- 1Department of Cardiovascular Medicine, Mayo Clinic, Jacksonville, FL, United States
- 2Center for Clinical and Translational Science, Mayo Clinic, Rochester, MN, United States
- 3Department of General Internal Medicine, Mayo Clinic, Jacksonville, FL, United States
- 4Department of Immunology, Mayo Clinic, Jacksonville, FL, United States
- 5Division of Cardiovascular Medicine, University of Florida, Gainesville, FL, United States
- 6Department of Otolaryngology, Mayo Clinic, Jacksonville, FL, United States
- 7Department of Quantitative Health Sciences, Mayo Clinic, Jacksonville, FL, United States
- 8Atwal Clinic, West Palm Beach, FL, United States
Background: In 2017 a revised clinical criterion for the diagnosis of hypermobile Ehlers-Danlos syndrome (hEDS) was proposed in order to better distinguish hEDS from other joint hypermobility disorders which are termed hypermobility spectrum disorders (HSD). The goal of this study was to determine whether patients with localized HSD (L-HSD) or historical HSD (H-HSD) differed in 100 symptoms/comorbidities from controls and/or patients diagnosed with hEDS or HSD.
Methods: In this study, we examined 100 self-reported symptoms/comorbidities from 2,695 patients diagnosed with hEDS, HSD, L-HSD/H-HSD, or controls.
Results: From November 1, 2019, to August 27, 2024, 2,695 patients filled out an Intake Questionnaire at the Mayo Clinic Florida EDS Clinic. Using the 2017 diagnostic criterion, 60.6% (n = 1,632) of patients were diagnosed with HSD, 18.3% (n = 493) hEDS, 10.7% (n = 289) with L-HSD or H-HSD, and 10.4% (n = 281) were controls without any of these diagnoses. We found that patients with L-HSD/H-HSD self-reported significantly more symptoms/comorbidities than controls for 62/100 (62%) of issues compared to 58/100 (58%) for HSD and 20/100 (20%) for hEDS. These findings suggest that L-HSD/H-HSD share similar symptoms and comorbidities to HSD. Interestingly, patients with L-HSD/H-HSD self-reported significantly more symptoms/comorbidities than patients diagnosed with hEDS or HSD for 20/100 (20%) of issues such as joint pain, muscle weakness, multiple sensitivities, wheezing/shortness of breath, gastroesophageal reflux disease (GERD), pain/cramps in the lower abdomen, constipation, heat and/or cold intolerance, hearing difficulties, attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), snoring, and narcolepsy. Symptoms/comorbidities that were significantly increased in L-HSD/H-HSD patients compared to controls (but not in hEDS or HSD compared to controls) and so were specific to this diagnosis included wheezing, hearing difficulties, narcolepsy, circadian rhythm disorders, and ASD.
Conclusion: We found that patients with L-HSD/H-HSD had many symptoms and comorbidities that closely resembled HSD suggesting that revised diagnostic criteria for hEDS and HSD should include L-HSD/H-HSD within a diagnosis of HSD. Additionally, our data further suggest that patients with HSD (including L-HSD/H-HSD) have more symptoms/comorbidities than patients with hEDS.
1 Introduction
Hypermobile Ehlers-Danlos syndrome (hEDS) and hypermobility spectrum disorders (HSD) are heritable collagen disorders with widespread distribution of fragile connective tissue in the skin, joints, ligaments, and internal organs (1). 255 million people worldwide (3%) are estimated to have hEDS or HSD (2). Unlike the more classical forms of EDS, gene variants unique to hEDS/HSD are unknown except for a recent preprint report of mutations in the kallikrein gene family in patients with hEDS (3). Diagnosis is based on strict, physical criteria developed by the International EDS Consortium (Table 1) (4). Patients diagnosed with hEDS or HSD have been reported to have significantly greater joint pain, musculoskeletal pain, and fatigue than the general population (5). Comorbidities/symptoms of hypermobile patients are often multiorgan, multifaceted, and potentially debilitating (1, 2, 6).
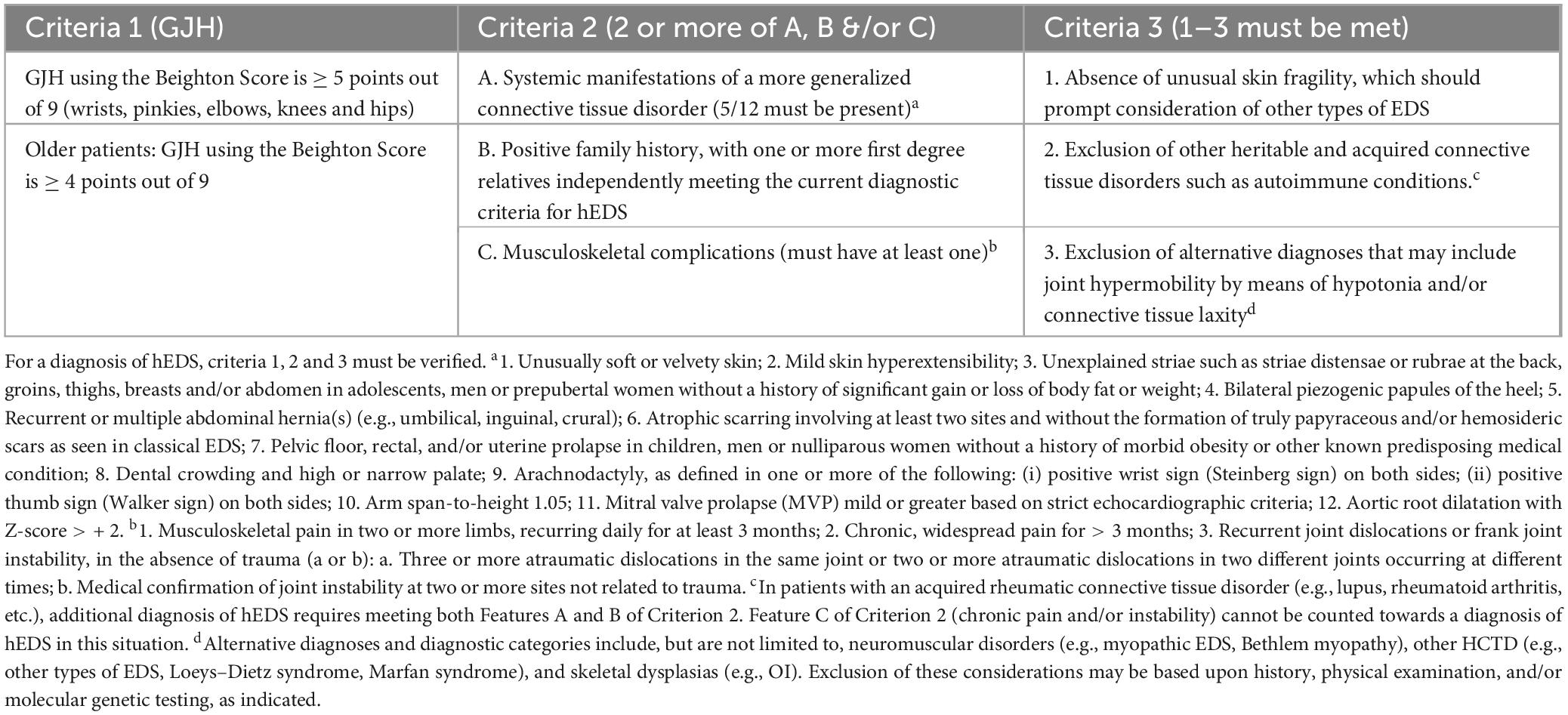
Table 1. Diagnostic criteria for hypermobile EDS (hEDS) based on Malfait et al. (4).
In 2017 a new clinical criterion for the diagnosis of hEDS was published that distinguished hEDS from HSD with a goal of better understanding the similarities and/or differences that exist within the spectrum of disease (4). The criteria for a diagnosis of hEDS briefly includes identification of generalized joint hypermobility (GJH) of specific joints using the Beighton Scale, evidence of a systemic connective tissue disorder, family history and/or musculoskeletal complications, and several exclusions (see Table 1) (4). The Beighton Scale assesses the joint hypermobility of only a few joints such as knees and elbows (4, 7). A Beighton Scale score of ≥ 5/9 after puberty or ≥ 4/9 after age 50 is a positive score (4). Patients are diagnosed with HSD if they do not meet the diagnostic criteria for hEDS, have a positive Beighton Scale score and also have evidence that the joint hypermobility is causing problems and it is not just an asymptomatic feature (feature C of the 2nd EDS criterion) (4, 7). Localized HSD (L-HSD) is diagnosed in patients that do not meet the diagnostic criteria for hEDS, do not have a positive Beighton Score (but their Beighton Score is not zero), and other areas of the body are hypermobile (7). Patients that do not meet the diagnostic criteria for hEDS, have a Beighton Score of zero, and experience hypermobility in other joints are termed historical HSD (H-HSD) (7).
In collaboration with the International Consortium’s working groups and the European Reference Networks, The Ehlers-Danlos Society aims to reassess the 2017 hEDS diagnostic criterion and formally define HSD (8). According to the Ehlers-Danlos Society website, part of their research goals include studying whether symptoms and comorbidities are similar and/or different between hEDS and HSD to determine whether the 2017 diagnostic criteria for hEDS should be revised (9, 10). Since the 2017 diagnostic criterion was established, several studies found either that symptoms and comorbidities between hEDS and HSD were essentially the same (9, 11–14) or that that both similarities and important differences exist (Table 2) (6, 8, 15, 16). A study by Aubry-Rozier et al. (13) examined 61 patients with hEDS and 36 with HSD using the 16-item Clinical Severity Score (CSS-16). They also assessed bone involvement, neuropathic pain (DN4) and symptoms of mast cell disorders (MCAS) as extra-articular manifestations. They found the two diagnoses were similar for most symptoms/comorbidities (Table 2) (13). They concluded from this study that patients with hEDS and HSD should receive the same clinical care. Martinez et al. examined 98 patients with hEDS and 27 with HSD using 7 health questionnaires (Short Form Health Survey (SF-36), Patient Health Questionnaire (PHQ)-15, Tampa Scale for Kinesiophobia (TSK), Fatigue Severity Scale (FSS), Epworth Sleepiness Scale (ESS), Gastro-Questionnaire, Composite Autonomic Symptom Score (COMPASS) 31) (14). They found that although a few differences between hEDS and HSD were observed, that the frequency and severity of most symptoms were indistinguishable between the two diagnoses (Table 2) (14). Ritelli et al. (8) examined 87 patients with hEDS and 126 with HSD for 90 symptoms/comorbidities and found that most occurred more often in patients with hEDS than HSD suggesting that hEDS may be more severe than HSD (Table 2) (8). Ritelli et al. (15) later reported a similar finding examining 9 symptoms/comorbidities in 94 patients with hEDS and 80 with HSD with more hEDS patients identified with these issues (Table 2) (15). Examining 423 patients with hEDS and 1,389 with HSD, we previously reported that most of 115 self-reported symptoms/comorbidities had extensive overlap between the two diagnoses, but that patients diagnosed with HSD reported more symptoms/comorbidities than patients with hEDS, suggesting that symptoms may be worse in patients with HSD (16). It is not clear why differences exist in the findings of different studies. Possible reasons include that most of the studies compared small numbers of patients (under 100/group), from different countries (i.e., races/ethnicities) and different ages (Table 2) (11–15). Importantly, none of these studies (aside from ours) indicated whether the patients diagnosed with HSD were HSD, L-HSD and/or H-HSD.
In previous manuscripts we had included L-HSD and H-HSD as part of our controls (patients attended the EDS Clinic but were not diagnosed with hEDS or HSD) (6, 17, 18). However, we published recently that patients with L-HSD/H-HSD were significantly different than controls that did not have any type of HSD for most of 115 symptoms/comorbidities (16). These findings led us to conduct this study. The goal of this study is to determine whether patients with L-HSD or H-HSD differ in 100 symptoms/comorbidities from controls and/or patients diagnosed with hEDS or HSD. An important issue that needs to be considered in future revisions of the EDS and HSD diagnostic criteria is the question of whether patients with HSD, L-HSD and/or H-HSD should be incorporated more fully into the diagnostic criteria. We found that patients with L-HSD/H-HSD had many symptoms and comorbidities that closely resembled HSD suggesting that revised diagnostic criteria for hEDS and HSD should include L-HSD/H-HSD within a diagnosis of HSD. Additionally, our data indicate that patients with HSD (including L-HSD/H-HSD) have more symptoms/comorbidities than patients with hEDS.
2 Materials and methods
2.1 Ethics statement
The Institutional Review Board (IRB# 19-010260) of Mayo Clinic approved the retrospective analysis of demographic and clinical data from medical records for this study and waived informed consent for all patients. The research conformed to the principles outlined in the Declaration of Helsinki.
2.2 Controls
Previously, we reported that patients seen at the EDS Clinic who were not diagnosed with HSD or hEDS using the 2017 diagnostic criteria were significantly different for most symptoms and comorbidities compared to diagnosed patients, (6) suggesting that this group may serve as appropriate controls. However, this control group contains patients diagnosed with L-HSD or H-HSD (7, 16). We published recently that patients with L-HSD/H-HSD were significantly different than controls that did not have any type of HSD for most of 115 symptoms/comorbidities (16). The goal of this study is to determine whether patients with L-HSD/H-HSD differ in 100 self-reported symptoms/comorbidities from controls and/or patients diagnosed with hEDS or HSD seen at the EDS Clinic.
2.3 Patients
Adult patients (≥ 18 years of age) were seen at the Mayo Clinic Florida EDS Clinic from November 1, 2019, to August 27, 2024 (n = 2,695) by self-referral or referrals from inside or outside Mayo Clinic. Patients were diagnosed with hEDS according to the 2017 diagnostic criteria (Table 1), (4) as previously (6, 16–18). hEDS does not have a recognized causative genetic variant compared to the other types of EDS (4). Briefly, the diagnostic criteria for hEDS includes identification of generalized joint hypermobility (GJH) of specific joints using the Beighton Scale (past puberty ≥ 5/9 and over 50 years of age ≥ 4/9), evidence of a systemic connective tissue disorder, family history and/or musculoskeletal complications, and several exclusions (Table 1) (4). HSD is diagnosed in patients that do not meet the diagnostic criteria for hEDS but have a positive Beighton Score (≥ 5/9 and ≥ 4/9 for older patients) and evidence that the joint hypermobility is causing problems and it is not just an asymptomatic feature (feature C of the 2nd EDS criterion) (4, 7). L-HSD is diagnosed in patients that do not meet the diagnostic criteria for hEDS, do not have a positive Beighton Score (but their Beighton Score was not zero), and other areas of the body are hypermobile (7). H-HSD is diagnosed in patients that do not meet the diagnostic criteria for hEDS, have a Beighton Score of zero, and other areas of the body are hypermobile (positive for the 5-point GJH questionnaire) (7, 19). The International Classification of Diseases, 10th Revision (ICD-10) codes used at Mayo Clinic for these conditions included: GJH (M24.80) (other specific joint derangements, unspecified site), hEDS (Q79.62), and HSD (M35.7) (hypermobility syndrome). HSD, L-HSD and H-HSD do not have specific ICD-10 codes, and M35.7 may be used for these conditions even though they have different diagnostic criteria (7). We track the specific EDS and HSD diagnosis of all patients seen at the EDS Clinic and so we were able to identify which patients received a diagnosis of L-HSD or H-HSD. Because we had very few H-HSD patients, we combined L-HSD and H-HSD patients for the analysis. Controls (n = 281) were patients that attended the EDS Clinic but were not diagnosed with hEDS (n = 493), HSD (n = 1,632), L-HSD or H-HSD (n = 289), as previously (16).
2.4 EDS Clinic data collection
Patient data were collected from November 1, 2019, to August 27, 2024 (n = 2,695). Patients received a 300-question REDCap Intake Questionnaire as standard of care prior to their first appointment at the Mayo Clinic Florida EDS Clinic. Self-reported data on 100 symptoms/comorbidities were obtained from the Intake Questionnaire of adult patients > 18 years of age. The Intake Questionnaire categorized questions by organ or system such as muscles, joints, allergy, neurological symptoms or gastrointestinal symptoms. Thus, data were organized in this manuscript according to those organ or system categories.
2.5 Statistical analysis
Continuous variables were summarized with the sample median and range. Categorical variables were summarized with number and percentage of subjects. Kruskal-Wallis rank sum test was used to compare the difference of continuous variables among groups. A χ2 test examined the association between two categorical variables. All the tests were two-tailed and p < 0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, United States). Graphs were created using GraphPad Prism (Version 10.3.1).
3 Results
3.1 Patient demographics
From the 2,695 patients who were assessed for hypermobility at the EDS Clinic using the 2017 diagnostic criteria (Table 1) and the Morlino and Castori definitions of HSD, (4, 7) we found that 60.6% (n = 1,632) were diagnosed with HSD, 18.3% (n = 493) with hEDS, 10.7% (n = 289) with L-HSD or H-HSD, and 10.4% (n = 281) were controls without any of these diagnoses.
We found that the average age of controls was significantly older (38.7) than patients diagnosed with hEDS or HSD of any type (34.0–35.1) (p < 0.001) (Table 3). Over 85% in each group self-reported as females with the highest percentage of females in patients diagnosed with HSD (p < 0.001) (Table 3). Most patients in each category self-reported as not Hispanic White; however, the highest percentage of White was in patients with HSD (89%) and the lowest in hEDS (83%) (p = 0.015) (Table 3). More patients with L-HSD/H-HSD self-reported as Native Hawaii/Pacific Islander than for other diagnoses (p = 0.004) (Table 3). The BMI of controls (25.2) and patients with hEDS (24.8) was lower than patients with HSD (26.1) or L-HSD/H-HSD (26.4) (p < 0.001) (Table 4). Alcohol consumption was self-reported as highest in patients diagnosed with hEDS compared to other groups (p = 0.04) (Table 4). Where differences between groups occurred, the demographics of patients with L-HSD/H-HSD most closely resembled patients with a diagnosis of HSD rather than hEDS or controls.
3.2 Joint and muscle weakness and easy bruising
For joint and muscle symptoms/comorbidities we found that patients with L-HSD/H-HSD self-reported more often than controls joint pain (p < 0.001), subluxations (p < 0.001), sprains (p < 0.001), easy bruising (p < 0.001), muscle weakness (p < 0.001), temporomandibular joint (TMJ) symptoms (p < 0.001), and dislocations (p < 0.001) (Table 5). Additionally, more L-HSD/H-HSD patients self-reported issues with joint pain (hEDS p < 0.001, HSD p = 0.037) and muscle weakness (hEDS p < 0.001, HSD p < 0.001) than patients diagnosed with hEDS or HSD (Table 5) indicating that L-HSD/H-HSD patients have significant joint and muscle symptoms/comorbidities.
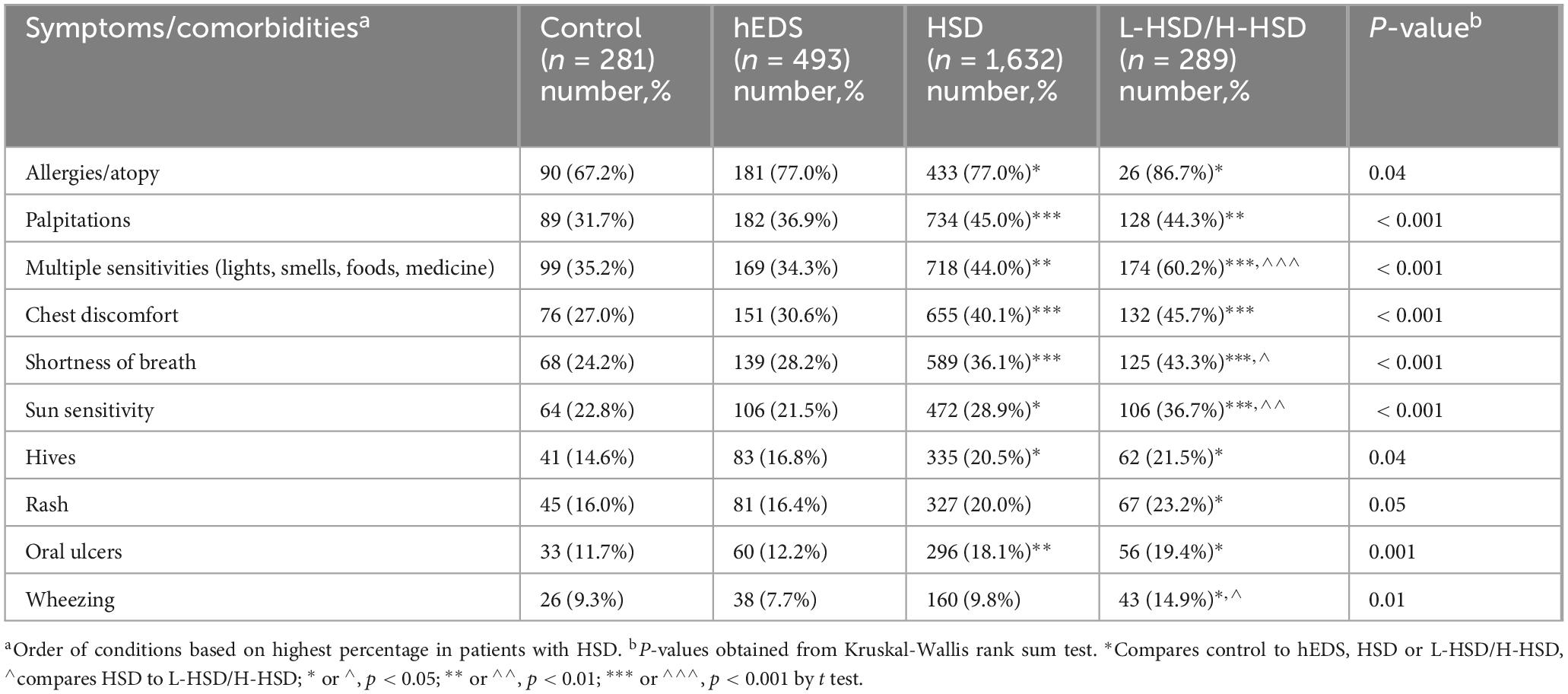
3.3 Asthma, allergy, and related symptoms
When we examined asthma and allergy symptoms, we found that all of the symptoms we examined were self-reported more often in patients with L-HSD/H-HSD than controls for allergy/atopy (p = 0.04), palpitations (p < 0.001), multiple sensitivities (p < 0.001), chest discomfort (p < 0.001), shortness of breath (p = 0.001), sun sensitivity (p < 0.001), hives (p = 0.039), rash (p = 0.048), oral ulcers (p = 0.001), and wheezing (p = 0.013) (Table 6). Additionally, more L-HSD/H-HSD patients self-reported issues with multiple sensitivities (hEDS p < 0.001, HSD p < 0.001), shortness of breath (hEDS p < 0.001, HSD p < 0.02), sun sensitivity (hEDS p < 0.001, HSD p < 0.008), and wheezing (hEDS p = 0.001, HSD p < 0.01) than patients diagnosed with hEDS or HSD (Table 6) indicating that patients with L-HSD/H-HSD have significant asthma/allergy symptoms and comorbidities.
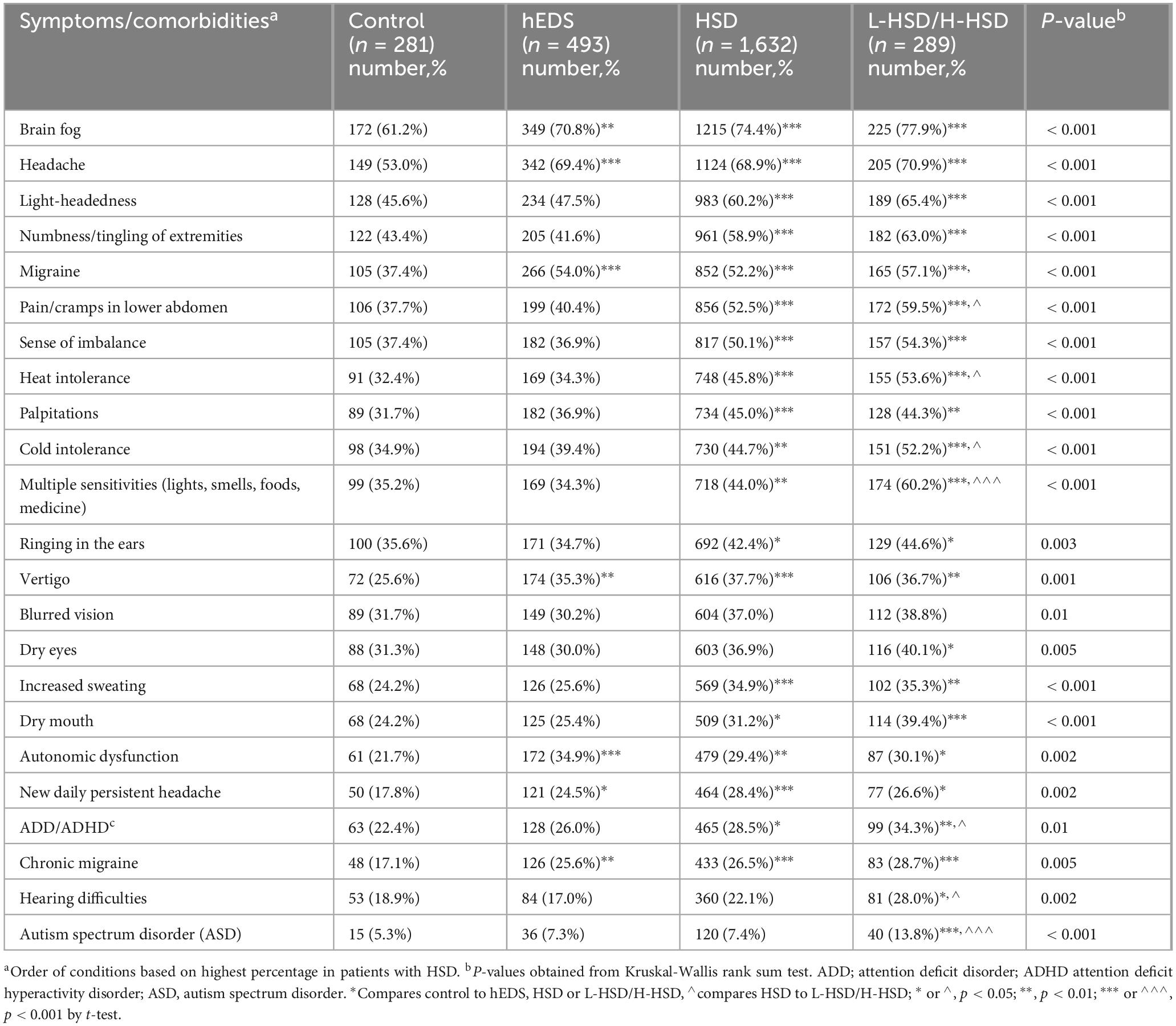
3.4 Neurological symptoms and comorbidities
When we examined neurological symptoms and comorbidities, we found that all of the symptoms we examined were self-reported more often in patients with L-HSD/H-HSD than controls including brain fog (p < 0.001), headache (p < 0.001), light-headedness (p < 0.001), numbness/tingling of extremities (p < 0.001), migraine (p < 0.001), pain/cramps in lower abdomen (p < 0.001), sense of imbalance (p < 0.001), heat intolerance (p < 0.001), palpitations (p < 0.001), cold intolerance (p < 0.001), multiple sensitivities (p < 0.001), ringing in the ears (p = 0.003), vertigo (p = 0.001), blurred vision (p = 0.013), dry eyes (p = 0.005), increased sweating (p < 0.001), dry mouth (p < 0.001), autonomic dysfunction (p = 0.002), new daily persistent headache (p = 0.002), ADD/ADHD (p = 0.011), chronic migraine (p = 0.005), hearing difficulties (p = 0.002), and ASD (p < 0.001) (Table 7). Additionally, more L-HSD/H-HSD patients self-reported issues with pain/cramps in lower abdomen (hEDS p < 0.001, HSD p = 0.026), heat intolerance (hEDS p < 0.001, HSD p < 0.014), cold intolerance (hEDS p < 0.001, HSD p < 0.018), multiple sensitivities (hEDS p < 0.001, HSD p < 0.001), ADD/ADHD (hEDS p = 0.014, HSD p = 0.047), hearing difficulties (hEDS p < 0.001, HSD p = 0.026), and ASD (hEDS p = 0.005, HSD p = 0.002) than patients diagnosed with hEDS or HSD (Table 7) indicating that patients with L-HSD/H-HSD have significant neurological symptoms/comorbidities.
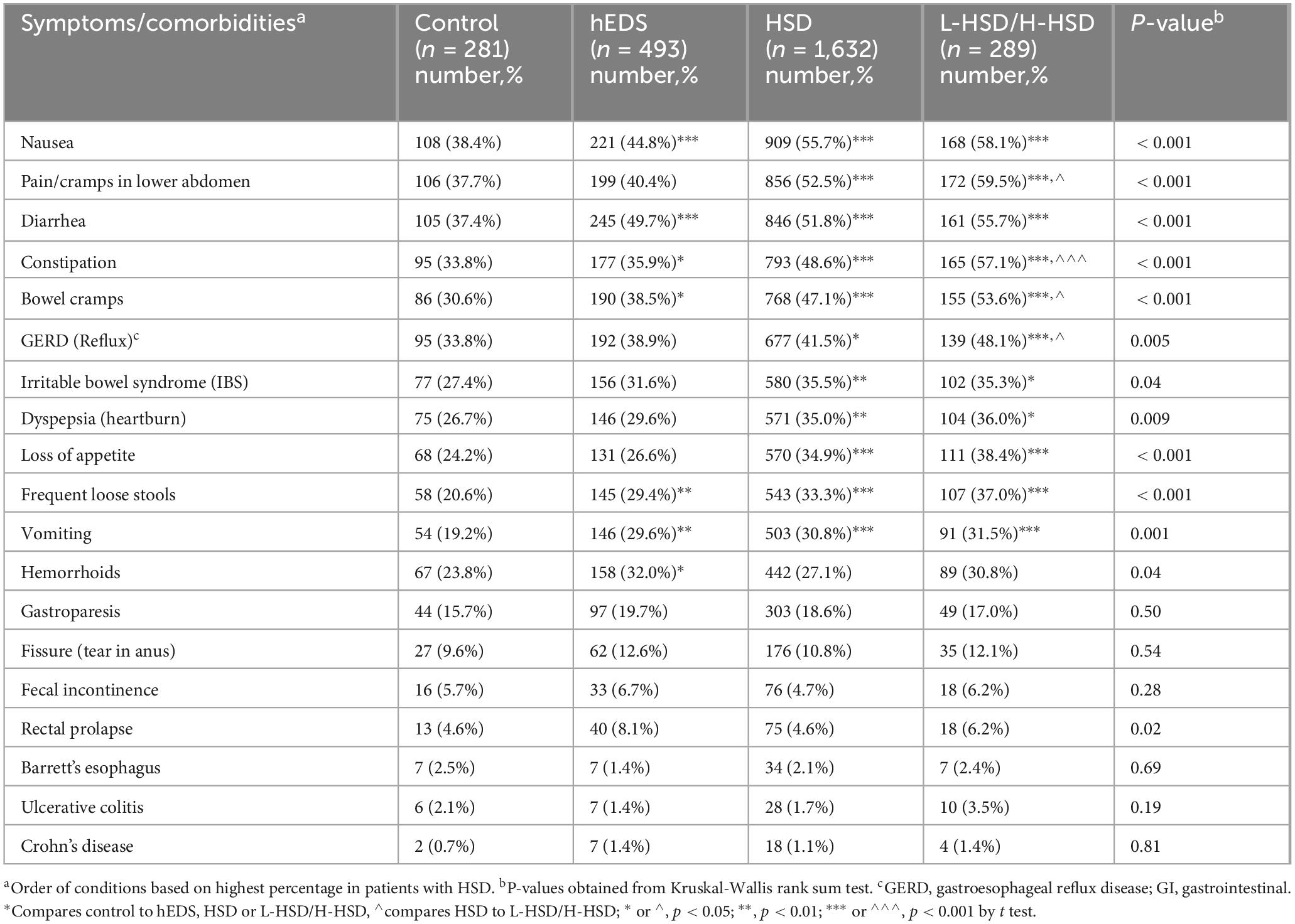
3.5 Gastrointestinal symptoms
When we examined gastrointestinal symptoms and comorbidities, we found that over half of the symptoms we examined were self-reported more often in patients with L-HSD/H-HSD than controls including pain/cramps in the lower abdomen (p < 0.001), diarrhea (p < 0.001), constipation (p < 0.001), bowel cramps (p < 0.001), GERD (p = 0.005), irritable bowel syndrome (IBS) (p = 0.035), dyspepsia (p = 0.009), loss of appetite (p < 0.001), frequent loose stools (p < 0.001), vomiting (p = 0.001), hemorrhoids (p = 0.042), and rectal prolapse (p = 0.02) (Table 8). Additionally, more L-HSD/H-HSD patients self-reported issues with pain/cramps in lower abdomen (hEDS p < 0.001, HSD p = 0.026), constipation (hEDS p < 0.001, HSD p = 0.008), bowel cramps (hEDS p < 0.001, HSD p = 0.039), and GERD (hEDS p = 0.012, HSD p = 0.036) than patients diagnosed with hEDS or HSD (Table 8) indicating that patients with L-HSD/H-HSD have significant gastrointestinal symptoms/comorbidities.
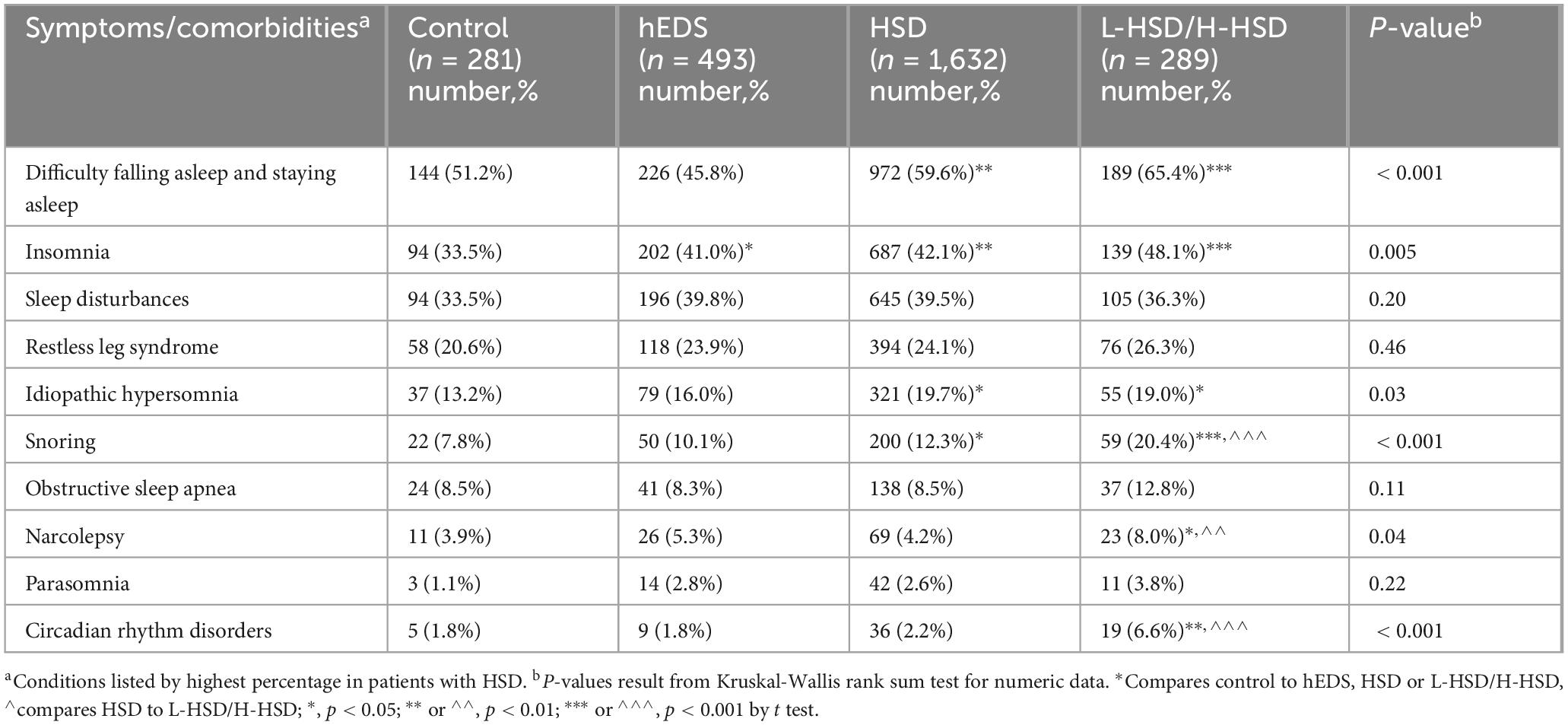
3.6 Sleep symptoms and comorbidities
When we examined sleep symptoms and comorbidities, we found that half of the symptoms we examined were self-reported more often in patients with L-HSD/H-HSD than controls including difficulty falling and staying asleep (p < 0.001), insomnia (p = 0.005), idiopathic hypersomnia (p = 0.032), snoring (p < 0.001), narcolepsy (p = 0.042), and circadian rhythm disorders (p < 0.001) (Table 9). Additionally, more L-HSD/H-HSD patients self-reported issues with snoring (hEDS p < 0.001, HSD p < 0.001) and narcolepsy (hEDS p = 0.14, HSD p = 0.006) than patients diagnosed with hEDS or HSD (Table 9) indicating that patients with L-HSD/H-HSD have some sleep symptoms and comorbidities.
3.7 Psychological conditions and abuse
When we examined psychological symptoms and comorbidities, we found that over half of the symptoms we examined were self-reported more often in patients with L-HSD/H-HSD than controls including anxiety (p < 0.001), depression (p < 0.001), depressed mood (p < 0.001), nervousness (p < 0.001), abuse (p = 0.042), emotional/verbal abuse (p < 0.001), PTSD (p = 0.001), ADD/ADHD (p = 0.011), sexual abuse (p = 0.038), and ASD (p < 0.001) (Table 10). As described above, more L-HSD/H-HSD patients self-reported issues with ADD/ADHD (hEDS p = 0.014, HSD p = 0.047) and ASD (hEDS p = 0.005, HSD p = 0.002) than patients diagnosed with hEDS or HSD (Table 10) indicating that patients with L-HSD/H-HSD may have more of these conditions. However, none of the other psychological symptoms/comorbidities or abuse that we examined were elevated in patients with L-HSD/H-HSD.
4 Discussion
To our knowledge, this is the first study to compare symptoms/comorbidities of L-HSD/H-HSD to controls or to patients diagnosed with hEDS and HSD. In this study, we found that patients with L-HSD/H-HSD had symptoms/comorbidities that closely resembled HSD suggesting that revised diagnostic criteria for hEDS and HSD should include L-HSD/H-HSD in the HSD diagnosis. Most of the symptoms/comorbidities that we examined in this study occurred in all patient groups; however, patients with HSD and L-HSD/H-HSD self-reported more symptoms/comorbidities than those diagnosed with hEDS or controls suggesting that patients with HSD, L-HSD and H-HSD may have more severe disease than patients with hEDS. We found that patients with L-HSD/H-HSD self-reported significantly more symptoms/comorbidities than controls for 62/100 (62%) of issues compared to 58/100 (58%) for HSD and 20/100 (20%) for hEDS. These findings indicate that L-HSD/H-HSD share similar symptoms and comorbidities to HSD and differ from hEDS and controls. Importantly, patients with L-HSD/H-HSD self-reported significantly more symptoms/comorbidities than patients diagnosed with hEDS or HSD for 20/100 (20%) of issues. These symptoms/comorbidities included joint pain, muscle weakness, multiple sensitivities, wheezing/shortness of breath, GERD, pain/cramps in the lower abdomen, constipation, heat and/or cold intolerance, hearing difficulties, ADHD, ASD, snoring, and narcolepsy (Figure 1). Additionally, some symptoms/comorbidities were self-reported exclusively more often in L-HSD/H-HSD patients compared to controls, but not in hEDS or HSD compared to controls, including wheezing, hearing difficulties, narcolepsy, circadian rhythm disorders, and ASD (Figure 1). These findings suggest that patients with L-HSD/H-HSD may have more symptoms/comorbidities than hEDS and HSD patients using current diagnostic definitions. However, it is possible that individuals diagnosed with L-HSD or H-HSD may be less likely to present to a specialty clinic unless they experience significant systemic symptoms (e.g., dysautonomia, GI distress, or neurocognitive issues). This referral or selection bias could contribute to the unexpectedly high symptom burden in this group relative to patients with hEDS or HSD, who may be referred based on more classical joint findings alone. Interestingly, patients with hEDS more closely resembled controls for many symptoms/comorbidities. Controls attended the EDS Clinic because they believed they could have hEDS but did not receive the diagnosis of hEDS, HSD, L-HSD or H-HSD. The controls consistently had fewer symptoms/comorbidities than HSD and L-HSD/H-HSD patients, but we do not know what their appropriate diagnosis is.
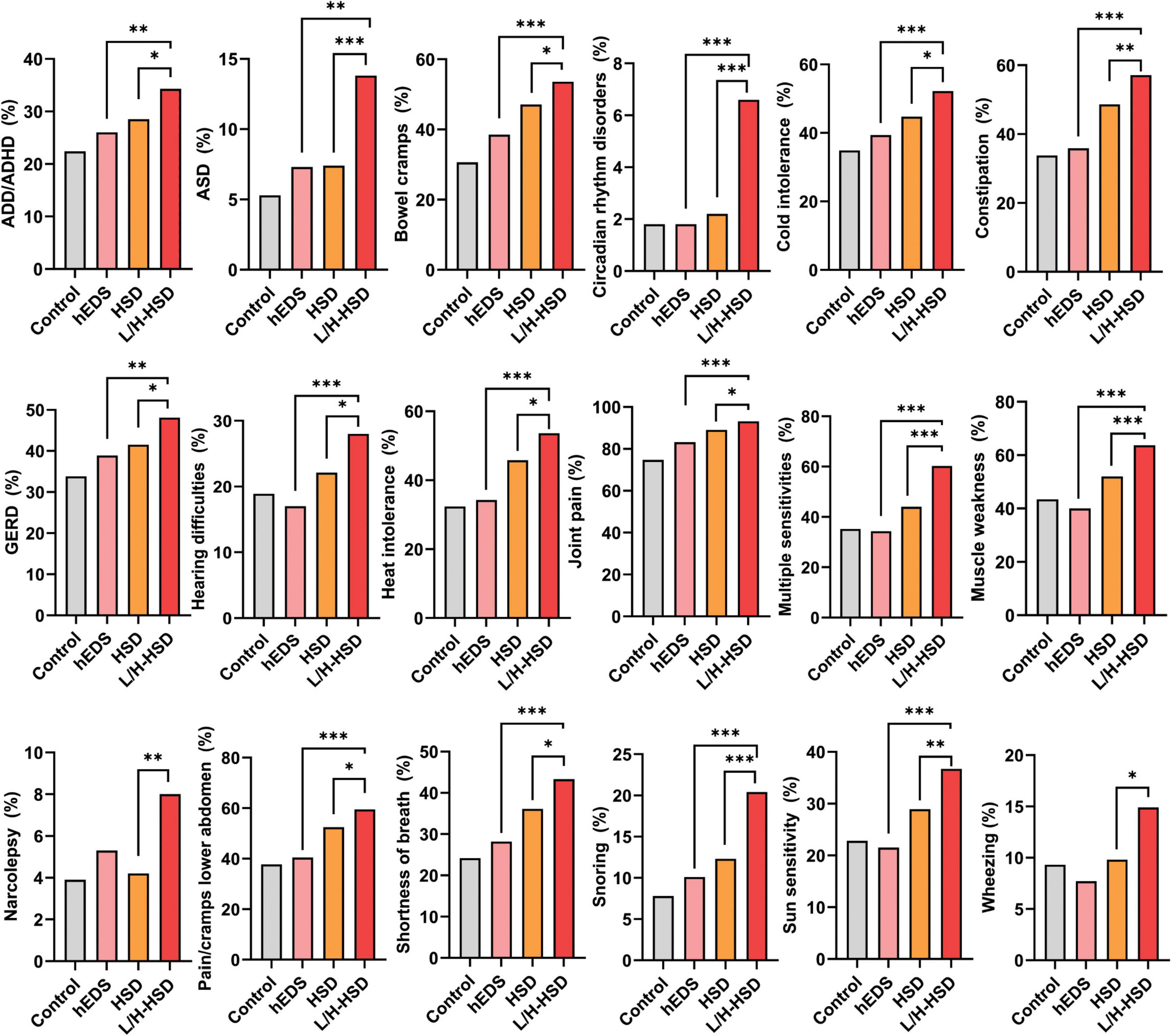
Figure 1. Symptoms and comorbidities that were significantly increased in patients with L-HSD/H-HSD compared to hEDS or HSD. Twenty out of 100 or 20% of the self-reported symptoms and comorbidities examined in this study were significantly higher in patients with L-HSD/H-HSD compared to other diagnoses indicating that these patients should be incorporated into the diagnostic criteria for HSD in the future (not all shown). *p < 0.05, **p < 0.01, ***p < 0.001 using Chi-square test comparing hEDS or HSD to L/H-HSD. Each graph was significant comparing 4 groups- see tables.
Since the development of the 2017 diagnostic criterion, a number of studies have compared hEDS to HSD to determine whether the two diagnoses represent a spectrum or are two distinct conditions. A study by Copetti et al. examined 105 patients diagnosed with hEDS or HSD for 59 characteristics and analyzed data using hierarchical clustering of principal components (11). They found that some patients within both diagnoses were considered more severe regardless of diagnosis and recommended a new severity score. This finding is similar to our previous report that around 70% of the patients seen at the Mayo Clinic Florida EDS Clinic were diagnosed with fibromyalgia, a condition characterized by widespread joint and muscle pain (6). This 70% included patients with L-HSD/H-HSD. We found that patients diagnosed with hEDS or HSD that also had a diagnosis of fibromyalgia (56% of patients) self-reported significantly more symptoms and comorbidities than those without a fibromyalgia diagnosis suggesting more severe disease (6). Copetti et al. (11) did not indicate whether the patients with greater severity in their study also had a diagnosis of fibromyalgia (11). Perhaps future revised hEDS/HSD diagnosis criteria could include an assessment/diagnosis of fibromyalgia.
We recently examined 2,088 patients at the Mayo Clinic Florida EDS Clinic using the 2017 diagnostic criteria comparing 115 self-reported symptoms/comorbidities between hEDS, HSD and controls to determine similarities and differences between the diagnoses (16). We found that all patients experienced some level of these symptoms/comorbidities and they were reported significantly more often in hEDS and HSD compared to controls. Importantly, several symptoms/comorbidities occurred at a high level in both diagnoses including allergies, subluxations, sprains, headache, and migraine (16). However, we found that most symptoms/comorbidities differed between the two diagnoses. Patients diagnosed with HSD self-reported 42 conditions significantly more often compared to hEDS (42/115 or 37%), while only 9 conditions occurred more often in patients with hEDS (9/115 or 8%) (16). One important finding was that the conditions reported more often in hEDS were “structural” like prolapses whereas conditions reported more often in HSD were “functional” like irritable bowel syndrome. These findings indicate that key features of hEDS/HSD like mast cell activation/allergy, subluxations/sprains and headache/migraine are common to both diagnoses, but many other symptoms/comorbidities occur more often in patients with HSD. The structural issues in hEDS patients are not surprising, because the diagnostic criterion selects patients with these issues. What stands out is that this diagnostic “selection” results in patients with fewer systemic symptoms/comorbidities. Our findings of overlap in allergy/mast cell symptoms in hEDS and HSD are congruent with the recent report by Ritelli et al. who discovered plasma biomarkers in both hEDS and HSD, but not in controls, that are the product of extracellular matrix (ECM) degradation (collagen I and fibronectin fragments) (15). Mast cell enzymes are principally responsible for the breakdown of ECM proteins, and so high mast cell activity in hEDS and HSD patients could result in a similar plasma ECM degradation profile. Mast cell hyperactivation that degrades collagen and ECM provides a mechanism for hypermobility and similar symptoms/comorbidities between the diagnoses. Mechanisms to explain the differences between symptoms/comorbidities between the diagnoses are an avid area of research.
In contrast to our findings, several previous studies did not find a significant difference between symptoms and comorbidities between hEDS and HSD (Table 2) (9, 11–14). Ritelli et al. (20) conducted RNA sequencing of skin fibroblast biopsies from 20 patients with hEDS, 20 with HSD and 40 controls using the 2017 criteria, and found that the gene profiles overlapped between hEDS and HSD (Table 2) (20). In contrast, studies by Ritelli et al. have found differences in symptoms/comorbidities between hEDS and HSD with more symptoms in hEDS patients suggesting that they could be more severe than patients with HSD (8, 15). Several explanations for the differences between the studies include age or race differences and/or that Ritelli et al. reported data obtained from the medical record and our studies include self-reported data. Overall, most studies examined a relatively small number of symptoms/comorbidities in a small number of patients (Table 2). Perhaps a larger number of patients is needed before differences between the diagnoses appear. An oral abstract submitted to the Ehlers-Danlos Society 2022 International Scientific Symposium on EDS and HSD reported an initial analysis of the worldwide Ehlers-Danlos Society Registry that examined 8,867 hEDS and 990 HSD patients for 8 symptoms/comorbidities including fatigue, gastrointestinal symptoms, headache, anxiety, depression, dysautonomia, gynae, bladder and mast cell activation disease (9). They did not find any differences in these 8 categories of self-reported symptoms/comorbidities between patients with hEDS or HSD (9). However, the published abstract does not provide information on whether all patients were diagnosed using the 2017 criteria, information on age, race/ethnicity or statistical information. Thus, it is difficult to compare this large number of patients to our findings. We are currently examining whether specific comorbidities obtained from the medical record differ by diagnosis to determine whether self-reported differences match medical record data.
In this study we examined 2,695 patients using the 2017 diagnostic criterion with 60.6% (n = 1,632) diagnosed with HSD, 18.3% (n = 493) hEDS, 10.7% (n = 289) L-HSD or H-HSD, and 10.4% (n = 281) were controls without any of these diagnoses. This study has several advantages including a large number of patients diagnosed using the 2017 criterion, and a relatively large number of controls seen at the EDS Clinic that were not diagnosed with hEDS, HSD or L-HSD/H-HSD. Although the controls were older on average than the other groups, otherwise their demographic data was relatively similar, consisting primarily of non-Hispanic White women (Tables 3, 4). We found that hEDS, HSD and L-HSD/H-HSD patients consistently self-reported more symptoms/comorbidities than controls. Importantly, patients with L-HSD/H-HSD self-reported symptoms/comorbidities at levels that were very similar to HSD but differed from hEDS, as we previously reported (16). One possible explanation for the differences in our findings compared to the initial reports from the Ehlers-Danlos Society Registry is that our population is almost 90% White non-Hispanic and their study included many races/ethnicities. Importantly, our findings indicate that features specific to L-HSD/H-HSD such as hypermobility in other areas of the body, which are not included in the current diagnostic criterion, should be incorporated into future revised diagnostic criteria. Our findings also mirror patient experience, where many patients that do not receive a diagnosis according to the current 2017 diagnostic criterion indicate that they suffer from many of the same symptoms/comorbidities as hEDS and HSD patients, and they are upset that they have not received a diagnosis. Receiving a diagnosis not only confirms that patients’ suffering has a cause/explanation, but it is needed for patients to receive appropriate medical care. Gaining a better understanding of similarities and differences between patients with hEDS, HSD, L-HSD and H-HSD may improve patient care and provide mechanistic insight into the pathogenesis of disease. Research into these mechanisms may lead to improved tailored therapies that reduce the progression of disease.
There are several limitations to the current study. The site of this study is a tertiary care center and findings from patients in this study may not represent other regions of the US or world. Additionally, symptoms/comorbidities were self-reported and not validated through another method. Future studies are needed to examine whether the key findings of this study can be verified when medical records are examined. A strength of this study is that hEDS/HSD patients were diagnosed using the most recent 2017 criterion by physician experts. An additional strength is the large study population that contained an internal control group which was not diagnosed with hEDS, HSD, L-HSD/H-HSD but had the same diagnostic process.
In conclusion, the contribution of this study to the field is that most centers seeing EDS patients do not include L-HSD or H-HSD in a diagnosis of HSD so that these patients may not receive the same level of care as patients diagnosed with hEDS and HSD. Additionally, these patients may not be included in research studies of HSD and we found that these patients self-reported similar, and in some cases higher, levels of symptoms/comorbidities as HSD patients. Thus, our data suggests that the new diagnostic criteria should incorporate these patients into the criteria for HSD so that the patients receive care and research.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Mayo Clinic IRB, Mayo Clinic, Rochester, MN, USA. The studies were conducted in accordance with the local legislation and institutional requirements. The Mayo Clinic IRB waived informed consent for this retrospective study.
Author contributions
DF: Writing – original draft, Supervision, Formal analysis, Resources, Writing – review & editing, Data curation, Project administration, Conceptualization, Visualization, Methodology, Funding acquisition, Validation, Investigation. KB: Writing – review & editing, Methodology, Data curation, Investigation. AD: Investigation, Writing – review & editing, Data curation. FW: Data curation, Writing – review & editing, Investigation. JF: Data curation, Writing – review & editing, Investigation. EM: Data curation, Investigation, Writing – review & editing. SK: Investigation, Data curation, Writing – review & editing. MS: Data curation, Writing – review & editing, Investigation. GW: Data curation, Investigation, Writing – review & editing. AP: Investigation, Writing – review & editing, Data curation. CH: Investigation, Writing – review & editing, Data curation. CH: Data curation, Investigation, Writing – review & editing. EW: Investigation, Writing – review & editing, Data curation. JG: Writing – review & editing, Investigation, Data curation. SM: Investigation, Writing – review & editing, Data curation. HS: Writing – review & editing, Formal analysis, Methodology. DH: Formal analysis, Methodology, Writing – review & editing. SG: Writing – review & editing, Investigation, Data curation. BM: Writing – review & editing, Data curation, Investigation. CS: Investigation, Writing – review & editing, Funding acquisition. PA: Writing – review & editing, Investigation. DK: Writing – review & editing, Funding acquisition, Methodology, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by funding from the Ehlers-Danlos Society MicroGrant to DF and DK; Mayo Clinic RACER and RACER Plus Award to DK; Mayo Clinic STARDOM Award to DF and DK; Mayo Clinic’s Division of General Internal Medicine to DF, DK, KB, the Ralph E. Pounds and Kathy Olesker Pounds Fund in Research Related to Chronic Pain to DF; American Heart Association 20TPA35490415 to DF and 23SCEFIA1153413 to KB, National Institutes of Health R01 HL164520, R21 AI154927, R21 AI152318, R21 AI145356 to DF, and National Institutes of Health R21 AI163302 and R21 AI180863 to KB.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Syx D, De Wandele I, Rombaut L, Malfait F. Hypermobility, the Ehlers-Danlos syndromes and chronic pain. Clin Exp Rheumatol. (2017) 35:116–22.
2. Tinkle B, Castori M, Berglund B, Cohen H, Grahame R, Kazkaz H, et al. Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome type iii and Ehlers-Danlos syndrome hypermobility type): clinical description and natural history. Am J Med Genet C Semin Med Genet. (2017) 175:48–69. doi: 10.1002/ajmg.c.31538
3. Gensemer C, Beck T, Guo L, Petrucci T, Morningstar J, Kornblau I, et al. Variants in the kallikrein gene family and hypermobile Ehlers-Danlos syndrome. Res Sq [Preprint]. (2024). doi: 10.21203/rs.3.rs-4547888/v1
4. Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. (2017) 175:8–26. doi: 10.1002/ajmg.c.31552
5. Scheper M, Juul-Kristensen B, Rombaut L, Rameckers E, Verbunt J, Engelbert R. Disability in adolescents and adults diagnosed with hypermobility-related disorders: a meta-analysis. Arch Phys Med Rehabil. (2016) 97:2174–87. doi: 10.1016/j.apmr.2016.02.015
6. Fairweather D, Bruno K, Darakjian A, Bruce B, Gehin J, Kotha A, et al. High overlap in patients diagnosed with hypermobile Ehlers-Danlos syndrome or hypermobile spectrum disorders with fibromyalgia and 40 self-reported symptoms and comorbidities. Front Med (Lausanne). (2023) 10:1096180. doi: 10.3389/fmed.2023.1096180
7. Morlino S, Castori M. Placing joint hypermobility in context: traits, disorders and syndromes. Br Med Bull. (2023) 147:90–107. doi: 10.1093/bmb/ldad013
8. Ritelli M, Chiarelli N, Cinquina V, Vezzoli M, Venturini M, Colombi M. Looking back and beyond the 2017 diagnostic criteria for hypermobile Ehlers-Danlos syndrome: a retrospective cross-sectional study from an Italian reference center. Am J Med Genet A. (2024) 194:174–94. doi: 10.1002/ajmg.a.63426
9. Hakim AK, Francomano C, Bloom L, Malfait F, Gandy W. The proportion of people reporting multisystemic morbidities is similar across the Ehlers-Danlos syndromes. Proceedings of the 2022 Ehlers-Danlos Society International Symposium on the EDS and HSD Abstract Booklet (Rome) (2024), 117, p. 81–83.
10. Hakim A, Francomano C. Update on the Diagnostic Criteria for hEDS. (2024) Available online at: https://www.ehlers-danlos.com/criteria-and-diagnostic-pathway-update/ (accessed September 16, 2024).
11. Copetti M, Morlino S, Colombi M, Grammatico P, Fontana A, Castori M. Severity classes in adults with hypermobile Ehlers-Danlos syndrome/hypermobility spectrum disorders: a pilot study of 105 Italian patients. Rheumatology (Oxford). (2019) 58:1722–30. doi: 10.1093/rheumatology/kez029
12. McGillis L, Mittal N, Santa Mina D, So J, Soowamber M, Weinrib A, et al. Utilization of the 2017 diagnostic criteria for hEDS by the Toronto GoodHope Ehlers-Danlos syndrome clinic: a retrospective review. Am J Med Genet A. (2020) 182:484–92. doi: 10.1002/ajmg.a.61459
13. Aubry-Rozier B, Schwitzguebel A, Valerio F, Tanniger J, Paquier C, Berna C, et al. Are patients with hypermobile Ehlers-Danlos syndrome or hypermobility spectrum disorder so different? Rheumatol Int. (2021) 41:1785–94. doi: 10.1007/s00296-021-04968-3
14. Martinez K, Mauss C, Andrews J, Saboda K, Huynh J, Sanoja A, et al. Subtle differences in autonomic symptoms in people diagnosed with hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders. Am J Med Genet A. (2021) 185:2012–25. doi: 10.1002/ajmg.a.62197
15. Ritelli M, Chiarelli N, Cinquina V, Bertini V, Piantoni S, Caproli A, et al. Bridging the diagnostic gap for hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders: evidence of a common extracellular matrix fragmentation pattern in patient plasma as a potential biomarker. Am J Med Genet A. (2025) 197:e63857. doi: 10.1002/ajmg.a.63857
16. Darakjian A, Bhutani M, Fairweather D, Kocsis S, Fliess J, Khatib S, et al. Similarities and differences in self-reported symptoms and comorbidities between hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders. Rheumatol Adv Pract. (2024) 8:rkae134. doi: 10.1093/rap/rkae134
17. Menton S, Fairweather D, Bruno K, Thompson C, Candelo E, Darakjian A, et al. Laryngological complaint prevalence in hypermobile Ehlers-Danlos or hypermobility spectrum disorders. Laryngoscope. (2024) 134:773–8. doi: 10.1002/lary.30964
18. Knight D, Bruno K, Singh A, Munipalli B, Gajarawala S, Solomon M, et al. Cardiac defects of hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders: a retrospective cohort study. Front Cardiovasc Med. (2024) 11:1332508. doi: 10.3389/fcvm.2024.1332508
19. Hakim A, Grahame R. A simple questionnaire to detect hypermobility: an adjunct to the assessment of patients with diffuse musculoskeletal pain. Int J Clin Pract. (2003) 57:163–6. doi: 10.1111/j.1742-1241.2003.tb10455.x
20. Ritelli M, Chiarelli N, Cinquina V, Zoppi N, Bertini V, Venturini M, et al. RNA-seq of dermal fibroblasts from patients with hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders supports their categorization as a single entity with involvement of extracellular matrix degrading and proinflammatory pathomechanisms. Cells. (2022) 11:4040. doi: 10.3390/cells11244040
Keywords: hypermobile Ehlers-Danlos syndrome, hypermobility spectrum disorders, localized hypermobility spectrum disorders, historical hypermobility spectrum disorders, diagnostic criteria, comorbidities, autism spectrum disorder
Citation: Fairweather D, Bruno KA, Darakjian AA, Wilson FC, Fliess JJ, Murphy EF, Kocsis SC, Strandes MW, Weigel GJ, Puls AM, Hartmoyer CJ, Hamilton C, Whelan ER, Gehin JM, Menton SM, Sledge H, Hodge DO, Gajarawala S, Munipalli B, Shufelt CL, Atwal PS and Knight DRT (2025) Localized and historical hypermobile spectrum disorders share self-reported symptoms and comorbidities with hEDS and HSD. Front. Med. 12:1594796. doi: 10.3389/fmed.2025.1594796
Received: 17 March 2025; Accepted: 02 July 2025;
Published: 13 August 2025.
Edited by:
Isabelle Brock, Independent Researcher, New York, NY, United StatesReviewed by:
David Saperstein, Center for Complex Neurology, EDS & POTS, United StatesSusan Mills, The Pennsylvania State University, United States
Copyright © 2025 Fairweather, Bruno, Darakjian, Wilson, Fliess, Murphy, Kocsis, Strandes, Weigel, Puls, Hartmoyer, Hamilton, Whelan, Gehin, Menton, Sledge, Hodge, Gajarawala, Munipalli, Shufelt, Atwal and Knight. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katelyn A. Bruno, a2F0ZWx5bi5icnVub0BtZWRpY2luZS51ZmwuZWR1; Dacre R. T. Knight, S25pZ2h0LkRhY3JlQG1heW8uZWR1
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
 DeLisa Fairweather
DeLisa Fairweather Katelyn A. Bruno
Katelyn A. Bruno Ashley A. Darakjian1,3†
Ashley A. Darakjian1,3† Frances C. Wilson
Frances C. Wilson S. Christian Kocsis
S. Christian Kocsis Emily R. Whelan
Emily R. Whelan Jessica M. Gehin
Jessica M. Gehin Stacey M. Menton
Stacey M. Menton David O. Hodge
David O. Hodge Bala Munipalli
Bala Munipalli Chrisandra L. Shufelt
Chrisandra L. Shufelt Dacre R. T. Knight
Dacre R. T. Knight