- School of International Pharmaceutical Business, China Pharmaceutical University, Nanjing, China
Objective: This study systematically compares the safety information presented on drug labels for antineoplastic and supportive care medications listed on the WHO Essential Medicines List (EML) across three major pharmaceutical markets: China, Japan, and the United States.
Methods: The safety information of the same drugs approved in the three countries was compared and analyzed, involving the calculation of the proportion of each safety information and the percentage of boxed warnings (PBW) to all information on the label, we also comparative analysis of pediatric medication information studied from each country.
Results: There were substantial differences in each safety information of the labels in three countries. Except for the proportion of information on use in the elderly, the proportion of each type of safety information differed significantly between countries, and the content also varied widely. Concordance of the presence or the absence of a BW on the label between China, Japan, and the US was 36% on the total labels. Of the 19 drugs, Japan had the most pediatric drug information with 13 drugs, followed by the US with nine drugs and China with eight drugs.
Conclusion: There were significant differences in the safety information on drug labels in China, Japan, and the US from the same manufacturer, which could pose a risk to patients. Regulatory agencies should better align their approaches to labeling to prevent confusion or misunderstanding across regions.
1 Introduction
In recent years, the number of cancer patients and the burden of cancer disease have increased worldwide (1). In 2019, the World Health Organization (WHO) released the latest WHO EML, adding 12 new cancer medicines to 48 antineoplastic and supportive medicines to help countries prioritize widely available and affordable cancer medicines. The global circulation and use of anticancer drugs have intensified, which has led to greater awareness of each other’s approaches, decision-making, and interactions seeking alignment among drug regulatory authorities around the world (2), which is essential as it allows for the assessment of drug safety and efficacy (3), as well as the oversight of drug labeling to provide health care professionals and patients with comprehensive information about the efficacy, safety, and use of drugs (4). Harmonized drug labels can ensure that everyone, regardless of region, receives the same comprehensive information necessary to make an informed decision (3).
However, there are differences in the content and regulation of labels from country to country, and researchers have tried to identify the causes of differences in drug labels between regions, with various results. One study found significant differences by comparing the safety information in labeling new molecular entities in the US, United Kingdom (UK), and Japan (5). Another study compared Food and Drug Administration (FDA) Package Inserts (PIs) and European Medicines Agency (EMA) Summary of Product Characteristics (SmPCs) and found poor consistency between the two regions (3). A comparison study of adverse drug reactions and boxed warnings on oncology drug labels in Japan and the US found significant differences in all sections of the labels between the two countries, due to differences in regulatory and historical factors in both local and global contexts (6). Some studies have found a low level of consistency of safety information in package inserts in Japan and the US and a low consistency between regulatory decisions on labeling changes and the timeliness of those changes (7, 8).
China consumes a lot of anti-cancer drugs every year, and the regulation and use of drug inserts deserve attention (9), but there are few studies on Chinese drug labels, so we chose the US and Japan, which are also member of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), to conduct a study on the comparison of anti-cancer drug labels. The three agencies conduct independent reviews of available data and have their guiding standards for labeling format and content; each regulatory agency’s approach, language, and recommendations for use may differ (10). Our objective was to compare Chinese, Japanese, and US labeling for the same drug to determine whether there are consistent similarities or differences and to provide an evidence base for better regulation of drug safety.
2 Methods
2.1 Data sources
This study was a cross-sectional study conducted in the period between June and October 2023, with data collection from June to August 2023. We searched which of the 60 antineoplastic and supportive drugs on the WHO EML were imported from China National Medical Products Administration (11) (NMPA). Then, according to the manufacturer of the drug imported in China, we went to the Pharmaceuticals and Medical Devices Agency (PMDA), and the DailyMed to find the drug label of the same manufacturer (CSL STYLE ERROR: reference with no printed form). If there is a drug from the same manufacturer that is not available in Japan or the US, we use the Japanese Orange Book or the FDA Orange Book to find the reference listed drug (RLD) labels to replace it (12, 13). The labels must be approved in NMPA and PMDA or the US FDA. Labels must be accessible in the public domain.
2.2 Variable definitions, evaluation, and analysis
We performed a direct comparison of the proportion of all information given to each safety information across the same drug and the proportion of the number of labels with box warnings or warnings to that of all labels in the three countries. For example, the proportion of adverse reaction information was calculated by dividing number of adverse reaction information words by the total number of safety information words. We defined the measures independently of language: for Chinese and English, we counted the number of words, and for Japanese, we counted the number of letters in the safety sections, and then divided this by the total number of words or letters on the label. Each number of safety information words was calculated based on the sections listed in Table 1 from each country.
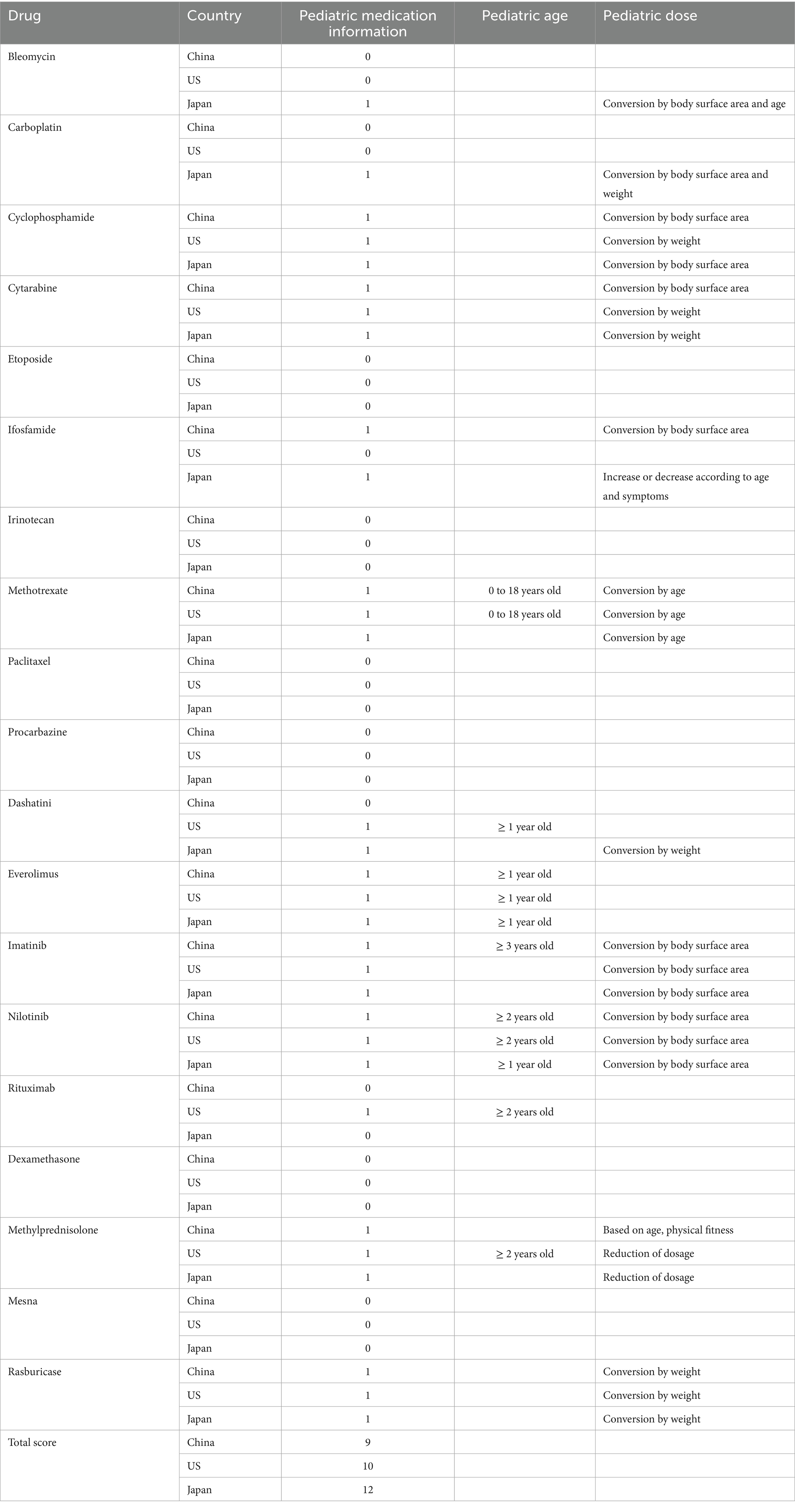
Pediatric use is often omitted in oncology labels. We narrowed the analysis to drugs with explicit pediatric information (dosage/age) in ≥1 country. We conducted a comparative analysis of pediatric medication information for 19 medicines across three countries, including information such as pediatric medication dosage and age on the label. If there is information on this drug for children, we mark it as 1, and if the trial was not conducted or the safety is unclear, we mark it as 0. Finally, we added up the scores of all 19 medicines in each country, and the higher the score, the more complete pediatric medication information in that country (Table 2).
Warnings convey important safety information in eye-catching fonts on the first page of labels, alerting healthcare professionals and patients to medication risks. We identified a boxed warning (BW) on Chinese, Japanese, and US drug labels through a manual search and measured PBW on all labels.
2.3 Comparison of drug label safety information
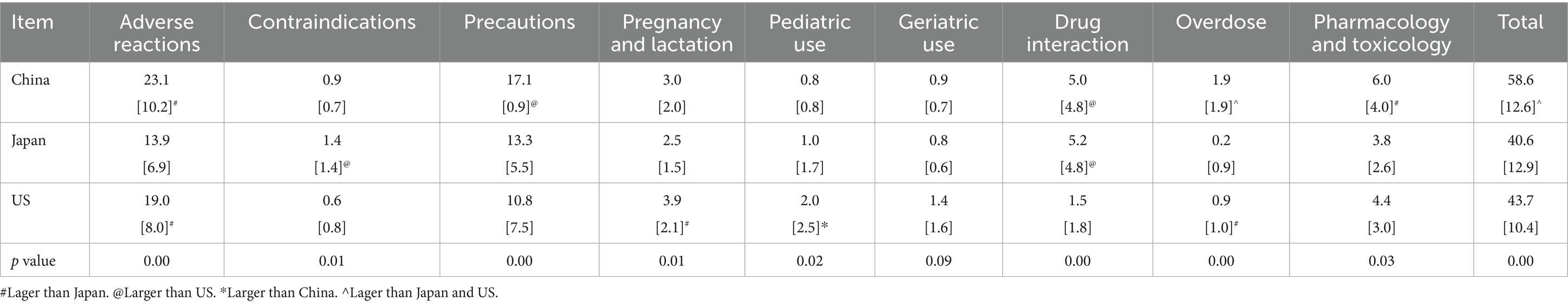
There is a possibility that the volume of text is different, but the information conveyed is the same (14). To understand the differences in the information provided across the three countries, we read the content of each safety information, gave the complete content a score of 1, the incomplete or missing content a score of 0, and then summed the scores of each item; the higher the score, the more complete the content of each safety information (Table 3).
2.4 Statistical analysis
Descriptive statistics were performed on the data obtained. Data were presented as the mean and standard deviation (SD). One-way analysis of variance (One-way ANOVA) was performed to find out the differences between groups followed by Scheffe’s post hoc test to determine which means were different with a level of significance set at p < 0.05. Data were analyzed using Statistical Analysis Software (SPSS 26.0).
3 Results
Of the 60 drugs, 35 drugs were imported into China, but 2 drugs were excluded because no labels were found for the drugs in China. According to the manufacturer of 33 drugs, we went to PMDA and DailyMed to find the drug label of the same manufacturer and found 15 drug labels. For an additional 18 drugs, we used the reference-listed drug labels to replace them. Finally, 33 drug groups were finally included in our study.
The total number of label words on the Chinese labels {16,003 (10178) [mean (standard deviation)]} was higher than the number of Japan [12,196 (6406)] and the US [12,278 (8998)] label words with a p-value> 0.05. The total number of safety words was higher on Chinese labels [8,903 (5019)] when compared to Japan [4,829 (2616)] and the US [5,304 (4194)] label safety words with a p-value < 0.05.
3.1 Proportion of each safety information
Table 4 shows the proportion of each safety information on drug labels in three countries. The proportion of each safety information was significantly different across the countries except for the proportion of geriatric use information.
The proportion of adverse reaction information on Chinese labels {23.1 (10.2) [mean (standard deviation)]} and US labels (19.0 [8.0]) was higher than on Japanese labels [13.9 (6.9)]. The proportion of contraindication information on Japanese labels [1.4 (1.4)] was higher than on US labels [0.6 (0.8)]. The proportion of precaution information on Chinese labels [17.1 (0.9)] was higher than on US labels [10.8 (7.5)]. The proportion of pregnancy and lactation information on US labels [3.9 (2.1)] was higher than on Japanese labels [2.5 (1.5)]. The proportion of pediatric use information on US labels [2.0 (2.5)] was higher than on Chinese labels [0.8 (0.8)]. The proportion of drug interaction information on Chinese labels [5.0 (4.8)] and Japanese labels [5.2 (4.8)] was higher than on US labels [1.5 (1.8)]. The proportion of overdose information on Chinese labels [1.9 (1.9)] was higher than on Japanese labels [0.2 (0.9)] and US labels [0.9 (1.0)]. The proportion of pharmacology and toxicology information on Chinese labels [6.0 (4.0)] was higher than on Japanese labels [3.8 (2.6)]. The proportion of total safety information on Chinese labels [58.6 (12.6)] was higher than on Japanese labels [40.6 (12.9)] and US labels [43.7 (10.4)] (Figure 1).
3.2 Comparison of drug label safety information
Table 5 shows a detailed comparative analysis of the content of each safety information. We sum the scores of each safety information and then get the total score for each item in three countries (Table 3). Chinese labels achieved the highest scores in overdose, pharmacology, and toxicology, suggesting that these sections are more comprehensively labeled. Japan scored highest in contraindications, precautions, and drug interactions, while the US received the highest scores in adverse reactions, pregnancy and lactation, pediatric use, and geriatric use. Overall, Chinese drug label safety information total scores are higher than those in the US and Japan.

Table 5. Comparative analysis of the content of each safety information in China, Japan and the USA.
3.3 Labels with a warning
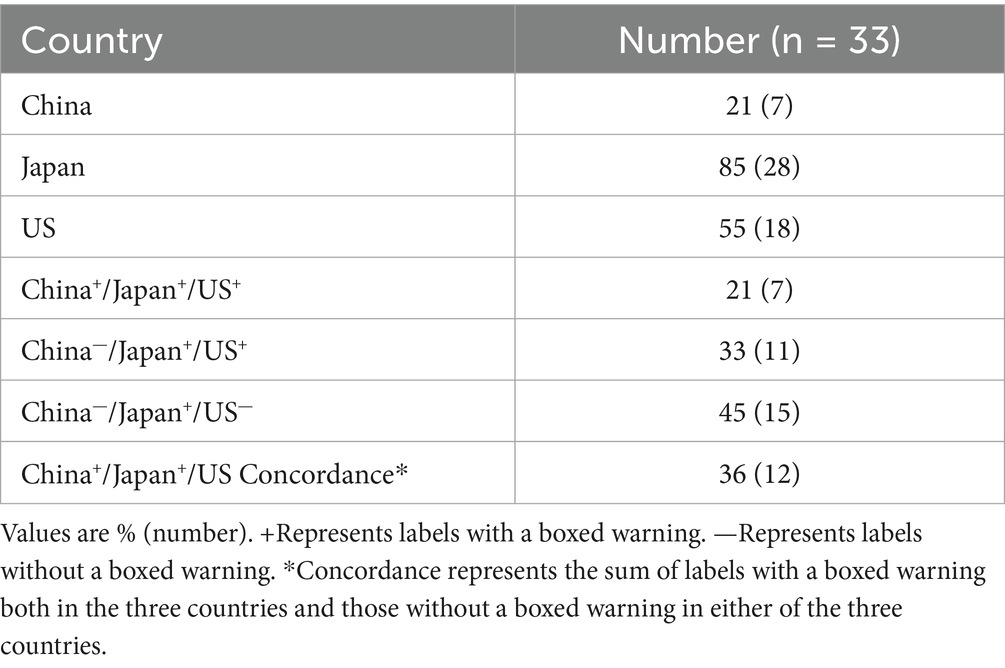
Table 6 shows the proportion of the number of labels with BW in China, Japan and the US. PBW on the Japanese labels (85%) was larger than that on the US labels (55%) and Chinese labels (21%). Concordance of the presence or the absence of a BW on the label between China, Japan, and the US was 36% on the total labels.
3.4 Comparative analysis of pediatric medication information
We compared and analyzed the differences in pediatric medication information for 19 medicines included in the WHO EMLc in three countries (Table 2). Of the 19 drugs, Japan had the most pediatric drug information with 13 drugs, followed by the US with nine drugs and China with eight drugs. And seven out of the 19 drugs have pediatric medication information available in all three countries.
4 Discussion
4.1 Proportion of each safety information
We found significant differences in the proportion of each safety information provided on the same drug label in the three countries, except for geriatric use information. Chinese labels have significantly more proportion in more safety information items than other countries, followed by the US and then Japan, and Chinese labels’ proportion of total safety information is significantly more than the US and Japan. In a previous study conducted on the labels of new molecular entities between the US, the UK, and Canada, it was found that significant differences among the mean proportion of total safety information (PSI) of all three countries (14).
In our study, we also found that there are significant differences among the mean PSI and proportion of many safety information items of all three countries, which is most likely due to the different guidelines set by these authorities for the preparation of drug labels (15–17), as different laws in different countries may influence regulatory decisions (18).
4.2 Comparison of drug label safety information
The proportion of each safety information was generated by counting words/characters, which are crude measurements that do not always indicate critical safety issues (5). So we compared the content of each label’s safety information and found that there were indeed differences between labels in three countries. We found that except for precautions, Chinese labels have significantly more words than Japanese labels, but the score is lower than that of Japan, that is, the information integrity is lower than that of Japan, and the other safety information is higher the number of words, the higher the score.
The Japanese guidelines for writing package labels require precautions to be divided into [precautions related to indications], [precautions related to use and dosage], [important basic precautions], [other precautions], [operational precautions], etc., while the Chinese guidelines for writing package labels do not require precautions to be divided, which may lead to a more complete content of precautions in Japanese package leaflets (15, 16).
We went on to study the section related to adverse reactions in China, Japan, and the US. The proportion of adverse reaction information on Chinese labels and US labels was higher than on Japanese labels and the adverse reaction score on Chinese labels and the US labels was also higher than those in Japanese labels. For the drug mesna, the proportion of adverse reaction information on Chinese labels (0.37) and US labels (0.12) was higher than on Japanese labels (0.05), and the Chinese label contained 11 adverse reactions, the US 15, and Japan only 10.
In the pregnancy and lactation, pediatric use, and geriatric use sections, we also found that the US, which had more words, scored higher. We found that the Chinese and Japanese labels contained vague information about the drug details for certain groups, including that the trial was not conducted, that there were no references, that it was not clear, and so on. For example, there is no data on the presence of everolimus or its metabolites in human milk, the effects of everolimus on the breastfed infant, or on milk production. The lack of human data relevant to pregnant and lactating populations, children and the elderly has long been identified as an area of significant public health need (19), mainly because the current patients participating in pre-marketing clinical trials of drugs are highly selected patients, only qualified patients can participate in clinical trials, and there will be a lack of clinical data for special populations (20).
4.3 Labels with a warning
The warning is displayed in a prominent font below the title of the drug label, which is about serious adverse reactions to medicines and their serious safety concerns, covering items such as contraindications and precautions. Our comparison shows that Japan has a higher proportion of BWs, followed by the US and China. In addition, the concordance of the presence or the absence of a BW on the label between China, Japan, and the US was 36% on the total labels, indicating a low level of coordination between the three countries, which could have implications for medical professionals and patients (3).
Both the US and Japan have stricter requirements for warnings, and contraindications or serious warnings, especially those that could lead to death or serious injury, must be included in boxed warnings. China’s Provisions for Drug Insert Sheets and Labels stipulate that drug manufacturers can take the initiative to add warnings to drug specifications or labels, but the regulations are not sufficiently clear on the legal responsibility for regulating warnings and do not stipulate that regulatory authorities, production and management companies have their responsibilities and must do so. The previous study found that the use of febuxostat decreased significantly in the US after the FDA added a boxed warning to the label for febuxostat (21). Physicians may have responded to the FDA’s warning by prescribing AAPs less often and adopting, instead, alternative therapeutic strategies for managing NPSs in elderly individuals with dementia (22). Therefore, many Chinese pharmaceutical companies do not actively add warnings to their drug labels so as not to affect drug sales.
4.4 Comparative analysis of pediatric medication information
We found that only 7 of the 19 WHO EMLc drugs in three countries had pediatric medication information on their use in children, confirming previous research that pediatric drug use is often missing due to lack of evidence or regulatory delays in label updates, which may put children at higher risk for side effects, adverse drug reactions, and medication errors (23).
We also found differences in pediatric dosing regimens of the same drug in different countries, for example, cytarabine for children with acute leukemia, China uses body surface area to convert drug doses, while the United States and Japan use body weight to convert drug doses. Historically, however, pediatric dosing regimens have often been derived from pharmacokinetic data in adults (24). This approach, although clinically widespread, is not supported by solid empirical evidence and may result in neonates and children being exposed to inappropriate systemic drug levels (25).
While pediatric drug development is international in scope, pediatric drug labeling information is not uniformly shared and is under the control of national regulatory authorities (4), requiring countries to increase cooperation, information sharing, dissemination, discussion, and efforts to reach consensus on regulatory “standards” for quality pediatric medicines.
4.5 Limitations
We acknowledge several limitations of our study. The most important limitation was the generalizability of our findings in antineoplastic and supportive medications to other types of medications. Also, this study included few drug samples, which may have affected the accuracy of the results.
5 Conclusion
There were significant differences in the safety information on drug labels in China, Japan, and the US from the same manufacturer, which could pose a risk to patients. The amount and content of information on drug labels vary from country to country. Regulatory agencies should better align their approaches to labeling to prevent confusion or misunderstanding across regions. This could be done in a variety of forums, including bilaterally as issues arise, starting with an understanding of the thinking underlying our differences, but eventually through standard-setting organizations.
Author contributions
CD: Data curation, Formal analysis, Visualization, Writing – original draft. CW: Data curation, Writing – original draft. PL: Funding acquisition, Project administration, Supervision, Writing – review & editing. JL: Writing - review & editing, Data Curation, Formal analysis, Writing - original draft, Software, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank Professor Eunjoo Pacifici of the D. K. Kim International Center for Regulatory Science at the University of Southern California, who generously provided us with important insights and comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
US, United States; WHO EML, World Health Organization Model List of Essential Medicines; PBW, percentage of boxed warnings; WHO, World Health Organization; UK, United Kingdom; FDA, Food and Drug Administration; PIs, Package Inserts; EMA, European Medicines Agency; SmPCs, Summary of Product Characteristics; ICH, The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; NMPA, National Medical Products Administration; PMDA, Pharmaceuticals and Medical Devices Agency; RLD, Reference Listed Drug; SD, Standard Deviation; PSI, Proportion of Total Safety Information.
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Borges, AP d S, Campos, MS d A, and Pereira, LRL. Evaluation of unlicensed and off-label antiepileptic drugs prescribed to children: Brazilian regulatory agency versus FDA. Int J Clin Pharm. (2013) 35:425–31. doi: 10.1007/s11096-013-9755-2
3. Seo, Y, and Pacifici, E. Elements of regulatory dissonance: examining FDA and EMA product labeling of new vaccines (2006–2018). Vaccine. (2020) 38:7485–9. doi: 10.1016/j.vaccine.2020.09.067
4. Song, Y, Han, N, Burckart, GJ, and Oh, JM. International coherence of pediatric drug labeling for drug safety: comparison of approved labels in Korea and the United States. Clin Pharmacol Ther. (2020) 107:530–40. doi: 10.1002/cpt.1640
5. Shimazawa, R, and Ikeda, M. Safety information in drug labeling: a comparison of the USA, the UK, and Japan. Pharmacoepidemiol Drug Saf. (2013) 22:306–18. doi: 10.1002/pds.3408
6. Jayaputra, K, and Ono, S. Differences between the United States and Japan in labels of oncological drugs. Pharmacoepidemiol Drug Saf. (2017) 26:143–51. doi: 10.1002/pds.4111
7. Hoshino, Y, and Narukawa, M. A comparison of safety information in drug labeling at the initial approval of new drugs approved both in Japan and the United States. Ther Innov Regul Sci. (2022) 56:839–47. doi: 10.1007/s43441-022-00431-y
8. Hoshino, Y, and Narukawa, M. A comparison of decision and timing of safety related labeling changes for new drugs approved both in Japan and the United States. Pharmacoepidemiol Drug Saf. (2021) 30:561–72. doi: 10.1002/pds.5203
9. Maomao, C, He, L, Dianqin, S, Siyi, H, Xinxin, Y, Fan, Y, et al. Current cancer burden in China: epidemiology, etiology, and prevention. Cancer Biol Med. (2022) 19:1121–38. doi: 10.20892/j.issn.2095-3941.2022.0231
10. Kappel, D, Sahin, L, Yao, L, Thor, S, and Kweder, S. A comparison of FDA and EMA pregnancy and lactation labeling. Clin Pharmacol Ther. (2023) 113:1251–7. doi: 10.1002/cpt.2843
11. China National Medical Products Administration Data Search. (2023). Available online at: https://www.nmpa.gov.cn/datasearch/home-index.html#category=yp (Accessed Sep 4, 2023).
12. Food and Drug Administration. Orange book: approved drug products with therapeutic equivalence evaluations. (2023). Available online at: https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm (Accessed Sep 4, 2023).
13. Pharmaceuticals and Medical Devices Agency. Japanese Orange Book home page. (2023). Available online at: http://www.jp-orangebook.gr.jp/english/ (Accessed Sep 4, 2023).
14. Alshammari, TM, Devadasu, VR, and Rathnam, RP. Comparison of the safety information on drug labels in three developed countries: the USA, UK and Canada. Saudi Pharm J. (2017) 25:1103–7. doi: 10.1016/j.jsps.2017.07.006
15. Center for Drug Evaluation, NMPA. General formats and drafting guidelines for instructions for chemical and biological products. (2022). Available online at: https://amr.hainan.gov.cn/himpa/HICDME/zdyz/yp/202207/t20220719_3232706.html (Accessed Nov 4, 2023).
16. Pharmaceuticals and Medical Devices Agency. Precautions for the description of package inserts, etc. for prescription drugs. (2017). Available online at: https://www.mhlw.go.jp/web/t_doc?dataId=00tc2704&dataType=1&pageNo=1 (Accessed Nov 30, 2023).
17. Research C for DE. Instructions for use — patient labeling for human prescription drug and biological products — content and format. (2022). Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/instructions-use-patient-labeling-human-prescription-drug-and-biological-products-content-and-format (Accessed Oct 4, 2023).
18. Shimazawa, R, and Ikeda, M. Differences in pharmacogenomic biomarker information in package inserts from the United States, the United Kingdom and Japan. J Clin Pharm Ther. (2013) 38:468–75. doi: 10.1111/jcpt.12089
19. Dinatale, M, Roca, C, Sahin, L, Johnson, T, Mulugeta, LY, Fletcher, EP, et al. The importance of clinical research in pregnant women to inform prescription drug labeling. J Clin Pharmacol. (2020) 60:S18–25. doi: 10.1002/jcph.1761
20. Sahin, L, Nallani, SC, and Tassinari, MS. Medication use in pregnancy and the pregnancy and lactation labeling rule. Clin Pharmacol Ther. (2016) 100:23–5. doi: 10.1002/cpt.380
21. Kim, SC, Neogi, T, Kim, E, Lii, J, and Desai, RJ. Trends in utilization of urate-lowering therapies following the FDA’S boxed warning to febuxostat in the U.S. Arthritis Rheumatol Hoboken NJ. (2021) 73:542–3. doi: 10.1002/art.41550
22. Rubino, A, Sanon, M, Ganz, ML, Simpson, A, Fenton, MC, Verma, S, et al. Association of the US Food and Drug Administration antipsychotic drug boxed warning with medication use and health outcomes in elderly patients with dementia. JAMA Netw Open. (2020) 3:e203630. doi: 10.1001/jamanetworkopen.2020.3630
23. Act (PREA) C on PSCU the BP for CA (BPCA) and the PRE, Policy B on HS, Medicine I of. Safe and effective medicines for children. Washington, DC: National Academies Press, US (2012).
24. Ahmed, U, Spyridis, N, Wong, ICK, Sharland, M, and Long, PFimproving Children’s Antibiotic Prescribing UK Research Network. Dosing of oral penicillins in children: is big child=half an adult, small child=half a big child, baby=half a small child still the best we can do? BMJ. (2011) 343:d7803. doi: 10.1136/bmj.d7803
Keywords: drug labels, antineoplastic and supportive medicines, NMPA, PMDA, FDA
Citation: Du C, Liu J, Wang C and Liu P (2025) A comparison of the labeling of antineoplastic and supportive medicines in China, Japan, and the US based on the WHO essential medicines list. Front. Med. 12:1600207. doi: 10.3389/fmed.2025.1600207
Edited by:
Morufu Olalekan Raimi, Federal University Otuoke, NigeriaReviewed by:
Shenbao Liang, Shanghai University of Finance and Economics, ChinaMinghong Yao, Sichuan University, China
Copyright © 2025 Du, Liu, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengcheng Liu, bGl1cGNtYWlsQDE2My5jb20=
 Chenyu Du
Chenyu Du Caiyi Wang
Caiyi Wang