Abstract
Background:
This meta-analysis systematically evaluates the analgesic efficacy of two regional anesthesia techniques - transversus abdominis plane block (TAPB) and erector spinae plane block (ESPB) in abdominal surgical procedures.
Methods:
This PRISMA-compliant meta-analysis systematically queried PubMed, Embase, Web of science, and Cochrane library. Eligible studies were controlled clinical trials comparing ESPB and TAPB for postoperative analgesia, documenting pain scales, opioid use, and safety outcomes. Methodological rigor was evaluated per Cochrane criteria, with quantitative synthesis conducted via RevMan 5.4 using effect magnitudes (SMD/MD) and risk ratios (RR). Evidence certainty was graded using GRADE methodology.
Result:
Pooled data from 21 RCTs (n = 1,293 patients) revealed better pain control during the 24-h postoperative period in the ESPB groups (2-h: MD = −0.68, 95% CI [−1.04, −0.32], p < 0.05). Also, postoperative opioid consumption was significantly reduced in the ESPB group (MD = −1.25; 95% CI [−1.66 to −0.85]; p < 0.05). No significant differences were observed in complication occurrence (RR = 1.13, 95% CI [0.75, 1.71], p > 0.05).
Conclusion:
Current evidence indicates that ESPB demonstrates superior postoperative analgesic efficacy and reduced opioid requirements compared to TAPB, while maintaining comparable safety profiles.
Systematic review registration:
Introduction
Abdominal surgical procedures constitute a cornerstone of global surgical practice, with epidemiologic reports indicating a steadily escalating procedure volume accounting for 20–35% of all operative interventions annually (1). Contemporary surgical approaches, ranging from minimally invasive laparoscopy to conventional laparotomy, continue to confront substantial postoperative nociceptive burden. Previous studies documented 38–42% incidence of moderate-to-severe acute postsurgical pain (Visual Analog Scale ≥4) within 48 h post-procedure (2), a critical clinical determinant associated with functional recovery impairment (3) and elevated 30-day complication risks (4).
While neuraxial analgesia maintains its status as the reference standard for abdominal pain management (5), technical constraints (e.g., anticoagulation contraindications, anatomical complexity) limit its universal applicability. The advent of fascial plane blocks has revolutionized regional anesthesia paradigms since the seminal description of transversus abdominis plane block (TAPB) by Rafi in 2001 (6). This ultrasound-guided interfascial technique deposits local anesthetic between the transversus abdominis and internal oblique muscle layers, achieving somatic analgesia through blockade of thoracolumbar nerve branches (T6-L1) (7). Nevertheless, its inherent anatomical confinement precludes visceral nociception modulation-a critical limitation given that visceral afferents mediate 68% of post-laparotomy pain components. Erector spinae plane block (ESPB), first conceptualized in 2016 for chronic thoracic pain management (8), has emerged as a versatile truncal analgesia modality. Clinical series have validated its efficacy across diverse surgical contexts, from thoracic to pelvic procedures (9–11). Recent meta-analyses comparing ESPB and TAPB present conflicting conclusions: Matthew et al. demonstrated ESPB’s superiority in opioid-sparing effects (12), whereas Lin’s analysis found ESPB does not provide better clinical analgesia than the TAPB (13). Furthermore, the above meta-analyses had sample sizes.
Thus, the purpose of this review is to compare the efficacy of the ESPB with the TAPB in patients undergoing abdominal surgeries.
Methods
Study design and registration
Conducted per PRISMA 2020 guidelines, this pre-registered meta-analysis (PROSPERO CRD42021275992) adhered to systematic review standards.
Information sources and search strategy
A systematic multistage search algorithm was executed across four electronic databases: PubMed, Embase, Cochrane library, and Web of science. The search chronology spanned from database inception to October 31, 2024, with no linguistic or publication status restrictions. The optimized Boolean syntax incorporated: MeSH terms: “NerveBlock” [Mesh], “Analgesia” [Mesh]; Free-text permutations: (erector spinae OR ESP) AND (plane block OR fascial block), (transversus abdominis OR TAP) AND (regional anesthesia OR nerve block); Procedure-specific filters: (“abdominal surgery” [tiab] OR laparotom [tiab] OR colectom*[tiab])*. An exemplar PubMed search strategy is detailed in Supplementary Data. Snowball searching was performed on included studies’ reference lists, supplemented by contact with corresponding authors for unpublished datasets.
Study selection criteria
The inclusion criteria were formulated according to PICOS framework with the following operational definitions: Population (P): Patients ≥18 years undergoing elective procedures under general anesthesia; Intervention (I): Ultrasound-guided ESPB; Comparator (C): Ultrasound-guided TAPB; Outcomes (O): Primary: Pain score at 2-h postoperative; Secondary: Pain scores at 4 h, 6 h, 8 h, 12 h, and 24 h during postoperative period; intraoperative opioid consumption; incidence of procedure-related complications (vascular puncture, local anesthetic systemic toxicity), and postoperative nausea and vomiting (PONV); Study design (S): Parallel-group RCTs with ≥20 participants per arm. Exclusion criteria comprised: (1) Non-randomized designs (case series, editorials, narrative reviews); (2) Conference abstracts without peer-reviewed full texts; (3) Ongoing trials without primary outcome data; (4) Combined regional techniques (e.g., ESPB with paravertebral block).
Data extraction protocol
Two researchers independently managed study selection: initial deduplication using EndNote; title/abstract screening for relevance; full-text review against inclusion criteria. Data extraction included: study characteristics (author, year, sample size); surgical/anesthesia details; complication rates (nerve block effects, and PONV). Discrepancies were resolved through consensus discussions.
Risk of bias and evidence quality assessment
The Cochrane Review Manager (version 5.3) was employed to assess potential study biases. Two independent reviewers appraised trials based on:
-
Selective outcome reporting
-
Incomplete outcome data
-
Evaluator/participant blinding status
-
Allocation concealment methods
-
Random sequence generation
-
Other potential biases
The GRADE framework evaluated evidence certainty through six domains: study design, risk of bias, imprecision, inconsistency, indirectness, and other considerations. Evidence quality was stratified into four levels: very low, low, moderate, or high.
Statistical analysis
Quantitative synthesis was performed using Review Manager 5.3. For dichotomous variables, pooled effects were expressed as risk ratios (RR) with 95% CIs. Continuous outcomes were analyzed through standardized mean differences (SMDs) or weighted mean differences (MDs), accompanied by 95% CIs. When studies reported continuous variables as medians with interquartile or min-max ranges, these values were converted to parametric measures using established transformation algorithms (14, 15).
The predefined statistical significance threshold was set at α = 0.05. Between-study heterogeneity was quantified using I2 statistics, with values exceeding 50% denoting substantial heterogeneity. Given the multiple sources of clinical heterogeneity arising from variations in surgical protocols and analgesic regimens, a random-effects model was uniformly implemented for pooled analyses irrespective of I2 statistic values. To explore potential sources of heterogeneity in primary outcome, we performed meta-regression analyses using a random-effects model. Covariates included: surgery type (upper abdominal surgery, upper abdominal surgery), TAPB approach (subcostal approach, lateral approach, and posterior approach), and local anesthetic type (bupivacaine, ropivacaine). Meta-regression analyses were performed by Stata 18.0 (Stata Statistical Software Release 18; StataCorp, College Station, TX, USA, 2023).
Results
Search results
The systematic retrieval across four biomedical databases (PubMed, Embase, Cochrane library, Web of science) yielded 268 candidate records as of October 31, 2024. First, we excluded 96 duplicate publications. Subsequent title/abstract screening eliminated 148 records due to: Non-target population (e.g., pediatric/emergency surgeries; n = 67), Intervention mismatch (combined regional techniques; n = 41), Study design ineligibility (non-RCTs; n = 40). Then, full-text appraisal of the remaining 24 articles applied the PICOS exclusion hierarchy: protocol violations (n = 1: mixed cardiac procedure) (16); insufficient outcome reporting (n = 1) (17); publication type exclusion (n = 1: conference abstract without peer review) (18). The final synthesis incorporated 21 RCTs spanning 2019–2024 (9–11, 19–36), with detailed selection dynamics visualized in the PRISMA 2020 flowchart (Figure 1).
Figure 1

The inclusion process of the literature search.
Risk of bias
All but one of the included studies explicitly reported the randomization methods employed (25). Seven studies (33.3%) inadequately documented concealment protocols, precluding assessment of selection bias mitigation. Eleven trials (52.4%) failed to implement double-blinding procedures, compromising participant-researcher blinding integrity. Five studies (23.8%) neglected to report outcome assessor blinding status, introducing potential measurement inaccuracies. Figure 2 presents a summary of the bias risk for the included studies.
Figure 2

The risk bias assessment of all included studies.
Outcomes
Primary outcome
Postoperative 2-h pain score
Thirteen trials reported postoperative 2-h pain score. The forest plot indicated a significant lower pain score in ESPB group (MD = −0.68, 95% CI [−1.04, −0.32], p < 0.05, I2 = 92%, Figure 3), highlighting substantial heterogeneity among the studies.
Figure 3

Forest plot of postoperative 2-h pain score between ESPB and TAPB groups. (ESPB, erector spinae plane block; TAPB, transversus abdominis plane block).
Secondary outcomes
Postoperative 4-h pain score
Data from 12 trials demonstrated a significant reduction in pain score for the ESPB group (MD = −0.93, 95% CI [−1.60, −0.26], p < 0.05), with substantial heterogeneity (I2 = 96%; Figure 4).
Figure 4

Forest plot of postoperative 4-h pain score between ESPB and TAPB groups. (ESPB, erector spinae plane block; TAPB, transversus abdominis plane block).
Postoperative 6-h pain score
Eight trials revealed superior analgesic efficacy in the ESPB group (MD = −1.47, 95% CI [−2.48, −0.46], p < 0.05), accompanied by significant heterogeneity (I2 = 96%; Figure 5).
Figure 5
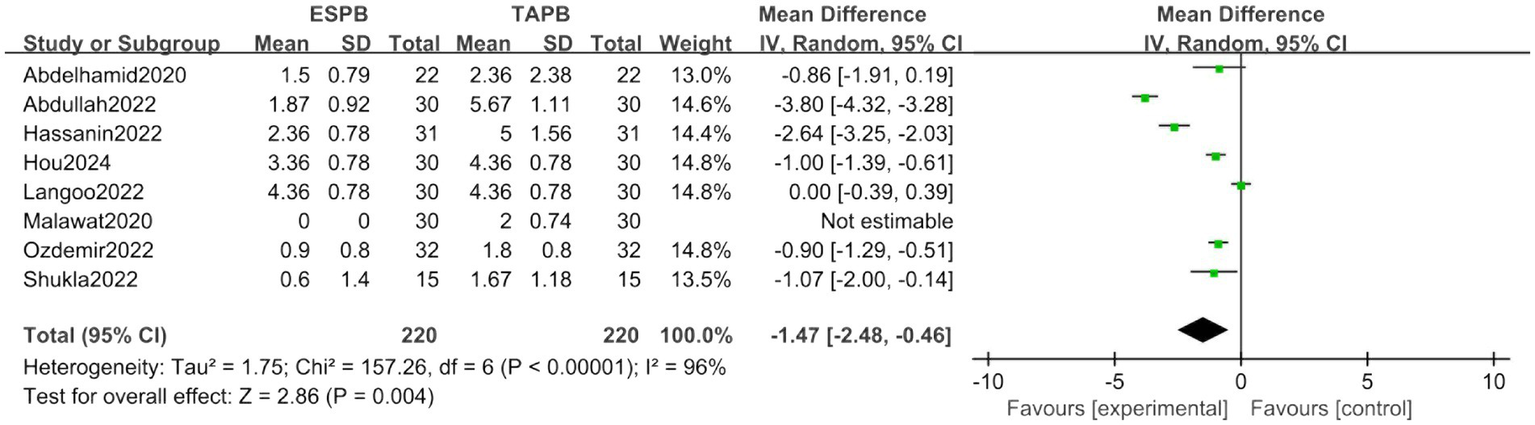
Forest plot of postoperative 6-h pain score between ESPB and TAPB groups. (ESPB, erector spinae plane block; TAPB, transversus abdominis plane block).
Postoperative 8-h pain score
Analysis of six trials confirmed sustained analgesic superiority of ESPB (MD = -0.98, 95% CI [−1.49, −0.47], p < 0.05), despite marked heterogeneity (I2 = 90%; Figure 6).
Figure 6
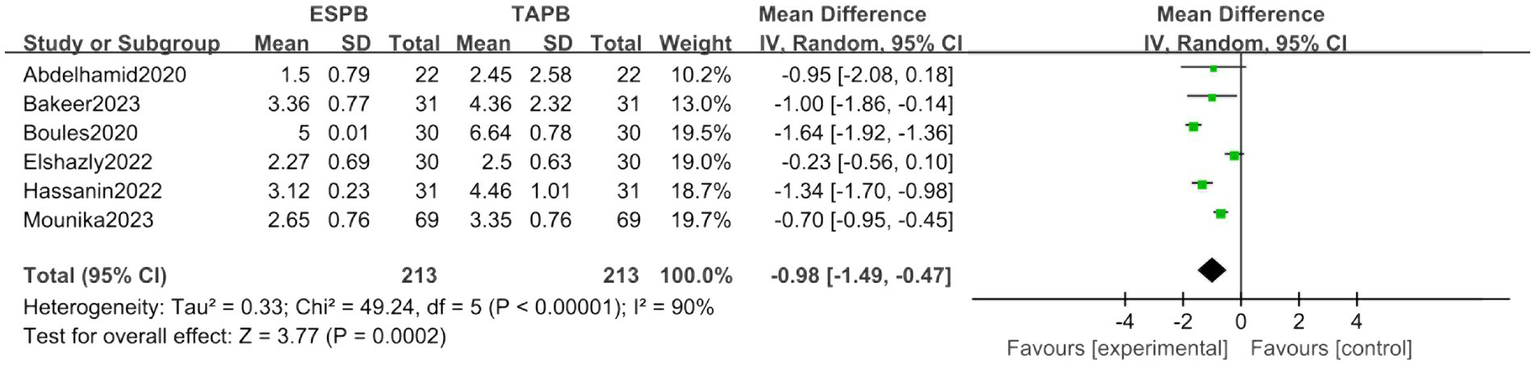
Forest plot of postoperative 8-h pain score between ESPB and TAPB groups. (ESPB, erector spinae plane block; TAPB, transversus abdominis plane block).
Postoperative 12-h pain score
Fourteen studies indicated reduced pain scores in the ESPB cohort (MD = −0.73, 95% CI [−1.32, −0.13], p < 0.05), with pronounced heterogeneity (I2 = 97%; Supplementary Figure 1).
Postoperative 24-h pain score
Persistent analgesic benefits were observed in 14 trials for ESPB (MD = −0.51, 95% CI [−0.82, −0.20], p < 0.05), maintaining high heterogeneity (I2 = 93%; Supplementary Figure 2).
Postoperative opioid consumption
Nineteen trials assessed postoperative opioid consumption. The forest plot revealed a significantly lower consumption in the ESPB group (SMD = −1.25, 95% CI [−1.66, −0.85], p < 0.05, I2 = 90%, Figure 7), indicating low heterogeneity among the studies.
Figure 7

Forest plot of postoperative opioid consumption between ESPB and TAPB groups. (ESPB, erector spinae plane block; TAPB, transversus abdominis plane block).
Adverse events
Ten trials examined the incidence of PONV. The forest plot demonstrated no significant incidence between two groups (RR = 1.13, 95% CI [0.75, 1.71], p > 0.05, I2 = 63%, Figure 8). No operative-related event was reported in both groups.
Figure 8

Forest plot of the incidence of PONV between ESPB and TAPB groups. (PONV, postoperative nausea and vomiting; ESPB, erector spinae plane block; TAPB, transversus abdominis plane block).
Meta-regression analysis
Meta-regression revealed no significant associations between the prespecified covariates (surgery type, TAPB approach, or local anesthetic) and heterogeneity in postoperative 2-h pain scores (all p-values > 0.05) (Table 1).
Table 1
| Study | Age | Sample size | ASA scale | Type of surgery | ESPB group | TAPB group | Anesthesia | PCIA |
|---|---|---|---|---|---|---|---|---|
| Abdelhamid et al. (19) | 18–59 | 44 | II-III | Laparoscopic sleeve gastrectomy | Location: 2–3 cm lateral to T9; Local anesthetic: 0.25% bupivacaine 15 mL on each side. |
Location: oblique subcostal approach; Local anesthetic: 30 mL of 0.25% bupivacaine on each side. |
General anesthesia | None |
| Abdullah et al. (20) | 18–65 | 60 | I-II | Ovarian cancer surgery | Location: 3 cm lateral to T10; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
Location: at the level of the umbilicus; Local anesthetic: 20 mL 0.25% bupivacaine on each side |
General anesthesia | Fentanyl |
| Altiparmak et al. (21) | 18–70 | 68 | I-II | Laparoscopic cholecystectomy | Location: 3 cm lateral to T7; Local anesthetic: 20 mL 0.375% bupivacaine on each side. |
Location: oblique subcostal approach; Local anesthetic: 20 mL 0.375% bupivacaine on each side. |
General anesthesia | Tramadol |
| Bakeer et al. (22) | 18–65 | 62 | II-III | Abdominal surgery | Not mentioned. | Not mentioned. | Not mentioned | Morphine |
| Boules et al. (23) | 18–40 | 60 | I-II | Cesarean | Location: 3 cm lateral to T10; Local anesthetic: 20 mL 0.5% bupivacaine on each side. |
Location: between the costal margin and iliac crest Local anesthetic: 20 mL 0.5% bupivacaine on each side. |
Spinal anesthesia | None |
| Eksteen et al. (11) | >18 | 66 | I-III | Cesarean | Location: 2–3 cm lateral to T9; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
Location: between the costal margin and iliac crest; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
Spinal anesthesia | Morphine |
| Elshazly et al. (24) | 18–60 | 60 | II-III | Laparoscopic cholecystectomy or paraumbilical hernia repair | Location: 3 cm lateral to T5; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
Location: oblique subcostal approach; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
General anesthesia | None |
| Ghielmini et al. (10) | >18 | 50 | I-II | Robot assisted hernia repair | Location: at the level of T10; Local anesthetic: 30 mL 0.2% ropivacaine on each side. |
Location: in the triangle of Peti; Local anesthetic: 30 mL 0.2% ropivacaine on each side. |
General anesthesia | None |
| Hassanin et al. (25) | 20–50 | 62 | I-III | Emergency laparotomy | Location: 3 cm lateral to T8; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
Location: between the costal margin and iliac crest; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
General anesthesia | None |
| Hou et al. (9) | 18–65 | 60 | I-III | Laparoscopic radical surgery | Location: 2–3 cm lateral to T9; Local anesthetic: 20 mL 0.2% ropivacaine on each side. |
Location: oblique subcostal approach; Local anesthetic: 20 mL 0.2% ropivacaine on each side. |
General anesthesia | Sufentani |
| Ibrahim et al. (26) | 20–60 | 42 | I-III | Laparoscopic cholecystectomy | Location: 3 cm lateral to L3; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
Location: oblique subcostal approach; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
General anesthesia | Tramadol |
| Kamel et al. (27) | 40–60 | 48 | I-II | Open total abdominal hysterectomy | Location: 3 cm lateral to T9; Local anesthetic: 20 mL 0.375% bupivacaine on each side. |
Location: between the costal margin and iliac crest; Local anesthetic: 20 mL 0.375% bupivacaine on each side. |
General anesthesia | None |
| Langoo et al. (28) | 18–40 | 60 | II | Cesarean | Location: 3 cm lateral to T10; Local anesthetic: 20 mL 0.2% ropivacaine on each side. |
Location: between the costal margin and iliac crest; Local anesthetic: 20 mL 0.2% ropivacaine on each side. |
Spinal anesthesia | Diclofenac |
| Malawat et al. (29) | 18–80 | 60 | I-III | Cesarean | Location: 3 cm lateral to T9; Local anesthetic: 0.2% ropivacaine 0.2 mL/kg on each side. |
Location: between the costal margin and iliac crest; Local anesthetic: 0.2% ropivacaine 0.2 mL/kg on each side. |
Spinal anesthesia | None |
| Mostafa et al. (30) | 18 | 60 | I-III | Open liver resection surgery | Location: 3 cm lateral to T7; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
Location: oblique subcostal approach; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
General anesthesia | None |
| Mounika et al. (31) | 18–70 | 138 | I-II | Laparoscopic cholecystectomy | Location: the level of T7; Local anesthetic: 20 mL 0.2% ropivacaine on each side. |
Location: oblique subcostal approach; Local anesthetic: 20 mL 0.2% ropivacaine on each side. |
General anesthesia | None |
| Ozdemir et al. (32) | 18–64 | 64 | I-III | Laparoscopic cholecystectomy | Location: 3 cm lateral to T7; Local anesthetic: 10 mL 0.25% bupivacaine and 10 mL of 2% prilocaine on each side. |
Location: oblique subcostal approach; Local anesthetic: 10 mL 0.25% bupivacaine and 10 mL of 2% prilocaine on each side. |
General anesthesia | None |
| Qi-Hong et al. (33) | 65 | 62 | I-III | Laparoscopic colorectal surgery | Location: 2–3 cm lateral to T9; Local anesthetic: 20 mL 0.25% ropivacaine on each side. |
Location: oblique subcostal approach; Local anesthetic: 20 mL 0.25% ropivacaine on each side. |
General anesthesia | Sufentanil |
| Sahu et al. (34) | 18–70 | 60 | I-II | Laparoscopic cholecystectomy | Location: 2–3 cm lateral to T7; Local anesthetic: 20 mL 0.2% ropivacaine on each side. |
Location: oblique subcostal approach; Local anesthetic: 20 mL 0.2% ropivacaine on each side. |
General anesthesia | None |
| Shukla et al. (35) | 35–60 | 30 | I-II | Open total abdominal hysterectomy | Location: 3 cm lateral to T9; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
Location: between the costal margin and iliac crest; Local anesthetic: 20 mL 0.25% bupivacaine on each side. |
General anesthesia | None |
| Warner et al. (36) | 18 | 77 | I-IV | Laparoscopic hysterectomy | 0.125% bupivacaine 20 mL at T8 and 20 mL at T12 on each side. | 0.125% bupivacaine 20 mL for the subcostal TAP and 20 mL for the posterior TAP on each side. | General anesthesia | None |
The details of included studies.
ASA, American society of anesthesiologists; ESPB, Erector spinae plane block; TAPB, Transversus abdominis plane block; PCIA, Patient controlled intravenous analgesia.
GRADE result
Table 2 shows the summary of the GRADE assessment.
Table 2
| Outcome | Included studies (n) | Patients (n) | Quality of evidence | Reasons |
|---|---|---|---|---|
| Postoperative 2-h pain score | 13 | 835 | ⨁⨁⨁◯ MODERATE |
“Inconsistency” was downgraded to “serious.” |
| Postoperative 4-h pain score | 12 | 750 | ⨁⨁⨁◯ MODERATE |
“Inconsistency” was downgraded to “serious.” |
| Postoperative 6-h pain score | 8 | 440 | ⨁⨁⨁◯ MODERATE |
“Inconsistency” was downgraded to “serious.” |
| Postoperative 8-h pain score | 6 | 426 | ⨁⨁⨁◯ MODERATE |
“Inconsistency” was downgraded to “serious.” |
| Postoperative 12-h pain score | 14 | 878 | ⨁⨁⨁◯ MODERATE |
“Inconsistency” was downgraded to “serious.” |
| Postoperative 24-h pain score | 14 | 878 | ⨁⨁⨁◯ MODERATE |
“Inconsistency” was downgraded to “serious.” |
| Postoperative opioid consumption | 19 | 1,185 | ⨁⨁◯◯ LOW |
“Imprecision” and “Inconsistency” were downgraded to “serious.” |
| Incidence of PONV | 10 | 581 | ⨁⨁⨁◯ MODERATE |
“Inconsistency” was downgraded to “serious.” |
The summary of GRADE for included studies.
PONV, postoperative nausea and vomiting.
Discussion
Our meta-analysis investigated the safety and effectiveness of ESPB in abdominal surgeries while comparing with TAPB. The results showed that ESPB significantly decreased postoperative pain scores and opioid consumption.
Abdominal surgical pain originates from multiple sources: incisional discomfort, visceral nociception, tissue trauma, CO₂ insufflation-induced shoulder pain, and phrenic nerve irritation (37). This multimodal pathophysiology results in concurrent somatic and visceral pain perception. Effective multimodal analgesia enhances patient satisfaction, accelerates functional recovery, reduces hospitalization duration, and decreases thromboembolic risks through improved early mobilization (38). Existing evidence confirms the analgesic efficacy of both TAPB and ESPB in abdominal surgical settings. However, contemporary meta-analyses present discordant conclusions regarding their comparative effectiveness. Thus, we conducted this systematic review with meta-analysis incorporating 21 randomized controlled trials (N = 1,293 patients) to compare the efficacy of ESPB versus TAPB for postoperative analgesia.
Our meta-analysis demonstrated the analgesic superiority of ESPB over TAPB, evidenced by significantly reduced postoperative pain scores and lower opioid consumption. While the precise mechanism of ESPB’s analgesic action remains debated within the scientific community, emerging cadaveric and radiological evidence suggests dual neural targeting - simultaneously engaging both ventral and dorsal rami of spinal nerves through fascial compartment diffusion (39, 40). This bidirectional blockade achieves comprehensive somatic-visceral pain control, a mechanistic advantage over TAPB’s limited anterior ramus inhibition.
We further evaluated the safety of ultrasound-guided ESPB, and none of the included studies reported procedure-related complications. Current literature suggests that severe complications occur in fewer than 0.02% (2 per 10,000) of cases (39), with documented adverse events involving motor nerve blockade, lung puncture (pneumothorax), accidental vascular puncture, and systemic toxic reactions. The procedure’s safety advantage stems from its anatomical approach, where injectates are deliberately positioned distal to vulnerable neurovascular structures like the spinal canal, pleural membranes, and major blood vessels.
Our study has limitations to consider. First, the significant differences in pain scores and opioid use across postoperative time points may stem from varied surgical and pain management approaches. Although we accounted for this using statistical methods, results should be interpreted carefully. Second, a small number of included studies exhibited a high risk of bias.
Conclusion
This systematic review and meta-analysis demonstrates that ultrasound-guided ESPB provides superior postoperative analgesia compared to TAPB. Our meta-regression did not identify surgery type, TAPB approach, or local anesthetic properties as sources of heterogeneity, future research should prioritize prospective studies with stratified designs to evaluate these covariates in homogenous surgical populations.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LQ: Conceptualization, Methodology, Writing – original draft. N-qH: Project administration, Resources, Supervision, Writing – original draft. Q-hS: Project administration, Resources, Supervision, Writing – original draft. KN: Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1595778/full#supplementary-material
References
1.
Ripollés-Melchor J Tomé-Roca JL Zorrilla-Vaca A Aldecoa C Colomina MJ Bassas-Parga E et al . Hemodynamic management guided by the hypotension prediction index in abdominal surgery: a multicenter randomized clinical trial. Anesthesiology. (2025) 142:639–54. doi: 10.1097/ALN.0000000000005355
2.
Wang L Qin F Zhen L Li R Tao S Li G . Development of a nomogram for predicting acute pain among patients after abdominal surgery: a prospective observational study. J Clin Nurs. (2024) 33:3586–98. doi: 10.1111/jocn.17031
3.
Ali F Wallin G Wahlin RR Montgomery A Rogmark P Sandblom G . Surgery for primary ventral hernias and risk of postoperative pain, nausea: a population-based register study. Hernia. (2025) 29:68. doi: 10.1007/s10029-025-03256-4
4.
Helden EV Kranendonk J Vermulst A Boer A Reuver P Rosman C et al . Early postoperative pain and 30-day complications following major abdominal surgery: a retrospective cohort study. Reg Anesth Pain Med. (2024) 4:rapm-2024-105277. doi: 10.1136/rapm-2024-105277
5.
Turan A Cohen B Elsharkawy H Maheshwari K Soliman LM Babazade R et al . Transversus abdominis plane block with liposomal bupivacaine versus continuous epidural analgesia for major abdominal surgery: the EXPLANE randomized trial. J Clin Anesth. (2022) 77:110640. doi: 10.1016/j.jclinane.2021.110640
6.
Rafi AN . Abdominal field block: a new approach via the lumbar triangle. Anaesthesia. (2001) 56:1024–6. doi: 10.1111/j.1365-2044.2001.2279-40.x
7.
Kim HC Park J Kim HS Hong YH Song Y Park JS . Comparative analysis of analgesic efficacy and functional recovery in open pancreaticoduodenectomy: a randomized controlled trial of local anesthetic wound infiltration, transversus abdominis plane block, and intramuscular electrical stimulation. Hepatobiliary Surg Nutr. (2024) 13:950–61. doi: 10.21037/hbsn-23-650
8.
Forero M Adhikary SD Lopez H Tsui C Chin KJ . The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. (2016) 41:621–7. doi: 10.1097/AAP.0000000000000451
9.
Hou P Liu W Chen R Mi H Jia S Lin J . Comparison of erector spinae plane block and transverse abdominis plane block in postoperative recovery after laparoscopic colorectal surgery: a randomized, double-blind, controlled trial. Perioper Med. (2024) 13:116. doi: 10.1186/s13741-024-00475-8
10.
Ghielmini EM Greco L Spampatti S Kubli R Saporito A La Regina D et al . Erector spinae plane block versus transversus abdominis plane block for robotic inguinal hernia repair: a blinded, active-controlled, randomized trial. Pain Phys. (2024) 27:27–34. doi: 10.36076/ppj.2024.27.27
11.
Eksteen A Wagner J Kleyenstuber T Kamerman P . Comparison of erector spinae plane and transversus abdominis plane block for postoperative analgesia after caesarean delivery under spinal anaesthesia: a randomised controlled trial. Int J Obstet Anesth. (2024) 60:104259. doi: 10.1016/j.ijoa.2024.104259
12.
Stewart M Tubog TD Johnson W Evans H . Bilateral erector spinae plane blocks versus bilateral transversus abdominis plane blocks in patients undergoing abdominal surgery: a systematic review and Meta-analysis. AANA J. (2023) 91:455–63. PMID:
13.
Liheng L Siyuan C Zhen C Changxue W . Erector spinae plane block versus transversus abdominis plane block for postoperative analgesia in abdominal surgery: a systematic review and Meta-analysis. J Investig Surg. (2022) 35:1711–22. doi: 10.1080/08941939.2022.2098426
14.
Luo D Wan X Liu J Tong T . Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
15.
Wan X Wang W Liu J Tong T . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
16.
Krishna SN Chauhan S Bhoi D Kaushal B Hasija S Sangdup T et al . Bilateral erector spinae plane block for acute post-surgical pain in adult cardiac surgical patients: a randomized controlled trial. J Cardiothorac Vasc Anesth. (2019) 33:368–75. doi: 10.1053/j.jvca.2018.05.050
17.
Chin KJ Adhikary S Sarwani N Forero M . The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair. Anaesthesia. (2017) 72:452–60. doi: 10.1111/anae.13814
18.
Sajna S Johnson E . Comparison of effectiveness of erector spinae plane block and transversus abdominis plane block in inguinal hernia repair for post operative analgesia-a prospective randomised single blinded study. Indian J Anaesth. (2020) 64:S35. doi: 10.4103/0019-5049.277903
19.
Abdelhamid BM Khaled D Mansour MA Hassan MM . Comparison between the ultrasound-guided erector spinae block and the subcostal approach to the transversus abdominis plane block in obese patients undergoing sleeve gastrectomy; a randomized controlled trial. Minerva Anestesiol. (2020) 86:816–26. doi: 10.23736/S0375-9393.20.14064-1
20.
Abdullah S Elshalakany N Farrag Y Abed S . The use of erector spinae versus transversus abdominis blocks in ovarian surgery: a randomized, comparative study. Colomb J Anesthesiol. (2022) 50:e1025. doi: 10.5554/22562087.e1025
21.
Altiparmak B Korkmaz Toker M Uysal AI Kuşçu Y Gümüş Demirbilek S . Ultrasound-guided erector spinae plane block versus oblique subcostal transversus abdominis plane block for postoperative analgesia of adult patients undergoing laparoscopic cholecystectomy: randomized, controlled trial. J Clin Anesth. (2019) 57:31–6. doi: 10.1016/j.jclinane.2019.03.012
22.
Bakeer AH Hamimy W Zaghloul A Shaban A Magdy M Ahmed MB . Analgesic efficacy of erector spinae plane block versus transversus abdominis plane block in laparotomies for cancer surgeries: a randomized blinded control study. Bali J Anesthesiol. (2023) 7:19–23. doi: 10.4103/bjoa.bjoa_229_22
23.
Boules ML Goda AS Abdelhady MA Abu el-Nour Abd el-Azeem SA Hamed MA . Comparison of analgesic effect between erector spinae plane block and transversus abdominis plane block after elective cesarean section: a prospective randomized single-blind controlled study. J Pain Res. (2020) 3:1073–80. doi: 10.2147/JPR.S253343
24.
Elshazly M el-Halafawy YM Mohamed DZ Wahab KAE Mohamed TMK . Feasibility and efficacy of erector spinae plane block versus transversus abdominis plane block in laparoscopic bariatric surgery: a randomized comparative trial. Korean J Anesthesiol. (2022) 75:502–9. doi: 10.4097/kja.22169
25.
Hassanin AAM Ali NS Elshorbagy HM . Efficacy of ultrasound-guided transversus abdominis plane block versus erector spinae plane block for postoperative analgesia in patients undergoing emergency laparotomies: a randomized, double-blinded, controlled study. Egypt J Anaesth. (2022) 38:521–8. doi: 10.1080/11101849.2022.2124660
26.
Ibrahim M . Erector spinae plane block in laparoscopic cholecystectomy, is there a difference? A randomized controlled trial. Anesth Essays Res. (2020) 14:119–26. doi: 10.4103/aer.AER_144_19
27.
Kamel AAF Amin OAI Ibrahem MAM . Bilateral ultrasound-guided erector spinae plane block versus transversus abdominis plane block on postoperative analgesia after Total abdominal hysterectomy. Pain Physician. (2020) 4;23:375–82. doi: 10.36076/ppj.2020/23/375
28.
Langoo SA Banoo F Jan S Ghulam R . Ultrasound guided erector spinae plane block versus transversus abdominis plane block for postoperative analgesia in patient undergoing cesarean section: a randomized controlled study. Eur J Mol Clin Med. (2022) 9:1303–12.
29.
Malawat A Verma K Jethava D Jethava DD . Erector spinae plane block and transversus abdominis plane block for postoperative analgesia in cesarean section: a prospective randomized comparative study. J Anaesthesiol Clin Pharmacol. (2020) 36:201–6. doi: 10.4103/joacp.JOACP_116_19
30.
Mostafa M Mousa MS Hasanin A Arafa AS Raafat H Ragab AS . Erector spinae plane block versus subcostal transversus abdominis plane block in patients undergoing open liver resection surgery: a randomized controlled trial. Anaesth Crit Care Pain Med. (2023) 42:101161. doi: 10.1016/j.accpm.2022.101161
31.
Mounika V Sahu L Mishra K Mohapatra PS . A comparative evaluation of post-operative pain management using erector spinae plane block and oblique transverse abdominis plane block in patients undergoing laparoscopic cholecystectomy. Cureus. (2023) 15:e35750. doi: 10.7759/cureus.35750
32.
Ozdemir H Araz C Karaca O Turk E . Comparison of ultrasound-guided erector spinae plane block and subcostal transversus abdominis plane block for postoperative analgesia after laparoscopic cholecystectomy: a randomized, controlled trial. J Investig Surg. (2022) 35:870–7. doi: 10.1080/08941939.2021.1931574
33.
Qi-Hong S Xu-Yan Z Xu S Yan-Jun C Ke L Rong W . Comparison of ultrasound-guided erector spinae plane block and oblique subcostal transverse abdominis plane block for postoperative analgesia in elderly patients after laparoscopic colorectal surgery: a prospective randomized study. Pain Ther. (2021) 10:1709–18. doi: 10.1007/s40122-021-00329-x
34.
Sahu L Behera SK Satapathy GC Saxena S Priyadarshini S Sahoo RK . Comparison of analgesic efficacy of erector spinae and oblique subcostal transverse abdominis plane block in laparoscopic cholecystectomy. J Clin Diagn Res. (2021) 15:UC13. doi: 10.7860/JCDR/2021/50795.15380
35.
Shukla U Yadav U Singh AK Tyagi A . Randomized comparative study between bilateral erector spinae plane block and transversus abdominis plane block under ultrasound guidance for postoperative analgesia after Total abdominal hysterectomy. Cureus. (2022) 14:e25227. doi: 10.7759/cureus.25227
36.
Warner M Yeap YL Rigueiro G Zhang P Kasper K . Erector spinae plane block versus transversus abdominis plane block in laparoscopic hysterectomy. Pain Manag. (2022) 12:907–16. doi: 10.2217/pmt-2022-0037
37.
Rasador ACD Balthazar da Silveira CA Pereira NP Nogueira R Malcher F Lima DL . Transversus abdominis plane (TAP) block for postoperative pain management after ventral hernia repair: an updated systematic review and meta-analysis. Hernia. (2025) 29:113. doi: 10.1007/s10029-025-03305-y
38.
Lee HJ Kim J Yoon SH Kong SH Kim WH Park DJ et al . Effectiveness of ERAS program on postoperative recovery after gastric cancer surgery: a randomized clinical trial. Int J Surg. (2025). doi: 10.1097/JS9.0000000000002328
39.
Harbell MW Langley NR Seamans DP Koyyalamudi V Kraus MB Carey FJ et al . Evaluating two approaches to the erector spinae plane block: an anatomical study. Reg Anesth Pain Med. (2023) 48:495–500. doi: 10.1136/rapm-2022-104132
40.
Schwartzmann A Peng P Maciel MA Forero M . Mechanism of the erector spinae plane block: insights from a magnetic resonance imaging study. Can J Anaesth. (2018) 65:1165–6. doi: 10.1007/s12630-018-1187-y
Summary
Keywords
transversus abdominis plane block, erector spinae plane block, meta-analysis, ESPB, TAPB
Citation
Qian L, Hu N-q, Shen Q-h and Ni K (2025) Comparison of the efficiency of ultrasound-guided ESPB and TAPB on postoperative analgesia: a system review and meta-analysis. Front. Med. 12:1595778. doi: 10.3389/fmed.2025.1595778
Received
18 March 2025
Accepted
08 May 2025
Published
26 May 2025
Volume
12 - 2025
Edited by
Domenico Pietro Santonastaso, Maurizio Bufalini Hospital, Italy
Reviewed by
Annabella De Chiara, Azienda Unità Sanitaria Locale (AUSL) della Romagna, Italy
Gabriele Melegari, University Hospital of Modena, Italy
Giorgio Ranieri, Ospedale Isola Tiberina - Gemelli Isola, Italy
Updates
Copyright
© 2025 Qian, Hu, Shen and Ni.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Ni, jkis_stone@sohu.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.