Abstract
Background:
Both domestic and foreign guidelines recommend that chronic hepatitis B virus (HBV) infection pregnant women in the immune tolerance phase (ITP) discontinue antiviral therapy after delivery, this study aimed to investigate the risk of postpartum drug withdrawal and the pros and cons of continuing treatment of tenofovir dipivoxil fumarate (TDF) in ITP pregnant women.
Methods:
The study group consisted of 116 naive pregnant women in ITP, the control group included 81 naive chronic hepatitis B (CHB) pregnant women with HBeAg-positive and high viral load. The study aimed to compare the risk of discontinue rebound within 48 weeks postpartum, the antiviral efficacy and bone and renal safety of continuing TDF treatment until 144 weeks postpartum between the two groups.
Results:
There was no significant difference in the reduction of HBV DNA levels between the ITP group and the CHB group prior to labor (p < 0.05), with a 100% success rate in mother-to-child transmission prevention for both cohorts. Postpartum, 38.8% (45/116) and 18.5% (15/81) of parturients in the ITP group and CHB group, respectively, discontinued TDF at various time intervals. Comparative analysis of the risk of viral rebound within 48 weeks postpartum revealed no significant difference between the two groups (p > 0.05). The ITP group had higher rates of suboptimal response and low viremia occurrence compared to the CHB group (p < 0.05) at 48 weeks postpartum, but following salvage therapy up to 144 weeks postpartum, the cumulative rate of complete virological response(CVR) in the ITP group was non-inferior to that in the CHB group (p > 0.05). There were no significant differences in the average eGFR and serum phosphorus levels between the two groups from baseline to 144 weeks after TDF treatment.
Conclusion:
Postpartum discontinuation of TDF poses significant risks for immunotolerant pregnant women, whereas continuing TDF treatment for 144 weeks postpartum demonstrates favorable antiviral efficacy, bone and renal safety profiles.
Introduction
Hepatitis B virus infection is a significant global public health issue, with mother-to-child transmission (MTCT) being a major route of transmission in China (1), antiviral prophylaxis during pregnancy in HBV-infected women is beneficial for disease control, prevention of vertical transmission, and ensuring maternal and neonatal safety (2), these efforts play a crucial role in achieving the World Health Organization’s global health sector strategy goal of eliminating viral hepatitis by 2030 (3). Currently, there is consensus on using pregnancy category B nucleotide analogues (NAs) for antiviral therapy in immunotolerant pregnant women with high viral loads. However, it is commonly believed that discontinuing therapy postpartum in immunotolerant mothers does not result in significant harm, and continuing therapy postpartum offers limited efficacy and increases the risk of resistance (4, 5). Therefore, guidelines both domestically and internationally suggest that immunotolerant pregnant women can discontinue therapy postpartum. However, in the current trend of expanding antiviral therapy for chronic HBV infection, there is a redefinition of indications for antiviral treatment in immunotolerant patients with normal ALT levels and high viral loads, especially those over 30 years old or with a family history of cirrhosis or hepatocellular carcinoma. This contradicts recommendations for postpartum therapy discontinuation in guidelines. Our research team believes it is necessary to assess the risks of postpartum discontinuation and the efficacy and safety of continuing treatment postpartum in immunotolerant pregnant women, to provide scientific evidence for clinical treatment decisions.
Materials and methods
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, with ethics approval number No. 2022-KY-(061). To ensure adherence to ethical standards, all research protocols were conducted in accordance with the guidelines outlined in the 1975 Helsinki Declaration, which was revised in 2013. Written informed consent was obtained from all participating patients.
Study patients
This study is a retrospective study enrolled a cohort of pregnant women with chronic HBV infection receiving TDF therapy, recruited from the outpatient clinic of the Department of Infectious Diseases, First Affiliated Hospital of Guangxi Medical University from January 2012 to December 2022. Inclusion criteria were as follows: (1) ITP group: HBsAg-positive, HBeAg-positive, HBV DNA > 2 × 107 IU/ml, and ALT within normal limits; (2) CHB group: HBsAg-positive, HBeAg-positive, HBV DNA > 2 × 105 IU/ml, with persistent or intermittent ALT elevation (> 50 U/L); (3) Initiation of TDF for antiviral prophylaxis or treatment during pregnancy, with follow-up extended to 48 weeks postpartum; (4) Continued follow-up to 144 weeks postpartum if complete virological response was not achieved. Exclusion criteria included: (1) Pregnant women previously treated with TDF or other NAs (e.g., LdT, TAF, ETV); (2) Pregnancy complications or organic damage other than HBV infection; (3) Pregnant women with concurrent viral infections other than HBV; (4) Pregnant women with chronic liver diseases such as alcoholic liver disease or cirrhosis. Patients in the ITP discontinuation group voluntarily ceased TDF therapy for more than 4 weeks postpartum within 0–12 weeks after delivery, in accordance with guidelines for CHB. Patients in the CHB discontinuation group were those who discontinued TDF therapy for more than 4 weeks within the same postpartum period without medical advice.
Study design
Patients in both groups initiated antiviral therapy during pregnancy. They took 300 mg of TDF daily, medication compliance was monitored through regular tablet counting, with monitoring conducted every 3 months for HBV DNA, HBV serological markers, liver and kidney function, blood phosphate, and AFP levels, and every 6 months with liver ultrasound examinations until 48 weeks postpartum. Follow-up was continued until 144 weeks postpartum for those who did not achieve complete virological response. Newborns received immunoglobulin injection at birth along with full hepatitis B vaccination, and were followed up for HBsAg testing at 7–12 months of age to assess MTCT prevention. The study roadmap is illustrated in Figure 1.
FIGURE 1
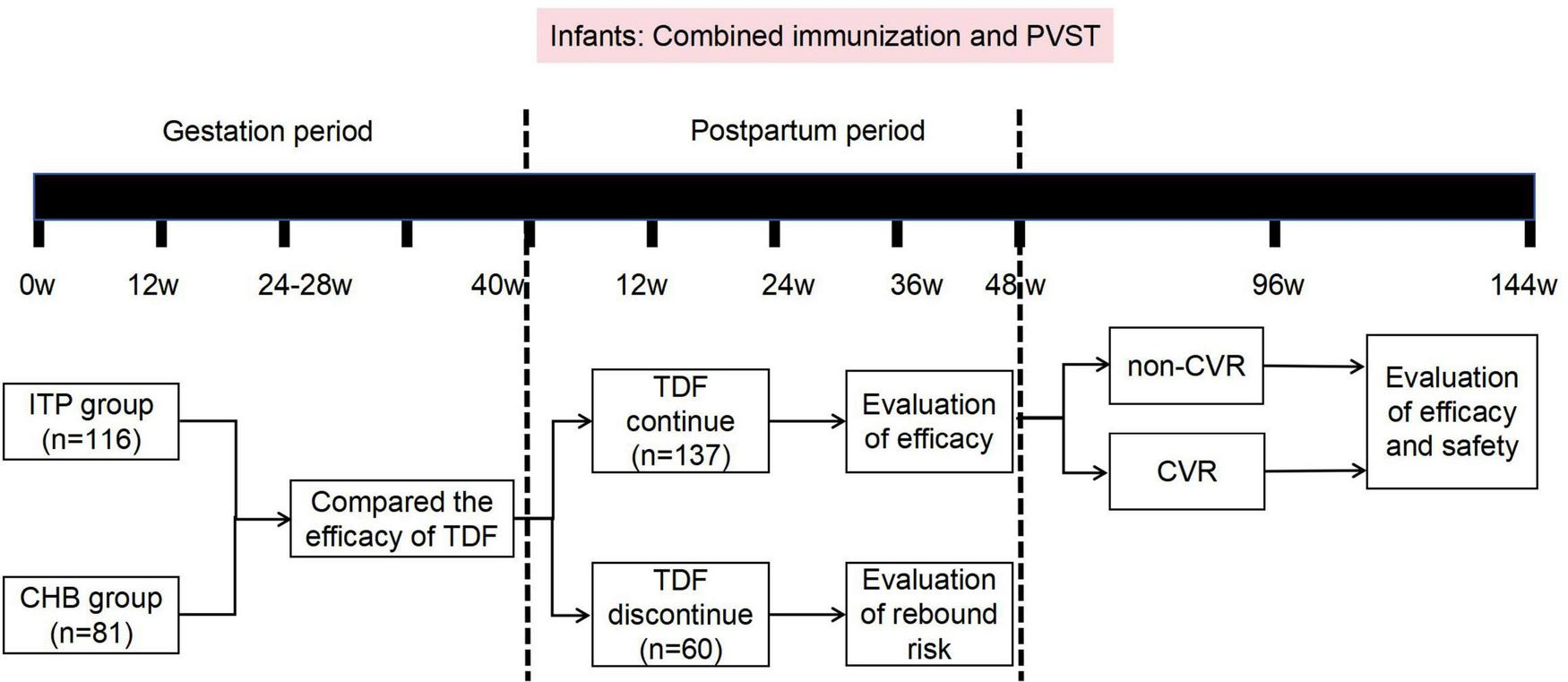
Research flowchart. ITP, immune tolerence phase; CHB, chronic hepatitis B; TDF, tenofovir dipivoxil fumarate; PVST, postpartum virological serological testing.
Statistical analysis
Data analysis was performed using SPSS26.0 statistical software (IBM, Armonk, NY, United States), and the pictures were plotted by GraphPad Prism8.0 software. For normally distributed metric data, mean ± standard deviation ( ± s) was used, while skewed metric data was expressed as median (M) (P25∼P75) or M (IQR). For inter-group comparisons of continuous variables that met normality and variance homogeneity assumptions, independent sample t-tests were employed. In case of heteroscedasticity, a t-test was used. One-way analysis of variance (ANOVA) and Kruskal-Wallis tests were used for inter-group comparisons, while R*C cross-table chi-squre test (χ2)was used for inter-group comparisons of unordered categorical variables. Statistical significance was set at p < 0.05. Comparisons of cumulative CVR rates between groups were performed using the Kaplan-Meier method and assessed with the Log-rank test. Additionally, the hazard ratio (HR) and its 95% confidence interval (CI) were calculated to quantify the risk difference in achieving CVR between the CHB and ITP groups.
Results
General characteristics of the study subjects
This study enrolled a total of 197 pregnant women with chronic HBV infection initiating antiviral therapy with TDF during pregnancy. The study group comprised 116 immunotolerant pregnant women, among whom 79.3% (92/116) were aged over 30 years or had a family history of hepatitis B-related liver cirrhosis or hepatocellular carcinoma. The control group included 81 pregnant women with HBeAg-positive and high viral load chronic hepatitis B. General patient characteristics are summarized in Table 1.
TABLE 1
| Observational content | ITP group (n = 116) |
CHB group (n = 81) |
|---|---|---|
| Gestational age (years), ( s) | 30.03 ± 4.25 | 30.09 ± 3.40 |
| Family history of hepatitis B (%) | 54.3% (63/116) | 60.5% (49/81) |
| Family history of cirrhosis and liver cancer (%) | 11.2% (13/116) | 17.3% (14/81) |
| Antiviral gestational age (weeks), ( s) | 25.51 ± 2.89 | 22.33 ± 6.47 |
| Baseline HBVDNA (log10 IU/ml), (s) | 8.08 ± 0.39 | 7.48 ± 1.03 |
| Baseline ALT (U/L), M (P25∼P75) | 17 (12∼23) | 50 (25∼121) |
| Course of TDF treatment until parturient (weeks), M (P25∼P75) | 14.71 (12.00∼15.79) | 15.07 (11.67∼19.03) |
| Postpartum withdrawal cases (n, %) | 45 (38.8%) | 15 (18.5%) |
| Treated until 48 weeks postpartum cases (n, %) | 71 (61.2%) | 66 (81.5%) |
The general information of chronic hepatitis B virus (HBV) infection in pregnant women.
Risk study of TDF discontinuation postpartum in immunotolerant patients
Following delivery, 38.8% (45/116) of immunotolerant patients and 18.5% (15/81) of chronic hepatitis B patients discontinued TDF at various time points. Subsequent follow-up continued until 48 weeks postpartum to compare virological and biochemical rebound rates (Figure 2a), rebound timing (Figure 2b), and rebound magnitude (Figure 2c) between the two groups, there was no significant difference in the risk of TDF discontinuation between the two groups of mothers.
FIGURE 2
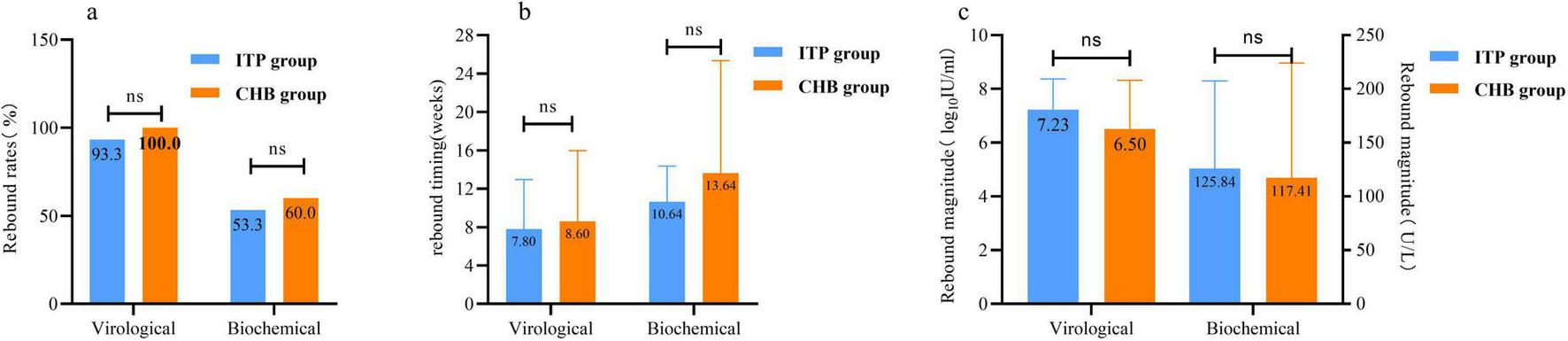
Comparison of the risk of rebound after tenofovir dipivoxil fumarate (TDF) withdrawal in 48 weeks postpartum between immune tolerence phase (ITP) group and chronic hepatitis B (CHB) group. (a) The comparison of withdrawal rebound rates. (b) The comparison of drug withdrawal rebound in time. (c) The comparison of drug withdrawal rebound level. Virological rebound: patients who discontinue TDF treatment, with hepatitis B virus (HBV) DNA levels rising by > 1 log10 IU/ml from the lowest point during treatment or at cessation, or converting from negative to positive and confirmed by repeat testing with the same assay 1 month later, with or without alanine aminotransferase (ALT) elevation. Biochemical rebound: patients on TDF therapy or after cessation, where ALT levels, having previously been below the limit of detection (9–50 U/L), rise again without other causes for ALT elevation, with or without HBV DNA increase. ns p > 0.05.
Further subgroup analysis of postpartum withdrawal among women in the ITP group (n = 45) revealed no significant differences in rebound risk between immediate withdrawal and withdrawal at 4–12 weeks postpartum, or between women aged ≤ 30 and > 30 years (p > 0.05),the results are shown in Table 2.
TABLE 2
| Observational content | Grouping by postpartum cessation time | Grouping by age at cessation | ||||
|---|---|---|---|---|---|---|
| 0-week group (n = 40) | 4–12-week group (n = 5) | P-value | ≤30 years group (n = 24) | >30 years group (n = 21) | P-value | |
| Virological rebound rate (n, %) | 87.5 (35/40) | 100.0 (5/5) | 0.539 | 91.7 (22/24) | 95.2 (20/21) | 0.551 |
| Virological rebound time (weeks), M (IQR) | 6.0 (8.0) | 6.0 (10.0) | 0.881 | 8.0 (8.0) | 4.0 (5.0) | 0.182 |
| Virological rebound level (log10 IU/ml), M (IQR) | 8.0 (1.0) | 8.0 (2.5) | 0.916 | 8.0 (1.0) | 8.0 (1.0) | 0.685 |
| Biochemical rebound rate (n, %) | 55.0 (22/40) | 40.0 (2/5) | 0.435 | 58.3 (14/24) | 52.4 (11/21) | 0.460 |
| Biochemical rebound time (weeks), M (IQR) | 3.5 (9.5) | 12.0 (4.0) | 0.392 | 4.0 (9.0) | 4.0 (10.0) | 0.481 |
| Biochemical rebound ALT level (U/L), M (IQR) | 86.5 (86.5) | 60.5 (7.0) | 0.195 | 87.0 (84.0) | 67.0 (96.0) | 0.289 |
The risk of postpartum discontinuation of tenofovir dipivoxil fumarate (TDF) in immunentolerant women.
Study on the benefits of continued TDF therapy in immune tolerant period patients postpartum
In pregnant women treated with TDF until peripartum, the average duration of antiviral therapy was shorter in the ITP group compared to the CHB group (p < 0.05). Prior to delivery, 81.0% (94/116) of pregnant women in the ITP group and 91.4% (74/81) in the CHB group achieved viral load reduction to low levels (HBV DNA ≤ 2 × 105 IU/ml). There was no significant difference in the extent of viral load reduction between the two groups prior to delivery [(3.81 ± 0.89) vs. (3.88 ± 1.29) log10 IU/ml, p > 0.05]. Both groups achieved a 100% rate of preventing MTCT. At 48 weeks postpartum with TDF treatment, both groups showed higher virological response rates, HBeAg seroconversion rates, and normalization of ALT compared to pre-delivery levels. The postpartum virological response rate in the ITP group was lower than that in the CHB group, with no statistically significant difference observed in HBeAg seroconversion rates between the two groups at 48 weeks postpartum. Detailed results are presented in Figure 3.
FIGURE 3
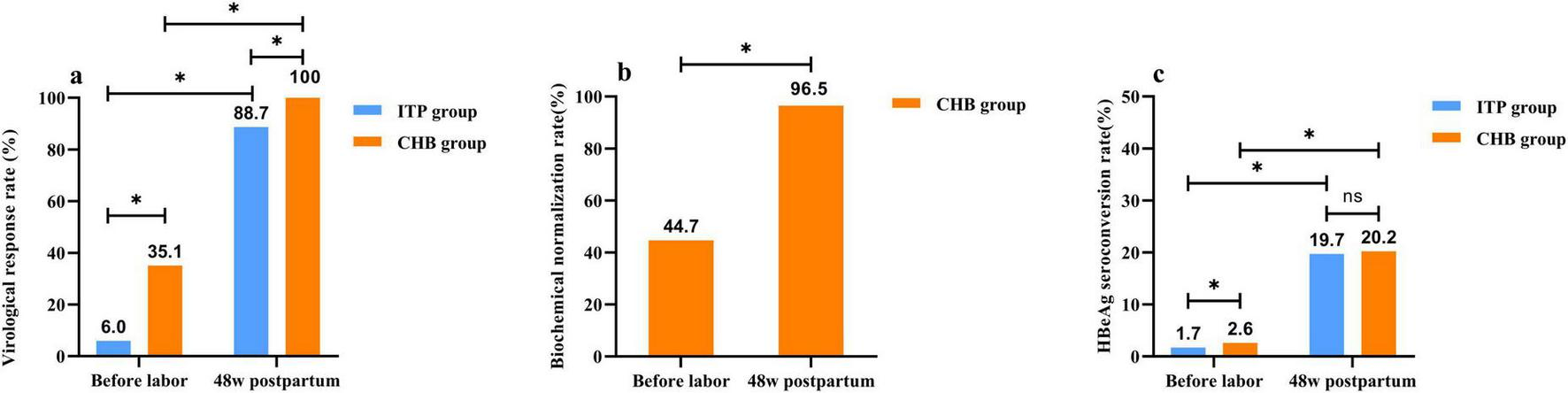
Two groups of patients with tenofovir dipivoxil fumarate (TDF) treatment to the curative effect of postpartum 48 weeks compared with before delivery. (a) The comparism of virological response rate, virological response was defined as achieving hepatitis B virus (HBV) DNA < 100 IU/ml after receiving TDF with good adherence, continuing treatment for 48 weeks or longer postpartum. (b) The comparism of biochemical normalization rate, biochemical normalization referred to the decrease in elevated alanine aminotransferase (ALT) levels to the detection limit after treatment initiation or during the course of TDF therapy. (c) The comparism of HBeAg seroconversion rate, HBeAg seroconversion denoted the disappearance of HBeAg in previously HBeAg-positive patients, accompanied by the appearance of HBeAb.*p < 0.05.ns p > 0.05.
Safety of postpartum TDF treatment in immunotolerant patients
At 48 weeks postpartum, no cases of virological breakthrough were observed in either group under TDF treatment. The ITP group exhibited lower virological response rates compared to the CHB group, with higher rates of suboptimal response and low viremia occurrence (Table 3). Patients with suboptimal response and low-level viremia in both groups were subjected to salvage therapy with TDF combined with entecavir (ETV) or switched to Tenofovir Amibufenamide (TMF). Survival analysis for CVR demonstrated that the CHB group had a significantly higher probability of achieving CVR compared to the ITP group [Hazard Ratio (HR) = 1.846, 95% CI: 1.292–2.693; Log-rank p < 0.001]. By 144 weeks postpartum, the time to achieve complete virological response (CVR) was later in the ITP group compared to the CHB group (Figure 4a). The cumulative rate of complete virological response (HBV DNA < 20 IU/mL) was lower in the ITP group [92.0% (46/50) vs. 98.3% (59/60), p > 0.05] than in the CHB group. However, with extended treatment duration, the cumulative complete virological response rate gradually increased and approached equivalence.
TABLE 3
| Observational content | ITP group (n = 71) |
CHB group (n = 66) |
P-value |
|---|---|---|---|
| Virological breakthrough rate (%) | 0.0% (0/71) | 0.0% (0/66) | – |
| Suboptimal response rate (%) | 18.3% (13/71) | 1.5% (1/66) | 0.000 |
| Virological response rate (%) | 81.7% (58/71) | 98.5% (65/66) | 0.010 |
| Low viremia rate (%) | 32.7% (16/49) | 4.5% (3/66) | 0.000 |
Comparison of virological response of tenofovir dipivoxil fumarate (TDF) treatment to 48 weeks postpartum.
Virological response: tenofovir dipivoxil fumarate (TDF) treatment showed good compliance, achieving hepatitis B virus (HBV) DNA < 100 IU/ml by 48 weeks postpartum. Virological breakthrough: under good compliance with TDF treatment, virological breakthrough was defined as an increase in HBV DNA levels > 1 log10 IU/ml from the lowest recorded level during treatment without treatment modification, or reversion from negative to positive HBV DNA, confirmed by repeat testing with the same assay 1 month later, with or without alanine aminotransferase (ALT) elevation. Suboptimal response: good compliance with TDF treatment resulted in HBV DNA > 100 IU/mL at 48 weeks postpartum. Low viremia: good compliance with TDF treatment resulted in HBV DNA levels between 20 and 100 IU/mL at 48 weeks postpartum and beyond.
FIGURE 4
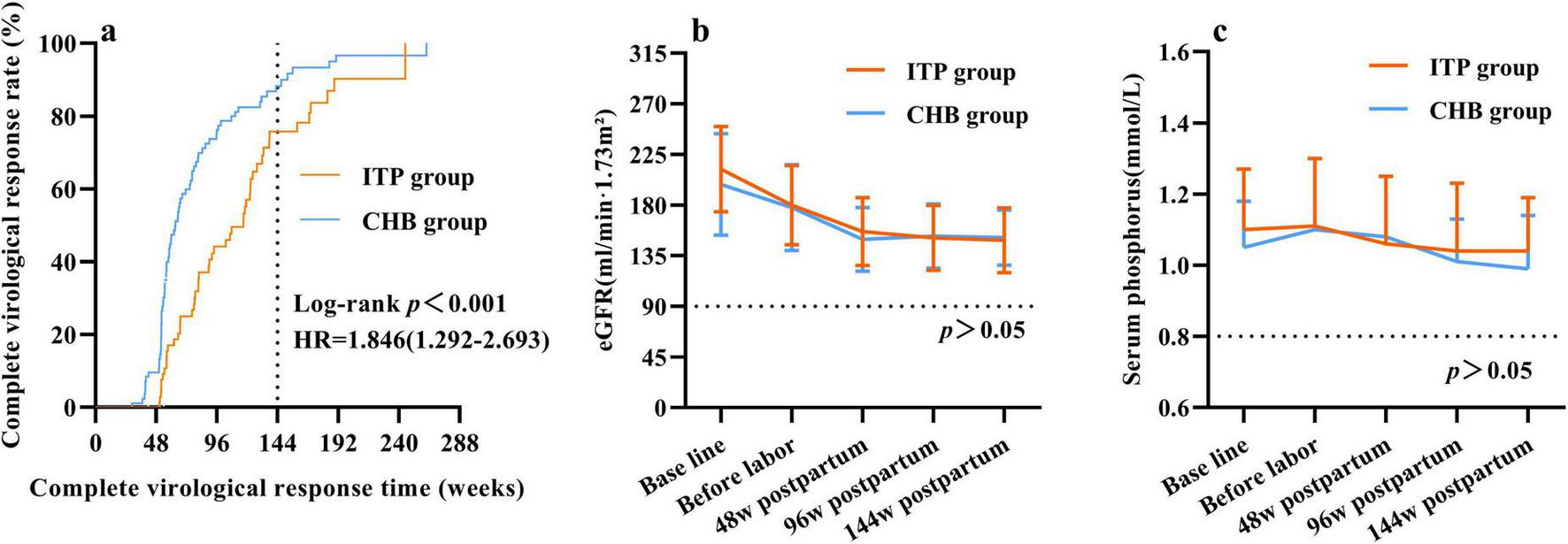
Safety study of postpartum continuation of tenofovir dipivoxil fumarate (TDF) up to 144 weeks in two groups. (a) Cumulative complete virologic response rate. “Time” was defined as the number of weeks from the initiation of TDF antiviral therapy until the achievement of a complete virological response. Data for subjects who did not achieve complete virological response (CVR) by the end of the study period were censored at their last follow-up visit. (b) Changes in eGFR levels. (c) Changes in serum phosphorus level.
There was no significant difference observed in the decline trend of eGFR levels between the groups, with both showing a decline in eGFR levels compared to baseline at 48 weeks postpartum (p < 0.05). Further follow-up at 96 and 144 weeks postpartum indicated no significant decline in eGFR levels compared to those at 48 weeks postpartum (Figure 4b). Comparison of serum phosphate levels between the ITP and CHB groups showed no significant differences in decline trends. At 48, 96, and 144 weeks postpartum, serum phosphate levels did not differ significantly from baseline levels (Figure 4c).
Discussion
High viral load of HBV is an independent risk factor for disease progression to liver cirrhosis, liver failure, and hepatocellular carcinoma in chronic HBV-infected patients (6), and remains the predominant risk factor for HBV MTCT (7). The population of immunotolerant patients is substantial (8), with those harboring high HBV viral loads being a priority group for MTCT prevention strategies (9). This study demonstrates that antiviral therapy with TDF during pregnancy significantly reduces maternal HBV DNA levels in both immunotolerant and chronic HBV-infected pregnant women with high viral loads, effectively achieving the goal of preventing MTCT. Clinically, a significant proportion of immunotolerant patients experience an indeterminate phase (10), with liver histopathology indicating progression (11, 12). Multiple studies indicate that chronic HBV-infected individuals with normal alanine aminotransferase (ALT) levels remain at risk of disease progression (13, 14). Immunotolerant patients who do not receive antiviral therapy face significantly higher risks of hepatocellular carcinoma (HCC), mortality, or transplantation compared to those regularly treated with NAs following HBV activity suppression (15, 16), underscoring the necessity of antiviral therapy in this patient population. This study confirms that postpartum continuation of TDF treatment among immunotolerant mothers achieves favorable antiviral efficacy, consistent with findings by Feng et al. (17).
However, guidelines both domestically and internationally generally assert that immunotolerant patients of HBV show no hepatic activity and thus do not meet indications for antiviral therapy. Furthermore, antiviral efficacy in these patients is inferior to that in those with chronic hepatitis B, with long-term medication increasing the risk of resistance. Advocating for discontinuation of therapy postpartum in immune-tolerant phase mothers has been proposed (7, 18-20). A prospective study of 330 pregnant women in a Chinese cohort showed that stopping tenofovir immediately at delivery did not increase the risk of virological relapse and retreatment compared with a longer duration of tenofovir treatment, suggesting that shortening the duration of peripartum antiviral prophylaxis from 12 weeks to immediately after delivery can be considered (4). However, all the subjects in this study were HBsag-positive pregnant women, and no comparison between immune tolerance and chronic hepatitis B groups was performed. Our study reveals that the risk of rebound after discontinuation is comparable between immunotolerant patients and those with CHB. For immunotolerant patients, regardless of immediate postpartum cessation or cessation at 4–12 weeks, and whether age at cessation is ≤ 30 years, the risk of rebound did not statistically differ, consistent with findings by Zhang et al. (21). This suggests that discontinuation of therapy postpartum in immune-tolerant phase mothers, even those who received antiviral prophylaxis during pregnancy to prevent vertical transmission, is unsafe.
Moreover, to achieve the goal of eliminating viral hepatitis as a public health threat by 2030, the treatment paradigm for chronic hepatitis B is shifting from “Treat only if…” to “Treat all…” (22-25). In our study, 79.3% of immune-tolerant phase patients belonged to high viral load, age over 30 years, or had a history of cirrhosis or liver cancer in the family, all of whom are also eligible for expanded antiviral therapy and thus should not discontinue therapy postpartum.
While the efficacy of TDF antiviral therapy in pregnant and postpartum women with chronic HBV infection has been well-established, concerns remain regarding its long-term safety on bone and kidney health, as well as the risk of virological breakthroughs, necessitating further clinical data. Our study results demonstrate good bone and kidney safety of TDF treatment up to 144 weeks postpartum, consistent with findings by Pietro et al. (26, 27). No cases of virological breakthrough were observed during the 48-week postpartum follow-up; however, there were instances of suboptimal response and low viremia patients, adjusting the treatment regimen to extend antiviral therapy duration resulted in virological response, highlighting the potential benefits of prolonged antiviral therapy in immune-tolerant phase patients. The survival analysis in our study dynamically illustrated the kinetics of CVR achievement in pregnant women with different clinical phases of HBV infection. We found that women in the CHB group had a significantly higher hazard of achieving CVR and a shorter median time to CVR compared to those in the ITP group. This result strongly suggests that for pregnant women in the immune-tolerant phase (ITP), who typically present with exceptionally high baseline viral loads, the virological response may be comparatively slower. Therefore, clinicians should reinforce patient education and adherence monitoring for ITP women, emphasizing the necessity and importance of persisting with the full course of TDF therapy and managing expectations regarding the rate of virological response. Our data provide a rationale for adopting a more patient and persistent management strategy for this specific subgroup of pregnant women.
However, this study was limited to pregnant women who received TDF treatment in the infectious disease outpatient department of our hospital, resulting in certain sample selection limitations and a restricted sample size. Incomplete clinical data were also noted. Additionally, due to factors such as regional disparities, the generalizability of our findings to national and international populations may be subject to bias. Further prospective, multicenter, large-sample studies are required to validate these results and evaluate long-term outcomes, thereby providing more robust data to guide antiviral therapy recommendations for pregnant women in the immune-tolerant phase.
In conclusion, postpartum discontinuation of TDF therapy poses a high risk of rebound in immunotolerant pregnant women. Continuing TDF therapy postpartum up to 144 weeks demonstrates both efficacy and safety. Immunotolerant pregnant women should therefore receive long-term antiviral management consistent with HBV-infected populations to reduce mother-to-child transmission rates. Postpartum continuation of antiviral therapy without cessation provides sustained virological suppression, reduces the risk of disease progression, and ultimately benefits patients.
Conclusion
Postpartum discontinuation of TDF poses significant risks for immunotolerant pregnant women, whereas continuing TDF treatment for 144 weeks postpartum demonstrates favorable antiviral efficacy, bone and renal safety profiles.
Statements
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HL: Writing – original draft, Data curation, Methodology, Investigation, Project administration, Writing – review & editing, Formal analysis. HkL: Software, Writing – original draft, Formal analysis, Data curation, Validation. BH: Data curation, Conceptualization, Writing – original draft, Software, Investigation. LH: Writing – original draft, Formal analysis, Validation, Data curation, Supervision. HqL: Writing – original draft, Project administration, Data curation, Visualization, Validation. MS: Supervision, Formal analysis, Writing – original draft, Validation, Software. RW: Methodology, Writing – original draft, Formal analysis. TS: Validation, Software, Investigation, Writing – original draft. QL: Methodology, Software, Formal analysis, Writing – original draft. YF: Validation, Writing – original draft, Data curation, Software, Methodology. JJ: Writing – review & editing, Formal analysis, Investigation, Writing – original draft, Validation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by grants from National Natural Science Foundation of China (Nos. 81960115, 82160123, 82260124), Guangxi Science and Technology Program under Grant (No. AD 25069077), the Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor (Guangxi Medical University), Ministry of Education (No. GKE-ZZ202107), and Guangxi Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor (No. GKE-ZZ202218).
Acknowledgments
We express their gratitude to the patients and their families for their valuable contribution to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HBV, chronic hepatitis B virus; ITP, immune tolerance phase; TDF, tenofovir dipivoxil fumarate; CHB, chronic hepatitis B; CVR, complete virological response; MTCT, mother-to-child transmission; Nas, nucleotide analogues; TMF, Tenofovir Amibufenamide; CVR, complete virological response.
References
1.
Tagkou N Kondylis G Cholongitas E . Pregnancy and viral hepatitis: current concepts.Curr Pharm Des. (2021) 27:3775–85. 10.2174/1381612826666201210113841
2.
Liu J Liu J Liu M Pang Q Wen Y . Prevalence of hepatitis B virus infection and its associated factors among 15,461 pregnant women in Yunnan province, China.Ann Epidemiol. (2020) 49:13–9. 10.1016/j.annepidem.2020.05.011
3.
World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016-2021. Towards Ending Viral Hepatitis. Geneva: World Health Organization (2016).
4.
Chen Y Mak L Tang M Yang J Chow C Tan A et al Immediate postpartum cessation of tenofovir did not increase risk of virological or clinical relapse in highly viremic pregnant mothers with chronic hepatitis B infection. JHEP Rep. (2024) 6:101050. 10.1016/j.jhepr.2024.101050
5.
Li M Sun F Bi X Lin Y Yang L Jiang T et al Effects of antiviral therapy and drug withdrawal on postpartum hepatitis in pregnant women with chronic HBV infection. Hepatol Int. (2023) 17:42–51. 10.1007/s12072-022-10412-w
6.
Rizzo G Cabibbo G Craxì A . Hepatitis B virus-associated hepatocellular carcinoma.Viruses. (2022) 14:986. 10.3390/v14050986
7.
Obstetrics group CSoOaG. [Clinical guidelines on prevention of mother-to-child transmission of hepatitis B virus (2020)]. Zhonghua Fu Chan Ke Za Zhi. (2020) 55:291–9. 10.3760/cma.j.cn112141-20200213-00101
8.
Razavi H . Polaris Observatory-supporting informed decision-making at the national, regional, and global levels to eliminate viral hepatitis.Antivir Ther. (2022) 27:13596535221083179. 10.1177/13596535221083179
9.
Lee L Lee G Mattar C Saw S Aw M . Maternal HBeAg positivity and viremia associated with umbilical cord blood hepatitis B viremia.Pediatr Neonatol. (2019) 60:517–22. 10.1016/j.pedneo.2019.01.002
10.
Yao K Liu J Wang J Yan X Xia J Yang Y et al Distribution and clinical characteristics of patients with chronic hepatitis B virus infection in the grey zone. J Viral Hepat. (2021) 28:1025–33. 10.1111/jvh.13511
11.
Duan M Chi X Xiao H Liu X Zhuang H . High-normal alanine aminotransferase is an indicator for liver histopathology in HBeAg-negative chronic hepatitis B.Hepatol Int. (2021) 15:318–27. 10.1007/s12072-021-10153-2
12.
Brown A Bartell N . Shedding light on the gray zone: hepatic fibrosis in chronic hepatitis B patients with fluctuating HBV DNA levels.Saudi J Gastroenterol. (2022) 28:397–8. 10.4103/sjg.sjg_336_22
13.
Schmilovitz-Weiss H Gingold-Belfer R Grossman A Issa N Boltin D Beloosesky Y et al Lowering the upper limit of serum alanine aminotransferase levels may reveal significant liver disease in the elderly. PLoS One. (2019) 14:e0212737. 10.1371/journal.pone.0212737
14.
Mason W Gill U Litwin S Zhou Y Peri S Pop O et al HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis b patients considered immune tolerant. Gastroenterology. (2016) 151: 986–98.e4. 10.1053/j.gastro.2016.07.012
15.
Lee H Kim S Baatarkhuu O Park J Kim D Ahn S et al Comparison between chronic hepatitis B patients with untreated immune-tolerant phase vs. those with virological response by antivirals. Sci Rep. (2019) 9:2508. 10.1038/s41598-019-39043-2
16.
Kim G Lim Y Han S Choi J Shim J Kim K et al High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. (2018) 67:945–52. 10.1136/gutjnl-2017-314904
17.
Feng Y Yao N Shi L Zhu Y Liu J He Y et al Efficacy and safety of long-term postpartum antiviral therapy in hepatitis B virus-infected mothers receiving prophylactic tenofovir disoproxil fumarate treatment. Eur J Gastroenterol Hepatol. (2023) 35:212–8. 10.1097/MEG.0000000000002476
18.
Hong Y Sheng W Taisheng L Xiaoyuan X Yameng S Yuemin N et al Guidelines for the prevention and treatment of chronic Hepatitis B (Version 2022). Infect Dis Immunity. (2023) 3:145–62. 10.14218/JCTH.2023.00320
19.
Kumar M Abbas Z Azami M Belopolskaya M Dokmeci A Ghazinyan H et al Asian Pacific association for the study of liver (APASL) guidelines: hepatitis B virus in pregnancy. Hepatol Int. (2022) 16:211–53. 10.1007/s12072-021-10285-5
20.
World Health Organization. Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy. Geneva: World Health Organization (2020).
21.
Zhang L Jiang T Yang Y Deng W Lu H Wang S et al Postpartum hepatitis and host immunity in pregnant women with chronic HBV infection. Front Immunol. (2023) 13:1112234. 10.3389/fimmu.2022.1112234
22.
Chinese Society of Hepatology, Chinese Medical Association. Expert opinion on expanding anti-HBV treatment for chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. (2022) 30:131–6. 10.3760/cma.j.cn501113-20220209-00060
23.
Viral Hepatitis Group HBoCRHA. Expert consensus on diagnosis and treatment of chronic hepatitis B virus with persistently normal alanine aminotransferase. J Clin Hepatol. (2021) 37:2286–91. 10.3969/j.issn.1001-5256.2021.10.007
24.
Wong R Kaufman H Niles J Kapoor H Gish R . Simplifying treatment criteria in chronic Hepatitis B: reducing barriers to elimination.Clin Infect Dis. (2023) 76:e791–800. 10.1093/cid/ciac385
25.
World Health Organization. Guidelines for the Prevention, Diagnosis, Care and Treatment for People with Chronic Hepatitis B Infection. Geneva: World Health Organization (2024).
26.
Lampertico P Berg T Buti M Pathil A Petersen J Ryder S et al Treatment with tenofovir disoproxil fumarate or entecavir in chronic hepatitis B virus-infected patients with renal impairment: results from a 7-year, multicentre retrospective cohort study. Aliment Pharmacol Ther. (2020) 52:500–12. 10.1111/apt.15901
27.
Woldemedihn G Aberra H Desalegn H Berhe N Belay D Rueegg C et al Renal safety of long-term tenofovir disoproxil fumarate treatment in patients with chronic Hepatitis B. Open Forum Infect Dis. (2023) 10:ofad404. 10.1093/ofid/ofad404
Summary
Keywords
immune tolerance phase, tenofovir disoproxil fumarate, discontinuation, risk, benefit
Citation
Liang H, Liang H, Hu B, Huang L, Liang H, Su M, Wang R, Su T, Li Q, Feng Y and Jiang J (2025) Assessment of the risk of discontinuation of tenofovir disoproxil fumarate after delivery and the benefit of continued treatment in patients with immune tolerance. Front. Med. 12:1597664. doi: 10.3389/fmed.2025.1597664
Received
21 March 2025
Accepted
27 October 2025
Published
07 November 2025
Volume
12 - 2025
Edited by
Juandy Jo, University of Pelita Harapan, Indonesia
Reviewed by
Nata Pratama Hardjo Lugito, University of Pelita Harapan, Indonesia
Rafael Rodrigues Dihl, Lutheran University of Brazil, Brazil
Ivet Suriapranata, Mochtar Riady Institute for Nanotechnology, Indonesia
Updates
Copyright
© 2025 Liang, Liang, Hu, Huang, Liang, Su, Wang, Su, Li, Feng and Jiang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianning Jiang, gxjianning@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.