- 1Department of Anesthesiology, Qingdao Municipal Hospital, Qingdao, China
- 2Second Clinical Medical College, Binzhou Medical University, Yantai, China
- 3Department of Anesthesiology, Haier Group Yingkang Life, Qingdao, China
- 4Department of Anesthesiology, Weifang Medical University, Weifang, China
Introduction: Postoperative delirium (POD) is a commonly occurring condition in the postoperative period. Therefore, the study intends to investigate the relationship between B2M and POD and the effect of B2M levels on three-year postoperative mortality in patients with POD.
Methods: Postoperatively, the Confusion Assessment Method (CAM) and the Monumental Delirium Assessment Scale (MDAS) were used to assess the incidence and severity of POD. Preoperative plasma B2M levels were measured utilizing a latex-enhanced immunoturbidimetric assay. Total tau protein (T-tau), phosphorylated tau protein (P-tau), and amyloid β plaque 42 (Aβ42) were detected in preoperative cerebrospinal fluid (CSF) by enzyme-linked immunosorbent assay. Logistic regression equations were applied to examine the risk factors linked to POD. Patients presenting with POD were grouped according to B2M level and followed up for 3 years postoperatively for their survival and Kaplan–Meier survival curves were plotted.
Results: The prevalence of POD was 7.23%. Serum B2M levels were higher in POD patients compared to non-POD (NPOD) patients (p = 0.01). The results of the logistic regression analysis indicated that B2M (OR = 1.394, 95% CI = 1.017–1.910, p = 0.002) and T-tau (OR = 1.006, 95% CI = 1.002–1.011, p = 0.007) posed a risk for POD. B2M and POD were partially associated through the mediation of CSF T-tau (10.0%). The K-M survival curves showed that patients with high B2M who developed POD had a higher mortality rate 3 years after surgery (p = 0.031).
Conclusion: In summary, B2M may be a risk factor for POD, which might be mediated in part by CSF T-tau.
Introduction
Postoperative delirium (POD) is defined as an acute impairment of attention and cognition (1). POD is very common in the postoperative period and its main clinical manifestations are altered consciousness, inattention, disorganized thinking, and decreased spatial orientation, occurring 1–7 days after surgery, most commonly 1–3 days after surgery (2, 3). POD is associated with prolonged intensive care, hospitalization, long-term cognitive dysfunction, and shorter survival, which not only affects the health of the patients themselves, but also places an enormous burden on their families and society (4). Therefore, identifying the risk factors associated with POD is particularly critical for early detection.
Although the causes of POD are poorly understood, a growing evidence indicates that oxidative stress, neuroinflammation, circadian rhythm or melatonin dysregulation, and cerebrospinal fluid (CSF) biomarkers play a critical role leading to the development of POD (5, 6). Within these mechanisms, the relationship between CSF biomarkers, which include β-amyloid plaques 42 (Aβ42), total tau protein (T-tau), as well as phosphorylated tau protein (P-tau), has been extensively studied. The biomarkers of β-amyloid (Aβ) and tau protein associated with POD in the CSF are associated with abnormal neurological function and are core proteins of POD (7, 8). Studies indicate that patients with POD have lower levels of Aβ protein and higher levels of tau protein in preoperative CSF, and that a decreased Aβ/tau ratio in preoperative CSF is associated with the development of POD (9, 10).
β2-microglobulin (B2M), which acts as the light chain of the major histocompatibility complex I (11). It is prevalent in nucleated cells (12, 13). As glomerular filtration rate decreases, B2M clearance is impaired, resulting in a gradual increase in its level (14). Smith et al. (15) demonstrated that B2M impairs learning and memory in mice. It has also been shown in animal studies that B2M causes cognitive deficits in mice (16, 17). Clinical studies have demonstrated a strong correlation between changes in the serum concentration of B2M and cognitive function in the perioperative period, whose mechanisms remain to be investigated (18, 19). There are currently no systematic studies to elucidate the relationship between B2M, POD and CSF biomarkers.
The present study hypothesizes that B2M may be associated with POD, and that CSF biomarkers may mediate this effect. The primary objective of this study was to investigate the relationship between B2M and CSF biomarkers and whether the relationship between B2M and POD is mediated by CSF biomarkers. Meanwhile, this study investigated the effect of B2M on 3 years postoperative survival in patients with POD. This cohort study has been reported in line with the STROCSS guidelines (20).
Methods
Participants
This prospective cohort study was conducted between November 2020 and December 2021, and all patients were followed up for 3 years postoperatively. This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee, which was registered on ClinicalTrials.gov. Then written informed consents from all subjects or legal surrogates were obtained.
Inclusion criteria: aged between 40 and 90 years; no gender restriction; ASA class I-II; patients undergoing knee and hip replacement surgeries under combined spinal-epidural anesthesia. Exclusion criteria were also specified as follow: (1) Patients with Minimental State Examination (MMSE) scores less than 24 (21); (2) Central nervous system infection, epilepsy, multiple sclerosis or other major nervous system diseases; (3) Major psychological disorders (such as depression, delirium, etc.); (4) Severe visual and hearing impairment; (5) Abnormal blood coagulation before operation; (6) renal dysfunction.
Cognitive measurements
Identical measures were utilized in the cohort studies. A neurologist evaluated patients’ preoperative cognitive function a day before surgery using MMSE, and those with MMSE scores <24 were excluded. Delirium was assessed by a trained anesthesiologist using the Confusion Assessment Method (CAM) (22) twice daily at 9.00–10.00 am and 2.00–3.00 pm for 1–7 days after surgery. The diagnostic criteria for POD were as follows: (1) acute onset with a fluctuating course; (2) inattention; (3) disordered thinking, and (4) changes in the level of consciousness (any state of consciousness other than fully conscious). The diagnosis of POD requires the presence of (1) and (2), accompanied by (3) or (4) or both. Patients who developed postoperative delirium (POD) underwent additional evaluation for delirium severity using the Memorial Delirium Assessment Scale (MDAS) (23). Subsequently, patients were divided into POD and non-POD (NPOD) groups based on their development of POD. The three-year postoperative survival of patients with POD was obtained using telephone follow-up.
Anesthesia and surgery
The study involved patients who had taken no medications preoperatively, fasted for at least 8 h, and abstained from water for at least 4 h (24) Healthcare providers routinely monitor the patient’s vital signs, such as electrocardiogram, pulse oximetry, and non-invasive blood pressure, before administering anesthesia. Peripheral venous access was established. Venous blood samples were collected from 6 am to 7 am on the day of surgery.
The patient received combined lumbar-rigid anesthesia. Firstly, 2 mL of cerebrospinal fluid samples were extracted from the subarachnoid space. Next, 2–2.5 mL of 0.66% ropivacaine was injected. The plane of anesthesia was adjusted at the level of thoracic 8 and oxygen was administered to the patients via a mask at a rate of 5 L/min during the operation. All patients were not sedated intraoperatively. Vasoactive drugs were administered to maintain the stability of vital signs based on the specific conditions of each patient during the operation. After surgery, patients were promptly transferred to the PACU and were closely monitored for 30 min prior to being sent back to the ward. The postoperative analgesic regimen used patient controlled intravenous analgesia (Butorphanol 10 mg + Tolansetron 5 mg + 0.9% saline solution 89 mL) to maintain a postoperative visual analog score (VAS) < 3, with non-steroidal analgesics administered as required.
Plasma B2M determination
Plasma B2M levels were measured with a latex-enhanced immunoturbidimetric assay using an automated biochemical analyzer (Beckman Coulter Automated Biochemistry Analyzer AU5800, United States), following standard clinical laboratory procedures. The assay was conducted after a 12-h overnight fast at the Qingdao Municipal Hospital’s clinical chemistry laboratory in China.
CSF biomarker assessments
The CSF samples were procured from participants and subjected to centrifugation at 2000 g for a duration of 10 min under room temperature. Thereafter, they were preserved at −80°C for supplementary analysis. The concentrations of Aβ42, T-tau and P-tau in the CSF were measured via enzyme-linked immunosorbent assay (ELISA) utilizing an enzyme labeling instrument (Thermo Scientific Multiskan MK3). The concentrations of Aβ42/T-tau and Aβ42/P-tau were calculated.
Sample size estimation
Following initial research, the study intended to include eight covariates. In addition, the incidence of POD was established to be 11% based on preliminary findings. It was assumed that the rate of patient loss to follow-up during the postoperative period would be 20%. Thus, the necessary sample size of the study required 910 cases [8 × 10 ÷ 11% ÷ (1–20%) = 910].
Statistical analysis
Participant characteristics are presented as either mean with standard deviation (SD), median and interquartile range (IQR 25th-75th percentile), or as a percentage (%). To test for normality, the Kolmogorov–Smirnov test was used for all variables. When data followed a normal distribution, independent samples t-tests were used to compare group differences. For continuous variables that were non-normally distributed, non-parametric methods were employed. The Mann–Whitney U test was utilized to compare inter-group differences, while the χ2 test was employed to compare categorical variables. Incidence of POD was denoted as a percentage.
The variables that were significant were included in univariate regression analyses and then multivariate logistic regression analyses were conducted after adjustment for ASA classification and sex. Two sensitivity analyses were then conducted. The first Adjusted for age, ASA, gender, years of education, BMI, smoking history, drinking history, MMSE scores, Hypertension, Diabetes, coronary heart disease to the multivariate logistic regression analysis, and the second added Duration of surgery, Duration of anesthesia, Intraoperative fluid, Estimated blood loss to the first. Linear regression analysis was utilized to correlate B2M levels with CSF biomarkers.
In order to investigate whether the relationship between the B2M and the POD was influenced by the presence or absence of POD pathology, we carried out a mediation analysis using the approach suggested by Baron and Kenny (25). To establish mediation, four key criteria had to be met simultaneously: (a) inclusion of the mediator (CSF biomarker) in the regression model attenuated the association between B2M and POD; (b) a notable correlation between CSF biomarker and POD was observed; (c) the correlation between B2M and POD was apparent; (d) a significant correlation between B2M and CSF biomarker existed.
The dynamic nomograms was used to determine the occurrence of POD in combination with other influencing factors (Age, Education, MMSE, ASA grade). This approach was used to substantiate the status of B2M as a risk factor for POD and to construct a corresponding clinical prediction model. Meanwhile, we plotted the ROC curve and assessed the diagnostic effect of the model by AUC. To assess calibration, a logistic model was fitted, and the fitted model provided the estimated probability of an observation conditional on the predicted risk. Observed and predicted risks were plotted against each other to generate flexible calibration curves. In addition, we utilize clinical decision curve (DCA) to evaluate the net benefit that the model provides to patients by influencing clinical decisions. Time-to-event results were analyzed with Kaplan–Meier survival analyses with log-rank tests.
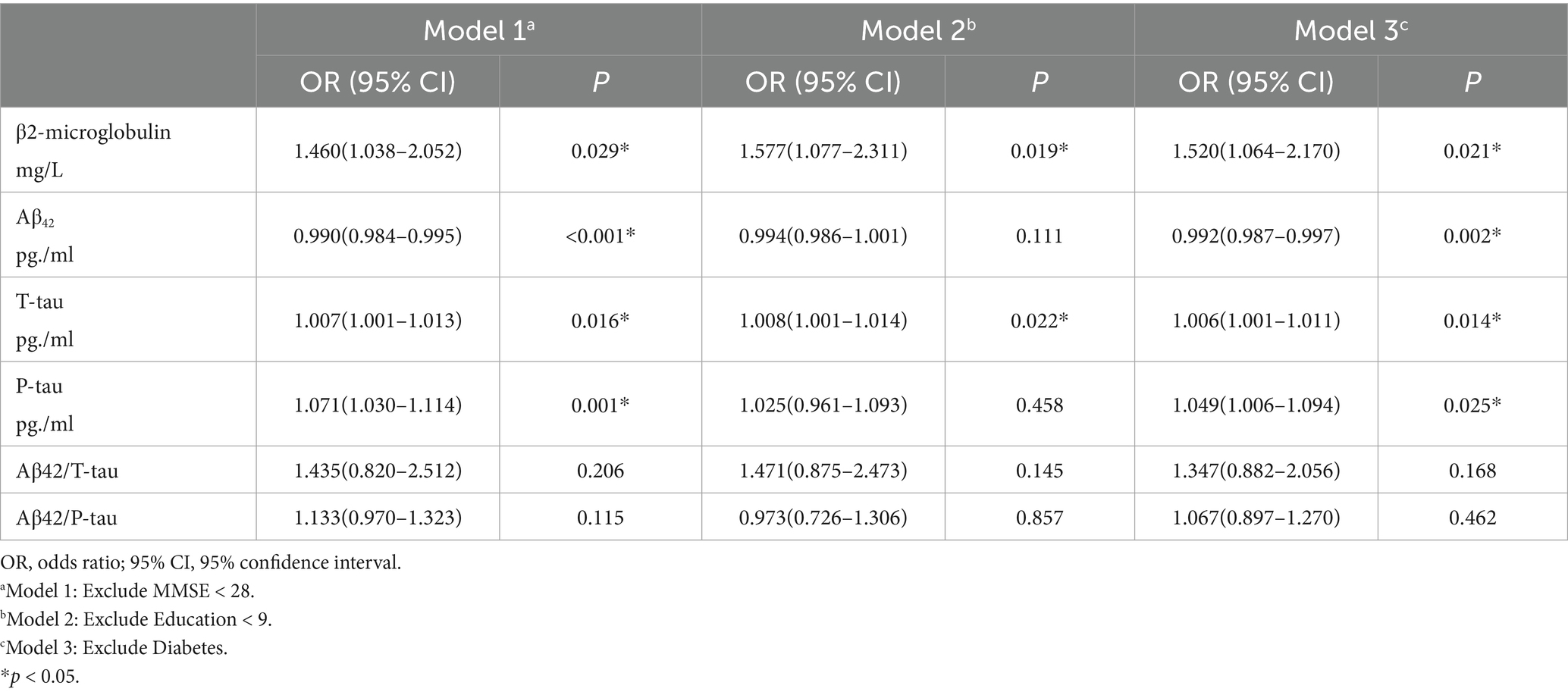
In a post hoc analysis, we replicated model 2b for the study and excluded patients with MMSE <28, education <9, and diabetes to assess whether our conclusions held true in a cognitively better, more educated, and non-diabetic population.
A statistically significant difference was defined as p < 0.05. All statistical techniques and graphs above were generated using SPSS (version 25.0), R (version 4.3.1), GraphPad Prism (version 9.4.2), and Stata (version 15.1).
Results
Patient characteristics
We enrolled a total of 910 participants, of whom 788 were eligible for this study and 122 were excluded. Figure 1 presents the reasons. Fifty-seven enrolled patients developed POD within 7 days postoperatively or before discharge. Demographic and clinical data for the study participants is also displayed in Table 1. The incidence of POD was 7.23%. Significant differences were found between POD and NPOD groups in terms of age, gender, education, hypertension, MDAS, ASA grade, and intraoperative fluid as determined via Mann–Whitney U test (p < 0.05). Additionally, there were significant differences in CSF Aβ42, T-tau, Aβ42/T-tau, Aβ42/P-tau between the two groups (p < 0.05).
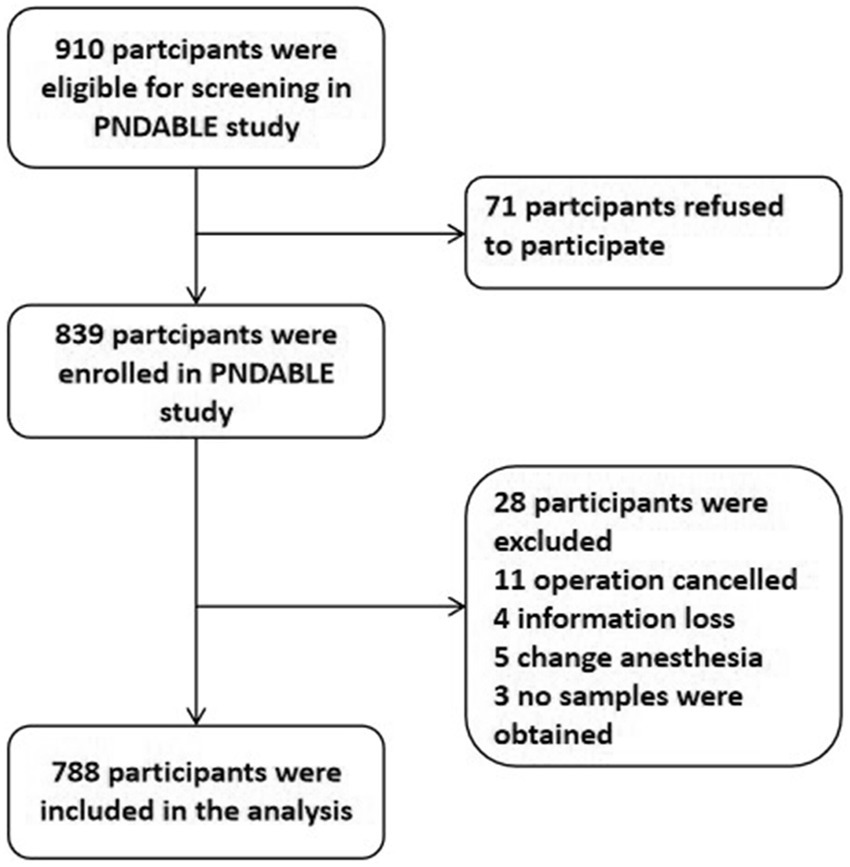
Figure 1. Flow diagram of the perioperative neurocognitive disorder and biomarker lifestyle (PNDABLE) study.
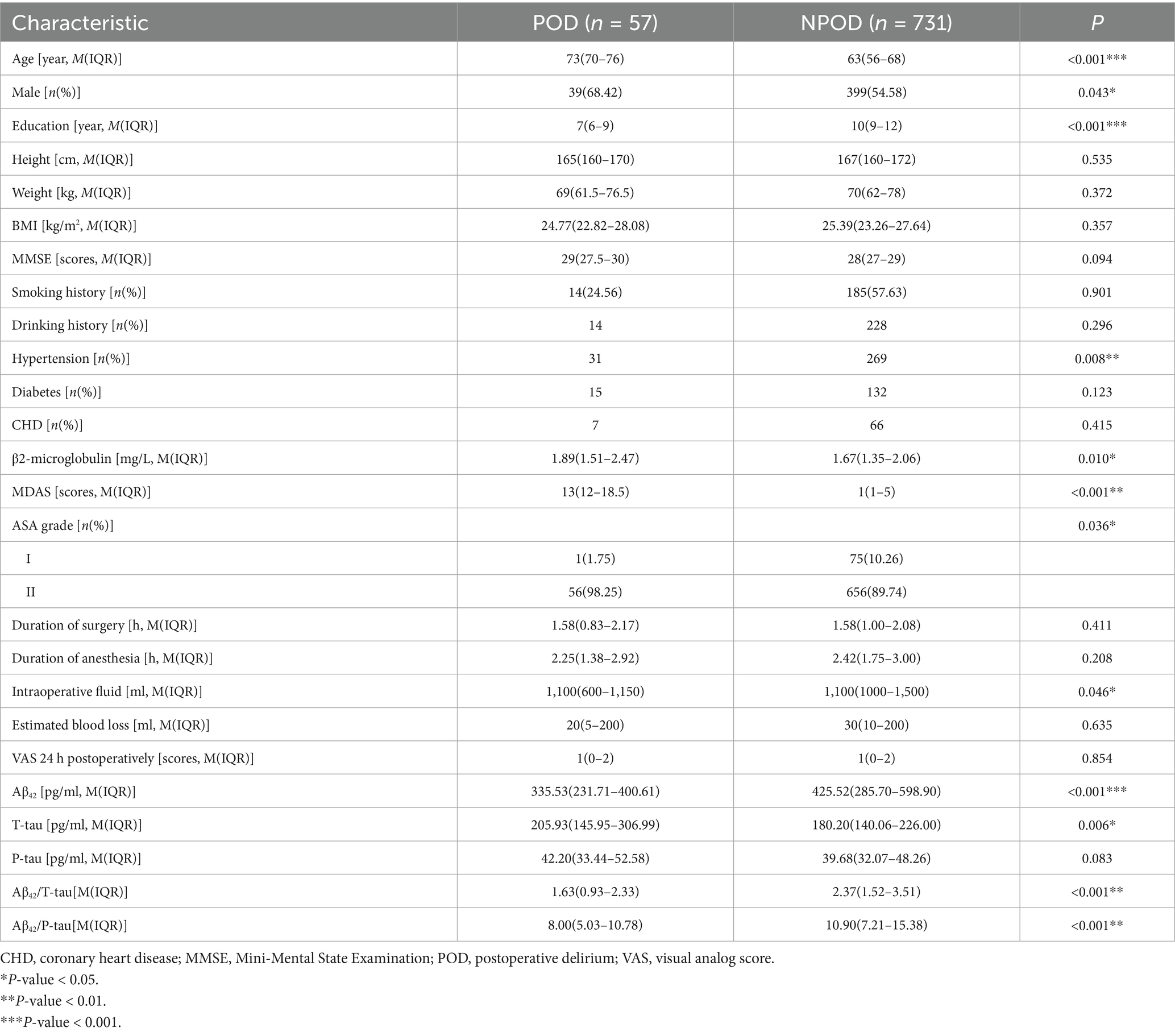
Table 1. Demographic and clinical characteristics of participants in the perioperative neurocognitive disorder and biomarker lifestyle (PNDABLE) study.
The correlation of B2M with POD
Multifactorial regression analysis adjusted for confounders showed that B2M (OR = 1.394, 95% CI = 1.017–1.910, p = 0.002) remained a risk factor for POD, as shown in Table 2. We also performed a two-step sensitivity analysis. Both sensitivity analysis steps were statistically significant.
Relationship between CSF biomarkers and POD
We compared the concentrations of the preoperative CSF biomarkers Aβ42, T-tau, and P-tau, as well as Aβ42/T-tau and Aβ42/P-tau in both POD and NPOD patients in addition to B2M. The Mann–Whitney test indicated significantly higher cerebrospinal fluid T-tau levels in POD patients compared to NPOD patients. In addition, CSF Aβ42 levels were significantly lower in POD patients than in NPOD patients. Unadjusted regression analysis indicated that all of the above CSF biomarkers were statistically significant; however, when adjusted multivariate regression was performed, Aβ42/T-tau (OR = 1.274, 95% CI = 0.830–1.956, p = 0.269) and Aβ42/P-tau (OR = 1.053, 95% CI = 0.906–1.224, p = 0.502) were not statistically significant. Nevertheless, T-tau (OR = 1.006, 95% CI = 1.002–1.011, p = 0.007), P-tau (OR = 1.038, 95% CI = 1.001–1.077, p = 0.043) and Aβ42 (OR = 0.993, 95% CI = 0.989–0.997, p = 0.002) remained statistically significant. Two rounds of sensitivity analysis showed that T-tau was a risk factor for POD, Aβ42 was a protective factor for POD, while P-tau was not statistically significant (Table 2).
The correlation of B2M with CSF biomarkers
Multiple linear regression analysis revealed a significant positive correlation (β = 0.469, p = 0.002) between B2M and CSF T-tau in POD patients, whereas no such correlation (β = 0.038, p = 0.360) was observed in NPOD patients. Aβ42 did not demonstrate the same relationship (Figure 2).

Figure 2. Linear regression of B2M with CSF biomarkers. Scatterplot representation of the relationship between B2M and CSF biomarkers: relationship between Aβ42, T-tau in different groups [POD (A,C), NPOD (B,D)]. CSF, cerebrospinal fluid; B2M, β2-microglobulin; Aβ42, amyloid-β42; T-tau, total Tau.
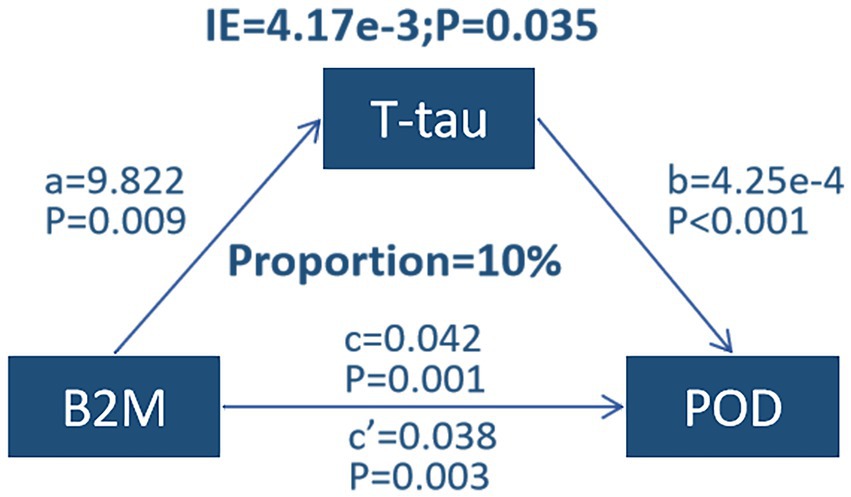
Mediation analyses
Mediation analyses revealed that the relationship between B2M and POD was partially mediated by a mediating effect of T-tau, with a proportion of mediators of approximately 10% (Figure 3).
Predictive model
The study showcased the predictive potential of B2M and the associated metrics (age, education, MMSE score, ASA grade) for POD through a Dynamic Nomogram. Results from the ROC curve strongly suggested that B2M and its associated metrics (AUC = 0.771) provided more precise predictions for POD than B2M alone (AUC = 0.528). Further supporting the predictive ability of the model, the calibration curve and DCA curves also displayed the effectiveness of the Dynamic Nomogram (Figure 4).

Figure 4. Dynamic nomogram (A), ROC curves (B), calibration curves (C) and decision curves (D). B2M, β2-microglobulin. Other factors: age, education, MMSE, ASA grade.
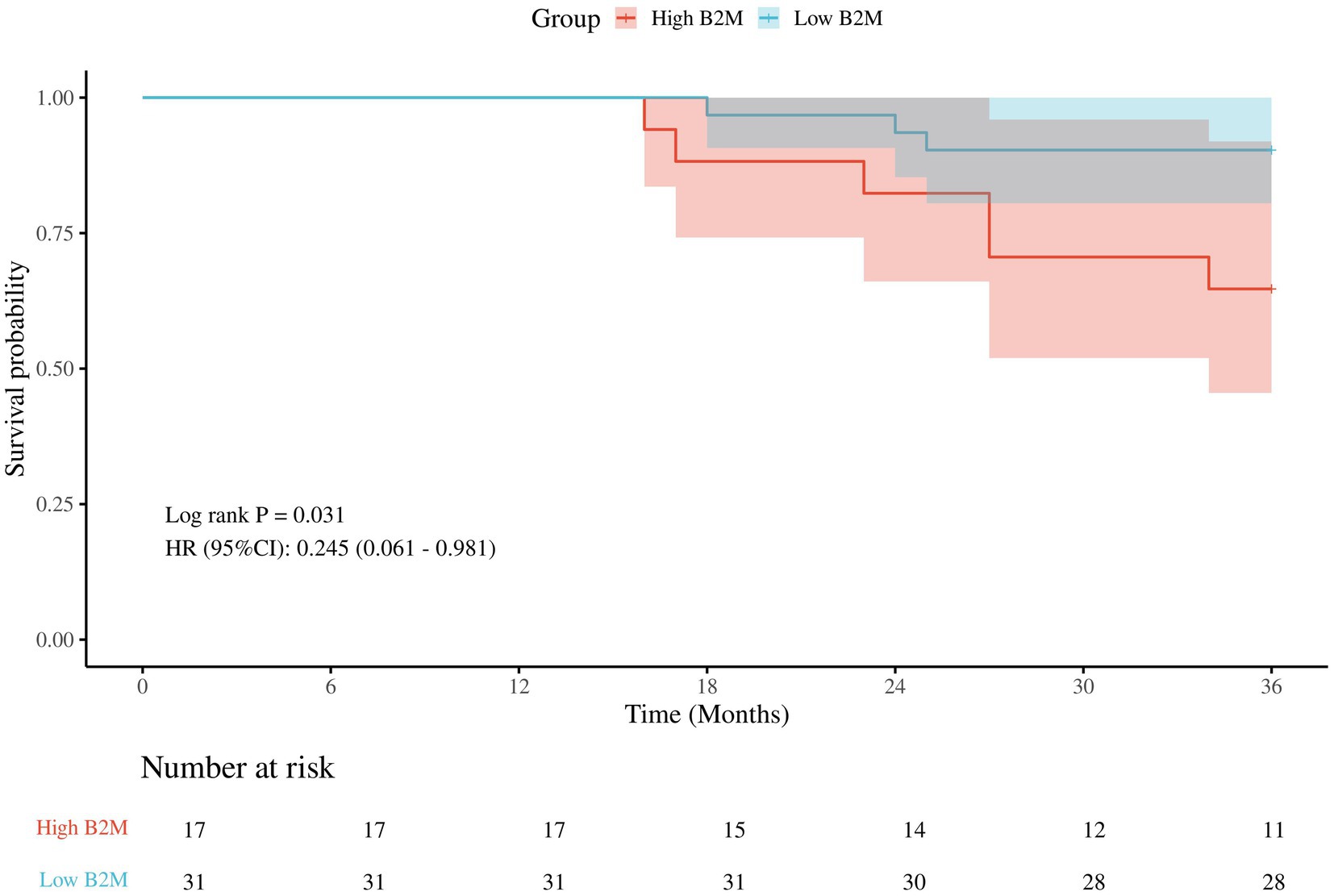
Kaplan–Meier survival analyses
There were a total of 57 patients with POD, of which 9 patients were lost to follow-up. There were 17 patients with high B2M and 6 (35.3%) deaths; 31 patients with low B2M and 3 (9.7%) deaths. Classification of POD patients into low B2M and high B2M groups based on 75% quartiles (2.1 mg/L). The Kaplan–Meier survival analysis demonstrated that the three-year postoperative survival rate was significantly lower in the high B2M group than in the low B2M group (p = 0.031), in Figure 5.
Post hoc analyses
We performed a replication of the study by excluding individuals with MMSE < 28, education level < 9, and diabetes. The results are respectively: Model 1 [B2M (OR = 1.460, 95% CI = 1.038–2.052, p = 0.029)]; Model 2 [B2M (OR = 1.577, 95% CI = 1.077–2.311, p = 0.019)]; Model3 [B2M (OR = 1.520, 95% CI = 1.064–2.170, p = 0.021)]. The results demonstrated that these exclusions had no impact on our findings, which reveals that B2M is a risk factor for POD (Table 3).
Discussion
Our study demonstrated that B2M may be a risk factor for POD, and CSF T-tau partially mediates this effect. In addition, it was found that patients with high B2M who developed POD had a higher three-year postoperative mortality rate. The model we developed by combining B2M and other relevant indicators effectively predicts POD, allowing for early detection and prevention, and ultimately reducing its incidence.
Our study concluded that B2M may be a risk factor for POD, which is consistent with the previous research linking B2M to cognitive impairment (17, 18). We strengthened the validity of our findings with a sensitivity analysis and a post hoc analysis. At the same time, T-tau as a risk factor for POD and Aβ42 as a protective factor for POD were also demonstrated in our study. Several mechanisms by which B2M causes cognitive dysfunction have been uncovered in the past. B2M is the light chain of MHC-I, with regulatory effects on brain development and synaptic plasticity, and is thought to contribute to cognitive deficits (26). Furthermore, the research has revealed that B2M has the capability to impact the characteristics of hippocampal neural progenitor cells (NPCs) potentially leading to cognitive dysfunction (27, 28). Examine has demonstrated the presence of immune responses (involving B2M) in the brain, which may lead to neuronal damage (29, 30). In conclusion, B2M may lead to cognitive dysfunction through its impact on NPCs activity, immune response, and related pathways. However, the mechanism that is responsible for B2M-induced POD remains unexplored.
Plasma B2M correlates with CSF biomarkers, including Aβ and tau proteins, which may play an important role in B2M’s association with cognitive impairment (31). Our results indicate that CSF T-tau levels were significantly higher in patients with POD compared to NPOD, whereas Aβ42 levels were significantly lower in POD patients compared to NPOD patients. Additionally, only in the POD cohort did we find a significant correlation between T-tau and B2M, while no such association between Aβ42 and B2M was found in both groups. We have found a significant correlation between B2M and POD, as well as a clear correlation between B2M and CSF T-tau. Therefore, we hypothesized that the correlation between B2M and POD may be mediated by T-tau.
Next, the mediating effect was implemented to substantiate our hypothesis. The association between B2M and POD was partly moderated by the mediating influence of T-tau (10%), while P-tau did not play a role as a risk factor for POD. This suggests that the effect of B2M on POD may be partially influenced by the blocking of tau protein. However, it is unclear how B2M facilitates T-tau in causing POD. Tau protein is abundant in axonal compartments of neurons and also presents at lower levels in oligodendrocytes and astrocytes (2). Its main functions include regulating microtubule assembly, nucleation, and bundling, as well as the regulation of axonal transport (32). In sum, additional research is necessary to determine the mechanism through which B2M facilitates T-tau, and POD subsequently. Currently, multiple studies have demonstrated that P-tau can induce pathological changes through a variety of mechanisms (33–35). Except phosphorylation, tau protein undergoes a variety of other post-translational modifications, such as acetylation, glycosylation (36), saccharification, deamidation, isomerization, nitration, methylation, ubiquitination, sumoylation, and truncation (37). Consequently, we hypothesized that the effect of B2M on POD may be mediated by tau proteins other than P-tau, which remains to be investigated in the future.
For patients diagnosed with chronic kidney disease, the management of hypertension, diabetes and other diseases that result in renal injury, followed by the enhancement of renal function, has been identified as a efficacious approach to reduce B2M (38). Moreover, for patients with end-stage renal disease, high-throughput hemodialysis or hemofiltration has been shown to be a more efficient method for the elimination of B2M (39). It has been demonstrated that immunotherapy (e.g., anti-tau antibodies) (40) or autophagy activators (e.g., rapamycin) (41) may accelerate aberrant degradation of tau proteins. These measures have the potential to reduce the occurrence of POD in patients and improve survival by lowering B2M or tau protein, but further validation through clinical trials is required to substantiate these findings.
The findings of this study indicated a substantially elevated three-year postoperative mortality rate in patients with elevated B2M levels on POD. Concurrent studies have established a correlation between B2M and arterial disease, as well as vascular structural changes, and have identified an association with inflammatory responses (42). Furthermore, B2M has been demonstrated to induce smooth muscle cell vitrification, which can subsequently result in atherosclerosis by means of a mediation process involving inflammatory response factors, including soluble viscous molecules. (43) In addition, the chemotactic effect of B2M on thrombogenic mononuclear macrophages accelerates thrombosis and is involved in the onset and development of atherosclerosis (44). It has been demonstrated that in patients with CKD, a 1 mg/L increase in serum B2M levels is associated with an 18% increased risk of all-cause mortality and a 22% increased risk of cardiovascular death (45). Another study has shown that elevated B2M levels are significantly associated with short-term mortality in patients with acute heart failure (46). In conclusion, elevated B2M levels have the potential to influence mortality through multi-organ interactions, involving the kidneys, cardiovascular system, immune system, and nervous system. Further research is required to elucidate the pathological mechanisms of B2M elevation using multi-omics analysis and other methodologies.
It is worth noting that microglia B2M may alter astrocyte function and phenotype, further affecting the blood–brain barrier (BBB), Central Nervous System (CNS) immune homeostasis, synaptic plasticity, and conventional neuronal communication (47). Reactive astrocytes overexpress glial fibrillary acidic protein (GFAP), which has also been found to play a role in the pathophysiology of tau proteins and amyloid in the brain (48). In addition, B2M not only acts as a component of GFAP (49). When MHC-I is destabilized, high levels of B2M overactivated reactive astrocytes, leading to astrocyte proliferation and elevated GFAP levels, which can lead to neuronal dysfunction and increased neurotoxicity, which can further affect tau protein pathology (50). Knockdown of MHC-I expression reduces astrocyte proliferation, whereas B2M silencing leads to astrocyte atrophy by reducing GFAP expression (51).
In this study, mediation analyses were performed to examine how CSF biomarkers mediate the correlation between B2M and POD. Several factors improved the reliability of the study. To begin with, we performed a variety of data validations, including sensitivity analyses and post hoc analyses. In addition, we developed a clinical prediction model that proved to be an important tool for predicting POD in patients and taking preventive measures in advance. In this study, nomograms were produced to facilitate clinical application, which can be used to predict POD risk by relevant indicators in patients before surgery. This facilitates preoperative risk assessment, improved intraoperative management of patients at high risk of POD, and early postoperative intervention. Finally, we followed POD patients for survival 3 years after surgery, which makes the study more valuable in the long term.
However, our study has limitations. Firstly, this study only investigated the correlation between B2M and POD, as well as the mediating role of CSF biomarkers. However, it would be beneficial for future research to explore other potential mechanisms of association between B2M and POD. Secondly, the study was a single-center design, which limits its generalisability. Consequently, the results of this study require greater generalisability, which can be achieved through prospective multicenter studies. In addition, the predictive model needs to be validated externally in order to test it. Furthermore, the incidence of POD in this study was lower than the 11% assumed in the sample size calculation, which may have a bearing on the reliability of the conclusions. Next, in this study, methods such as propensity score matching were not employed to balance the variables between the POD and non-POD groups. However, multivariate logistic regression was utilized to compensate for the potential limitations of not using propensity score matching. In future studies, we will apply the propensity score matching method by developing better inclusion and exclusion criteria, lowering the loss-to-follow-up rate, and increasing the sample size in order to validate the current findings. Finally, it is acknowledged that the study may have been influenced by a number of potential confounders. In subsequent studies, the potential for bias reduction may be enhanced by the inclusion of a more extensive and heterogeneous group of participants.
Conclusion
In summary, B2M may be a risk factor for POD, which might be mediated in part by CSF T-tau. The prediction model we constructed by combining B2M and other related indicators can effectively predict the occurrence of POD, which will be helpful for us to detect and prevent POD in advance and thus reduce the occurrence of POD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Qingdao Municipal Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YW: Investigation, Writing – original draft. QH: Data curation, Writing – original draft. KF: Data curation, Writing – review & editing. YB: Investigation, Writing – original draft, Writing – review & editing. BW: Investigation, Writing – original draft, Writing – review & editing. WK: Data curation, Writing – review & editing. SH: Data curation, Writing – review & editing. JK: Data curation, Writing – review & editing. XS: Data curation, Writing – review & editing. HG: Formal analysis, Writing – review & editing. JW: Formal analysis, Writing – review & editing. CL: Formal analysis, Writing – review & editing. YL: Formal analysis, Writing – review & editing. XL: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (91849126).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
POD, Postoperative delirium; B2M, β2-microglobulin; PNDABLE, Perioperative Neurocognitive Disorder and Biomarkers Lifestyle; MMSE, Mini Mental State Examination; CAM, Confusion Assessment Method; MDAS, Monumental Delirium Assessment Scale; ELISA, enzyme-linked immunosorbent assay; T-tau, total tau protein; P-tau, phosphorylated tau protein; Aβ42, amyloid β plaque 42; CSF, cerebrospinal fluid; NPOD, non-POD; DCA, decision curves; PACU, post-anesthetic care unit.
References
1. Oh, S-T, and Park, JY. Postoperative delirium. Korean J Anesthesiol. (2019) 72:4–12. doi: 10.4097/kja.d.18.00073.1
2. Ayers, JI, Giasson, BI, and Borchelt, DR. Prion-like spreading in Tauopathies. Biol Psychiatry. (2018) 83:337–46. doi: 10.1016/j.biopsych.2017.04.003
3. Zhao, B, Ni, Y, and Tian, X. Low plasma cholinesterase activity is associated with postoperative delirium after noncardiac surgery in elderly patients: a prospective observational study. Psychosomatics. (2019) 60:190–6. doi: 10.1016/j.psym.2018.06.014
4. Jin, Z, Hu, J, and Ma, D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. (2020) 125:492–504. doi: 10.1016/j.bja.2020.06.063
5. Cunningham, EL, McGuinness, B, McAuley, DF, Toombs, J, Mawhinney, T, O’Brien, S, et al. CSF Beta-amyloid 1–42 concentration predicts delirium following elective arthroplasty surgery in an observational cohort study. Ann Surg. (2019) 269:1200–5. doi: 10.1097/SLA.0000000000002684
6. Xu, Y, Ma, Q, Du, H, Yang, C, and Lin, G. Postoperative delirium in neurosurgical patients: recent insights into the pathogenesis. Brain Sci. (2022) 12:1371. doi: 10.3390/brainsci12101371
7. Nizynski, B, Dzwolak, W, and Nieznanski, K. Amyloidogenesis of tau protein: tau aggregation. Protein Sci. (2017) 26:2126–50. doi: 10.1002/pro.3275
8. Wang, Y, and Mandelkow, E. Tau in physiology and pathology. Nat Rev Neurosci. (2016) 17:22–35. doi: 10.1038/nrn.2015.1
9. Fong, TG, Vasunilashorn, SM, Libermann, T, Marcantonio, ER, and Inouye, SK. Delirium and A lzheimer disease: a proposed model for shared pathophysiology. Int J Geriatr Psychiatry. (2019) 34:781–9. doi: 10.1002/gps.5088
10. Idland, A-V, Wyller, TB, Støen, R, Eri, LM, Frihagen, F, Ræder, J, et al. Preclinical amyloid-β and axonal degeneration pathology in delirium. J Alzheimers Dis. (2016) 55:371–9. doi: 10.3233/JAD-160461
11. Floege, J, and Ketteler, M. β2-microglobulin–derived amyloidosis: an update. Kidney Int. (2001) 59:S164–71. doi: 10.1046/j.1523-1755.2001.59780164.x
12. Ploegh, HL, Orr, HT, and Strominger, JL. Major histocompatibility antigens: the human (HLA-A,-B,-C) and murine (H-2K, H-2D) class I molecules. Cell. (1981) 24:287–99. doi: 10.1016/0092-8674(81)90318-4
13. Saper, MA, Bjorkman, PJ, and Wiley, DC. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 Å resolution. J Mol Biol. (1991) 219:277–319. doi: 10.1016/0022-2836(91)90567-P
14. Drüeke, TB, and Massy, ZA. Beta2-Microglobulin. Semin Dial. (2009) 22:378–80. doi: 10.1111/j.1525-139X.2009.00584.x
15. Smith, LK, He, Y, Park, J-S, Bieri, G, Snethlage, CE, Lin, K, et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. (2015) 21:932–7. doi: 10.1038/nm.3898
16. Chen, F, Liu, J, Li, F-Q, Wang, S-S, Zhang, Y-Y, Lu, Y-Y, et al. β2-microglobulin exacerbates neuroinflammation, brain damage, and cognitive impairment after stroke in rats. Neural Regen Res. (2023) 18:603. doi: 10.4103/1673-5374.350204
17. Zhao, Y, Zheng, Q, Hong, Y, Gao, Y, Hu, J, Lang, M, et al. β2-microglobulin coaggregates with aβ and contributes to amyloid pathology and cognitive deficits in Alzheimer’s disease model mice. Nat Neurosci. (2023) 26:1170–84. doi: 10.1038/s41593-023-01352-1
18. Cui, Z, Wang, S, Hao, Y, and Chen, Y. Higher serum β2-microglobulin is a predictive biomarker for cognitive impairment in spinal cord injury. PeerJ. (2023) 11:e15372. doi: 10.7717/peerj.15372
19. Załęska-Kocięcka, M, Jezierski, P, Grabowski, M, Kuśmierski, K, Dąbrowski, M, Piotrowska, K, et al. Role of β2-microglobulin in postoperative cognitive decline. Biomark Med. (2017) 11:245–53. doi: 10.2217/bmm-2016-0274
20. Rashid, R, Sohrabi, C, Kerwan, A, Franchi, T, Mathew, G, Nicola, M, et al. The STROCSS 2024 guideline: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg. (2024) 110:3151–65. doi: 10.1097/JS9.0000000000001268
21. Folstein, MF. The Mini-mental state examination. Arch Gen Psychiatry. (1983) 40:812. doi: 10.1001/archpsyc.1983.01790060110016
22. Inouye, SK. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. (1990) 113:941–8. doi: 10.7326/0003-4819-113-12-941
23. De, J, and Wand, APF. Delirium screening: a systematic review of delirium screening tools in hospitalized patients. Geront. (2015) 55:1079–99. doi: 10.1093/geront/gnv100
24. Joshi, GP, Abdelmalak, BB, Weigel, WA, Harbell, MW, Kuo, CI, Soriano, SG, et al. 2023 American Society of Anesthesiologists Practice Guidelines for preoperative fasting: carbohydrate-containing clear liquids with or without protein, chewing gum, and pediatric fasting duration—a modular update of the 2017 American Society of Anesthesiologists Practice Guidelines for preoperative fasting*. Anesthesiology. (2023) 138:132–51. doi: 10.1097/ALN.0000000000004381
25. Baron, RM, and Kenny, DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. (1986) 51:1173–82. doi: 10.1037/0022-3514.51.6.1173
26. Cebrián, C, Loike, JD, and Sulzer, D. Neuronal MHC-I expression and its implications in synaptic function, axonal regeneration and Parkinson’s and other brain diseases. Front Neuroanat. (2014) 8:114. doi: 10.3389/fnana.2014.00114
27. Das, MM, Godoy, M, Chen, S, Moser, VA, Avalos, P, Roxas, KM, et al. Young bone marrow transplantation preserves learning and memory in old mice. Commun Biol. (2019) 2:73. doi: 10.1038/s42003-019-0298-5
28. Yang, R, Fu, S, Zhao, L, Zhen, B, Ye, L, Niu, X, et al. Quantitation of circulating GDF-11 and β2-MG in aged patients with age-related impairment in cognitive function. Clin Sci. (2017) 131:1895–904. doi: 10.1042/CS20171028
29. Bien, CG, Bauer, J, Deckwerth, TL, Wiendl, H, Deckert, M, Wiestler, OD, et al. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in rasmussen’s encephalitis. Ann Neurol. (2002) 51:311–8. doi: 10.1002/ana.10100
30. Pereira, RA, and Simmons, A. Cell surface expression of H2 antigens on primary sensory neurons in response to acute but not latent herpes simplex virus infection in vivo. J Virol. (1999) 73:6484–9. doi: 10.1128/JVI.73.8.6484-6489.1999
31. Huang, Y-M, Ma, Y-H, Gao, P-Y, Wang, Z-B, Huang, L-Y, Hou, J-H, et al. Plasma β2-microglobulin and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology in cognitively intact older adults: the CABLE study. Alz Res Therapy. (2023) 15:69. doi: 10.1186/s13195-023-01217-6
32. Guo, T, Noble, W, and Hanger, DP. Roles of tau protein in health and disease. Acta Neuropathol. (2017) 133:665–704. doi: 10.1007/s00401-017-1707-9
33. Dickey, CA, Kamal, A, Lundgren, K, Klosak, N, Bailey, RM, Dunmore, J, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. (2007) 117:648–58. doi: 10.1172/JCI29715
34. Hoover, BR, Reed, MN, Su, J, Penrod, RD, Kotilinek, LA, Grant, MK, et al. Tau Mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. (2010) 68:1067–81. doi: 10.1016/j.neuron.2010.11.030
35. Ittner, LM, Ke, YD, and Götz, J. Phosphorylated tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. J Biol Chem. (2009) 284:20909–16. doi: 10.1074/jbc.M109.014472
36. Min, S-W, Cho, S-H, Zhou, Y, Schroeder, S, Haroutunian, V, Seeley, WW, et al. Acetylation of tau inhibits its degradation and contributes to Tauopathy. Neuron. (2010) 67:953–66. doi: 10.1016/j.neuron.2010.08.044
37. Martin, L, Latypova, X, and Terro, F. Post-translational modifications of tau protein: implications for Alzheimer’s disease. Neurochem Int. (2011) 58:458–71. doi: 10.1016/j.neuint.2010.12.023
38. Levey, AS, and Coresh, J. Chronic kidney disease. Lancet. (2012) 379:165–80. doi: 10.1016/S0140-6736(11)60178-5
39. Locatelli, F, Martin-Malo, A, Hannedouche, T, Loureiro, A, Papadimitriou, M, Wizemann, V, et al. Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol. (2009) 20:645–54. doi: 10.1681/ASN.2008060590
40. Yanamandra, K, Jiang, H, Mahan, TE, Maloney, SE, Wozniak, DF, Diamond, MI, et al. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann Clin Transl Neurol. (2015) 2:278–88. doi: 10.1002/acn3.176
41. Silva, MC, Nandi, GA, Tentarelli, S, Gurrell, IK, Jamier, T, Lucente, D, et al. Prolonged tau clearance and stress vulnerability rescue by pharmacological activation of autophagy in tauopathy neurons. Nat Commun. (2020) 11:3258. doi: 10.1038/s41467-020-16984-1
42. Wilson, AM, Kimura, E, Harada, RK, Nair, N, Narasimhan, B, Meng, X-Y, et al. Β2-microglobulin as a biomarker in peripheral arterial disease: proteomic profiling and clinical studies. Circulation. (2007) 116:1396–403. doi: 10.1161/CIRCULATIONAHA.106.683722
43. Yılmaz, A, Yılmaz, B, and Küçükseymen, S. β-2 microglobulin level is negatively associated with global left ventricular longitudinal peak systolic strain and left atrial volume index in patients with chronic kidney disease not on dialysis. Anatol J Cardiol. (2016) 16:844–9. doi: 10.14744/AnatolJCardiol.2015.6691
44. Huo, Q, Dong, W, Gao, Y, Zhang, Y, Liu, X, Yang, L, et al. Effect of β2-microglobulin in evaluating the severity and prognosis of brain injury: a clinical study. BMC Neurol. (2022) 22:327. doi: 10.1186/s12883-022-02850-8
45. Foster, MC, Coresh, J, Hsu, C-Y, Xie, D, Levey, AS, Nelson, RG, et al. Serum β-trace protein and β2-microglobulin as predictors of ESRD, mortality, and cardiovascular disease in adults with CKD in the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. (2016) 68:68–76. doi: 10.1053/j.ajkd.2016.01.015
46. Kawai, K, Kawashima, S, Miyazaki, T, Tajiri, E, Mori, M, Kitazaki, K, et al. Serum beta2-microglobulin concentration as a novel marker to distinguish levels of risk in acute heart failure patients. J Cardiol. (2010) 55:99–107. doi: 10.1016/j.jjcc.2009.10.003
47. Santello, M, Toni, N, and Volterra, A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci. (2019) 22:154–66. doi: 10.1038/s41593-018-0325-8
48. Ferrari-Souza, JP, Ferreira, PCL, Bellaver, B, Tissot, C, Wang, Y-T, Leffa, DT, et al. Astrocyte biomarker signatures of amyloid-β and tau pathologies in Alzheimer’s disease. Mol Psychiatry. (2022) 27:4781–9. doi: 10.1038/s41380-022-01716-2
49. Ignarro, RS, Bombeiro, AL, Chiarotto, GB, Cartarozzi, LP, Coser, LDO, Ghizoni, E, et al. Interferon-beta induces major histocompatibility complex of class I (MHC-I) expression and a proinflammatory phenotype in cultivated human astrocytes. Differentiation. (2022) 128:43–56. doi: 10.1016/j.diff.2022.10.004
50. Sánchez-Juan, P, Valeriano-Lorenzo, E, Ruiz-González, A, Pastor, AB, Rodrigo Lara, H, López-González, F, et al. Serum GFAP levels correlate with astrocyte reactivity, post-mortem brain atrophy and neurofibrillary tangles. Brain. (2024) 147:1667–79. doi: 10.1093/brain/awae035
Keywords: β2-microglobulin, postoperative delirium, mediation analysis, prediction model, mortality rate
Citation: Wang Y, He Q, Fu K, Bi Y, Wang B, Kong W, Hua S, Kong J, Xu S, Gong H, Wang J, Li C, Lin Y and Lin X (2025) Correlation of β2-microglobulin with postoperative delirium and 3-year mortality undergoing knee or hip replacement surgery: a prospective cohort study. Front. Med. 12:1597764. doi: 10.3389/fmed.2025.1597764
Edited by:
Theodoros Aslanidis, Agios Pavlos General Hospital, GreeceReviewed by:
Pingping Zhao, University of California, Los Angeles, United StatesYi-Ming Huang, Capital Medical University, China
Qiuying Li, Guangdong Provincial People’s Hospital, China
Copyright © 2025 Wang, He, Fu, Bi, Wang, Kong, Hua, Kong, Xu, Gong, Wang, Li, Lin and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Lin, bGlueHVfZ3JlZW5AMTI2LmNvbQ==
†These authors have contributed equally to this work
 Yuanlong Wang1,2†
Yuanlong Wang1,2† Yanlin Bi
Yanlin Bi Shanling Xu
Shanling Xu Yanan Lin
Yanan Lin Xu Lin
Xu Lin