- 1Department of Cardiothoracic Surgery, Cixi People Hospital Medical Health Group (Cixi People Hospital), Ningbo, Zhejiang, China
- 2Department of Nephrology, Cixi People Hospital Medical Health Group (Cixi People Hospital), Ningbo, Zhejiang, China
- 3Department of Medical Imaging (Radiology), Cixi People Hospital Medical Health Group (Cixi People Hospital), Ningbo, Zhejiang, China
- 4Department of Endocrinology, Ningbo Medical Center LiHuiLi Hospital, Ningbo, Zhejiang, China
- 5Department of Endocrinology, Ningbo Medical Center LiHuiLi Hospital, Ningbo, Zhejiang, China
- 6Department of Gastroenterology, Cixi People Hospital Medical Health Group (Cixi People Hospital), Ningbo, Zhejiang, China
- 7Department of Endocrinology, Cixi People Hospital Medical Health Group (Cixi People Hospital), Ningbo, Zhejiang, China
Background: Iatrogenic Cushing’s syndrome (ICS) is frequently observed as a side effect of long-term steroid treatment. While it is most commonly linked to the administration of oral steroids, rare instances have been reported following the inadvertent use of topical steroids, which can lead to severe clinical consequences.
Case summary: This case report details the case of a psoriasis patient who exhibited Cushing-like symptoms, such as central obesity, violaceous striae, moon face, and hypertension, following the long-term use of a so-called “herbal cream” containing clobetasol propionate. Laboratory investigations revealed hypokalemia, a disrupted cortisol circadian rhythm, and suppressed plasma ACTH levels. Additionally, bone mineral density analysis indicated a reduction in bone density.
Conclusion: This case underscores the critical need for clinicians to remain vigilant about the potential systemic adverse effects of topical glucocorticoids, particularly in psoriasis patients, where even minimal concentrations of clobetasol propionate can precipitate ICS. Furthermore, identifying the source of exogenous glucocorticoids in ICS necessitates a thorough review of the patient’s medication history, encompassing over-the-counter drugs and supplements. It is important that medication use be guided by specialist consultation and rigorous monitoring, rather than unsupervised self-medication.
Introduction
Iatrogenic corticosteroid use is the most frequent cause of cushingoid characteristics, while certain herbal treatments can also raise corticosteroid levels, which can result in iatrogenic Cushing’s syndrome (ICS) (1). Topical corticosteroid related ICS is rarely seen in adults, often with significant clinical consequences. The development of ICS depends on the dose, duration, as well as the potency of corticosteroids (2).
Clobetasol propionate is a superpotent topical corticosteroid widely utilized in the treatment of various dermatological conditions, including psoriasis, due to its potent anti-inflammatory, antipruritic, vasoconstrictive, and antiproliferative properties (3). Psoriasis, a chronic inflammatory skin disorder, often necessitates long-term therapeutic intervention (4). Safety studies have indicated that clobetasol propionate is safer when used over shorter durations; however, prolonged application can lead to adverse side effects include Cushing-like syndrome, adrenal hypophysial axis suppression (3).
The case presented herein highlights a patient with psoriasis who developed ICS following the prolonged use of a so-called “herbal cream” containing clobetasol propionate. This case not only underscores the potential risks associated with topical glucocorticoids but also emphasizes the importance of a thorough medication history, including over-the-counter products, in diagnosing ICS.
Case presentation
A 25-year-old male was admitted on December 25, 2019, with a chief complaint of blood pressure (190/110 mmHg) for 1 week. The patient’s blood pressure was measured at 140/102 mmHg during a routine physical examination 3 years ago, without dizziness headache, blurred vision, and did not pursue further evaluation or treatment. Notably, he had a history of psoriasis for more than 10 years. During the first year of diagnosis, he received intermittent intravenous dexamethasone therapy for one year, which was discontinued due to side effects including weight gain and facial acne. For the past three years, he had been taking oral acitretin capsules (10 mg twice daily) and denied the use of any oral or intravenous glucocorticoids. He denied any history of known drug or food allergies, as well as smoking, or alcohol abuse.
Upon admission, physical examination showed the height of the patients was 1.8 m, the weight was 89 kg, and the BMI was 27.4 kg/m2, pulse rate was 68 beats per minute, arterial blood pressure was 192/125 mmHg. He had a moon face, buffalo hump, central obesity, facial acne (Figure 1A). The skin was visibly red, thin and scaly. Broad violaceous striae on the skin of chest, back, abdomen and proximal thighs (Figure 1B). Male secondary sexual characteristics were normal.
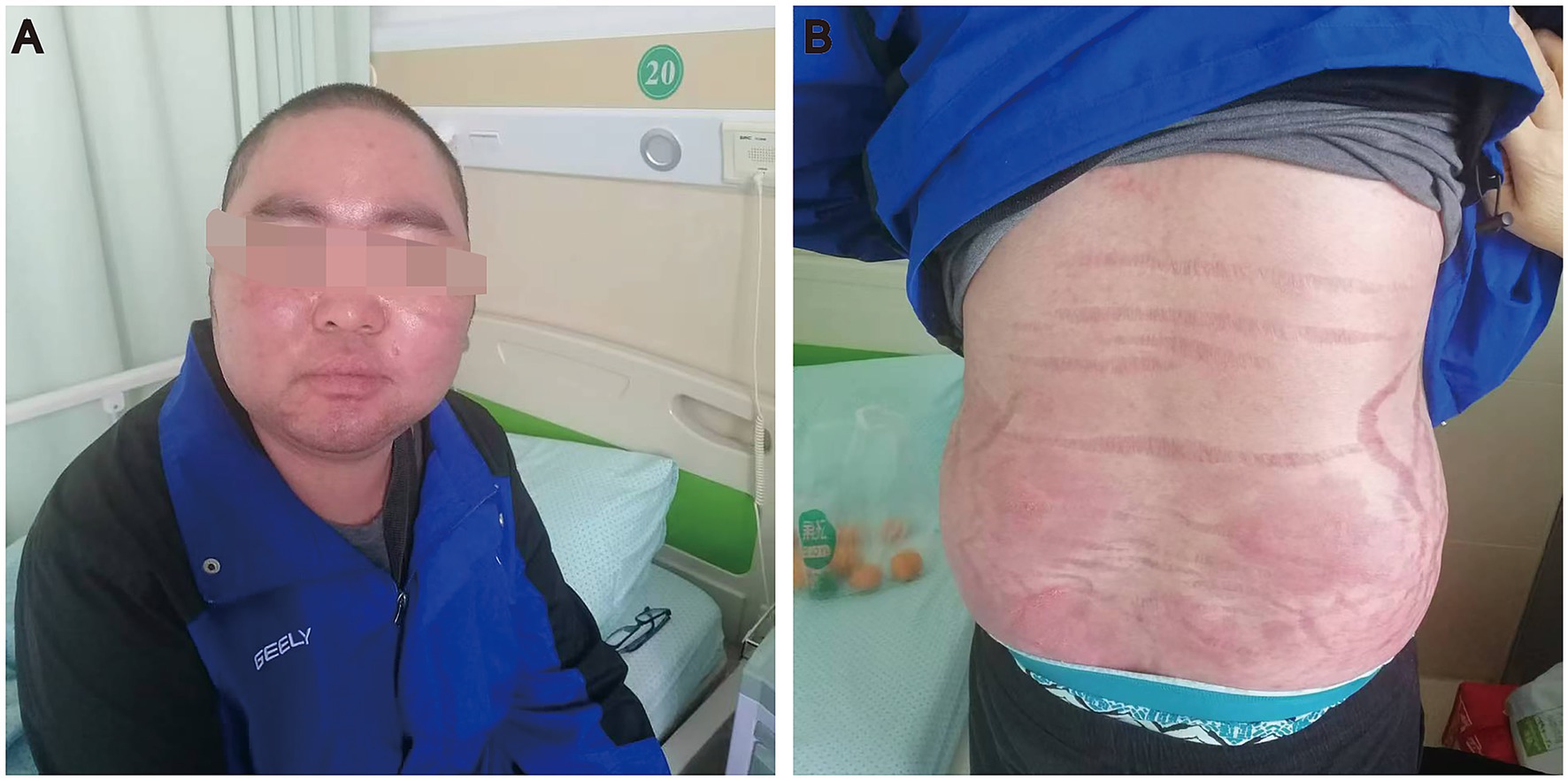
Figure 1. A case of iatrogenic Cushing’s syndrome due to the administration of topical clobetasol propionate. (A) He had a moon face and facial acne. (B) Broad violaceous striae on the waist and abdomen.
Laboratory tests indicated eosinophil ratio (1.9%, ref: 0.4–8.0%), blood potassium (3.48 mmol/L, ref: 3.5–5.1 mmol/L), thyroid-stimulating hormone (TSH, 0.4 μIU/L, ref: 0.55–4.78 μIU/L). The plasma adrenocorticotropic hormone (ACTH) levels were <5.0 pg/mL at 08:00 a.m. (ref: 0–46 pg/mL), <5.0 pg/mL at 04:00 p.m. The cortisol (COR) levels were <5.0 ng/mL at 08:00 a.m. (ref: 52.7–224.5 ng/mL), <5.0 ng/mL at 04:00 p.m. (ref: 34.4–167.6 ng/mL). The 24-h urinary free cortisol (UFC) was measured at <5.0 μg/24 h (ref: 19.3–317.5 μg/24 h). Hemogram, liver and kidney functions, blood glucose levels, blood lipids, serum electrolyte and urine examination were within normal limits. The upright aldosterone-to-renin ratio, 24 h urine vanillylmandelic acid were normal. An analysis of bone mineral density revealed decreased bone density in the hip joint as well as lumbar vertebrae. Color Doppler ultrasound did not reveal apparent abnormalities in the renal arteries, kidneys, and adrenal glands. Adrenal CT (computed tomography), and pituitary MRI (magnetic resonance imaging) showed no abnormality.
The circadian rhythm of cortisol was disrupted, and ACTH levels were markedly suppressed. Detailed history-taking revealed the patient had a history of using “Compendium of Materia Medica Herbal Antibacterial Cream” (6 g/day) for psoriasis within the past 5 years. Additionally, over the past month, he had intermittently used a “antibacterial ointment” (classified as non-medical product). At this point, we strongly suspected that these topical products might be the cause of his condition. Both creams were sent for laboratory analysis, and both were found to contain clobetasol propionate. Specifically, the “Compendium of Materia Medica Herbal Antibacterial Cream” contained approximately 0.02% clobetasol propionate. Due to the limited sample volume of the “Antibacterial Ointment,” its clobetasol propionate content could not be quantified. Based on these findings, the patient was definitively diagnosed with ICS.
The best therapy for ICS is to taper exogenous steroids (1). Sudden withdrawal may precipitate adrenal crisis, therefore, we recommended that the patient gradually reduce the use of the “cream.” The dosage is halved every 3 days until discontinuation. Concurrently, the patient was prescribed take 150 mg of irbesartan and 30 mg of nifedipine controlled release tablets daily for blood pressure management.
After discharge, the patient was advised to gradually taper off the dosage of clobetasol propionate-containing creams and regularly attend outpatient clinics for monitoring of blood cortisol, ACTH, electrolytes, and blood pressure. However, due to concerns about the condition, the patient abruptly discontinued the “cream” on his own. The patient returned for a follow-up seven months post-discharge. Blood pressure was within normal limits, and laboratory tests revealed the following results: the COR levels were 38.61 ng/mL at 08:00 a.m., 38.68 ng/mL at 04:00 p.m.; the ACTH levels were 50.50 pg/mL at 08:00 a.m., 31.70 pg/mL at 04:00 p.m.
Discussion
Prolonged exposure to inappropriately high levels of exogenous glucocorticoids can lead to ICS, most commonly associated with the use of oral steroids. ICS may also result from the inadvertent ingestion of substances containing glucocorticoids, such as some over-the-counter medications, including herbal products (5). Which is often unexpected and insidious, many patients without overt complications may remain undiagnosed (6), and the diagnosis is frequently delayed until severe complications, such as adrenal crises or sepsis, arise (2, 6).
The patient developed ICS following prolonged unsupervised use of a topical herbal ointment containing clobetasol propionate. To differentiate from endogenous causes of hypercortisolism—such as Cushing’s disease, adrenal cortisol-producing tumors, ectopic ACTH secretion, or apparent mineralocorticoid excess—laboratory evaluation revealed suppressed morning ACTH and COR levels, and reduced urinary free cortisol. Additionally, normal aldosterone-to-renin ratio helped exclude primary aldosteronism. The biochemical profile is consistent with exogenous glucocorticoid-induced hypothalamic-pituitary-adrenal (HPA) axis suppression, mediated by chronic negative feedback inhibition that reduces pituitary ACTH secretion. Consequently, the observed clinical features of hypercortisolism are attributable to the external glucocorticoid exposure, while the biochemical findings reflect impaired endogenous cortisol production due to HPA axis suppression.
Clobetasol propionate, a potent topical corticosteroid, can be absorbed through intact skin (7) and has demonstrated effective and rapid healing of psoriasis (8). Its use must be well instructed, considering factors such as dosage, body surface area coverage, frequency of application, site of application, age, and skin quality (9). Children and infants have a higher body surface area-to-weight ratio hence are more likely develop adrenal suppression due to systemic absorption (10, 11). Moreover, the use in the skin fold areas may further increase the risk of local side effects (10). Studies suggest that daily application of 20 mg clobetasol propionate may exert more significant systemic effects than 60 mg of oral prednisone (12). Allenby et al. (13) found that application of more than 50 g per week of clobetasol propionate cream leads to secondary adrenal failure, while Ohman et al. (14) found that adrenal suppression following low-dose topical clobetasol propionate. The topical use of clobetasol propionate at a dose as low as 2 g/day (0.05% cream) can cause HPA suppression within a few days (8). A single application of 25 g clobetasol propionate in psoriasis patients resulted in suppressed cortisol levels persisting for up to 96 h (15). Additionally, van Velsen et al. (7) reported that daily application of 20–30 mg of 0.05% clobetasol propionate cream may led to serum drug concentrations of 0.173–4.504 ng/mL and adrenal suppression. Ghirardo et al. (16) recommend that the safe blood concentration of clobetasol propionate should be below 0.41 ng/mL, or more conservatively, below 0.173 ng/mL. However, the safe dosages of topical corticosteroids remain understudied (17). In the present case, the patient applied approximately 1.2 mg of clobetasol propionate daily to psoriatic lesions involving more than 30% of body surface area, including the trunk, limbs, and face. The impaired skin barrier in plaque psoriasis, combined with the high permeability of facial skin—where the stratum corneum is thin and follicular density is elevated—significantly enhanced systemic absorption. The extensive and permeable treatment area, coupled with prolonged use, resulted in sufficient systemic exposure to cause HPA axis suppression and ICS.
The patient presented with central obesity, striae, moon face, hypertension, hypokalemia, osteoporosis, and HPA axis suppression. Although topical steroid-induced hypertension is rarely reported (17), similar cases—such as infants applying potent steroids like clobetasol over weeks to months—have demonstrated adrenal suppression and systemic complications, with HPA axis recovery typically occurring within several months (18). Following discharge, the patient was placed on a combined topical regimen consisting of calcipotriol ointment for long-term maintenance and dienestrol cream, which was gradually tapered off. Due to the patient’s diagnosis of moderate-to-severe plaque psoriasis, secukinumab was later introduced as systemic therapy to achieve sustained disease control.
Laboratory analysis showed that the presence of clobetasol propionate in this “herbal cream,” which may be the active ingredient of glucocorticoid in the herbal medicine or the incorporation of exogenous steroid hormones. Many products marketed as “natural herbal remedies” may contain undeclared pharmaceutical ingredients, including non-steroidal anti-inflammatory drugs, oral hypoglycemic agents, antihistamines, and sildenafil, with glucocorticoids being the most common (19). In this instance, the patient initially chose this “herbal cream” based on the belief that it was “natural” and therefore safe. Although instructed to taper the cream gradually after discharge and to return for regular monitoring of COR, ACTH, electrolytes, and blood pressure, he discontinued use abruptly due to anxiety regarding his condition. This deviation from medical guidance reflects emotional distress and underscores the need for improved patient education on the risks of unregulated topical products.
In conclusion, physicians must remain vigilant about the potential side effects of potent corticosteroids, particularly in patients with psoriasis. Even low-concentration clobetasol propionate cream can be absorbed through the skin, leading to ICS. Medication needs to be taken in consultation with specialists and closely monitored, including with herbal medicines, rather than resorting to unsupervised self-medication. Furthermore, we also call for stricter regulation of herbal and over-the-counter products to ensure patient safety.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Cixi People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual (s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JC: Writing – original draft. JS: Writing – original draft. CW: Data curation, Writing – original draft. HZ: Data curation, Resources, Writing – original draft. WL: Methodology, Resources, Writing – review & editing. SW: Data curation, Validation, Writing – original draft. SD: Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the University-Level Higher Education Teaching Reform Project of Wenzhou Medical University in 2024 (No. JG2024117).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chaudhry, HS, and Singh, G. Cushing syndrome In: StatPearls. Treasure Island, FL: StatPearls Publishing (2025)
2. Sahıp, B, Celık, M, Ayturk, S, Kucukarda, A, Mert, O, Dıncer, N, et al. Iatrogenic Cushing’s syndrome after topical steroid therapy for psoriasis. Indian J Dermatol. (2016) 61:120. doi: 10.4103/0019-5154.174094
3. Nair, AB, Kumar, S, Dalal, P, Nagpal, C, Dalal, S, Rao, R, et al. Novel dermal delivery cargos of clobetasol propionate: an update. Pharmaceutics. (2022) 14:383. doi: 10.3390/pharmaceutics14020383
4. Rendon, A, and Schäkel, K. Psoriasis pathogenesis and treatment. Int J Mol Sci. (2019) 20:1475. doi: 10.3390/ijms20061475
5. Dunn, C, Amaya, J, and Green, P. A case of iatrogenic Cushing’s syndrome following use of an over-the-counter arthritis supplement. Case Rep Endocrinol. (2023) 2023:1–3. doi: 10.1155/2023/4769258
6. Cristante, J, and Chabre, O. Factitious, or iatrogenic but unexpected Cushing’s syndrome. Ann Endocrinol. (2023) 84:370–2. doi: 10.1016/j.ando.2023.03.007
7. van Velsen, SGA, De Roos, MP, Haeck, IM, Sparidans, RW, and Bruijnzeel-Koomen, CAFM. The potency of clobetasol propionate: serum levels of clobetasol propionate and adrenal function during therapy with 0.05% clobetasol propionate in patients with severe atopic dermatitis. J Dermatol Treat. (2012) 23:16–20. doi: 10.3109/09546634.2010.534127
8. Sidgiddi, S, Naqvi, SMH, Shenoy, M, Balraj, DN, Kothari, J, Gupta, S, et al. Efficacy and safety of novel formulation of clobetasol propionate 0.025% cream in Indian moderate-to-severe psoriasis patients: phase-2a, randomized 3-arm study. Dermatol Ther. (2021) 11:1717–32. doi: 10.1007/s13555-021-00591-z
9. Pels, R, Sterry, W, and Lademann, J. Clobetasol propionate—where, when, why? Drugs Today. (2008) 44:547–57. doi: 10.1358/dot.2008.44.7.1122221
10. Kalb, RE, Bagel, J, Korman, NJ, Lebwohl, MG, Young, M, Horn, EJ, et al. Treatment of intertriginous psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. (2009) 60:120–4. doi: 10.1016/j.jaad.2008.06.041
11. Coondoo, A, Phiske, M, Verma, S, and Lahiri, K. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol Online J. (2014) 5:416–25. doi: 10.4103/2229-5178.142483
12. Negrini, S, Murdaca, G, Ferone, D, and Borro, M. Adult iatrogenic Cushing’s syndrome induced by topical skin corticosteroid misuse. Therapies. (2019) 74:547–9. doi: 10.1016/j.therap.2019.03.005
13. Allenby, CF, Main, RA, Marsden, RA, and Sparkes, CG. Effect on adrenal function of topically applied clobetasol propionate (dermovate). Br Med J. (1975) 4:619–21. doi: 10.1136/bmj.4.5997.619
14. Ohman, EM, Rogers, S, Meenan, FO, and McKenna, TJ. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. (1987) 80:422–4. doi: 10.1177/014107688708000709
15. Hehir, M, Du Vivier, A, Eilon, L, Danie, MJ, and Shenoy, EV. Investigation of the pharmacokinetics of clobetasol propionate and clobetasone butyrate after a single application of ointment. Clin Exp Dermatol. (1983) 8:143–51. doi: 10.1111/j.1365-2230.1983.tb01758.x
16. Ghirardo, S, Nardi, LD, Tommasini, A, Barbi, E, and Tornese, G. Topical clobetasol: an overlooked cause of Cushing syndrome. Endocr Metab Immune Disord Drug Targets. (2021) 21:2300–2. doi: 10.2174/1871530321666210426131423
17. Sachdeva, S, Arora, P, Kulhari, A, and Sardana, K. Iatrogenic Cushing’s syndrome due to topical steroid abuse in a child with psoriasis presenting as septicaemia. Dermatol Ther. (2020) 33:e13514. doi: 10.1111/dth.13514
18. Kilci, F, and Sarıkaya, E. Pediatric iatrogenic Cushing’s syndrome: a series of seven cases induced by topical corticosteroid use. J Pediatr Endocrinol Metab. (2025) 38:863–7. doi: 10.1515/jpem-2025-0032
Keywords: iatrogenic Cushing’s syndrome, clobetasol propionate, psoriasis, corticosteroids, herbal cream
Citation: Chen J, Sun J, Wang C, Zhao H, Li W, Wang S and Du S (2025) Iatrogenic Cushing’s syndrome induced by topical steroid abuse in a patient with psoriasis: a case report. Front. Med. 12:1591869. doi: 10.3389/fmed.2025.1591869
Edited by:
Sergio Crovella, Qatar University, QatarReviewed by:
David Croitoru, University of Toronto, CanadaEmre Sarıkaya, Kocaeli City Hospital, Türkiye
Copyright © 2025 Chen, Sun, Wang, Zhao, Li, Wang and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shujun Wang, YW5uaWVtb3RoZXJAMTYzLmNvbQ==; Sina Du, ZHVzaW5hMTk4NUAxNjMuY29t
†These authors have contributed equally to this work
 Jianwei Chen1†
Jianwei Chen1† Sina Du
Sina Du