- 1Fujian Provincial Key Laboratory on Hematology, Fujian Institute of Hematology, Fujian Medical University Union Hospital, Fuzhou, China
- 2School of Basic Medical Science, Fujian Medical University, Fuzhou, China
Objective: This study aimed to evaluate the prognostic impact of methylation-related gene mutations in older patients with acute myeloid leukemia (AML).
Methods: We conducted a retrospective analysis of clinical characteristics in 645 patients aged ≥ 60 years diagnosed with AML at Fujian Medical University Union Hospital between July 2016 and December 2024.
Results: Methylation-related gene mutations—specifically DNMT3A, TET2, IDH1, and IDH2—were identified in 24.0%, 22.5%, 9.1%, and 13.8% of cases, respectively. Patients with single mutations in DNMT3A or TET2 exhibited similar long-term survival outcomes compared to those without these mutations, with median survival times of 25.2 and 22.3 months, respectively (p = 0.9639). However, patients with concurrent DNMT3A and TET2 mutations demonstrated the poorest treatment response and prognosis, achieving a complete remission (CR) rate of 35.5% and a median survival of only 6.2 months. In contrast, patients with IDH1/IDH2 mutations responded better to treatment, achieving a CR rate of 69.6% and a median survival of 34.7 months. Treatment regimens combining azacitidine and venetoclax did not provide additional improvement in treatment response for patients with methylation-related gene mutations compared to intensive chemotherapy (IC).
Conclusion: Concurrent mutations in DNMT3A and TET2 were associated with significantly poorer treatment response and survival outcomes. These common methylation-related gene mutations did not influence the choice between IC and azacitidine plus venetoclax combination therapy in elderly AML patients.
1 Introduction
Acute myeloid leukemia (AML) is one of the most common subtypes of leukemia in the elderly population (1, 2). Regrettably, the survival rates for this age group remain alarmingly low, largely due to the high prevalence of adverse cytogenetic prognostic factors (3–5), with five-year survival rates reported to be below 10% (2, 6, 7). Current treatment strategies for elderly AML patients are tailored based on factors including age, physical fitness, and the presence of adverse genetic prognostic markers (8, 9). Approximately 40% to 60% of physically fit patients treated with standard intensive chemotherapy (IC) regimens achieve complete remission (CR) (10). For patients unsuitable for IC, a combination therapy of hypomethylating agents (HMAs) and venetoclax is recommended as the frontline treatment (9, 11).
Methylation alterations play a pivotal role in the pathogenesis of cancer by modulating gene expression through changes in chromatin structure and the accessibility of transcription factors to DNA (12–14). In leukemia, aberrant methylation patterns, including hypermethylation of tumor suppressor genes or hypomethylation of oncogenes, can disrupt normal gene expression, thereby contributing to disease development (15, 16). Several methylation-related gene mutations have been identified in AML, with DNMT3A, TET2, IDH1, and IDH2 being the most frequently affected (17–20). However, the prognostic implications of these gene mutations in elderly AML patients, as well as their potential impact on the efficacy of IC or combined HMA and venetoclax regimens, are not well elucidated and necessitate further study.
In light of these considerations, this study aims to evaluate the impact of prevalent methylation-related gene mutations on the prognosis of elderly AML patients. Additionally, we seek to determine whether these mutations should influence the decision-making process when selecting between IC and combination treatment regimens incorporating HMAs. This research is critically important for refining therapeutic strategies tailored to the practical needs of elderly AML patients.
2 Methods
2.1 Study population
This retrospective study included a cohort of 645 elderly patients (aged 60 years and above) who were diagnosed with AML, spanning from July 2016 to December 2024 at the Fujian Medical University Union Hospital. Patients with available genetic mutation profiles were included in this study, whereas those lacking documented molecular analysis data were systematically excluded. A retrospective analysis of patient medical records enabled systematic compilation of multidimensional clinical datasets encompassing, including hematologic parameters and biochemical profiles, genetic aberrations, therapeutic protocols, objective treatment responses and long-term survival. This study was conducted in accordance with the principles of the Declaration of Helsinki. This retrospective cohort study utilizing anonymized clinical data was classified as non-interventional research under Declaration of Helsinki. In accordance with ICH-GCP guidelines regarding secondary data use, the requirement for written informed consent was formally waived by the Institutional Review Board of Fujian Medical University Union Hospital (approval no. 2024KY278), as the research posed no additional risks and preserved participant confidentiality.
2.2 Definition of AML and genetic mutations
The diagnosis of AML was established in accordance with the criteria provided in the 2016 revision of the World Health Organization (WHO) Classification of Tumours of Hematopoietic and Lymphoid Tissues, specifically pertaining to myeloid neoplasms and acute leukemia (21). Secondary AML (s-AML) was defined as AML that arose from pre-existing myeloproliferative disorders, such as myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPN), and chronic myelomonocytic leukemia (CMML). Patients with the blast phase of chronic myeloid leukemia (CML) were not included in this study. Therapy-related AML (t-AML) was identified as AML that developed following prior chemotherapy or radiation therapy for another malignancy. The chromosomal karyotype analysis was performed using R-banding technique in our center, and a complex karyotype was defined as the presence of three or more chromosomal aberrations. Gene mutations analysis were mainly conducted through targeted next-generation sequencing (NGS) using a custom-designed myeloid-focused gene panel (Kangsheng Global Medical Technology, Beijing) covering clinically actionable genes. Sequencing was performed on the Illumina NovaSeq X Plus platform, and the bioinformatic processing utilized an automated pipeline for hematologic neoplasm genomic reporting. The specific gene regions analyzed for mutation detection are summarized in Supplementary Table 1.
2.3 Treatment protocols
In this study, the “IC” regimen denotes the standard 3 + 7 protocol. Following the achievement of complete remission (CR) or complete remission with incomplete recovery (CRi) after IC treatment, intermediate or high-dose cytarabine was used as a consolidation therapy. Hypomethylating agent (HMA)-based therapies were administered either as monotherapy or in combination with low-dose chemotherapy regimens. For patients who achieved CR with HMA-based treatments, the same regimen was continued for consolidation. The azacitidine-venetoclax regimen utilized the standard dosage and administration. For patients who achieved CR with this combination, the concurrent administration of AZA and VEN was sustained until disease progression or intolerable adverse effects arose. A detailed summary of the initial treatment protocols for these patients, including the specific therapeutic approaches utilized, is provided in Supplementary Table 2.
2.4 Definition of response and outcomes
The effectiveness of therapeutic interventions was assessed in accordance with the 2022 edition (5th) of the European LeukemiaNet (ELN) response criteria for AML (8). Complete blood count measurements were obtained at the commencement of each treatment cycle, and bone marrow aspirations were conducted at intervals of one or two cycles to evaluate the therapeutic response. The overall response rate (ORR) was determined by combining the rates of CR, CR with incomplete hematologic recovery (CRi), and partial remission (PR). To identify minimal residual disease (MRD) in AML, an 8-color flow cytometric protocol was utilized at our center, employing the FACSCanto flow cytometer from BD company, United States. The protocol was designed with a lower limit of detection set at 10−4. Relapse was defined as the return of leukemic cells in the bone marrow or peripheral blood after achieving CR/CRi, indicated by blast cell percentages greater than 5%, or the appearance of extramedullary lesions. The duration of remission (DOR) was calculated as the time span from the achievement of CR/CRi or PR to the occurrence of relapse or disease progression. Overall survival (OS) was defined as the time period from the date of diagnosis to the date of death or the last date of follow-up.
2.5 Statistical analysis
The statistical evaluation of differences between patient subgroups was carried out using the chi-square test for categorical variables, the t-test for continuous data that were normally distributed, or nonparametric tests for data that did not conform to the assumptions of parametric tests. The Kaplan–Meier method was used for univariate analysis to assess the prognostic impact of methylation-related gene mutations compared to wild-type and other clinical and laboratory indicators. Log-rank tests were conducted to compare differences between groups. All statistical computations and graphical illustrations were performed with GraphPad Prism 9.5 (GraphPad Software, San Diego, CA, United States). Statistical significance was established at a p-value of less than 0.05.
3 Results
3.1 Clinical characteristics
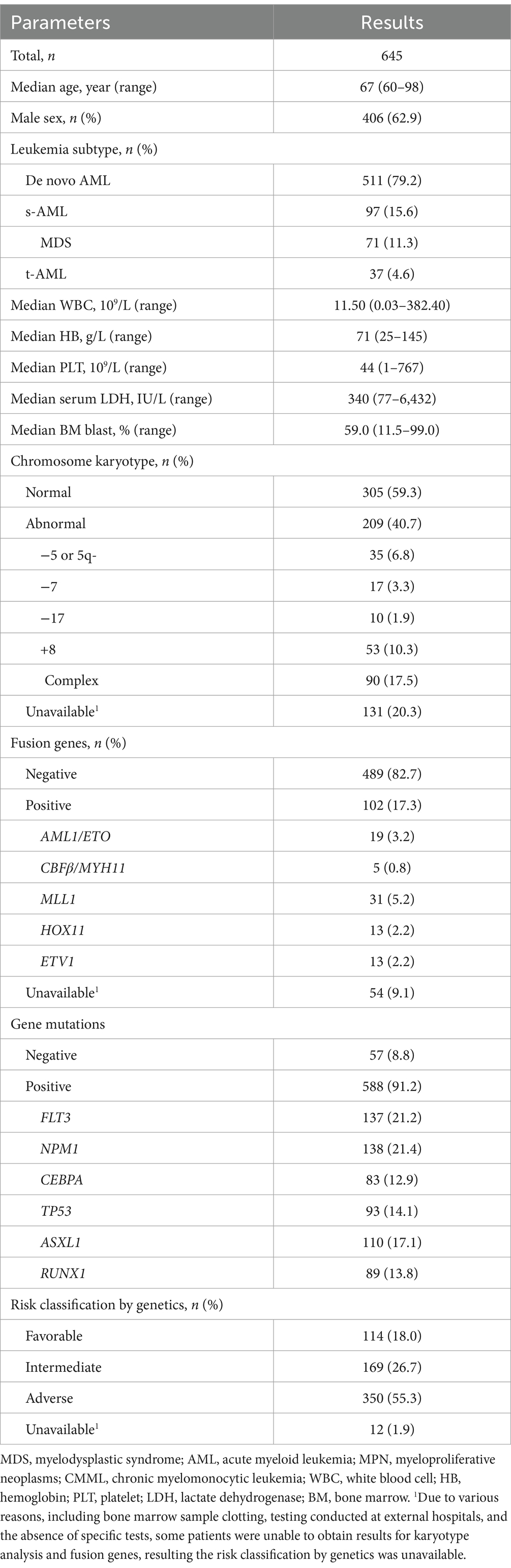
The study cohort comprised 645 individuals, with a male-to-female ratio of approximately 1.70:1, including 406 males (62.9%) and 239 females (37.1%). The age of the patients ranged from 60 to 98 years, with a median age of 67 years. Among these patients, 511 (79.2%) had de novo AML, 97 (15.0%) had s-AML, and 37 (5.7%) had t-AML. Myelodysplastic syndromes (MDS) were the most frequent precursors of s-AML, representing 73.2% of cases.
Cytogenetic analysis results were available for a subset of patients with 40.7% displaying abnormal chromosome karyotypes. Specific abnormalities included deletions of chromosome 5 or 5q- in 6.8%, chromosome 7- in 3.3%, chromosome 17- in 1.9%, chromosome +8 in 10.3%, and complex karyotypes in 17.5% of cases. As depicted in Figure 1, methylation-related gene mutations, including DNMT3A, TET2, IDH1, and IDH2, were observed in 24.0%, 22.5%, 9.1%, and 13.8% of patients, respectively. The incidence of IDH1 gene mutations was more occurred in De novo AML compared to s-AML/t-AML, and there was no significant difference in the occurrence rates of DNMT3A, TET2, and IDH2 gene mutations between these two AML subtypes. FLT3 gene mutations were found in 21.2% of patients, and other commonly detected mutations included those in NPM1, CEBPA, TP53, ASXL1, and RUNX1, with positive rates of 21.4%, 12.9%, 14.1%, 17.1%, and 13.8%, respectively. Based on genetic risk classification, the adverse group was the most prevalent, accounting for 55.3%, while the favorable and intermediate groups represented 18.0% and 26.7% of the patients, respectively. A comprehensive summary of the patients’ characteristics is provided in Table 1.
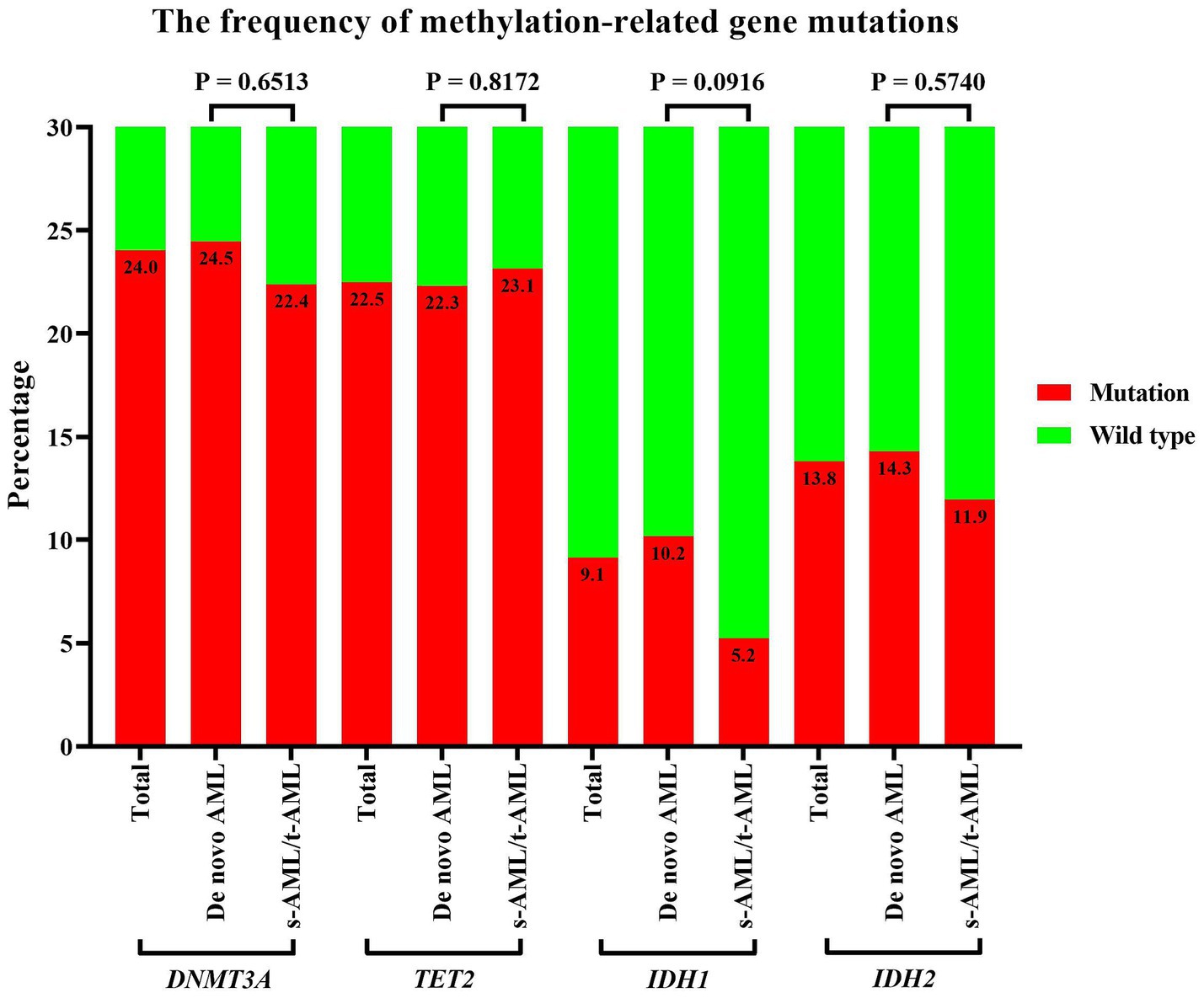
Figure 1. Incidence of methylation-related gene mutations in older patients with AML. Incidence of methylation-related gene (DNMT3A, TET2, IDH1/2) mutations stratified by disease subtype: de novo AML vs. therapy-related/secondary AML. Statistical comparisons used Fisher’s exact test, with p-values: DNMT3A = 0.651, TET2 = 0.817, IDH1 = 0.092, IDH2 = 0.574. AML, acute myeloid leukemia.
3.2 Treatment strategies and outcomes
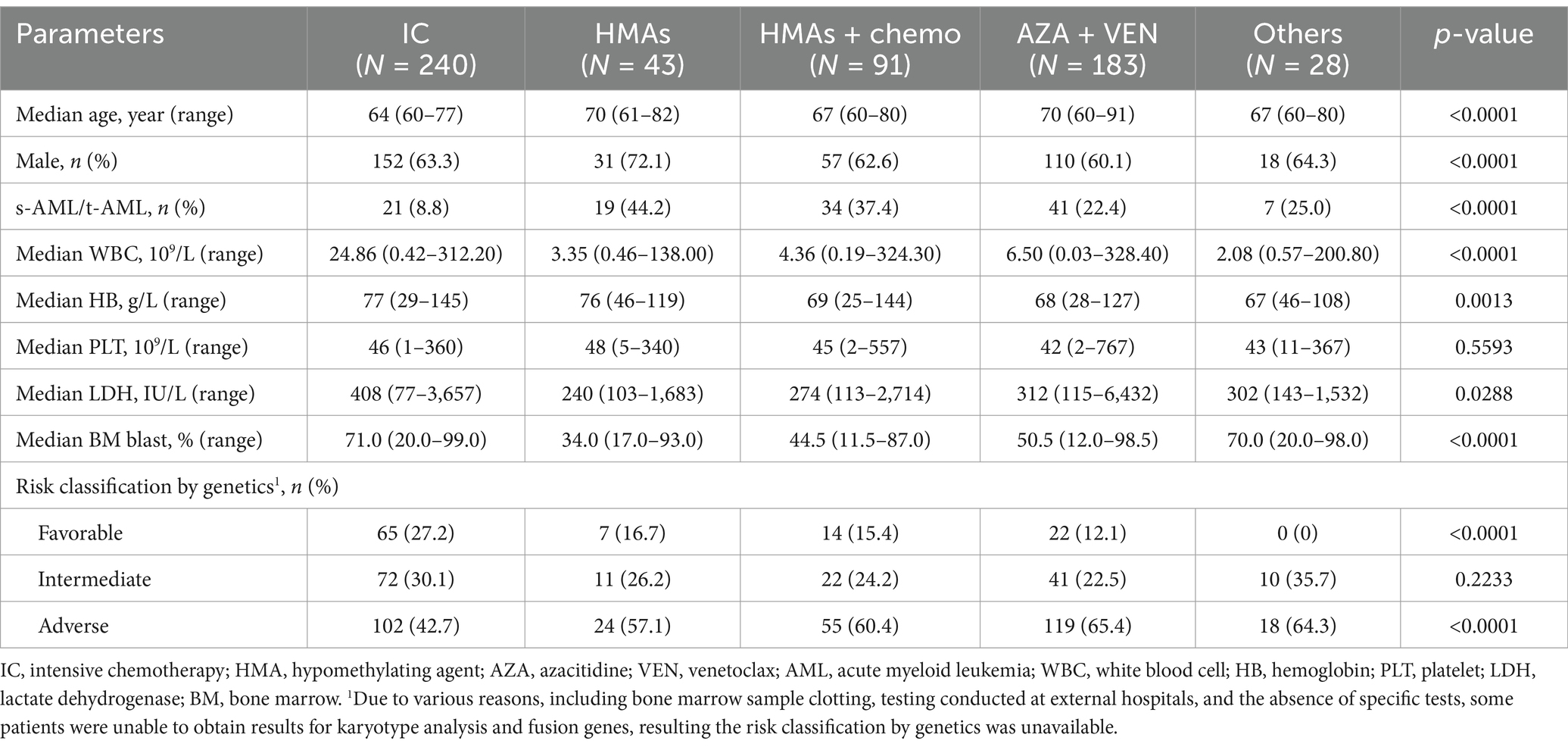
Of the 645 patients in the study cohort, 585 (90.7%) received further anti-leukemia treatment following diagnosis. The treatments included 240 patients (41.0%) undergoing IC, 43 patients (7.4%) receiving HMAs as monotherapy, 91 patients (15.6%) treated with a combination of HMAs and low-dose chemotherapy, 183 patients (31.3%) administered AZA in combination with VEN, and 28 patients (4.8%) managed with other therapeutic regimens. The baseline characteristics of patients receiving various treatment regimens were summarized in Table 2. From the table, it could be observed that patients in the IC group were relatively younger compared to those in other groups. Additionally, the proportions of s-AML/t-AML and adverse cytogenetics in the IC group were significant lower than those in the other treatment groups.
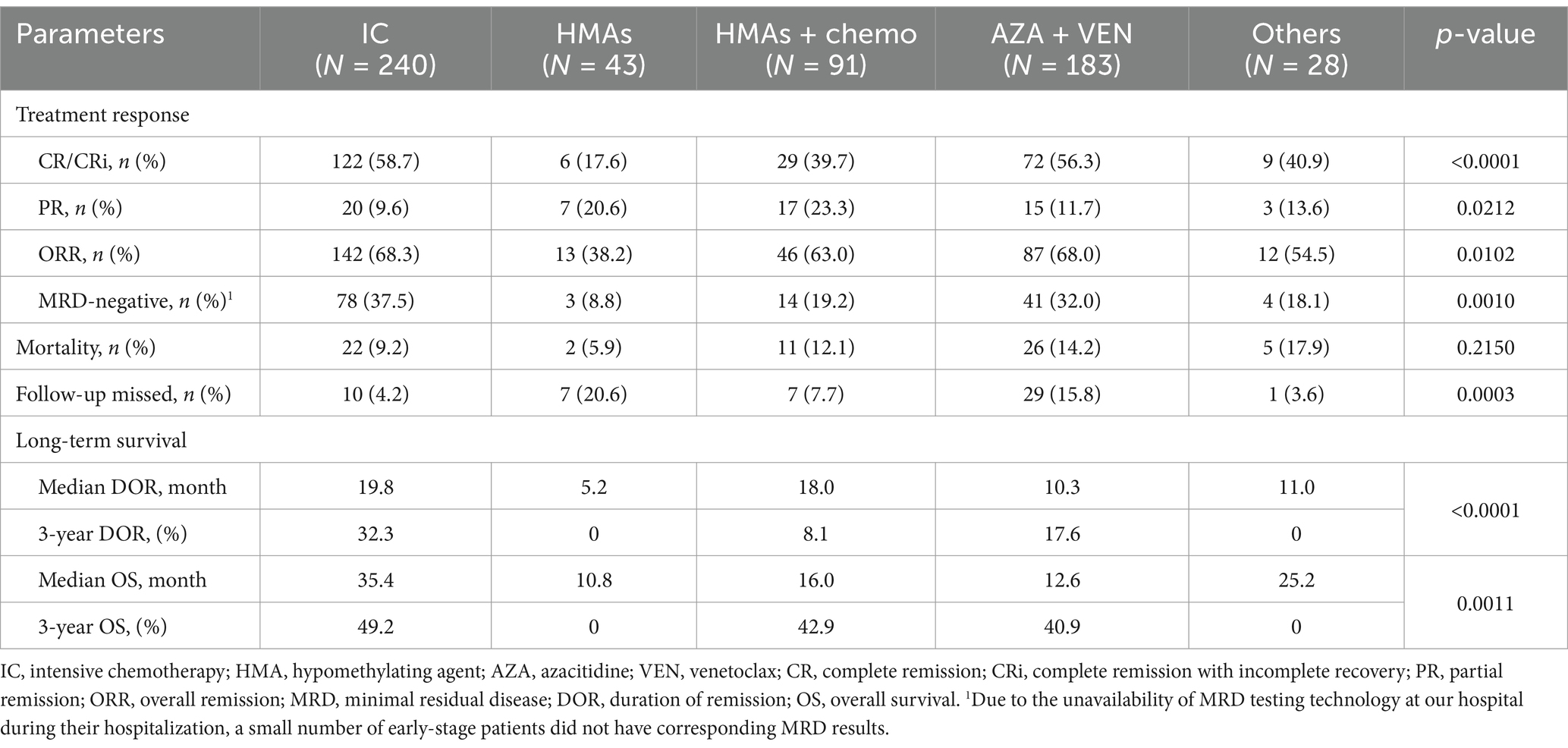
Among the 585 patients who received anti-leukemia therapy, 54 (9.2%) were lost to follow-up, and 66 (11.3%) died during the induction phase. As illustrated in Figure 2, the median DOR was 16.0 months, with 1-year, 3-year, and 5-year DOR rates of 55.4, 22.8, and 15.6%, respectively. The median OS was 21.2 months, with corresponding 1-year, 3-year, and 5-year OS rates of 58.0%, 40.6%, and 30.4%. Across the entire cohort, the induction treatment CR rate was 51.2%, the PR rate was 13.3%, the ORR rate was 64.5%, and the MRD-negative rate was 30.1%. Obviously, patients treated with IC showed the most favorable treatment response and long-term outcomes, with a CR rate of 58.7% and median DOR and OS of 19.8 months and 35.4 months, respectively, and a 5-year OS of 38.2%. The AZA and VEN combination therapy also resulted in a satisfactory response, with a CR rate of 56.3%, which was comparable to the IC group (p = 0.7331). HMA monotherapy had the least favorable outcomes, with a CR rate of 17.6% and median DOR and OS of only 5.2 months and 10.8 months, respectively. In contrast, the combination of HMAs with low-dose chemotherapy was more effective, with a CR rate of 39.7% and median DOR and OS of 18.0 and 16.0 months, respectively. The highest proportions of MRD-negative responses were observed in patients treated with IC and AZA + VEN, at 37.5% and 32.0%, respectively, while all other treatment protocols resulted in MRD-negative rates of less than 20%. The comprehensive treatment response and prognosis for patients undergoing various therapeutic protocols were summarized in Table 3.
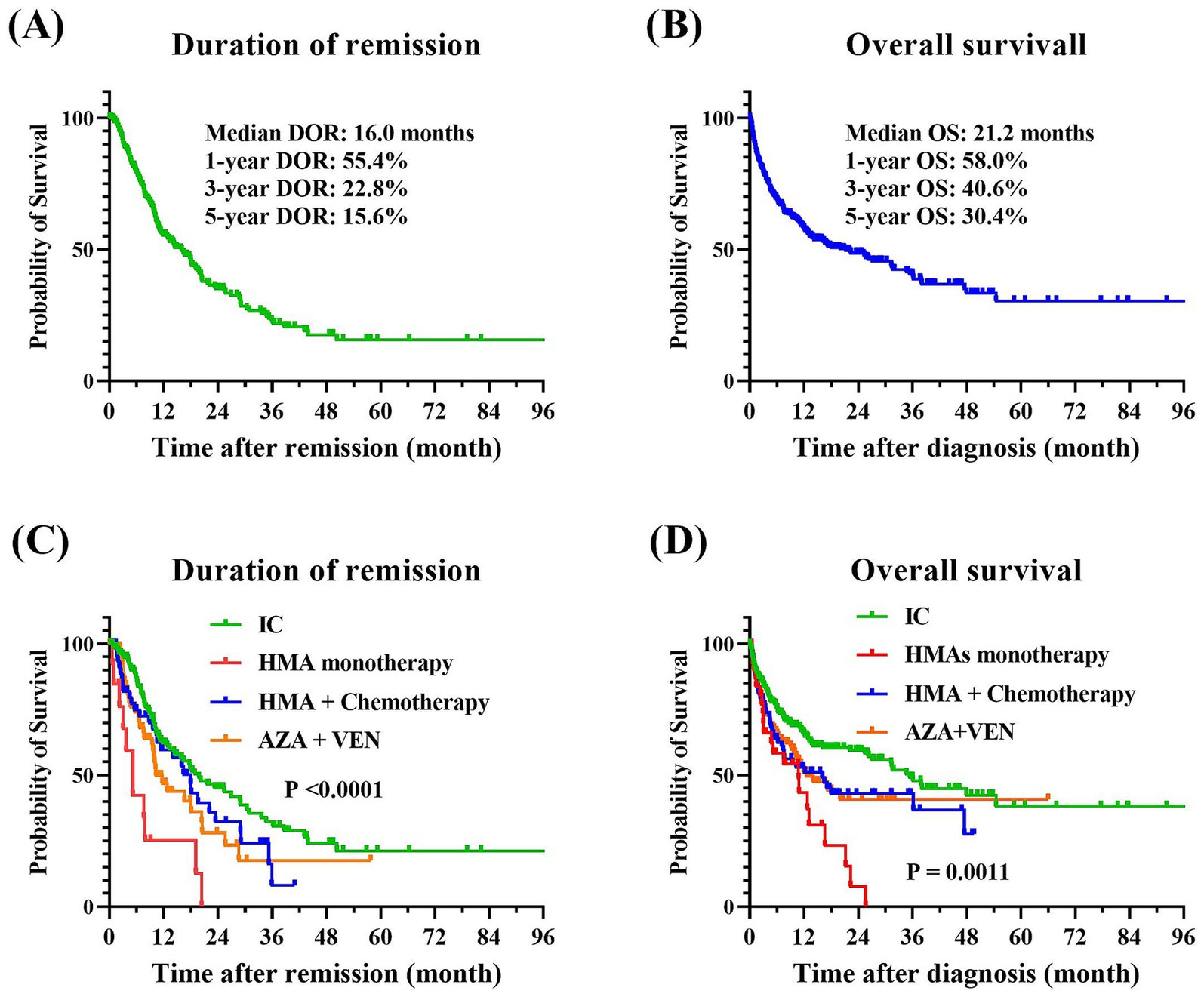
Figure 2. Treatment outcomes of older patients with AML. (A) DOR for the entire cohort. (B) OS for the entire cohort. (C) Comparative DOR among various treatment groups (p < 0.0001). (D) Comparative OS among various treatment groups (p = 0.0011). The Kaplan–Meier method was employed to perform univariate analysis of the prognostic impact of treatment regimens, and the log-rank tests were utilized to assess the differences between the groups. AML, acute myeloid leukemia; DOR, duration of remission; OS, overall survival.
3.3 Prognostic impact of methylation-related gene mutations
To delve deeper into the impact of demethylating gene mutations on the prognostic outcomes of elderly AML patients, as well as their influence on the therapeutic efficacy of the IC and AZA plus VEN regimen, we performed a univariate prognostic analysis. As they were showed at Figure 3, our findings suggested that mutations in DNMT3A and TET2 were inclined to correlate with an adverse prognosis. Specifically, the median OS for patients with DNMT3A mutations, as opposed to those without, were 12.8 months and 22.3 months, respectively, yielding a p-value of 0.1922. Similarly, individuals harboring TET2 mutations experienced a median survival of 13.7 months, in contrast to 21.2 months for their mutation-negative counterparts, with a p-value of 0.2081. Moreover, the poorest prognostic outcomes were observed in patients with concurrent DNMT3A and TET2 mutations, exhibiting a median survival of merely 6.2 months and a 2-year overall survival rate of 23.9%. In stark contrast, patients with IDH1 and IDH2 mutations exhibited a trend toward improved long-term prognoses. Those with IDH1 mutations had a median survival of 25.2 months, while the median survival for IDH2 mutation carriers had not yet been reached, compared to 18.0 months for wild-type patients, with a p-value of 0.1709.
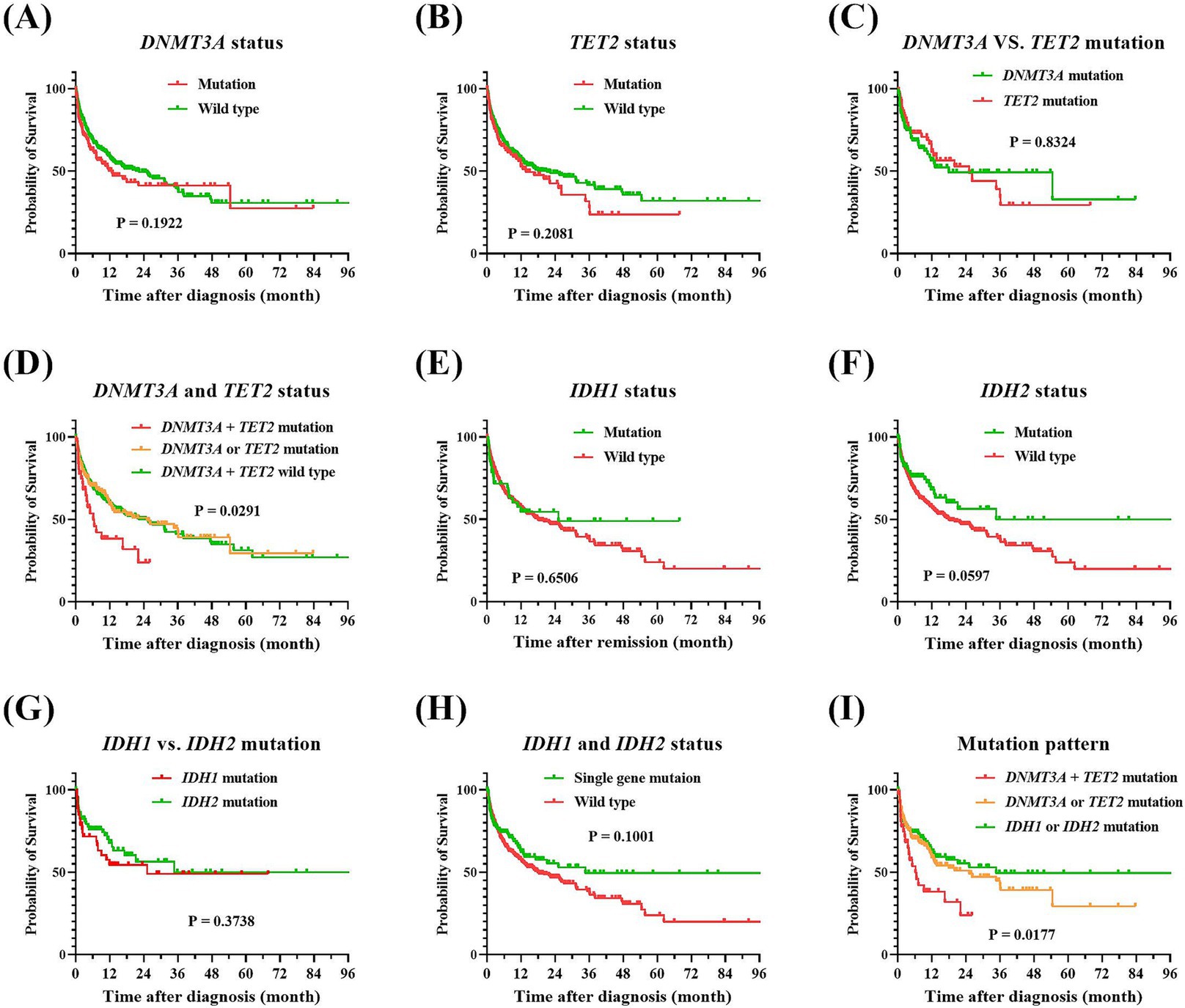
Figure 3. Comparison of overall survival of patients with AML according to their methylation-related gene profiles. (A) DNMT3A mutation vs. wild-type, p = 0.1922. (B) TET2 mutation vs. wild-type, p = 0.2081. (C) DNMT3A vs. TET2 mutations, p = 0.8324. (D) DNMT3A and TET2 mutation vs. wild-type, p = 0.0291. (E) IDH1 mutation vs. wild-type, p = 0.6506. (F) IDH2 mutation vs. wild-type, p = 0.0597. (G) IDH1 vs. IDH2 mutations, p = 0.3738. (H) IDH1 and IDH2 single mutation vs. wild type, p = 0.1001. (I) Comparative overall survival by methylation-related gene mutations clusters, p = 0.0177. Kaplan–Meier analysis was employed to compare overall survival between patients harboring methylation-related gene mutations vs. wild-type counterparts, and the log-rank tests were utilized to assess the differences between the groups. AML, acute myeloid leukemia.
As presented at Figure 4, patients harboring the DNMT3A mutation exhibited an induction remission rate that was nearly identical to that of their counterparts without the mutation, with respective complete remission (CR) rates of 53.6% and 50.6%, yielding a p-value of 0.5868. The therapeutic efficacy of the IC regimen and the combination of azacitidine with venetoclax were found to be equivalent, with CR rates of 58.0% and 59.0%, respectively, (p > 0.9999). Patients with the TET2 mutation experienced a CR rate of 42.2%, which was lower than the 53.9% observed in non-mutated patients (p = 0.0594). Additionally, the induction CR rate in the IC group was 56.5%, surpassing the 37.0% rate in the azacitidine plus venetoclax group, with a p-value of 0.1468. Notably, patients who carried both DNMT3A and TET2 mutations had an induction CR rate of merely 35.5%, which below the rates for those with isolated mutations of either DNMT3A or TET2 (p = 0.0974). The effectiveness of the IC regimen was similar with the azacitidine plus venetoclax regimen (CR rate: 41.2% vs. 37.5%, p > 0.9999). In contrast, patients with IDH1 or IDH2 mutations demonstrated a markedly higher induction CR rate compared to wild-type patients, with CR rates reaching 71.1% for IDH1 mutation carriers and 68.8% for IDH2 mutation carriers. For IDH1 mutation carriers treated with the IC regimen, the CR rate was as high as 82.4%, accompanied by a MRD-negative rate of 70.6%. Among IDH2 mutation carriers, the induction CR rate reached 68.8%, with CR rates of 68.0 and 76.9% for the IC and azacitidine plus venetoclax regimen regimens, respectively (p = 0.5414).
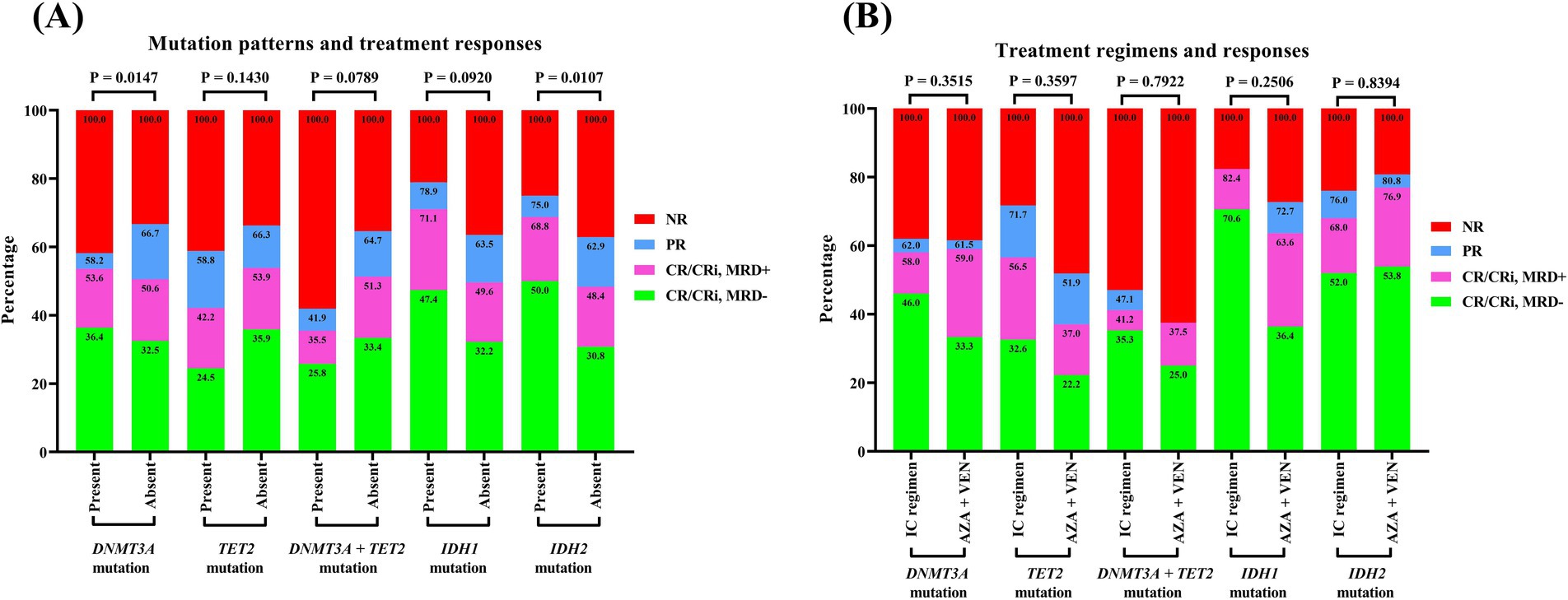
Figure 4. Treatment responses of AML patients with methylation-related gene mutations. (A) Mutation patterns and treatment response. (B) Treatment regimens and response. A Fisher’s exact test was performed to compare the gene mutation rates between groups. AML, acute myeloid leukemia.
4 Discussion
Older patients with AML typically exhibit a poor prognosis, with previous studies reporting a 5-year survival rate of less than 10% (2, 6, 7). In a prior study conducted at our center, we detailed the treatment outcomes of elderly AML patients managed before 2016, when conventional chemotherapy was the sole therapeutic option. Among this cohort, patients who received standard IC or low-dose chemotherapy achieved a CR rate of 42.7% during the induction phase. However, the 5-year survival rate for this population was only 13.5%, with a median survival duration of 9.2 months, underscoring the urgent need for more effective therapeutic strategies (22). In contrast, our current data demonstrate that the widespread adoption of novel agents, particularly HMAs and venetoclax, has significantly improved outcomes in elderly AML patients. The overall CR rate increased to 51.3%, the median OS extended to 21.2 months, and the 5-year survival rate reached 30.4%. These findings highlight the substantial advancements in efficacy and prognosis for elderly AML patients in the era of novel therapies compared to the era of traditional chemotherapy alone.
In real-world clinical practice, a patient’s ability to tolerate standard chemotherapy is a key determinant in selecting treatment regimens, such as IC, HMA-based therapy, or the combination of azacitidine and venetoclax. Consequently, in our study, the IC treatment group primarily comprised fit patients, while the HMA- or venetoclax-based treatment groups included older or less fit patients. Our data revealed that 58.7% of patients treated with IC achieved CR, with an ORR of 68.3%, and exhibited superior long-term survival compared to other regimens. This suggests that IC remains the preferred option for fit patients. Conversely, the combination of azacitidine and venetoclax is currently recommended as the first-line regimen for patients unsuitable for IC or those with adverse molecular cytogenetic profiles. The efficacy of this regimen has been validated in numerous clinical trials (23–25). Our study further demonstrated that treatment responses, including CR rates and MRD-negative rates, were comparable between the IC and azacitidine + venetoclax groups and significantly higher than those of other treatment options.
Cytogenetic abnormalities play a pivotal role in risk stratification for AML and are a critical factor in guiding treatment decisions, including the incorporation of targeted therapies (26–29). Methylation-related gene mutations are highly prevalent in elderly AML patients (30). However, the impact of these mutations on prognosis and whether HMAs confer additional benefits in this population remain unclear. In our study, patients with DNMT3A mutations exhibited similar initial induction remission rates compared to wild-type patients but had poorer long-term outcomes. In contrast, patients with TET2 mutations showed both lower induction response rates and worse long-term survival than wild-type patients. Notably, we observed that concurrent mutations in DNMT3A and TET2 were associated with markedly inferior treatment responses and prognosis, a finding not previously reported. On the other hand, patients with IDH1 and IDH2 mutations demonstrated significantly better treatment responses and prognosis compared to wild-type patients.
Furthermore, we compared the efficacy of treatment regimens—IC vs. azacitidine combined with venetoclax—in patients with methylation-related gene mutations. Our analysis revealed that patients harboring DNMT3A or IDH2 mutations achieved comparable remission rates with either the IC regimen or the azacitidine + venetoclax combination. In contrast, patients with TET2 or IDH1 mutations exhibited higher remission rates, including CR and MRD-negative rates, when treated with the IC regimen compared to the azacitidine + venetoclax regimen. Notably, for patients with concurrent DNMT3A and TET2 mutations, the overall treatment response rate was below 50% regardless of the treatment regimen, although the IC group demonstrated a slightly higher response rate than the azacitidine plus venetoclax group. These findings suggest that treatment regimens incorporating HMAs did not provide additional benefits for patients with methylation-related gene mutations. In other words, the presence of these mutations does not necessitate special consideration when choosing between the IC regimen and a combination regimen incorporating hypomethylating agents (HMAs) and venetoclax.
This study has several limitations. Firstly, as a single-center, retrospective design and extended observation period, some degree of information bias was unavoidable. Secondly, the heterogeneity in the number of treatment cycles across patients should be taken into account when interpreting the results. Thirdly, given the limited sample size and the heterogeneity of testing methods stemming from the extended duration of data collection, we were unable to carry out further subgroup analysis according to the mutation loci of each methylation-related gene mutation. Therefore, further prospective studies are warranted to validate and refine our findings.
5 Conclusion
Methylation-related gene mutations are commonly observed in older AML patients. Patients with co-occurring DNMT3A and TET2 mutations showed notably poor treatment responses and survival outcomes. These common methylation-related gene mutations do not significantly influence the choice between IC and azacitidine plus venetoclax regimen.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval for the study was granted by the Ethics Committee of the Fujian Medical University Union Hospital, under the protocol number 2024KY278. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because given that this study was a retrospective case analysis, which does not interfere with the patients’ treatment and had no impact on the prognosis of the patients, the requirement for written informed consent from the patients has been waived.
Author contributions
YiC: Conceptualization, Data curation, Writing – original draft, Software, Investigation, Methodology, Funding acquisition, Formal analysis. ZWu: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft. YaC: Methodology, Investigation, Writing – original draft, Formal analysis. ZWa: Investigation, Writing – original draft, Data curation. RC: Methodology, Writing – original draft, Investigation. YW: Funding acquisition, Conceptualization, Writing – review & editing, Project administration, Resources. JZ: Writing – review & editing, Conceptualization, Validation, Visualization, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Department of Hematology, National Key Clinical Specialty Construction Project (2128300238), the Joint Funds for the Innovation of Science and Technology, Fujian Province (2023Y9128), Fujian Provincial Natural Science Foundation of China (2024J01587), and Fujian Provincial Department of Finance Special Fund Project (2023CZ003).
Acknowledgments
We greatly appreciate all patients with AML who supported this study, and we are also grateful for the diligent efforts of all our departmental colleagues who concurrently contributed to the diagnosis and treatment of these leukemia patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1594784/full#supplementary-material
References
1. Yi, M, Li, A, Zhou, L, Chu, Q, Song, Y, and Kongming, W. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: estimates based on the global burden of disease study 2017. J Hematol Oncol. (2020) 13:72. doi: 10.1186/s13045-020-00908-z
2. Shallis, RM, Wang, R, Davidoff, A, Ma, X, and Zeidan, AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. (2019) 36:70–87. doi: 10.1016/j.blre.2019.04.005
3. Leone, G, Mele, L, Pulsoni, A, Equitani, F, and Pagano, L. The incidence of secondary leukemias. Haematologica. (1999) 84:937–45.
4. Metzeler, KH, Herold, T, Rothenberg-Thurley, M, Amler, S, Sauerland, MC, Görlich, D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. (2016) 128:686–98. doi: 10.1182/blood-2016-01-693879
5. Oliai, C, and Schiller, G. How to address second and therapy-related acute myelogenous leukaemia. Br J Haematol. (2020) 188:116–28. doi: 10.1111/bjh.16354
6. Herold, T, Rothenberg-Thurley, M, Grunwald, VV, Janke, H, Goerlich, D, Sauerland, MC, et al. Validation and refinement of the revised 2017 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia. (2020) 34:3161–72. doi: 10.1038/s41375-020-0806-0
7. Oran, B, and Weisdorf, DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. (2012) 97:1916–24. doi: 10.3324/haematol.2012.066100
8. Döhner, H, Wei, AH, Appelbaum, FR, Craddock, C, DiNardo, CD, Dombret, H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. (2022) 140:1345–77. doi: 10.1182/blood.2022016867
9. Pollyea, D, Altman, JK, Assi, R, Bixby, D, Fathi, AT, Foran, JM, et al. Acute myeloid leukemia, version 3.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2023) 21:503–13. doi: 10.6004/jnccn.2023.0025
10. Döhner, H, Estey, E, Grimwade, D, Amadori, S, Appelbaum, FR, Büchner, T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. (2017) 129:424–47. doi: 10.1182/blood-2016-08-733196
11. El Chaer, F, Hourigan, CS, and Zeidan, AM. How I treat AML incorporating the updated classifications and guidelines. Blood. (2023) 141:2813–23. doi: 10.1182/blood.2022017808
12. Lee, AV, Nestler, KA, and Chiappinelli, KB. Therapeutic targeting of DNA methylation alterations in cancer. Pharmacol Ther. (2024) 258:108640. doi: 10.1016/j.pharmthera.2024.108640
13. Davalos, V, and Esteller, M. Cancer epigenetics in clinical practice. CA Cancer J Clin. (2023) 73:376–424. doi: 10.3322/caac.21765
14. Skvortsova, K, Stirzaker, C, and Taberlay, P. The DNA methylation landscape in cancer. Essays Biochem. (2019) 63:797–811. doi: 10.1042/EBC20190037
15. Yang, X, Wong, MPM, and Ng, RK. Aberrant DNA methylation in acute myeloid leukemia and its clinical implications. Int J Mol Sci. (2019) 20:4576. doi: 10.3390/ijms20184576
16. Rahmani, M, Talebi, M, Hagh, MF, Hosseinpour, AA, and Feizi, SS. Aberrant DNA methylation of key genes and acute lymphoblastic leukemia. Biomed Pharmacother. (2018) 97:1493–500. doi: 10.1016/j.biopha.2017.11.033
17. Ley, TJ, Ding, L, Walter, MJ, McLellan, MD, Lamprecht, T, Larson, DE, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. (2010) 363:2424–33. doi: 10.1056/NEJMoa1005143
18. Pan, X'a, Chang, Y, Ruan, G, Zhou, S, Jiang, H, Jiang, Q, et al. TET2 mutations contribute to adverse prognosis in acute myeloid leukemia (AML): results from a comprehensive analysis of 502 AML cases and the beat AML public database. Clin Exp Med. (2024) 24:35. doi: 10.1007/s10238-024-01297-0
19. Megías-Vericat, JE, Ballesta-López, O, Barragán, E, and Montesinos, P. IDH1-mutated relapsed or refractory AML: current challenges and future prospects. Blood Lymphat. Cancer. (2019) 9:19–32. doi: 10.2147/BLCTT.S177913
20. Babakhanlou, R, DiNardo, C, and Borthakur, G. IDH2 mutations in acute myeloid leukemia. Leuk Lymphoma. (2023) 64:1733–41. doi: 10.1080/10428194.2023.2237153
21. Arber, D, Orazi, A, Hasserjian, R, Thiele, J, Borowitz, M, Beau, M, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. (2016) 127:2391–405. doi: 10.1182/blood-2016-03-643544
22. Chen, Y, Yang, T, Zheng, X, Yang, X, Zheng, Z, Zheng, J, et al. The outcome and prognostic factors of 248 elderly patients with acute myeloid leukemia treated with standard-dose or low-intensity induction therapy. Medicine (Baltimore). (2016) 95:e4182. doi: 10.1097/MD.0000000000004182
23. DiNardo, CD, Pratz, K, Pullarkat, V, Jonas, BA, Arellano, M, Becker, PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. (2019) 133:7–17. doi: 10.1182/blood-2018-08-868752
24. DiNardo, CD, Jonas, BA, Pullarkat, V, Thirman, MJ, Garcia, JS, Wei, AH, et al. Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. (2020) 383:617–29. doi: 10.1056/NEJMoa2012971
25. Döhner, H, Pratz, KW, DiNardo, CD, Wei, AH, Jonas, BA, Pullarkat, VA, et al. Genetic risk stratification and outcomes among treatment-naive patients with AML treated with venetoclax and azacitidine. Blood. (2024) 144:2211–22. doi: 10.1182/blood.2024024944
26. Grimwade, D, and Hills, RK. Independent prognostic factors for AML outcome. Hematology Am Soc Hematol Educ Program. (2009) 2009:385–95. doi: 10.1182/asheducation-2009.1.385
27. Erba, HP. Prognostic factors in elderly patients with AML and the implications for treatment. Hematol Am Soc Hematol Educ Program. (2007) 2007:420–8. doi: 10.1182/asheducation-2007.1.420
28. Liersch, R, Müller-Tidow, C, Berdel, WE, and Krug, U. Prognostic factors for acute myeloid leukaemia in adults--biological significance and clinical use. Br J Haematol. (2014) 165:17–38. doi: 10.1111/bjh.12750
29. Ciurea, SO, Chilkulwar, A, Saliba, RM, Chen, J, Rondon, G, Patel, KP, et al. Prognostic factors influencing survival after allogeneic transplantation for AML/MDS patients with TP53 mutations. Blood. (2018) 131:2989–92. doi: 10.1182/blood-2018-02-832360
Keywords: elderly, acute myeloid leukemia, methylation, mutation, prognosis
Citation: Chen Y, Wu Z, Chen Y, Wang Z, Cai R, Wu Y and Zheng J (2025) Prognostic impact of methylation-related gene mutations in elderly acute myeloid leukemia: a real-world retrospective analysis. Front. Med. 12:1594784. doi: 10.3389/fmed.2025.1594784
Edited by:
Håkon Reikvam, University of Bergen, NorwayReviewed by:
Moon Nyeo Park, Kyung Hee University, Republic of KoreaAhmed Mahmoud Khattab, Ain Shams University, Egypt
Copyright © 2025 Chen, Wu, Chen, Wang, Cai, Wu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Wu, d3V5b25nOTE5NUAxMjYuY29t; Jing Zheng, ZHJ6ajEyMzQ1NkAxNjMuY29t
†These authors have contributed equally to this work
 Yi Chen
Yi Chen Zhengjun Wu
Zhengjun Wu Yanxin Chen
Yanxin Chen Zhengyang Wang2
Zhengyang Wang2