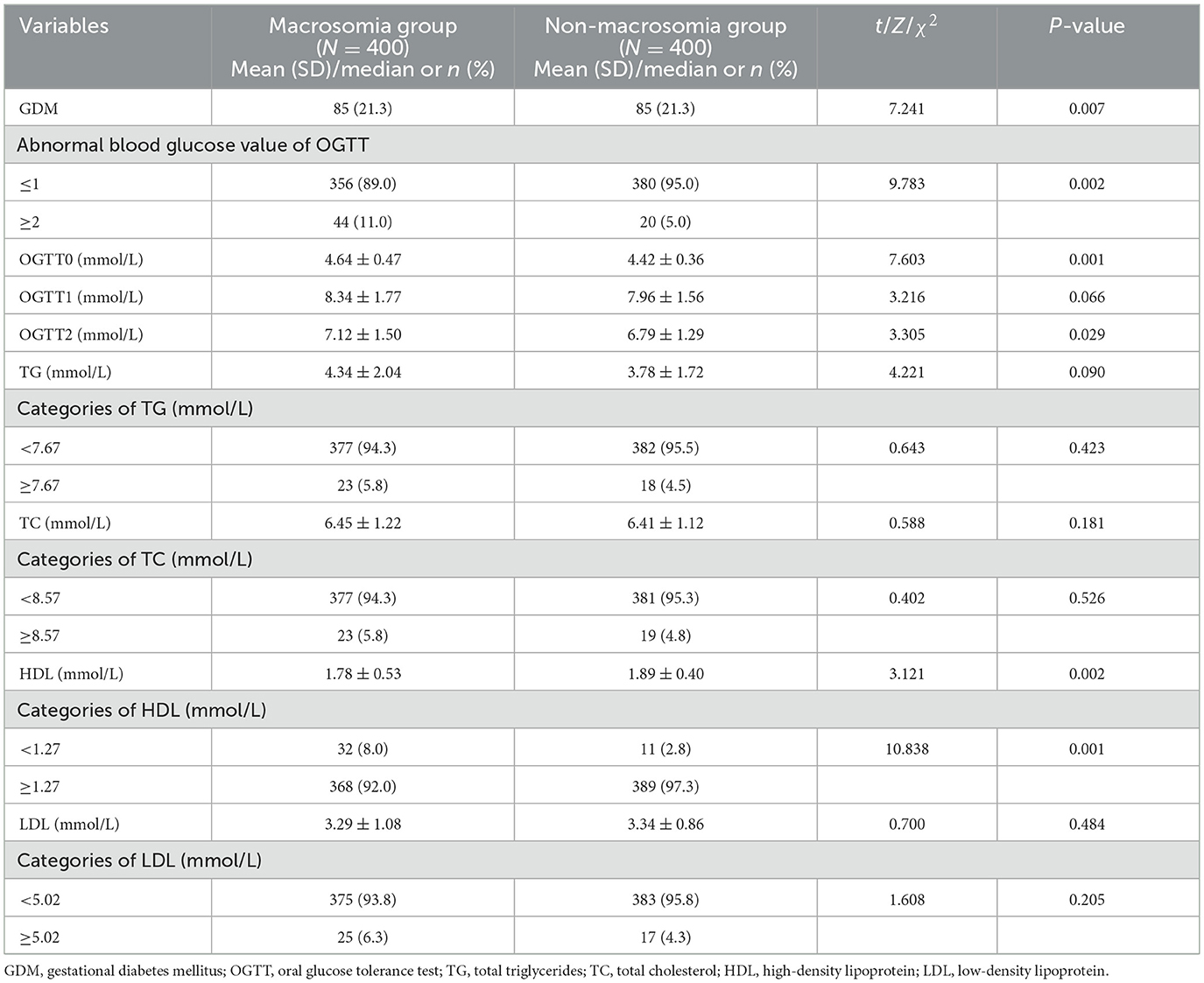
Table 2. Comparisons of the level of blood glucose of OGTT and blood lipids at late pregnancy between the macrosomia and non-macrosomia groups.
3.3 Comparisons of fetal growth indicators at the last ultrasound examination before delivery between the macrosomia and non-macrosomia groups
To evaluate the predictive value of BPD, HC, AC, and FL at the last ultrasound examination before delivery for macrosomia, these measurements were compared between the two groups. The macrosomia group had higher mean values for BPD, HC, AC, and FL than the non-macrosomia group. Additionally, the macrosomia group exhibited significantly higher proportions of fetuses with BPD ≥ 10 cm, HC ≥ 35 cm, AC ≥ 36 cm, and FL ≥ 7.5 cm than the non-macrosomia group (Table 3).
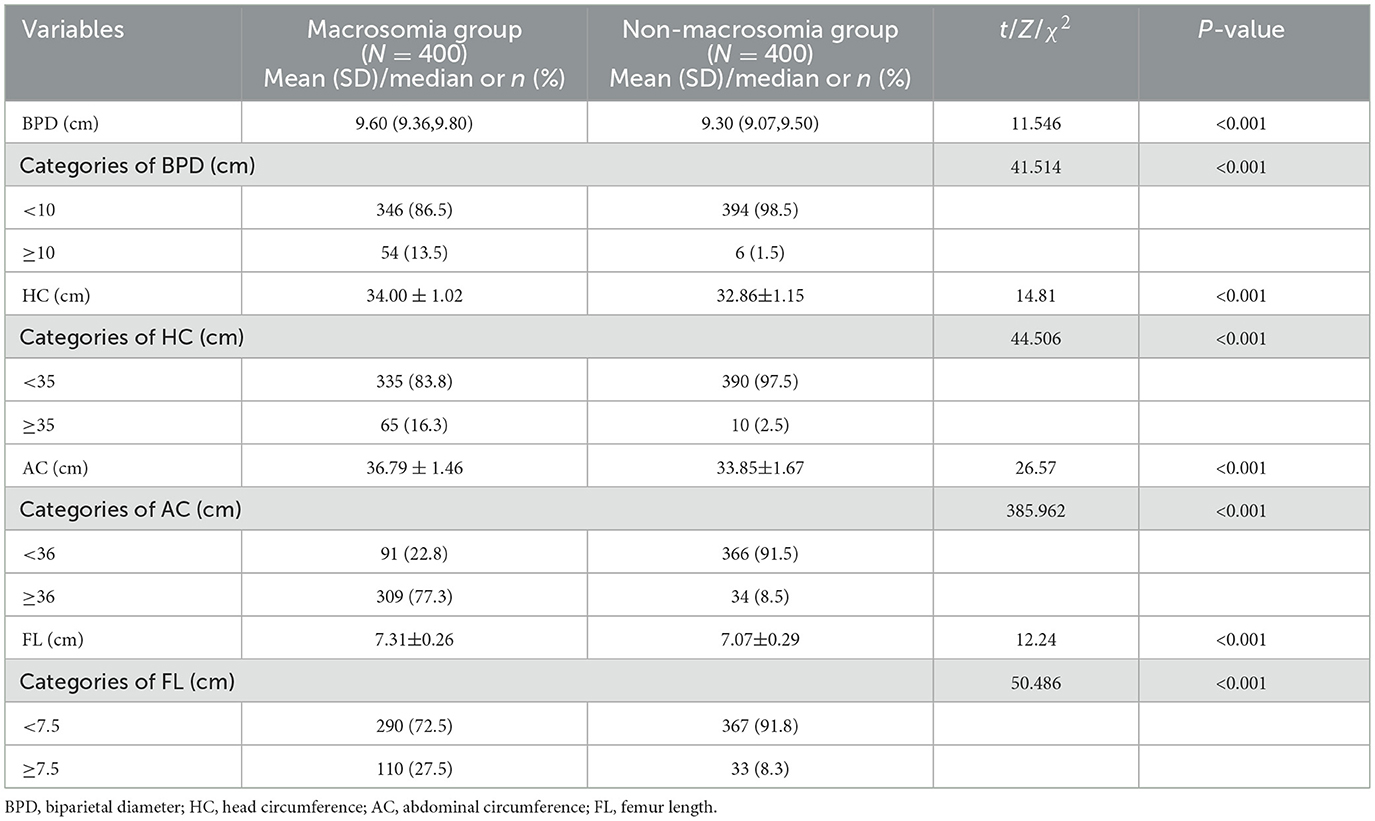
Table 3. Comparisons of fetal growth indicators at the last ultrasound examination before delivery between the macrosomia and non-macrosomia groups.
3.4 Comparison of pregnancy outcomes between the macrosomia and non-macrosomia groups
Intraoperative or intrapartum hemorrhage and PPH are severe complications associated with the delivery of macrosomic infants. PPH incidence rates, intraoperative or intrapartum hemorrhage volume, and PPH volume within 120 min post-delivery were higher in the macrosomia group than in the non-macrosomia group. Women who delivered macrosomic infants had an increased risk of prenatal anemia, cesarean section, shoulder dystocia, and need for the administration of potent oxytocin. However, no significant differences were observed in the incidence rates of neonatal jaundice, neonatal asphyxia, or hospitalization in the neonatal intensive care unit between the two groups. These results are presented in Table 4.
3.5 Logistic regression analysis of the risk factors for macrosomia and construction of the macrosomia prediction model
3.5.1 Construction of the macrosomia prediction model in singleton fetuses during mid-to-late pregnancy
Multiple logistic regression analysis was conducted to identify risk factors for macrosomia and develop a predictive model in singleton fetuses during mid-to-late pregnancy, with macrosomia as the dependent variable. Statistically significant variables from univariate regression analyses in early and mid-pregnancy were included for the multivariate logistic regression analysis, such as maternal height, pre-pregnancy BMI, GWG rates in the first and second trimesters, GDM, and abnormal blood glucose levels, as shown in Table 5. The results showed that macrosomia was significantly associated with maternal height ≥ 165 cm [odds ratio (OR): 2.303, 95% confidence interval (CI): 1.232–4.305], pre-pregnancy overweight (OR: 2.166, 95% CI: 1.119–3.83), pre-pregnancy obesity (OR: 3.189, 95% CI: 1.020–9.968), excessive GWG in the second trimester (OR: 2.083, 95% CI: 1.250–3.470), and at least two abnormal blood glucose values in the OGTT (OR: 5.267, 95% CI: 1.814–15.29; Supplementary Table S1). Based on the multiple regression analysis, the prediction model was defined as follows: logit(P) = −0.731 + 0.834 × maternal height + 0.039 × pre-pregnancy underweight + 0.773 × pre-pregnancy overweight + 1.160 × pre-pregnancy obesity + 0.374 × excessive GWG in the first trimester −0.561 × insufficiently excessive GWG in the first trimester + 0.734 × excessive GWG in the second trimester −0.568 × insufficiently excessive GWG in the second trimester + 1.661 × at least two abnormal OGTT values.
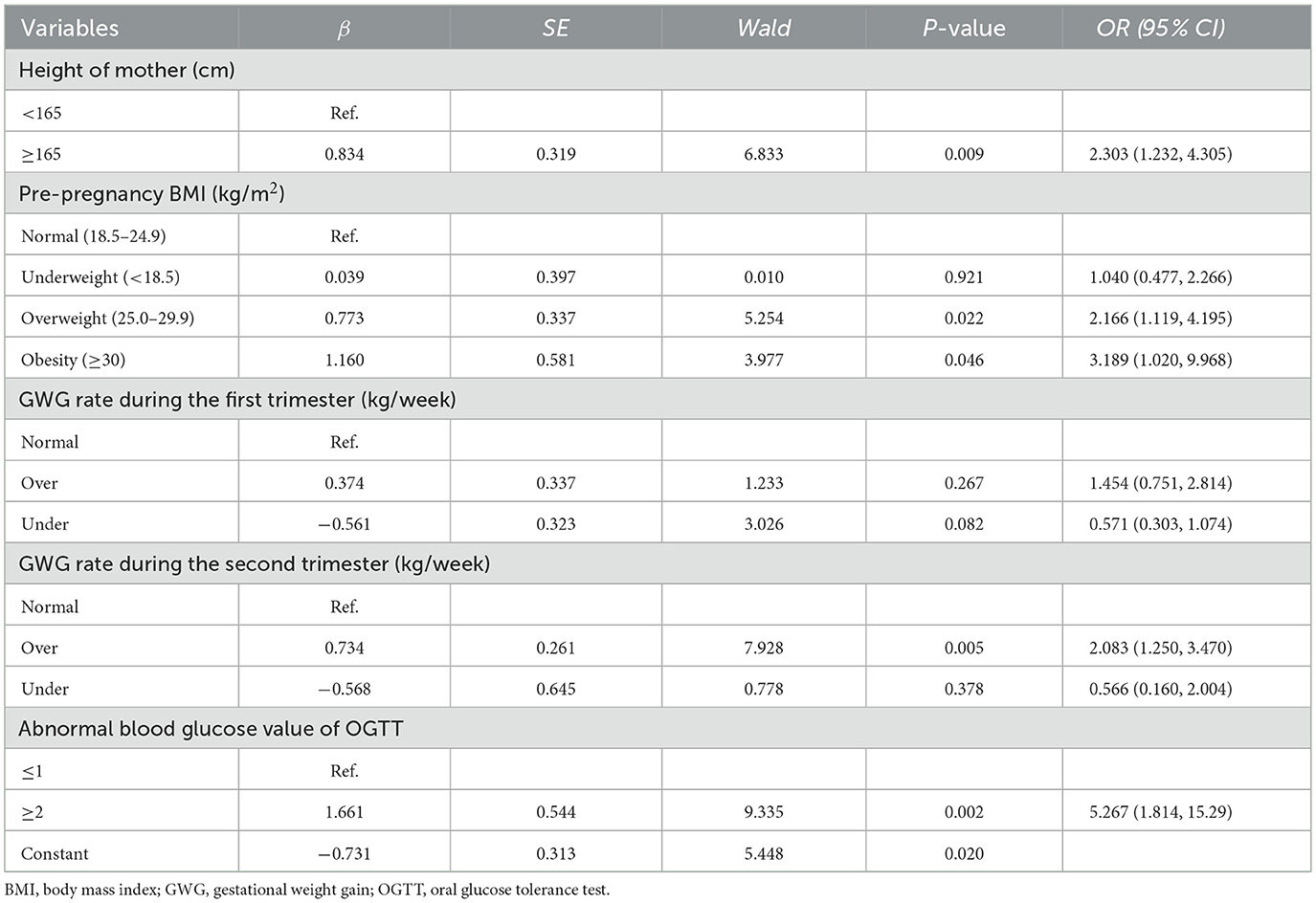
Table 5. Multivariate logistic regression analysis results of risk factors in early and mid-pregnancy of macrosomia.
ROC curve analysis was performed to examine the model's predictive performance. The area under the ROC curve (AUC) for predicting macrosomia was 0.719 (Figure 1). Additionally, the sensitivity, specificity, Youden index, positive predictive value (PPV), and negative predictive value (NPV) were 75.3, 57.6%, 0.329, 65.1, and 68.9%, respectively (Table 6). The Hosmer–Lemeshow test indicated no significant difference in the calibration of the model (Hosmer–Lemeshow χ2 = 3.534, P = 0.897), suggesting strong agreement between the model predictions and observed data.
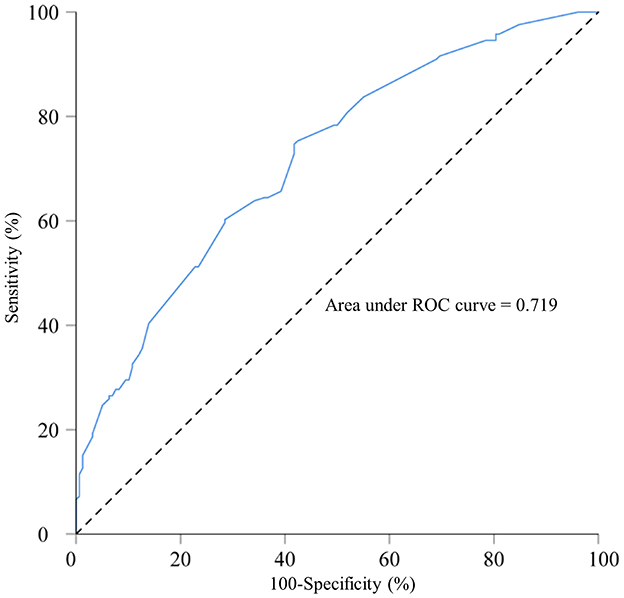
Figure 1. Receiver operating characteristic curve for the macrosomia prediction model in singleton fetuses during mid-to-late pregnancy; area under the curve: 0.719. A total of 800 singleton pregnant women who delivered in 2022 at Fujian Maternity and Child Health Hospital and Quanzhou Women and Children's Hospital.
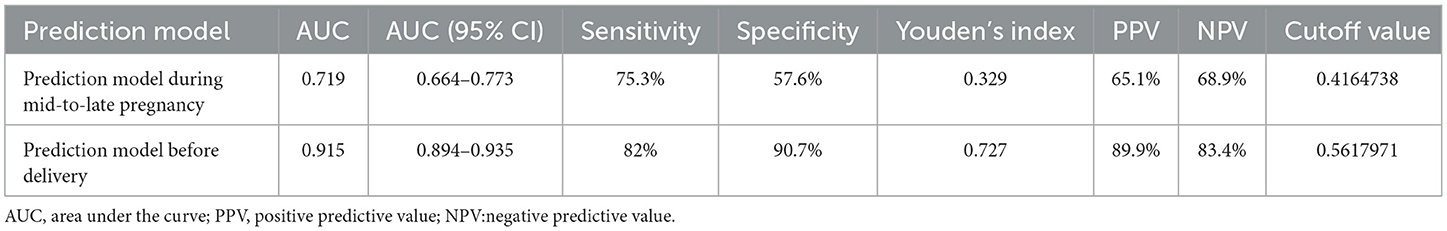
Table 6. Receiver operating characteristic curve for the macrosomia prediction model in singleton fetuses during mid-to-late pregnancy and before delivery.
3.5.2 Construction of macrosomia prediction model in singleton fetuses before delivery
To guide the timing, mode of delivery, and related risks of macrosomia, we performed a multiple logistic regression analysis to validate the risk factors for macrosomia. Additionally, we developed a prediction model for macrosomia in singleton fetuses before delivery. We incorporated the significant factors identified from univariate regression analyses during late pregnancy for further multivariate logistic regression analysis, as shown in Table 7. A significant association was found between macrosomia and maternal height ≥ 165 cm (OR: 1.729, 95% CI: 1.232–4.305), gestational age at delivery ≥ 40 weeks (OR: 1.996, 95% CI: 1.284–3.104), at least two abnormal OGTT values (OR: 5.267, 95% CI: 1.814–15.29), maternal AC plus fundal length at pre-delivery ≥ 140 cm (OR: 6.283, 95% CI: 3.976–9.927), fetal BPD ≥ 10 cm (OR: 3.373, 95% CI: 1.103–10.31), fetal HC ≥ 35 cm (OR: 3.473, 95% CI: 1.334–9.041), and fetal AC ≥ 36 cm (OR: 23.46, 95% CI: 14.81–37.16; Supplementary Table S2). The prediction model, derived from the multiple regression analysis, is as follows: logit(P) = −2.465 + 0.547 × maternal height + 0.691 × gestational age at delivery ≥ 40 weeks + 1.065 × at least two abnormal OGTT values + 1.838 × maternal AC plus fundal length at pre-delivery ≥ 140 cm + 1.216 × fetal BPD ≥ 10 cm +1.245 × fetal HC ≥ 35 cm + 3.155 × AC ≥ 36 cm.
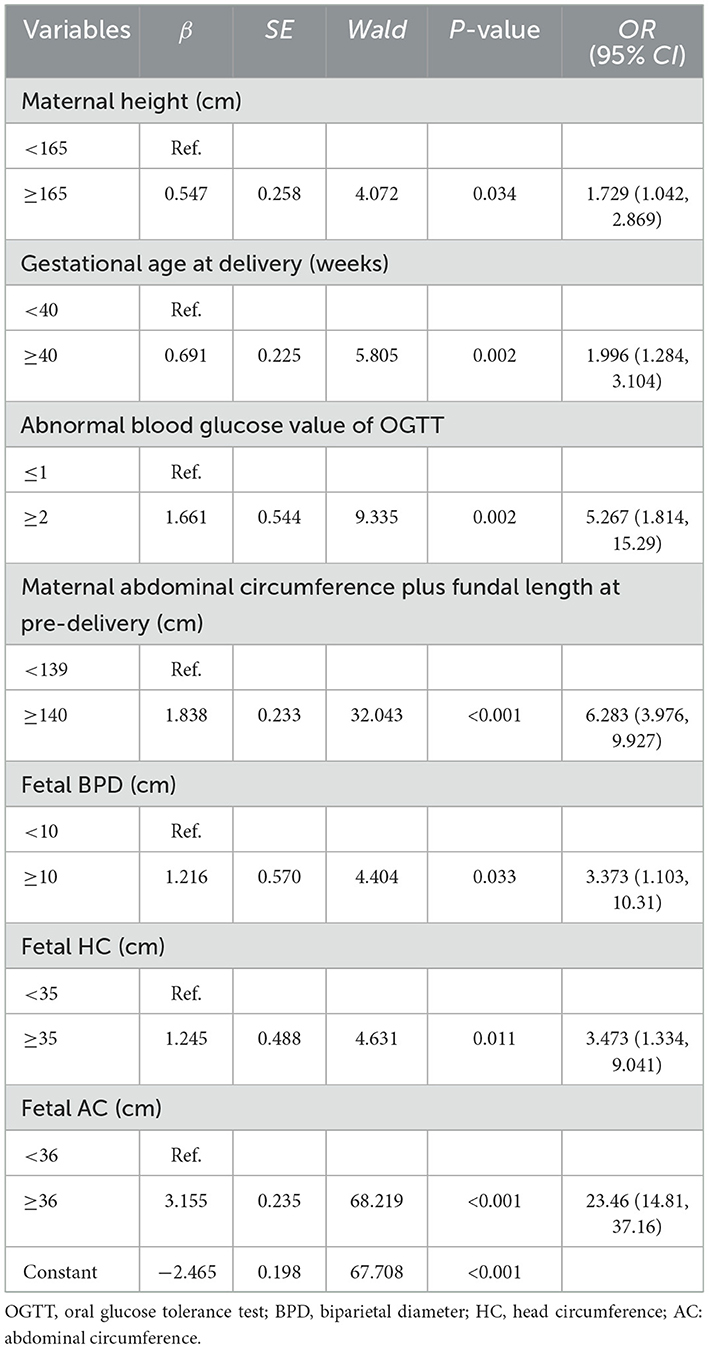
Table 7. Multivariate logistic regression analysis results of risk factors at late pregnancy before delivery of macrosomia.
ROC curve analysis was conducted to assess the model's predictive capability. The AUC for predicting macrosomia was 0.915 (Figure 2). Additionally, the sensitivity, specificity, Youden index, PPV, and NPV were 82.0%, 90.7%, 0.727, 89.9%, and 83.4%, respectively (Table 6). The Hosmer–Lemeshow test showed no significant difference in the calibration of the model (Hosmer–Lemeshow χ2 = 10.99, P = 0.089), indicating a strong agreement between the model and observed data.
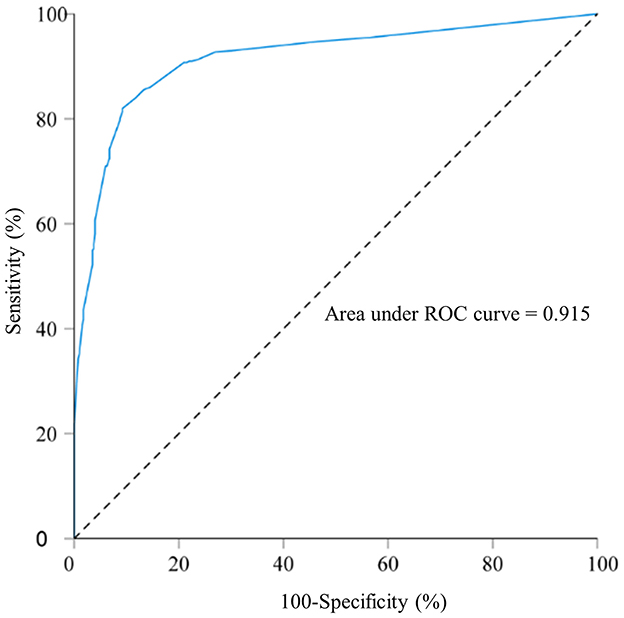
Figure 2. Receiver operating characteristic curve for the macrosomia prediction model in singleton fetuses before delivery; area under the curve: 0.915. A total of 800 singleton pregnant women who delivered in 2022 at Fujian Maternity and Child Health Hospital and Quanzhou Women and Children's Hospital.
4 Discussion
Macrosomia arises from the complex interaction of various environmental and genetic factors, thereby making the identification of risk factors and prediction more challenging. In clinical practice, these factors are categorized as unchangeable or changeable. Unchangeable factors include gravidity, parity, maternal height, and fetal sex, whereas changeable factors encompass pre-pregnancy BMI, gestational age before delivery, and GDM (31, 32). In this population-based case-control study, the relationship between macrosomia and 20 maternal and seven fetal characteristics was investigated to establish predictive models for fetal macrosomia. Maternal height ≥ 165 cm, pre-pregnancy overweight/obesity, excessive GWG during the second trimester, at least two abnormal blood glucose values on OGTT ≥ 2, gestational age at delivery ≥ 40 weeks, and maternal AC plus fundal length at pre-delivery ≥ 140 cm were identified as independent risk factors for macrosomia. The predictive model for macrosomia in singleton fetuses during mid-to-late pregnancy and before delivery showed high predictive performance.
Previous studies have revealed comparable associations between macrosomia and pre-pregnancy overweight/obesity. However, inconsistencies exist in the ORs compared with those of a normal pre-pregnancy BMI. Pre-pregnancy obesity has been associated with a 7.69-fold higher risk of macrosomia (18). Studies have demonstrated that women with obesity face a 1.5–2.3 times greater risk of macrosomia than those with a normal BMI (33, 34). In this study, we observed that women with pre-pregnancy obesity and overweight have a 3.189-fold and 2.166-fold higher risk of macrosomia, respectively, than those with a normal BMI. Excessive GWG also increases the risk of macrosomia, as confirmed by previous research (17, 35). The GWG rate during the first and second trimesters was higher in the macrosomia group than in the non-macrosomia group. Several studies have revealed GDM as an independent risk factor for macrosomia (4, 18, 36). Elevated blood sugar levels in pregnant women with GDM can be transferred to the fetus through the placenta. Since fetal pancreatic function is inadequate and maternal insulin cannot cross the placental barrier, the fetus experiences sustained elevated blood sugar levels. The synergistic effects of hyperinsulinemia and hyperglycemia in the fetus lead to enhanced glucose utilization and increased synthesis of fat and protein tissues. Reportedly, managing blood glucose levels in pregnant women with GDM through medications or dietary interventions can decrease the likelihood of macrosomia by 73% (37). Macrosomia rates in pregnant women with GDM and well-controlled blood sugar levels are comparable to those in pregnant women with normal blood sugar levels (38). Our study confirms the association between macrosomia and other risk factors, including maternal height, fetal sex (male), and gestational age ≥ 40 weeks. These findings align with those of previous studies (39–41).
Maternal blood lipid levels—TC, TG, HDL-C, and LDL-C— increase significantly at the beginning of the 12th week of gestation, particularly during the second and third trimesters (42, 43). Previous cohort studies have revealed that elevated maternal TG and reduced HDL-C levels in late pregnancy are associated with an increased risk of macrosomia (16, 44). In this study, mean HDL-C levels and the proportions of pregnant women with HDL-C ≤ 1.27 mmol/L were significantly lower in the macrosomia group than in the non-macrosomia group. However, no significant differences were observed in TG, TC, or LDL-C levels between the two groups. The discrepancies between our findings and those of earlier studies may be due to variations in methodology. All participants in this study were from the same region, ensuring comparability between the case and control groups regarding prenatal care quality and eliminating the influence of ethnic/racial factors.
While the effect of maternal obesity and overweight on excessive BW is well-known, their significant role in predicting the risk of macrosomia underscores the importance of improving the nutritional status of women to facilitate timely interventions during early and mid-pregnancy, thereby mitigating the risk of adverse neonatal outcomes. Maternal overweight or obesity is also a well-documented risk factor for GDM, which further increases the likelihood of fetal macrosomia. To clarify the relevant high-risk factors and their predictive value for macrosomia, a prediction model based on maternal height, pre-pregnancy underweight, pre-pregnancy overweight, pre-pregnancy obesity, excessive GWG during the first and second trimesters, and at least two abnormal OGTT values was developed in this study. The model achieved an AUC value of 0.719, with sensitivity, specificity, and Youden index of 75.3, 57.6%, and 0.329, respectively, indicating its predictive value. Monitoring and managing maternal weight and blood sugar levels during pre-pregnancy, early pregnancy, and mid-pregnancy is crucial for reducing the risk of macrosomia. The sensitivity and specificity are relatively low, which is mainly related to the types of variables included in the model. Specifically, this model is established based on the clinical and metabolic characteristics of the mother during the second trimester and does not incorporate the ultrasound indicators from the third trimester of pregnancy; therefore, its predictive ability is limited. Despite this, the model can identify some high-risk groups in the second trimester of pregnancy and has a certain clinical early warning value. We plan to incorporate more biological and imaging indicators in future studies and validate them in multi-center cohorts to further enhance the model's predictive performance.
Developing a predictive model for macrosomia before delivery is crucial for guiding the timing and mode of delivery. Therefore, creating efficient tools to alert healthcare professionals to the potential risks of accelerated fetal growth and macrosomia is crucial. Macrosomia was previously predicted using ultrasound and maternal physical examination (45). However, ultrasound and clinical measurements have limitations in accurately predicting newborn weight individually (2, 46, 47). In this study, we combined BPD, HC, AC, and FL from the last ultrasound examination with clinical data from pregnant women to predict macrosomia before delivery. The model achieved an AUC value of 0.915, with sensitivity, specificity, and Youden index of 82.0, 90.7%, and 0.727, respectively, indicating its high predictive value. Consequently, integrating this predictive model into clinical practice can reduce the incidence of delivery complications.
Regarding clinical application, in mid-pregnancy—especially after completing the OGTT—we can use a prediction model to predict the risk of macrosomia, and subsequently make some interventions (e.g., dietary counseling, closer glucose monitoring, ultrasound follow-ups) to prevent macrosomia at the time of delivery. When approaching the delivery time, the model can be used again before delivery to further evaluate the risk of macrosomia and decide the mode of delivery. For instance, a patient was 166 cm tall, weighed 68 kg before pregnancy, and had a pre-pregnancy BMI of 24.68 kg/m2. The weight gain rate was normal in both the first and second trimesters. Additionally, the OGTT at 24 weeks was 5.2–10.5–8.1 mmol/L. The patient underwent diet intervention and engaged in exercise to control the blood glucose to normal, and the GWG was 12 kg. Maternal AC plus fundal length at pre-delivery was 138 cm. The ultrasound result at 40 weeks of gestation indicated BPD, HC, AC, and FL of 9.8, 35.5, 35.5, and 7.4 cm, respectively. Notably, the patient vaginally delivered a baby weighing 3.5 kg without complications. The rate of macrosomia at 24 weeks of gestation was 92.7%, according to the equation: logit(P) = −0.731 + 0.834 × 1 + 0.039 × 0 + 0.773 × 1 + 1.160 × 0 + 0.374 × 0 – 0.561 × 0 + 0.734 × 0 – 0.568 × 0 + 1.661 × 1 = 2.54. Furthermore, the rate of macrosomia at 39 weeks of gestation is 74.7%, based on the equation: logit(P) = −2.465 + 0.547 × 1 + 0.691 × 1 + 1.065 × 1 + 1.838 × 0 + 1.216 × 0 + 1.245 × 1 + 3.155 × 0 = 1.08. This implies that when the rate of macrosomia is significantly high in mid-pregnancy, interventions should be implemented to decrease the rate before delivery, thereby increasing the likelihood of a successful vaginal birth.
This study has some limitations. First, as a retrospective case-control study, it is inherently subject to selection bias. Second, the study focused exclusively on the Chinese population due to ethnic differences, which may limit the generalizability of its findings to other racial groups. Third, the predictive model was derived and tested using a two-center dataset of Fujian province, without external validation. Additionally, the study did not include data on the history of macrosomia or the dietary intake of pregnant women, which could increase the risk of macrosomia. Therefore, future research should focus on several key areas to deepen the understanding of macrosomia. These include conducting external validation in independent cohorts of multiple centers, different regions, and different populations to comprehensively evaluate the universality and clinical application value of the model, as well as exploring additional risk factors unaddressed in this study, such as maternal diet, lifestyle factors, and environmental exposures.
In conclusion, this study identifies key high-risk factors for macrosomia during the perinatal period. We also developed a macrosomia prediction model in singleton fetuses during mid-to-late pregnancy and before delivery. ROC curve analysis showed that the model had a strong predictive value for macrosomia. We expect that this model will help doctors predict the occurrence of macrosomia early and implement interventions to mitigate adverse perinatal outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics Review Committee of Fujian Provincial Maternity and Child Hospital approval number: 2023KY046. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
JL: Data curation, Formal analysis, Methodology, Writing – original draft. WH: Data curation, Formal analysis, Methodology, Writing – original draft. SL: Investigation, Methodology, Writing – original draft. LD: Data curation, Formal analysis, Writing – original draft. LL: Data curation, Formal analysis, Investigation, Writing – original draft. QL: Conceptualization, Writing – review & editing. JY: Conceptualization, Writing – review & editing. JZ: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Fujian Provincial Natural Science Foundation of China (2023J011233) and the National Key Clinical Specialty Construction Program of China (Obstetrics).
Acknowledgments
We thank the patients and their families for their contribution to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1590283/full#supplementary-material
Abbreviations
PPH, postpartum hemorrhage; BW, birth weight; OR, odds ratio; ROC, receiver operating characteristic; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the receiver operating characteristic curve; GWG, gestational weight gain; GDM, gestational diabetes mellitus; CI, confidence interval; OGTT, oral glucose tolerance test; TG, total triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; BPD, biparietal diameter; HC, head circumference; AC, abdominal circumference; FL, femur length.
References
1. Beta J, Khan N, Khalil A, Fiolna M, Ramadan G, Akolekar R. Maternal and neonatal complications of fetal macrosomia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2019) 54:308–18. doi: 10.1002/uog.20279
2. Macrosomia: ACOG practice bulletin, number 216. Obstet Gynecol. (2020) 135:e18–35. doi: 10.1097/AOG.0000000000003606
3. Koyanagi A, Zhang J, Dagvadorj A, Hirayama F, Shibuya K, Souza JP, et al. Macrosomia in 23 developing countries: an analysis of a multicountry, facility-based, cross-sectional survey. Lancet. (2013) 381:476–83. doi: 10.1016/S0140-6736(12)61605-5
4. Song X, Chen L, Zhang S, Liu Y, Wei J, Wang T, et al. Gestational diabetes mellitus and high triglyceride levels mediate the association between pre-pregnancy overweight/obesity and macrosomia: a prospective cohort study in central China. Nutrients. (2022) 14:3347. doi: 10.3390/nu14163347
5. Zhao LJ, Li HT, Zhang YL, Zhou YB, Liu JM. Mobile terminal-based survey on the birth characteristics for Chinese newborns. Beijing Da Xue Xue Bao Yi Xue Ban. (2019) 51:813–8. doi: 10.19723/j.issn.1671-167X.2019.05.004
6. Juan J, Yang H, Wei Y, Song G, Su R, Chen X, et al. Prevalence and characteristics of macrosomia in the first and subsequent pregnancy: a multi-center retrospective study. Chin Med J. (2022) 135:1492–4. doi: 10.1097/CM9.0000000000002077
7. Wang D, Hong Y, Zhu L, Wang X, Lv Q, Zhou Q, et al. Risk factors and outcomes of macrosomia in China: a multicentric survey based on birth data. J Matern Fetal Neonatal Med. (2017) 30:623–7. doi: 10.1080/14767058.2016.1252746
8. Badr DA, Carlin A, Kadji C, Kang X, Cannie MM, Jani JC. Timing of induction of labor in suspected macrosomia: retrospective cohort study, systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2024) 64:443–52. doi: 10.1002/uog.27643
9. Badr DA, Cannie MM, Kadji C, Kang X, Carlin A, Jani JC. Reducing macrosomia-related birth complications in primigravid women: ultrasound- and magnetic resonance imaging–based models. Am J Obstet Gynecol. (2024) 230:557.e1–8. doi: 10.1016/j.ajog.2023.10.011
10. Åberg K, Norman M, Pettersson K, Ekéus C. Vacuum extraction in fetal macrosomia and risk of neonatal complications: a population-based cohort study. Acta Obstet Gynecol Scand. (2016) 95:1089–96. doi: 10.1111/aogs.12952
11. Schellong K, Schulz S, Harder T, Plagemann A. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS One. (2012) 7:e47776. doi: 10.1371/journal.pone.0047776
12. Collier A, Abraham EC, Armstrong J, Godwin J, Monteath K, Lindsay R. Reported prevalence of gestational diabetes in Scotland: the relationship with obesity, age, socioeconomic status, smoking and macrosomia, and how many are we missing? J Diabetes Investig. (2017) 8:161–7. doi: 10.1111/jdi.12552
13. Hua Q, Tan J, Liu ZH, Liu RK, Yang Z. A cohort study on the correlation between birth weight, simple obesity, blood lipids, blood glucose and blood pressure from childhood to adolescence. Zhonghua Nei Ke Za Zhi. (2007) 46:923–5.
14. Van Lieshout RJ, Savoy CD, Ferro MA, Krzeczkowski JE, Colman I. Macrosomia and psychiatric risk in adolescence. Eur Child Adolesc Psychiatry. (2020) 29:1537–45. doi: 10.1007/s00787-019-01466-7
15. Li C, Ju L, Yang M, Zhang Q, Sun S, Cao J, et al. Prenatal air pollution exposure increases the risk of macrosomia: evidence from a prospective cohort study in the coastal area of China. Environ Sci Pollut Res. (2022) 29:5144–52. doi: 10.1007/s11356-021-16054-z
16. Yuan X, Han X, Jia C, Long W, Wang H, Yu B, et al. (2022). Investigation and application of risk factors of macrosomia based on 10,396 Chinese pregnant women. Front. Endocrinol. 13:837816. doi: 10.3389/fendo.2022.837816
17. Pereda, J, Bove, I, Pineyro, MM. Excessive Maternal Weight and Diabetes Are Risk Factors for Macrosomia: A Cross-Sectional Study of 42,663 Pregnancies in Uruguay. Front Endocrinol. (2020) 11:588443. doi: 10.3389/fendo.2020.588443
18. Song X, Shu J, Zhang S, Chen L, Diao J, Li J, et al. Pre-pregnancy body mass index and risk of macrosomia and large for gestational age births with gestational diabetes mellitus as a mediator: a prospective cohort study in central China. Nutrients. (2022) 14:1072. doi: 10.3390/nu14051072
19. Aji AS, Lipoeto NI, Yusrawati Y, Malik SG, Kusmayanti NA, Susanto I, et al. Association between pre-pregnancy body mass index and gestational weight gain on pregnancy outcomes: a cohort study in Indonesian pregnant women. BMC Pregnancy Childbirth. (2022) 22:492. doi: 10.1186/s12884-022-04815-8
20. Rao J, Fan D, Wu S, Lin D, Zhang H, Ye S, et al. Trend and risk factors of low birth weight and macrosomia in south China, 2005–2017: a retrospective observational study. Sci Rep. (2018) 8:3393. doi: 10.1038/s41598-018-21771-6
21. Heus P, Damen JAAG, Pajouheshnia R, Scholten RJPM, Reitsma JB, Collins GS, et al. Poor reporting of multivariable prediction model studies: towards a targeted implementation strategy of the TRIPOD statement. BMC Med. (2018) 16:120. doi: 10.1186/s12916-018-1099-2
22. Steyerberg EW, Moons KGM, van der Windt DA, Hayden JA, Perel P, Schroter S, et al. Prognosis research strategy (PROGRESS) 3: prognostic model research. PLoS Med. (2013) 10:e1001381. doi: 10.1371/journal.pmed.1001381
23. Cobo T, Aldecoa V, Figueras F, Herranz A, Ferrero S, Izquierdo M, et al. Development and validation of a multivariable prediction model of spontaneous preterm delivery and microbial invasion of the amniotic cavity in women with preterm labor. Am J Obstet Gynecol. (2020) 223:421.e1–14. doi: 10.1016/j.ajog.2020.02.049
24. Ciobanu A, Rouvali A, Syngelaki A, Akolekar R, Nicolaides KH. Prediction of small for gestational age neonates: screening by maternal factors, fetal biometry, and biomarkers at 35–37 weeks' gestation. Am J Obstet Gynecol. (2019) 220:486.e1–11. doi: 10.1016/j.ajog.2019.01.227
25. Tarca AL, Taran A, Romero R, Jung E, Paredes C, Bhatti G, et al. Prediction of preeclampsia throughout gestation with maternal characteristics and biophysical and biochemical markers: a longitudinal study. Am J Obstet Gynecol. (2022) 226:126.e1–22. doi: 10.1016/j.ajog.2021.01.020
26. Wastlund D, Moraitis A, Thornton J, Sanders J, White I, Brocklehurst P, et al. The cost-effectiveness of universal late-pregnancy screening for macrosomia in nulliparous women: a decision analysis. BJOG. (2019) 126:1243–50. doi: 10.1111/1471-0528.15809
27. Boulet S, Salihu H, Alexander G. Mode of delivery and birth outcomes of macrosomic infants. J Obstet Gynaecol. (2004) 24:622–9. doi: 10.1080/01443610400007828
28. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a world health organization guideline. Diabetes Res Clin Pract. (2014) 103:341–63. doi: 10.1016/j.diabres.2013.10.012
29. Xue R, Yun Z. An evaluation of macrosomia risk factors in a cohort of Chinese women and neonates. J Int Med Res. (2025) 53:3000605251365806. doi: 10.1177/03000605251365806
30. Bao C, Zhou Y, Jiang L, Sun C, Wang F, Xia W, et al. Reasons for the increasing incidence of macrosomia in Harbin, China. BJOG. (2011) 118:93–8. doi: 10.1111/j.1471-0528.2010.02776.x
31. Henriksen T. The macrosomic fetus: a challenge in current obstetrics. Acta Obstet Gynecol Scand. (2008) 87:134–45. doi: 10.1080/00016340801899289
32. Walsh JM, McAuliffe FM. Prediction and prevention of the macrosomic fetus. Eur J Obstet Gynecol Reprod Biol. (2012) 162:125–30. doi: 10.1016/j.ejogrb.2012.03.005
33. Dai R, He X-J, Hu C-L. Maternal pre-pregnancy obesity and the risk of macrosomia: a meta-analysis. Arch Gynecol Obstet. (2018) 297:139–45. doi: 10.1007/s00404-017-4573-8
34. Vinturache AE, Chaput KH, Tough SC. Pre-pregnancy body mass index (BMI) and macrosomia in a Canadian birth cohort. J Matern Fetal Neonatal Med. (2017) 30:109–16. doi: 10.3109/14767058.2016.1163679
35. Uchinuma H, Tsuchiya K, Sekine T, Horiuchi S, Kushima M, Otawa S, et al. Gestational body weight gain and risk of low birth weight or macrosomia in women of Japan: a nationwide cohort study. Int J Obes. (2021) 45:2666–74. doi: 10.1038/s41366-021-00947-7
36. Foussard N, Cougnard-Grégoire A, Rajaobelina K, Delcourt C, Helmer C, Lamireau T, et al. Skin autofluorescence of pregnant women with diabetes predicts the macrosomia of their children. Diabetes. (2019) 68:1663–9. doi: 10.2337/db18-0906
37. Wei J, Heng W, Gao J. Effects of low glycemic index diets on gestational diabetes mellitus. Medicine. (2016) 95:e3792. doi: 10.1097/MD.0000000000003792
38. Juan J, Wei Y, Song G, Su R, Chen X, Shan R, et al. Risk factors for macrosomia in multipara: a multi-center retrospective study. Children. (2022) 9:935. doi: 10.3390/children9070935
39. Adugna DG, Enyew EF, Jemberie MT. Prevalence and associated factors of macrosomia among newborns delivered in university of Gondar comprehensive specialized hospital, Gondar, Ethiopia: an institution-based cross-sectional study. Pediatric Health Med Ther. (2020) 11:495–503. doi: 10.2147/PHMT.S289218
40. Spada E, Chiossi G, Coscia A, Monari F, Facchinetti F. Effect of maternal age, height, BMI and ethnicity on birth weight: an Italian multicenter study. J Perinat Med. (2018) 46:1016–21. doi: 10.1515/jpm-2017-0102
41. Fang F, Zhang Q-Y, Zhang J, Lei X-P, Luo Z-C, Cheng H-D. Risk factors for recurrent macrosomia and child outcomes. World J Pediat. (2019) 15:289–96. doi: 10.1007/s12519-019-00249-z
42. Lu Y, Jia Z, Su S, Han L, Meng L, Tang G, et al. Establishment of trimester-specific reference intervals of serum lipids and the associations with pregnancy complications and adverse perinatal outcomes: a population-based prospective study. Ann Med. (2021) 53:1632–41. doi: 10.1080/07853890.2021.1974082
43. Bartels Ä, Egan N, Broadhurst DI, Khashan AS, Joyce C, Stapleton M, et al. Maternal serum cholesterol levels are elevated from the 1st trimester of pregnancy: a cross-sectional study. J Obstet Gynaecol. (2012) 32:747–52. doi: 10.3109/01443615.2012.714017
44. Jin W-Y, Lin S-L, Hou R-L, Chen X-Y, Han T, Jin Y, et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth. (2016) 16:60. doi: 10.1186/s12884-016-0852-9
45. Nguyen MT, Ouzounian JG. Evaluation and management of fetal macrosomia. Obstet Gynecol Clin North Am. (2021) 48:387–99. doi: 10.1016/j.ogc.2021.02.008
46. Kayem G, Grangé G, Bréart G, Goffinet F. Comparison of fundal height measurement and sonographically measured fetal abdominal circumference in the prediction of high and low birth weight at term. Ultrasound Obstet Gynecol. (2009) 34:566–71. doi: 10.1002/uog.6378
Keywords: macrosomia, predictive model, pre-pregnancy body mass index, gestational weightgain, gestational diabetes mellitus, risk factors
Citation: Luo J, Huang W, Luo S, Deng L, Lin L, Liao Q, Yan J and Zhou J (2025) Assessing the risk factors and establishing multivariable prediction models for singleton macrosomia. Front. Med. 12:1590283. doi: 10.3389/fmed.2025.1590283
Received: 09 March 2025; Accepted: 02 September 2025;
Published: 24 September 2025.
Edited by:
Javier Diaz-Castro, University of Granada, SpainReviewed by:
Sixtus Aguree, Indiana University, United StatesKristjana Einarsdottir, Curtin University, Australia
Copyright © 2025 Luo, Huang, Luo, Deng, Lin, Liao, Yan and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinfu Zhou, emhvdTgxMTIwM0Bmam11LmVkdS5jbg==; Jianying Yan, eWFuankyMDE5QGZqbXUuZWR1LmNu; Qiuping Liao, bGlhb3FpdXBpbmdAZmptdS5lZHUuY24=
†These authors have contributed equally to this work
 Jinying Luo
Jinying Luo Wenyan Huang1,4†
Wenyan Huang1,4† Lihua Lin
Lihua Lin Qiuping Liao
Qiuping Liao Jianying Yan
Jianying Yan Jinfu Zhou
Jinfu Zhou