- 1The Second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Department of Nephrology, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
Background: To investigate the clinicopathological features and prognostic factors of idiopathic membranous nephropathy (IMN) patients with renal arteriolosclerosis, providing evidence for individualized clinical management.
Methods: A retrospective analysis was conducted on 597 biopsy-confirmed IMN patients at Guangdong Provincial Hospital of Chinese Medicine from January 1, 2012, to December 31, 2022. Patients were stratified into two groups based on the presence of renal arteriolosclerosis. Clinical and pathological characteristics were compared between groups. Kaplan–Meier curves were used to assess cumulative renal remission rates, and Cox regression analysis was performed to identify risk factors for composite endpoint events in IMN patients with arteriolosclerosis.
Results: In a cohort of 597 IMN patients (55.6% male), significant baseline differences were observed in Serum Sodium, triglycerides, membranous nephropathy (MN) stage, mesangial proliferation, interstitial fibrosis, and IgG deposition between the arteriolosclerosis and non-arteriolosclerosis groups (p < 0.05). Kaplan–Meier analysis demonstrated markedly lower renal survival in the arteriolosclerosis group (Log-rank χ2 = 8.296, p = 0.004). Multivariate Cox regression identified age (HR = 1.022, 95% CI 1.003–1.042; p = 0.022), serum creatinine (SCr) (HR = 1.010, 95% CI 1.002–1.018; p = 0.017), IgM 3 + deposition (HR = 4.718, 95% CI 1.003–1.042; p < 0.001), and interstitial fibrosis (HR > 1, p < 0.05) as independent risk factors for composite endpoint events, Compared to their respective reference groups, C1q (3+) and tubular atrophy (≥50%) have a protective effect against adverse renal outcomes (HR < 1, p < 0.05).
Conclusion: Renal arteriolosclerosis portends poorer prognosis in IMN, with distinct clinicopathological features and accelerated renal function decline. Age, elevated creatinine, intense immune complex deposition, and advanced tubular-interstitial damage represent critical risk markers, highlighting the need for early vascular assessment and histology-guided risk stratification in this population.
1 Introduction
Idiopathic membranous nephropathy (IMN) is one of the leading causes of nephrotic syndrome in adults. Over the past two decades, the incidence of IMN has increased approximately 1.5-fold, with a global incidence rate of 1–2 cases per 100,000 person-years. However, the incidence in Asian populations is significantly higher than in Western countries, which may be attributed to genetic predisposition, environmental factors, and improved diagnostic capabilities (1). In China, the rise in IMN prevalence has been particularly pronounced. Epidemiological studies indicate that IMN now accounts for 23.7% of primary glomerular diseases, up from 15.3%, making it one of the most common primary glomerulopathies (2).
Despite advances in PLA2R antibody detection guiding risk stratification, approximately 30–40% of IMN patients progress to end-stage kidney disease within 5–15 years, with relapses occurring in 35–45% of remitted cases under calcineurin inhibitors (3, 4). Current biomarkers fail to predict treatment resistance in 30% of high-titer patients, while comorbidities like diabetes exacerbate therapeutic challenges by impairing glycemic control during immunosuppression (3, 4). Identifying prognostic risk factors for IMN is critical for personalized therapeutic strategies and improving patient outcomes. Furthermore, although histological factors are associated with lower remission rates and more rapid renal function decline, they have not been adequately studied (5). Current research on IMN prognosis has paid limited attention to renal arteriolosclerosis. The clinicopathological characteristics and prognostic implications of IMN patients with concomitant renal arteriolosclerosis remain poorly defined, necessitating further investigation (6, 7).
Therefore, in this study, we retrospectively analyzed the clinicopathological characteristics of patients with IMN complicated by renal arteriolosclerosis, identified factors influencing renal prognosis, and proposed potential management strategies based on these correlations.
2 Materials and methods
2.1 Study population
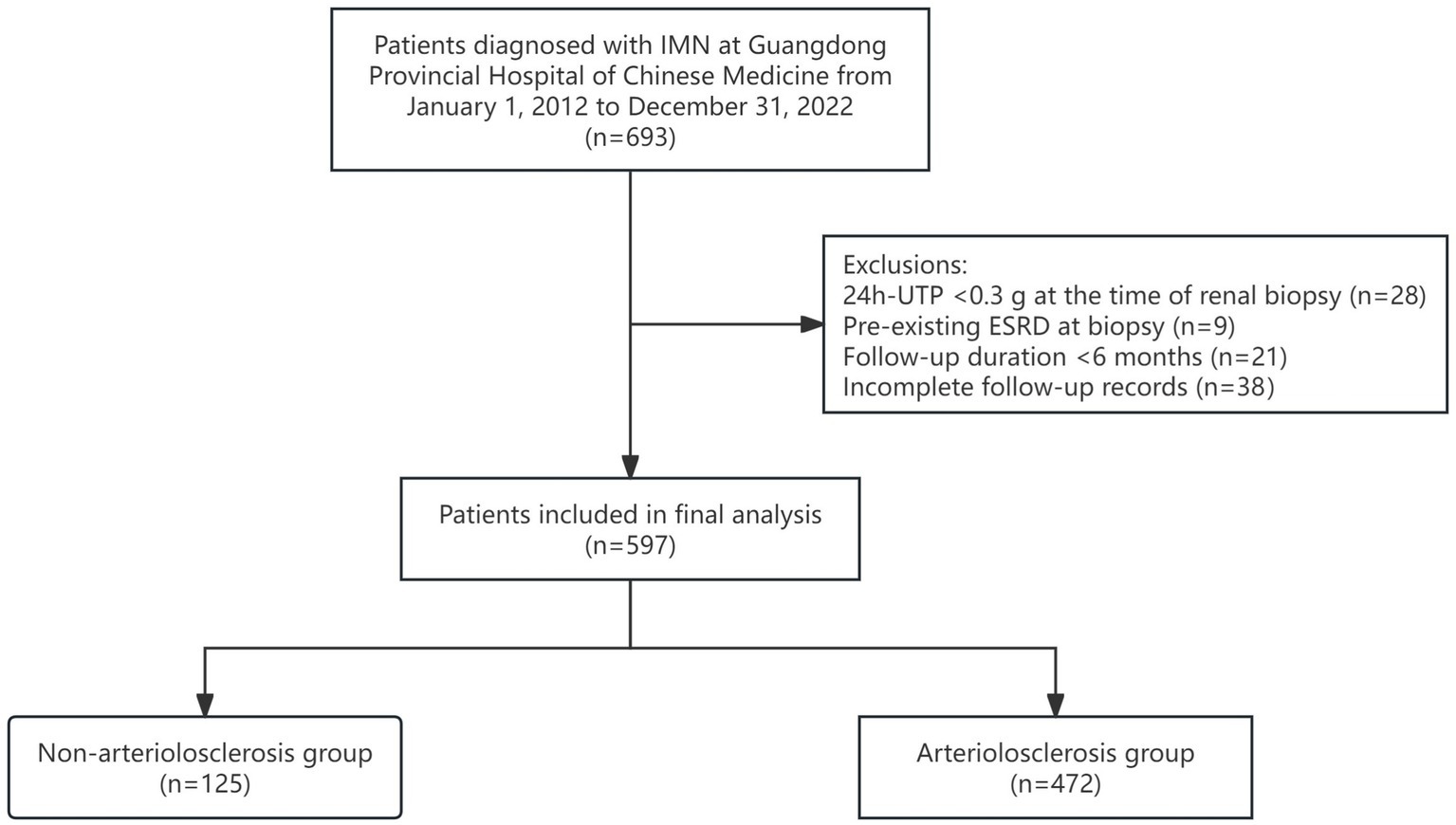
A retrospective analysis was conducted on 597 patients diagnosed with IMN via renal biopsy at Guangdong Provincial Hospital of Chinese Medicine between January 1, 2012, and December 31, 2022 (Figure 1).
2.1.1 Inclusion criteria
(1) Age ≥18 years, regardless of sex;
(2) Pathologically confirmed IMN by renal biopsy;
(3) Complete medical records available.
2.1.2 Exclusion criteria
(1) Severe psychiatric disorders or other conditions impairing cooperation;
(2) Clinical diagnosis of secondary membranous nephropathy;
(3) 24-h urinary protein excretion (24 h-UTP) < 0.3 g at the time of renal biopsy;
(4) Pre-existing End-stage renal disease (ESRD) at biopsy;
(5) Follow-up duration <6 months.
Patients were categorized into two groups based on the presence of renal arteriolosclerosis: the non-arteriolosclerosis group (n = 125) and the arteriolosclerosis group (n = 472). This study was approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (Ethics approval number: YE 2024-266-01).
2.2 Methods
2.2.1 Data collection
Demographic data included sex, age, and comorbidities such as hypertension (HTN), diabetes mellitus (DM), and hyperuricemia (HUA). Laboratory data comprised 24 h-UTP, white blood cell count (WBC), lymphocyte count (LYM), hemoglobin (Hb), platelet count (PLT), blood urea nitrogen (BUN), total carbon dioxide combining rate (TCO2), serum uric acid (SUA), serum creatinine (SCr), estimated glomerular filtration rate (eGFR), serum albumin (ALB), triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), serum potassium (K+), serum sodium (Na+), and serum calcium (Ca2+). Renal pathological data included findings from light microscopy, electron microscopy, and immunofluorescence studies.
2.2.2 Key definitions
(1) The follow-up cutoff date was December 31, 2024. A composite endpoint event was defined as any of the following: a ≥ 30% decline in eGFR from baseline, all-cause mortality, progression to ESRD, or failure to achieve partial or complete remission of IMN during follow-up.
(2) Renal arteriolosclerosis in this study included arteriolar wall thickening, luminal stenosis or occlusion, and hyaline degeneration. Arteriolar wall thickening was assessed according to the criteria established by Zou et al. defined as a ratio of arteriolar internal diameter to external diameter <0.5 (8).
(3) Complete remission of IMN was defined as stable renal function and 24 h urinary protein < 0.3 g; partial remission is defined as 24 h urinary protein decreased by more than 50% from the baseline (with stable renal function) and < 3.5 g; non—remission is defined as persistent proteinuria with a 24 h urinary protein quantitative reduction < 50% or renal function progression (9).
2.2.3 Statistical analysis
All statistical analyses were performed using R software (version 4.4.2). Continuous variables were tested for normality using the Shapiro–Wilk test. Normally distributed data were expressed as mean ± standard deviation (SD) and compared using independent samples t-tests. Non-normally distributed data were expressed as median (interquartile range, IQR) and compared using the Mann–Whitney U test. Categorical variables were expressed as frequencies and compared using the chi-square test, corrected chi-square test, or Fisher’s exact test, as appropriate.
For prognosis and outcome analysis, Kaplan–Meier curves were plotted, and the log-rank test was used to compare survival rates between groups. Univariate and multivariate Cox regression analyses were performed to identify factors influencing the composite endpoint in IMN patients with renal arteriolosclerosis. Missing data were handled using multiple imputation methods. A two-sided p-value <0.05 was considered statistically significant.
3 Results
3.1 Comparison of baseline characteristics
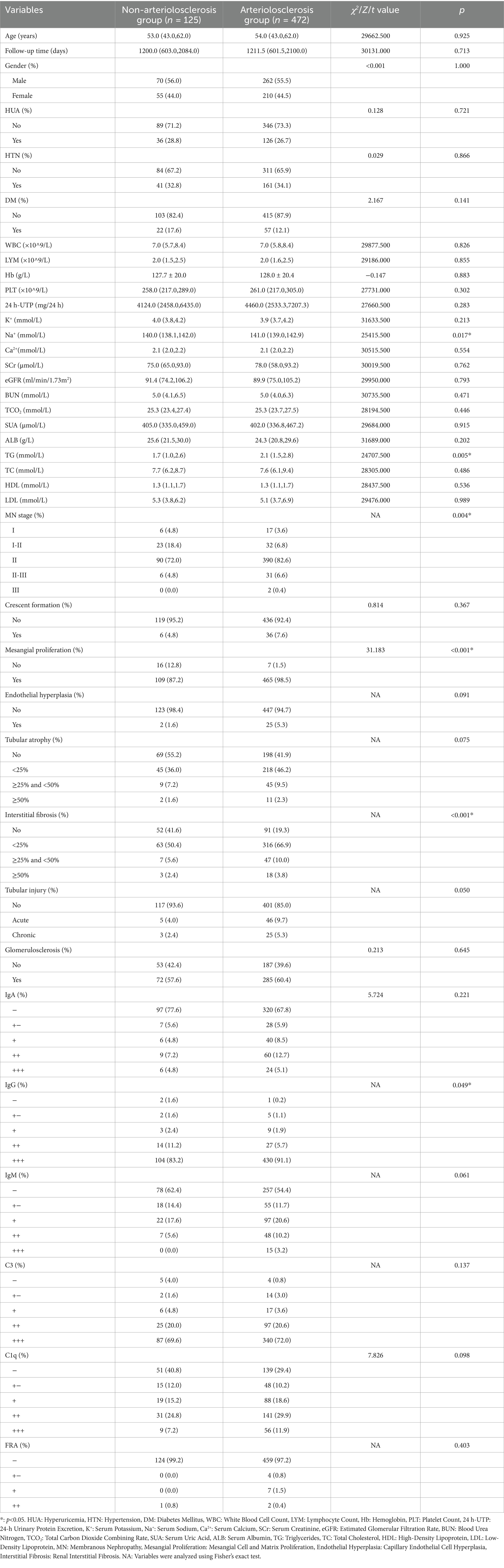
Among the 597 IMN patients, 265 (44.4%) were female, and 332 (55.6%) were male. Significant differences were observed between the non-arteriolosclerosis and arteriolosclerosis groups only in Na+, TG, MN stage, mesangial cell and matrix proliferation, renal interstitial fibrosis, and IgG deposition (p < 0.05) (Table 1).
3.2 Analysis of prognostic factors
Kaplan–Meier survival curves were plotted, revealing that the survival curve of the non-arteriolosclerosis group was consistently higher than that of the arteriolosclerosis group throughout the follow-up period. Moreover, the cumulative renal survival rate declined more significantly in the arteriolosclerosis group, with the difference being statistically significant (Log-rank test: χ2 = 8.296, p = 0.004) (Figure 2).
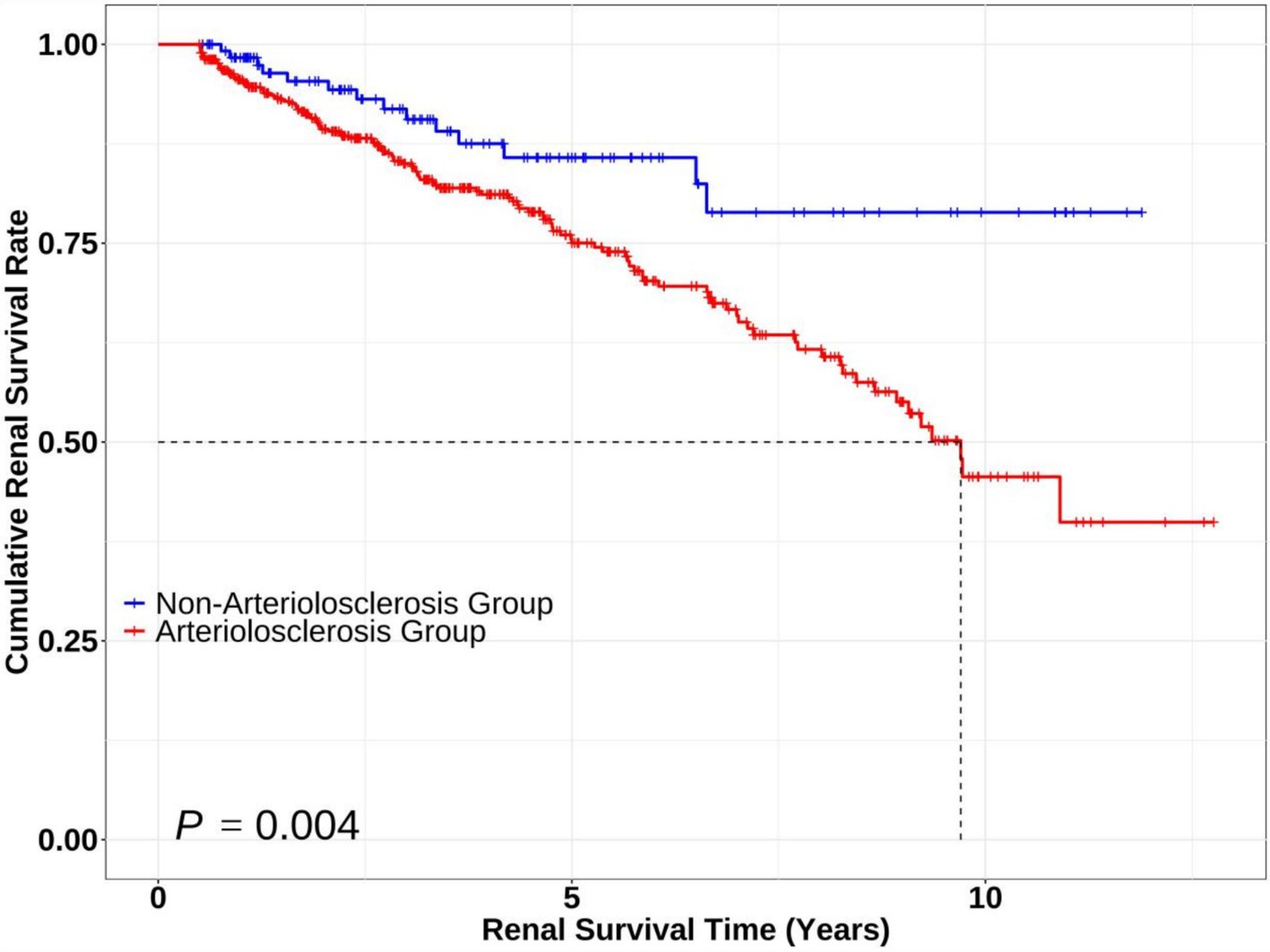
Figure 2. Kaplan–Meier survival curves comparing renal survival between patients with and without arteriolosclerosis. The Kaplan–Meier curves illustrate renal survival probabilities over time for the Non-Arteriolosclerosis Group (n = 125, blue line) and Arteriolosclerosis Group (n = 472, red line). Statistical significance between groups was assessed using the log-rank test (p = 0.004, Log-rank χ2 = 8.296).
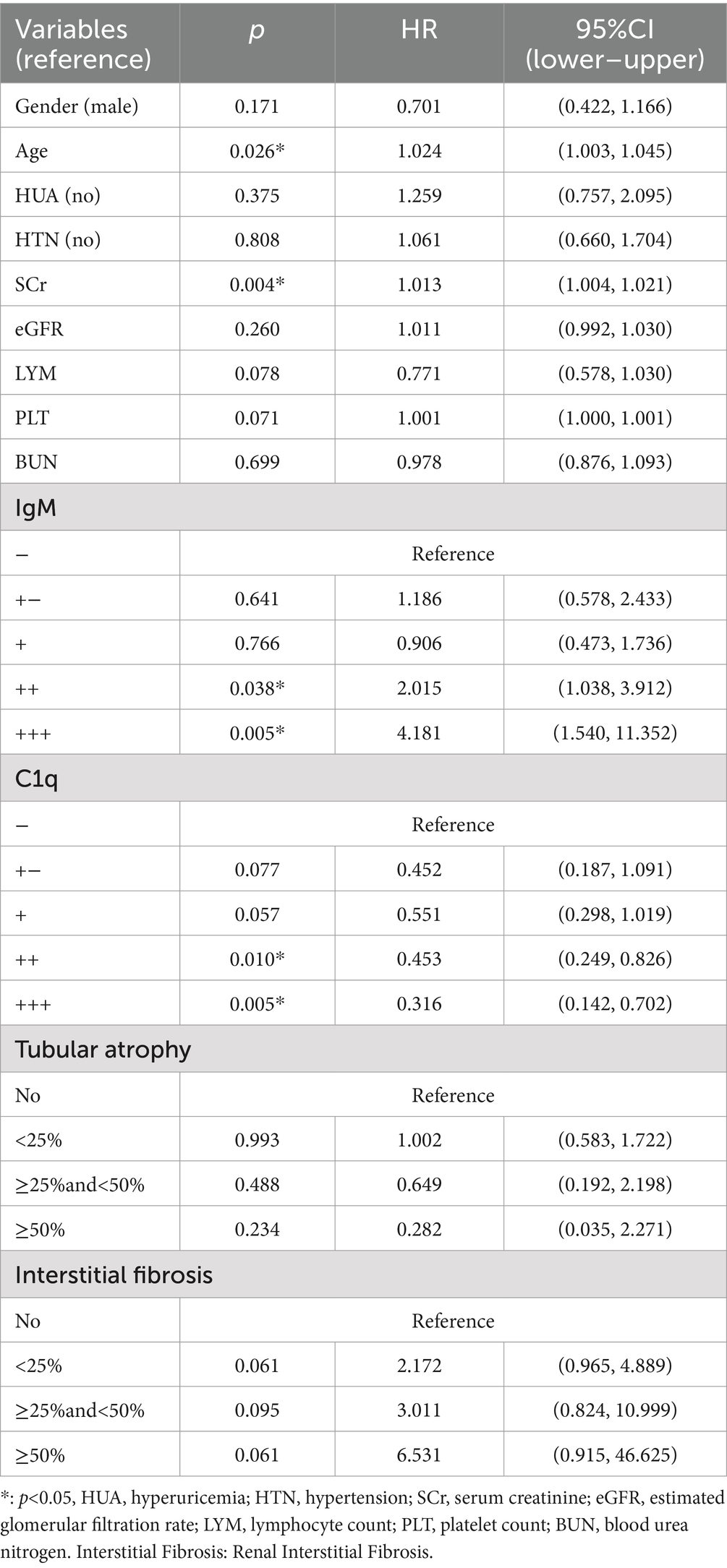
Univariate and multivariate Cox regression analyses were performed on 472 patients with renal arteriolosclerosis. Univariate Cox regression identified sex, age, HUA, HTN, SCr, eGFR, LYM, PLT, BUN, IgM, C1q, tubular atrophy, and renal interstitial fibrosis significantly influenced the prognosis of IMN with renal arteriolosclerosis (p < 0.05). Multivariate Cox regression of significant univariate predictors showed that age and SCr were independent risk factors for endpoint events (HR = 1.022, 95% CI 1.003–1.042, p = 0.022; HR = 1.010, 95% CI 1.002–1.018, p = 0.017). Compared with IgM (−) deposition, IgM (3+) was associated with a higher risk of poor outcomes, whereas C1q (3+) was protective versus C1q (−) (p < 0.05). Compared to the no tubular atrophy group, patients with ≥50% tubular atrophy had a significantly decreased likelihood of endpoint events (p = 0.039). Compared to no renal interstitial fibrosis, all severity levels of renal interstitial fibrosis were associated with a significantly higher risk of endpoint events (p < 0.05) (Table 2).
3.3 Subgroup and sensitivity analyses
Figure 3 displays the re-stratified Kaplan–Meier curves, demonstrating lower cumulative renal remission rates in the following subgroups: advanced age cohort (≥60 years), elevated serum creatinine group (SCr ≥ 133 μmol/L), high IgM deposition group (IgM: +++), and patients with renal interstitial fibrosis.
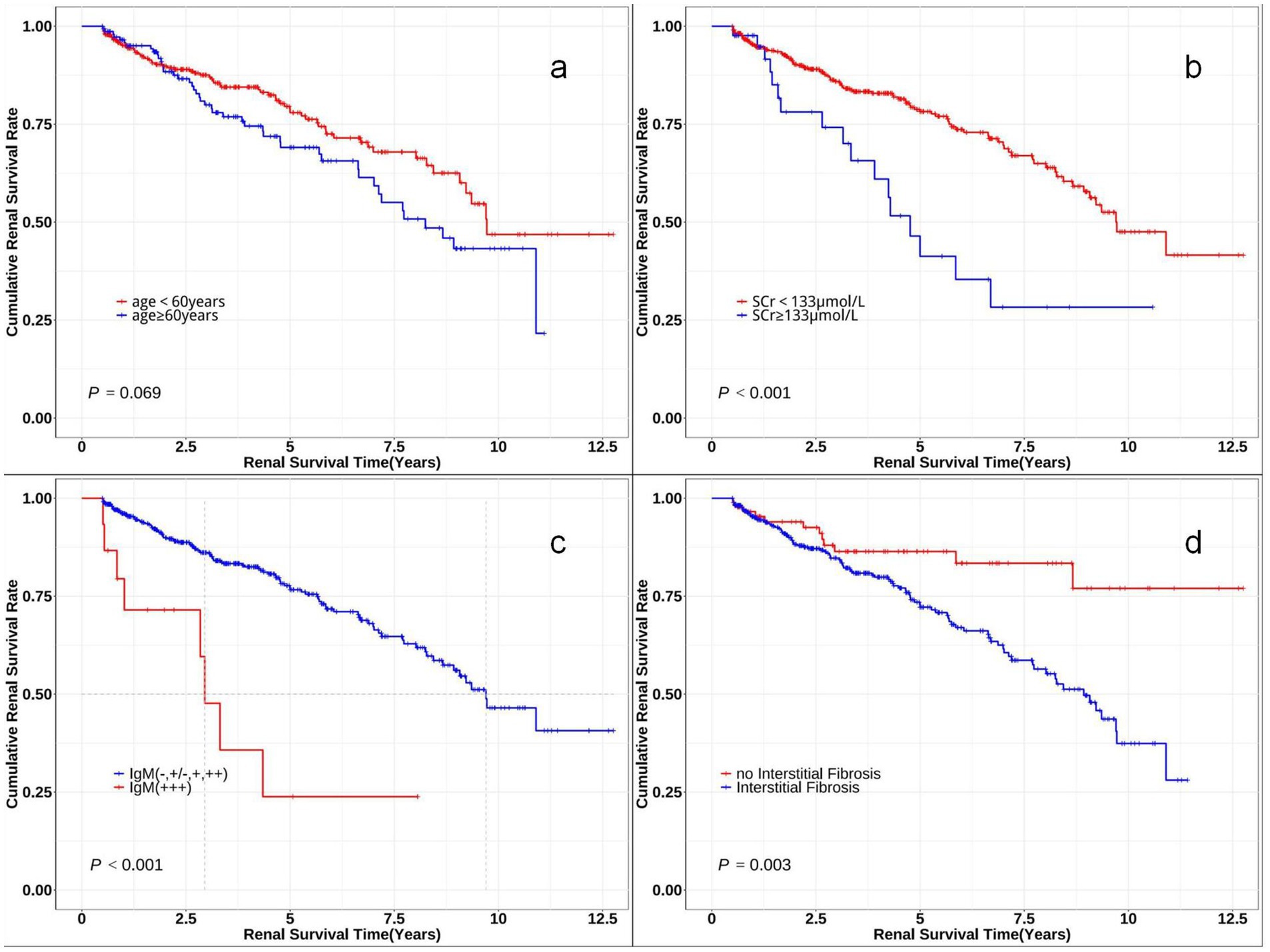
Figure 3. Kaplan–Meier survival curves comparing renal survival of subgroups. The Kaplan–Meier curves for time to renal survival in different subclassifications. (a) Probability of renal survival in different age levels (log-rank χ2 = 3.315, p = 0.069). (b) Probability of renal survival in different serum creatinine (SCr) levels (log-rank χ2 = 13.722, p < 0.001). (c) Probability of renal survival in different IgM levels (log-rank χ2 = 18.635, p < 0.001). (d) Probability of renal survival with or without interstitial fibrosis (log-rank χ2 = 8.702, p = 0.003).
We performed a sensitivity analysis by excluding participants with follow-up duration <1 year, resulting in a final cohort of 404 patients. The refined multivariable Cox proportional hazards model demonstrated comparable results to the primary analysis. Notably, renal interstitial fibrosis lost statistical significance in this long-term follow-up cohort (Table 3).
4 Discussion
Renal arteriolosclerosis is closely associated with various kidney diseases, and studies have reported its coexistence with hypertensive nephropathy, diabetic nephropathy, IgA nephropathy, chronic kidney disease (CKD) and IMN (10–14). Renal arteriolosclerosis is a vascular lesion characterized by pathological thickening of the arteriolar wall and luminal stenosis, primarily affecting the afferent arterioles and interlobular arteries, leading to abnormal renal hemodynamics and localized ischemic injury (15). Arteriolosclerosis-induced hemodynamic alterations, particularly glomerular hyperfiltration resulting from arteriolar stenosis, enhance mechanical stress on the glomerular basement membrane. This process promotes proteinuria and foot process effacement, which are hallmark features of IMN progression (16). Currently, there is limited research on IMN patients with renal arteriolosclerosis, and most studies have included IMN with vascular lesions or arteriolar intimal thickening as subgroups.
4.1 Key findings
In this study of 597 IMN patients (2012–2022), 79% showed concurrent renal arteriolosclerosis, significantly exceeding the previously reported prevalence of 55% (17). The arteriolosclerosis group demonstrated a male predominance (male-to-female ratio: 1.23: 1) and a higher median age compared to the non-arteriolosclerosis group, consistent with the clinical profile of vascular sclerosis susceptibility in elderly males (17, 18). These observations support androgen-mediated vascular inflammatory mechanisms in disease progression (19, 20).
Patients with renal arteriolosclerosis exhibited significantly elevated TG and Na+ levels compared to controls, the findings are consistent with those of previous studies (21, 22). A higher proportion of Churg stage II lesions (p = 0.003) was observed in this group, likely attributable to hemodynamic disturbances causing metabolic dysregulation of the glomerular basement membrane (23, 24). Prior studies have established associations between renal arteriolosclerosis and renal interstitial fibrosis, mesangial cell proliferation, matrix expansion, and immune complex deposition, which may exacerbate vascular pathology by increasing intraglomerular pressure, oxidative stress, and localized inflammatory responses—consistent with the heightened tubular injury observed in this cohort (24, 25).
After a median follow-up of 1,211.5 days, the arteriolosclerosis group demonstrated a composite endpoint event rate of 23.9%, significantly exceeding the 11.2% rate in the non-arteriolosclerosis group, with reduced renal survival. Multivariate Cox regression analysis identified age, SCr, IgM and C1q (3+) immune deposits, renal interstitial fibrosis, and tubular atrophy ≥50% as independent factors for adverse renal outcomes in IMN patients with arteriolosclerosis.
4.2 Mechanistic insights
The pathological interplay between renal arteriolosclerosis and IMN is fundamentally orchestrated by ischemia and hypoxia, a central driver that integrates hemodynamic, oxidative, and metabolic perturbations to amplify renal damage. Specifically, arteriolar luminal stenosis disrupts renal microcirculation, leading to glomerular hyperfiltration and tubulointerstitial ischemia, thereby creating a hypoxic microenvironment that bridges vascular and glomerular injury (26–28). Notably, this hypoxic state triggers hypoxia-inducible factor-1α (HIF-1α) activation, which subsequently upregulates the Wnt/β-catenin pathway to promote renal fibrosis through epithelial-mesenchymal transition (EMT) in tubular cells and interstitial fibroblast activation (29).
Moreover, this pathway further exacerbates disease progression by driving renin-angiotensin system (RAS) hyperactivation. As a result, increased angiotensin II production induces arteriolar vasoconstriction and disrupts podocyte slit diaphragms, facilitating subepithelial immune complex retention (30, 31). Importantly, the clinical relevance of this mechanism is strongly supported by evidence demonstrating that RAS blockade with maximum-dose Angiotensin-Converting Enzyme (ACE) inhibitors/Angiotensin II Receptor Blockers (ARBs) achieves comparable 5-year outcomes to immunosuppressive therapy in terms of proteinuria reduction and renal function preservation, as shown by Dikow et al. (32). This finding underscores the dual role of RAS inhibition in mitigating both hemodynamic and non-hemodynamic injury pathways.
Beyond the hypoxia-RAS axis, renal arteriolosclerosis synergistically interacts with IMN through oxidative stress-immune and microbiota-metabolite dysregulation pathways. On one hand, aberrant NOX2/NOX4 activation in sclerotic arterioles generates reactive oxygen species (ROS) that oxidatively modify podocyte PLA2R, exposing cryptic epitopes for IgG4 binding and complement-mediated damage. Additionally, PM2.5-induced extracellular vesicles carrying PLA2R and oxidative stress markers propagate injury through ERK1/2-mediated podocyte cytoskeletal disassembly, establishing a pathogenic “lung-kidney axis” (33). On the other hand, IMN-associated depletion of Lactobacillus and Bifidobacterium species reduces synthesis of AhR-antagonistic indole metabolites, leading to unopposed AhR activation that destabilizes podocytes and recruits inflammatory cells via CXCL16/CXCR6 chemotaxis. Simultaneously, impaired renal clearance of pro-fibrotic metabolites accelerates collagen deposition through myofibroblast activation, creating a self-perpetuating cycle of fibrosis (34). Notably, the identification of C1q (3+) and IgM (3+) deposition as independent factors for adverse renal outcomes prompts mechanistic consideration of how these immune deposits may influence prognosis. We hypothesize that IgM may activate Fc receptors on mesangial cells, inducing pro-inflammatory cytokine release (e.g., IL-6) that could exacerbate arteriolosclerosis-induced ischemia. The apparent paradox of reduced adverse renal outcomes in C1q (3+) compared to no C1q deposition may reflect a threshold effect of complement activation intensity. This potentially indicates more effective regulation of the alternative pathway amplification loop, which becomes critical when classical pathway activation exceeds threshold levels. These hypotheses would require validation through future studies employing electron microscopy for deposit localization, serial measurement of complement split products, and in vitro modeling of IgM/C1q-endothelial interactions.
4.3 Clinical implications and therapeutic strategies
The coexistence of renal arteriolosclerosis and IMN likely amplifies Cardiovascular Disease (CVD) risk through shared risk factors and cardiorenal interactions. Both conditions are strongly associated with hypertension and dyslipidemia, which are key drivers of atherosclerotic CVD (10). Furthermore, the activation of RAAS in arteriolosclerosis induces myocardial remodeling and sodium retention, while IMN-related hypoalbuminemia and volume overload exacerbate cardiac stress, forming a vicious cycle of cardiorenal syndrome (CRS) with elevated mortality (16, 35).
The prognostic heterogeneity observed across studies regarding renal arteriolosclerosis as a risk factor in IMN stems from three interrelated factors that warrant careful consideration in clinical practice. First, population characteristics play a crucial role, as evidenced by the predominance of elderly patients with advanced vascular fibrosis and multiple comorbidities in our cohort. This demographic profile differs significantly from younger populations in other studies, potentially amplifying ischemia-hypoxia injury and skewing risk estimates (36). Second, inconsistencies in pathological assessment criteria present substantial challenges. The histological spectrum of arteriolosclerosis, ranging from subtle intimal thickening to full-layer hyalinosis, lacks universal diagnostic standards, while variable thresholds for defining severe fibrosis further complicate cross-study comparisons (37). Third, the critical importance of follow-up duration cannot be overstated. Our 3.3-year median follow-up period was necessary to capture the insidious progression of ischemia-driven renal dysfunction, which shorter-term analyses might overlook entirely.
For optimal management of IMN with concurrent arteriolosclerosis, a comprehensive, multimodal therapeutic approach addressing both vascular and immune components is essential. A study of 489 IMN patients showed that the efficacy of immunosuppressive therapy differed among patient groups. Patients <65 years old, female, with proteinuria ≥4.0 g/g or eGFR ≥60 mL/min/1.73 m2 had better responses and higher 12-month remission rates, suggesting that renal function and proteinuria levels significantly impact treatment efficacy for IMN patients with renal arteriolosclerosis (38). This could be due to enhanced antigen–antibody reactions in the glomeruli, driven by the impact of arteriolosclerosis on renal microcirculation and subsequent changes in podocyte. The foundation of treatment remains tailored immunosuppressive therapy, with glucocorticoid-cyclophosphamide combinations demonstrating a 65.7% remission rate in experience (36). However, special consideration must be given to elderly patients and those with advanced tubulointerstitial damage (≥30% atrophy), who require careful dose individualization guided by serial PLA2R antibody monitoring and repeat histopathological assessments to balance efficacy against infection risks.
Vascular-protective strategies should be implemented concomitantly. RAS inhibition, particularly with ACE inhibitors or angiotensin receptor blockers, serves dual purposes: reducing proteinuria through intraglomerular pressure modulation while simultaneously countering angiotensin II-induced oxidative stress via NOX4/ROS pathway inhibition (31). Emerging evidence suggests potential adjunctive benefits from antioxidants like N-acetylcysteine, which have shown promising results in preclinical models for attenuating ROS-mediated podocyte injury, though clinical validation studies are still needed (33).
The gut-kidney axis presents novel therapeutic opportunities. Targeted microbiota interventions, including specific probiotic strains like Lactobacillus plantarum or dietary modifications to restore aryl hydrocarbon receptor (AhR)-antagonistic bacterial populations, may help modulate tubulointerstitial inflammation. This approach gains credence from preclinical models demonstrating renal protection through fecal microbiota transplantation (39). These strategies should be guided by integrated assessments, which examine renal biopsy findings, particularly arteriolar lesions and immune complex deposition patterns, in conjunction with advanced serological markers.
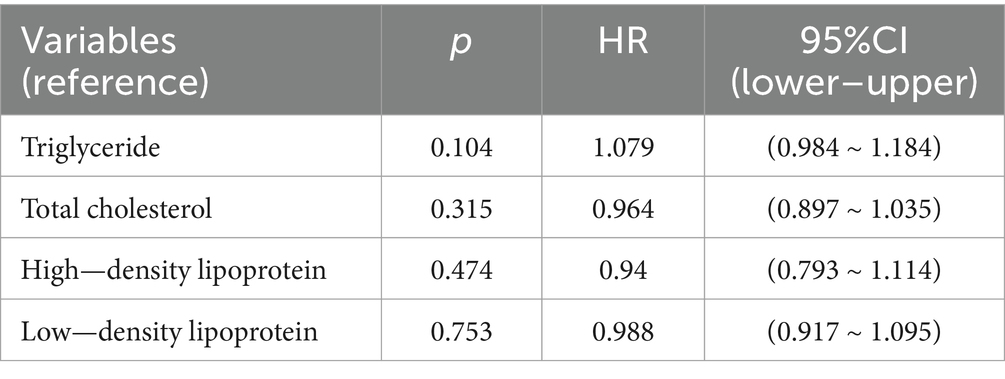
Regarding lipid management, our analysis of Table 4 revealed several noteworthy findings. Among 473 arteriolosclerosis patients, 152 (32.1%) received statin therapy while only 12 (2.5%) were treated with fibrates, with no cases of familial hyperlipidemia identified. The aggregate 34.7% utilization rate of lipid-lowering medications likely explains the lack of significant association between lipid parameters and composite endpoints in Cox regression analysis. This suggests that contemporary lipid management protocols may be effectively maintaining lipid levels below nephrotoxic thresholds in approximately one-third of patients. However, the potential benefits of more aggressive lipid control in high-risk subgroups—particularly those with extensive vascular pathology or progressive renal dysfunction—merit further investigation through prospective studies with protocolized treatment algorithms and longer follow-up periods. These findings underscore the importance of individualized risk stratification when considering therapeutic intensity for dyslipidemia in this complex patient population.
4.4 Limitations
This study has several limitations. First, as a single-center retrospective study with a population from a geographically limited area, the findings may not be generalizable to all ethnic groups. Second, due to the retrospective nature of the study, some early-stage patients lacked PLA2R antibody testing results and certain serological immunological markers at the time of renal biopsy, and were therefore excluded. Finally, data regarding renal artery atherosclerosis were not collected due to the inherent limitations of retrospective study design. We look forward to future multicenter, large-cohort studies to further explore the relationship between renal arteriolosclerosis and IMN.
5 Conclusion
This study demonstrates that renal arteriolosclerosis is a critical determinant of adverse outcomes in patients with IMN, characterized by distinct clinicopathological features and accelerated renal function decline. In a cohort of 597 biopsy-confirmed IMN patients, those with arteriolosclerosis exhibited significantly elevated Na+, TG, advanced MN stages, mesangial proliferation, interstitial fibrosis, and IgG deposition compared to non-arteriolosclerosis counterparts. Kaplan–Meier analysis revealed markedly reduced renal survival in the arteriolosclerosis group (log-rank χ2 = 8.296, p = 0.004), with a composite endpoint event rate of 23.9% versus 11.2% in controls. Multivariate Cox regression identified age, elevated SCr, intense IgM (3+) deposition, and interstitial fibrosis as independent risk factors for adverse renal outcomes. Conversely, C1q (3+) deposition and tubular atrophy (≥50%) demonstrated protective effects, suggesting a complex interplay between complement activation thresholds and tubular adaptive responses.
These findings underscore the necessity of integrating vascular assessment into the diagnostic and prognostic evaluation of IMN. Early identification of arteriolosclerosis, coupled with histology-guided risk stratification, may enable personalized therapeutic strategies targeting both immune-mediated injury and vascular pathology. Clinicians should prioritize aggressive management of modifiable risk factors, such as optimizing blood pressure control via RAS inhibition and mitigating oxidative stress, while tailoring immunosuppressive regimens based on histological severity. Future multicenter studies are warranted to validate these associations, explore mechanistic links between complement deposition and vascular remodeling, and evaluate the efficacy of novel interventions, including microbiota modulation and targeted antifibrotic therapies, in this high-risk population.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements (Ethics approval number: YE 2024-266-01). The participants provided informed consent to participate in the study.
Author contributions
RJ: Writing – original draft, Data curation. LL: Data curation, Writing – review & editing. YY: Writing – review & editing, Methodology. HL: Writing – review & editing, Software, Supervision. RY: Formal analysis, Writing – review & editing. YH: Writing – review & editing, Formal analysis. LW: Writing – review & editing, Funding acquisition, Project administration, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (82104779), the Open Project of State Key Laboratory of Dampness Syndrome of Chinese Medicine (Co-constructed by the Ministry of Science and Technology and Guangdong Province) (SZ2021KF03), the Yang Nizhi National Famous Veteran Traditional Chinese Medicine Expert Inheritance Studio of the National Administration of Traditional Chinese Medicine (document no. 167, 2016), the Academic Experience Inheritance Studio of Zhang Daning, Guangdong Provincial Hospital of Chinese Medicine (E43714, no. 102 [2018] of the Second Hospital of Chinese Medicine), and the Traditional Chinese Medicine Bureau of Guangdong Province (grant number: 20232051).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Wang, Y, Zhang, L, Yuan, L, Xie, Q, Liu, S, and Hao, CM. Changes in the spectrum of biopsy-proven renal diseases over 11 years: a single-center study in China. Ren Fail. (2024) 46:2381614. doi: 10.1080/0886022X.2024.2381614
3. Bi, J, Guo, W, Ji, P, Wang, X, and Xie, Y. Diagnosis and treatment of membranous nephropathy in integrative medicine. Integrative Medicine in Nephrology and Andrology. (2024) 11:11. doi: 10.1097/IMNA-D-23-00014
4. Wang, Y, Cui, C, Tian, X, Wang, L, Ma, X, Ge, H, et al. The combination therapy of glucocorticoids, tacrolimus, and mycophenolate mofetil in primary membranous nephropathy coexisting with type 2 diabetes mellitus: a retrospective study. Integrative Medicine in Nephrology and Andrology. (2023) 10:10. doi: 10.1097/IMNA-D-22-00010
5. Zhang, W, Hu, A, Wang, J, Wang, Y, and Yu, X. Use of chinese herbal medicine to inhibit podocyte damage as therapeutic strategy for membranous nephropathy. Integrative Medicine in Nephrology and Andrology. (2023) 10:10. doi: 10.1097/IMNA-D-23-00004
6. Zhou, K, Zhou, J, Zhou, L, Xue, J, Liu, B, Zhang, Z, et al. Predictive value of the domain specific pla2r antibodies for clinical remission in patients with primary membranous nephropathy: a retrospective study. PLoS One. (2024) 19:e302100. doi: 10.1371/journal.pone.0302100
7. Wang, YR. Distribution patterns of TCM syndromes, clinicopathological characteristics, and serum anti-PLA2R antibody levels in elderly patients with primary nephrotic syndrome. China: GTZYY University (2023).
9. Work Group KDIGO. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100:S1–S276. doi: 10.1016/j.kint.2021.05.021
10. Goto, H, Iseri, K, and Hida, N. Fibrates and the risk of cardiovascular outcomes in chronic kidney disease patients. Nephrol Dial Transplant. (2024) 39:1016–22. doi: 10.1093/ndt/gfad248
11. Miyaoka, Y, Okada, T, Tomiyama, H, Morikawa, A, Rinno, S, Kato, M, et al. Structural changes in renal arterioles are closely associated with central hemodynamic parameters in patients with renal disease. Hypertens Res. (2021) 44:1113–21. doi: 10.1038/s41440-021-00656-8
12. Ruan, Y, Hong, F, Lin, M, Wang, C, Lian, F, Cao, F, et al. Clinicopathological characteristics, risk factors and prognostic value of intrarenal vascular lesions in Iga nephropathy. Eur J Intern Med. (2023) 117:91–7. doi: 10.1016/j.ejim.2023.07.007
13. Shinzato, Y, Zamami, R, Oshiro, N, Nakamura, T, Ishida, A, Ohya, Y, et al. The association of smoking and hyperuricemia with renal arteriolosclerosis in Iga nephropathy. Biomedicines. (2023) 11:11. doi: 10.3390/biomedicines11072053
14. Li, J, Guo, L, Shi, S, Zhou, X, Zhu, L, Liu, L, et al. The role of complement in microangiopathic lesions of Iga nephropathy. Kidney Int Rep. (2022) 7:1219–28. doi: 10.1016/j.ekir.2022.03.028
15. Kimura, N, Kimura, H, Takahashi, N, Hamada, T, Maegawa, H, Mori, M, et al. Renal resistive index correlates with peritubular capillary loss and arteriosclerosis in biopsy tissues from patients with chronic kidney disease. Clin Exp Nephrol. (2015) 19:1114–9. doi: 10.1007/s10157-015-1116-0
16. Zhao, BR, Hu, XR, Wang, WD, and Zhou, Y. Cardiorenal syndrome: clinical diagnosis, molecular mechanisms and therapeutic strategies. Acta Pharmacol Sin. (2025). doi: 10.1038/s41401-025-01476-z [Epubh a head of print].
17. Cheng, Y, Li, W, Chen, J, Qi, D, Guan, M, Cao, T, et al. Correlation analysis between intrarenal small artery intimal thickening and clinicopathological features and prognosis in primary membranous nephropathy patients. Nephron Clin Pract. (2024) 148:95–103. doi: 10.1159/000533414
18. Chen, Y, Chen, XX, and Huang, CX. Clinicopathological characteristics of idiopathic membranous nephropathy in the elderly. Chin J Integr Trad West Nephrol. (2015) 16:985–6. Available at: https://kns.cnki.net/kcms2/article/abstract?v=jNHD1hIvxn2JvcQ5q69fvdpRS2pXsqD1bdaGUsqLqjS8TR6F4V9mHGTUkikXA_9n9tTGY9Q_E_q0PnmuQnhDbdSq1mBUU5iY7SoZ2uDDDfG0o6U4qmEGRwgTl121PuWY6vv2DEZC8QUSflQY-Jv-a5Y5IXz9h0wK86AY0Fmx3M8jMxbvr54MhF7DQJ9SpWExMCKEhn-4dgc=&uniplatform=NZKPT&language=CHS
19. Liu, WX, Dong, YZ, and Yang, HR. Clinical and pathological analysis of 65 elderly patients with idiopathic membranous nephropathy. J Clin Nephrol. (2017) 17:90–4. Available at: https://kns.cnki.net/kcms2/article/abstract?v=tOz5m-jLbAWbYobdYRgsBg40WuA81ei0Rm3-OlyglhjUATcbjcBpCnpvYPUojZr_-AtFHVlZfxvgvWoK43MkUe1Oi3VluezIY_8s8Hxo3UDgUqCNgRhY-OleHnYz1XF8GsHPHPL4iwkyl_uhiAjv5rn_k6Iuu8TD314eHoeYl5Sw8fVx5TXDYJtxxSq8RLK-3YxSLImo6bg=&uniplatform=NZKPT&language=CHS
20. Chen, J, Liao, YJ, Jiang, HY, Li, JS, Li, LH, and Li, J. Clinicopathological features and prognosis of idiopathic membranous nephropathy in adults: a single-center study. J Clin Nephrol. (2019) 19:245–50. Available at: https://kns.cnki.net/kcms2/article/abstract?v=tOz5m-jLbAU8aN2QVp9AR553sBJebOUYDg2ymtsJAS-NFgzjTEh2ZSc1ouqg83Tt2tmGI-tlIak8UlrDpSgfMTbh2E-kFeMrbuRRJQ2OoSyhghMYWix7PYDe5tVwWsxKyOyJYMtYnkl5AdGG9ZbBmbwLH_YPOBzX08mgt51slsD0Xxx0-VkbyiHGMpi5bdqk0ujRHZzCAtA=&uniplatform=NZKPT&language=CHS
21. Guo, WY, An, XP, Sun, LJ, Dong, HR, Xu, XY, Cheng, WR, et al. Clinicopathological characteristics and prognosis of IgA nephropathy with renal arteriolosclerosis. Chin J Nephrol. (2023) 39:209–14. doi: 10.3760/cma.j.cn441217-20220621-00638
22. Oguchi, H, Sasamura, H, Shinoda, K, Morita, S, Kono, H, Nakagawa, K, et al. Renal arteriolar injury by salt intake contributes to salt memory for the development of hypertension. Hypertension. (2014) 64:784–91. doi: 10.1161/HYPERTENSIONAHA.113.02973
23. Bazzi, C, Stivali, G, Rachele, G, Rizza, V, Casellato, D, and Nangaku, M. Arteriolar hyalinosis and arterial hypertension as possible surrogate markers of reduced interstitial blood flow and hypoxia in glomerulonephritis. Nephrology (Carlton). (2015) 20:11–7. doi: 10.1111/nep.12339
24. Sugiura, N, Moriyama, T, Miyabe, Y, Karasawa, K, and Nitta, K. Severity of arterial and/or arteriolar sclerosis in Iga nephropathy and the effects of renin-angiotensin system inhibitors on its prognosis. J Pathol Clin Res. (2021) 7:616–23. doi: 10.1002/cjp2.234
25. Liao, XL, Yin, YS, Ou, J, Qin, XF, Wei, JZ, and Yang, YZ. Clinical, pathological features and prognosis of 326 cases of idiopathic membranous nephropathy. Huaxia Med J. (2020) 33:155–60. doi: 10.19296/j.cnki.1008-2409.2020-05-040
26. Chen, Y, Tang, L, Feng, Z, Cao, X, Sun, X, Liu, M, et al. Pathological predictors of renal outcomes in nephrotic idiopathic membranous nephropathy with decreased renal function. J Nephrol. (2014) 27:307–16. doi: 10.1007/s40620-014-0057-0
27. Guo, C, Cui, Y, Jiao, M, Yao, J, Zhao, J, Tian, Y, et al. Crosstalk between proximal tubular epithelial cells and other interstitial cells in tubulointerstitial fibrosis after renal injury. Front Endocrinol (Lausanne). (2023) 14:1256375. doi: 10.3389/fendo.2023.1256375
28. Singh, AK, Kolligundla, LP, Francis, J, and Pasupulati, AK. Detrimental effects of hypoxia on glomerular podocytes. J Physiol Biochem. (2021) 77:193–203. doi: 10.1007/s13105-021-00788-y
29. Qian, YL, and Luo, PL. Mechanisms of hypoxia-induced renal tubular injury. World Latest Med Inform. (2018) 18:103–4. doi: 10.19613/j.cnki.1671-3141.2018.80.044
30. Miao, H, Wang, YN, Su, W, Zou, L, Zhuang, SG, Yu, XY, et al. Sirtuin 6 protects against podocyte injury by blocking the renin-angiotensin system by inhibiting the wnt1/beta-catenin pathway. Acta Pharmacol Sin. (2024) 45:137–49. doi: 10.1038/s41401-023-01148-w
31. Hoxha, E, Harendza, S, Pinnschmidt, H, Panzer, U, and Stahl, RA. Pla2r antibody levels and clinical outcome in patients with membranous nephropathy and non-nephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PLoS One. (2014) 9:e110681. doi: 10.1371/journal.pone.0110681
32. Dikow, R, Quentmeier, P, Schwenger, V, Waldherr, R, Andrassy, K, Ritz, E, et al. Optimal blood pressure control versus additional immunosuppressive therapy in idiopathic membranous nephropathy—a retrospective analysis. Clin Nephrol. (2009) 72:366–72. doi: 10.5414/cnp72366
33. Zhang, W, Chen, J, Yuan, Y, Luo, J, Zhou, Z, and Wang, G. Pm2.5-induced oxidative stress upregulates pla2r expression in the lung and is involved in the pathogenesis of membranous nephropathy through extracellular vesicles. Front Pharmacol. (2024) 15:1516111. doi: 10.3389/fphar.2024.1516111
34. Miao, H, Wang, YN, Yu, XY, Zou, L, Guo, Y, Su, W, et al. Lactobacillus species ameliorate membranous nephropathy through inhibiting the aryl hydrocarbon receptor pathway via tryptophan-produced indole metabolites. Br J Pharmacol. (2024) 181:162–79. doi: 10.1111/bph.16219
35. Chou, C, Chiu, H, Hsu, Y, Yu, SM, Liou, T, and Sung, L. Impact of chronic kidney disease and end-stage renal disease on the mid-term adverse outcomes in diabetic patients with cardiovascular diseases. Sci Rep. (2024) 14:15770. doi: 10.1038/s41598-024-66655-0
36. Liang, J, Hao, W, Xia, F, Zhao, Z, Wu, Y, Yu, F, et al. Clinicopathological features and outcome in elderly patients with idiopathic membranous nephropathy. Ren Fail. (2023) 45:45. doi: 10.1080/0886022X.2023.2212081
37. Shiiki, H, Saito, T, Nishitani, Y, Mitarai, T, Yorioka, N, Yoshimura, A, et al. Prognosis and risk factors for idiopathic membranous nephropathy with nephrotic syndrome in Japan. Kidney Int. (2004) 65:1400–7. doi: 10.1111/j.1523-1755.2004.00518.x
38. Choi, J, Chin, HJ, Lee, H, Jeon, Y, Lim, J, Jung, H, et al. Effect of immunosuppressive agents on clinical outcomes in idiopathic membranous nephropathy. Kidney Res Clin Pract. (2024) 43:635–47. doi: 10.23876/j.krcp.22.255
Keywords: idiopathic membranous nephropathy, arteriolar nephrosclerosis lesion, renal arteriolosclerosis, clinicopathologic, prognosis
Citation: Jiang R, Li L, Yan Y, Li H, Yu R, Huang Y and Wang L (2025) Renal arteriolosclerosis impact on clinicopathological features and outcomes of idiopathic membranous nephropathy: a retrospective cohort analysis. Front. Med. 12:1594990. doi: 10.3389/fmed.2025.1594990
Edited by:
Ying-Yong Zhao, Northwest University, ChinaReviewed by:
Ling Chen, Shanghai Municipal Hospital of Traditional Chinese Medicine, ChinaZhongjie Qu, Third Affiliated Hospital of Zhejiang Chinese Medical University, China
Olimkhon Sharapov, Republican Specialized Scientific Practical Medical Center of Nephrology and Kidney Transplantation, Uzbekistan
Ana Luisa Correia, Centro Hospitalar do Tâmega e Sousa, Portugal
Copyright © 2025 Jiang, Li, Yan, Li, Yu, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixin Wang, d2FuZ2xpeGluMTIxMEAxNjMuY29t
 Rui Jiang
Rui Jiang Li Li
Li Li Yunfei Yan
Yunfei Yan Hucai Li
Hucai Li Ruizhi Yu
Ruizhi Yu Yang Huang
Yang Huang Lixin Wang
Lixin Wang