Abstract
Objective:
This study aimed to investigate the correlation between decreased partial pressure of arterial carbon dioxide (PaCO2) and thrombus location, as well as its association with 90-day prognosis in patients with acute pulmonary embolism (PE).
Method:
A retrospective analysis was conducted on the risk factors, clinical characteristics, and diagnostic findings of PE patients presenting with decreased PaCO2 at our hospital.
Results:
Multivariate logistic regression analysis revealed that decreased PaCO2 in PE was significantly associated with thrombosis in the right pulmonary artery, thrombosis in the left lower lobe pulmonary arteries, and pulmonary hypertension (PH). Pearson correlation analysis showed a weak negative correlation between decreased PaCO2 and PASP and PVOI. Spearman correlation analysis indicated a weak negative correlation between decreased PaCO2 and computed tomography severity (CTS)-defined risk PE. Kaplan–Meier survival analysis demonstrated that decreased PaCO2 was not associated with 90-day mortality (χ2 = 0.263, p = 0.608).
Conclusion:
A significant proportion of PE patients presented with decreased PaCO2, which was correlated with thrombosis in the right pulmonary artery and left lower lobe pulmonary arteries. However, decreased PaCO2 was not associated with 90-day mortality in PE patients.
1 Introduction
Arterial partial pressure of carbon dioxide (PaCO2) is a common parameter in blood gas analysis, reflecting lung ventilation function. Hyperventilation leads to a decrease in PaCO2, while insufficient ventilation results in increased PaCO2. In pulmonary embolism (PE) patients, decreased PaCO2 is often observed and is currently attributed to pulmonary artery blockage by thrombi, which reduces alveolar surfactant, lung compliance, and alveolar diffusion capacity. This disrupts the ventilation/perfusion (V/Q) ratio, leading to pulmonary arteriovenous shunting, local atelectasis, pleural effusion, hypoxemia, and compensatory hyperventilation (resulting in decreased PaCO2) (1, 2). However, we observed that some PE patients exhibited decreased PaCO2, while others did not, even in cases with smaller thrombi. This suggests that other factors (e.g., nerve distribution in the lungs) may contribute to decreased PaCO2. Pulmonary vessels have sympathetic and vagus nerve distribution. When the thrombus stimulates the pulmonary vessel wall, the sympathetic nerve is excited, which leads to vasoconstriction and increased pulmonary vascular pressure. Nevertheless, whether the stimulation of pulmonary nerves by thrombus leads to decreased PaCO2, and the relationship between thrombus location and decreased PaCO2 in PE patients is limited research.
This study retrospectively analyzed data from PE patients with decreased PaCO2 to explore the causes of decreased PaCO2 and its correlation with thrombus location and 90-day prognosis.
2 Methods
2.1 Subjects
A total of 801 PE patients (418 males and 383 females, aged 22–94 years) diagnosed via computed tomography pulmonary angiography (CTPA) at the Department of Respiratory and Critical Care Medicine of Shanghai Sixth People’s Hospital between September 2018 and April 2024 were included. Arterial blood gas analysis (with nasal catheter oxygen inhalation in some patients) was performed within 4 h of PE diagnosis. Patients were stratified into two groups based on PaCO2 levels: a decreased group (< 35 mmHg, 332 cases) and a non-decreased group (≥ 35 mmHg, 469 cases).
The inclusion criteria followed the diagnostic guidelines for pulmonary thromboembolism published by the Chinese Thoracic Society (CTS) in 2018 (3). Exclusion criteria included: patients diagnosed with CTPA at other hospitals who sought further treatment at our hospital; patients with incomplete medical records or missing critical research data; patients unable to undergo CTPA due to factors such as obesity, severe renal insufficiency, or contrast allergy; patients who underwent pulmonary perfusion/ventilation imaging or other diagnostic methods; patients with severe cardiomyopathy, valvular heart disease, acute coronary syndrome, acute infection, acute cerebrovascular disease, or chronic pulmonary thromboembolism; and patients without arterial blood gas analysis within 4 h of PE diagnosis.
2.2 Treatment methods
Following PE diagnosis, anticoagulant therapy was initiated. Low-molecular-weight heparin (100 IU/kg every 12 h) was administered subcutaneously to 369 patients. Warfarin (2.5 mg) was introduced orally 24–48 h after the start of low-molecular-weight heparin. Both low-molecular-weight heparin and warfarin were used concurrently for 4–5 days. Once prothrombin time (PT) and international normalized ratio (INR) reached 2.0–3.0 for two consecutive days, low-molecular-weight heparin was discontinued, but warfarin was continued orally. When INR was less than 2.0, warfarin doses was increased 0.625 mg. When INR was more than 3.0, warfarin doses should be reduced or even discontinued. In 432 patients, rivaroxaban was used (15 mg orally twice daily for 3 weeks, followed by 20 mg once daily for 9 weeks).
This study was approved by the Ethics Committee of Shanghai Sixth People’s Hospital, Shanghai Jiao Tong University [No.: 2023-KY-138(K)]. All procedures adhered to the ethical standards of the institutional and national research committees and the principles of the 1964 Helsinki Declaration and its amendments. Informed consent was waived due to the retrospective design.
2.3 Data collection
The following data were retrospectively collected and analyzed: gender, age, body mass index (BMI), smoking history, underlying diseases (hypertension, coronary heart disease, stroke, diabetes, recent fractures within the past 4 weeks, recent fixation or surgery, malignancy, chronic pulmonary disease), major symptoms (dyspnea, chest pain, shortness of breath, etc.), arterial blood gas analysis, plasma D-dimer (D-D), N-terminal pro-Brain Natriuretic Peptide (NT-proBNP), cardiac troponin I (cTNI), electrocardiogram findings, pulmonary artery systolic pressure (PASP; measured via echocardiography), ultrasound findings of lower extremity veins, thrombus location, pulmonary hypertension (PH; PASP > 40 mmHg), pulmonary vascular obstruction index (PVOI) (4), CTS-defined risk PE (low risk, intermediate-low risk, intermediate-high risk, high risk), simplified Pulmonary Embolism Severity Index (sPESI; scores 0, 1, 2, ≥3), treatment modalities, and outcomes.
2.4 Statistical analysis
Statistical analysis was performed using SPSS version 17.0. The Mann–Whitney U test was used for intergroup comparisons. Normally distributed data were expressed as mean ± standard deviation (Mean ± SD) and compared using the t-test. Categorical data were presented as frequencies (%) and compared using the χ2 test. A logistic regression model was constructed with decreased PaCO2 (+)/(−) as the dependent variable and gender, age, risk factors, underlying diseases, arterial blood gas parameters, D-dimer, cTNI, NT-proBNP, and thrombus location as independent variables to identify predictors of decreased PaCO2. Pearson correlation was used to assess relationships between PaCO2, PASP, and PVOI. Spearman’s correlation was used to analyze associations between PaCO2 and CTS-defined risk PE or sPESI scores. Kaplan–Meier survival analysis was performed to evaluate the correlation between decreased PaCO2 and 90-day mortality. A p-value < 0.05 was considered statistically significant.
3 Result
3.1 Basic information
The proportion of PE patients presenting with decreased PaCO2 was 41.39% (332/801). The mean age of PE patients in the decreased group was 66.2 ± 14.82 years, while in the non-decreased group, it was 66.88 ± 13.59 years. In the decreased group, 50.6% (168/164) were males, compared to 53.3% (250/219) in the non-decreased group. No statistically significant differences were observed in basic information between the decreased and non-decreased groups (p > 0.05). Additionally, no significant differences were noted in underlying diseases, symptoms, arterial blood gas parameters, NT-proBNP levels, time of onset, or elevated cTNI levels between the two groups (p > 0.05). Detailed results are presented in Tables 1, 2.
Table 1
| General information | Decreased PaCO2 group (n = 332 cases) | Non-decreased group (n = 469 cases) | F/χ2 value | p-value |
|---|---|---|---|---|
| Male/female (cases) | 168/164 | 250/219 | 1.181 | 0.277 |
| Age (years) | 66.2 ± 14.82 | 66.88 ± 13.59 | 3.06 | 0.810 |
| BMI (kg/m2) | 23.01 ± 3.35 | 23.08 ± 3.13 | 2.086 | 0.149 |
| Smoking history (cases) | 90 (27.1%) | 133 (28.35%) | 0.265 | 0.607 |
| Symptom duration (days) | 5.59 ± 4.48 | 5.70 ± 4.59 | 3.241 | 0.072 |
| Oral contraceptive use (cases) | 0 | 0 | – | – |
| Long-haul travel history (cases) | 0 | 0 | – | – |
| Atrial fibrillation (cases) | 0 | 0 | – | – |
| Hypertension (cases) | 96 (28.9%) | 138 (29.4%) | 0.097 | 0.755 |
| Coronary heart disease (cases) | 81 (24.3%) | 125 (26.65%) | 2.961 | 0.086 |
| Diabetes mellitus (cases) | 42 (12.6%) | 52 (11.08%) | 1.821 | 0.178 |
| Malignant tumor (cases) | 44 (13.2%) | 66 (14.07%) | 0.030 | 0.862 |
| Surgery or trauma (cases) | 35 (10.5%) | 42 (8.95%) | 2.916 | 0.088 |
| Stroke history (cases) | 31 (9.33%) | 39 (8.31%) | 1.013 | 0.315 |
| Chronic pulmonary disease (cases) | 85 (25.6%) | 112 (23.8%) | 1.227 | 0.268 |
| Symptoms | ||||
| Cough | 70 (21.08%) | 99 (21.1%) | 0.001 | 0.987 |
| Fever | 37 (11.14%) | 49 (10.4%) | 0.392 | 0.531 |
| Chest pain | 52 (15.66%) | 77 (16.4%) | 0.329 | 0.567 |
| Syncope | 25 (7.53%) | 43 (9.16%) | 2.708 | 0.100 |
| Chest distress | 109 (32.8%) | 142 (30.2%) | 2.852 | 0.092 |
| SBP at admission (mmHg) | 130.45 ± 13.43 | 129.97 ± 15.16 | 3.46 | 0.063 |
| DBP at admission (mmHg) | 78.43 ± 10.97 | 78.86 ± 10.51 | 1.685 | 0.195 |
| Heart rate at admission (bpm) | 89.05 ± 19.47 | 86.59 ± 18.63 | 3.376 | 0.067 |
Comparison of basic information (mean ± SD) (n, %).
*p < 0.05. BMI, body mass index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; bpm, Beats per min.
Table 2
| General information | Decreased group(n = 332 cases) | Non-decreased group(n = 469 cases) | F/χ2 values | p-values |
|---|---|---|---|---|
| sPESI Class (cases) | 3.694 | 0.296 | ||
| 0 | 79 (23.79%) | 131 (27.9%) | ||
| 1 | 127 (38.2%) | 177 (37.73%) | ||
| 2 | 86 (25.9%) | 121 (25.79%) | ||
| ≥ 3 | 40 (12.04%) | 40 (8.52%) | ||
| cTNI (ug/L) | 0.106 ± 0.199 | 0.116 ± 0.410 | 1.792 | 0.181 |
| Pro-BNP (ng/L) | 922.89 ± 1143.12 | 754.79 ± 1009.58 | 0.864 | 0.353 |
| PaO2 (mmHg) | 77.22 ± 17.44 | 82.25 ± 19.91 | 6.73 | <0.001* |
| PaCO2 (mmHg) | 31.58 ± 2.86 | 41.13 ± 4.82 | 42.25 | <0.001* |
| D-Dimer (mg/L) | 6.72 ± 5.44 | 6.06 ± 5.70 | 1.419 | 0.223 |
| PASP (mmHg) | 34.24 ± 18.64 | 29.84 ± 9.06 | 17.966 | <0.001* |
| PVOI (%) | 29.65 ± 15.90 | 27.90 ± 15.95 | 0.087 | 0.768 |
| Thrombus site (cases) | 74.018 | <0.001* | ||
| Main pulmonary artery | 62 (18.6%) | 56 (11.9%) | ||
| Left pulmonary artery | 141 (42.4%) | 148 (31.5%) | ||
| Right pulmonary artery | 167 (50.3%) | 168 (35.8%) | ||
| Left superior pulmonary artery | 136 (40.9%) | 167 (35.6%) | ||
| Left inferior pulmonary artery | 209 (62.9%) | 120 (25.5%) | ||
| Right superior pulmonary artery | 146 (43.9%) | 205 (43.7%) | ||
| Right middle lobe pulmonary artery | 134 (40.3%) | 196 (41.7%) | ||
| Right inferior pulmonary artery | 157 (47.2%) | 291 (62.04%) | ||
| CTS-defined risk PE | 12.922 | 0.002* | ||
| Low-risk patients | 103 (31.02%) | 189 (40.2%) | ||
| Intermediate-low-risk patients | 133 (40.06%) | 191 (40.7%) | ||
| Intermediate-high-risk patients | 96 (28.9%) | 89 (18.9%) | ||
| DVT (cases) | 71 (21.38%) | 101 (21.53%) | 0.01 | 0.919 |
| PH (cases) | 100 (30.21%) | 75 (15.9%) | 89.602 | <0.001* |
| Pleural effusion (cases) | 63 (18.9%) | 78 (16.63%) | 3.485 | 0.062 |
| Minor bleeding (cases) | 10 (3.01%) | 11 (2.34%) | 1.35 | 0.246 |
| Hospitalization duration (days) | 9.8 ± 3.28 | 9.03 ± 3.13 | 1.141 | 0.286 |
| 90-day mortality (cases) | 9 (2.71%) | 10 (2.13%) | 1.717 | 0.424 |
Comparison of laboratory examination (mean ± SD) (n, %).
*p < 0.05. TNI, troponin I; BNP, B-type natriuretic peptide; PVOI, pulmonary vascular obstruction index; PaCO2, partial pressure of carbon dioxide; PaO2, oxygen partial pressure; DVT, deep vein thrombosis, PASP, Pulmonary artery systolic pressure; PH, Pulmonary hypertension; sPESI, simplified Pulmonary embolism severity index.
3.2 Univariate and multivariate logistic regressions
Univariate logistic regression analyses were conducted to evaluate the associations between clinical variables and decreased PaCO2 in PE patients. The results indicated that decreased PaCO2 was associated with intermediate-high-risk PE, pulmonary hypertension (PH), pulmonary artery systolic pressure (PASP), and thrombosis in the left or right pulmonary artery, as well as thrombosis in the left or right inferior pulmonary artery (p < 0.05).
Multivariate logistic regression analysis was performed for variables that showed statistically significant differences in the univariate analysis. It revealed that the risk of decreased PaCO2 in PE patients was linked to thrombosis in the right pulmonary artery (OR = 1.737, 95% CI: 1.093–2.760, p = 0.020), thrombosis in the left inferior pulmonary artery (OR = 2.447, 95% CI: 1.743–3.434, p < 0.001), and was positively correlated with PH (OR = 3.506, 95% CI: 1.263–9.726, p = 0.016). Additionally, the risk of decreased PaCO2 was linked to PaO2 (OR = 0.988, 95% CI: 0.98–0.997, p = 0.003), intermediate-high-risk PE (OR = 0.371, 95% CI: 0.148–0.926, p = 0.034), and thrombosis in the right inferior pulmonary artery (OR = 0.416, 95% CI: 0.297–0.582, p < 0.001), showing a negative correlation.
3.3 Pearson correlation analysis
The decrease in PaCO2 demonstrated a weak negative correlation with PASP (Rs = −0.248, p < 0.001) and pulmonary vascular obstruction index (PVOI) (Rs = −0.086, p = 0.015). These results are illustrated in Figures 1, 2.
Figure 1
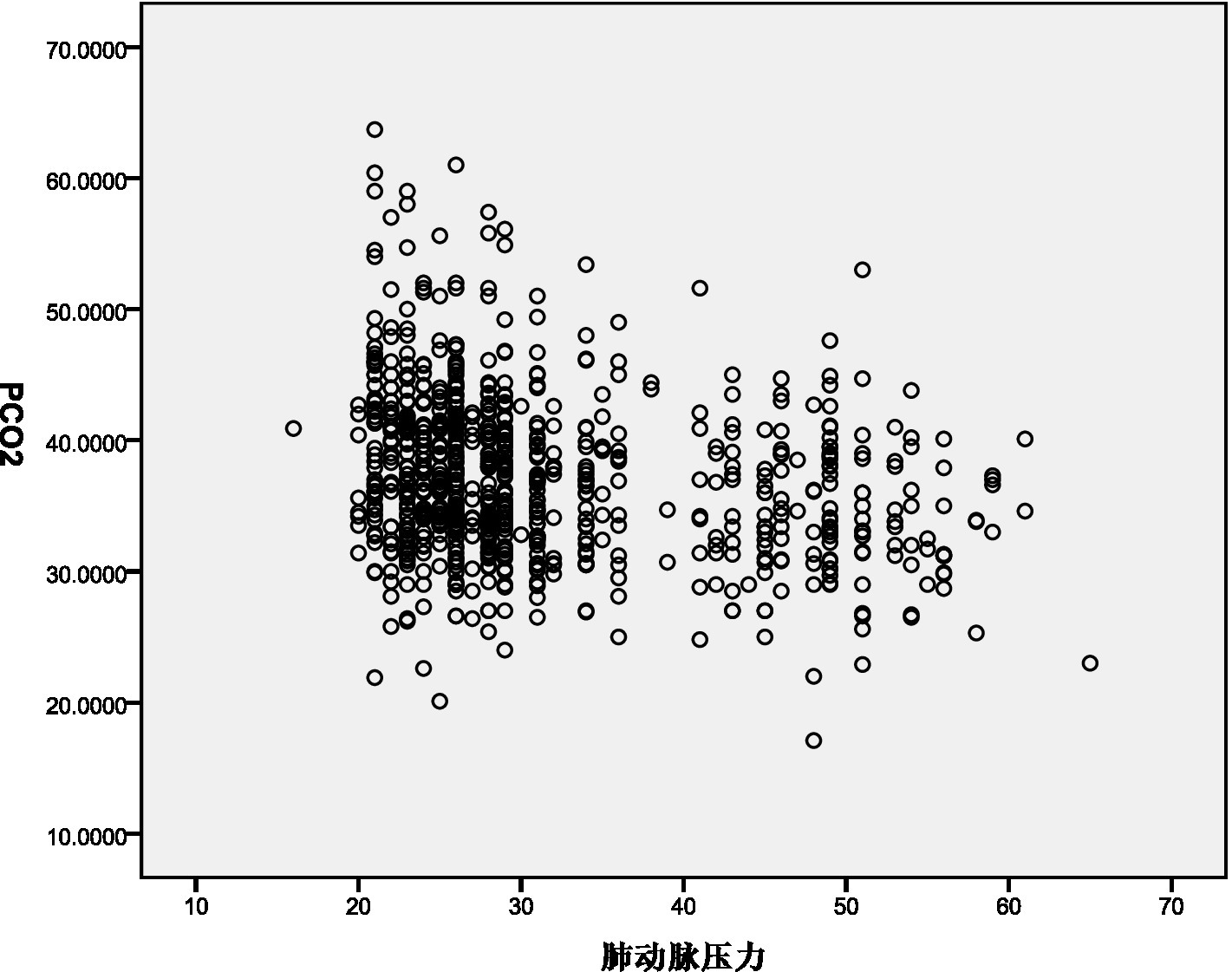
Pearson correlation coefficient assess the association between PaCO2 and PASP.
Figure 2
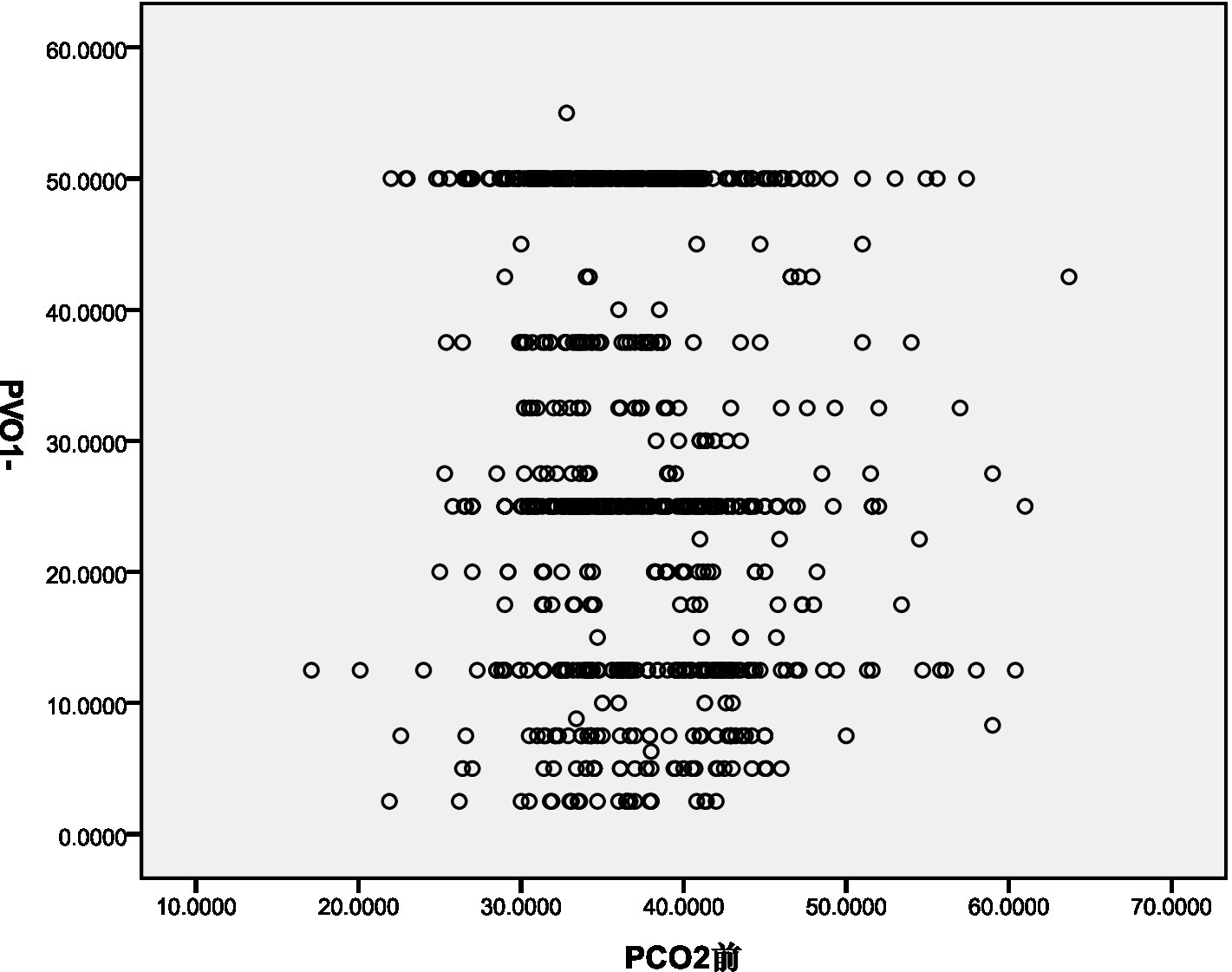
Pearson correlation coefficient assess the association between PaCO2 and PVOI.
3.4 Spearman correlation analysis
The decrease in PaCO2 demonstrated a weak negative correlation with CTS-defined risk PE (Rs = −0.124, p < 0.001). However, no correlation was observed between decreased PaCO2 and the simplified Pulmonary Embolism Severity Index (sPESI) (Rs = 0.041, p = 0.243).
3.5 Kaplan–Meier survival analysis
Kaplan–Meier survival analysis indicated that decreased PaCO2 was not associated with 90-day mortality (χ2 = 0.263, p = 0.608). These results are shown in Figure 3.
Figure 3
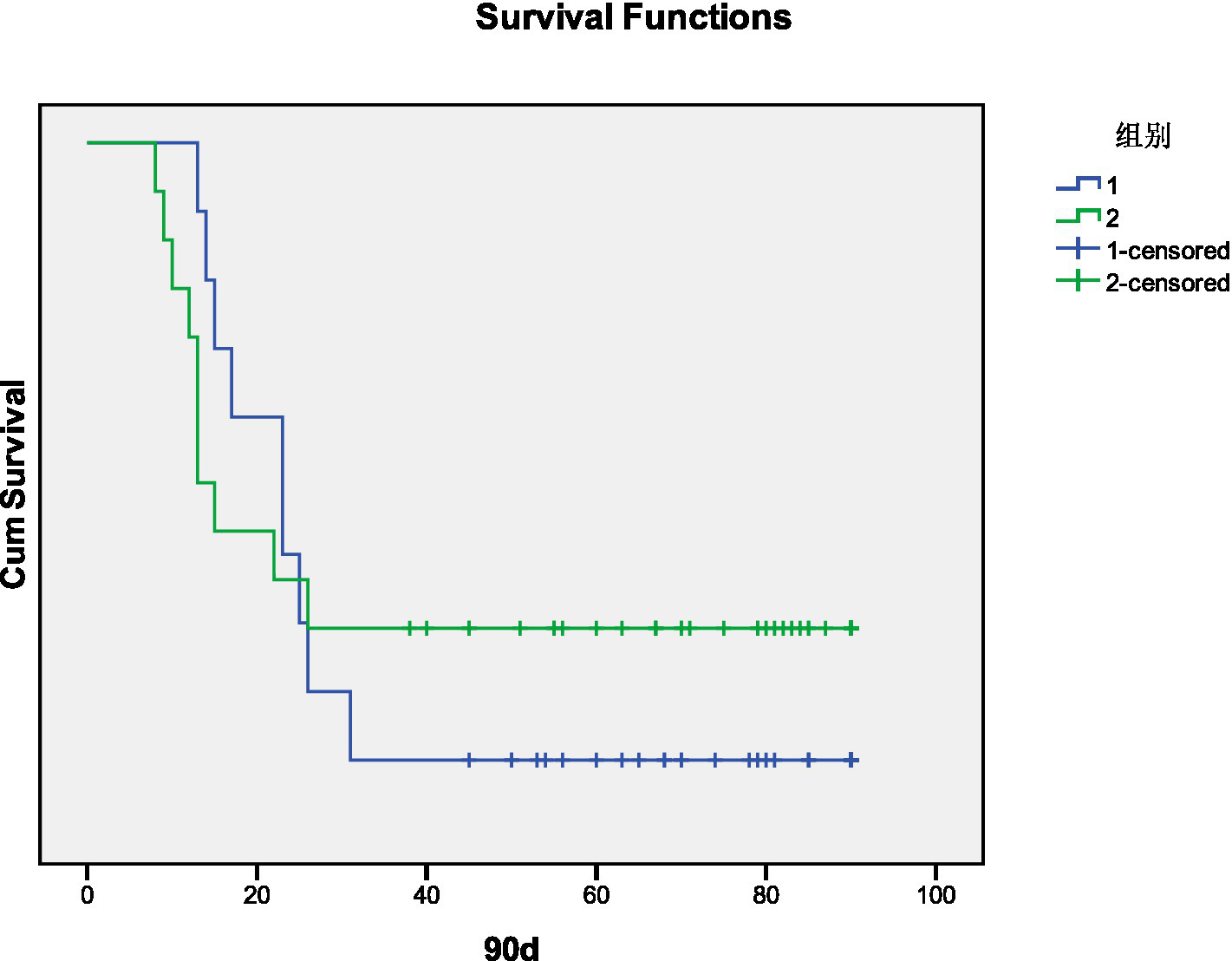
Kaplan–Meier curves for 90-day mortality after decreased PaCO2 in days (1. Decreased group, 2. Non-Decreased group).
4 Discussion
There are many factors affecting arterial partial pressure of carbon dioxide (PaCO2). Changes in PaCO2 may result from cardiopulmonary diseases or neurological and mental disorders. Chronic lung diseases such as chronic obstructive pulmonary disease (COPD) can lead to PaCO2 retention, while idiopathic pulmonary fibrosis (IPF) can cause a decrease in PaCO2. In this study, no statistically significant differences were observed in chronic lung diseases between the two groups. Additionally, vagus nerve (VN) excitation can increase PaCO2, whereas sympathetic nerve (SN) excitation can increase the respiratory rate and decrease PaCO2.
The lungs are innervated by both the sympathetic and parasympathetic nervous systems. The sympathetic nerves originate from the thoracolumbar segment of the spinal cord (T1–L2), emit postganglionic fibers after the sympathetic trunk, and form the anterior and posterior pulmonary plexuses with the vagus nerve. These nerves descend from the hilum of the lung along the trachea and pulmonary vessels, distributing around the bronchi and pulmonary vessel walls. Zhou et al. (5) found that the canine pulmonary sympathetic nerve bundle was primarily distributed in the posterior wall of the pulmonary trunk and the left pulmonary artery, with minimal distribution in the anterior wall of the pulmonary trunk and the right pulmonary artery. The longest and shortest distances between pulmonary artery nerve endings and the pulmonary artery lumen were 4,148 μm and 823 μm, respectively. Zhang et al. (6) reported that there were 2,160 sympathetic nerves around the pulmonary artery in dogs, with the largest concentration at the proximal end of the pulmonary artery bifurcation, accounting for 70 to 84% of the total. Most sympathetic nerves were distributed 1 to 3 mm below the intima at the beginning of the left and right pulmonary arteries and under the intima of the wall after the pulmonary artery bifurcation. Huang et al. (7) confirmed the presence of a large number of sympathetic nerves in the posterior wall of the main pulmonary trunk, the right wall, and the posterior wall of the pulmonary artery bifurcations. Wang et al. (8) found that the sympathetic nerve was overactivated in an animal model of pulmonary thrombosis, with significant increases in sympathetic neurotransmitters such as tyrosine hydroxylase and neuropeptide Y in lung tissue.
The results of this study showed that pulmonary thrombi were distributed in the right pulmonary arteries and/or the left lower pulmonary artery in the decreased PaCO2 group, consistent with the distribution of pulmonary sympathetic nerves. Multivariate logistic regression analysis identified thrombosis in the right pulmonary artery and left lower lobe pulmonary arteries as risk factors for decreased PaCO2. This may be due to the abundance of sympathetic nerves in the branches of the right and left pulmonary arteries, which are close to the vascular intima. Mechanical stimulation of the vascular wall by thrombi may activate para-pulmonary sympathetic nerves, leading to hyperventilation and decreased PaCO2. However, this study was a retrospective case analysis, and sympathetic neurotransmitters such as tyrosine hydroxylase, neuropeptide Y, and norepinephrine were not measured in PE patients with decreased PaCO2. Therefore, decreased PaCO2 cannot be considered a predictor of pulmonary vascular sympathetic nerve excitability.
Pearson correlation analysis revealed a weak negative correlation between PASP and decreased PaCO2. This may be due to the decrease in PaCO2 leading to a reduction in H+ concentration in the blood, stimulating chemoreceptors and causing reflex vasoconstriction, thereby increasing PASP (9, 10). Additionally, Pearson correlation analysis showed a negative correlation between the pulmonary vascular obstruction index (PVOI) and decreased PaCO2. Metafratzi et al. (11) demonstrated that a PaCO2 value of 30 mmHg or lower was associated with an arterial bed obstruction index of >50%. The greater the degree of thrombus blockage, the higher the pulmonary vascular pressure, and the more pronounced the vascular sympathetic nerve and mechanical stimulation, which may contribute to the decrease in PaCO2.
Spearman correlation analysis revealed a weak negative correlation between decreased PaCO2 and CTS-defined risk PE. However, no correlation was observed between decreased PaCO2 and the simplified Pulmonary Embolism Severity Index (sPESI). This may be because PASP and PVOI were higher in patients with decreased PaCO2 compared to the non-decreased group, indicating larger thrombus volume and greater pulmonary artery blockage, resulting in impaired cardiac function and higher risk stratification. However, as a retrospective study, systolic blood pressure and heart rate could not be recorded in real time, and some patients had received oxygen therapy at the time of diagnosis, which may explain the lack of correlation between decreased PaCO2 and sPESI scores. From these correlation studies, it is evident that decreased PaCO2 can indicate increased PVOI blockage, leading to elevated PASP and higher CTS-defined risk PE.
Kotsiou et al. (9) followed PE patients with decreased PaCO2 for 6 months and found that their 6-month survival rate was lower than that of patients with normal PaCO2. In our study, patients with decreased PaCO2 exhibited no hemodynamic instability, and the 90-day mortality in the decreased PaCO2 group was 9 cases, compared to 10 cases in the non-decreased PaCO2 group. Kaplan–Meier survival analysis indicated no correlation between decreased PaCO2 and 90-day mortality. The discrepancy with previous findings may be due to the shorter observation period in this study. Additionally, the 90-day mortality in both groups primarily involved patients with chronic end-stage pulmonary diseases, such as interstitial pulmonary disease combined with pulmonary hypertension, right heart failure, and chronic obstructive pulmonary disease with poor pulmonary function. These patients were bed-bound for extended periods, making them more susceptible to pulmonary thrombosis or infection, which likely contributed to the increased mortality.
However, this study has several limitations. First, its retrospective, single-center design limits the generalizability of the findings. To strengthen the results, further research with a larger, prospective, multicenter cohort, incorporating comprehensive medical histories and long-term follow-up data, is necessary. Second, because the number of patients in high-risk was small, and many patients could not be diagnosed by CTPA examination, so it was excluded from this study. Third, Many PE patients have COPD, or pneumonia, interstitial lung disease, pleural effusion, etc., which may have an impact on arterial blood gas, but it is difficult to the inability to accurately quantify the extent or scope of lung lesions prevented an analysis of their impact on PaCO2, so we did not include CT images in the manuscript.
5 Conclusion
The prevalence of decreased PaCO2 was elevated among PE patients. Decreased PaCO2 was associated with thrombosis in the right pulmonary artery and left lower lobe pulmonary arteries, consistent with the distribution of pulmonary sympathetic nerves. However, it remains unclear whether decreased PaCO2 can indicate increased sympathetic sensitivity. Decreased PaCO2 was negatively correlated with PVOI, PASP, and CTS-defined risk PE, indicating greater pulmonary vascular obstruction and higher risk stratification. However, decreased PaCO2 did not correlate with 90-day mortality.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shanghai Sixth People’s Hospital, Shanghai Jiao Tong University [No.: 2023-KY-138(K)]. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because informed consent was waived due to the retrospective design. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. All procedures adhered to the ethical standards of the institutional and national research committees and the principles of the 1964 Helsinki Declaration and its amendments.
Author contributions
DY: Resources, Validation, Writing – review & editing, Formal analysis, Supervision, Project administration, Funding acquisition, Software, Data curation, Methodology, Visualization, Investigation, Conceptualization. YL: Investigation, Software, Conceptualization, Validation, Data curation, Resources, Formal analysis, Writing – original draft, Supervision, Funding acquisition, Writing – review & editing, Project administration, Methodology, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study received support from the Shanghai Sixth People’s Hospital Affiliated to Jiao Tong University School of Medicine Hospital-level project Fund (grant number: ynhg202317). This study was supported by the Shanghai Pudong New Area Science and Economic Commission Minsheng Technology Special Fund (grant number: PKJ2019-Y01).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Glazier CR Baciewicz FA . Epidemiology, etiology, and pathophysiology of pulmonary embolism. Int J Angiol. (2024) 33:76–81. doi: 10.1055/s-0044-1785487
2.
Higashiya K Ford J Yoon HC . Variation in positivity rates of computed tomography pulmonary angiograms for the evaluation of acute pulmonary embolism among emergency department physicians. Perm J. (2022) 26:58–63. doi: 10.7812/TPP/21.019
3.
Pulmonary Embolism and Pulmonary Vascular Diseases Group of Chinese Thoracic Society, Pulmonary Embolism and Pulmonary Vascular Disease Working Committee of Chinese Association of Chest Physicians, National Cooperation Group on Prevention and Treatment of Pulmonary Embolism and Pulmonary Vascular Disease . Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism. Zhonghua Yi Xue Za Zhi. (2018) 98:1060–87. doi: 10.3760/cma.j.issn.0376-2491.2018.14.007
4.
Cildag MB Gok M Karaman CZ . Pulmonary artery obstruction index and right ventricular dysfunction signs in initial and follow up pulmonary computed tomography angiography in acute pulmonary embolism. J Clin Diagn Res. (2017) 11:TC21–5. doi: 10.7860/JCDR/2017/28740.10296
5.
Zhou L Zhang J Jiang XM et al . Pulmonary artery denervation attenuates pulmonary arterial modeling in dogs with pulmonary arterial hypertension induced by dehydrogenized monocrotaline. JACC Cardiovasc Interv. (2015) 8:2013–23. doi: 10.1016/j.jcin.2015.09.015
6.
Zhang Y , ChenWXuYLiuHChenYYangHet al. Never distribution of canine pulmonary arteries and potential clinical implications. Am J Transl Res. (2016) 8:365–374
7.
Huang Y , LiuYWPanHZZhangXLLiJXiangLet al. Transthoracic pulmonaryartery denervation for pulmonary arterial hypertension sympatheticnever distribution and pulmonary artery remodeling. Arterioscler Thromb Vasc Biol. (2019) 39:704–718, doi: 10.1161/ATVBAHA.118.311992
8.
Wang Y Yu D Yu Y Zou W Zeng X Hu L et al . Potential role of sympathetic activity on the pathogenesis of massive pulmonary embolism with circulatory shock in rabbits. Respir Res. (2019) 20:97. doi: 10.1186/s12931-019-1069-z
9.
Kotsiou OS Karadontas V Daniil Z Zakynthinos E Gourgoulianis KI . Transcutaneous carbon dioxide monitoring as a predictive tool for all-cause 6-month mortality after acute pulmonary embolism. Eur J Intern Med. (2019) 68:44–50. doi: 10.1016/j.ejim.2019.07.030
10.
Pilcher J Perrin K Beasley R . The effect of high concentration oxygen therapy on PaCO2 in acute and chronic respiratory disorders. Transl Respir Med. (2013) 1:8. doi: 10.1186/2213-0802-1-8
11.
Metafratzi ZM Vassiliou MP Maglaras GC Katzioti FG Constantopoulos SH Katsaraki A et al . Acute pulmonary embolism: correlation of CT pulmonary artery obstruction index with blood gas values. AJR Am J Roentgenol. (2006) 186:213–9. doi: 10.2214/AJR.04.1320
Summary
Keywords
pulmonary embolism, PaCO2, prognosis, relevant factors, D-dimer
Citation
Yang D and Liu Y (2025) Correlation between decreased PaCO2 and thrombus site and 90d prognosis in patients with acute pulmonary embolism. Front. Med. 12:1601169. doi: 10.3389/fmed.2025.1601169
Received
27 March 2025
Accepted
04 June 2025
Published
17 June 2025
Corrected
16 July 2025
Volume
12 - 2025
Edited by
Carmine Siniscalchi, University of Parma, Italy
Reviewed by
Omer Iqbal, Loyola University Chicago, United States
Gaurav Manek, MedStar Health, United States
Updates
Copyright
© 2025 Yang and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Liu, liuyi830401@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.