- 1Taizhou Hospital, Shanghai University of Traditional Chinese Medicine, Taizhou, China
- 2Taizhou Hospital of Traditional Chinese Medicine, Taizhou, China
This case report describes a case of exogenous lipoid pneumonia (ELP) due to medical aspiration of paraffin oil. An 87-year-old male was hospitalized with bedridden, dysphagic dysphagia. Two days after being given nasal paraffin oil, the patient developed high fever and respiratory distress. Blood gas analysis showed a PaCO2 of 33 mmHg in the room air. CT scan of the chest showed multiple ground glass opacity with solid lesions. The patient then underwent bronchoscopy, and large quantities of oily turbid fluid was found in the bronchoalveolar lavage fluid (BALF). Further cytological analysis of the BALF showed 35% phagocytes, 60% neutrophils, and 5% lymphocytes. The patient was diagnosed with ELP based on a history of paraffin oil exposure, CT imaging of the chest, and cytological examination. Despite our aggressive anti-inflammatory and anti-infective treatment, the patient eventually passed away due to advanced age and multiple complications. Aspiration of oily substances is the most important risk factor for ELP. For people at high risk of misadministration, a suitable naso-intestinal tube is more appropriate for feeding and medication.
Background
Lipoid pneumonia, characterized by the presence of lipid-laden macrophages or foam cells in the alveolar walls and interstitial tissues, can be divided into exogenous and endogenous lipoid pneumonia (1). Endogenous lipoid pneumonia, also known as cholesterol pneumonia, commonly occurs in chronic inflammatory diseases of the lungs. Localized inflammation leads to cell membrane disruption and intracellular lipid extravasation, which attracts macrophage aggregates that in turn affect lipid metabolism (2). Exogenous lipoid pneumonia (ELP) results from aspiration of lipid-rich substances, such as polyethylene glycol, paraffin oil, and diesel fuel, into the terminal fine bronchioles and alveoli (3). Lipids are phagocytosed by macrophages to form lipid-rich vacuolated cells, leading to sustained inflammatory response (4). Cryptogenic Organizing Pneumonia (COP) may be confused with ELP because of its wandering solid lesions, but COP has no history of lipid exposure and no lipid droplets in bronchoalveolar lavage fluid (BALF). Alveolar protein deposition resembles the “paving stone sign” on imaging, but the BALF is milky white. Therefore, it can be differentiated. Due to the lack of specificity in clinical symptoms and laboratory tests, many cases require chest imaging combined with a history of lipid aspiration to confirm the diagnosis. According to the literature, ELP is mostly induced by occupational exposure, repeated use of nasal drops, and autonomous use of drugs. While, medically induced aspiration is uncommon. Here, we report a case of medically induced lipoid pneumonia.
Case presentation
An 87- year-old male was admitted to the rehabilitation department of our hospital. He had a history of an old hip fracture and had been bedridden for more than 2 years. At the same time, the patient had a comorbid history of hypertension, Alzheimer’s disease, chronic bronchitis with emphysema, and chronic heart failure. Routine rehabilitation, including motor function exercises and swallowing training, was performed after admission to the hospital. On the 15th day of treatment, the condition worsened with abdominal pain and difficulty in defecation. Abdominal X-ray showed abundant contents in the intestinal cavity and scattered air-filled shadows. Therefore, a nasogastric tube was inserted through which 50 mL of paraffin oil was administered daily for laxative purposes. The patient passed a small amount of feces, but the nausea and vomiting did not subside. Two days later, the patient started to develop a fever with a maximum temperature of 38.9°C, accompanied by cough, expectoration and shortness of breath. Auxiliary examinations showed a high-sensitivity C-reactive protein of 127.79 mg/L, a white blood cell count of 9.5 × 109/L, and a calcitoninogen of 0.239 ng/mL. Blood gas analysis showed a PaCO2 of 33 mmHg in the room air. Sputum culture showed the presence of Pseudomonas aeruginosa infection. However, after 2 days of treatment with ceftriaxone (2.0 g qd IV) prescribed by his physician, the clinical symptoms remained unrelieved. Surprisingly, a CT scan of the chest showed multiple ground glass opacity with solid lesions that were significantly worse than at the time of hospitalization. The CT measurements ranged between −120 and −50 Hounsfield Units (HU), with no change on enhanced examination. Both lungs were involved and there was an asymmetric distribution in both lower lungs, with small amounts of bilateral pleural effusion, consistent with acute ELP (Figure 1). After requesting a consultation with the Department of Pulmonary and Critical Care Medicine, the patient underwent bronchoscopy. As shown in Figures 2, 3, large quantities of oily turbid fluid were found in the BALF. Cytological analysis of the BALF showed 35% phagocytes, 60% neutrophils, and 5% lymphocytes. To exclude the possibility of fungal infection, we performed fungal culture and identification, galactomannan detection test, and fungal D-glucan detection test on BALF, all of which were negative. We also recommended pathological examination of BALF; regrettably, the patient’s family refused further testing. In summary, based on the patient’s medical history, chest CT imaging and cytological findings, we consider this to be a case of ELP. For treatment, we changed the nasogastric tube and treatment regimen, which included: methylprednisolone 40 mg bid IV, cefoperazone sulbactam 3.0 g q12h IV combined with levofloxacin 0.5 g IV. Despite aggressive treatment, the patient unfortunately passed away after the 12th day of the change in treatment regimen due to advanced age, worsening respiratory symptoms, and complications from an acute myocardial infarction.
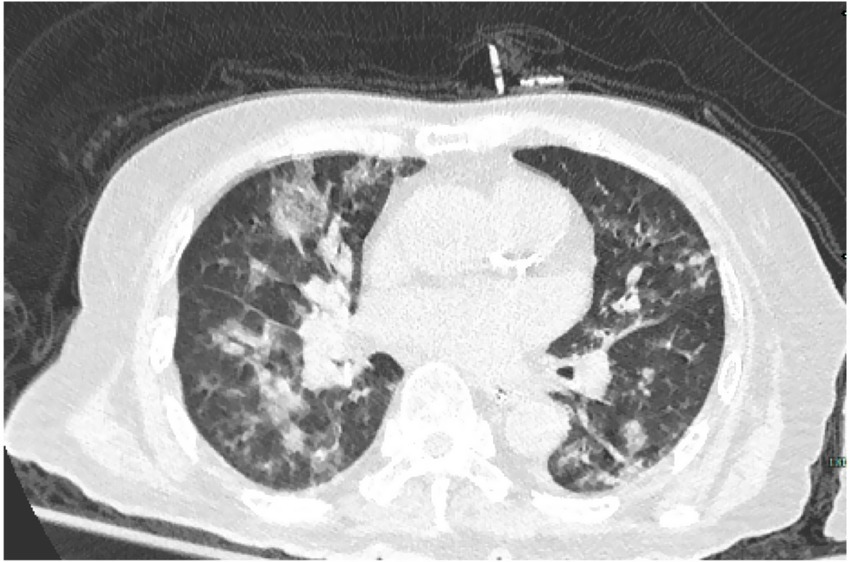
Figure 1. The chest CT scan showed patchy ground-glass lesions in the lungs, mainly along the bronchi.
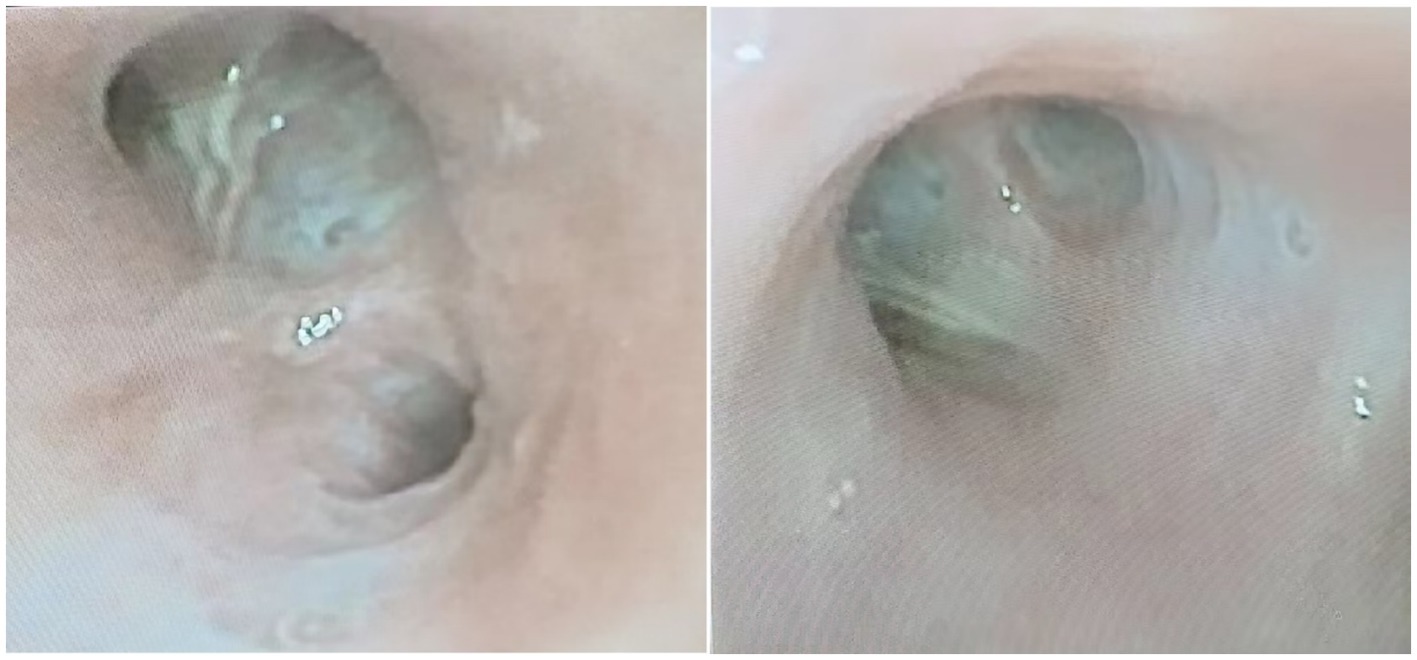
Figure 2. Bedside bronchoscopy showed large amounts of lipoidal mucus secretions distributed in the tracheal lumen.
Discussion and conclusions
Swallowing dysfunction is known to be a high-risk factor for aspiration, which was further exacerbated in this case by the patient’s persistent nausea and vomiting. In clinical practice, all patients with nasogastric tubes should be assessed for the risk of aspiration. Tube placement should be carefully considered if the patient has any of the following conditions, including inability to protect the airway, mechanical ventilation, age >70 years, diminished consciousness, poor oral care, inadequate nurse-to-patient ratio, prolonged bed rest, neurologic deficits, and gastroesophageal reflux (5). In terms of nursing care, doctors should closely monitor the patient’s status and promptly address conditions such as nausea, vomiting, respiratory distress or fever (6). Therefore, a detailed history, such as the exposure to lipids, should be taken during the clinical consultation. The CT examination of ELP shows various manifestations, which can be a mass similar to lung cancer with unclear boundary; it can also be distributed bilaterally, mainly in the posterior segment of the right upper lobe, the right middle lobe and the two lower lobes, while pleural effusions are often described in acute ELP (7). Low-density solid lesions or nodules (CT values ranging from −150 to −30 HU) are characteristic of lipoid pneumonia (8). However, superimposed peripheral inflammation can lead to more prominent areas of fat hypodensity, resulting in solid or nodular shadows that are not readily observable in some patients, making diagnostic imaging more difficult (9). As with other diseases, pathological examination is the gold standard for the diagnosis of ELP. Currently, CT-guided percutaneous puncture lung biopsy is an effective test. The pathological manifestations of ELP include widening of the alveoli, the appearance of lipid vacuoles, and foam cells in the lumen of the fine bronchioles and alveolar septa, accompanied by lymphocytic infiltration and possibly fibrosis (10).
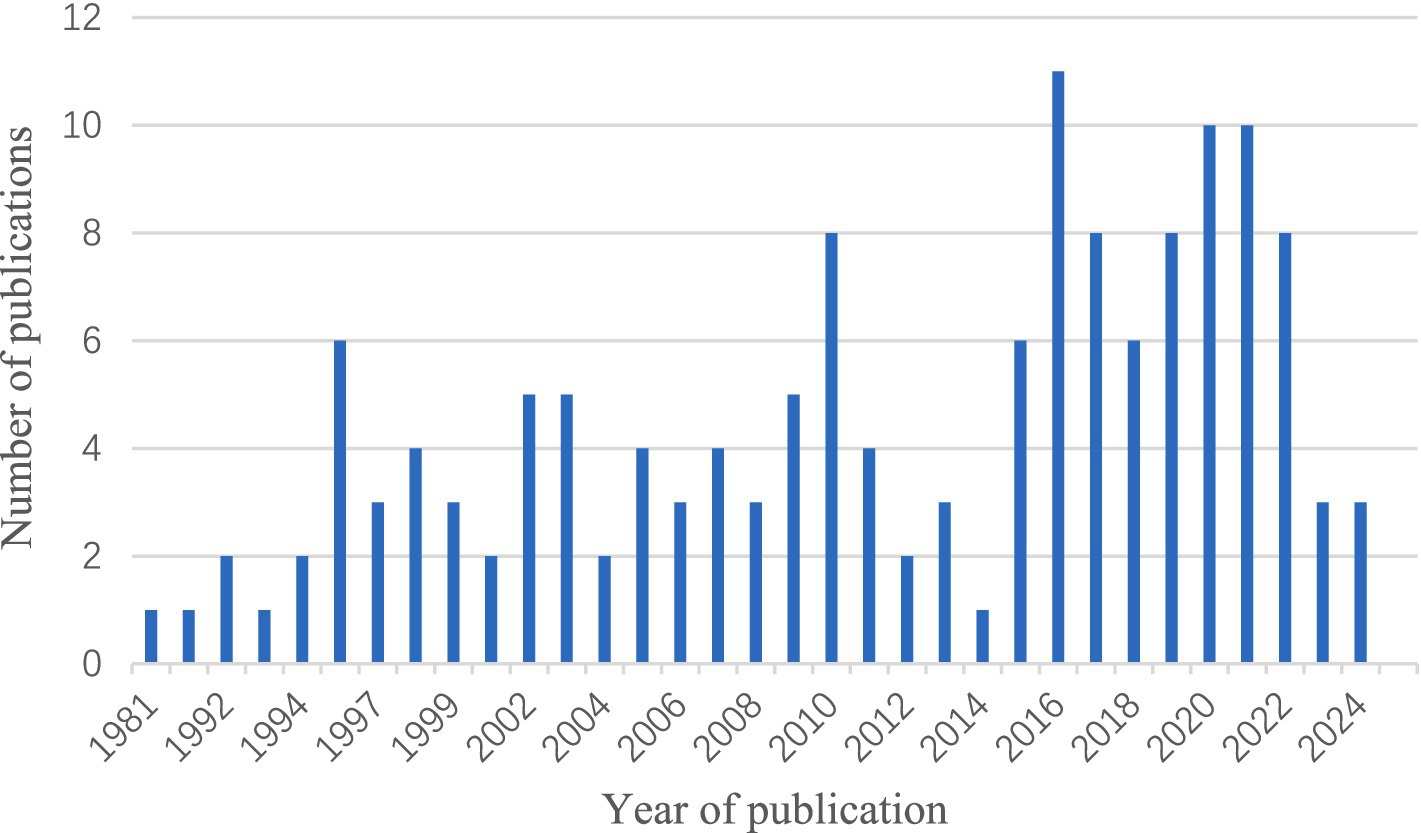
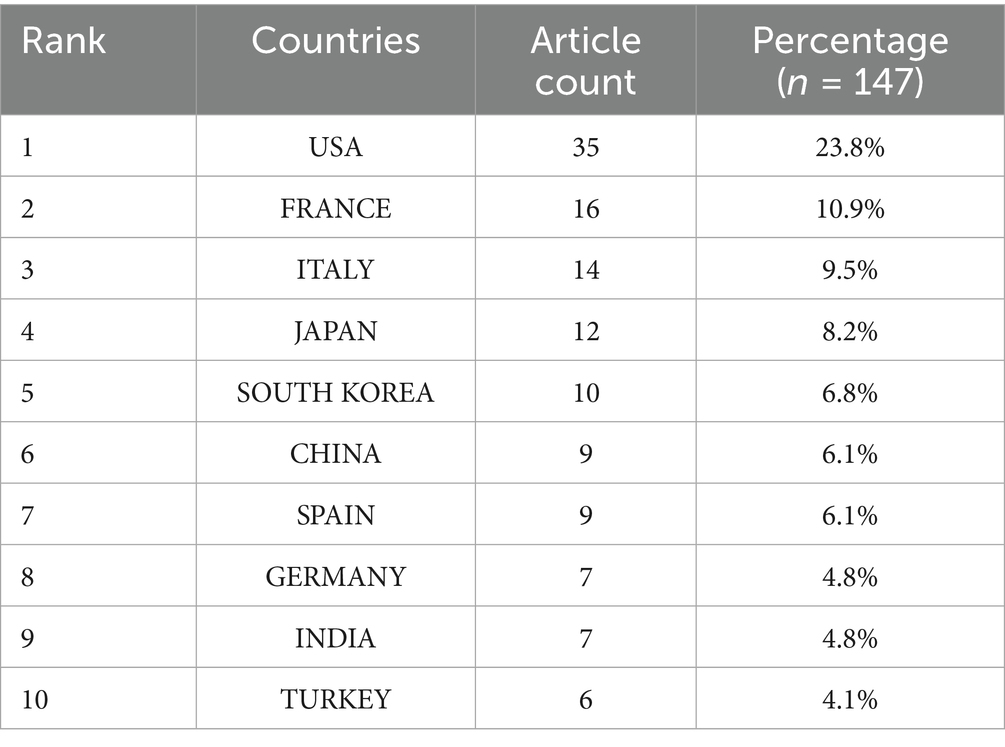
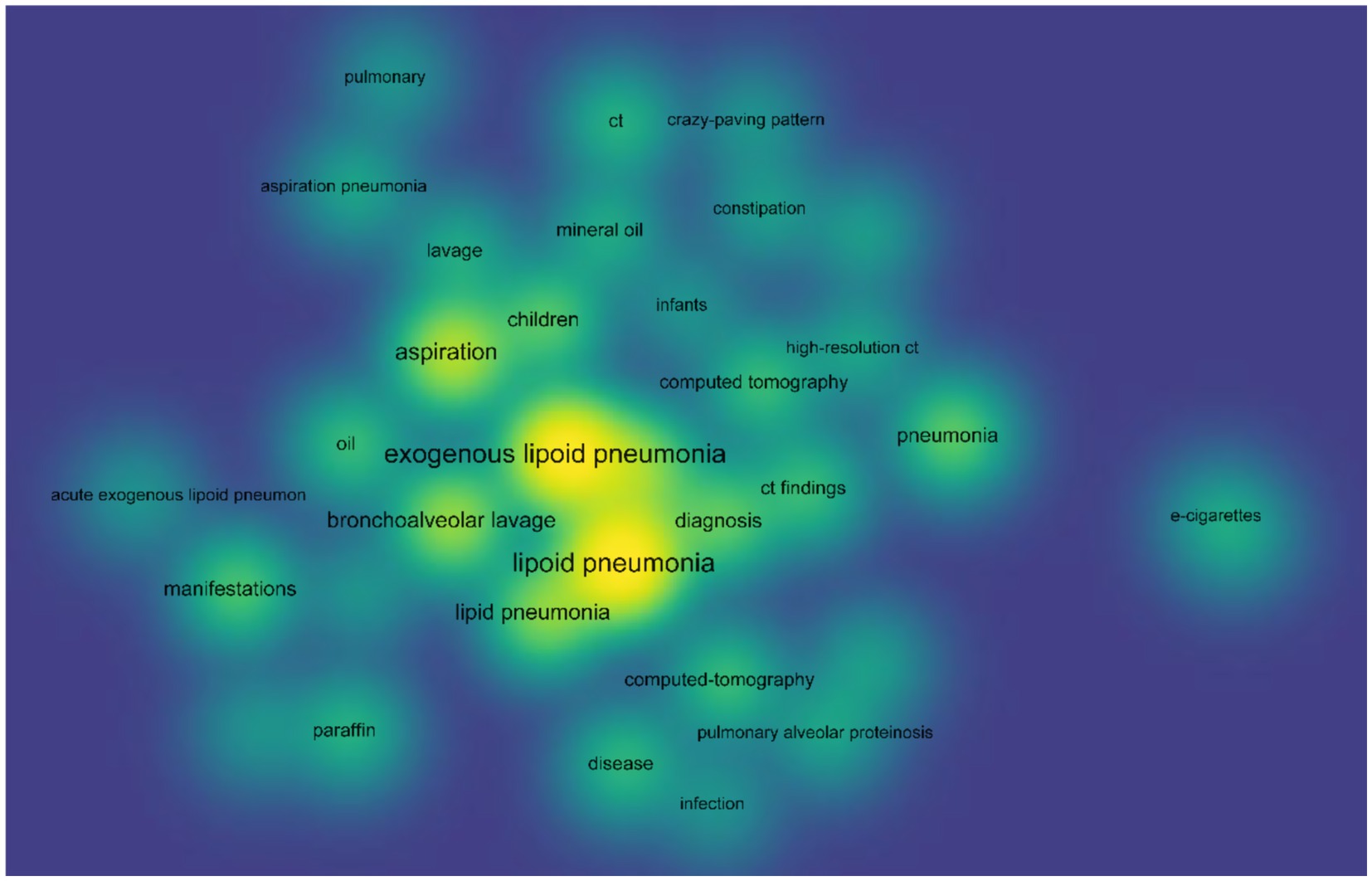
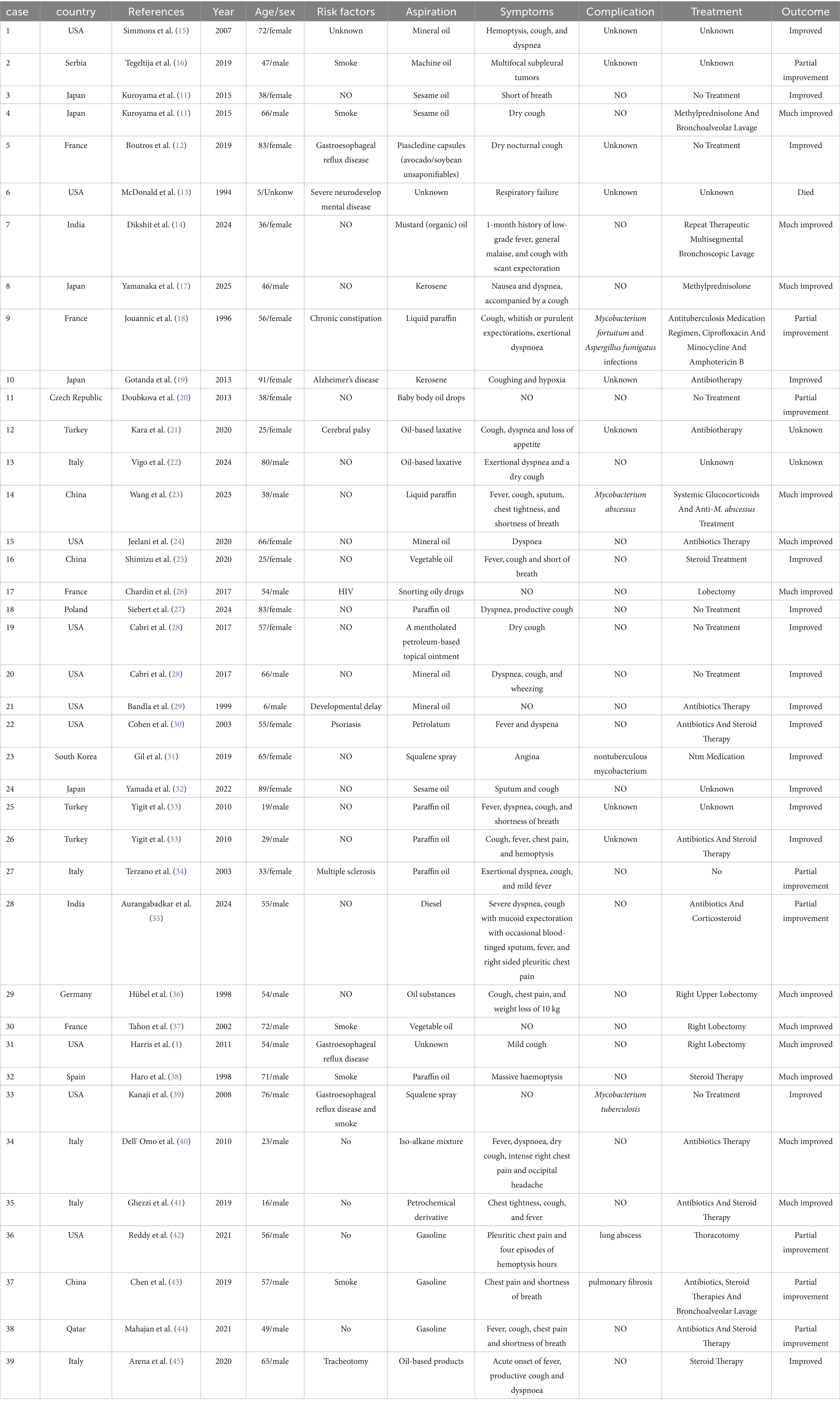
In order to get a deeper understanding of the etiology of lipoid pneumonia, we conducted a retrospective study of literature. The keywords “lipoid pneumonia” or “exogenous lipoid pneumonia” or “endogenous lipoid pneumonia” were used to search for papers indexed in the Web of Science database between 1981 and 2024. After removing commentaries, book chapters and meeting papers, a total of 147 literatures were obtained. As can be seen in Figure 4, the number of articles on lipoid pneumonia has risen each year, with 11 papers published in 2017, indicating that clinicians are becoming more sensitive to lipoid pneumonia diagnosis. The top three countries with the highest number of publications were the United States of America, France and Italy, contributing 65 articles and 44% of the total (Table 1). CiteSpace analysis of the keywords revealed that “exogenous lipoid pneumonia” accounted for the largest proportion of cases, mostly due to aspiration of oily substances. It has also been noted that children are susceptible to this disease (Figure 5). We re-screened the above 147 articles, using “exogenous lipoid pneumonia” and “case report” as keywords, and obtained a total of 58 articles. After removing irrelevant and incomplete clinical data, we found 39 cases (Table 2).
Summarized in Table 2, we found that ELP has no specific clinical manifestations and is difficult to distinguish from common respiratory diseases. Acute ELP is due to inhalation of large amounts of oily substances in a short period of time. It is often characterized by fever and shortness of breath. Chronic ELP has a long history of exposure to oily substances. Symptoms are dominated by chest pain, dry cough, or even no obvious symptoms, and usually do not manifest as fever or coughing up sputum. Chronic ELP is challenging to diagnose and is easily confused with lung cancer and idiopathic pulmonary fibrosis. We have found that nontuberculous mycobacterial (NTM) infections are susceptible to secondary ELP, mainly Mycobacterium choriogenes and Mycobacterium kansasii infections (11, 12). Repeated inhalation can lead to pulmonary fibrosis and even hypoxic respiratory failure (13, 14).
There is no uniform treatment protocol for ELP. In addition to immediate stopping of exposure to oily substances, application of steroids has been chosen in most cases. The use of antibiotics has not been found to be more beneficial in the absence of combined bacterial infections. Anti-NTM drugs are used when combined with NTM infection. Surgical resection was chosen for treatment in some cases when the lesion was severe or could not be differentiated from lung cancer. For asymptomatic patients with only imaging manifestations, some cases remain partially improved clinically without treatment. Therefore, in clinical work, we should analyze specific cases and choose appropriate treatment to maximize clinical efficacy.
From Table 2, we found that the prognosis is good for patients without underlying disease. Most clinical symptoms resolve despite slow or even no image absorption. In children, mostly with congenital dysplasia, the disease progresses rapidly and the prognosis is poor. Elderly patients with complex underlying disease or immunodeficiency also have a poorer prognosis. There is currently one case of ELP combined with lung abscess, which underwent open heart surgery and has a fair prognosis. It is worth noting that application of paraffin oil due to constipation was the main cause of the disease. Therefore, risk factors must be strictly evaluated when applying paraffin oil. For people at high risk of aspiration, including dysphagia and gastroesophageal reflux, the use of paraffin oil should be avoided as much as possible.
In conclusion, an 87-year-old male with a history of exposure to paraffin oil was diagnosed with ELP based on radiological and cytological findings. This is the first reported case of ELP due to medical aspiration. Because of the diverse clinical presentation of ELP, its diagnosis requires a combination of risk factor exposure history, radiologic imaging, cytology, and histopathology. For people at high risk of misadministration, a suitable naso-intestinal tube is more appropriate for feeding and medication.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because this study is a case report and the data presented were obtained from the treatment process. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ML: Conceptualization, Writing – original draft. CR: Writing – review & editing. CC: Conceptualization, Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Harris, K, Chalhoub, M, Maroun, R, Abi-Fadel, F, and Zhao, F. Lipoid pneumonia: a challenging diagnosis. Heart Lung. (2011) 40:580–4. doi: 10.1016/j.hrtlng.2010.12.003
2. Morita, H, Arai, S, Kurasawa, K, Okada, H, Tanaka, A, Yamazaki, R, et al. Intensive immunosuppressive therapy for endogenous lipoid pneumonia associated with rheumatoid arthritis. Mod Rheumatol. (2018) 28:1044–8. doi: 10.1080/14397595.2016.1193270
3. Marchiori, E, Zanetti, G, Mano, CM, and Hochhegger, B. Exogenous lipoid pneumonia. Clinical and radiological manifestations. Respir Med. (2011) 105:659–66. doi: 10.1016/j.rmed.2010.12.001
4. Marangu, D, Gray, D, Vanker, A, and Zampoli, M. Exogenous lipoid pneumonia in children: a systematic review. Paediatr Respir Rev. (2020) 33:45–51. doi: 10.1016/j.prrv.2019.01.001
5. McClave, SA, DeMeo, MT, DeLegge, MH, DiSario, JA, Heyland, DK, Maloney, JP, et al. North American summit on aspiration in the critically ill patient: consensus statement. JPEN J Parenter Enteral Nutr. (2002) 26:S80–5. doi: 10.1177/014860710202600613
6. Wang, ZY, Chen, JM, and Ni, GX. Effect of an indwelling nasogastric tube on swallowing function in elderly post-stroke dysphagia patients with long-term nasal feeding. BMC Neurol. (2019) 19:83. doi: 10.1186/s12883-019-1314-6
7. Cheng, ML, Thomas, V, Vaz, N, Hammer, MM, Nishino, M, Vargas, SO, et al. Lipid pneumonia associated with mineral oil use presenting as fluorine-18-fluorodeoxy-D-glucose-avid lung mass. JTCVS Tech. (2022) 15:192–4. doi: 10.1016/j.xjtc.2022.08.004
8. Baron, SE, Haramati, LB, and Rivera, VT. Radiological and clinical findings in acute and chronic exogenous lipoid pneumonia. J Thorac Imaging. (2003) 18:217–24. doi: 10.1097/00005382-200310000-00002
9. Lee, KS, Muller, NL, Hale, V, Newell, JD Jr, Lynch, DA, and Im, JG. Lipoid pneumonia: CT findings. J Comput Assist Tomogr. (1995) 19:48–51. doi: 10.1097/00004728-199501000-00009
10. Guo, M, Liu, J, and Jiang, B. Exogenous lipid pneumonia in old people caused by aspiration: two case reports and literature review. Respir Med Case Rep. (2019) 27:100850. doi: 10.1016/j.rmcr.2019.100850
11. Kuroyama, M, Kagawa, H, Kitada, S, Maekura, R, Mori, M, and Hirano, H. Exogenous lipoid pneumonia caused by repeated sesame oil pulling: a report of two cases. BMC Pulm Med. (2015) 15:135. doi: 10.1186/s12890-015-0134-8
12. Boutros, J, Muzzone, M, Benzaquen, J, Levraut, M, Marquette, CH, Rocher, F, et al. A case report of exogenous lipoid pneumonia associated with avocado/soybean unsaponifiables. BMC Pulm Med. (2019) 19:234. doi: 10.1186/s12890-019-0997-1
13. McDonald, JW, Roggli, VL, and Bradford, WD. Coexisting endogenous and exogenous lipoid pneumonia and pulmonary alveolar proteinosis in a patient with neurodevelopmental disease. Pediatr Pathol. (1994) 14:505–11. doi: 10.3109/15513819409024280
14. Dikshit, N, Gupta, M, Nigam, N, and Nath, A. Rare cause of chronic cough in a young healthcare worker - a case of exogenous lipoid pneumonia! J Family Med Prim Care. (2025) 14:487–90. doi: 10.4103/jfmpc.jfmpc_1233_24
15. Simmons, A, Rouf, E, and Whittle, J. Not your typical pneumonia: a case of exogenous lipoid pneumonia. J Gen Intern Med. (2007) 22:1613–6. doi: 10.1007/s11606-007-0280-7
16. Tegeltija, D, Lovrenski, A, Vasiljevic, T, Samardzija, G, and Kuhajda, I. Exogenous lipoid pneumonia mimicking multifocal subpleural tumors. Srp Arh Celok Lek. (2020) 148:207–10. doi: 10.2298/SARH180410070T
17. Yamanaka, M, Nisiyama, Y, Fujishima, N, Yokoyama, A, and Komiya, K. An acute case of exogenous lipoid pneumonia which developed in tent sauna: a case report. Intern Med. (2025). 0954-6820:1–6. doi: 10.2169/internalmedicine.5634-25
18. Jouannic, I, Desrues, B, Lena, H, Quinquenel, ML, Donnio, PY, and Delaval, P. Exogenous lipoid pneumonia complicated by Mycobacterium fortuitum and Aspergillus fumigatus infections. Eur Respir J. (1996) 9:172–4. doi: 10.1183/09031936.96.09010172
19. Gotanda, H, Kameyama, Y, Yamaguchi, Y, Ishii, M, Hanaoka, Y, Yamamoto, H, et al. Acute exogenous lipoid pneumonia caused by accidental kerosene ingestion in an elderly patient with dementia: a case report. Geriatr Gerontol Int. (2013) 13:222–5. doi: 10.1111/j.1447-0594.2012.00896.x
20. Doubkova, M, Doubek, M, Moulis, M, and Skrickova, J. Exogenous lipoid pneumonia caused by chronic improper use of baby body oil in adult patient. Rev Port Pneumol. (2013) 19:233–6. doi: 10.1016/j.rppneu.2013.05.002
21. Kara, BY, Özyurt, S, Metin, Y, Karadogan, D, and Sahin, Ü. A rare case of non-resolving pneumonia: lipoid pneumonia. Med Bull Haseki. (2020) 58:115–7. doi: 10.4274/haseki.galenos.2019.5427
22. Vigo, B, Rinaldo, RF, Sanfilippo, CM, Pettenon, F, Torre, O, Mondoni, M, et al. When exogenous lipoid pneumonia mocks lung cancer. Minerva Respir Med. (2024) 63:84–9. doi: 10.23736/S2784-8477.23.02100-9
23. Wang, H, Lu, S, Li, H, and Wang, Y. Mycobacterium infection secondary to exogenous lipoid pneumonia caused by nasal drops: a case report and literature review. BMC Pulm Med. (2023) 23:47. doi: 10.1186/s12890-022-02265-8
24. Jeelani, HM, Sheikh, MM, Sheikh, B, Mahboob, H, and Bharat, A. Exogenous lipoid pneumonia complicated by mineral oil aspiration in a patient with chronic constipation: a case report and review. Cureus J Med Sci. (2020) 12:e9294. doi: 10.7759/cureus.9294
25. Shimizu, T, Nakagawa, Y, Iida, Y, Hayashi, K, Sato, Y, Maruoka, S, et al. The diagnosis of exogenous lipoid pneumonia caused by the silent aspiration of vegetable oil using a Lipidomic analysis. Intern Med. (2020) 59:409–14. doi: 10.2169/internalmedicine.3676-19
26. Chardin, D, Nivaggioni, G, Viau, P, Butori, C, Padovani, B, Grangeon-Chapon, C, et al. False positive 18FDG PET-CT results due to exogenous lipoid pneumonia secondary to oily drug inhalation: a case report. Medicine. (2017) 96:e6889. doi: 10.1097/MD.0000000000006889
27. Siebert, K, Jassem, E, Porzezinska, M, Jelitto, M, and Bernard, W. Lipoid pneumonia induced by aspiration of liquid paraffin. Ann Agric Environ Med. (2024) 31:144–6. doi: 10.26444/aaem/168783
28. Cabri, AE, King, A, Morrow, L, and Malesker, MA. Pharmacists can help prevent lipoid pneumonia: two case reports. J Am Pharm Assoc. (2017) 57:616–8. doi: 10.1016/j.japh.2017.05.012
29. Bandla, HP, Davis, SH, and Hopkins, NE. Lipoid pneumonia: a silent complication of mineral oil aspiration. Pediatrics. (1999) 103:E19. doi: 10.1542/peds.103.2.e19
30. Cohen, MA, Galbut, B, and Kerdel, FA. Exogenous lipoid pneumonia caused by facial application of petrolatum. J Am Acad Dermatol. (2003) 49:1128–30. doi: 10.1016/S0190-9622(03)00445-6
31. Gil, BM, Chung, MH, Kim, YD, Kim, YH, Kang, HS, and Kim, IJ. Aggressive mycobacterium abscessus on repeated exogenous lipoid pneumonia in the right middle lobe. Ann Transl Med. (2019) 7:206. doi: 10.21037/atm.2019.04.02
32. Yamada, A, Kagawa, T, Nishimoto, Y, Sugawara, R, Arai, T, Inoue, Y, et al. Exogenous lipoid pneumonia caused by gargling with sesame oil: a case report. J Thorac Imaging. (2022) 37:W97–W100. doi: 10.1097/RTI.0000000000000669
33. Yigit, O, Bektas, F, Sayrac, AV, and Senay, E. Fire-eater's pneumonia: two case reports of accidentally aspirated paraffin oil. J Emerg Med. (2012) 42:417–9. doi: 10.1016/j.jemermed.2010.11.025
34. Terzano, C, Ricci, A, Petroianni, A, Laurendi, G, Mammarella, A, Paoletti, V, et al. Lipoid pneumonia in multiple sclerosis: an insidious complication--case report. Adv Ther. (2003) 20:138–42. doi: 10.1007/BF02850200
35. Aurangabadkar, GM, Choudhary, SS, and Khan, SM. Lipoid pneumonia secondary to diesel aspiration: an occupational Hazard. Cureus. (2024) 16:e58509. doi: 10.7759/cureus.58509
36. Hübel, K, Schmitz, S, Diehl, V, and Engert, A. Tentative diagnosis of lung cancer in a patient with lipoid pneumonia. Onkologie. (1998) 21:245–7. doi: 10.1159/000026824
37. Tahon, F, Berthezene, Y, Hominal, S, Blineau, N, Guerin, JC, Cinotti, L, et al. Exogenous lipoid pneumonia with unusual CT pattern and FDG positron emission tomography scan findings. Eur Radiol. (2002) 12:S171–3. doi: 10.1007/s00330-002-1659-9
38. Haro, M, Murcia, I, Nunez, A, Julia, E, and Valer, J. Massive haemoptysis complicating exogenous lipid pneumonia. Eur Respir J. (1998) 11:507–8. doi: 10.1183/09031936.98.11020507
39. Kanaji, N, Bandoh, S, Takano, K, Kadota, K, Haba, R, Matsunaga, T, et al. Positron emission tomography-positive squalene-induced lipoid pneumonia confirmed by gas chromatography-mass spectrometry of bronchoalveolar lavage fluid. Am J Med Sci. (2008) 335:310–4. doi: 10.1097/MAJ.0b013e31811ec1a0
40. Dell' Omo, M, Murgia, N, Chiodi, M, Giovenali, P, Cecati, A, and Gambelunghe, A. Acute pneumonia in a fire-eater. Int J Immunopathol Pharmacol. (2010) 23:1289–92. doi: 10.1177/039463201002300437
41. Ghezzi, M, Odoni, M, Testagrossa, O, Messina, D, Ruocco, JD, Lovati, C, et al. Pneumonia in a teenager hiding a fire-eating stunt. Pediatr Emerg Care. (2019) 35:e147–9. doi: 10.1097/PEC.0000000000001316
42. Reddy, R, Baek, J, Perone, HR, Chen, K, and Lichtstein, DM. The hurricane lung: a case of hydrocarbon pneumonitis with abscess formation following fuel siphoning. Cureus. (2021) 13:e14807. doi: 10.7759/cureus.14807
43. Chen, YJ, Hsu, CC, and Chen, KT. Hydrocarbon pneumonitis following fuel siphonage: a case report and literature review. World J Emerg Med. (2019) 10:69–74. doi: 10.5847/wjem.j.1920-8642.2019.02.001
44. Mahajan, PS, Kolleri, JJ, and Farghaly, H. Report of a rare case of computed tomography diagnosis of hydrocarbon pneumonitis. Cureus. (2021) 13:e19914. doi: 10.7759/cureus.19914
Keywords: paraffin oil, respiratory aspiration, intubation, gastrointestinal, exogenous lipoid pneumonia
Citation: Li M, Ren C and Chen C (2025) Exogenous lipoid pneumonia due to medical aspiration of paraffin oil: a case report and literature review. Front. Med. 12:1596160. doi: 10.3389/fmed.2025.1596160
Edited by:
Yong-Xiao Wang, Albany Medical College, United StatesReviewed by:
Kelly Robert Redeker, University of York, United KingdomPalagan Senopati Sewoyo, Udayana University, Indonesia
Copyright © 2025 Li, Ren and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Conglin Ren, MjAxOTExMDEwODExMDM1QHpjbXUuZWR1LmNu
†ORCID: Mingshuang Li, orcid.org/0000-0002-4651-212X
 Mingshuang Li
Mingshuang Li Conglin Ren
Conglin Ren Cangsong Chen1
,2
Cangsong Chen1
,2