- 1Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, Arcavacata di Rende, Italy
- 2Nephrology, Dialysis and Transplant Unit, “SS. Annunziata” Hospital, Cosenza, Italy
Advances in kidney transplantation have made significant progress, yet challenges remain in managing both the pre- and post-transplantation phases, which have a direct impact on long-term allograft survival and comorbidities experienced by kidney transplant recipients (KTRs). Among the common immunosuppression-related complications, malignancies are a notable concern, and endocrine tumors are frequently observed. These tumors exhibit heterogeneous pathogenesis, prognosis, and treatment responses but existing literature is limited, and prevalence studies often compare KTRs to the general population. Thyroid cancers (particularly papillary thyroid cancer) have a high incidence in KTRs, whereas rare endocrine malignancies (such as neuroendocrine tumors, adrenal cortical carcinomas, pheochromocytomas, paragangliomas, and parathyroid carcinoma) are mostly reported in isolated case reports, and no clinical trials have been performed to assess the impact of different immunosuppressive treatments on their onset and development. However, current guidelines for the management of post-transplant malignancies suggest reducing or withdrawing immunosuppressive therapy whereas a switch from calcineurin inhibitors to mammalian target of rapamycin (mTOR) inhibitors is currently not recommended due to limited supporting data. Notably, the pathogenic role of transplantation and the timeline for endocrine malignancies onset in KTRs are poorly defined. To address these challenges, a multicenter and interdisciplinary approach is critical to improve our understanding of the epidemiology and pathogenesis of endocrine malignancies in KTRs. Additionally, specific guidelines for early diagnosis and treatment are necessary to ensure safe and effective management of these tumors in this vulnerable population. This mini-review aims to synthesize the available data and current insights into this important issue.
1 Endocrine malignancies in kidney transplant recipients: challenges and considerations
Kidney transplantation is the preferred treatment option for patients with end-stage kidney disease (ESKD), and its incidence is continually increasing (1). Based on data from 2018, the global incidence of kidney transplantation was approximately 14 per million people, and the average prevalence of ESKD requiring replacement therapy is predicted to double by 2030 (2).
Although a better overall early graft survival rate has been achieved due to significant progress in graft preservation methodologies (including machine perfusion), transplantation surgical techniques, and management/personalization of the immunosuppressive protocols, long-term allograft survival is still not optimal, and the rate of comorbidities, including tumors, remains high (3–7).
Compared with the general population, the overall risk of developing tumors is 2–4 times greater in kidney transplant recipients (KTRs), with a cumulative incidence of solid organ cancers that increases during the follow-up period, ranging from 4%−11% after 10 years (8–11) to 12%−37% 20 years after transplantation (10, 12, 13).
Multiple factors may contribute to the increased risk of tumors in this fragile patient population, including advanced recipient age, prolonged time on dialysis before transplantation (14), previous cancer, type of transplant (kidneys from deceased donors or from expanded criteria donors) (15), high reactive antibody panel (PRA) (16), acute rejection (17), and oncogenic viral infections (5, 7, 18).
Viral-associated malignancies are the most frequent type of tumor in KTRs, with a standardized incidence ratio (SIR) exceeding 11 (19). The most common types are Kaposi sarcoma (human herpesvirus 8), post-transplant lymphoproliferative disorder (PTLD; Epstein–Barr virus), hepatocellular carcinoma, and lip and anal cancers (human papillomavirus, HPV) (20).
There are also cancers that may cause ESKD and are therefore commonly seen in KTRs (e.g., myeloma and renal cell carcinoma) (21).
Additionally, non-infection-related cancers with high incidence in KTRs include non-melanoma skin cancer, non-Hodgkin lymphoma, lip and oral cavity, lung, thyroid, kidney, and prostate cancer (9, 12, 22–25).
However, the increase is not consistent across all studies or cancer sites. Some malignancies with high incidence rates in the general population, such as breast and prostate cancer, showed no increase following kidney transplantation in certain studies (7, 12, 19, 22, 26–31), while others even reported a slight decrease (9, 24, 25, 32, 33).
This discrepancy may result from variations in cancer incidence among populations, differences in data collection methods, cancer ascertainment processes, and the sex and age distribution of KTRs and the reference population (19, 34, 35).
Moreover, immunosuppression may affect the onset and development of cancer. This therapy impairs the ability of patients to control viral infections, thereby increasing the risk of infection-associated cancer. It also weakens tumor surveillance, allowing cancers to grow rapidly. However, as reported in the 2009 KDIGO guidelines, the role of these medications seems to be related to an overall inefficient immune response against tumor growth rather than the activation of specific drug-related biological/molecular mechanisms (21).
Furthermore, the degree of renal dysfunction affects cancer development in both the pre- and post-transplant periods, beginning to increase at a glomerular filtration rate of 55 ml/min and reaching a maximum three-fold increased risk with GFR ≤ 40 ml/min per 1.73 m2 (mainly lung and urinary) independently of other known risk factors, such as age and smoking (36).
In the presence of advanced kidney impairment, the state of chronic systemic inflammation, imbalance of oxidative stress, impairment of DNA repair, accumulation of carcinogenic compounds, excessive parathyroid hormone (PTH), and changes in intestinal microbiota contribute to tumorigenesis and are partially responsible for the increased incidence of some tumors (37).
On the other hand, we cannot rule out that the increased prevalence of some tumors (e.g., breast, prostate, lung, colorectal and thyroid cancers) in patients with ESKD could be partly due to over-diagnosis (mainly incidentally by imagine techniques) (5, 38) as well as in KTRs underlying more frequent health care access than the general population, particularly during the first year after surgery.
In fact, the American Society of Transplantation and the European Best Practice Guidelines recommend following the current cancer screening practices for common cancer types such as colorectal, breast, cervical, and prostate cancers as per the general population (39), while screening for kidney and lung cancer is not recommended (40, 41).
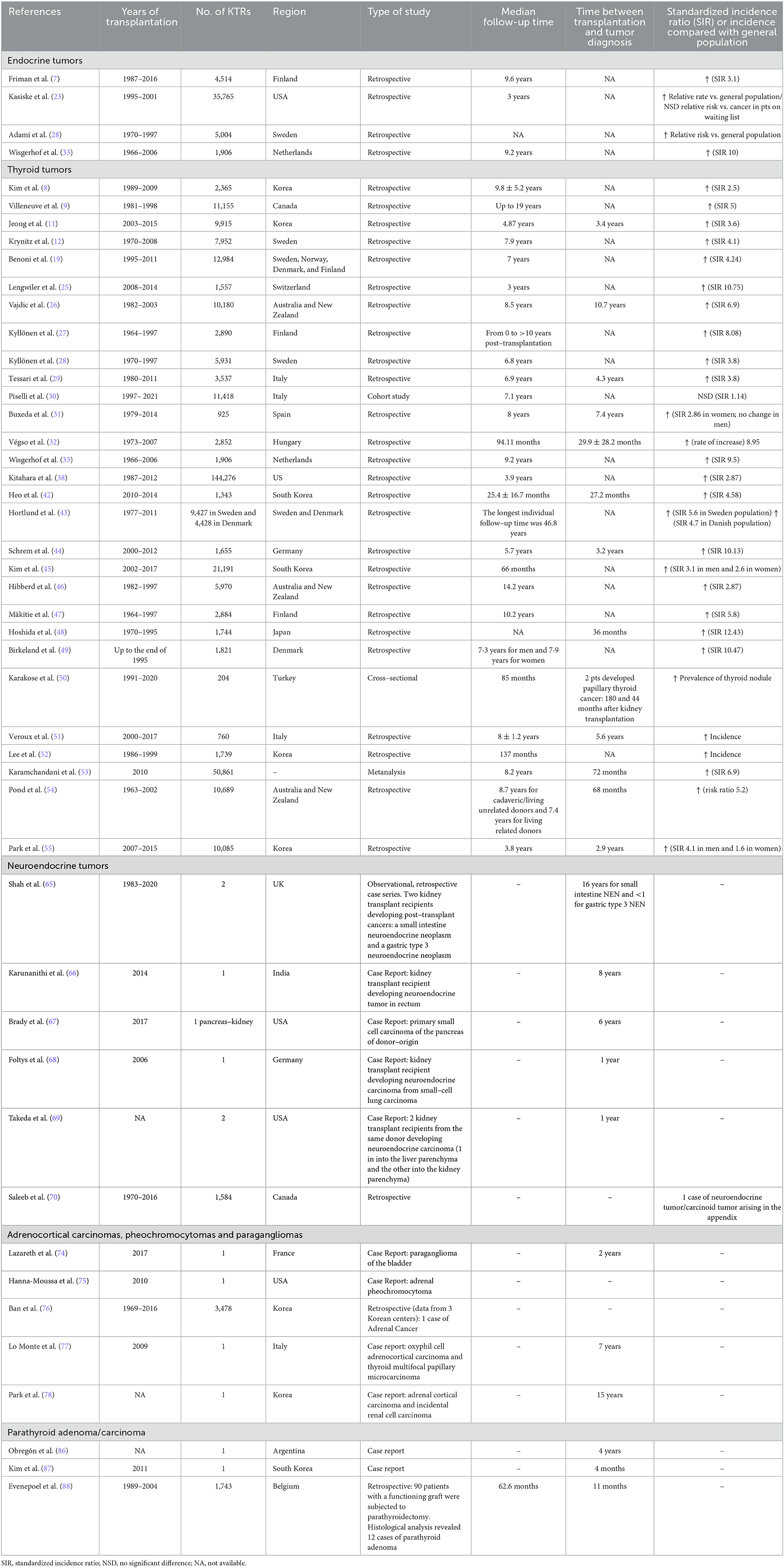
However, the overall incidence of endocrine and thyroid tumors in KTRs has been evaluated by several studies, which have shown a risk of up to 10 times greater than in the general population (Table 1) (7–9, 25, 27, 32, 33, 38, 42–54), but no differences when compared with ESKD patients on waiting list (23, 26, 28).
Although well-performed and including large patient populations, these studies have analyzed the overall prevalence of “endocrine tumors” in the absence of a clear specification of the endocrine glands involved and a clear distinction between benign tumors and histological subtypes. Therefore, this review specifically focuses on endocrine tumors in KTRs.
2 Thyroid malignancies
Thyroid tumors are the most extensively studied endocrine malignancies in KTRs with a high incidence (SIR >4) (Table 1) (8, 9, 11, 12, 19, 25–33, 38, 42–55). Nonetheless, a more recent Italian study, performed on a large population of ~17,000 KTRs over a 25-year period, found no difference compared to the general population (SIR 1.14) (30). The large heterogeneity in these studies is due to differences in the follow-up time, pre-transplant disease status, and post-operative immune therapy regimen.
Notably, almost all thyroid carcinomas in KTRs are differentiated microcarcinomas that are generally diagnosed incidentally, and they are almost exclusively papillary thyroid cancer (PTC) (38, 51, 53, 54, 56–58) whereas follicular carcinomas are rare (51, 57) and medullary and anaplastic carcinomas are even more rare (38).
The average time between kidney transplantation and the diagnosis of these cancers is 6 years (51, 53, 54), with a peak occurrence occurring within the first year (38, 48).
Additionally, even if there are contrasting data (38), aggressive loco-regional involvement has been reported, with the presence of lateral cervical lymph node metastases at diagnosis in almost half of the cases and variable recurrence of loco-regional disease (16.7%−75%) during a follow-up period of 94–137 months (51, 52).
Histologically solid organ transplant recipients with PTC were more likely to have multifocality and central compartment lymph node metastasis, although their tumors were smaller in size (52, 59).
It has been speculated that thyroid tumors are mainly CKD-related and dialysis-associated (with a higher incidence in KTRs and in patients with more than 5 years of dialysis vintage) (26, 38, 51).
Instead, the impact of immunosuppression on their onset and development is debated. Some authors did not find any differences in thyroid cancer incidence by immunosuppressive regimen (51, 59), although cyclosporine and azathioprine were associated with reduced thyroid cancer incidence, while tacrolimus and mycophenolate mofetil correlated with a greater risk of thyroid carcinomas, especially those with regional or distant extension (38). mTOR inhibitors (mTOR-Is) act on the primary pathogenic pathway of thyroid carcinoma (60) and could exert protective effects (61). Rapamycin-based immunosuppressive regimens in solid organ transplant recipients have been associated with a reduced incidence of thyroid cancer (38). In addition, patients with PTC undergoing this therapy showed unilateral cancer and absence of lymph node metastasis (59).
Phase 2 clinical trials have, in fact, demonstrated that oncological treatment with mTOR-Is has permitted disease stability in ~65%−76% of patients with thyroid carcinoma of different histology (62). However, no data is available on the role of mTOR-Is as immunosuppressive therapy in the incidence of thyroid malignancies in KTRs.
In a US data-linkage cohort study, the risk of thyroid cancer was increased in KTRs who underwent CD52-targeting monoclonal antibody alemtuzumab induction therapy and decreased in patients receiving muromonab-CD3 compared with those receiving no induction therapy (63). However, these results were not confirmed in the subsequent registry data analysis, which included more than 200,000 U.S. solid organ transplant recipients and 356 thyroid cancers (38).
The development of thyroid tumors is also not associated with gland functionality and the presence of anti-thyroglobulin antibodies and anti-thyroid peroxidase antibodies (50, 57) even if these studies did not differentiate benign from malignant nodules.
Further prospective studies are necessary to better define the rate of thyroid tumors in KTRs (most of them undiagnosed) in the pre- and post-transplant period, to identify the clinical, biological, and pathological fingerprints associated with their onset, and to select early diagnostic and therapeutic strategies.
In the absence of any extensive and conclusive data, the current available guidelines provide some indications for transplantation in patients on the waitlist (no waiting time for follicular/papillary tumors < 2 cm of low grade histology; at least 2 years wait for Stage 2; at least 5 years wait for Stage 3; kidney transplant contraindicated for Stage 4 or anaplastic carcinomas) (39) whereas some studies recommended specific therapeutic management of thyroid carcinomas after transplantation (64) and, as for other types of cancers, a minimization of immunosuppressive therapy. This clinical decision should take into account the risk of allograft rejection and/or severe adverse events (21). However, available case reports did not reveal significant short-time deterioration of renal function or increase of proteinuria levels after reduction of immunosuppressive therapy (50, 51). Instead, no data was provided on the long-term effect of these therapeutic changes.
Finally, in high-risk patients and those with previously recognized pre-transplant malignancy, close follow-up using specific laboratory tests and imaging technologies and monitoring of the immunosuppression therapy should be undertaken to minimize the risk of de-novo or recurrent cancers.
3 Neuroendocrine tumors
Neuroendocrine tumors (NET) are rare and most frequently occur in the intestines (65, 66), pancreas (67), lungs (68, 69), and appendix (70), accounting for 4.7% of all donor-derived cancers (71) (Table 1).
In particular, they are mostly neuroendocrine of the lung, diagnosed at a median transplant age of 10 months, with distant metastases already present at the time of diagnosis in ~73% of cases (71). This suggests the necessity to screen donors for these conditions, especially in the case of donors at high risk due to smoking, premalignant illnesses, or advanced age (72).
In a study performed on a large Canadian population of KTRs, an overall increased risk of gastrointestinal malignancies was described, with a single case of low grade neuroendocrine tumor/carcinoid tumor arising in the appendix (73).
Treatment of transmitted cancer requires graft removal and the suspension of immunosuppressive therapy, with or without chemotherapy (71).
However, some authors have suggested switching patients from calcineurin inhibitors to mTOR-Is as immunosuppression medications already employed for the treatment of well- or moderately-differentiated pancreatic NETs, G1/G2 Gastrointestinal NETs and lung NETs (65, 72, 73).
4 Adrenal cortical carcinomas, pheochromocytomas, and paragangliomas
Adrenal carcinomas, pheochromocytomas, and paragangliomas in KTRs are very rare disorders that have been only partially described in kidney transplantation, as in case reports, with an unclear causative role of kidney transplantation/immunosuppression (Table 1) (10, 74–78).
In an observational study performed on more than 3,700 KTRs, among the 259 cases of de novo malignancies, only one case of adrenocortical carcinoma was described (76).
In a case report, 79 months after the transplantation, a patient simultaneously developed papillary thyroid carcinoma and oxyphilic cell adrenal carcinoma treated with surgical eradications without adjuvant therapy (77). Another rare event is the incidental renal cell carcinoma after en-bloc resection for adrenal carcinoma (78). To minimize the risk of progression immunosuppression was minimized and mTOR-I was introduced (78).
5 Hyperparathyroidism and parathyroid adenoma/carcinoma
Hyperparathyroidism is a frequent complication at the time of kidney transplantation which often resolves within 1 year post-transplant. However, if persistent [particularly in KTRs with a long dialysis vintage, dysmetabolism/obesity, use of high doses of calcimimetic, high pre-transplant levels of PTH and hypercalcemia at the time of kidney transplantation (79)] it may have a significant impact on the graft function.
Patients with PTH >70 pg/ml 1 year after transplantation had a 1.37-fold higher risk of all-cause graft loss and a 1.6-fold higher risk of death-censored graft loss compared with patients without post-transplant hyperparathyroidism (80). Likewise, PTH >150 pg/ml at 3 months after kidney transplantation was an independent predictor of long-term allograft functional impairment (81).
Although the mechanisms involved in this condition are still under investigation, some studies have suggested that high levels of PTH can induce endothelial damage and cause structural vascular alterations, making those vessels less responsive to changes in blood flow and blood pressure (82, 83).
Additionally, hyperparathyroidism may induce nephrocalcinosis with consequent risk of severe impairment of graft function (84, 85).
Possible therapies for hyperthyroidism include vitamin D and vitamin D analogs, use of calcimimetics, and partial or total parathyroidectomy (39).
However, parathyroid carcinoma is rare in KTRs (86), and only a few case reports have described histological findings of parathyroid carcinoma or adenoma after parathyroidectomy for tertiary hyperparathyroidism (87, 88) (Table 1).
6 Conclusion
To date, a well-standardized clinical approach to endocrine tumors in KTRs remains an unmet need, and no specific guidelines have been proposed to guide the diagnosis and treatment of these conditions. In addition, the impact of immunosuppression on the onset and development of these tumors is poorly understood. Most of the studies were performed on the general population, and the small number of patients did not allow us to draw definitive conclusions.
In addition, collaborative networking (including endocrinologists, surgeons, and molecular pathologists) is needed to early identify and treat KTRs affected by these complex, often rare, cancers. Finally, larger multicenter international studies are needed to define the impact on the health system and to standardize the management of these clinical conditions in kidney transplant recipients.
Author contributions
BP: Writing – original draft. FL: Writing – original draft. RG: Writing – original draft. MM: Writing – review & editing. MP: Writing – review & editing. GZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Recovery and Resilience Plan (NRRP), Mission 4, Component 2, Investment 1.1, Call for tender No. 104 published on 2-2-2022 by the Italian Ministry of University and Research (MUR), funded by the European Union—NextGenerationEU—Project Title DEvelopment and Testing of an artifiCial intelligence Tool for predicting end-Stage kidney disease (Prot. 2022FH7889).
Conflict of interest
The author(s) declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mudiayi D, Shojai S, Okpechi I, Christie EA, Wen K, Kamaleldin M, et al. Global estimates of capacity for kidney transplantation in world countries and regions. Transplantation. (2022) 106:1113–22. doi: 10.1097/TP.0000000000003943
2. Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. (2015) 385:1975–82. doi: 10.1016/S0140-6736(14)61601-9
3. Poggio ED, Augustine JJ, Arrigain S, Brennan DC, Schold JD. Long-term kidney transplant graft survival-making progress when most needed. Am J Transplant. (2021) 21:2824–32. doi: 10.1111/ajt.16463
4. Malhotra D, Jethwani P. Preventing rejection of the kidney transplant. J Clin Med. (2023) 12:5938. doi: 10.3390/jcm12185938
5. Massicotte-Azarniouch D, Noel JA, Knoll GA. Epidemiology of cancer in kidney transplant recipients. Semin Nephrol. (2024) 44:151494. doi: 10.1016/j.semnephrol.2024.151494
6. Tessari G, Maggiore U, Zaza G, Momo REN, Nacchia F, Boschiero L, et al. Mortality from cancer is not increased in elderly kidney transplant recipients compared to the general population: a competing risk analysis. J Nephrol. (2020) 33:1309–19. doi: 10.1007/s40620-020-00847-5
7. Friman TK, Jäämaa-Holmberg S, Åberg F, Helanterä I, Halme M, Pentikäinen MO, et al. Cancer risk and mortality after solid organ transplantation: a population-based 30-year cohort study in Finland. Int J Cancer. (2022) 150:1779–91. doi: 10.1002/ijc.33934
8. Kim JH, Kim SO, Han DJ, Park SK. Post-transplant malignancy: a burdensome complication in renal allograft recipients in Korea. Clin Transplant. (2014) 28:434–42. doi: 10.1111/ctr.12328
9. Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. (2007) 7:941–8. doi: 10.1111/j.1600-6143.2007.01736.x
10. Fuhrmann JD, Valkova K, von Moos S, Wüthrich RP, Müller TF, Schachtner T. Cancer among kidney transplant recipients >20 years after transplantation: post-transplant lymphoproliferative disorder remains the most common cancer type in the ultra long-term. Clin Kidney J. (2022) 15:1152–9. doi: 10.1093/ckj/sfac013
11. Jeong S, Lee HS, Kong SG, Kim DJ, Lee S, Park MJ, et al. Incidence of malignancy and related mortality after kidney transplantation: a nationwide, population-based cohort study in Korea. Sci Rep. (2020) 10:21398. doi: 10.1038/s41598-020-78283-5
12. Krynitz B, Edgren G, Lindelöf B, Baecklund E, Brattström C, Wilczek H, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008–a Swedish population-based study. Int J Cancer. (2013) 132:1429–38. doi: 10.1002/ijc.27765
13. McCaughan JA, Courtney AE. The clinical course of kidney transplant recipients after 20 years of graft function. Am J Transplant. (2015) 15:734–40. doi: 10.1111/ajt.13041
14. Wong G, Turner RM, Chapman JR, Howell M, Lim WH, Webster AC, et al. Time on dialysis and cancer risk after kidney transplantation. Transplantation. (2013) 95:114–21. doi: 10.1097/TP.0b013e31827743b4
15. Ma MK, Lim WH, Turner RM, Chapman JR, Craig JC, Wong G. The risk of cancer in recipients of living-donor, standard and expanded criteria deceased donor kidney transplants: a registry analysis. Transplantation. (2014) 98:1286–93. doi: 10.1097/TP.0000000000000375
16. Lim WH, Chapman JR, Wong G. Peak panel reactive antibody, cancer, graft, and patient outcomes in kidney transplant recipients. Transplantation. (2015) 99:1043–50. doi: 10.1097/TP.0000000000000469
17. Lim WH, Turner RM, Chapman JR, Ma MK, Webster AC, Craig JC, et al. Acute rejection, T-cell-depleting antibodies, and cancer after transplantation. Transplantation. (2014) 97:817–25. doi: 10.1097/01.TP.0000442773.38510.32
18. Sprangers B, Nair V, Launay-Vacher V, Riella LV, Jhaveri KD. Risk factors associated with post-kidney transplant malignancies: an article from the Cancer-Kidney International Network. Clin Kidney J. (2018) 11:315–29. doi: 10.1093/ckj/sfx122
19. Benoni H, Eloranta S, Dahle DO, Svensson MHS, Nordin A, Carstens J, et al. Relative and absolute cancer risks among Nordic kidney transplant recipients-a population-based study. Transpl Int. (2020) 33:1700–10. doi: 10.1111/tri.13734
20. Al-Adra D, Al-Qaoud T, Fowler K, Wong G. De novo malignancies after kidney transplantation. Clin J Am Soc Nephrol. (2022) 17:434–43. doi: 10.2215/CJN.14570920
21. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. (2009) 9(Suppl 3):S1–155 doi: 10.1111/j.1600-6143.2009.02834.x
22. Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. (2010) 10:1889–96. doi: 10.1111/j.1600-6143.2010.03181.x
23. Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. (2004) 4:905–13. doi: 10.1111/j.1600-6143.2004.00450.x
24. Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. (2011) 306:1891–901. doi: 10.1001/jama.2011.1592
25. Lengwiler E, Stampf S, Zippelius A, Salati E, Zaman K, Schäfer N, et al. Solid cancer development in solid organ transplant recipients within the Swiss Transplant Cohort Study. Swiss Med Wkly. (2019) 149:w20078. doi: 10.4414/smw.2019.20078
26. Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. (2006) 296:2823–31. doi: 10.1001/jama.296.23.2823
27. Kyllönen L, Salmela K, Pukkala E. Cancer incidence in a kidney-transplanted population. Transpl Int. (2000) 13:S394–8. doi: 10.1111/j.1432-2277.2000.tb02068.x
28. Adami J, Gäbel H, Lindelöf B, Ekström K, Rydh B, Glimelius B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. (2003) 89:1221–7. doi: 10.1038/sj.bjc.6601219
29. Tessari G, Naldi L, Boschiero L, Minetti E, Sandrini S, Nacchia F, et al. Incidence of primary and second cancers in renal transplant recipients: a multicenter cohort study. Am J Transplant. (2013) 13:214–21. doi: 10.1111/j.1600-6143.2012.04294.x
30. Piselli P, Serraino D, Cimaglia C, Furian L, Biancone L, Busnach G, et al. Variation in post-transplant cancer incidence among italian kidney transplant recipients over a 25-year period. Cancers. (2023) 15:1347. doi: 10.3390/cancers15041347
31. Buxeda A, Redondo-Pachón D, Pérez-Sáez MJ, Bartolomé Á, Mir M, Pascual-Dapena A, et al. Gender differences in cancer risk after kidney transplantation. Oncotarget. (2019) 10:3114–28. doi: 10.18632/oncotarget.26859
32. Végso G, Tóth M, Hídvégi M, Toronyi E, Langer RM, Dinya E, et al. Malignancies after renal transplantation during 33 years at a single center. Pathol Oncol Res. (2007) 13:63–9. doi: 10.1007/BF02893443
33. Wisgerhof HC, van der Geest LG, de Fijter JW, Haasnoot GW, Claas FH, le Cessie S, et al. Incidence of cancer in kidney-transplant recipients: a long-term cohort study in a single center. Cancer Epidemiol. (2011) 35:105–11. doi: 10.1016/j.canep.2010.07.002
34. Au E, Wong G, Chapman JR. Cancer in kidney transplant recipients. Nat Rev Nephrol. (2018) 14:508–20. doi: 10.1038/s41581-018-0022-6
35. Wang Y, Lan GB, Peng FH, Xie XB. Cancer risks in recipients of renal transplants: a meta-analysis of cohort studies. Oncotarget. (2017) 9:15375–85. doi: 10.18632/oncotarget.23841
36. Wong G, Hayen A, Chapman JR, Webster AC, Wang JJ, Mitchell P, et al. Association of CKD and cancer risk in older people. J Am Soc Nephrol. (2009) 20:1341–50. doi: 10.1681/ASN.2008090998
37. Hu M, Wang Q, Liu B, Ma Q, Zhang T, Huang T, et al. Chronic kidney disease and cancer: inter-relationships and mechanisms. Front Cell Dev Biol. (2022) 10:868715. doi: 10.3389/fcell.2022.868715
38. Kitahara CM, Yanik EL, Ladenson PW, Hernandez BY, Lynch CF, Pawlish KS, et al. Risk of thyroid cancer among solid organ transplant recipients. Am J Transplant. (2017) 17:2911–21. doi: 10.1111/ajt.14310
39. Chadban SJ, Ahn C, Axelrod DA, Foster BJ, Kasiske BL, Kher V, et al. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation. (2020) 104(4S1 Suppl 1):S11–103 doi: 10.1097/TP.0000000000003136
40. Acuna SA, Huang JW, Scott AL, Micic S, Daly C, Brezden-Masley C, et al. Cancer screening recommendations for solid organ transplant recipients: a systematic review of clinical practice guidelines. Am J Transplant. (2017) 17:103–14. doi: 10.1111/ajt.13978
41. Cheung CY, Tang SCW. An update on cancer after kidney transplantation. Nephrol Dial Transplant. (2019) 34:914–20. doi: 10.1093/ndt/gfy262
42. Heo J, Noh OK, Oh YT, Chun M, Kim L. Cancer risk after renal transplantation in South Korea: a nationwide population-based study. BMC Nephrol. (2018) 19:311. doi: 10.1186/s12882-018-1110-3
43. Hortlund M, Arroyo Mühr LS, Storm H, Engholm G, Dillner J, Bzhalava D. Cancer risks after solid organ transplantation and after long-term dialysis. Int J Cancer. (2017) 140:1091–101. doi: 10.1002/ijc.30531
44. Schrem H, Schneider V, Kurok M, Goldis A, Dreier M, Kaltenborn A, et al. Independent pre-transplant recipient cancer risk factors after kidney transplantation and the utility of G-chart analysis for clinical process control. PLoS ONE. (2016) 11:e0158732. doi: 10.1371/journal.pone.0158732
45. Kim B, Kang M, Kim Y, Lee HS, Kim B, Lee JJ, et al. De novo cancer incidence after kidney transplantation in South Korea from 2002 to 2017. J Clin Med. (2021) 10:3530. doi: 10.3390/jcm10163530
46. Hibberd AD, Trevillian PR, Wlodarczyk JH, Kemp DG, Stein AM, Gillies AH, et al. Effect of immunosuppression for primary renal disease on the risk of cancer in subsequent renal transplantation: a population-based retrospective cohort study. Transplantation. (2013) 95:122–7. doi: 10.1097/TP.0b013e3182782f59
47. Mäkitie AA, Lundberg M, Salmela K, Kyllönen L, Pukkala E. Head and neck cancer in renal transplant patients in Finland. Acta Otolaryngol. (2008) 128:1255–8. doi: 10.1080/00016480801901725
48. Hoshida Y, Tsukuma H, Yasunaga Y, Xu N, Fujita MQ, Satoh T, et al. Cancer risk after renal transplantation in Japan. Int J Cancer. (1997) 71:517–20. doi: 10.1002/(SICI)1097-0215(19970516)71:4<
49. Birkeland SA, Løkkegaard H, Storm HH. Cancer risk in patients on dialysis and after renal transplantation. Lancet. (2000) 355:1886–7. doi: 10.1016/S0140-6736(00)02298-4
50. Karakose S, Cordan I, Gonulalan G, Karakose M, Kurtgoz PO, Baloglu I, et al. Thyroid disorders prevalence in a cohort of kidney transplant recipients. Acta Endocrinol. (2020) 16:324–8. doi: 10.4183/aeb.2020.324
51. Veroux M, Giuffrida G, Lo Bianco S, Cannizzaro MA, Corona D, Giaquinta A, et al. Thyroid disease and cancer in kidney transplantation: a single-center analysis. BMC Surg. (2019) 18:80. doi: 10.1186/s12893-018-0408-1
52. Lee J, Jeong JJ, Lee YS, Nam KH, Chang HS, Chung WY, et al. Incidence and clinical behavior of papillary thyroid carcinoma in renal allograft recipients: a single center experience. Transplant Proc. (2008) 40:3751–4. doi: 10.1016/j.transproceed.2008.07.127
53. Karamchandani D, Arias-Amaya R, Donaldson N, Gilbert J, Schulte KM. Thyroid cancer and renal transplantation: a meta-analysis. Endocr Relat Cancer. (2010) 17:159–67. doi: 10.1677/ERC-09-0191
54. Pond F, Serpell JW, Webster A. Thyroid cancer in the renal transplant population: epidemiological study. ANZ J Surg. (2005) 75:106–9. doi: 10.1111/j.1445-2197.2005.03311.x
55. Park B, Yoon J, Choi D, Kim HJ, Jung YK, Kwon OJ, et al. De novo cancer incidence after kidney and liver transplantation: results from a nationwide population based data. Sci Rep. (2019) 9:17202. doi: 10.1038/s41598-019-53163-9
56. Hu L, Wu Y, Ju F, Zhang Y, Wang W. Effects of solid organ transplantation on the risk of developing thyroid cancer: a systematic review and meta-analysis. Gland Surg. (2022) 11:710–9. doi: 10.21037/gs-22-137
57. Łebkowska U, Małyszko JS, Małyszko J, Dziecioł J, Walecki J, Myśliwiec M. Thyroid function and incidentalomas in kidney transplant recipients. Med Sci Monit. (2003) 9:MT8–11.
58. Tisset H, Kamar N, Faugeron I, Roy P, Pouteil-Noble C, Klein M, et al. Is thyroid cancer recurrence risk increased after transplantation? J Clin Endocrinol Metab. (2013) 98:3981–8. doi: 10.1210/jc.2013-1357
59. Hu L, Wu Y, Ju F, Zhang Y, Wang W. Analysis of clinicopathological features of papillary thyroid carcinoma in solid organ transplant recipients: a retrospective study. Gland Surg. (2022) 11:1404–14. doi: 10.21037/gs-22-431
60. Derwich A, Sykutera M, Bromińska B, Andrusiewicz M, Ruchała M, Sawicka-Gutaj N. Clinical implications of mTOR expression in papillary thyroid cancer-a systematic review. Cancers. (2023) 15:1665. doi: 10.3390/cancers15061665
61. Hellström VC, Enström Y, von Zur-Mühlen B, Hagberg H, Laurell A, Nyberg F, et al. Malignancies in transplanted patients: multidisciplinary evaluation and switch to mTOR inhibitors after kidney transplantation - experiences from a prospective, clinical, observational study. Acta Oncol. (2016) 55:774–81. doi: 10.3109/0284186X.2015.1130855
62. Oh Y, Park JH, Djunadi TA, Shah Z, Chung LI, Chae YK. Deep response to a combination of mTOR inhibitor temsirolimus and dual immunotherapy of nivolumab/ipilimumab in poorly differentiated thyroid carcinoma with PTEN mutation: a case report and literature review. Front Endocrinol. (2024) 15:1304188. doi: 10.3389/fendo.2024.1304188
63. Hall EC, Engels EA, Pfeiffer RM, Segev DL. Association of antibody induction immunosuppression with cancer after kidney transplantation. Transplantation. (2015) 99:1051–7. doi: 10.1097/TP.0000000000000449
64. Romagnoli J, Tagliaferri L, Acampora A, Bianchi V, D'Ambrosio V, D'Aviero A, et al. Management of the kidney transplant patient with cancer: report from a multidisciplinary consensus conference. Transplant Rev. (2021) 35:100636. doi: 10.1016/j.trre.2021.100636
65. Shah HA, Faulkes R, Coldham C, Shetty S, Shah T. Effects of transplantation-related immunosuppression on co-existent neuroendocrine tumours. QJM. (2022) 115:661–4. doi: 10.1093/qjmed/hcac036
66. Karunanithi S, Roy SG, Sharma P, Yadav R, Bal C, Kumar R. Metastatic neuroendocrine tumour in a renal transplant recipient: dual-tracer PET-CT with (18)F-FDG and (68)Ga-DOTANOC in this rare setting. Nucl Med Mol Imaging. (2015) 49:57–60. doi: 10.1007/s13139-014-0297-x
67. Brady JE Jr, Tamburro L, Joy AG, Ugarte RM. Donor origin neuroendocrine cancer: a case report and literature review. Transplant Direct. (2023) 9:e1524. doi: 10.1097/TXD.0000000000001524
68. Foltys D, Linkermann A, Heumann A, Hoppe-Lotichius M, Heise M, Schad A, et al. Organ recipients suffering from undifferentiated neuroendocrine small-cell carcinoma of donor origin: a case report. Transplant Proc. (2009) 41:2639–42. doi: 10.1016/j.transproceed.2009.06.026
69. Takeda K, Mittenzwei R, Geisinger KR, Datto MB, Rebellato LM. Donor-derived neuroendocrine carcinoma transmission to two kidney transplant recipients demonstrated by short tandem repeat analysis: a case report. Transplant Proc. (2021) 53:1337–41. doi: 10.1016/j.transproceed.2021.03.002
70. Saleeb R, Faragalla H, Yousef GM, Stewart R, Streutker CJ. Malignancies in a renal transplant population: the St. Michael's Hospital experience. Urol Ann. (2016) 8:163–7. doi: 10.4103/0974-7796.165712
71. Eccher A, Girolami I, Motter JD, Marletta S, Gambaro G, Momo REN, et al. Donor-transmitted cancer in kidney transplant recipients: a systematic review. J Nephrol. (2020) 33:1321–32. doi: 10.1007/s40620-020-00775-4
72. Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2020) 31:844–60. doi: 10.1016/j.annonc.2020.03.304
73. Dantal J, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, et al. Sirolimus for secondary prevention of skin cancer in kidney transplant recipients: 5-year results. J Clin Oncol. (2018) 36:2612–20. doi: 10.1200/JCO.2017.76.6691
74. Lazareth H, Cohen D, Vasiliu V, Tinel C, Martinez F, Grünfeld JP, et al. Paraganglioma of the bladder in a kidney transplant recipient: a case report. Mol Clin Oncol. (2017) 6:553–5. doi: 10.3892/mco.2017.1182
75. Hanna-Moussa A, Kurukulasuriya LR, Sowers JR. Malignant pheochromocytoma presenting with uncontrolled hypertension after kidney transplant. J Clin Hypertens. (2010) 12:105–8. doi: 10.1111/j.1751-7176.2009.00221.x
76. Ban TH, Park WY, Jin K, Han S, Chung BH, Park SC, et al. Changing pattern and safety of pretransplant malignancy in kidney transplant recipients. Kidney Res Clin Pract. (2019) 38:509–16. doi: 10.23876/j.krcp.19.047
77. Lo Monte AI, Palumbo VD, Damiano G, Maione C, Florena AM, Gioviale MC, et al. Double endocrine neoplasia in a renal transplant recipient: case report and review of the literature. Transplant Proc. (2011) 43:1201–5. doi: 10.1016/j.transproceed.2011.02.040
78. Park P, Kim WY, Lee JB, Choi SB, Kim WB, Choi SY. Incidental renal cell carcinoma originating from a native kidney after en-bloc resection for adrenal carcinoma in a kidney transplant recipient. Transplant Proc. (2014) 46:637–9. doi: 10.1016/j.transproceed.2013.11.078
79. Sutton W, Chen X, Patel P, Karzai S, Prescott JD, Segev DL, et al. Prevalence and risk factors for tertiary hyperparathyroidism in kidney transplant recipients. Surgery. (2022) 171:69–76. doi: 10.1016/j.surg.2021.03.067
80. Crepeau P, Chen X, Udyavar R, Morris-Wiseman LF, Segev DL, McAdams-DeMarco M, et al. Hyperparathyroidism at 1 year after kidney transplantation is associated with graft loss. Surgery. (2023) 173:138–45. doi: 10.1016/j.surg.2022.07.031
81. Isakov O, Ghinea R, Beckerman P, Mor E, Riella LV, Hod T. Early persistent hyperparathyroidism post-renal transplantation as a predictor of worse graft function and mortality after transplantation. Clin Transplant. (2020) 34:e14085. doi: 10.1111/ctr.14085
82. Barenbrock M, Hausberg M, Kosch M, Kisters K, Hoeks AP, Rahn KH. Effect of hyperparathyroidism on arterial distensibility in renal transplant recipients. Kidney Int. (1998) 54:210–5. doi: 10.1046/j.1523-1755.1998.00963.x
83. Suwelack B, Gerhardt U, Witta J, Hillebrandt U, Hohage H. Effect of parathyroid hormone levels on carotid intima-media thickness after renal transplantation. Am J Hypertens. (2001) 14:1012–8. doi: 10.1016/S0895-7061(01)02144-6
84. Levi M, Ellis MA, Berl T. Control of renal hemodynamics and glomerular filtration rate in chronic hypercalcemia. Role of prostaglandins, renin-angiotensin system, and calcium. J Clin Invest. (1983) 71:1624–32. doi: 10.1172/JCI110918
85. Gwinner W, Suppa S, Mengel M, Hoy L, Kreipe HH, Haller H, et al. Early calcification of renal allografts detected by protocol biopsies: causes and clinical implications. Am J Transplant. (2005) 5:1934–41. doi: 10.1111/j.1600-6143.2005.00938.x
86. Obregón LM, Taylor MF, Mir G, Pereyra CA, Pianzola H, Petrone H, et al. Parathyroidectomy for parathyroid carcinoma in renal transplantation. Transplant Proc. (2005) 37:973–6. doi: 10.1016/j.transproceed.2004.12.305
87. Kim BS Ryu HS, Kang KH, Park SJ. Parathyroid carcinoma in tertiary hyperparathyroidism. Asian J Surg. (2016) 39:255–9. doi: 10.1016/j.asjsur.2013.01.007
Keywords: endocrine malignancies, kidney transplantation, thyroid cancer, adrenal cortical carcinomas, pheochromocytomas, paragangliomas
Citation: Pellegrini B, Leone F, Greco R, Maggiolini M, Provenzano M and Zaza G (2025) Endocrine malignancies: a still neglected issue in kidney transplantation. Front. Med. 12:1598168. doi: 10.3389/fmed.2025.1598168
Received: 22 March 2025; Accepted: 23 May 2025;
Published: 17 June 2025.
Edited by:
Angela Gonzalez, Hospital Clinic of Barcelona, SpainReviewed by:
Giulia Vanessa Re Sartò, University of Milan, ItalyCopyright © 2025 Pellegrini, Leone, Greco, Maggiolini, Provenzano and Zaza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gianluigi Zaza, Z2lhbmx1aWdpLnphemFAdW5pY2FsLml0
 Bianca Pellegrini1
Bianca Pellegrini1 Francesca Leone
Francesca Leone Marcello Maggiolini
Marcello Maggiolini Gianluigi Zaza
Gianluigi Zaza