- 1Science and Technology Innovation Center, Institute of Gastroenterology, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
- 2School of Chinese Materia Medica, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
Background: Inflammatory bowel disease (IBD), encompassing both Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic, inflammatory, and immune-mediated disorder of the gastrointestinal tract. If left inadequately treated, IBD can lead to disease progression, resulting in severe long-term complications, including irreversible structural damage to the intestinal tissues. While clinical symptoms are traditionally used to assess treatment efficacy, they do not always align with the underlying mucosal inflammation, particularly in CD. This limitation underscores the importance of exploring alternative treatment strategies. To address this gap, the present study evaluates the effectiveness of non-pharmacological treatments (NPTs) for IBD through a network meta-analysis (NMA), providing a thorough assessment of the available evidence.
Methods: We systematically reviewed randomized controlled trials (RCTs) from the following databases: PubMed, Embase, Springer, Cochrane Controlled Register of Trials (CENTRAL), and Web of Science, comparing various NPTs for IBD, including Cognitive Behavioral Therapy (CBT), diet interventions (DI), fecal microbiota transplantation (FMT), physical training (PT), and acupuncture and moxibustion (APMX). Outcomes assessed included clinical remission, disease activity, quality of life (QOL), serum biomarkers (fecal calprotectin [FC] and C-reactive protein [CRP]), and adverse effects. The quality assessment was assessed by Cochrane Handbook and GRADEpro software. The risk ratio (RR) was calculated for dichotomous outcomes while standardized mean difference (SMD) was used for continuous variables with 95% credible intervals (CI). Funnel plot was performed to evaluate publication bias. Surface under the cumulative ranking curve (SUCRA) was conducted to rank the included interventions. Data were analyzed with STATA 15.0 and Review Manager 5.3.
Results: A total of 62 eligible RCTs were identified in this NMA. The results showed that standard medical therapy (SMT) exhibited the highest probability in inducing clinical remission, as expected. Among non-pharmacological interventions, APMX, a traditional Chinese medicine involving acupuncture and moxibustion, showed promising results in both animal models and clinical trials, reducing serum TNF-α levels and improving intestinal health. DI was most effective in maintaining clinical remission and reducing serum FC levels. FMT emerged as the most effective treatment for reducing serum CRP levels and ranked second in terms of clinical remission induction.
Conclusion: APMX, DI, and FMT represent promising non-pharmacological options for managing IBD. APMX was the most effective for clinical remission and symptom relief, while DI was best for maintaining remission, and FMT showed promise in reducing inflammation. Further high-quality clinical trials are needed to strengthen the evidence and guide clinical practice in IBD management.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024596233, CRD42024596233.
Introduction
Inflammatory bowel disease (IBD), encompassing both ulcerative colitis (UC) and Crohn’s disease (CD), refers to a group of chronic, inflammatory disorders of the gastrointestinal tract. The global prevalence of IBD has been steadily rising, with approximately 6.8 million individuals affected worldwide. This growing burden incurs substantial healthcare costs, amounting to billions annually, placing significant strain on healthcare systems globally (1). While the exact etiology of IBD remains unknown, it is believed to result from a combination of genetic predisposition, environmental factors, and gut microbiota dysbiosis (2, 3). Dysbiosis of the gut microbiota can activate the innate immune system, leading to the excessive secretion of pro-inflammatory cytokines (such as TNF-α, IL-6), which in turn drives chronic inflammatory cascade reactions in animal models (4). In addition, a recent prospective cohort study conducted on patients with ulcerative colitis (UC) found that patients with higher stress reactivity exhibited significant differences in their gut microbiota and metabolite profiles. These microbial and metabolic biomarkers were able to effectively predict the risk of disease relapse within the next 6 to 24 months, suggesting that the gut-brain-microbiota interactions play a crucial role in stress-related UC activity (5). Meanwhile, dysfunction of the gut-brain axis may explain the high prevalence of psychological comorbidities such as anxiety and depression in IBD patients (approximately 1/3 of patients with anxious, and 1/4 with depressed) (6), Psychological stress can further exacerbate intestinal inflammation through the vagus nerve-immune pathway, creating a vicious cycle (7–10). Despite the widespread use of traditional IBD treatment regimens in clinical practice, they all have corresponding limitations. 5-aminosalicylic acid (5-ASA) drugs have limited efficacy in Crohn’s disease and may cause side effects such as headaches and nausea, with long-term use potentially leading to kidney damage (11). Corticosteroids, while effective in inducing inflammation remission, are ineffective for maintaining long-term remission (12), and may cause adverse reactions such as bone loss, hyperglycemia, and edema (13). Immunosuppressants have a slow onset of action and may cause side effects such as leukopenia and liver damage, with long-term use potentially increasing the risk of cancer (14). Biologics, while highly effective in severe cases, carry a risk of serious infections (15) and come with high treatment costs. These limitations highlight the need for developing safer and more effective therapeutic options. Furthermore, traditional treatment plans pay insufficient attention to the mental health of IBD patients, while comorbidities such as anxiety and depression can increase the risk of disease recurrence by 1.6 times and the hospitalization rate by 42% (16). With the increasing attention to the impact of IBD on mental health, non-pharmacological therapies (NPTs) are gradually gaining importance, becoming a crucial supplement to the comprehensive management of IBD.
Non-pharmacological therapies (NPTs), Such as psychological interventions (including cognitive behavioral therapy [CBT], mindfulness-based therapy, acceptance and commitment therapy [ACT], etc.), diet interventions (DI), fecal microbiota transplantation (FMT), physical training (PT), and acupuncture and moxibustion (APMX), have been proposed as adjunctive treatments for IBD (17–21).
Systematic reviews have assessed the efficacy of these interventions. For example, a meta-analysis by Seaton et al. (22) found that emotional interventions could effectively improve inflammation markers (such as fecal calprotectin and C-reactive protein), with therapies like CBT showing better efficacy than physical exercise. A study by El et al. (23) demonstrated that fecal microbiota transplantation (FMT) provides significant clinical and endoscopic benefits in the short-term treatment of active ulcerative colitis (UC), with good safety. The latest systematic review by Yang et al. (24) also suggested that acupuncture may have potential anti-inflammatory effects (24). In an updated 2023 systematic review by Limketkai et al. (25), it was pointed out that despite the widespread attention on dietary interventions, the existing evidence quality remains low and is characterized by high uncertainty. Additionally, earlier Cochrane reviews explored the roles of dietary interventions (18) and fecal microbiota transplantation (26) in inducing and maintaining IBD remission, but their scope was limited to single intervention types and did not compare different non-pharmacological therapies across studies.
The assessment of IBD involves multiple levels of indicators. In addition to traditional clinical symptom scores, recent clinical research has placed greater emphasis on the combination of subjective and objective outcome measures. Previous studies have shown that there is often an inconsistency between the subjective symptoms reported by IBD patients and the objective degree of mucosal inflammation, particularly in patients with Crohn’s disease (CD) (27, 28). Therefore, a single clinical symptom score often cannot comprehensively reflect the disease activity. In recent years, biomarkers (such as C-reactive protein, fecal calprotectin), quality of life scales (such as IBDQ, SIBDQ), and composite scoring tools have been widely used to assist in evaluation. Based on this, this study incorporates multiple outcome indicators, including clinical remission, disease activity, quality of life, biomarkers, and adverse reactions, in order to provide a more comprehensive evaluation of the effects of non-pharmacological interventions.
For this purpose, this study aims to conduct a network meta-analysis to evaluate the efficacy and safety of five non-pharmacological interventions: CBT, DI, FMT, PT, and APMX in treating IBD. By examining a range of therapeutic options, this review seeks to provide a comprehensive overview of non-pharmacological interventions that may complement conventional treatments and improve the overall management of IBD. This study represents the first network meta-analysis comparing the efficacy of multiple non-pharmacological interventions, aiming to systematically elucidate their relative advantages, address existing evidence deficiencies in this field, and offer evidence-based guidance for clinical decision-making regarding therapeutic selection as well as future research prioritization.
Materials and methods
This study was conducted in accordance with the Cochrane criteria, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (29) and relevant meta-analysis guidance. The protocol has been registered with PROSPERO under the registration number CRD42024596233.
Search strategy and study selection
A comprehensive literature search was conducted from inception through 6 November 2024, in the following databases: PubMed, Embase, Springer, Cochrane Controlled Register of Trials (CENTRAL), and Web of Science (Supplementary File 1). There were no restrictions on language or publication date. Randomized controlled trials (RCTs) meeting the PICO (Population, Intervention, Comparison, Outcome) methodology were eligible for inclusion: (1) Participants: Individuals with a confirmed diagnosis of IBD; (2) Interventions: Any non-pharmacological therapy for IBD treatment, including Cognitive Behavioral Therapy (CBT), dietary interventions (DI), fecal microbiota transplantation (FMT), physical training (PT), and acupuncture and moxibustion (APMX), the combination therapies were also included; (3) Comparisons: Comparison with usual conventional treatments, placebos, or other non-pharmacological interventions. Any study in which there is a clear difference in treatment methods between the intervention and control groups may be included for comparison. In some studies, “usual treatment” was defined as standard medical therapy (SMT), which refers to conventional pharmacological regimens for IBD (e.g., mesalazine, corticosteroids, or biologics). SMT was treated solely as a comparator and not as a non-pharmacological intervention in this analysis; (4) Outcomes: Clinical remission, disease activity, gastrointestinal symptoms, inflammation biomarkers (C-reactive protein [CRP], fecal calprotectin [FC]), quality of life (QOL), and adverse effects. Studies were excluded based on the following criteria: meeting abstracts (Since these typically do not include complete methods and results data, making it difficult to extract data and assess bias.); incomplete or imprecise data; ambiguous treatment protocols; unavailable full texts; cross-sectional studies; or reviews. Studies with a Jadad score of ≤ 3 were also excluded.
Data extraction
Two authors independently performed data extraction from the included studies. Any discrepancies were resolved by discussion between the two independent investigators. Adjudication was performed as needed by a third author (MG). Extracted data included the study design, population characteristics, intervention, comparator, duration of interventions and follow-up, outcomes, timing, setting, the method of handling missing data, funding source, and potential conflicts of interest. In cases where data is incomplete or information is unclear in the literature, we proactively contacted the corresponding author to obtain Supplementary Information.
Quality evaluation
Risk of bias for RCTs was independently assessed by two authors using the Cochrane Risk of Bias tool (30). The overall certainty of evidence was independently assessed by two authors for each stratified outcome using the GRADE methodology (31). Inconsistencies were resolved by a third author. Risk of bias was evaluated based on standard definitions used in Cochrane systematic reviews, with domains including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, and selective reporting. Each domain was classified as “low risk,” “unclear risk,” or “high risk.” Heterogeneity was initially assessed qualitatively by considering differences in study populations (e.g., age, sex, race), research settings, methods of dietary interventions, intervention durations, and definitions or thresholds for remission. For studies exhibiting qualitative homogeneity, statistical heterogeneity was assessed using the Chi2 test, with a P-value < 0.10 indicating statistically significant heterogeneity.
Data synthesis and statistical analysis
Evidence of direct and indirect multiple-intervention comparisons is obtained by network meta-analysis, and performing this analysis with the Bayesian framework can improve the accuracy of the results. For binary outcomes, we calculated the risk ratio (RR) with corresponding 95% confidence interval (CI). For continuous outcomes, we calculated the mean difference (MD) and corresponding 95% CI. The random effects model will be utilized for combining data if there is statistically significant variation across studies, or the fixed effects model will be chosen if there was no statistically significant heterogeneity across the studies. Given the potential clinical or methodological heterogeneity in population characteristics, intervention methods, and study designs, among other aspects, across the included studies, this study uniformly applies a random-effects model for data pooling analysis to improve the robustness of the estimated results and ensure consistency in model selection. A funnel plot was applied to evaluate the existence of publication bias. The surface under the cumulative ranking curve (SUCRA) was calculated to rank the probability of interventions. The SUCRA value ranges from 0% to 100%, with higher values indicating that the intervention ranks higher and has a better effect in all comparisons. Given that most studies did not report outcome measures stratified by gender, we did not conduct gender-based subgroup analysis. STATA 15.0 and Review manager 5.3 software was used for conducting the meta-analysis.
Results
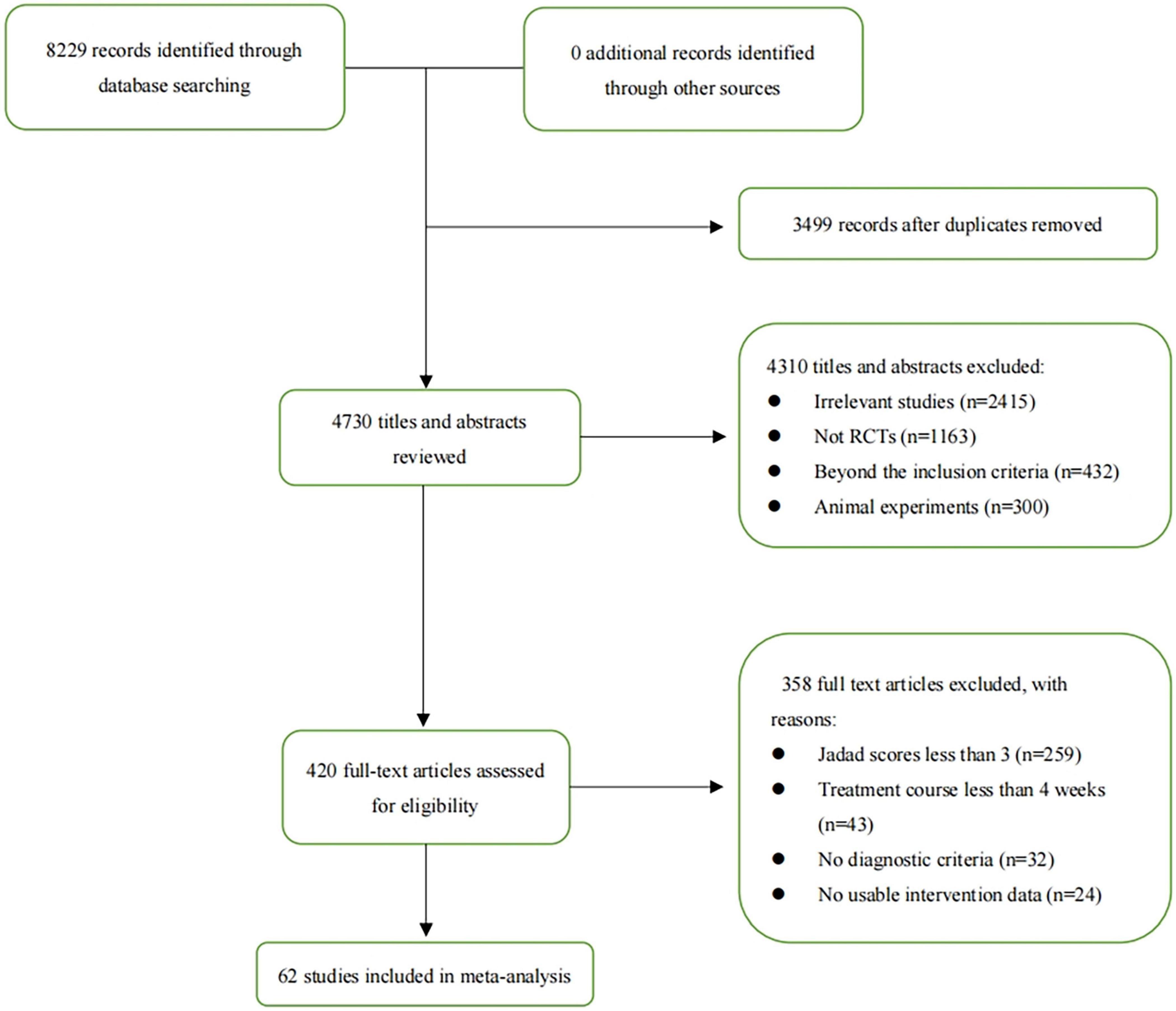
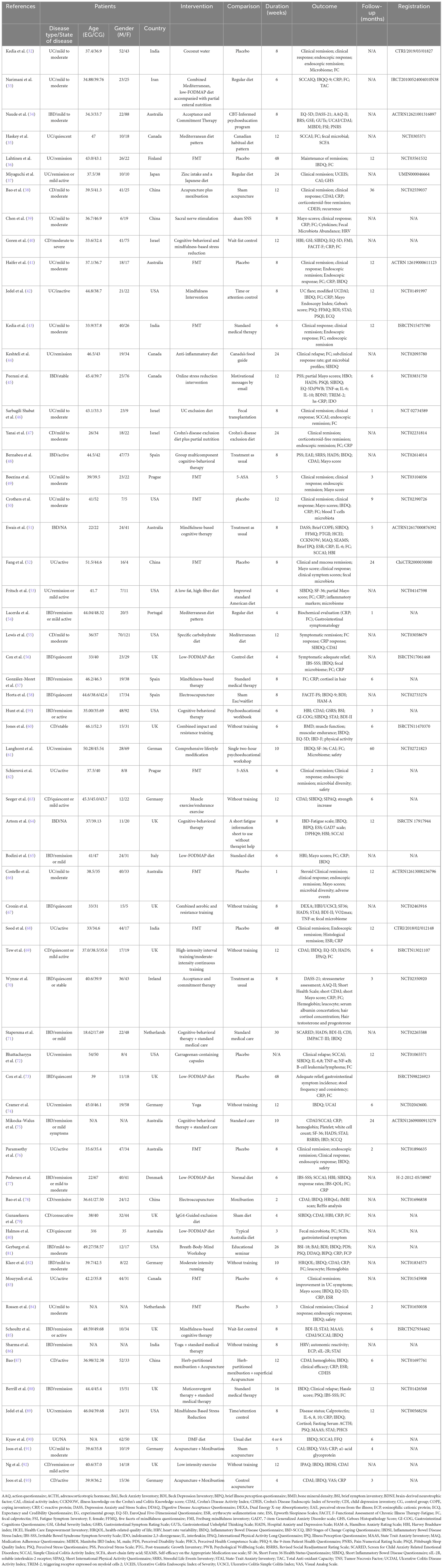
The comprehensive literature search identified 8,229 records from the following databases: PubMed, Embase, Springer, Cochrane Controlled Register of Trials (CENTRAL), and Web of Science (the search strategies are provided in Supplementary File 1). After careful screening, 62 studies met the inclusion criteria and were deemed eligible for further quantitative analysis (32–93) is (32–57, 59–95). A flow diagram of the specific screening procedures is shown in Figure 1. The baseline characteristics of the included studies were summarized in Table 1. Totally, 7 interventions were enrolled: FMT, PT, CBT, DI, APMX, standard medical therapy (SMT) such as 5-aminosalicylic acid (5-ASA), and placebo.
Risk of bias and publication bias
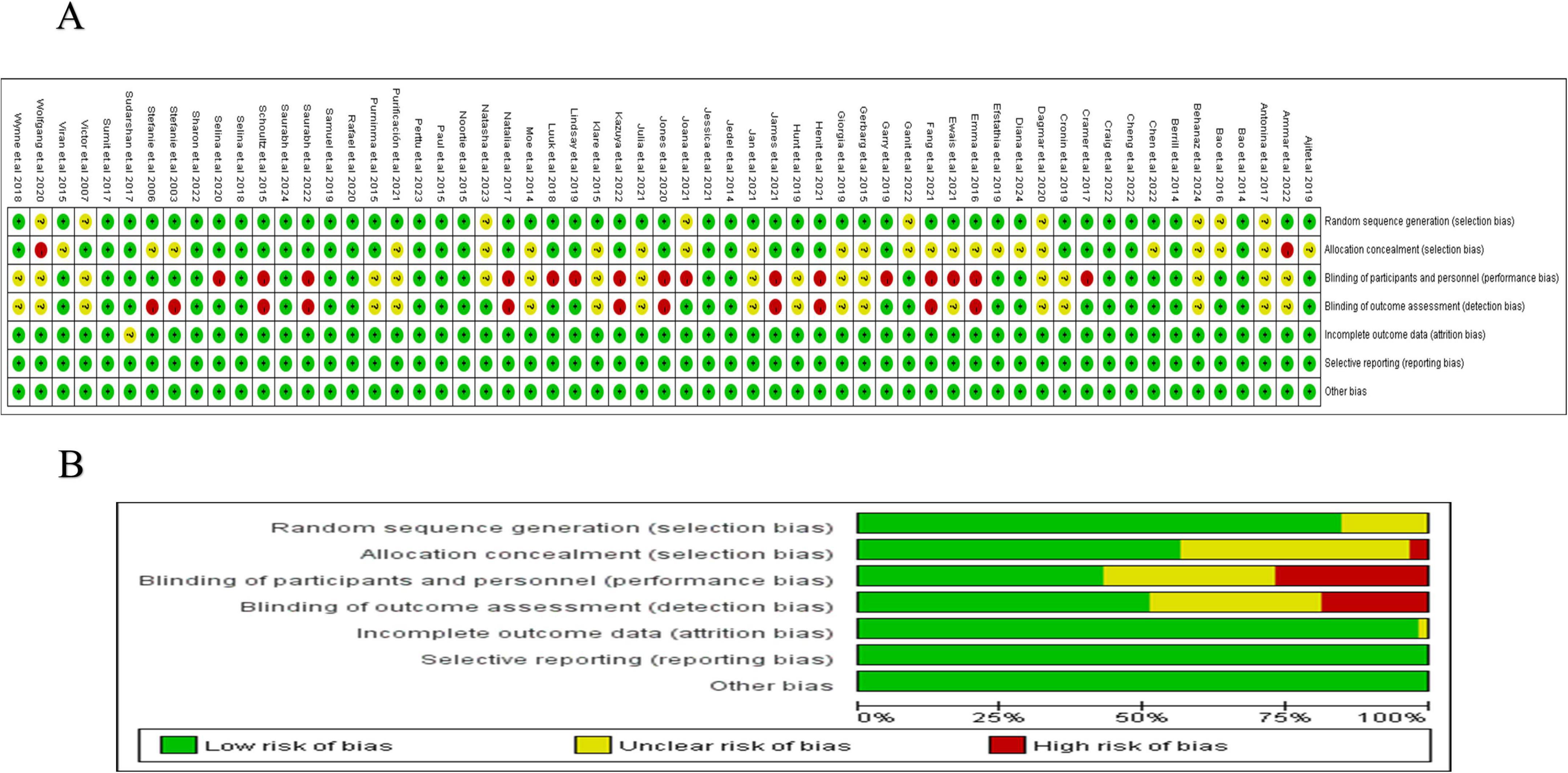
Among the 62 included studies, 20 were rated as having a high risk of bias in one or more domains, primarily in the areas of blinding of participants and personnel, and blinding of outcome assessment. Additionally, 25 studies were rated as having an unclear risk of bias in two or more domains. A detailed risk of bias assessment is provided in Figure 2. Figure 2A systematically presents the specific assessment of each study across seven risk dimensions, while Figure 2B further summarizes the overall risk distribution for each dimension. Overall, most studies showed low risks in random sequence generation, allocation concealment, and outcome reporting, suggesting a certain level of quality assurance in study design and data reporting. However, some non-pharmacological interventions, due to the limitations of the intervention characteristics (e.g., difficulty in implementing double-blinding), exhibited relatively higher bias risks in blinding-related dimensions, which could impact the internal validity of some outcomes. To further verify the robustness of the results, we conducted a sensitivity analysis. After excluding each study one by one, the changes in the pooled effect size of the main outcomes were minimal, indicating that the study results are stable. The heterogeneity analysis revealed high consistency across studies. The I2 value was 1.09%, with P = 0.580, suggesting strong reliability of the pooled analysis results (Figure 3A). Combined with Egger’s regression test results (P > 0.1), no significant publication bias was found (Figure 3B). The primary outcome measures showed minimal variation in the sensitivity analyses, indicating that the overall conclusions of the study remained robust and stable (Figure 3C). Consistency and inconsistency analysis indicate that the data reliability is high, and there is no significant inconsistency or bias in the comparison between treatment methods. In conclusion, the overall bias risk of this study is controllable, heterogeneity is low, and the results demonstrate strong robustness and credibility.
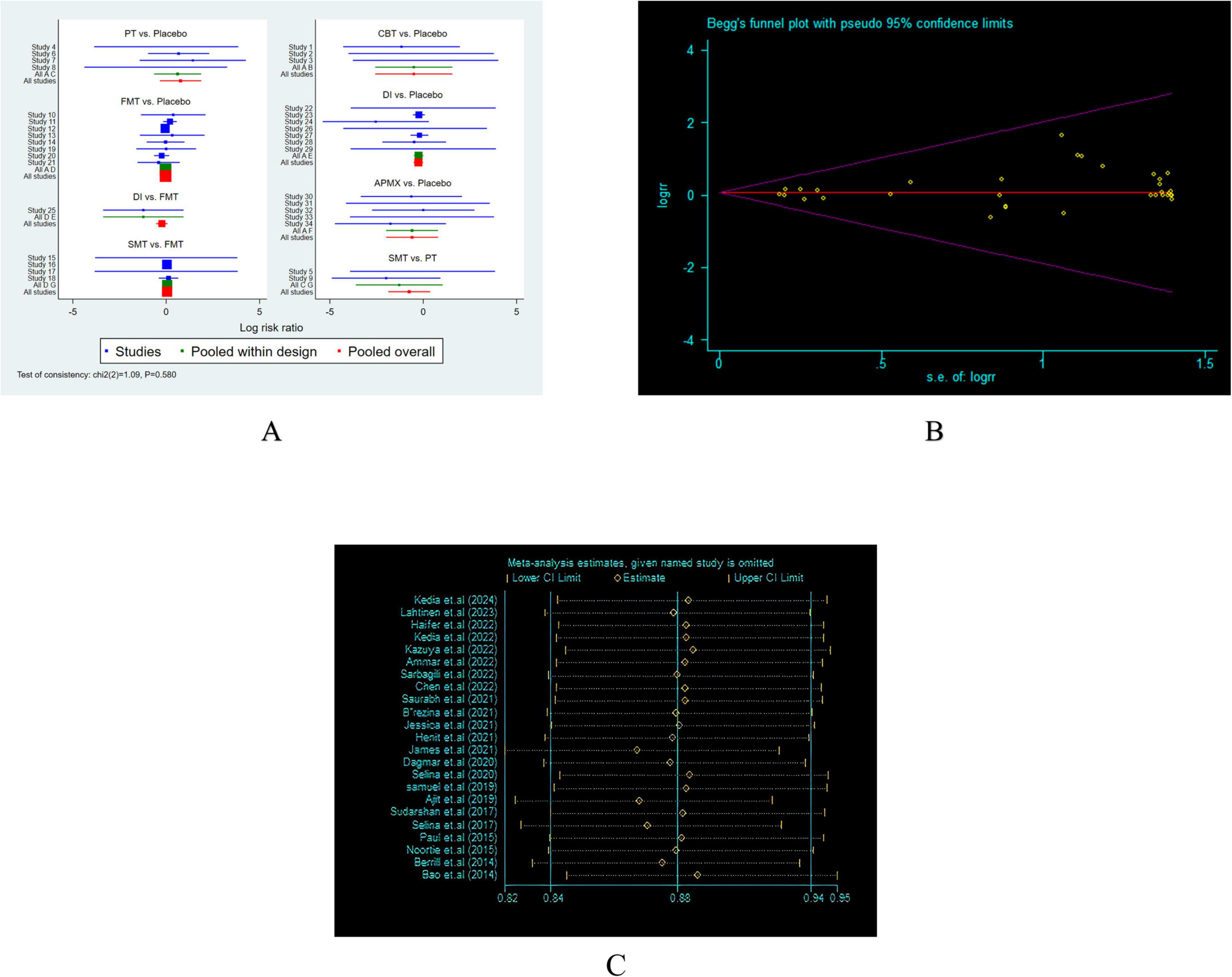
Figure 3. Heterogeneity analysis and Funnel plot. (A) Heterogeneity analysis; (B) Egger’s regression test. (C) Sensitivity analysis.
Clinical remission
Induction of remission
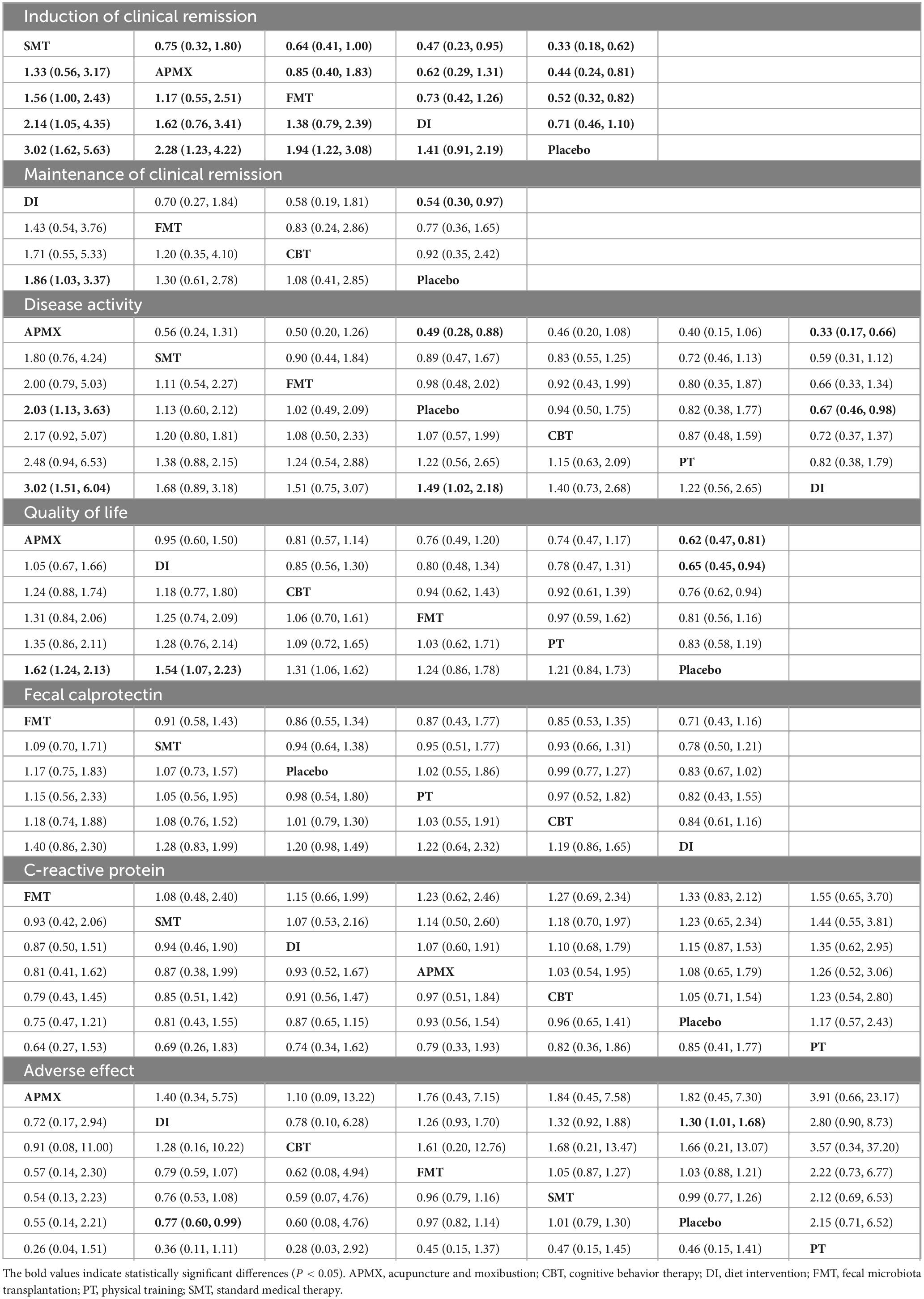
A total of 19 studies involving 1,120 participants with active IBD were included in the assessment of clinical remission induction (Figure 4A). The results indicated that SMT (RR = 3.02, 95% CI 1.62–5.63), APMX (RR = 2.28, 95% CI 1.23–4.22), and FMT (RR = 1.94, 95% CI 1.22–3.08) therapies were significantly more effective than placebo in inducing clinical remission (Table 2). Additionally, SMT was found to be superior to DI (RR = 2.14, 95% CI 1.05–4.35). The SUCRA plot (Figure 4B) demonstrated that SMT (92.5%) was the most favorable treatment for inducing remission in patients with active IBD, followed by APMX (70.4%) and FMT (56.0%).
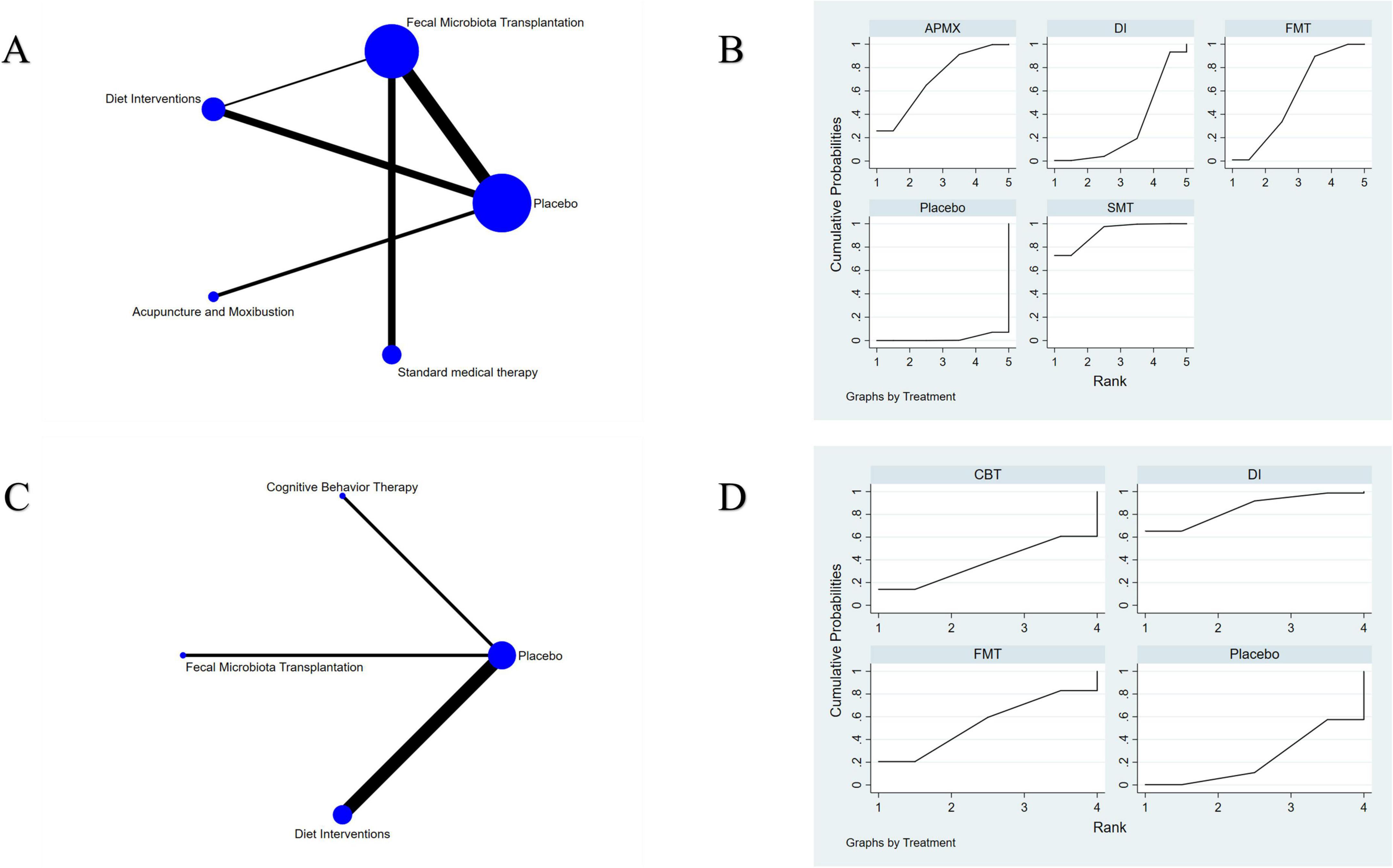
Figure 4. Network meta-analysis of clinical remission. (A) network evidence of induction of remission; (B) SCURA of induction of remission; (C) network evidence of maintenance of remission; (D) SCURA of maintenance of remission. The SUCRA results indicate that SMT and DI was the best intervention for the induction and maintenance of clinical remission, respectively.
Maintenance of remission
Seven studies involving 365 patients with inactive IBD evaluated the maintenance of remission (Figure 4C). The results suggested that DI was more effective than placebo in maintaining clinical remission (RR = 1.86, 95% CI 1.03–3.37) (Table 2). The SUCRA plot (Figure 4D) indicated that DI ranked highest (85.6%), followed by FMT (61.2%) and CBT (50.3%).
Consistency analysis and GRADE estimates
The results of consistency and inconsistency analysis were shown in Supplementary File 2. The test of consistency showed that chi2(1) = 1.78, P = 0.182, suggesting good consistency of the model. The node splitting results also showed no inconsistency (all P > 0.05). The quality of estimate based on GRADE criteria for clinical remission and maintenance remission (Figure 5) was “moderate,” which was possibly derived from the direct and indirect comparisons within RCTs, leading to imprecision and unclear risk of bias.
Disease activity
A total of 30 studies involving 1,400 participants assessed disease activity (Figure 6A). Among them, 13 studies used the Crohn’s Disease Activity Index (CDAI), 10 used the Simple Clinical Colitis Activity Index (SCCAI), 3 used the Clinical Activity Index (CAI), and 2 used the UC Disease Activity Index (UCDAI). The network meta-analysis (NMA) results (Table 2) revealed that APMX was superior to placebo (SMD = 2.03, 95% CI 1.13–3.63) and diet interventions (SMD = 3.02, 95% CI 1.51–6.04) in alleviating disease activity. The SUCRA plot (Figure 7A) indicated that APMX (95.9%) was the most effective non-pharmacological treatment for reducing disease activity in IBD, followed by SMT (67.6%) and CBT (52.1%).
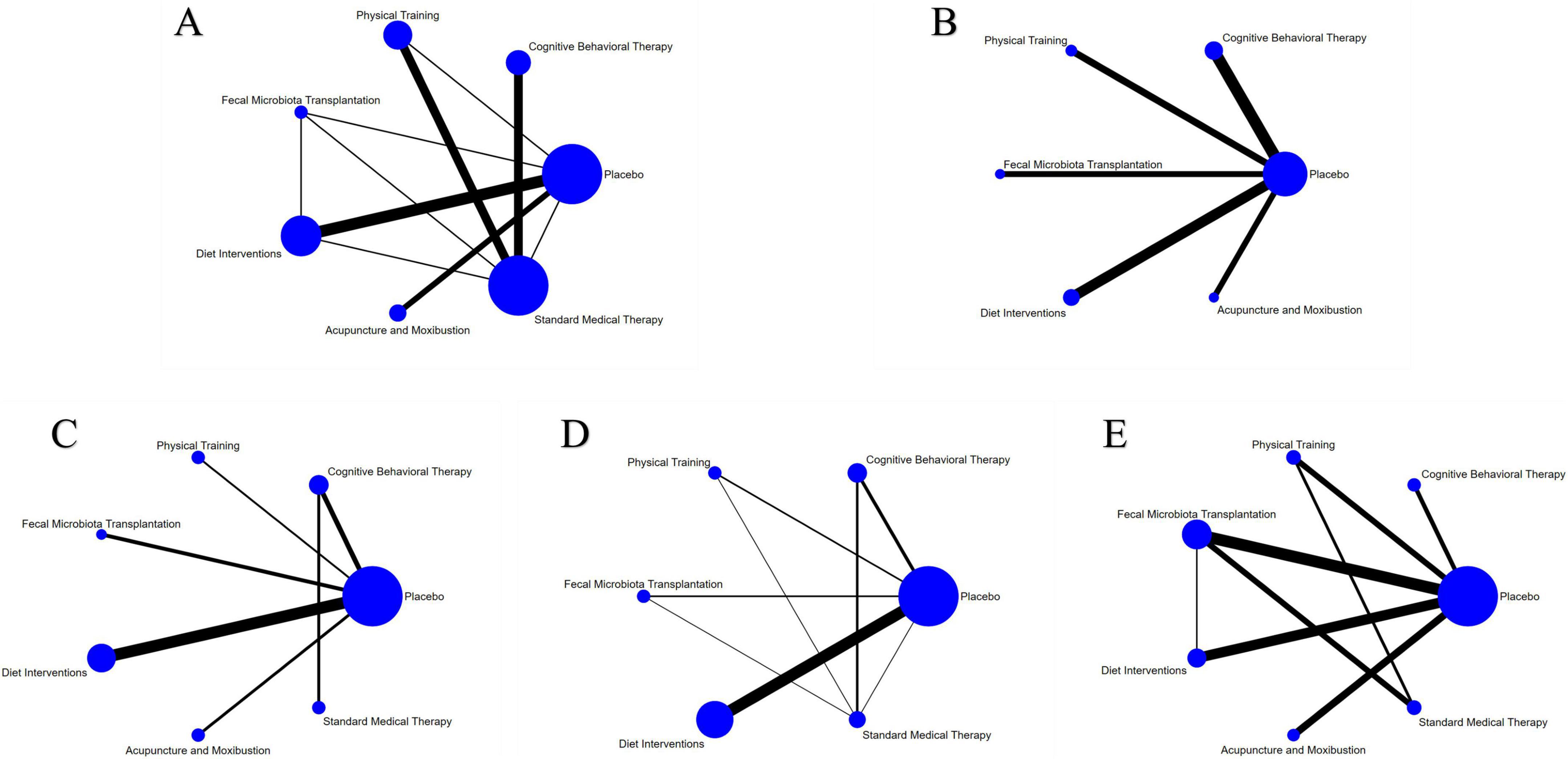
Figure 6. Network meta-analysis of clinical outcomes. (A) Disease activity; (B) quality of life; (C) C-reactive protein; (D) fecal calprotectin; (E) adverse effects.
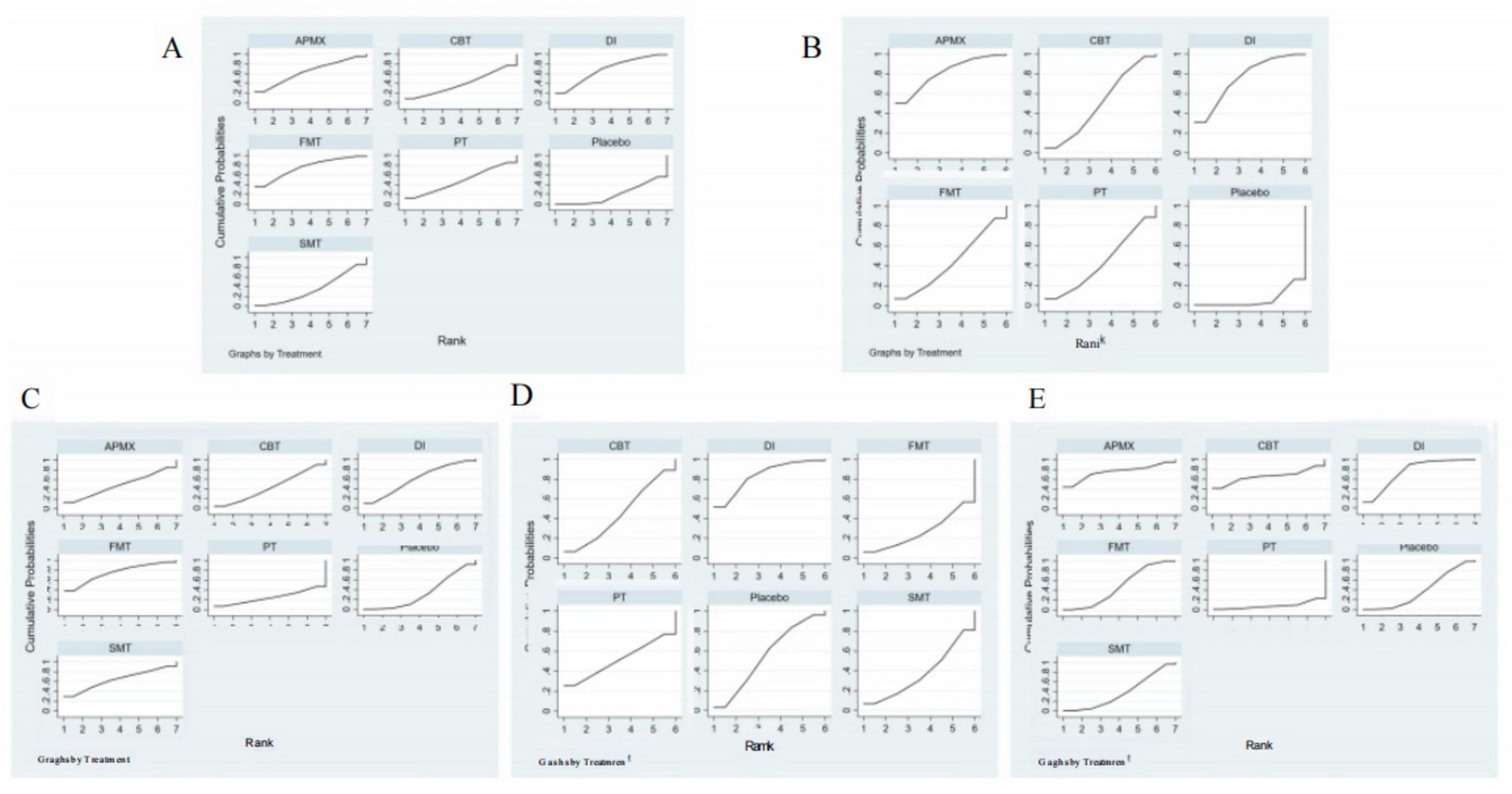
Figure 7. SUCRA of clinical outcomes. (A) Disease activity; (B) Quality of life; (C) C-reactive protein; (D) Fecal calprotectin; (E) Adverse effects. The SUCRA results indicate that APMX was the best intervention for alleviating disease activity and improving quality of life, while FMT and DI was the most effective treatments for reducing CRP and FC, respectively.
Quality of life
Thirty-seven studies involving 2,312 participants assessed changes in quality of life (Figure 6B). Most studies used the IBD Questionnaire (IBDQ), while 8 studies used the Short IBDQ (SIBDQ), and 2 used the IBDQ-9. The NMA results (Table 2) showed that APMX (SMD = 1.62, 95% CI 1.24–2.13) and DI (SMD = 1.54, 95% CI 1.07–2.23) were more effective than placebo in improving quality of life scores. The SUCRA plot (Figure 7B) suggested that APMX (84.8%) was the most effective non-pharmacological option for improving quality of life in IBD patients, followed by DI (76.0%) and CBT (51.8%). The definition of clinical outcomes were shown in Supplementary File 3.
Biomarkers of inflammation
C-reactive protein (CRP) was reported in 30 studies with 1,450 patients, while fecal calprotectin (FC) was reported in 26 studies with 1,213 patients (Figures 6C, D). No significant differences in CRP or FC changes were observed between the various treatments (Table 2). However, the SUCRA plots recommended FMT (74.7%) and DI (84.1%) as the most effective treatments for reducing CRP and FC, respectively (Figures 7C, D).
Adverse effects
Thirty-five studies involving 1,906 participants reported adverse effects (Figure 6E). Compared with placebo, DI was associated with a lower incidence of adverse effects (OR = 0.77, 95% CI 0.60–0.99). No significant differences were found among the non-pharmacological therapies, suggesting that all interventions were similarly safe (Table 2 and Figure 7E).
Discussion
UC and CD are progressive diseases, and without timely and effective intervention, they can result in irreversible long-term complications (96). Clinical symptoms were once considered a primary factor in evaluating treatment efficacy; however, there is a clear disconnect between clinical symptoms and active mucosal inflammation in IBD, especially in Crohn’s disease (CD) (27, 28). To address this, various diagnostic and monitoring tools have been developed, including clinical symptom-based scoring systems, patient-reported outcomes, serum biomarkers, stool biomarkers, imaging modalities, and ileo-colonoscopy (97).
In this study, we evaluated the following outcomes: clinical remission, disease activity, quality of life (QOL), serum biomarkers (fecal calprotectin [FC] and C-reactive protein [CRP]), and adverse effects. Clinical remission was defined using symptom scoring systems, primarily the Crohn’s Disease Activity Index (CDAI) for CD and the Mayo score for ulcerative colitis (UC) (Supplementary File 2). Disease activity was measured using the Simple Clinical Colitis Activity Index (SCCAI), CDAI, Ulcerative Colitis Disease Activity Index (UCDAI), and Clinical Activity Index (CAI). These scores are simple to implement in clinical practice and are useful for monitoring symptoms over time. In addition to the physical damage caused by IBD, psychosocial issues often arise, with low QOL scores and high disability levels being associated with increased indirect medical costs (98). The most widely used QOL measurement tools in IBD are the IBD Questionnaire (IBDQ) and its shortened version, the Short IBDQ (SIBDQ), both of which were predominantly evaluated in this NMA. Serum biomarkers such as CRP and FC are commonly used non-invasive markers of inflammation in IBD. CRP is generally used to monitor inflammation but has only a moderate correlation with endoscopic disease activity, exhibiting high specificity but low sensitivity for endoscopically active disease (pooled specificity 0.92, 95% CI 0.72–0.96; pooled sensitivity 0.49, 95% CI 0.34–0.64) (99). In contrast, FC has been shown to accurately differentiate between active and quiescent disease in both UC and CD, making it an excellent surrogate marker of mucosal inflammation (99).
The results of NMA show that APMX is the most effective non-pharmacological intervention for inducing clinical remission, alleviating disease activity, and improving patients’ quality of life. Acupuncture and moxibustion are two widely used treatments forms of traditional Chinese medicine and have been employed extensively for the prevention and treatment of IBD, particularly in Asia (38). These treatments are general regarded as natural and safe (100). Acupuncture involves the insertion of slender needles into specific anatomical locations on the body, while Moxibustion applies heat from dried moxa plants to targeted skin areas. Both acupuncture and moxibustion have been reported to alleviate intestinal inflammation, regulate gut microbiota, and relieve IBD symptoms in both animal models and clinical trials (38). In IBD mouse models, APMX has been shown to increase levels of anti-inflammatory cytokines such as interleukin (IL)-10, while decreasing levels of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α), nuclear factor kappa B (NF-κB), IL-6, IL-1β, and IL-17 (101). Electroacupuncture (EA), a form of acupuncture, has been observed to improve colitis severity by maintaining epithelial tight junction proteins and vasoactive intestinal peptide (VIP) receptors, especially VPAC2 (102). Another study found that EA can stimulate neurogenic inflammation, as indicated by reduced levels of substances P, hyaluronic acid, bradykinin, and prostacyclin in the skin of rats with colitis, specifically in the L6 DRG region (103). Clinical studies have demonstrated that APMX can significantly reduce serum TNF-α levels and alter the fecal microbiota composition in UC patients (39). In CD, APMX can enhance the abundance of anti-inflammatory bacteria, improve the intestinal barrier, and regulate circulating Th1/Th17 cytokines (38). In line with the former studies, this NMA show the promising therapeutic effects of APMX in the treatment of IBD, reducing patients’ clinical symptoms and improving their quality of life.
DI were also found to be highly effective, particularly in the maintenance of clinical remission. This aligns with findings from a recent meta-analysis (104). The relationship between diet and IBD is gaining increasing attention, as specific dietary components are recognized for their potential to influence gut health and microbial balance. In this NMA, the most commonly studied dietary interventions included the Mediterranean diet and the low-FODMAP diet. These diets are characterized by low-fat, high-fiber, moderate protein intake (rich in Omega-3 fatty acids), and a reduction in processed foods and sugars (105, 106). The International Organization for the Study of inflammatory bowel disease (IOIBD) has issued guidelines emphasizing the importance of increasing Omega-3 fatty acid intake, particularly from sources like fish oil and fresh fish (107). Omega-3 fatty acids are known for their anti-inflammatory properties and have been shown to alleviate symptoms and promote gut health in UC patients (108). For CD patients, a diet rich in fruits and vegetables, and a reduction in saturated fats, trans fats, dairy fats, additives like polysorbate 80, and artificial sweeteners such as sucralose and saccharin have been recommended (107).
The role of gut microbiota in the development of IBD has recently been emphasized, thus several therapeutic strategies have focused on manipulation of the gut microbiota. Probiotics and antibiotics have been widely used for the treatment of IBD by controlling the growth of pathological organisms, but the results were controversial (109–112). In contrast to both antibiotics and probiotics, FMT may represent a more robust method of manipulating the gut microbiota as a therapy for patients with. Unlike antibiotics, FMT increases the diversity of fecal bacterial populations in recipients, likely contributing to its success in C. difficile infection (113, 114). Furthermore, unlike probiotics, evidence suggests that FMT results in long-term engraftment in recipients with C. difficile infection (115). Many studies had reported therapeutic effect of FMT in inducing short-term clinical remission/response and changes in disease activity indices, biochemical indicators, and microbial diversity indices for IBD (116). All together, these factors suggest that FMT may be a more promising therapy for IBD than either antibiotics or probiotics. However, donor screening standardization (e.g., microbiome diversity thresholds) route of administration (colonoscopy vs. oral capsules), and regulatory hurdles (e.g., FDA classification as an investigational drug) currently limit widespread adoption.
Although this study found no significant intergroup differences in reducing CRP and FC with NPTs, the SCURA ranking suggests that FMT and DI are the best choices for regulating CRP and FC, respectively. As a systemic inflammation marker, CRP is easily influenced by extrinsic factors (such as infections and stress), and its short half-life (approximately 19 h) may dilute the signal of treatment efficacy (117). Although FC is highly sensitive to local intestinal inflammation, its changes lag behind the improvement of clinical symptoms, and some studies with insufficient follow-up periods (≤ 4 weeks) or inconsistent testing time points may weaken the effect size (118). In addition, FMT inhibits systemic inflammation by transplanting functional microbiota, but its effect on CRP may take longer (≥ 8 weeks) to manifest (76); DI (such as a low FODMAP diet) directly reduces FC release by decreasing intestinal fermentation substrates, and polyphenolic substances (such as oleuropein from olive oil) can quickly inhibit the NF-κB pathway, reducing neutrophil infiltration (56). Overall, the effects of FMT and DI on CRP and FC still need to be further validated in the future.
Another explanation for the lack of significant changes in inflammatory markers (such as CRP) in our study is that we believe these interventions may be more focused on improving symptoms (such as pain and diarrhea) and quality of life, rather than directly reducing baseline inflammation levels. Existing research has shown that the correlation between self-reported symptoms and inflammatory markers is often weak. For example, Gracie et al. (119) noted that the clinical disease activity index does not strongly correlate with endoscopic mucosal inflammation and FCP levels. Seaton et al. (22) also found that psychological factors (such as depression) significantly affect self-reported symptoms, while these symptoms are not closely related to inflammatory markers (22). Similar studies have suggested that, despite no significant changes in inflammatory markers such as FCP, improvements in symptoms and quality of life can still be significant (120–122). Future studies could further explore the mechanisms behind this discrepancy, particularly the role of psychological factors in symptom perception. Such research could help optimize treatment strategies for IBD by integrating these factors.
While our study does incorporate cognitive behavioral therapy (CBT) as part of the psychological intervention, we acknowledge that there may be some differences compared to other studies in the specific application or methodology of CBT. In our study, CBT was combined with other non-pharmacological interventions, such as acupuncture/moxibustion, dietary interventions, and fecal microbiota transplantation. Although these interventions have shown positive results in improving clinical symptoms and disease activity, they may not have had a significant impact on inflammatory markers (such as CRP and FCP) due to differences in the mechanisms of action compared to the interventions described in other studies.
Although we used CBT in our study, there may be differences in terms of the intensity of the intervention, its duration, or the characteristics of the participants when compared to formal study (21). These differences could explain why the impact of CBT on CRP and FCP in our study was not as pronounced as in studies that specifically focused on emotional and psychological health.
Additionally, as noted by Riggott, the effects of psychological interventions can vary significantly depending on study design, participant characteristics, and the intervention methods used (123). While CBT is beneficial in alleviating psychological symptoms such as anxiety and depression, more intensive or longer-term interventions may be necessary to observe significant changes in inflammatory markers. The intervention protocol in our study may have differed in these respects from other studies.
This NMA is, to our knowledge, the first study to compare and summarize the effectiveness and safety of non-pharmacological treatments in IBD patients. It was registered in PROSPERO prior to commencement and followed PRISMA guidelines. All the studies included in this analysis were randomized controlled trials (RCTs), with Jadad scores above 3 points, ensuring a high level of evidence. The outcomes assessed incorporated both clinical manifestations and patient-reported outcomes, combining objective measures with subjective descriptions, allowing for a more comprehensive assessment of treatment efficacy.
However, several limitations must be acknowledged. Firstly, the heterogeneity among the different interventions, variations in treatment courses, indirect comparisons, and differences in outcome measures are notable limitations. While statistical homogeneity was observed across studies, clinical heterogeneity in patient characteristics (e.g., disease duration, baseline severity), treatment protocols (e.g., APMX modalities, FMT donor selection criteria), and outcome definitions (e.g., varying thresholds for clinical remission) may limit the generalizability of our findings. Future research should standardize core outcome measures for NPTs in IBD to enhance cross-trial comparability. Secondly, the sample sizes in many studies were relatively small, and nearly half of the studies were assessed as having an “unclear or high risk of bias” in terms of selection, performance, and detection biases. Thirdly, this study mainly explores the short-term to mid-term efficacy of NPTs, so the long-term effects of NPTs needed further investigation. Additionally, a key limitation of our study is the broad categorization of interventions. Future research should refine the intervention types (such as different dietary patterns, psychological therapies, and exercise intensities) to assess the specific efficacy of each intervention on IBD. This approach will not only help to reveal the differences in the effects of various interventions but will also provide more targeted guidance for non-pharmacological treatments of IBD. Besides, One of the main limitations of this network meta-analysis is the heterogeneity in the reporting and definitions of adverse effects and biomarker outcomes across the included studies. Different studies used varying definitions of adverse events and biomarker measurements, making it difficult to directly compare results across studies. Additionally, some studies did not report certain adverse effects or biomarkers, further complicating our analysis. Due to insufficient sample size, a detailed subgroup analysis could not be performed. The absence of subgroup analysis may limit our assessment of the applicability and external validity of the treatment effects across different patient populations. Despite these limitations, our study provides valuable insights into the potential benefits of non-pharmacological interventions for IBD. SUCRA ranking provides only a relative ranking of treatments. In clinical practice, it should be considered alongside the quality of evidence for each outcome, the risk of bias, and the clinical significance of the treatment effects for a comprehensive evaluation. By addressing these limitations in future research, we can better understand the nuances of each intervention and improve the precision of treatment recommendations, ultimately enhancing patient care and outcomes in IBD management.
Conclusion
This NMA provides comprehensive insights into the efficacy and safety of common non-pharmacological interventions for IBD. The results highlight that APMX is the most effective treatment for inducing clinical remission, alleviating disease activity, and improving quality of life. DI are best for maintaining clinical remission and reducing serum FC levels, while FMT was found to be the most effective at reducing serum CRP levels. These findings underscore the promising potential of non-pharmacological treatments in managing IBD and improving patient outcomes. Although APMX shows potential as a non-pharmacological intervention for inflammatory bowel disease, the current evidence is limited by methodological shortcomings and heterogeneity among studies. Therefore, further high-quality, rigorously designed randomized controlled trials are warranted to confirm its efficacy and safety. In the meantime, the current evidence should be applied cautiously in clinical decision-making. While caution is warranted in the clinical application of APMX due to the current limitations in evidence quality, it is encouraging that several other non-pharmacological interventions demonstrated promising efficacy. These findings offer patients a broader range of scientifically supported treatment options, laying the groundwork for personalized care and empowering individuals to make informed decisions based on their unique needs and preferences.
Strength and limitations
1. To our knowledge, this is the first network meta-analysis comparing the effectiveness and safety of non-pharmacological treatment for IBD. The study evaluated a wide range of outcomes, including clinical remission, disease activity, quality of life (QOL), serum biomarkers (FC and CRP), and adverse effects, offering a holistic view of treatment efficacy and safety.
2. The use of network meta-analysis (NMA) allowed for direct and indirect comparisons of multiple NPTs, providing a robust ranking of interventions (e.g., APMX, DI, FMT) based on their effectiveness across diverse outcomes.
3. The application of Cochrane Handbook guidelines and GRADEpro software for quality assessment, along with funnel plots to evaluate publication bias, enhanced the reliability and transparency of the findings. This study emphasizes the management of mental health issues in IBD patients, evaluating the effectiveness of psychological interventions like cognitive behavioral therapy, reflecting the growing importance of comprehensive care for IBD patients, and aligning with the current trends in IBD management.
4. Variability in study designs, patient populations, and intervention protocols across the included RCTs may have introduced heterogeneity, potentially affecting the consistency and generalizability of the findings. Despite the use of funnel plots, the possibility of unpublished negative results or selective reporting in the included studies could not be entirely ruled out, potentially skewing the findings.
5. The reliance on aggregated data from published RCTs limited the ability to perform patient-level analyses or adjust for confounding factors that may have varied across studies.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JJ: Software, Investigation, Writing – review and editing, Project administration. Y-bW: Writing – review and editing, Writing – original draft, Software, Project administration, Methodology. S-wL: Software, Writing – review and editing, Project administration. W-jC: Validation, Supervision, Writing – review and editing. R-lL: Supervision, Validation, Writing – review and editing. Y-lB: Validation, Writing – review and editing, Supervision, Visualization. LH: Supervision, Conceptualization, Funding acquisition, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Nos. 81774238, 81373563 and 30772689).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1593483/full#supplementary-material
Supplementary File 1 Search strides.
Supplementary File 2 Consistency and inconsistency analysis.
Supplementary File 3 Definition of clinical outcomes.
References
1. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. (2020) 5:17–30. doi: 10.1016/S2468-125330333-4
2. Singh N, Bernstein C. Environmental risk factors for inflammatory bowel disease. U Eur Gastroenterol J. (2022) 10:1047–53. doi: 10.1002/ueg2.12319
3. Khor B, Gardet A, Xavier R. Genetics and pathogenesis of inflammatory bowel disease. Nature. (2011) 474:307–17. doi: 10.1038/nature10209
4. Ni J, Wu G, Albenberg L, Tomov V. Gut microbiota and IBD: Causation or correlation? Nat Rev Gastroenterol Hepatol. (2017) 14:573–84. doi: 10.1038/nrgastro.2017.88
5. Jacobs J, Sauk J, Ahdoot A, Liang F, Katzka W, Ryu H, et al. Microbial and metabolite signatures of stress reactivity in ulcerative colitis patients in clinical remission predict clinical flare risk. Inflamm Bowel Dis. (2024) 30:336–46. doi: 10.1093/ibd/izad185
6. Barberio B, Zamani M, Black C, Savarino E, Ford A. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2021) 6:359–70. doi: 10.1016/S2468-125300014-5
7. Foster J, McVey N. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. (2013) 36:305–12. doi: 10.1016/j.tins.2013.01.005
8. Lamb C, Kennedy N, Raine T, Hendy P, Smith P, Limdi J, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. (2019) 68:s1–106. doi: 10.1136/gutjnl-2019-318484
9. Subramanian V, Saxena S, Kang J, Pollok R. Preoperative steroid use and risk of postoperative complications in patients with inflammatory bowel disease undergoing abdominal surgery. Am J Gastroenterol. (2008) 103:2373–81. doi: 10.1111/j.1572-0241.2008.01942.x
10. Muller A, Stevens P, McIntyre A, Ellison H, Logan R. Experience of 5-aminosalicylate nephrotoxicity in the united kingdom. Aliment Pharmacol Ther. (2005) 21:1217–24. doi: 10.1111/j.1365-2036.2005.02462.x
11. Moss J, Parry C, Holt R, McWilliam S. 5-ASA induced interstitial nephritis in patients with inflammatory bowel disease: A systematic review. Eur J Med Res. (2022) 27:61. doi: 10.1186/s40001-022-00687-y
12. Liu J, Di B, Xu L. Recent advances in the treatment of IBD: Targets, mechanisms and related therapies. Cytokine Growth Factor Rev. (2023) 71-72:1–12. doi: 10.1016/j.cytogfr.2023.07.001
13. Ford A, Bernstein C, Khan K, Abreu M, Marshall J, Talley N, et al. Glucocorticosteroid therapy in inflammatory bowel disease: Systematic review and meta-analysis. Am J Gastroenterol. (2011) 106:590–9. doi: 10.1038/ajg.2011.70
14. Kandiel A, Fraser A, Korelitz B, Brensinger C, Lewis J. Increased risk of lymphoma among inflammatory Bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. (2005) 54:1121–5. doi: 10.1136/gut.2004.049460
15. Bickston S, Muniyappa K. Natalizumab for the treatment of crohn’s disease. Expert Rev Clin Immunol. (2010) 6:513–9. doi: 10.1586/eci.10.38
16. Graff L, Walker J, Lix L, Clara I, Rawsthorne P, Rogala L, et al. The relationship of inflammatory bowel disease type and activity to psychological functioning and quality of life. Clin Gastroenterol Hepatol. (2006) 4:1491–501. doi: 10.1016/j.cgh.2006.09.027
17. Wang X, Zhao N, Sun Y, Bai X, Si J, Liu J, et al. Acupuncture for ulcerative colitis: A systematic review and meta-analysis of randomized clinical trials. BMC Complement Med Ther. (2020) 20:309. doi: 10.1186/s12906-020-03101-4
18. Limketkai B, Iheozor-Ejiofor Z, Gjuladin-Hellon T, Parian A, Matarese L, Bracewell K, et al. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst Rev. (2019) 2:CD012839. doi: 10.1002/14651858.CD012839.pub2
19. Imdad A, Nicholson M, Tanner-Smith E, Zackular J, Gomez-Duarte O, Beaulieu D. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. (2018) 11:CD012774. doi: 10.1002/14651858.CD012774.pub2
20. Jones K, Kimble R, Baker K, Tew G. Effects of structured exercise programmes on physiological and psychological outcomes in adults with inflammatory bowel disease (IBD): A systematic review and meta-analysis. PLoS One. (2022) 17:e0278480. doi: 10.1371/journal.pone.0278480
21. Naude C, Skvarc D, Knowles S, Russell L, Evans S, Mikocka-Walus A. The effectiveness of mindfulness-based interventions in inflammatory bowel disease: A systematic review & meta-analysis. J Psychosom Res. (2023) 169:111232. doi: 10.1016/j.jpsychores.2023.111232
22. Seaton N, Hudson J, Harding S, Norton S, Mondelli V, Jones A, et al. Do interventions for mood improve inflammatory biomarkers in inflammatory bowel disease?: A systematic review and meta-analysis. Ebiomedicine. (2024) 100:104910. doi: 10.1016/j.ebiom.2023.104910
23. El H, Ghoneim S, Shah S, Chahine A, Mourad F, Francis F, et al. Efficacy of fecal microbiota transplantation in the treatment of active ulcerative colitis: A systematic review and meta-analysis of double-blind randomized controlled trials. Inflamm Bowel Dis. (2023) 29:808–17. doi: 10.1093/ibd/izac135
24. Yang X, He M, Tang Q, Wang Z, Jin D, Wu X, et al. Assessment of anti-inflammatory efficacy of acupuncture in patients with inflammatory bowel disease: A systematic review and meta-analysis. Complement Ther Med. (2023) 74:102946. doi: 10.1016/j.ctim.2023.102946
25. Limketkai B, Godoy-Brewer G, Parian A, Noorian S, Krishna M, Shah N, et al. Dietary interventions for the treatment of inflammatory bowel diseases: An updated systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2023) 21:2508–25. doi: 10.1016/j.cgh.2022.11.026
26. Imdad A, Pandit N, Zaman M, Minkoff N, Tanner-Smith E, Gomez-Duarte O, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. (2023) 4:CD012774. doi: 10.1002/14651858.CD012774.pub3
27. Jones J, Loftus E, Panaccione R, Chen L, Peterson S, McConnell J, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. (2008) 6:1218–24. doi: 10.1016/j.cgh.2008.06.010
28. Cellier C, Sahmoud T, Froguel E, Adenis A, Belaiche J, Bretagne J, et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The groupe d’etudes therapeutiques des affections inflammatoires digestives. Gut. (1994) 35:231–5. doi: 10.1136/gut.35.2.231
29. Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
30. Savovic J, Weeks L, Sterne J, Turner L, Altman D, Moher D, et al. Evaluation of the Cochrane collaboration’s tool for assessing the risk of bias in randomized trials: Focus groups, online survey, proposed recommendations and their implementation. Syst Rev. (2014) 3:37. doi: 10.1186/2046-4053-3-37
31. Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
32. Kedia S, Virmani S, Bajaj A, Markandey M, Singh N, Madan D, et al. Coconut water induces clinical remission in mild to moderate ulcerative colitis: Double-blind placebo-controlled trial. Clin Gastroenterol Hepatol. (2024) 22:1295–306. doi: 10.1016/j.cgh.2024.01.013
33. Narimani B, Sadeghi A, Daryani N, Shahrokh S, Nilghaz M, Ghods M, et al. Effectiveness of a novel diet in attenuation of clinical activity of disease in patients with ulcerative colitis: A randomized, clinical trial. Sci Rep. (2024) 14:13791. doi: 10.1038/s41598-024-64512-8
34. Naude, C, Skvarc D, Maunick B, Evans S, Romano D, Chesterman S, et al. Acceptance and commitment therapy for adults living with inflammatory bowel disease and distress: A randomized controlled trial. Am J Gastroenterol. (2024). doi: 10.14309/ajg.0000000000003032 [Epub ahead of print].
35. Haskey N, Estaki M, Ye J, Shim R, Singh S, Dieleman L, et al. A mediterranean diet pattern improves intestinal inflammation concomitant with reshaping of the bacteriome in ulcerative colitis: A randomised controlled trial. J Crohns Colitis. (2023) 17:1569–78. doi: 10.1093/ecco-jcc/jjad073
36. Lahtinen P, Jalanka J, Mattila E, Tillonen J, Bergman P, Satokari R, et al. Fecal microbiota transplantation for the maintenance of remission in patients with ulcerative colitis: A randomized controlled trial. World J Gastroenterol. (2023) 29:2666–78. doi: 10.3748/wjg.v29.i17.2666
37. Miyaguchi K, Tsuzuki Y, Ichikawa Y, Shiomi R, Ohgo H, Nakamoto H, et al. Positive zinc intake and a Japanese diet rich in n-3 fatty acids induces clinical remission in patients with mild active ulcerative colitis: A randomized interventional pilot study. J Clin Biochem Nutr. (2023) 72:82–8. doi: 10.3164/jcbn.22-72
38. Bao C, Wu L, Wang D, Chen L, Jin X, Shi Y, et al. Acupuncture improves the symptoms, intestinal microbiota, and inflammation of patients with mild to moderate Crohn’s disease: A randomized controlled trial. Eclinicalmedicine. (2022) 45:101300. doi: 10.1016/j.eclinm.2022.101300
39. Chen Z, Li J, Ma Q, Pikov V, Li M, Wang L, et al. Anti-inflammatory effects of two-week sacral nerve stimulation therapy in patients with ulcerative colitis. Neuromodulation. (2022) 27:360–71. doi: 10.1016/j.neurom.2023.01.019
40. Goren G, Schwartz D, Friger M, Banai H, Sergienko R, Regev S, et al. Randomized controlled trial of cognitive-behavioral and mindfulness-based stress reduction on the quality of life of patients with crohn disease. Inflamm Bowel Dis. (2022) 28:393–408. doi: 10.1093/ibd/izab083
41. Haifer C, Paramsothy S, Kaakoush N, Saikal A, Ghaly S, Yang T, et al. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (lotus): A randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. (2022) 7:141. doi: 10.1016/S2468-125300400-3
42. Jedel S, Beck T, Swanson G, Hood M, Voigt R, Gorenz A, et al. Mindfulness intervention decreases frequency and severity of flares in inactive ulcerative colitis patients: Results of a phase ii, randomized, placebo-controlled trial. Inflamm Bowel Dis. (2022) 28:1872–92. doi: 10.1093/ibd/izac036
43. Kedia S, Virmani S, Vuyyuru S, Kumar P, Kante B, Sahu P, et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-aid) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: A randomised controlled trial. Gut. (2022) 71:2401–13. doi: 10.1136/gutjnl-2022-327811
44. Keshteli A, Valcheva R, Nickurak C, Park H, Mandal R, van Diepen K, et al. Anti-inflammatory diet prevents subclinical colonic inflammation and alters metabolomic profile of ulcerative colitis patients in clinical remission. Nutrients. (2022) 14:3294. doi: 10.3390/nu14163294
45. Peerani F, Watt M, Ismond K, Whitlock R, Ambrosio L, Hotte N, et al. A randomized controlled trial of a multicomponent online stress reduction intervention in inflammatory bowel disease. Ther Adv Gastroenterol. (2022) 15:1098310214. doi: 10.1177/17562848221127238
46. Sarbagili Shabat C, Scaldaferri F, Zittan E, Hirsch A, Mentella M, Musca T, et al. Use of faecal transplantation with a novel diet for mild to moderate active ulcerative colitis: The craft UC randomised controlled trial. J Crohns Colitis. (2022) 16:369–78. doi: 10.1093/ecco-jcc/jjab165
47. Yanai H, Levine A, Hirsch A, Boneh R, Kopylov U, Eran H, et al. The crohn’s disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn’s disease (CDED-ad): An open-label, pilot, randomised trial. Lancet Gastroenterol Hepatol. (2022) 7:49. doi: 10.1016/S2468-125300299-5
48. Bernabeu P, Van-der Hofstadt C, Rodríguez-Marín J, Gutierrez A, Alonso M, Zapater P, et al. Effectiveness of a multicomponent group psychological intervention program in patients with inflammatory bowel disease: A randomized trial. Int J Environ Res Public Health. (2021) 18:5439. doi: 10.3390/ijerph18105439
49. Březina J, Bajer L, Wohl P, Ďuricová D, Hrabák P, Novotný A, et al. Fecal microbial transplantation versus mesalamine enema for treatment of active left-sided ulcerative colitis–results of a randomized controlled trial. J Clin Med. (2021) 10:2753. doi: 10.3390/jcm10132753
50. Crothers J, Chu N, Nguyen L, Phillips M, Collins C, Fortner K, et al. Daily, oral fmt for long-term maintenance therapy in ulcerative colitis: Results of a single-center, prospective, randomized pilot study. Bmc Gastroenterol. (2021) 21:281. doi: 10.1186/s12876-021-01856-9
51. Ewais T, Begun J, Kenny M, Hay K, Houldin E, Chuang K, et al. Mindfulness based cognitive therapy for youth with inflammatory bowel disease and depression – findings from a pilot randomised controlled trial. J Psychosom Res. (2021) 149:110594. doi: 10.1016/j.jpsychores.2021.110594
52. Fang H, Fu L, Li X, Lu C, Su Y, Xiong K, et al. Long-term efficacy and safety of monotherapy with a single fresh fecal microbiota transplant for recurrent active ulcerative colitis: A prospective randomized pilot study. Microb Cell Fact. (2021) 20:18. doi: 10.1186/s12934-021-01513-6
53. Fritsch J, Garces L, Quintero M, Pignac-Kobinger J, Santander A, Fernandez I, et al. Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Clin Gastroenterol Hepatol. (2021) 19:1189–99. doi: 10.1016/j.cgh.2020.05.026
54. Lacerda J, Lagos A, Carolino E, Silva-Herdade A, Silva M, Sousa Guerreiro C. Functional food components, intestinal permeability and inflammatory markers in patients with inflammatory bowel disease. Nutrients. (2021) 13:642. doi: 10.3390/nu13020642
55. Lewis J, Sandler R, Brotherton C, Brensinger C, Li H, Kappelman M, et al. A randomized trial comparing the specific carbohydrate diet to a mediterranean diet in adults with Crohn’s disease. Gastroenterology. (2021) 161:837–52. doi: 10.1053/j.gastro.2021.05.047
56. Cox S, Lindsay J, Fromentin S, Stagg A, McCarthy N, Galleron N, et al. Effects of low fodmap diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology. (2020) 158:176–88. doi: 10.1053/j.gastro.2019.09.024
57. González-Moret R, Cebolla A, Cortés X, Baños R, Navarrete J, de la Rubia JE, et al. The effect of a mindfulness-based therapy on different biomarkers among patients with inflammatory Bowel disease: A randomised controlled trial. Sci Rep. (2020) 10:6071. doi: 10.1038/s41598-020-63168-4
58. Horta D, Lira A, Sanchez-Lloansi M, Villoria A, Teggiachi M, Garcia-Rojo D, et al. A prospective pilot randomized study: Electroacupuncture vs. Sham procedure for the treatment of fatigue in patients with quiescent inflammatory bowel disease. Inflamm Bowel Dis. (2020) 26:484–92. doi: 10.1093/ibd/izz091
59. Hunt M, Loftus P, Accardo M, Keenan M, Cohen L, Osterman M. Self-help cognitive behavioral therapy improves health-related quality of life for inflammatory bowel disease patients: A randomized controlled effectiveness trial. J Clin Psychol Med Settings. (2020) 27:467–79. doi: 10.1007/s10880-019-09621-7
60. Jones K, Baker K, Speight R, Thompson N, Tew G. Randomised clinical trial: Combined impact and resistance training in adults with stable Crohn’s disease. Aliment Pharmacol Ther. (2020) 52:964–75. doi: 10.1111/apt.16002
61. Langhorst J, Schols M, Cinar Z, Eilert R, Kofink K, Paul A, et al. Comprehensive lifestyle-modification in patients with ulcerative colitis-a randomized controlled trial. J Clin Med. (2020) 9:3087. doi: 10.3390/jcm9103087
62. Schierová D, Březina J, Mrázek J, Fliegerová K, Kvasnová S, Bajer L, et al. Gut microbiome changes in patients with active left-sided ulcerative colitis after fecal microbiome transplantation and topical 5-aminosalicylic acid therapy. Cells. (2020) 9:2283. doi: 10.3390/cells9102283
63. Seeger W, Thieringer J, Esters P, Allmendinger B, Stein J, Schulze H, et al. Moderate endurance and muscle training is beneficial and safe in patients with quiescent or mildly active crohn’s disease. U Eur Gastroenterol J. (2020) 8:804–13. doi: 10.1177/2050640620936383
64. Artom M, Czuber-Dochan W, Sturt J, Proudfoot H, Roberts D, Norton C. Cognitive-behavioural therapy for the management of inflammatory bowel disease-fatigue: A feasibility randomised controlled trial. Pilot Feasibil Stud. (2019) 5:145. doi: 10.1186/s40814-019-0538-y
65. Bodini G, Zanella C, Crespi M, Lo Pumo S, Demarzo M, Savarino E, et al. A randomized, 6-wk trial of a low fodmap diet in patients with inflammatory bowel disease. Nutrition. (2019) 67-68:110542. doi: 10.1016/j.nut.2019.06.023
66. Costello S, Hughes P, Waters O, Bryant R, Vincent A, Blatchford P, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis. JAMA. (2019) 321:156. doi: 10.1001/jama.2018.20046
67. Cronin O, Barton W, Moran C, Sheehan D, Whiston R, Nugent H, et al. Moderate-intensity aerobic and resistance exercise is safe and favorably influences body composition in patients with quiescent inflammatory bowel disease: A randomized controlled cross-over trial. BMC Gastroenterol. (2019) 19:29. doi: 10.1186/s12876-019-0952-x
68. Sood A, Mahajan R, Singh A, Midha V, Mehta V, Narang V, et al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: A pilot study. J Crohns Colitis. (2019) 13:1311–7. doi: 10.1093/ecco-jcc/jjz060
69. Tew G, Leighton D, Carpenter R, Anderson S, Langmead L, Ramage J, et al. High-intensity interval training and moderate-intensity continuous training in adults with crohn’s disease: A pilot randomised controlled trial. BMC Gastroenterol. (2019) 19:19. doi: 10.1186/s12876-019-0936-x
70. Wynne B, McHugh L, Gao W, Keegan D, Byrne K, Rowan C, et al. Acceptance and commitment therapy reduces psychological stress in patients with inflammatory Bowel diseases. Gastroenterology. (2019) 156:935–45. doi: 10.1053/j.gastro.2018.11.030
71. Stapersma L, van den Brink G, van der Ende J, Szigethy E, Beukers R, Korpershoek T, et al. Effectiveness of disease-specific cognitive behavioral therapy on anxiety, depression, and quality of life in youth with inflammatory bowel disease: A randomized controlled trial. J Pediatr Psychol. (2018) 43:967–80. doi: 10.1093/jpepsy/jsy029
72. Bhattacharyya S, Shumard T, Xie H, Dodda A, Varady K, Feferman L, et al. A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity. Nutr Healthy Aging. (2017) 4:181–92. doi: 10.3233/NHA-170023
73. Cox S, Prince A, Myers C, Irving P, Lindsay J, Lomer M, et al. Fermentable carbohydrates [fodmaps] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: A randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. (2017) 11:1420–9. doi: 10.1093/ecco-jcc/jjx073
74. Cramer H, Schäfer M, Schöls M, Köcke J, Elsenbruch S, Lauche R, et al. Randomised clinical trial: Yoga vs written self-care advice for ulcerative colitis. Aliment Pharmacol Ther. (2017) 45:1379–89. doi: 10.1111/apt.14062
75. Mikocka-Walus A, Bampton P, Hetzel D, Hughes P, Esterman A, Andrews J. Cognitive-behavioural therapy for inflammatory bowel disease: 24-month data from a randomised controlled trial. Int J Behav Med. (2017) 24:127–35. doi: 10.1007/s12529-016-9580-9
76. Paramsothy S, Kamm M, Kaakoush N, Walsh A, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet. (2017) 389:1218–28. doi: 10.1016/S0140-673630182-4
77. Pedersen N, Ankersen D, Felding M, Wachmann H, Vegh Z, Molzen L, et al. Low-fodmap diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J Gastroenterol. (2017) 23:3356–66. doi: 10.3748/wjg.v23.i18.3356
78. Bao C, Liu P, Liu H, Jin X, Calhoun V, Wu L, et al. Different brain responses to electro-acupuncture and moxibustion treatment in patients with Crohn’s disease. Sci Rep. (2016) 6:36636. doi: 10.1038/srep36636
79. Gunasekeera V, Mendall M, Chan D, Kumar D. Treatment of Crohn’s disease with an igg4-guided exclusion diet: A randomized controlled trial. Dig Dis Sci. (2016) 61:1148–57. doi: 10.1007/s10620-015-3987-z
80. Halmos E, Christophersen C, Bird A, Shepherd S, Muir J, Gibson P. Consistent prebiotic effect on gut microbiota with altered fodmap intake in patients with Crohn’s disease: A randomised, controlled cross-over trial of well-defined diets. Clin Transl Gastroenterol. (2016) 7:e164. doi: 10.1038/ctg.2016.22
81. Gerbarg P, Jacob V, Stevens L, Bosworth B, Chabouni F, DeFilippis E, et al. The effect of breathing, movement, and meditation on psychological and physical symptoms and inflammatory biomarkers in inflammatory bowel disease. Inflamm Bowel Dis. (2015) 21:2886–96. doi: 10.1097/MIB.0000000000000568
82. Klare P, Nigg J, Nold J, Haller B, Krug A, Mair S, et al. The impact of a ten-week physical exercise program on health-related quality of life in patients with inflammatory bowel disease: A prospective randomized controlled trial. Digestion. (2015) 91:239–47. doi: 10.1159/000371795
83. Moayyedi P, Surette M, Kim P, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. (2015) 149:102–9. doi: 10.1053/j.gastro.2015.04.001
84. Rossen N, Fuentes S, van der Spek M, Tijssen J, Hartman J, Duflou A, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. (2015) 149:110–8. doi: 10.1053/j.gastro.2015.03.045
85. Schoultz M, Atherton I, Watson A. Mindfulness-based cognitive therapy for inflammatory bowel disease patients: Findings from an exploratory pilot randomised controlled trial. Trials. (2015) 16:379. doi: 10.1186/s13063-015-0909-5
86. Sharma P, Poojary G, Dwivedi S, Deepak K. Effect of yoga-based intervention in patients with inflammatory bowel disease. Int J Yoga Ther. (2015) 25:101–12. doi: 10.17761/1531-2054-25.1.101
87. Bao C. Randomized controlled trial: Moxibustion and acupuncture for the treatment of crohn’s disease. World J Gastroenterol. (2014) 20:11000. doi: 10.3748/wjg.v20.i31.11000
88. Berrill J, Sadlier M, Hood K, Green J. Mindfulness-based therapy for inflammatory Bowel disease patients with functional abdominal symptoms or high perceived stress levels. J Crohns Colitis. (2014) 8:945–55. doi: 10.1016/j.crohns.2014.01.018
89. Jedel S, Hoffman A, Merriman P, Swanson B, Voigt R, Rajan K, et al. A randomized controlled trial of mindfulness-based stress reduction to prevent flare-up in patients with inactive ulcerative colitis. Digestion. (2014) 89:142–55. doi: 10.1159/000356316
90. Kyaw M, Moshkovska T, Mayberry J. A prospective, randomized, controlled, exploratory study of comprehensive dietary advice in ulcerative colitis. Eur J Gastroenterol Hepatol. (2014) 26:910–7. doi: 10.1097/MEG.0000000000000127
91. Joos S, Wildau N, Kohnen R, Szecsenyi J, Schuppan D, Willich S, et al. Acupuncture and moxibustion in the treatment of ulcerative colitis: A randomized controlled study. Scand J Gastroenterol. (2009) 41:1056–63. doi: 10.1080/00365520600580688
92. Ng V, Millard W, Lebrun C, Howard J. Low-intensity exercise improves quality of life in patients with Crohn’s disease. Clin J Sport Med. (2007) 17:384–8. doi: 10.1097/JSM.0b013e31802b4fda
93. Joos S, Brinkhaus B, Maluche C, Maupai N, Kohnen R, Kraehmer N, et al. Acupuncture and moxibustion in the treatment of active Crohn’s disease: A randomized controlled study. Digestion. (2004) 69:131–9. doi: 10.1159/000078151
94. Horta D, Lira A, Sanchez-Lloansi M, Villoria A, Teggiachi M, García-Rojo D, et al. A prospective pilot randomized study: Electroacupuncture vs. Sham procedure for the treatment of fatigue in patients with quiescent inflammatory bowel disease. Inflamm Bowel Dis. (2019) 26:484–92. doi: 10.1093/ibd/izz091
95. Papada E, Amerikanou C, Torović L, Kalogeropoulos N, Tzavara C, Forbes A, et al. Plasma free amino acid profile in quiescent inflammatory bowel disease patients orally administered with mastiha (pistacia lentiscus); A randomised clinical trial. Phytomedicine. (2019) 56:40–7. doi: 10.1016/j.phymed.2018.08.008
96. Le Berre C, Ananthakrishnan A, Danese S, Singh S, Peyrin-Biroulet L. Ulcerative colitis and Crohn’s disease have similar burden and goals for treatment. Clin Gastroenterol Hepatol. (2020) 18:14–23. doi: 10.1016/j.cgh.2019.07.005
97. Plevris N, Lees C. Disease monitoring in inflammatory bowel disease: Evolving principles and possibilities. Gastroenterology. (2022) 162:1456–75. doi: 10.1053/j.gastro.2022.01.024
98. Holko P, Kawalec P, Mossakowska M, Pilc A. Health-related quality of life impairment and indirect cost of crohn’s disease: A self-report study in Poland. PLoS One. (2016) 11:e0168586. doi: 10.1371/journal.pone.0168586
99. Mosli M, Zou G, Garg S, Feagan S, MacDonald J, Chande N, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: A systematic review and meta-analysis. Am J Gastroenterol. (2015) 110:802–19. doi: 10.1038/ajg.2015.120
100. Picardo S, Altuwaijri M, Devlin S, Seow C. Complementary and alternative medications in the management of inflammatory bowel disease. Ther Adv Gastroenterol. (2020) 13:320850882. doi: 10.1177/1756284820927550
101. Yang X, He M, Cao J, Tang Q, Yang B, Li T, et al. Acupuncture and moxibustion for inflammatory Bowel disease: Regulatory mechanisms revealed by microbiome and metabolomic analysis. Am J Chin Med. (2024) 52:1891–923. doi: 10.1142/S0192415X24500745
102. Liu G, Zhuo X, Huang Y, Liu H, Wu R, Kuo C, et al. Alterations in gut microbiota and upregulations of vpac2 and intestinal tight junctions correlate with anti-inflammatory effects of electroacupuncture in colitis mice with sleep fragmentation. Biology (Basel). (2022) 11:962. doi: 10.3390/biology11070962
103. Wang Y, Zhu H, Lv X, Ren X, Peng Y, Qu J, et al. Electroacupuncture zusanli (st36) relieves somatic pain in colitis rats by inhibiting dorsal root ganglion sympathetic-sensory coupling and neurogenic inflammation. Neural Plast. (2023) 2023:9303419. doi: 10.1155/2023/9303419
104. Herrador-Lopez M, Martin-Masot R, Navas-Lopez V. Dietary interventions in ulcerative colitis: A systematic review of the evidence with meta-analysis. Nutrients. (2023) 15:4194. doi: 10.3390/nu15194194
105. Prince A, Myers C, Joyce T, Irving P, Lomer M, Whelan K. Fermentable carbohydrate restriction (low fodmap diet) in clinical practice improves functional gastrointestinal symptoms in patients with inflammatory bowel disease. Inflamm Bowel Dis. (2016) 22:1129–36. doi: 10.1097/MIB.0000000000000708
106. Chicco F, Magri S, Cingolani A, Paduano D, Pesenti M, Zara F, et al. Multidimensional impact of mediterranean diet on IBD patients. Inflamm Bowel Dis. (2021) 27:1–9. doi: 10.1093/ibd/izaa097
107. Levine A, Rhodes J, Lindsay J, Abreu M, Kamm M, Gibson P, et al. Dietary guidance from the international organization for the study of inflammatory Bowel diseases. Clin Gastroenterol Hepatol. (2020) 18:1381–92. doi: 10.1016/j.cgh.2020.01.046
108. Cabre E, Manosa M, Gassull M. Omega-3 fatty acids and inflammatory bowel diseases –a systematic review. Br J Nutr. (2012) 107:S240–52. doi: 10.1017/S0007114512001626
109. Khan K, Ullman T, Ford A, Abreu M, Abadir A, Marshall J, et al. Antibiotic therapy in inflammatory bowel disease: A systematic review and meta-analysis. Am J Gastroenterol. (2011) 106:661–73. doi: 10.1038/ajg.2011.72
110. Prantera C, Lochs H, Campieri M, Scribano M, Sturniolo G, Castiglione F, et al. Antibiotic treatment of Crohn’s disease: Results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment Pharmacol Ther. (2006) 23:1117–25. doi: 10.1111/j.1365-2036.2006.02879.x
111. Mardini H, Grigorian A. Probiotic mix vsl#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: A meta-analysis. Inflamm Bowel Dis. (2014) 20:1562–7. doi: 10.1097/MIB.0000000000000084
112. Guslandi M. Saccharomyces boulardii plus rifaximin in mesalamine-intolerant ulcerative colitis. J Clin Gastroenterol. (2010) 44:385. doi: 10.1097/MCG.0b013e3181cb4233
113. Khoruts A, Dicksved J, Jansson J, Sadowsky M. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent clostridium difficile-associated diarrhea. J Clin Gastroenterol. (2010) 44:354–60. doi: 10.1097/MCG.0b013e3181c87e02
114. Hamilton M, Weingarden A, Unno T, Khoruts A, Sadowsky M. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. (2013) 4:125–35. doi: 10.4161/gmic.23571
115. Weingarden A, Gonzalez A, Vazquez-Baeza Y, Weiss S, Humphry G, Berg-Lyons D, et al. Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome. (2015) 3:10. doi: 10.1186/s40168-015-0070-0
116. Zhou S, Cui Y, Zhang Y, Zhao T, Cong J. Fecal microbiota transplantation for induction of remission in Crohn’s disease: A systematic review and meta-analysis. Int J Colorectal Dis. (2023) 38:62. doi: 10.1007/s00384-023-04354-4
117. Sproston N, Ashworth J. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. (2018) 9:754. doi: 10.3389/fimmu.2018.00754
118. Xiang B, Jiang M, Sun M, Dai C. Optimal range of fecal calprotectin for predicting mucosal healing in patients with inflammatory bowel disease: A systematic review and meta-analysis. Visc Med. (2021) 37:338–48. doi: 10.1159/000514196
119. Gracie D, Williams C, Sood R, Mumtaz S, Bholah M, Hamlin P, et al. Poor correlation between clinical disease activity and mucosal inflammation, and the role of psychological comorbidity, in inflammatory bowel disease. Am J Gastroenterol. (2016) 111:541–51. doi: 10.1038/ajg.2016.59
120. Yang Y, Rao K, Zhan K, Shen M, Zheng H, Qin S, et al. Clinical evidence of acupuncture and moxibustion for irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. Front Public Health. (2022) 10:1022145. doi: 10.3389/fpubh.2022.1022145
121. Vickers A, Vertosick E, Lewith G, MacPherson H, Foster N, Sherman K, et al. Acupuncture for chronic pain: Update of an individual patient data meta-analysis. J Pain. (2018) 19:455–74. doi: 10.1016/j.jpain.2017.11.005
122. Falvey J, Hoskin T, Meijer B, Ashcroft A, Walmsley R, Day A, et al. Disease activity assessment in IBD: Clinical indices and biomarkers fail to predict endoscopic remission. Inflamm Bowel Dis. (2015) 21:824–31. doi: 10.1097/MIB.0000000000000341
Keywords: inflammatory bowel disease, non-pharmacological therapies, network meta-analysis, effectiveness, safety
Citation: Jia J, Wu Y-b, Liu S-w, Chen W-j, Li R-l, Bai Y-l and Hu L (2025) Effectiveness and safety of non-pharmacological therapies for the treatment of inflammatory bowel disease: a network meta-analysis. Front. Med. 12:1593483. doi: 10.3389/fmed.2025.1593483
Received: 14 March 2025; Accepted: 09 June 2025;
Published: 30 June 2025.
Edited by:
Thomas Brzozowski, Jagiellonian University Medical College, PolandReviewed by:
Antonella Smeriglio, University of Messina, ItalyYomna Ali, Alexandria University, Egypt
Apurva Jadhav, Bharati Vidyapeeth Deemed University, India
Tiancheng Wang, Anhui University of Chinese Medicine, China
Shreyashi Pal, Birla Institute of Technology, Mesra, India
Copyright © 2025 Jia, Wu, Liu, Chen, Li, Bai and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-long Bai, eWxiYWlAZ3p1Y20uZWR1LmNu; Ling Hu, ZHJodWxpbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jing Jia
Jing Jia Si-wei Liu1
Si-wei Liu1 Ling Hu
Ling Hu