Abstract
Purpose:
Sedentary behavior and impaired thyroid hormone sensitivity are linked to a variety of comorbid conditions; however, the exact nature of their relationship remains inadequately studied. This study sought to examine the association between sedentary time and thyroid hormone sensitivity.
Methods:
Utilizing a cross-sectional design, the study analyzed data from U.S. participants in the National Health and Nutrition Examination Survey (NHANES) conducted between 2007 and 2012. The Least Absolute Shrinkage and Selection Operator (LASSO) regression and the Boruta algorithm were employed to screen out confounding factors closely associated with sedentary time and the parametric thyroid feedback quantile-based index (PTFQI). Multivariate linear regression models were applied to analyze the association between sedentary time and indicators of thyroid hormone sensitivity. After adjusting for all confounding factors, restricted cubic spline (RCS) curves were utilized to further explore the potential non-linear relationship between sedentary time and indicators of thyroid hormone sensitivity. Additionally, subgroup analyses and interaction tests were conducted to further explore this association.
Results:
A total of 22 significant confounding factors were identified through LASSO regression and the Boruta algorithm. Among these potential confounding factors, body mass index (BMI) occupied a central position, and it partially mediated the association between sedentary time and the PTFQI. RCS analysis indicated that, after adjusting for all covariates, there was a significant linear association between sedentary time and PTFQI in men (P for overall = 0.002, P for non-linear = 0.085). In contrast, in women, the relationship presented an “inverted U-shaped” curve, which was not statistically significant (P for overall > 0.05). Moreover, the results of the interaction analysis revealed a significant interaction effect of race on the association between sedentary time and PTFQI (P for interaction = 0.004).
Conclusions:
In this study, we found a positive association between impaired thyroid hormone sensitivity and sedentary time in men after adjusting for confounders, and BMI partially mediated this positive association. Additionally, the factor of race exhibited a significant interaction effect on the association between sedentary time and the PTFQI.
Introduction
In both developed and developing nations, social, economic, and environmental transitions have precipitated a pervasive rise in sedentary behavior (SB) (1). Sedentary behavior (SB) has emerged as a prominent aspect of contemporary life, garnering considerable attention due to its implications for health (2). The physical activity (PA) guidelines promulgated by the World Health Organization in 2020 define sedentary behavior as “any behavior in a sitting, reclining, or lying position during wakefulness, with an energy expenditure of 1.5 metabolic equivalents or less” encompassing activities such as prolonged sitting at work, television viewing, and the use of electronic devices (3).
Thyroid hormones play a crucial role in regulating the body's metabolism and energy expenditure and are key factors in maintaining metabolic homeostasis. Thyroid dysfunction is closely associated with the occurrence of various metabolic diseases (4). Impaired thyroid hormone sensitivity refers to a weakened response of the body to thyroid hormones, which may manifest as resistance to or a decrease in sensitivity to the effects of thyroid hormones, thus having a wide-ranging impact on an individual's health. Previous research has demonstrated that thyroid-stimulating hormone (TSH) levels alone do not provide an objective measure of thyroid function, as TSH secretion is inversely regulated by peripheral free thyroxine (FT4) concentrations (5). Quantitative indicators such as thyrotrophin thyroxine resistance index (TT4RI), thyroid-stimulating hormone index (TSHI), and parametric thyroid feedback quantile-based index (PTFQI) are employed to assess the sensitivity of the pituitary-central axis to thyroid hormones (6–8). The PTFQI, in particular, utilizes a mathematical model incorporating TSH and FT4 levels to reflect the feedback regulation state of the pituitary-thyroid axis, thereby quantifying thyroid hormone sensitivity. Compared to TSHI and TT4RI, PTFQI offers a more precise quantification of changes in thyroid hormone sensitivity and demonstrates superior stability (9, 10).
The interrelationship between sedentary behavior and impaired thyroid hormone sensitivity encompasses a range of comorbidities (Figure 1). Contemporary research has substantiated that sedentary behavior serves as a significant catalyst for metabolic and endocrine disorders, with its detrimental effects on public health warranting considerable attention (11). Sedentary behavior is particularly associated with an increased incidence of cardiovascular diseases (12, 13), liver diseases (14, 15), kidney diseases (16, 17), tumorigenesis and tumor progression (18, 19), obesity (20), compromised bone health (21, 22), and metabolic syndrome (23). Concurrently, impaired thyroid hormone sensitivity is intricately linked to metabolic syndrome, hypertension, hyperuricemia, vitamin D deficiency, diabetes, and related conditions (24–28). Sun et al. (29) have demonstrated that reduced sensitivity to thyroid hormones in individuals with subclinical hypothyroidism is linked to an elevated risk of cardiovascular disease and obesity. Among elderly individuals with normal thyroid function, diminished thyroid hormone sensitivity is correlated with an increased incidence of osteoporosis and fractures, independent of traditional risk factors (30, 31). Furthermore, impaired sensitivity to thyroid hormones is associated with a heightened risk of thyroid cancer and cervical lymph node metastasis (32, 33).
Figure 1

Sedentary behavior is associated with a series of comorbid conditions related to impaired thyroid hormone sensitivity.
Nevertheless, the relationship between impaired thyroid hormone sensitivity and sedentary behavior remains inadequately understood. Consequently, this study utilized data from the National Health and Nutrition Examination Survey (NHANES) spanning 2007–2012 to investigate the independent association between thyroid hormone sensitivity and sedentary behavior.
Materials and methods
Study population
The NHANES is a nationally representative survey employing a multi-stage, stratified sampling methodology. Ethical approval for the NHANES study was obtained from the Institutional Review Board of the National Center for Health Statistics (NCHS) in the United States, with all participants providing informed consent prior to participation. This study utilized NHANES data from three survey cycles: 2007–2008, 2009–2010, and 2011–2012.
Initially, a total of 30,442 participants were enrolled in this study. We excluded the following individuals: (1) participants with missing thyroid function indices (n = 20,039); (2) participants with missing data on sedentary time or those with extreme values of sedentary time data (n = 984); (3) participants aged over 60 years old (n = 4,076), as the characteristics of thyroid function change significantly in the elderly population (34, 35); (4) participants with missing demographic or relevant health variables (n = 894); and (5) participants with missing results of routine blood tests (n = 13), standard biochemical tests (n = 14), those who were taking estrogen (n = 228), pregnant women (n = 56), or individuals with thyroid diseases (n = 157). Ultimately, 3,981 participants were included (Figure 2).
Figure 2

Participant flow diagram.
Sedentary time
In the NHANES, the physical activity questionnaire is derived from the Global Physical Activity Questionnaire and encompasses a range of queries pertaining to daily activities, leisure pursuits, and sedentary behaviors. The sedentary time considered in this study excludes sleep duration and specifically pertains to activities such as sitting at a desk for occupational purposes, socializing with friends, commuting by car, bus, or train, reading, playing cards, watching television, and using a computer.
Thyroid hormone sensitivity indicator
Thyroid hormone sensitivity indices were calculated by PTFQI, TSHI, and TT4RI. During the physical examination, serum FT4 levels were quantified using a two-step enzyme immunoassay, while serum TSH levels were measured using a third-generation two-site immunoenzymatic assay. In order to provide an index that can be calculated for any new value or adapted to other populations, an approximation with the same range and interpretation, the PTFQI can be obtained from FT4 in pmol/L and TSH in mIU/L using the standard normal cumulative distribution function as follows: Φ ((FT4 – μ FT4)/σ FT4) – (1 – Φ ((ln TSH – μln TSH)/σln TSH)), where μ FT4 = 10.075, σ FT4 = 2.155, μln TSH = 0.4654, and σln TSH = 0.7744 for the U.S. population (28). The PTFQI score exhibits a negative correlation with thyroid hormone sensitivity, indicating that an increase in the PTFQI score corresponds to a decrease in thyroid hormone sensitivity. TSHI was calculated as ln TSH (mIU/L) + 0.1345 × FT4 (pmol/L) (5). TT4RI was calculated as FT4 (pmol/L) × TSH (mIU/L) (36). The indices PTFQI, TSHI, and TT4RI are all constructed based on mathematical models of TSH and FT4 levels. Notably, PTFQI demonstrates more outstanding performance in terms of sensitivity and stability (9, 10).
Covariates
In this study, covariates encompass demographic information, pertinent health variables, and laboratory test data. The demographic information includes gender, age, race, educational attainment (categorized as ≤ high school or >high school), marital status, and the poverty income ratio (PIR). Pertinent health variables comprise body mass index (BMI), smoking status, drinking status, and the presence of hypertension and diabetes. Smoking status is classified into smokers and non-smokers based on whether an individual has smoked more than 100 cigarettes in their lifetime. Drinking status is categorized into drinkers and non-drinkers according to whether a person has consumed more than 12 alcoholic beverages within 1 year. In this study, a diagnosis of diabetes is established if any of the following criteria are satisfied: (1) glycated hemoglobin A1c level ≥6.5% (47.5 mmol/mol); (2) fasting plasma glucose level ≥126 mg/dl (7.0 mmol/L); (3) random plasma glucose ≥200 mg/dl (11.1 mmol/L); (4) blood glucose level in a 2-h oral glucose tolerance test ≥200 mg/dl (11.1 mmol/L); (5) use of hypoglycemic drugs; and (6) participants with a self-reported diabetes diagnosis (37). Hypertension is diagnosed if any of the following criteria are met: (1) a medical professional has informed the individual of a hypertension diagnosis; (2) the use of antihypertensive medications; or (3) a systolic blood pressure of ≥140 mmHg or a diastolic blood pressure of ≥90 mmHg. Moderate-to-vigorous physical activity (MVPA) is composed of four indicators, namely vigorous recreational activities, moderate recreational activities, high-intensity work, and moderate-intensity work. Laboratory examination data included complete blood count and standard biochemistry tests.
Statistical analysis
In accordance with the analytical guidelines set forth by the National Center for Health Statistics (NCHS), the complex survey design elements of the National Health and Nutrition Examination Survey (NHANES), such as weighting, clustering, and stratification, were meticulously considered. Data conforming to either a normal or skewed distribution were reported as “mean ± standard deviation”, while categorical variables were represented by the number of subjects and their respective percentages. The data were stratified into four quartile intervals based on sedentary time: Q1 (≤ 2 h), Q2 (2 < Q2 ≤ 4 h), Q3 (4 < Q3 ≤ 6 h), and Q4 (>6 h). The LASSO regression and the Boruta algorithm were used to screen covariates, aiming to identify the confounding factors that were closely associated with sedentary time and PTFQI. Multiple linear regression analysis and RCS analysis were employed to explore the association between sedentary time and PTFQI. In addition, subgroup analyses were conducted for categories such as age, sex, and body mass index (BMI), with all covariates except the variable of interest itself being adjusted. An interaction term was incorporated to test the heterogeneity of these associations. A two-sided P value threshold of < 0.05 was considered statistically significant. All analyses were performed using R Studio (version 4.4.2, United States).
Results
Baseline characteristics of participants
This study analyzed data from 3,981 participants drawn from the NHANES conducted in the United States between 2007 and 2012. Participants' baseline characteristics were categorized into four quartiles based on sedentary time (Table 1). Among the different quartile groups of sedentary time, significant differences were observed in sex, educational level, the ratio of family poverty to income (PIR), the prevalence of hypertension, BMI, TT4RI, TSHI, and PTFQI (all P values < 0.05). As sedentary time increased, TT4RI, TSHI, and PTFQI showed an upward trend, suggesting that sedentary behavior may have an adverse impact on thyroid hormone sensitivity.
Table 1
| Characteristic | Overall (n = 3,981) | Q1 (n = 1,016) | Q2 (n = 1,152) | Q3 (n = 819) | Q4 (n = 994) | P value |
|---|---|---|---|---|---|---|
| Gender, n(%) | ||||||
| Male | 2,189 (56%) | 546 (55%) | 693 (61%) | 455 (54%) | 495 (52%) | 0.001 |
| Female | 1,792 (44%) | 470 (45%) | 459 (39%) | 364 (46%) | 499 (48%) | |
| Age, Mean (95% CI) | 39 (29, 49) | 39 (30, 48) | 38 (29, 49) | 40 (29, 50) | 40 (30, 50) | 0.541 |
| Race, n(%) | ||||||
| Non-Hispanic Whites | 1,695 (66%) | 316 (54%) | 506 (67%) | 396 (71%) | 477 (71%) | < 0.001 |
| Non-Hispanic Black | 792 (11%) | 187 (13%) | 214 (10%) | 171 (11%) | 220 (11%) | |
| Mexican American | 752 (9.9%) | 301 (19%) | 240 (11%) | 102 (6.1%) | 109 (4.9%) | |
| Others | 742 (13%) | 212 (14%) | 192 (12%) | 150 (11%) | 188 (13%) | |
| Education, n(%) | ||||||
| ≤ High school | 1,997 (42%) | 681 (60%) | 625 (47%) | 363 (38%) | 328 (27%) | < 0.001 |
| >High school | 1,984 (58%) | 335 (40%) | 527 (53%) | 456 (62%) | 666 (73%) | |
| Marital status, n(%) | ||||||
| Married or cohabiting | 2,413 (63%) | 651 (66%) | 730 (64%) | 458 (61%) | 574 (62%) | 0.111 |
| Living alone | 604 (13%) | 166 (15%) | 165 (13%) | 128 (13%) | 145 (13%) | |
| Single | 964 (24%) | 199 (19%) | 257 (22%) | 233 (26%) | 275 (25%) | |
| PIR, n(%) | ||||||
| ≤ 1.0 | 973 (17%) | 306 (25%) | 285 (17%) | 203 (18%) | 179 (12%) | < 0.001 |
| 1.0–3.0 | 1,607 (34%) | 483 (43%) | 477 (36%) | 309 (34%) | 338 (27%) | |
| ≥3.0 | 1,401 (49%) | 227 (33%) | 390 (47%) | 307 (49%) | 477 (61%) | |
| Smoking, n(%) | ||||||
| Yes | 1,783 (45%) | 435 (47%) | 526 (47%) | 385 (45%) | 437 (43%) | 0.686 |
| No | 2,198 (55%) | 581 (53%) | 626 (53%) | 434 (55%) | 557 (57%) | |
| Drinking, n(%) | ||||||
| Yes | 3,057 (81%) | 728 (76%) | 897 (81%) | 636 (81%) | 796 (84%) | 0.015 |
| No | 924 (19%) | 288 (24%) | 255 (19%) | 183 (19%) | 198 (16%) | |
| Hypertension, n(%) | ||||||
| Yes | 1,039 (24%) | 252 (22%) | 269 (21%) | 227 (26%) | 291 (29%) | < 0.001 |
| No | 2,942 (76%) | 764 (78%) | 883 (79%) | 592 (74%) | 703 (71%) | |
| Diabetes, n(%) | ||||||
| Yes | 455 (8.6%) | 120 (8.3%) | 109 (7.3%) | 104 (9.1%) | 122 (9.7%) | 0.418 |
| No | 3,526 (91%) | 896 (92%) | 1,043 (93%) | 715 (91%) | 872 (90%) | |
| BMI, Mean (95% CI) | 27 (24, 32) | 27 (24, 31) | 27 (24, 31) | 28 (24, 32) | 28 (24, 32) | 0.025 |
| TT4RI, Mean (95% CI) | 15 (10, 22) | 14 (9, 22) | 15 (10, 22) | 15 (11, 22) | 16 (11, 23) | 0.020 |
| TSHI, Mean (95% CI) | 1.77 (1.38, 2.17) | 1.68 (1.29, 2.15) | 1.77 (1.38, 2.19) | 1.80 (1.41, 2.15) | 1.81 (1.41, 2.19) | 0.015 |
| PTFQI, Mean (95% CI) | −0.03 (−0.24, 0.19) | −0.06 (−0.28, 0.14) | −0.05 (−0.25, 0.18) | 0.00 (−0.24, 0.19) | 0.00 (−0.21, 0.21) | 0.001 |
Baseline characteristics of the participants.
For categorical variables, the P value was determined using the weighted chi-square test. For continuous variables, data are presented as mean ± standard error (SE), and the P value was calculated using the weighted Kruskal–Wallis test. Bolded values indicate statistically significant differences. Sedentary time was divided into four quartile intervals: Q1 ( ≤ 2 h), Q2 (2 < Q2 ≤ 4 h), Q3 (4 < Q3 ≤ 6 h), and Q4 (>6 h).
PIR, Poverty Income Ratio; BMI, body mass index; PTFQI: parametric thyroid feedback quantile-based index; TSHI, thyroid-stimulating hormone index; TT4RI, thyrotrophin thyroxine resistance index.
Screening of confounding variables
In this study, the LASSO regression and the Boruta machine learning algorithm were utilized to systematically identify the potential confounding variables between sedentary time and thyroid hormone sensitivity. In the LASSO regression analysis, the minimum lambda value was determined through 10-fold cross-validation. This lambda value achieved a balance between model complexity and prediction error, effectively preventing the model from being overfitted or oversimplified (Figures 3a, b).
Figure 3

Confounder selection and the association between sedentary time and thyroid hormone sensitivity. (a) LASSO Coefficient distribution map for all confounding variables; (b) variables determined by LASSO analysis; (c) feature importance of confounding variables selected by LASSO regression assessed using the Boruta algorithm; (d) mediating effect of BMI on the association between sedentary time and PTFQI; (e) RCS curve of sedentary time and PTFQI for all participants; (f) RCS curve of sedentary time and PTFQI for male participants; (g) RCS curve of sedentary time and PTFQI for female participants. BMI, body mass index; Hb, hemoglobin; Cl, chloride; TBil, total bilirubin; RBC, red blood cell count; Alb, Albumin; UA, uric acid; Fe, Iron; Glu, serum Glucose; Cr, creatinine; P, phosphorus; LYM#, lymphocyte number; TG, triglyceride; BUN, blood urea nitrogen; MPV, mean platelet volume; TP, total protein; AST, aspartate aminotransferase; PLT, platelet count; K, potassium; NEU#, segmented neutrophils number; HCO3, bicarbonate; EOS%, eosinophils percent; MCHC, mean corpuscular hemoglobin concentration; MON%, monocyte percent; Chol, cholesterol; BAS%, basophils percent; PTFQI, parametric thyroid feedback quantile-based index.
Subsequently, the covariates screened out by the LASSO regression were further incorporated into the unweighted Boruta algorithm analysis. The Boruta algorithm evaluates the importance of features by introducing shadow variables, thus precisely identifying the covariates that have a significant impact on the dependent variable. After 200 iterations, the Boruta algorithm screened out 22 confounding factors of significant importance, which were included in the subsequent study (Figure 3c). It is worth noting that BMI ranked first among the importance scores of these features. Further mediation analysis revealed that BMI played a mediating role in the association between sedentary time and PTFQI (β = 0.0006, 95% CI: 0.0003–0.0011), and the proportion of the mediating effect was 7.99% (Figure 3d).
Association between thyroid hormone sensitivity and sedentary time
The scatter plots and linear fitting curves demonstrated direct links between sedentary time and thyroid hormone-related indicators such as PTFQI, TSHI, TT4RI, and TSH (Supplementary Figure 1). By using three times the standard deviation as a threshold, it was found that TSHI, TT4RI, and TSH had more outliers compared to PTFQI. These outliers suggested that the relationships between sedentary time and TSHI, TT4RI, and TSH might contain abnormal data that could influence the results, whereas PTFQI was less affected by outliers.
In Table 2, it is demonstrated that, after adjusting for all confounding variables in Model 3, the PTFQI level in the highest quartile among men was significantly elevated compared to that in the lowest quartile (β = 0.09, 95% CI: 0.04–0.13, P < 0.001, P for trend < 0.001). Supplementary Table 1 illustrates that this association persisted even when MVPA was included as a covariate in Model 3 (β = 0.07, 95% CI: 0.00–0.14, P = 0.043, P for trend = 0.034). Conversely, in women, the association between sedentary time and PTFQI was not statistically significant (all P values > 0.05). Following adjustment for all covariates, restricted cubic spline (RCS) analysis was conducted to investigate the potential non-linear relationship between sedentary time and thyroid hormone sensitivity. In the total population, the curve depicting the association between sedentary time and PTFQI initially rose and then leveled off (Figure 3e, P for overall = 0.003, P for non-linear = 0.053). The findings indicate a significant linear association between sedentary time and PTFQI among men (Figure 3f, P for overall = 0.002, P for non-linear = 0.085). In contrast, an “inverted U-shaped” relationship was observed among women, although this relationship did not reach statistical significance (Figure 3g, P for overall = 0.247). Supplementary Figure 2 illustrates the non-linear relationship between sedentary time and TSHI in male participants (P for overall = 0.012, P for non-linear = 0.038), whereas no statistically significant association was observed in female participants.
Table 2
| Characteristic | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P -value | β (95% CI) | P -value | β (95% CI) | P -value | |
| Overall | ||||||
| Q1 | Ref | Ref | Ref | Ref | Ref | Ref |
| Q2 | 0.03 (0.00, 0.06) | 0.049 | 0.03 (−0.00, 0.06) | 0.091 | 0.03 (−0.00, 0.06) | 0.072 |
| Q3 | 0.05 (0.01, 0.08) | 0.014 | 0.04 (0.01, 0.08) | 0.025 | 0.04 (0.01, 0.08) | 0.021 |
| Q4 | 0.07 (0.04, 0.11) | < 0.001 | 0.06 (0.03, 0.10) | < 0.001 | 0.06 (0.02, 0.09) | 0.003 |
| P trend | < 0.001 | < 0.001 | < 0.001 | |||
| Male | ||||||
| Q1 | Ref | Ref | Ref | Ref | Ref | Ref |
| Q2 | 0.03 (0.00, 0.07) | 0.053 | 0.03 (−0.00, 0.07) | 0.078 | 0.04 (0.00, 0.07) | 0.037 |
| Q3 | 0.04 (−0.00, 008) | 0.057 | 0.03 (0.01, 0.07) | 0.088 | 0.04 (0.00, 0.08) | 0.051 |
| Q4 | 0.10 (0.05, 0.15) | < 0.001 | 0.09 (0.05, 0.14) | < 0.001 | 0.09 (0.04, 0.13) | < 0.001 |
| P trend | < 0.001 | < 0.001 | < 0.001 | |||
| Female | ||||||
| Q1 | Ref | Ref | Ref | Ref | Ref | Ref |
| Q2 | 0.02 (−0.02, 007) | 0.293 | 0.02 (−0.03, 0.06) | 0.415 | 0.02 (−0.02, 0.06) | 0.352 |
| Q3 | 0.05 (−0.01, 0.10) | 0.096 | 0.04 (−0.01, 0.10) | 0.128 | 0.04 (−0.02, 0.09) | 0.165 |
| Q4 | 0.05 (−0.02, 0.11) | 0.160 | 0.03 (−0.03, 0.10) | 0.301 | 0.03 (−0.03, 0.09) | 0.265 |
| P trend | 0.143 | 0.252 | 0.263 | |||
Correlation between sedentary time and PTFQI.
Model 1 did not adjust for confounding factors. Model 2 was adjusted according to demographic and relevant health variables, including age, race, education, smoking, diabetes and BMI. Model 3 was adjusted according to demographic information, relevant health variables, and laboratory test variables, including age, race, education, smoking, diabetes, BMI, hematocrit, serum chloride, total bilirubin, red blood cell count, albumin, uric acid, serum iron, serum glucose, serum creatinine, phosphorus, lymphocyte count, triglycerides, blood urea nitrogen, mean platelet volume, total protein and aspartate aminotransferase. Bolded values indicate statistically significant differences. The P trend was obtained by converting sedentary time from a continuous variable to an ordinal categorical variable (Q1, Q2, Q3, and Q4) and performing regression analysis using the median of each category.
PTFQI, parametric thyroid feedback quantile-based index.
Analysis of subgroups and interaction tests
In Figure 4, subgroup analyses were performed to explore the associations between sedentary time and PTFQI levels across diverse populations stratified by gender, age, race, educational attainment, marital status, PIR, BMI, smoking status, alcohol consumption, hypertension, and diabetes. The analysis revealed a significant positive correlation between sedentary time and PTFQI within subgroups such as males and individuals with obesity. Furthermore, interaction analysis demonstrated that race significantly moderated the association between sedentary time and PTFQI (P for interaction = 0.004). Specifically, a positive correlation was identified among non-Hispanic whites and other ethnic groups, whereas this association was not significant among non-Hispanic blacks and Mexican Americans.
Figure 4
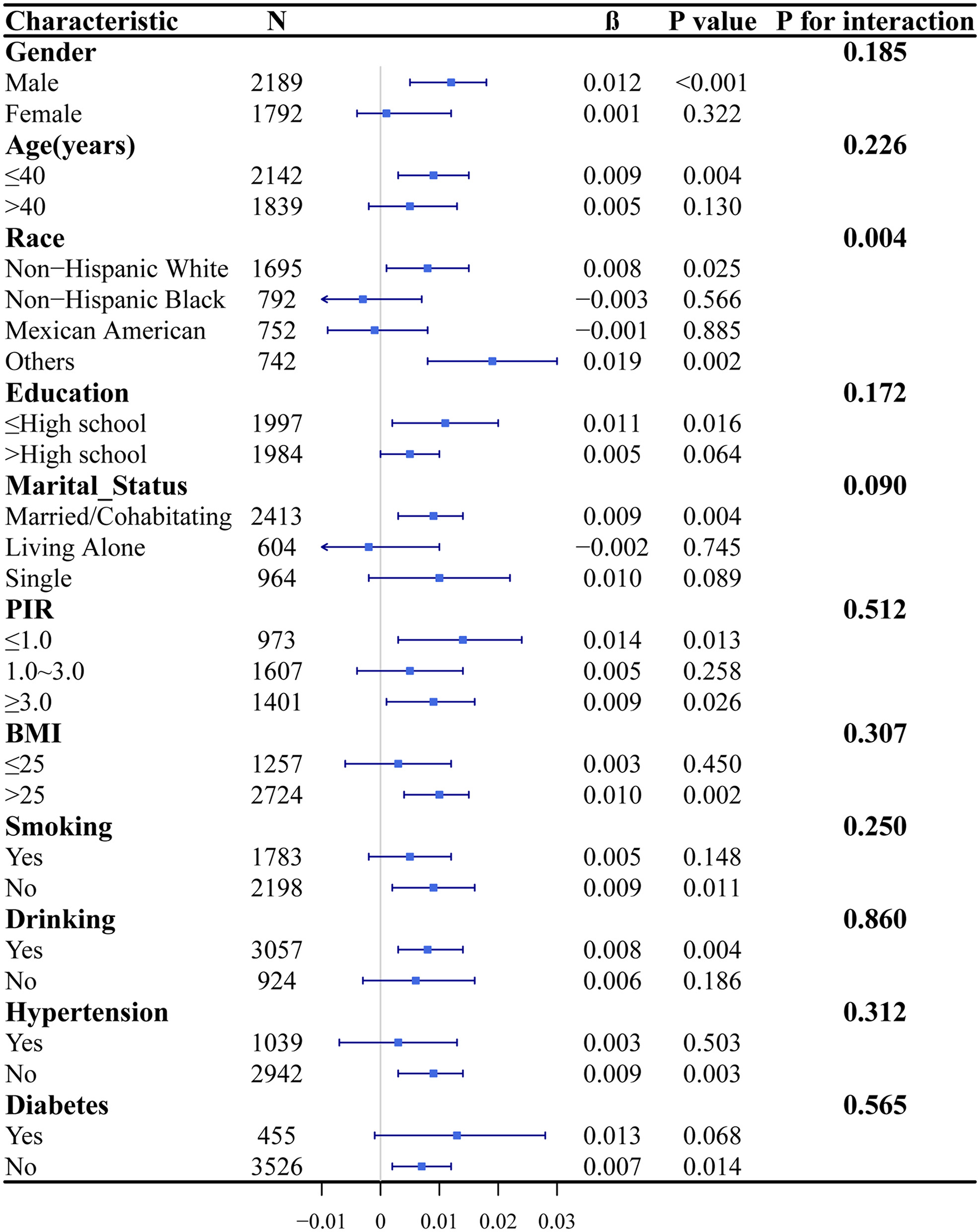
Subgroup analyses and interaction tests between sedentary time and PTFQI. In subgroup analyses, all covariates were adjusted, with the exception of the variable of interest.
Discussion
The association between thyroid health and sedentary behavior represents a relatively under-explored area in current research, warranting further investigation. Thyroid hormones, as crucial endocrine hormones, can regulate the body's energy metabolism, influence carbohydrate, protein, and fat metabolism, and participate in cardiovascular regulation (38, 39). The reduced energy expenditure and metabolic deceleration associated with sedentary behavior may disrupt the normal functioning of thyroid hormones, thereby impacting the body's metabolic state and energy equilibrium (40, 41). Furthermore, there is a significant positive correlation between sedentary behavior and elevated levels of C-reactive protein, abnormal lipid metabolism, insulin resistance index, and insulin concentration (42). These metabolic and inflammatory factors are likely linked to thyroid dysfunction (43). A Mendelian randomization study by Zhang et al. (44) found that higher genetically-influenced TSH levels are linked to increased leisure screen time. Additionally, an epidemiological study showed that sitting for over 6 h daily raises the risk of 12 chronic diseases, including thyroid issues, by 26%. Reducing sedentary time to under 6 h could prevent 3.7%−22.1% of these diseases (45). Common diseases linked to both sedentary behavior and impaired thyroid sensitivity include cardiovascular diseases, cancer, and metabolic syndrome, among others (Figure 1). However, there is currently a lack of direct evidence to definitively establish the correlation between these factors.
In this study, we integrated the LASSO regression and the Boruta algorithm to establish a dual feature screening framework. The LASSO regression, through L1 regularization, achieved efficient dimensionality reduction, meticulously selecting core covariates to form a parsimonious variable set. The Boruta algorithm, relying on the random forest mechanism, generated shadow variables to thoroughly uncover non-linear relationships and interaction effects among variables. This approach effectively circumvented the inherent flaws of path dependence and multicollinearity in stepwise regression, while also overcoming the limitations of the linear assumptions in LASSO regression. The synergistic application of these two methods established a robust system for controlling confounding factors in the exploration of the association between sedentary time and thyroid hormone sensitivity.
Among the numerous confounding factors involved in this study, BMI took the primary position, and BMI partially mediated the association between sedentary time and the PTFQI. This phenomenon may be attributed to several factors. Firstly, previous research has demonstrated an intrinsic link between thyroid hormone resistance and obesity, with this resistance being particularly pronounced in obese individuals (46). Secondly, BMI is closely associated with various metabolic parameters, including insulin resistance and dyslipidemia. Alterations in these metabolic parameters may potentially influence the mechanism of action of thyroid hormones (47). Thirdly, BMI may also indirectly affect thyroid hormone sensitivity by influencing the body's inflammatory state. Generally, obesity is often accompanied by chronic low-grade inflammation, and this inflammatory state is highly likely to alter the metabolic process and action effect of thyroid hormones (48).
The integrated examination of sedentary behavior and physical activity has been extensively utilized in the investigation of various chronic diseases (49, 50). The influence of physical activity on thyroid function is complex, with distinct effects observed between occupational physical activity and leisure-time exercise. Research indicates that occupational physical activity is correlated with a reduction in thyroid function and an increase in thyroid autoimmunity, whereas leisure-time exercise is associated with decreased TSH levels (51). Tian et al. (52) have demonstrated that the daily physical activity levels of American adults are significantly linked to modifications in thyroid function, including variations in thyroid hormone levels and the prevalence of thyroid disorders. Furthermore, the impact of exercise on thyroid function may also be mediated by myokines secreted by muscles (53).
In the investigation of the relationship between sedentary behavior and thyroid hormone sensitivity, a significant positive correlation was identified in males, whereas no significant association was detected in females. Despite both genders possessing the hypothalamic-pituitary-thyroid (HPT) axis to regulate thyroid hormone secretion, there are notable gender-specific differences in the regulatory mechanisms. For instance, during distinct physiological phases such as the menstrual cycle and pregnancy, women experience adaptive changes in the HPT axis (54). Studies indicate that thyroid hormone levels are associated with variations in the concentrations of sex hormone-binding globulin and albumin, which may manifest differently in males and females (55). Furthermore, disparities in lifestyle and environmental factors may also influence thyroid hormone sensitivity across genders (56, 57). The interaction effect of race on the relationship between sedentary time and PTFQI may be attributed to genetic variations within the thyroid hormone metabolic pathway, suggesting that genetic factors contribute to differences in thyroid hormone sensitivity among various racial groups (58).
In this study, we identified several key limitations that may impact the interpretation and generalizability of our findings. Firstly, the cross-sectional design of our study constrains our ability to establish a causal relationship between sedentary behavior and thyroid hormone sensitivity. For instance, individuals with hypothyroidism may exhibit a reduction in metabolic rate and sympathetic excitability, potentially influencing their sedentary behavior (59). Secondly, a significant and challenging source of bias arises from the possibility that participants reporting a sedentary lifestyle may decrease their physical activity due to undiagnosed or subclinical conditions (44). Lastly, the data on sedentary time were obtained through self-reported questionnaires, introducing a degree of subjectivity. Future research should consider employing more objective methods to measure sedentary time, thereby minimizing measurement errors and other biases associated with self-reported recall.
Conclusion
In this study, we found a positive association between impaired thyroid hormone sensitivity and sedentary time in men after adjusting for confounders, and BMI partially mediated this positive association. Additionally, the factor of race exhibited a significant interaction effect on the association between sedentary time and the PTFQI.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be foundaspx at: https://wwwn.cdc.gov/nchs/nhanes/Default.
Ethics statement
The studies involving humans were approved by the ethics committee review board for this study is the National Center for Health Statistics Research Ethics Review Board (NCHS Research Ethics Review Board, ERB). It is affiliated with the National Center for Health Statistics (NCHS), which is under the jurisdiction of the Centers for Disease Control and Prevention (CDC) of the United States. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HY: Methodology, Data curation, Investigation, Resources, Software, Writing – original draft. JK: Investigation, Conceptualization, Formal analysis, Supervision, Validation, Writing – review & editing. LD: Formal analysis, Investigation, Writing – original draft. ZL: Investigation, Writing – original draft, Conceptualization, Data curation. QL: Conceptualization, Data curation, Investigation, Writing – original draft, Methodology. BW: Methodology, Formal analysis, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Shanghai Jiao Tong University Interdisciplinary Research Fund in Medicine and Engineering (Grant number YG2023LC10).
Acknowledgments
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1596669/full#supplementary-material
References
1.
Owen N Healy GN Dempsey PC Salmon J Timperio A Clark BK et al . Sedentary behavior and public health: integrating the evidence and identifying potential solutions. Annu Rev Public Health. (2020) 41:265–87. 10.1146/annurev-publhealth-040119-094201
2.
Smith LP Ng SW Popkin BM . No time for the gym? Housework and other non-labor market time use patterns are associated with meeting physical activity recommendations among adults in full-time, sedentary jobs. Soc Sci Med. (2014) 120:126–34. 10.1016/j.socscimed.2014.09.010
3.
Ding D Mutrie N Bauman A Pratt M Hallal PRC Powell KE . Physical activity guidelines 2020: comprehensive and inclusive recommendations to activate populations. Lancet. (2020) 396:1780–2. 10.1016/S0140-6736(20)32229-7
4.
Lee JJ Pedley A Marqusee E Sutherland P Hoffmann U Massaro JM et al . Thyroid function and cardiovascular disease risk factors in euthyroid adults: a cross-sectional and longitudinal study. Clin Endocrinol. (2016) 85:932–41. 10.1111/cen.13124
5.
Jostel A Ryder WDJ Shalet SM . The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH Index. Clin Endocrinol. (2009) 71:529–34. 10.1111/j.1365-2265.2009.03534.x
6.
Laclaustra M Moreno-Franco B Lou-Bonafonte JM Mateo-Gallego R Casasnovas JA Guallar-Castillon P et al . Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care. (2019) 42:303–10. 10.2337/dc18-1410
7.
Yang S Lai S Wang Z Liu A Wang W Guan H . Thyroid feedback quantile-based index correlates strongly to renal function in euthyroid individuals. Ann Med. (2021) 53:1945–55. 10.1080/07853890.2021.1993324
8.
van der Spek AH Fliers E Boelen A . The classic pathways of thyroid hormone metabolism. Mol Cell Endocrinol. (2017) 458:29–38. 10.1016/j.mce.2017.01.025
9.
Wu Z Jiang Y Li P Wang Y Zhang H Li Z et al . Association of impaired sensitivity to thyroid hormones with hyperuricemia through obesity in the euthyroid population. J Transl Med. (2023) 21:436. 10.1186/s12967-023-04276-3
10.
Liu B Wang Z Fu J Guan H Lyu Z Wang W . Sensitivity to thyroid hormones and risk of prediabetes: a cross-sectional study. Front Endocrinol. (2021) 12:657114. 10.3389/fendo.2021.657114
11.
Kerr NR Booth FW . Contributions of physical inactivity and sedentary behavior to metabolic and endocrine diseases. Trends Endocrinol Metab. (2022) 33:817–27. 10.1016/j.tem.2022.09.002
12.
Dunstan DW Dogra S Carter SE Owen N . Sit less and move more for cardiovascular health: emerging insights and opportunities. Nat Rev Cardiol. (2021) 18:637–48. 10.1038/s41569-021-00547-y
13.
Young DR Hivert M-F Alhassan S Camhi SM Ferguson JF Katzmarzyk PT et al . Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation. (2016) 134:e262–279. 10.1161/CIR.0000000000000440
14.
Konyn P Ahmed A Kim D . Causes and risk profiles of mortality among individuals with nonalcoholic fatty liver disease. Clin Mol Hepatol. (2023) 29:S43–57. 10.3350/cmh.2022.0351
15.
Romero-Gómez M Zelber-Sagi S Trenell M . Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. (2017) 67:829–46. 10.1016/j.jhep.2017.05.016
16.
Oh W Cho M Jung SW Moon J-Y Lee S-H Hwang Y-C et al . High physical activity alleviates the adverse effect of higher sedentary time on the incidence of chronic kidney disease. J Cachexia Sarcopenia Muscle. (2023) 14:622–31. 10.1002/jcsm.13167
17.
Hara M Nishida Y Tanaka K Shimanoe C Koga K Furukawa T et al . Moderate-to-vigorous physical activity and sedentary behavior are independently associated with renal function: a cross-sectional study. J Epidemiol. (2023) 33:285–93. 10.2188/jea.JE20210155
18.
Kerr J Anderson C Lippman SM . Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. (2017) 18:e457–71. 10.1016/S1470-2045(17)30411-4
19.
Yahoo N Dudek M Knolle P Heikenwälder M . Role of immune responses in the development of NAFLD-associated liver cancer and prospects for therapeutic modulation. J Hepatol. (2023) 79:538–51. 10.1016/j.jhep.2023.02.033
20.
Zhang X Ha S Lau HC-H Yu J . Excess body weight: novel insights into its roles in obesity comorbidities. Semin Cancer Biol. (2023) 92:16–27. 10.1016/j.semcancer.2023.03.008
21.
Gabel L Macdonald HM Nettlefold L McKay HA . Physical activity, sedentary time, and bone strength from childhood to early adulthood: a mixed longitudinal HR-pQCT study. J Bone Miner Res. (2017) 32:1525–36. 10.1002/jbmr.3115
22.
Koedijk JB van Rijswijk J Oranje WA van den Bergh JP Bours SP Savelberg HH et al . Sedentary behaviour and bone health in children, adolescents and young adults: a systematic review. Osteoporos Int. (2017) 28:2507–19. 10.1007/s00198-017-4076-2
23.
Neeland IJ Lim S Tchernof A Gastaldelli A Rangaswami J Ndumele CE et al . Metabolic syndrome. Nat Rev Dis Primers. (2024) 10:77. 10.1038/s41572-024-00563-5
24.
Shi C Liu X Du Z Tian L . Impaired sensitivity to thyroid hormones is associated with the risk of diabetic nephropathy in euthyroid patients with type 1 diabetes mellitus. Diabetes Metab Syndr Obes. (2024) 17:611–8. 10.2147/DMSO.S449870
25.
Mehran L Delbari N Amouzegar A Hasheminia M Tohidi M Azizi F . Reduced sensitivity to thyroid hormone is associated with diabetes and hypertension. J Clin Endocrinol Metab. (2022) 107:167–76. 10.1210/clinem/dgab646
26.
Zhao X Sun J Xu X Xin S Zhang X . The effect of central and peripheral thyroid resistance indices on diabetic retinopathy: a study of hospitalized euthyroid patients with T2DM in China. Ann Med. (2023) 55:2249017. 10.1080/07853890.2023.2249017
27.
Lv F Cai X Li Y Zhang X Zhou X Han X et al . Sensitivity to thyroid hormone and risk of components of metabolic syndrome in a Chinese euthyroid population. J Diabetes. (2023) 15:900–10. 10.1111/1753-0407.13441
28.
Lu Y Wang J An Y Liu J Wang Y Wang G et al . Impaired sensitivity to thyroid hormones is associated with hyperuricemia in a Chinese euthyroid population. Front Endocrinol. (2023) 14:1132543. 10.3389/fendo.2023.1132543
29.
Sun Y Teng D Zhao L Shi X Li Y Shan Z et al . Impaired sensitivity to thyroid hormones is associated with hyperuricemia, obesity, and cardiovascular disease risk in subjects with subclinical hypothyroidism. Thyroid. (2022) 32:376–84. 10.1089/thy.2021.0500
30.
Chen S Huang W Zhou G Sun X Jin J Li Z . Association between sensitivity to thyroid hormone indices and bone mineral density in US males. Int J Endocrinol. (2022) 2022:2205616. 10.1155/2022/2205616
31.
Liu C Hua L Liu K Xin Z . Impaired sensitivity to thyroid hormone correlates to osteoporosis and fractures in euthyroid individuals. J Endocrinol Invest. (2023) 46:2017–29. 10.1007/s40618-023-02035-1
32.
Sun J Liu J Wu T Gu Z Zhang X . Sensitivity to thyroid hormone indices are associated with papillary thyroid carcinoma in Chinese patients with thyroid nodules. BMC Endocr Disord. (2023) 23:126. 10.1186/s12902-023-01381-8
33.
Muhanhali D Deng L Ai Z Ling Y . Impaired thyroid hormone sensitivity increases the risk of papillary thyroid cancer and cervical lymph node metastasis. Endocrine. (2023) 83:659–70. 10.1007/s12020-023-03508-2
34.
de Rezende LFM Rey-López JP Matsudo VKR do Carmo Luiz O . Sedentary behavior and health outcomes among older adults: a systematic review. BMC Public Health. (2014) 14:333. 10.1186/1471-2458-14-333
35.
Duntas LH . Thyroid function in aging: a discerning approach. Rejuvenation Res. (2018) 21:22–8. 10.1089/rej.2017.1991
36.
Yagi H Pohlenz J Hayashi Y Sakurai A Refetoff S . Resistance to thyroid hormone caused by two mutant thyroid hormone receptors beta, R243Q and R243W, with marked impairment of function that cannot be explained by altered in vitro 3,5,3′-triiodothyroinine binding affinity. J Clin Endocrinol Metab. (1997) 82:1608–14. 10.1210/jc.82.5.1608
37.
Cheng YJ Kanaya AM Araneta MRG Saydah SH Kahn HS Gregg EW et al . Prevalence of diabetes by race and ethnicity in the United States, 2011-2016. JAMA. (2019) 322:2389. 10.1001/jama.2019.19365
38.
Yamakawa H Kato TS Noh JY Yuasa S Kawamura A Fukuda K et al . Thyroid hormone plays an important role in cardiac function: from bench to bedside. Front Physiol. (2021) 12:606931. 10.3389/fphys.2021.606931
39.
Köhrle J . The colorful diversity of thyroid hormone metabolites. Eur Thyroid J. (2019) 8:115–29. 10.1159/000497141
40.
Tremblay MS Aubert S Barnes JD Saunders TJ Carson V Latimer-Cheung AE et al . Sedentary behavior research network (SBRN) - terminology consensus project process and outcome. Int J Behav Nutr Phys Act. (2017) 14:75. 10.1186/s12966-017-0525-8
41.
Wu J Zhang H Yang L Shao J Chen D Cui N et al . Sedentary time and the risk of metabolic syndrome: a systematic review and dose-response meta-analysis. Obes Rev. (2022) 23:e13510. 10.1111/obr.13510
42.
Zhou Q Zhang LY Dai MF Li Z Zou CC Liu H . Thyroid-stimulating hormone induces insulin resistance in adipocytes via endoplasmic reticulum stress. Endocr Connect. (2024) 13:e230302. 10.1530/EC-23-0302
43.
León-Latre M Moreno-Franco B Andrés-Esteban EM Ledesma M Laclaustra M Alcalde V et al . Sedentary lifestyle and its relation to cardiovascular risk factors, insulin resistance and inflammatory profile. Rev Esp Cardiol. (2014) 67:449–55. 10.1016/j.rec.2013.10.015
44.
Zhang C Han Y Gao X Teng W Shan Z . Thyroid function, physical activity and sedentary behaviour: a bidirectional two-sample Mendelian randomisation study. J Glob Health. (2024) 14:04154. 10.7189/jogh.14.04154
45.
Cao Z Xu C Zhang P Wang Y . Associations of sedentary time and physical activity with adverse health conditions: outcome-wide analyses using isotemporal substitution model. EClinicalMedicine. (2022) 48:101424. 10.1016/j.eclinm.2022.101424
46.
Wang Z Yu H Wang K Han J Song Y . Association between thyroid hormone resistance and obesity: a cross-sectional study and mouse stimulation test. Obesity. (2024) 32:1483–93. 10.1002/oby.24084
47.
Roef GL Rietzschel ER Van Daele CM Taes YE De Buyzere ML Gillebert TC et al . Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid. (2014) 24:223–31. 10.1089/thy.2013.0314
48.
Ferrannini E Iervasi G Cobb J Ndreu R Nannipieri M . Insulin resistance and normal thyroid hormone levels: prospective study and metabolomic analysis. Am J Physiol Endocrinol Metab. (2017) 312:E429–36. 10.1152/ajpendo.00464.2016
49.
Chen J Ruan X Fu T Lu S Gill D He Z et al . Sedentary lifestyle, physical activity, and gastrointestinal diseases: evidence from Mendelian randomization analysis. EBioMedicine. (2024) 103:105110. 10.1016/j.ebiom.2024.105110
50.
Lavie CJ Ozemek C Carbone S Katzmarzyk PT Blair SN . Sedentary behavior, exercise, and cardiovascular health. Circ Res. (2019) 124:799–815. 10.1161/CIRCRESAHA.118.312669
51.
Vuletić M Kaličanin D Barić ŽiŽić A Cvek M Sladić S Škrabić V et al . Occupational physical activity and regular exercise are inversely correlated with thyroid function in patients with Hashimoto's thyroiditis. Diseases. (2024) 12:281. 10.3390/diseases12110281
52.
Tian L Lu C Teng W . Association between physical activity and thyroid function in American adults: a survey from the NHANES database. BMC Public Health. (2024) 24:1277. 10.1186/s12889-024-18768-4
53.
Samy DM Ismail CA Nassra RA . Circulating irisin concentrations in rat models of thyroid dysfunction – effect of exercise. Metabolism. (2015) 64:804–13. 10.1016/j.metabol.2015.01.001
54.
Brown EDL Obeng-Gyasi B Hall JE Shekhar S . The thyroid hormone axis and female reproduction. Int J Mol Sci. (2023) 24:9815. 10.3390/ijms24129815
55.
Guay AT Traish AM Hislop-Chestnut DT Doros G Gawoski JM . Are there variances of calculated free testosterone attributed to variations in albumin and sex hormone-binding globulin concentrations in men?Endocr Pract. (2013) 19:236–42. 10.4158/EP12113.OR
56.
Persky V Piorkowski J Turyk M Freels S Chatterton R Dimos J et al . Associations of polychlorinated biphenyl exposure and endogenous hormones with diabetes in post-menopausal women previously employed at a capacitor manufacturing plant. Environ Res. (2011) 111:817–24. 10.1016/j.envres.2011.05.012
57.
Lee YK Shin DY Shin H Lee EJ . Sex-specific genetic influence on thyroid-stimulating hormone and free thyroxine levels, and interactions between measurements: KNHANES 2013-2015. PLoS ONE. (2018) 13:e0207446. 10.1371/journal.pone.0207446
58.
Vlaeminck-Guillem V Espiard S Flamant F Wémeau J-L . TRα receptor mutations extend the spectrum of syndromes of reduced sensitivity to thyroid hormone. Presse Med. (2015) 44:1103–12. 10.1016/j.lpm.2015.07.022
59.
Ragusa F Fallahi P Elia G Gonnella D Paparo SR Giusti C et al . Hashimotos' thyroiditis: epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab. (2019) 33:101367. 10.1016/j.beem.2019.101367
Summary
Keywords
NHANES, sedentary, PTFQI, sensitivity to thyroid hormones, thyroid hormone
Citation
Yang H, Kang J, Dong L, Lin Z, Lin Q and Wu B (2025) Association between impaired sensitivity to thyroid hormones and sedentary behavior: a cross-sectional study. Front. Med. 12:1596669. doi: 10.3389/fmed.2025.1596669
Received
20 March 2025
Accepted
15 May 2025
Published
06 June 2025
Volume
12 - 2025
Edited by
Chenyu Sun, The Second Affiliated Hospital of Anhui Medical University, China
Reviewed by
Maoming Xiong, First Affiliated Hospital of Anhui Medical University, China
Bo Xu, Guangzhou First People's Hospital, China
Rui Pang, Harbin Medical University Cancer Hospital, China
Updates
Copyright
© 2025 Yang, Kang, Dong, Lin, Lin and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Wu wubodr@sjtu.edu.cn
†These authors have contributed equally to this work
‡ORCID: Bo Wu orcid.org/0000-0002-9209-5777
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.