Abstract
Background:
This study aimed to evaluate the efficacy and safety of tenofovir alafenamide fumarate (TAF) in nucleos(t)ide analogue (NA)-experienced patients with chronic hepatitis B (CHB) who exhibited partial virological response (PRT) or low-level viremia (LLV).
Methods:
This single-center, retrospective, real-world study enrolled NA-experienced CHB patients who were switched to TAF treatment. Patients were categorized into the PRT (HBV DNA > 2,000 IU/mL) or LLV (20 IU/ mL < HBV DNA ≤ 2,000 IU/mL) groups according to baseline HBV DNA levels. The dynamic changes in HBV DNA, HBsAg, HBeAg, ALT, and APRI were analyzed after switching to TAF.
Results:
A total of 91 CHB patients with prior NA treatment and detectable HBV DNA after at least 48 weeks of therapy were enrolled and subsequently switched to TAF. Among them, 24 patients had PRT, and 67 patients had LLV. The complete virological response rate (HBV DNA < 20 IU/mL) in the PRT group was 29.1% at week 24 and 75.0% at week 48; in the LLV group, it was 76.1% and 88.1%, respectively. Both groups showed a decline in HBeAg levels from baseline to week 24 and 48. In the PRT group, HBsAg levels decreased by 9.0% and 5.0% at week 24 and 48, respectively; in the LLV group, the reductions were 2.1% and 3.6%. The ALT normalization rate increased by 24.2% at week 48 compared with baseline. Additionally, eGFR levels improved after switching to TAF. No serious adverse events (SAEs) or deaths related to adverse events were observed.
Conclusion:
This real-world study suggests that switching to TAF is an effective and well-tolerated therapeutic strategy for NA-experienced CHB patients with PRT or LLV, offering a promising approach for treatment optimization.
1 Introduction
It is estimated that approximately 86 million people in China are living with persistent hepatitis B virus (HBV) infection, among whom around 28 million are diagnosed with chronic hepatitis B (CHB) (1, 2). Persistent HBV infection significantly increases the risk of developing liver cirrhosis (LC) and hepatocellular carcinoma (HCC) (3). A high serum HBV-DNA load is an important factor in the development of HCC in patients with persistent HBV infection (4, 5). Meanwhile, serum hepatitis B surface antigen (HBsAg) levels are also an important determinant of the risk of HCC in HBV-infected patients, especially in CHB patients with serum HBsAg levels of 1,000 IU/mL or higher, where the cumulative incidence of HCC is significantly higher than in patients with serum HBsAg levels < 1,000 IU/mL (6). Therefore, reducing serum HBV DNA and HBsAg levels is key to preventing the progression of chronic serious complications such as LC and HCC in patients with persistent HBV infection.
Currently, nucleoside analogue/nucleotide analogue (NA) treatment can significantly reduce HBV DNA levels. However, low-level viremia (LLV) remains a common issue in some patients undergoing long-term NA therapy. For instance, some patients treated with entecavir (ETV) are still in a state of LLV and poor response (PRT) to antiviral therapy (7). Moreover, ETV has limited efficacy in reducing serum HBsAg levels (8, 9). Studies have shown that the incidence of disease progression and adverse outcomes in this population is higher than in patients with complete virologic response (10); and long-term use of tenofovir disoproxil fumarate (TDF) can cause bone and kidney damage (11, 12).
Tenofovir alafenamide fumarate (TAF), approved in China in November 2018, is recommended as a first-line antiviral therapy in the 2019 edition of the Chinese guidelines for the prevention and treatment of chronic hepatitis B (13). The guidelines also recommend TAF as a switching strategy for CHB patients with LLV or PRT. Therefore, this single-center, retrospective real-world study aims to evaluate the efficacy and safety of switching to TAF in treatment-experienced CHB patients with LLV or PRT in western China.
2 Materials and methods
2.1 Patients
This retrospective study included 95 treatment-experienced patients from the Department of Liver Diseases at the Second Hospital of Lanzhou University, between January 2019 and January 2021. All patients met the following inclusion criteria: (1) chronic HBV infection was diagnosed in accordance with the criteria of “Chinese guidelines for the prevention and treatment of chronic hepatitis B” (14); (2) prior NA treatment duration ≥ 48 weeks; (3) HBV DNA > 20 IU/mL; (4) age ≥ 18 years.
Exclusion criteria included the following: (1) co-infection with other hepatitis viruses (hepatitis A, C, D, or E); (2) presence of other liver diseases, including alcoholic liver disease, non-alcoholic fatty liver disease, drug-induced liver injury, autoimmune liver disease, or inherited metabolic disorders; (3) severe liver conditions, such as acute or chronic liver failure; (4) coexisting severe cardiovascular disease, chronic renal failure [estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2], or hematologic disorders; (5) co-infection with human immunodeficiency virus (HIV); (6) pregnancy.
2.2 Measurements
Serum HBV DNA were measured by Roche Cobas AmpliPrep/CobasTaqMan (lower limit of detection is 20 IU/mL), serum HBsAg and HBeAg quantification were measured by Roche Cobas AmpliPrep/CobasTaqMan, serum liver biochemistry indicators were detected by Roche Cobas 8000 automatic biochemical instrument (normal range of ALT: 0–50 U/L, normal range of AST: 0–40 U/L), blood test were detected by Mindray BC-6900 blood cell analyzer, eGFR was determined by the Cockcroft-Gault method.
2.3 Study subgroups
2.3.1 PRT group
CHB patients showed serum HBV DNA > 2,000 IU/mL with NA treatment for at least 48 weeks; LLV group: CHB patients showed serum HBV DNA < 2,000 IU/mL but still detectable (detection limit of 20 IU/mL) with NA treatment for at least 48 weeks.
2.4 Clinical outcomes
2.4.1 Primary endpoints
(1) Complete response (CVR) defined as serum HBV DNA < 20 IU/mL at 24 ± 2w and 48 ± 2w of TAF treatment; (2) subgroup analysis of the rate of complete virological response (CVR); the degree of serum HBV DNA level decreased from baseline in PRT and LLV groups; (3) the degree of serum HBeAg decreased from baseline and the rate of HBeAg negative/seroconversion in all patients; (4) subgroup analysis of the degree of serum HBeAg decreased from baseline and the rate of HBeAg negative/seroconversion in the PRT and LLV groups.
2.4.2 Secondary endpoints
(1) The degree of serum HBsAg decreased from baseline and the rate of HBsAg negative/seroconversion in all patients; (2) subgroup analysis of degree of serum HBsAg decreased from baseline and the rate of HBsAg negative/seroconversion in the PRT and LLV groups; (3) comparing the decreased degree of ALT and ALT normalization rate; (4) comparison of the aspartate aminotransferase to platelet ratio index [APRI = AST/ULN × 100/PLT (×109/L)].
Primary safety endpoint: the occurrence of adverse reactions (AEs) during treatment during the treatment.
Secondary safety endpoint: the changes in renal function, measured by eGFR.
2.5 Statistical analysis
SPSS 25.0 software was applied for the statistical analysis of the data. The χ2 test and Fisher’s exact test were used for the counting data. Use the median and upper and lower quartiles [M (Q1, Q3)] for data that does not conform to the normal distribution. The differences between the three groups were compared using the Friedman rank sum test, and the differences between the two groups were compared using the Wilcoxon rank sum test. p < 0.05 indicates that the difference is statistically significant, We adjusted the p-values for multiple comparisons using the Bonferroni method, p < 0.05 indicates that the difference is statistically significant. Use GraphPad Prime 8.0 to draw graphics.
3 Results
3.1 Baseline characteristics
A total of 95 CHB treatment-experienced patients who met the criteria were enrolled, of whom 4 dropped out of the study. The number of patients in the PRT and LLV groups was 24 and 67, and the baseline characteristics of patients were showed in Table 1. The LLV group had a lower proportion of male sex, lower HBV DNA levels, lower HBeAg levels, lower ALT levels, and a lower APRI score, while a higher proportion of ETV experienced treatment (p < 0.05).
Table 1
| Baseline characteristics | Total (n = 91) | PRT group (n = 24) | LLV group (n = 67) | p-value |
|---|---|---|---|---|
| Age, years | 38.0 ± 10.0 | 38.1 ± 11.5 | 39.5 ± 9.6 | 0.610 |
| Male sex, n (%) | 56 (61.5) | 19 (79.2) | 37 (52.2) | 0.039 |
| Comorbidity | ||||
| HCC, n (%) | 1 (1.1) | 1 (4.2) | 0 (0) | NA |
| Type 2 diabetes, n (%) | 3 (3.3) | 2 (8.3) | 1 (1.5) | NA |
| Hypertension, n (%) | 2 (2.2) | 1 (4.2) | 1 (1.5) | NA |
| CKD, n (%) | 1 (1.1) | 1 (4.2) | 0 (0) | NA |
| Hypothyroidism, n (%) | 1 (1.1) | 1 (4.2) | 0 (0) | NA |
| Experienced ETV therapy, n (%) | 65 (71.4) | 8 (33.3) | 57 (85.1) | <0.001 |
| HBV DNA, log IU/mL | 2.2 (1.7, 3.6) | 6.1 (4.2, 7.3) | 1.9 (1.8, 2.4) | <0.001 |
| HBsAg, log IU/mL | 3.6 (3.3, 3.9) | 3.6 (3.2, 4.2) | 3.6 (3.3, 3.9) | 0.449 |
| HBeAg, log IU/mL | 0.7 (−0.9, 2.0) | 1.8 (0.4, 2.9) | 0.5 (−1.0, 1.8) | 0.031 |
| HBeAg positive, n (%) | 62 (68.1) | 19 (79.2) | 43 (64.2) | 0.176 |
| ALT, U/L | 35.0 (20.0, 73.0) | 75. 0 (46.0, 146.3) | 25.0 (18.0, 49.0) | <0.001 |
| eGFR, mL/min/1.73m2 | 105.6 (98.6, 112.6) | 104.4 (94.6, 110.8) | 106.6 (100.0, 113.4) | 0.397 |
| APRI score | 0.5 (0.3, 1.0) | 1.0 (0.5, 2.1) | 0.4 (0.3, 0.7) | 0.002 |
Baseline characteristics of enrolled patients.
3.2 Virologic response
Compared with baseline, the levels of HBV DNA at 24 ± 2w and 48 ± 2w showed a significant downward trend (χ2 = 116.462, p < 0.001) after switching to TAF treatment, among which, the differences were statistically significant at 24 ± 2w and 48 ± 2w compared with baseline (Z = −7.145, p < 0.001 adjusted p-value < 0.001; Z = −7.948, p < 0.001, adjusted p-value < 0.001). The differences were statistically significant 48 ± 2w at compared with 24 ± 2w (adjusted p-value < 0.05). The decrease degree of serum HBV DNA at 24 ± 2w and 48 ± 2w compared with baseline was 44.0 and 51.8% (Table 2 and Figure 1a). The CVR rates were 63.7 and 84.6% at 24 ± 2w and 48 ± 2w (Table 2 and Figure 1b).
Table 2
| Treatment response | Total (n = 91) | PRT group (n = 24) | LLV group (n = 67) | p-value |
|---|---|---|---|---|
| HBV DNA (log10 IU/mL) | ||||
| Baseline | 2.2 (1.7, 3.6) | 6.1 (4.2, 7.3) | 2.2 (1.8, 2.4) | <0.001 |
| 24 ± 2w | 1.3 (1.3, 1.7)** | 1.8 (1.3, 2.8)** | 1.3 (1.3, 1.9)** | 0.017 |
| 48 ± 2w | 1.3 (1.3, 1.3)** | 1.3 (1.3, 1.3)** | 1.3 (1.3, 1.3)** | <0.001 |
| Decrease degree of HBV DNA (%) | ||||
| Baseline | — | — | — | |
| 24 ± 2w | 44.0 | 64.8 | 22.6 | |
| 48 ± 2w | 51.8 | 72.0 | 30.0 | |
| CVR rate (n, %) | ||||
| Baseline | — | — | — | |
| 24 ± 2w | 58 (63.7) | 7 (29.2)* | 51 (76.1)* | <0.001 |
| 48 ± 2w | 77 (84.6) | 18 (75.0)* | 59 (88.1)* | 0.185 |
| HBeAg (log10 IU/mL) | ||||
| Baseline | 0.7 (−0.9, 2.0) | 1.8 (0.4, 2.9) | 0.5 (−1.0, 1.8) | 0.031 |
| 24 ± 2w | 0.6 (−0.9, 1.8)* | 1.6 (0.1, 2.0)* | 0.4 (−1.0, 1.5) | 0.021 |
| 48 ± 2w | 0.6 (−0.4, 1.5)* | 1.1 (0.3, 1.8)* | 0.4 (−0.4, 1.3) | 0.058 |
| Decrease degree of HBeAg (%) | ||||
| Baseline | — | — | — | |
| 24 ± 2w | 22.2 | 17.7 | 44.6 | |
| 48 ± 2w | 26.7 | 41.0 | 47.3 | |
| HBsAg (log10 IU/mL) | ||||
| Baseline | 3.6 (3.3, 3.9) | 3.6 (3.2, 4.2) | 3.6 (3.3, 3.9) | 0.449 |
| 24 ± 2w | 3.5 (3.1, 3.9)** | 3.4 (2.9, 3.7)* | 3.6 (3.1, 3.9)** | 0.211 |
| 48 ± 2w | 3.5 (3.1, 3.8)** | 3.5 (3.0, 3.8)* | 3.6 (3.1, 3.9)** | 0.836 |
| Decrease degree of HBsAg (%) | ||||
| Baseline | — | — | — | |
| 24 ± 2w | 4.0 | 9.0 | 2.1 | |
| 48 ± 2w | 4.1 | 5.0 | 2.1 | |
Virological and serological response of TAF treatment.
*Denotes p < 0.05 compared with baseline level. **Denotes p < 0.001 compared with baseline level.
Figure 1
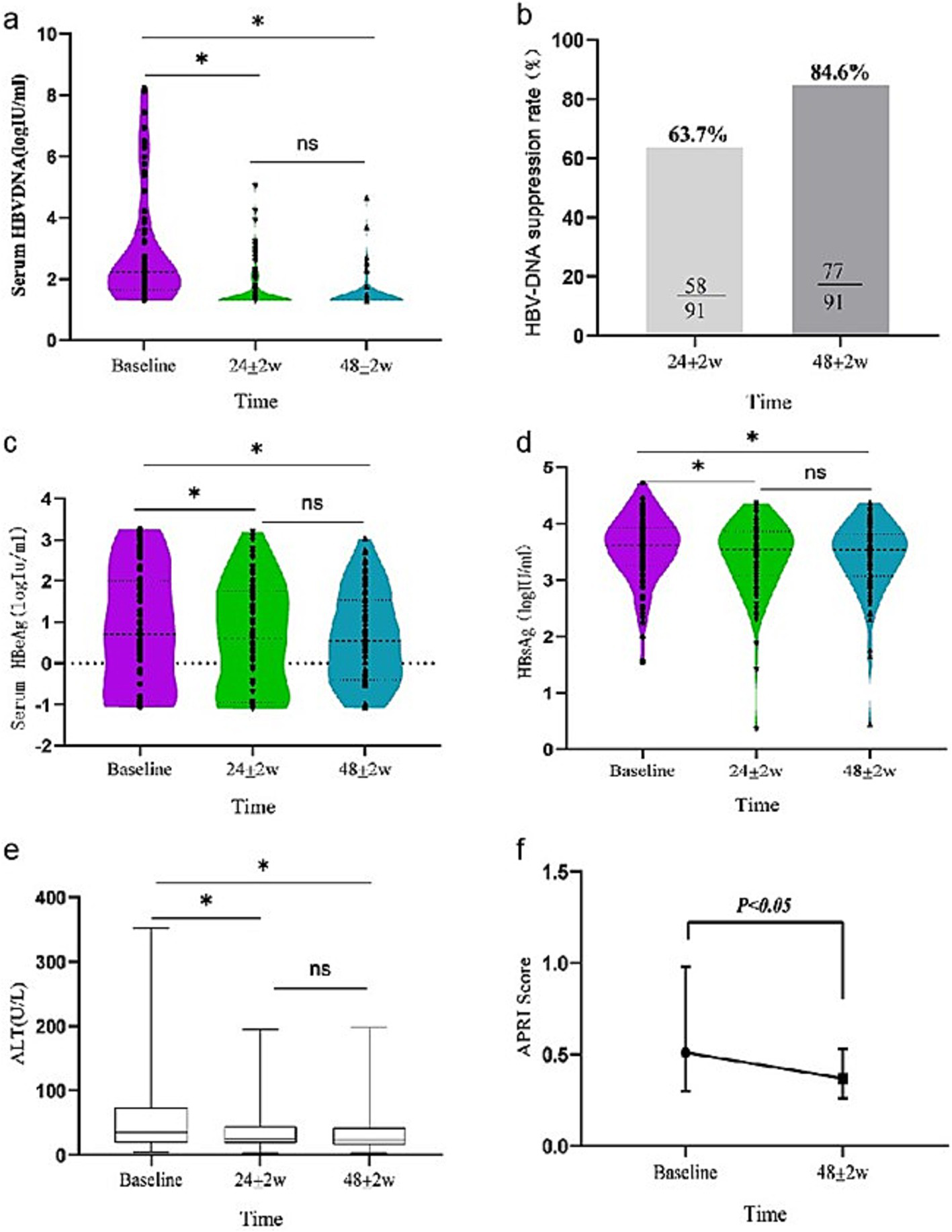
Dynamic change of virological [(a) serum HBV DNA level, (b) HBV DNA suppression rate], serological [(c) serum HBeAg level, (d) serum HBsAg level], and biochemical biomarker [(e) ALT level, (f) APRI index] response during 24 ± 2w and 48 ± 2w of TAF therapy in all patients.
Subgroup analysis showed that, in the PRT group, compared with baseline, HBV DNA was reduced significantly in both timepoints at 24 ± 2w and 48 ± 2w (χ2 = 43.888, p < 0.001) (Table 2 and Figure 2a). The CVR rates were 29.1 and 75.0% at 24 ± 2w and 48 ± 2w of switch therapy (Table 2 and Figure 2c). In the LLV group, HBV DNA level was significantly decreased at 24 ± 2w and 48 ± 2w (χ2 = 87.484, p < 0.001) (Table 2 and Figure 2a). The CVR rates were 76.1 and 88.1% at 24 ± 2w and 48 ± 2w (Table 2 and Figure 2c). As of the HBV DNA decreased degree comparison, in the PRT group, HBV DNA decreased degrees were 64.8 and 72.0% at 24 ± 2w and 48 ± 2w. In the LLV group, the decreased degree was 22.6 and 30.0% (Table 2).
Figure 2
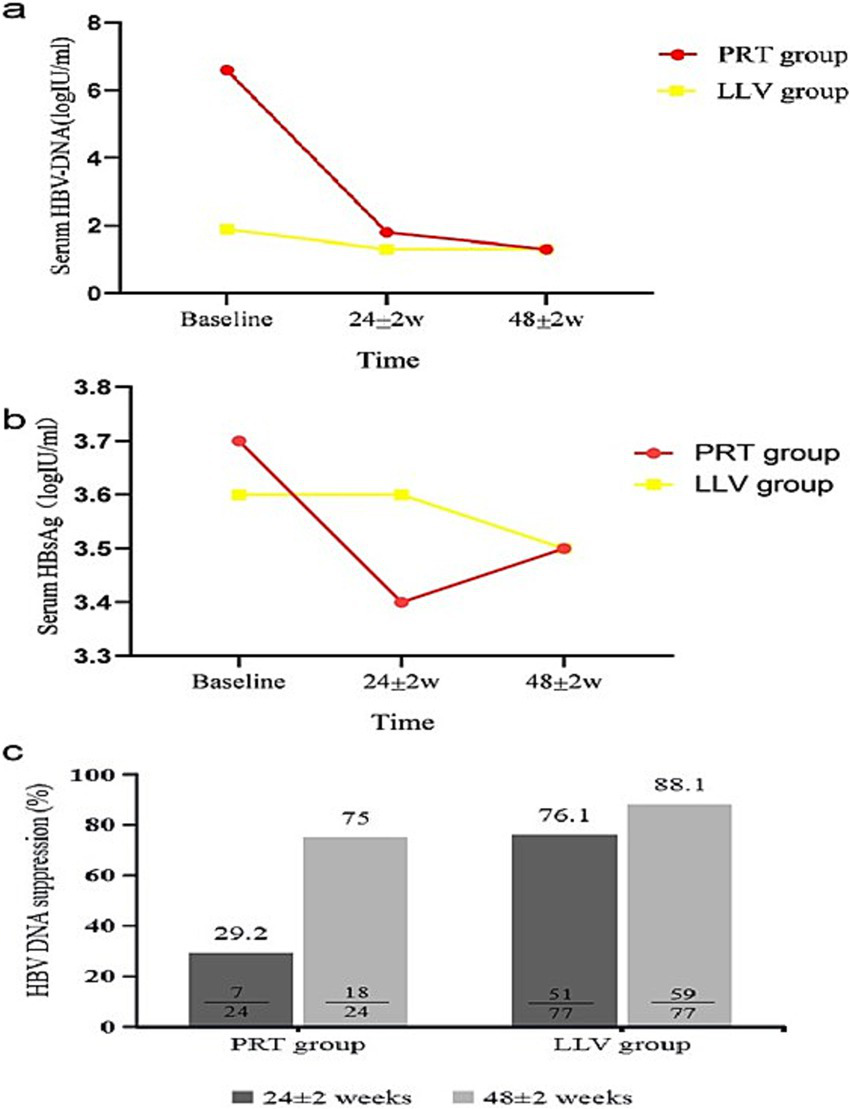
Dynamic change of virological [(a) serum HBV DNA level, (c) HBV DNA suppression rate] and serological biomarker [(b) serum HBsAg level] response during 24 ± 2w and 48 ± 2w of TAF therapy in PRT group and LLV group.
3.3 Serological response
In all enrolled patients, HBeAg showed a decreasing trend at 24 ± 2w and 48 ± 2w (χ2 = 8.715, p = 0.013) compared with baseline. The differences were statistically significant at 24 ± 2w and 48 ± 2w compared with baseline (Z = −3.688 p < 0.001, adjusted p-value = 0.049; Z = −2.581 p = 0.010 adjusted p-value = 0.079). The differences were not statistically significant 48 ± 2w at compared with 24 ± 2w (adjusted p-value = 0.042). The decreased degrees of HBeAg were 22.2 and 26.7% at 24 ± 2w and 48 ± 2w. HBeAg negative rates were 3.2 and 8.1% at 24 ± 2w and 48 ± 2w (Table 2 and Figure 1c).
Subgroup analysis showed that (Table 2), in the PRT group, HBeAg was decreased significantly at 24 ± 2w and 48 ± 2w (χ2 = 13.000, p = 0.002) compared with baseline. At 24 ± 2w and 48 ± 2w, HBeAg decreased by 17.7 and 41.0%. In the LLV group, HBeAg were also decreased significantly (χ2 = 3.630, p = 0.163), while HBeAg decreased degree were 44.6 and 47.3% at 24 ± 2w and 48 ± 2w.
As for the HBsAg response, the change in serum HBsAg after switching to TAF treatment was significant (χ2 = 28.424, p < 0.001). Compared with baseline, serum HBsAg decreased by 4.0 and 4.1% in 24 ± 2w and 48 ± 2w. Only one patient experienced HBsAg clearance, HBsAg rate was 1.1% (Table 2 and Figure 1d).
Subgroup analysis showed that (Table 2 and Figure 2b), the differences of HBsAg were statistically significant in the PRT group (χ2 = 13.083, p < 0.001). Compared with baseline, HBsAg decreased by 9.0 and 5.0% at 24 ± 2w and 48 ± 2w. In the LLV group, the differences in HBsAg at 24 ± 2w and 48 ± 2w were both statistically significant (Z = −4.446 p < 0.001, Z = −6.513, p < 0.001) compared with baseline. HBsAg decreased degrees were 2.1 and 3.6% at 24 ± 2w and 48 ± 2w.
3.4 Biochemical change
All included patients showed significant changes in ALT levels after switching to TAF treatment (Table 2 and Figure 1e). Compared with baseline, ALT levels decreased at 24 ± 2w and 48 ± 2w (χ2 = 28.424, p < 0.001). The differences were statistically significant at 24 ± 2w and 48 ± 2w compared with baseline (adjusted p-value < 0.005; adjusted p-value < 0.05). The differences were not statistically significant 48 ± 2w at compared with 24 ± 2w (adjusted p-value > 0.05). After 48 ± 2w of TAF treatment, the ALT normalization rate increased by 24.2% from baseline.
3.5 APRI change
The APRI scores at 24 ± 2w and 48 ± 2w were 0.5 (0.3, 1.0) and 0.4 (0.3, 0.5). There was a statistically significant decrease in APRI scores compared to baseline at 48 ± 2w of TAF replacement treatment (Z = 3.495, p = 0.001) (Figure 1f).
3.6 Safety
The median eGFR at baseline, 24 ± 2w and 48 ± 2w were 105.6 (98.6, 112.6), 104.8 (98.3, 112.5), and 108.8 (101.6, 119.3) mL/min/1.73 m2. There were significantly differences in eGFR at the three observation time points (χ2 = 20.738, p < 0.001), while eGFR levels were significantly higher at 48 ± 2w compared to 24 ± 2w (Z = −4.470, p < 0.001 adjusted p-value < 0.0001).
The overall incidence of adverse reactions at 48 weeks of TAF therapy was 3.2% (3/91), the main adverse reactions including: fatigue (1.1%), loss of appetite (1.1%), and facial erythema (1.1%). There was one patient who discontinued the drug at 12 weeks due to facial erythema and subsided after switching to TDF. No SAEs were observed in this study.
4 Discussion
In the guidelines of all countries, HBV DNA replication should be greatly inhibited to prevent disease progression and HCC occurrence, which lead to improving quality of life and prolonging the survival time of CHB patients, which are the main goals of antiviral therapy (13, 14). However, in real-world clinical practice, only a small portion of patients had detectable HBV DNA after long-term antiviral treatment. According to the Chinese guidelines for the prevention and treatment of chronic hepatitis B (2019 version), patients with HBV DNA levels >2,000 IU/mL after 48 weeks of first-line nucleos(t)ide analogue therapy—after excluding poor adherence and testing error—are considered to have a “poor response” and should be considered for treatment adjustment (13). The American Association for the Study of Liver Diseases (AASLD) 2018 update defines low-level viremia (LLV) as detectable HBV DNA < 2000 IU/mL (detection limit of 10 IU/mL) after 48 weeks of antiviral therapy, but it does not recommend immediate treatment modification in these patients (14). More recently, the European Association for the Study of the Liver (EASL) 2025 guidelines emphasise that patients with persistent detectable HBV DNA after prolonged therapy should undergo a comprehensive assessment—adherence, resistance testing, fibrosis progression—and consideration of treatment switch or intensification, especially in the presence of liver damage or other risk factors (15). These evolving definitions and recommendations across guidelines underline the need for further clinical evidence to guide management of PRT and LLV.
Recently, there were many studies focused on the NA switch to the TAF strategy. Ogawa et al. (16) found that CHB patients treated with ETV switched to TAF for 48 weeks, the virological response rate increased from 75.9 to 96.9%, and eGFR also improved (+0.40 mL/min/1.73m2). In the study of Nguyen et al. (17) found that after an average of 6 years on ETV, switching to TAF treatment increased CVR from 91.9 to 97.2% after 96 weeks. These studies suggested that TAF has promising antiviral efficacy in NA experienced CHB patients. The results of our study can further illustrate that the switch to TAF treatment in CHB treated patients could further inhibit the replication of HBV DNA and increase the CVR rate to 63.7% in 24 ± 2w and 84.6% at 48 ± 2w.
In the study of Li et al. (18), CHB patients with persistent or intermittent LLV status after ETV treatment who switched to TAF could increase the CVR rate by 62.7% for 24 weeks, compared with continuing ETV group, the CVR rate was only 9.3%, suggesting that LLV patients have a higher possibility to achieve CVR after switching to TAF treatment. In our study, we found that the CVR rate was 76.1% in the LLV group after 24 ± 2 weeks of switching to TAF therapy, which was higher than the study by Li et al. (18), and the CVR rate of 48 ± 2 weeks in the LLV group could be further increased to 88.1%. In the PRT group, the CVR significantly increased at 48 ± 2 weeks compared with 24 ± 2 weeks (26.2, 75.0%). Therefore, CHB patients with PRT or LLV status could both benefit from switching to TAF therapy.
The serological response was another major efficacy indicator of antiviral treatment. After switching to TAF therapy, the decreased degree of serum HBeAg was 22.2% for 24 ± 2 weeks, and gradually increased to 26.7% at 48 ± 2 weeks. In the PRT and LLV groups, we found that the decrease degree in the LLV group at 24 ± 2 weeks (44.6%) was significantly higher than that in the PRT group (17.7%), while the decreased degree in the PRT group increased rapidly to 41.0% at 48 ± 2 weeks. Lampertico et al. (19) reported that of 78 HBeAg positive patients who were treatment experienced, after switching to TAF, 6 patients (8%) had HBeAg clearance at 48 weeks. Our study observed a similar result, the HBeAg clearance rate was 8.1% after 48 ± 2 weeks of TAF treatment.
In the study by Uchida et al. (20), the decrease in HBsAg during treatment with ETV in CHB patients was approximately 0.041 log10 IU/mL, and the decreased degree was increased to 0.068 log10 IU/mL after switching to TAF for 48 weeks. Meanwhile, the effect of TAF in reducing serum HBsAg was more significant in patients with baseline HBsAg < 800 IU/mL. In our study, the decreased degree of serum HBsAg was 4.1% after switching to TAF at 48 ± 2 weeks. One patient has achieved HBsAg loss. Subgroup analysis suggested that some patients in the PRT group showed an increase in HBsAg during TAF treatment, but the magnitude of the increase was small, which was considered a fluctuation caused by the test reagent of HBsAg. It can also be affected by the immune status of the host (21). Many factors can influence the level of HBsAg and HBeAg, such as drugs, immune status, which can change during long-term suppression of HBV. Further studies are needed to confirm the changes in serum HBsAg and HBeAg in patients who consistently obtained CVR after switching to TAF treatment.
The serum ALT level reflected the degree of hepatocellular damage. In this study, the ALT normalization rate was significantly increased after 48 ± 2 weeks of TAF treatment, which was similar to the results of the TAF global phase III clinical trial (22). Our further analysis revealed that ALT levels were significantly lower than baseline at week 24 ± 2w, suggesting that TAF can significantly alleviate hepatocyte inflammation while rapidly suppressing viral replication. In addition, we also observed that the APRI score was significantly lower after 48 ± 2 weeks of TAF treatment.
TAF has a better renal safety profile compared to TDF (22, 23). Kaneko et al. (24) found that in CHB patients who developed renal impairment during TDF treatment, the renal function could be improved after switching to TAF, the eGFR levels increased significantly at week 4 and week 24 of TAF treatment. Lampertico et al. (19) also found that TAF improved renal tubular function in CHB patients. In our study, most patients had normal renal function at baseline. Although eGFR levels showed a mild numerical increase after switching to TAF for 48 ± 2 weeks, no renal adverse events were observed. Given the normal baseline renal function, this slight change in eGFR is likely of limited clinical significance, but it further supports the favorable renal safety profile of TAF in treatment-experienced CHB patients.
In addition, as this was a retrospective, single-arm analysis, no concurrent control group was included. The primary objective was to compare virological efficacy among patients with different baseline viral statuses. However, we have incorporated historical control data from our previous LLV cohort, in which the CVR rate at 48 ± 2 weeks without switching was only 16.2% (25), to provide context for our findings.
There are some limitations to our study. Firstly, the patients enrolled in this study were from a single center, resulting in a small number of patients in each subgroup, which may affect the effectiveness of the observed clinical results. Secondly, the observation period of this study was 48 ± 2 weeks, which was insufficient to fully evaluate the efficacy and safety of long-term antiviral treatment with TAF, and further follow-up was needed. Finally, large-scale, multicenter, randomized controlled trials were needed to explore the efficacy and safety of TAF in real-world settings to provide more valuable evidence for the optimized strategy of antiviral regimens for CHB experienced with PRT and LLV.
5 Conclusion
In conclusion, our study observed the efficacy and safety of the TAF therapy in NA-experienced CHB patients with PRT or LLV status in western China and found that switching to TAF could further suppress HBV DNA replication, increase the CVR rate, reduce the HBsAg and HBeAg levels, improve ALT normalization, and renal function. Switching to TAF treatment is a promising choice for an optimized strategy of NA experienced CHB patients with PRT or LLV.
Statements
Author’s note
Part of the result were presented in the form of poster at the 2022 Annual Meeting of the Asian Pacific Association for the Study of the Liver (APASL).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Lanzhou University Second Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XH: Resources, Data curation, Methodology, Writing – review & editing, Investigation, Software, Writing – original draft, Formal analysis, Conceptualization. YK: Writing – review & editing, Investigation, Resources. YLiu: Investigation, Writing – review & editing, Data curation. TL: Data curation, Methodology, Writing – review & editing. AM: Data curation, Investigation, Resources, Writing – review & editing. YLi: Investigation, Writing – review & editing, Resources, Data curation. YZ: Writing – review & editing, Investigation, Data curation, Resources. JL: Data curation, Writing – review & editing, Investigation. LZ: Conceptualization, Project administration, Supervision, Writing – review & editing. GL: Writing – review & editing, Project administration, Conceptualization, Visualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Natural Science Foundation of Gansu Province (21JR1RA146 and 25JRRA608); Lanzhou University Second Hospital “Cuiying Technology Innovation” Program (CY2018-BJ17 and CY2021-QN-A18).
Acknowledgments
The authors want to thank the clinic nurses, clinical laboratory technicians and medical doctors from Lanzhou University Second Hospital for their cooperation with patient’s follow-up during the treatment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
World Health Organization . (2017). Global hepatitis report, 2017. Available online at: https://www.who.int/publications/i/item/9789241565455. (Accessed April 19, 2017)
2.
Razavi-Shearer D Gamkrelidze I Nguyen MH Chen DS van Damme P Abbas Z et al . Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. (2018) 3:383–403. doi: 10.1016/s2468-1253(18)30056-6
3.
Forner A Reig M Bruix J . Hepatocellular carcinoma. Lancet. (2018) 391:1301–14. doi: 10.1016/s0140-6736(18)30010-2
4.
Chen CJ Yang HI Su J Jen CL You SL Lu SN et al . Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. (2006) 295:65–73. doi: 10.1001/jama.295.1.65
5.
Yip TC-F Wong GL-H Chan HL-Y Tse Y-K Lam KL-Y Lui GC-Y et al . HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J Hepatol. (2019) 70:361–70. doi: 10.1016/j.jhep.2018.10.014
6.
Tseng T-C Liu CT Yang HJ Su T-H Wang C-C Chen C-L et al . High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. (2012) 142:1140–1149.e3. doi: 10.1053/j.gastro.2012.02.007
7.
Wong GL-H Wong VW-S Chan H-Y Tse PC-H Wong J Chim AM-L et al . Undetectable HBV DNA at month 12 of entecavir treatment predicts maintained viral suppression and HBeAg-seroconversion in chronic hepatitis B patients at 3 years. Aliment Pharmacol Ther. (2012) 35:1326–35. doi: 10.1111/j.1365-2036.2012.05098.x
8.
Murata K Asano M Matsumoto A Sugiyama M Nishida N Tanaka E et al . Induction of IFN-λ3 as an additional effect of nucleotide, not nucleoside, analogues: a new potential target for HBV infection. Gut. (2018) 67:362–71. doi: 10.1136/gutjnl-2016-312653
9.
Arends P Sonneveld MJ Zoutendijk R Carey I Brown A Fasano M et al . Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: limited role for risk scores in Caucasians. Gut. (2014) 64:1289–95. doi: 10.1136/gutjnl-2014-307023
10.
Lee HW Kim BK . How does low-level viremia affect the prognosis of patients with chronic hepatitis B?Clin Mol Hepatol. (2020) 26:376–7. doi: 10.3350/cmh.2020.0121
11.
Hall AJ Hendry BM Nitsch D Connolly JE . Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. (2011) 57:773–80. doi: 10.1053/j.ajkd.2011.01.022
12.
Maggi P Montinaro V Leone A Fasano M Volpe A Bellacosa C et al . Bone and kidney toxicity induced by nucleotide analogues in patients affected by HBV-related chronic hepatitis: a longitudinal study. J Antimicrob Chemother. (2014) 70:1150–4. doi: 10.1093/jac/dku502
13.
Wang G Duan Z . Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for prevention and treatment of chronic hepatitis B.J Clin Transl Hepatol. (2021) 9:769–91. doi: 10.14218/JCTH.2021.00209
14.
Terrault NA Lok ASF McMahon BJ Chang K‐M Hwang JP Jonas MM et al . Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. (2018) 67:1560–99. doi: 10.1002/hep.29800
15.
European Association for the Study of the Liver . EASL clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. (2025) 83:502–83. doi: 10.1016/j.jhep.2025.03.018
16.
Ogawa E Nomura H Nakamuta M Furusyo N Koyanagi T Dohmen K et al . Tenofovir alafenamide after switching from entecavir or nucleos(t)ide combination therapy for patients with chronic hepatitis B. Liver Int. (2020) 40:1578–89. doi: 10.1111/liv.14482
17.
Nguyen MH Atsukawa M Ishikawa T Yasuda S Yokohama K Trinh HN et al . Outcomes of sequential therapy with tenofovir alafenamide after long-term entecavir. Am J Gastroenterol. (2021) 116:1264–73. doi: 10.14309/ajg.0000000000001157
18.
Li Z Li L Niu X Li ZB Niu XX Chen SH et al . Switching from entecavir to tenofovir alafenamide for chronic hepatitis B patients with low-level viraemia. Liver Int. (2021) 41:1254–64. doi: 10.1111/liv.14786
19.
Lampertico P Buti M Fung S Ahn SH Chuang WL Tak WY et al . Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in virologically suppressed patients with chronic hepatitis B: a randomised, double-blind, phase 3, multicentre non-inferiority study. Lancet Gastroenterol Hepatol. (2020) 5:441–53. doi: 10.1016/S2468-1253(19)30421-2
20.
Uchida Y Nakao M Tsuji S Uemura H Kouyama J‐i Naiki K et al . Significance of switching of the nucleos(t)ide analog used to treat Japanese patients with chronic hepatitis B virus infection from entecavir to tenofovir alafenamide fumarate. J Med Virol. (2019) 92:329–38. doi: 10.1002/jmv.25644
21.
Hou J Zhao W Lee C Hou J-L Hann H-W Peng C-Y et al . Outcomes of long-term treatment of chronic HBV infection with entecavir or other agents from a randomized trial in 24 countries. Clin Gastroenterol Hepatol. (2020) 18:457–467.e21. doi: 10.1016/j.cgh.2019.07.010
22.
Brunetto M Lim YS Gane E Seto WK Osipenko M Ahn SH et al . A phase 3 study comparing tenofovir alafenamide to tenofovir disoproxil fumarate in patients with HBeAg-negative, chronic hepatitis B: efficacy and safety results at week 96. J Hepatol. (2017) 66:S25–6. doi: 10.1016/s0168-8278(17)30313-6
23.
Ray AS Fordyce MW Hitchcock MJM . Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antivir Res. (2016) 125:63–70. doi: 10.1016/j.antiviral.2015.11.009
24.
Kaneko S Kurosaki M Tamaki N Itakura J Hayashi T Kirino S et al . Tenofovir alafenamide for hepatitis B virus infection including switching therapy from tenofovir disoproxil fumarate. J Gastroenterol Hepatol. (2019) 34:2004–10. doi: 10.1111/jgh.14686
25.
Liu Y Kong Y Liu Y Liu T Ma A Zhang Y et al . Different switching strategies in nucleos(t)ide analogue–experienced chronic hepatitis B patients with low-level viremia: a single-center retrospective study. J Prac Hepatol. (2022) 25:633–6. doi: 10.3969/j.issn.1672-5069.2022.05.007
Summary
Keywords
chronic hepatitis B, tenofovir alafenamide fumarate, antiviral treatment, low-level viremia, partial virological response
Citation
Hu X, Kong Y, Liu Y, Liu T, Ma A, Li Y, Zhang Y, Li J, Zhang L and Li G (2025) Optimized strategy of switching to tenofovir alafenamide fumarate treatment for nucleos(t)ide analogue experienced patients with chronic hepatitis B. Front. Med. 12:1592998. doi: 10.3389/fmed.2025.1592998
Received
13 March 2025
Accepted
30 October 2025
Published
14 November 2025
Volume
12 - 2025
Edited by
Diego Ripamonti, Papa Giovanni XXIII Hospital, Italy
Reviewed by
Esteban Panozzo Zenere, National University of Rosario, Argentina
Toni Herta, Charité University Medicine Berlin, Germany
Updates
Copyright
© 2025 Hu, Kong, Liu, Liu, Ma, Li, Zhang, Li, Zhang and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangming Li, liggming@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.