- 1The First Hospital of Jilin University, Changchun, China
- 2Changchun Central Hospital Microbiology Laboratory, Changchun, China
- 3Department of Critical Care Medicine, Guangdong Institute of Cardiovascular Diseases, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
Background: Acute respiratory distress syndrome (ARDS) is a prevalent condition in the respiratory department and intensive care unit that considerably influences prognosis. Prior research has demonstrated that angiotensin-converting enzyme inhibitors (ACEIs) or dexmedetomidine can improve the prognosis of ARDS. Nonetheless, the combinatorial effect of ACEIs and dexmedetomidine on the prognosis of ARDS remains to be investigated.
Method: A retrospective study was conducted using data from 696 patients with ARDS collected from the Medical Information Mart for Intensive Care IV database. Subsequently, a Cox regression model was constructed to screen for meaningful variables. Moreover, a multi-model Cox regression was constructed to exclude the interference of confounding factors and explore the effects of ACEIs alone, dexmedetomidine alone, and ACEIs combined with dexmedetomidine on the prognosis of patients with ARDS. Finally, it was verified by plotting the Kaplan–Meier survival curve.
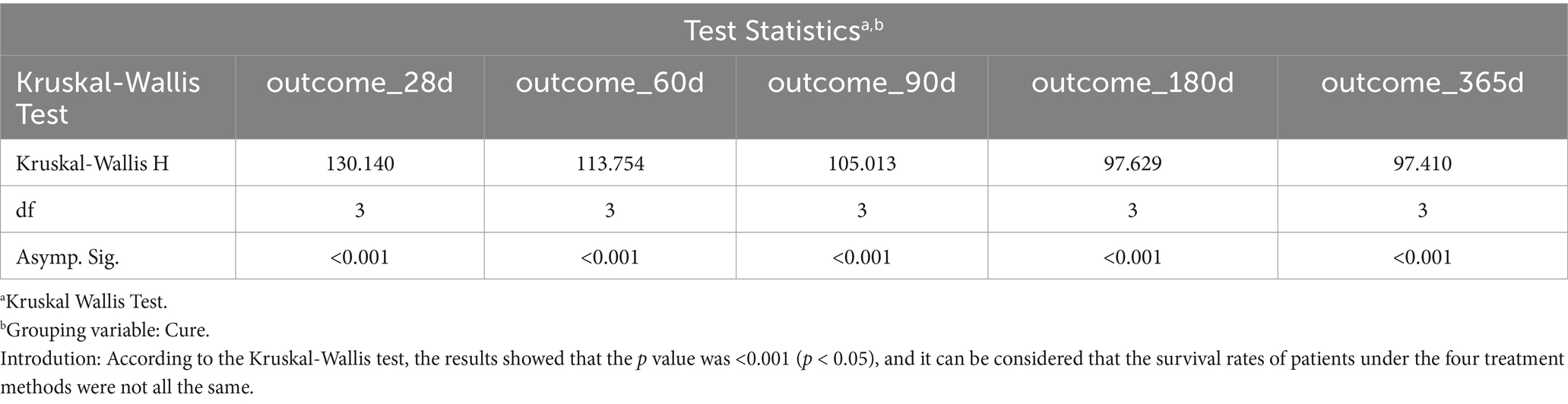
Result: The survival rates of patients with ARDS within days 28, 60, 90, 180, and 365 after admission were 61.6, 57.0, 55.9, 53.7, and 51.3%, respectively. The results of the multi-model Cox regression revealed that compared with the application of ACEIs or dexmedetomidine alone, ACEIs combined with dexmedetomidine have a synergistic effect on reducing the risk of death in patients with ARDS. The conclusion of the Kaplan–Meier survival curve is consistent with that of the Cox regression.
Conclusion: In terms of reducing the risk of death in patients with ARDS, the combined application of dexmedetomidine and ACEIs may have a better effect than monotherapy.
1 Introduction
Acute respiratory distress syndrome (ARDS) is a serious clinical syndrome and a common disease in intensive care units (ICUs). Its pathological features include alveolar–capillary barrier injury, alveolar collapse, ventilator-blood flow ratio imbalance, inflammatory response, oxidative stress imbalance, secondary pulmonary hypertension, and right heart dysfunction (1, 2). The incidence of ARDS is approximately 10.4% (3), and it is associated with high mortality rates in ICUs (4, 5). ARDS is the main cause of respiratory failure and death in patients with ARDS, and its significance is reflected in the high incidence and mortality of ARDS, multiple organ dysfunction, complexity of treatment, and long-term health problems that ARDS survivors may face, such as pulmonary insufficiency, cognitive dysfunction, and decline in quality of life. These long-term effects have had a significant impact on patient health, the medical system, and the social economy (6, 7). Currently, the diagnosis of ARDS is primarily based on the Berlin criteria developed in 2012, which is constantly updated (8, 9). According to these criteria, the in-hospital mortality rate for ARDS is approximately 50% (10). In treating ARDS, the primary goal is to resolve acute respiratory failure. Previous studies have confirmed that angiotensin-converting enzyme inhibitors (ACEIs) can improve the prognosis of outpatients with acute respiratory diseases (11) and reduce the in-hospital mortality rate of patients with acute respiratory failure (12, 13). Additionally, dexmedetomidine has been shown to improve patient prognosis (14–16). There have been relevant studies on the individual application mechanisms and effects of the two drugs on ARDS, which can provide a theoretical basis for potential combined medication. Based on the existing literature search results, no clinical studies have directly explored the combination of ACEIs and dexmedetomidine for ARDS. Currently, the vast majority of clinical studies have focused on the impact of single drugs on the prognosis of patients; however, this does not conform to the actual clinical situation. In clinical practice, it is rare to treat patients with only one drug. Therefore, exploring the impact of combined medication on the prognosis of patients can not only help understand whether there is a synergistic effect of combined medication but also investigate whether the combined application of drugs will exacerbate the side effects of drugs and the probability of complications.
To explore whether there is a synergistic or antagonistic effect between ACEIs and dexmedetomidine on improving the survival rate of patients with ARDS, we aimed to use the Medical Information Mart for Intensive Care (MIMIC IV) database to fill this research gap.
2 Methods
2.1 Database
This study is based on the MIMIC IV database, which contains information on all patients who received treatment in this institution as well as laboratory records. This information can be used to identify independent risk factors and clinical features of certain diseases (17), thereby assisting clinical decision-making and providing a basis for the formulation of clinical guidelines (18). Diseases were summarized and extracted according to the ninth (ICD-9) and tenth (ICD-10) codes of the International Classification of Diseases (19). One of the authors (Dr. Tang) completed a CITI Data Research course (study ID: 12467279) and obtained access to the MIMIC IV database. In the MIMIC database, since patient information is anonymized, informed consent from patients is not required (20).
2.2 Study population
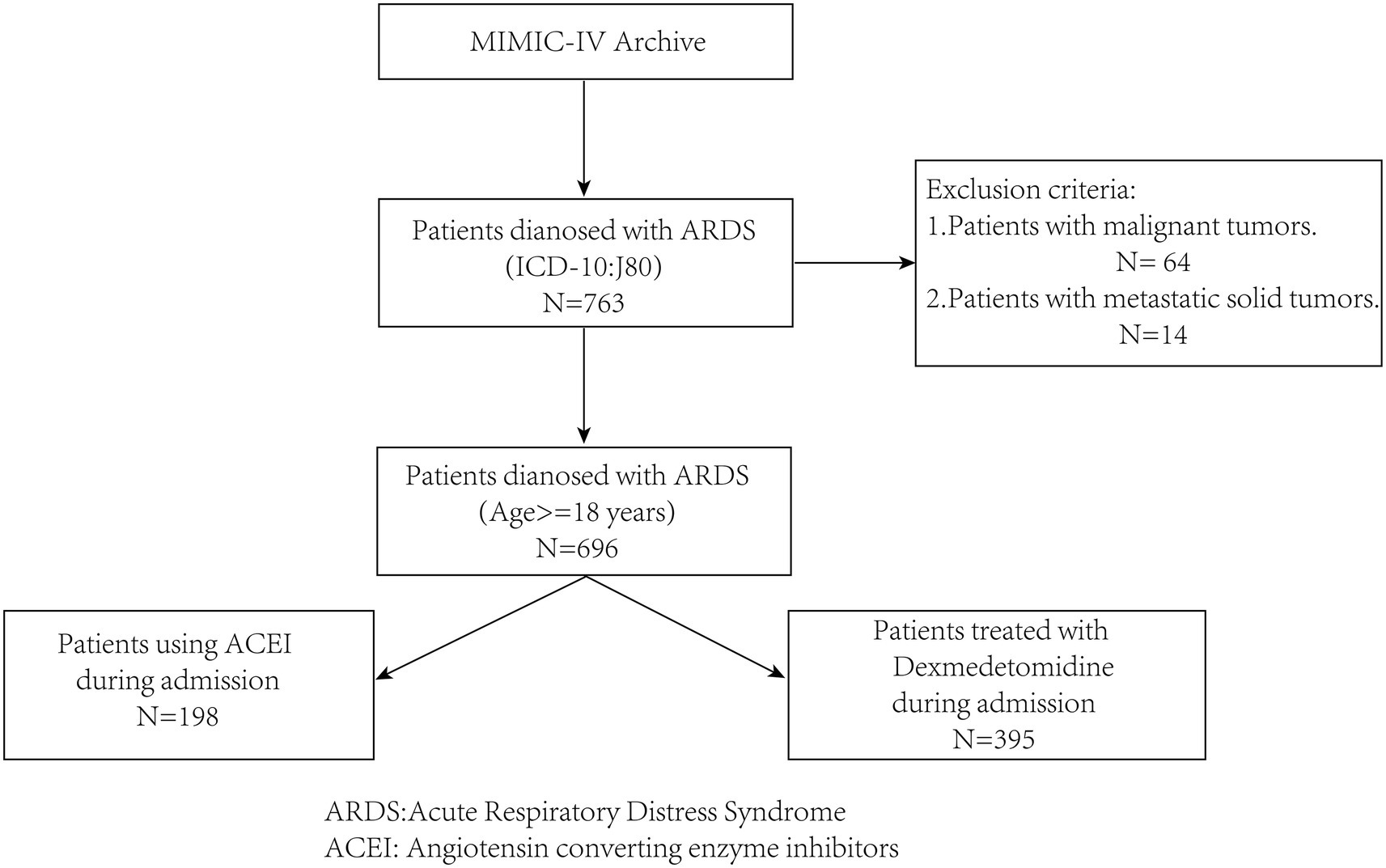
Patients with ARDS were identified in the database according to the disease code, and patient data were screened according to the following inclusion and exclusion criteria (Figure 1).
2.2.1 Inclusion criteria
The inclusion criteria included: (1) patients with acute respiratory distress syndrome who were admitted to Beth Israel Deaconess Medical Center for the first time, and (2) being at least 18 years old.
2.2.2 Exclusion criteria
The exclusion criteria included: (1) patients with combined malignant and metastatic solid tumors that may significantly impact prognosis, and (2) patients with incomplete information on ACEIs and dexmedetomidine use during hospitalization.
2.3 Data processing and analysis
The research data were mainly extracted from Navicat Premium version 16.0, which mainly included the medication information of ACEI and dexmedetomidine, comorbidiasis, and the survival period of patients. The survival period of the patient is calculated from the time of admission. The types of ACEI used in this study include captopril, ramipril, benazepril, quinopril, fosinopril, benazepril, nasepril, perindopril maleate, quinopril, linopril and mosipril.
The cleaned data were imported into R software (version 4.4), and IBM SPSS Statistics (version 25) was used for analysis. First, the Kruskal–Wallis test was performed to compare survival rates across different treatment methods, aiming to evaluate statistical significance (Table 1).
Cox regression analysis was conducted using the survival package in R. Subsequently, a stepwise Cox regression analysis was conducted. The effects of different treatment methods on the risk of patient death were compared by eliminating interference from covariates. Finally, the R package of survminer was used to plot the Kaplan–Meier survival curve to compare survival rate differences in patients with ARDS under different treatment methods (21).
3 Results
3.1 Population characteristics
After sorting and cleaning, 696 patients with ARDS were included in this study. Among them, 217 (42.7%) were female. There were 351 (50.4%) patients who were aged 60 years or older. A total of 429, 397, 389, 374, and 357 patients were alive at 28, 60, 90, 180, and 365 days after admission, respectively. On days 28, 60, 90, 180, and 365, survival rates were 61.6, 57.0, 55.9, 53.7, and 51.3%, respectively. There were 198 patients who used ACEIs, 395 patients who used dexmedetomidine, and 113 patients who used ACEIs and dexmedetomidine during admission.
Regarding complications, 98 (14.1%) patients had myocardial infarction. Thirty-five patients (19.4%) had congestive heart failure. Eighty-three patients (11.9%) had cerebrovascular disease. A total of 224 (32.2%) patients were diagnosed with diabetes, accounting for 32.2%. Additionally, 129 patients (18.5%) had kidney disease, and 57 patients had severe liver disease (9.1%).
3.2 Cox regression analysis and Kaplan–Meier curve
Multivariate Cox regression analysis showed that treatment methods and severe liver disease were associated with the prognosis of patients with ARDS on days 28, 60, and 90 after admission. Treatment methods, severe liver disease, and kidney disease were associated with outcomes at days 180 and 365 (Tables 2–6).
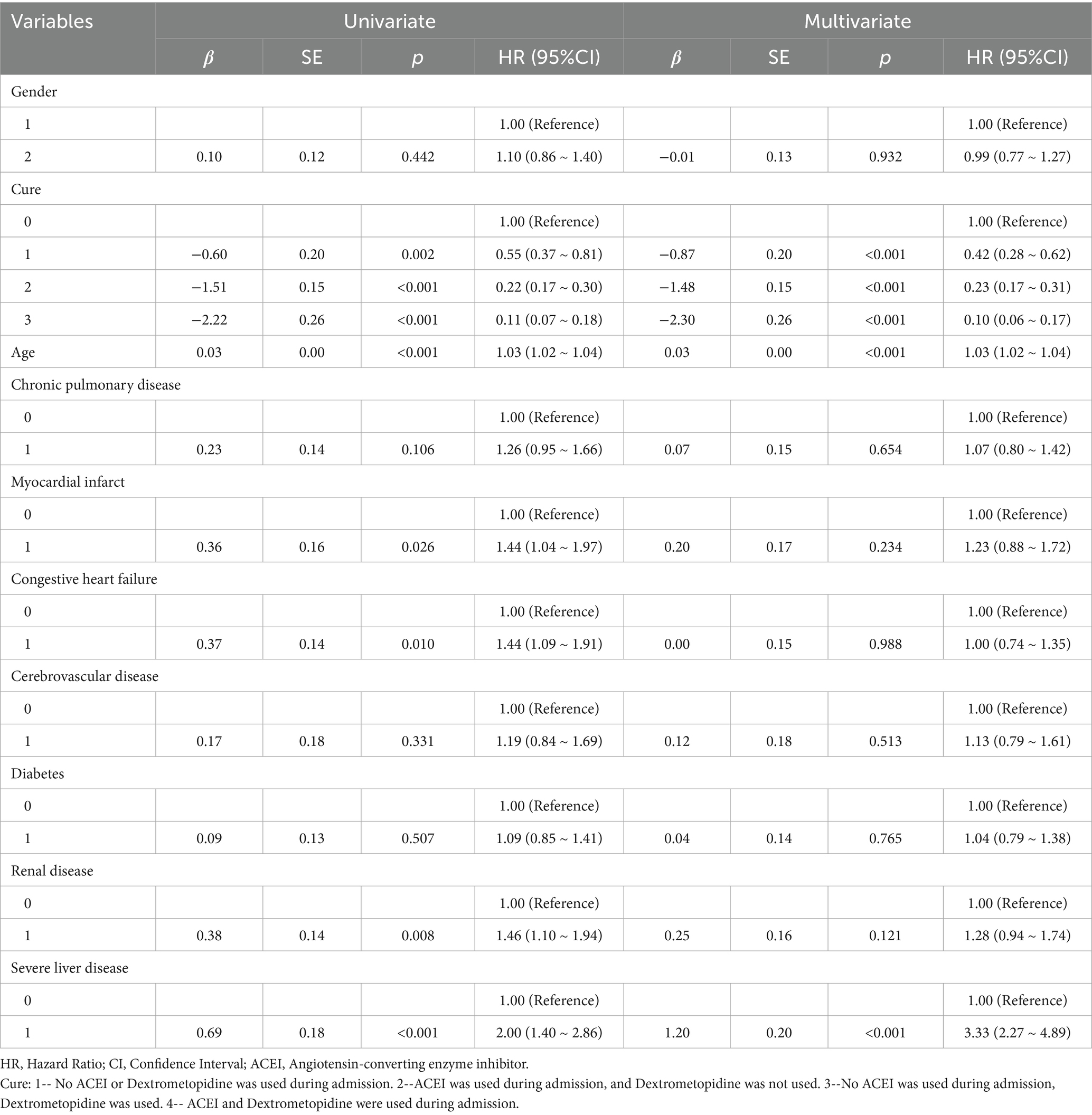
Table 2. Results of univariate and multifactorial cox proportional risk model affecting 28-day survival.
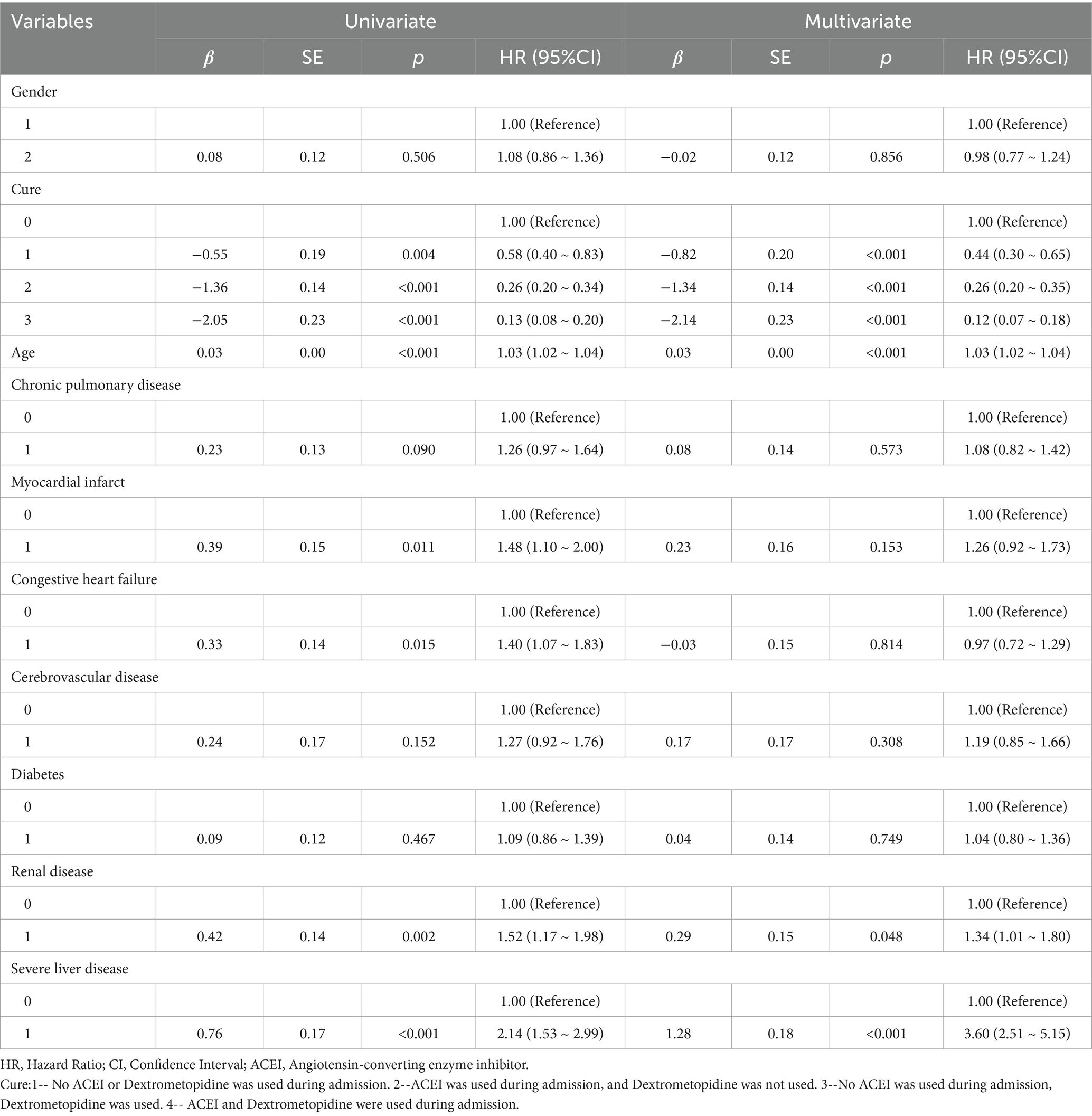
Table 3. Results of univariate and multifactorial cox proportional risk model affecting 60-day survival.
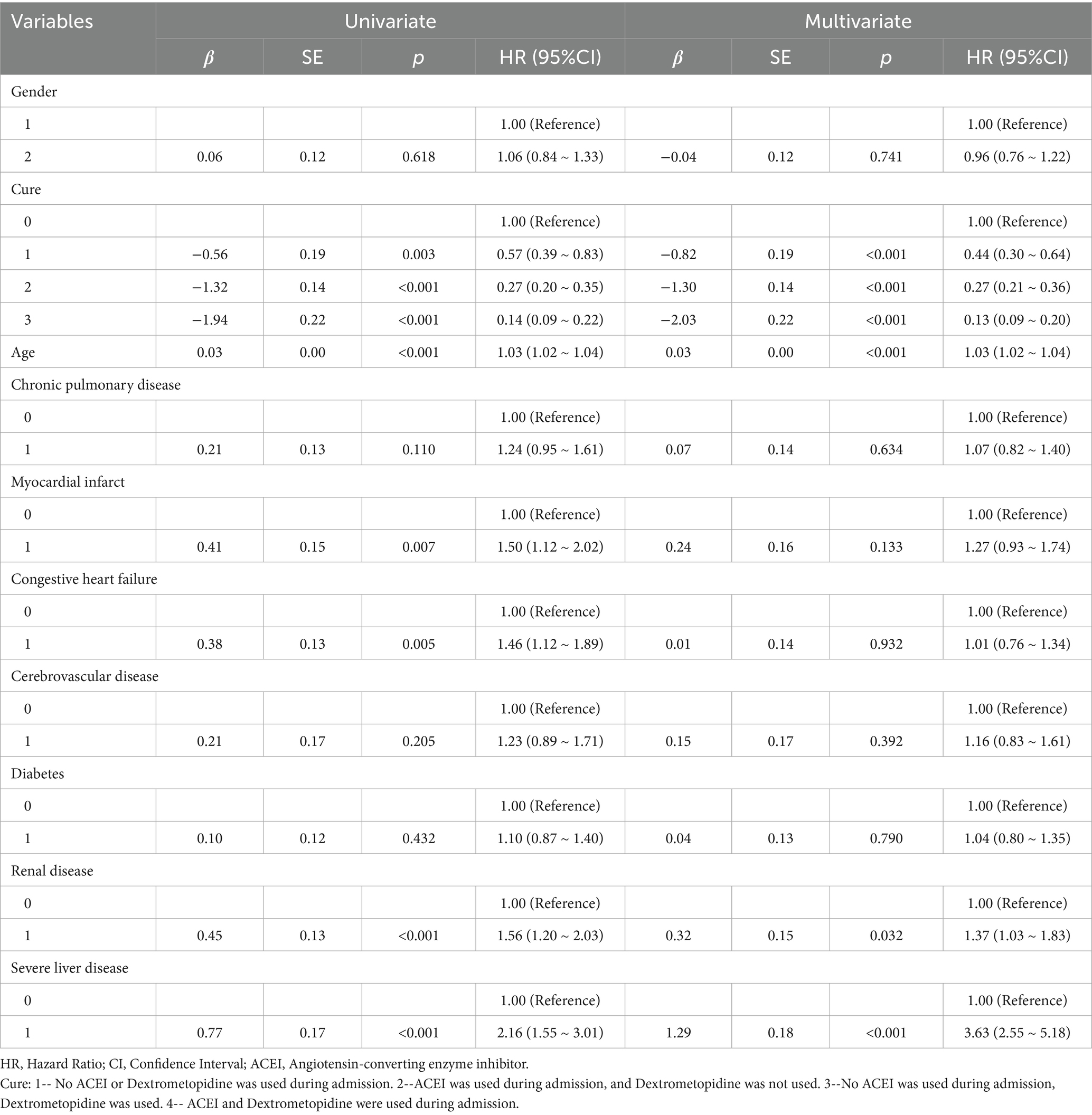
Table 4. Results of univariate and multifactorial cox proportional risk model affecting 90-day survival.
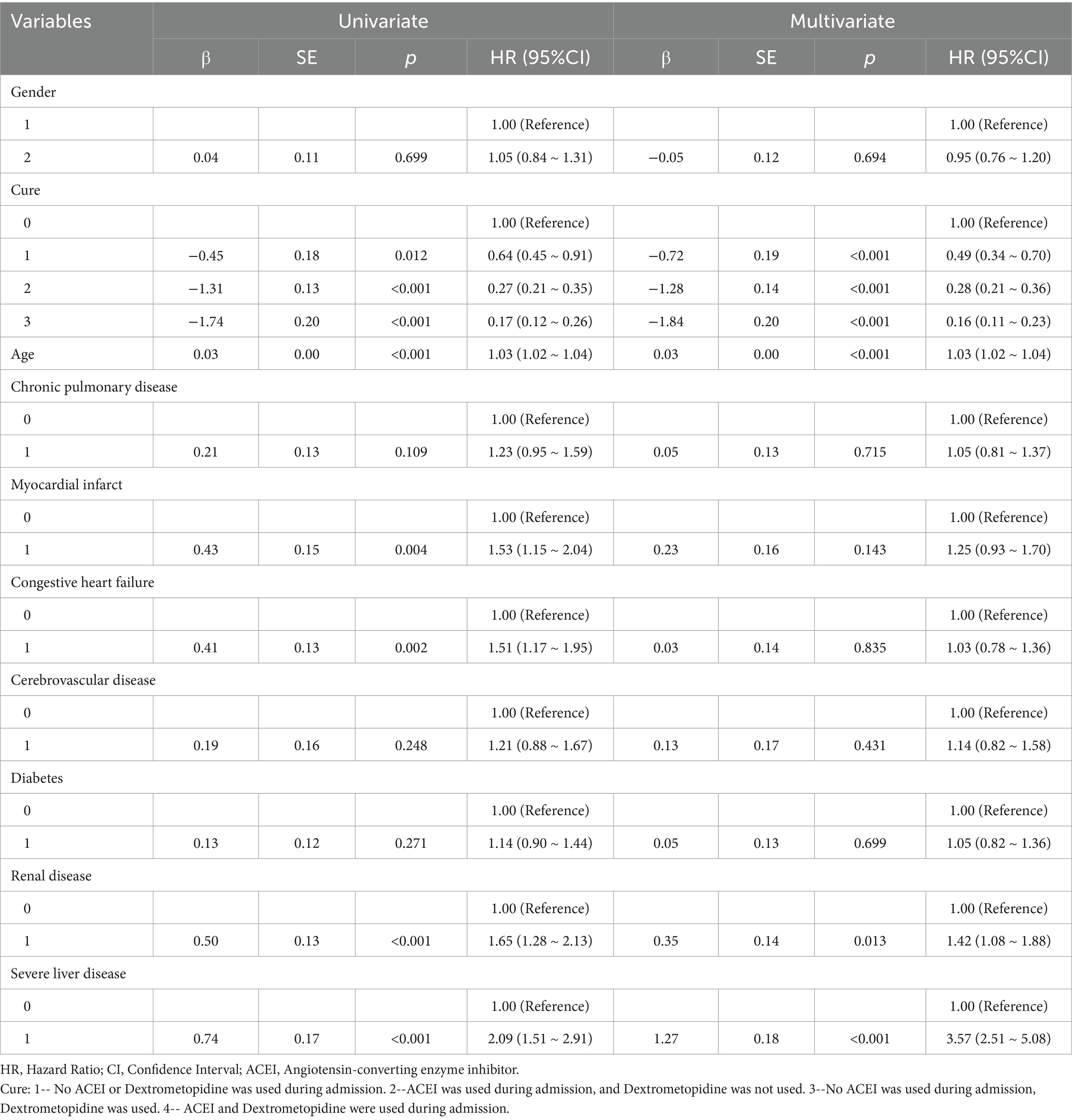
Table 5. Results of univariate and multifactorial cox proportional risk model affecting 180-day survival.
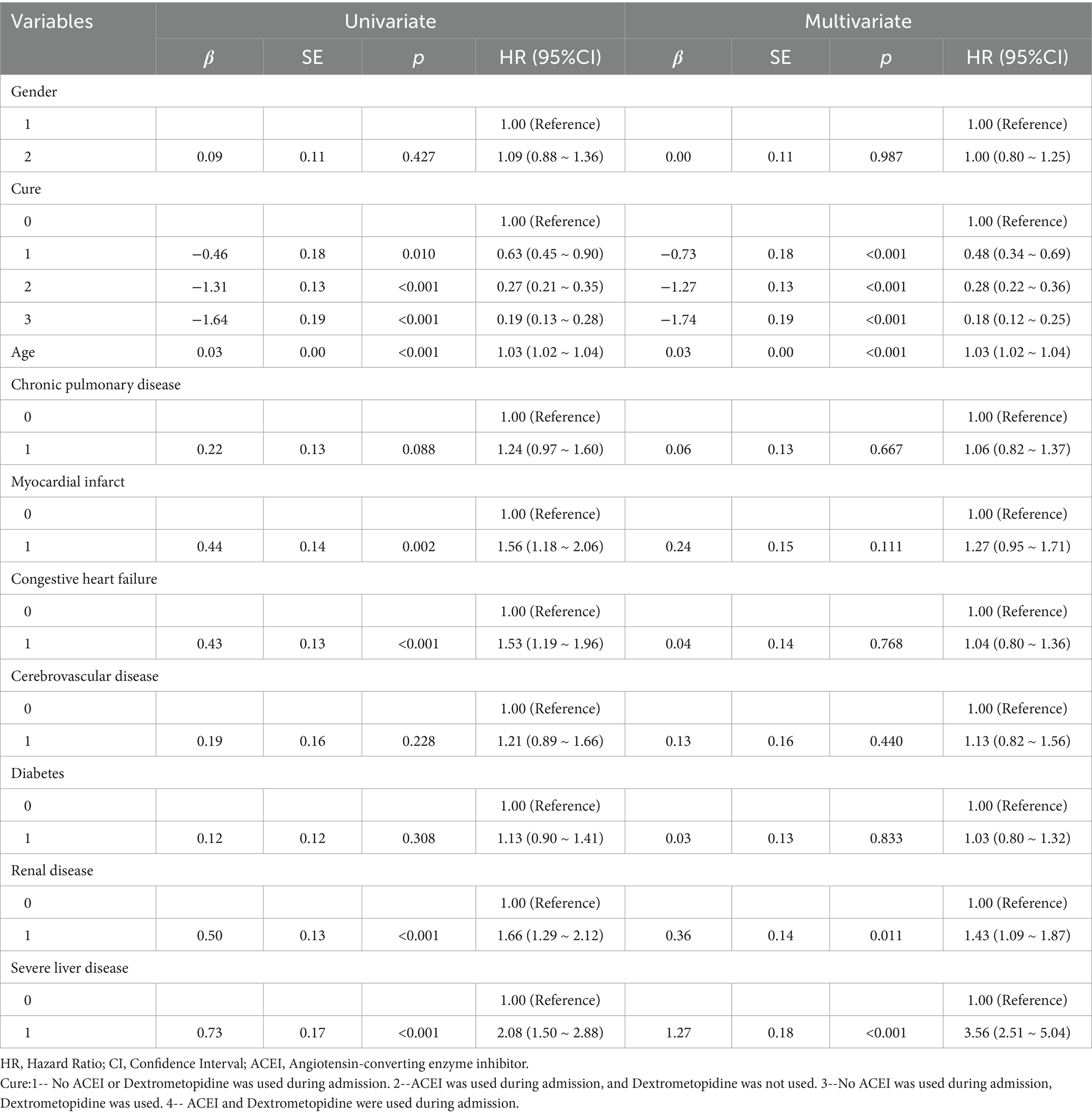
Table 6. Results of univariate and multifactorial cox proportional risk model affecting 365-day survival.
Subsequently, multi-model Cox regression was used to exclude the interference of serious liver disease, kidney disease, and other factors in prognosis, and the adjusted model showed that patients with ARDS who received a combination of ACEIs and dexmedetomidine had the best prognosis. Following treatment with dexmedetomidine alone, patients treated with neither dexmedetomidine nor ACEIs had the worst prognosis. Among them, the risk of death in patients with ARDS treated with ACEIs combined with dexmedetomidine was reduced by 88.7, 86.6, 85.0, 82.4, and 80.6% on days 28, 60, 90, 180, and 365, respectively (Tables 7–11). By drawing the Kaplan–Meier curve, the results showed that, compared to patients treated with ACEI monotherapy or dexmedetomidine monotherapy, the survival rate of patients with ARDS treated with ACEIs and dexmedetomidine was the highest, and the difference was statistically significant (p < 0.05) (Figure 2).
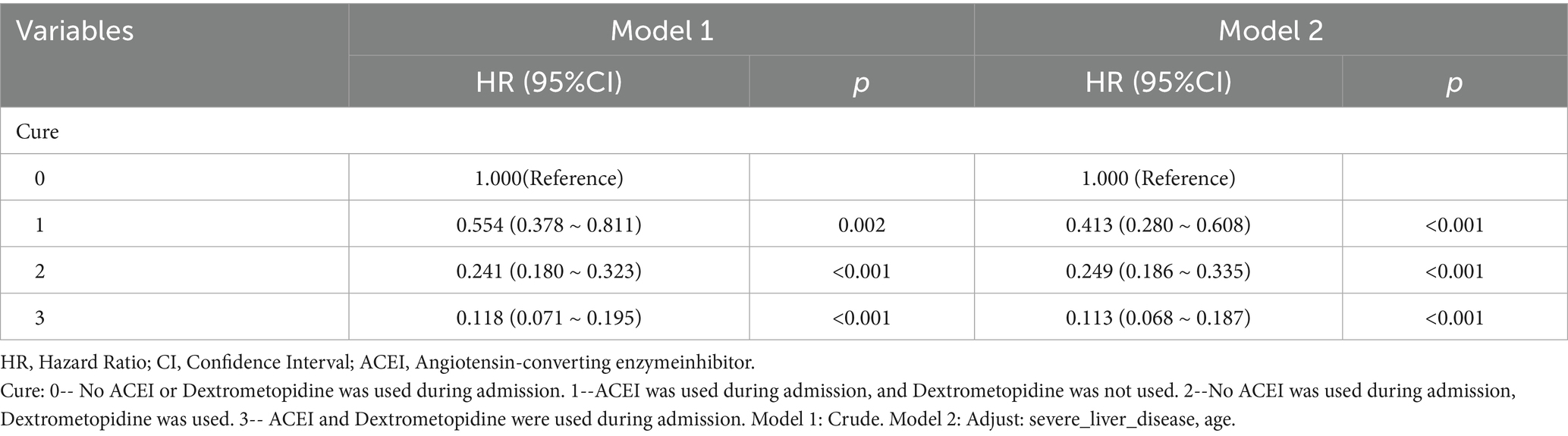
Table 7. Cox regression model and correction model affecting mortality of ARDS patients within 28 days after admission.
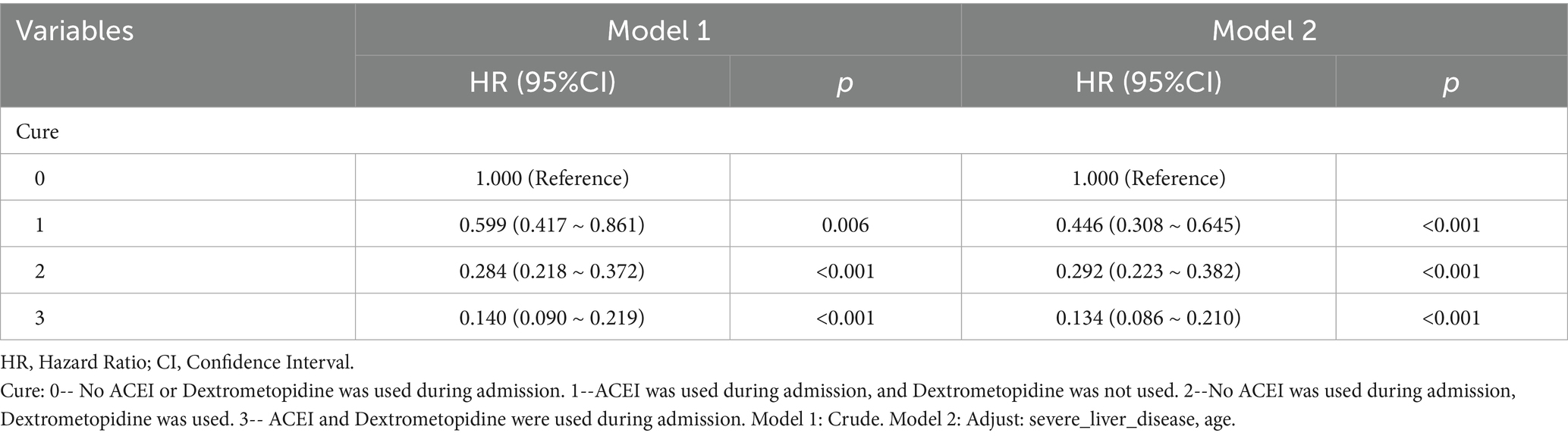
Table 8. Cox regression model and correction model affecting mortality of ARDS patients within 60 days after admission.
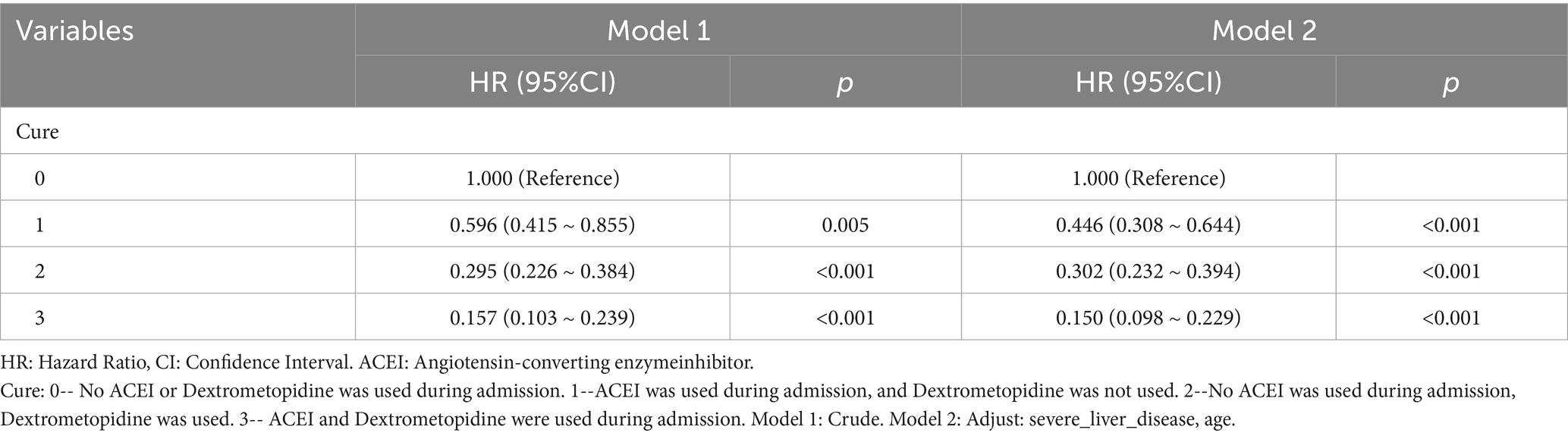
Table 9. Cox regression model and correction model affecting mortality of ARDS patients within 90 days after admission.
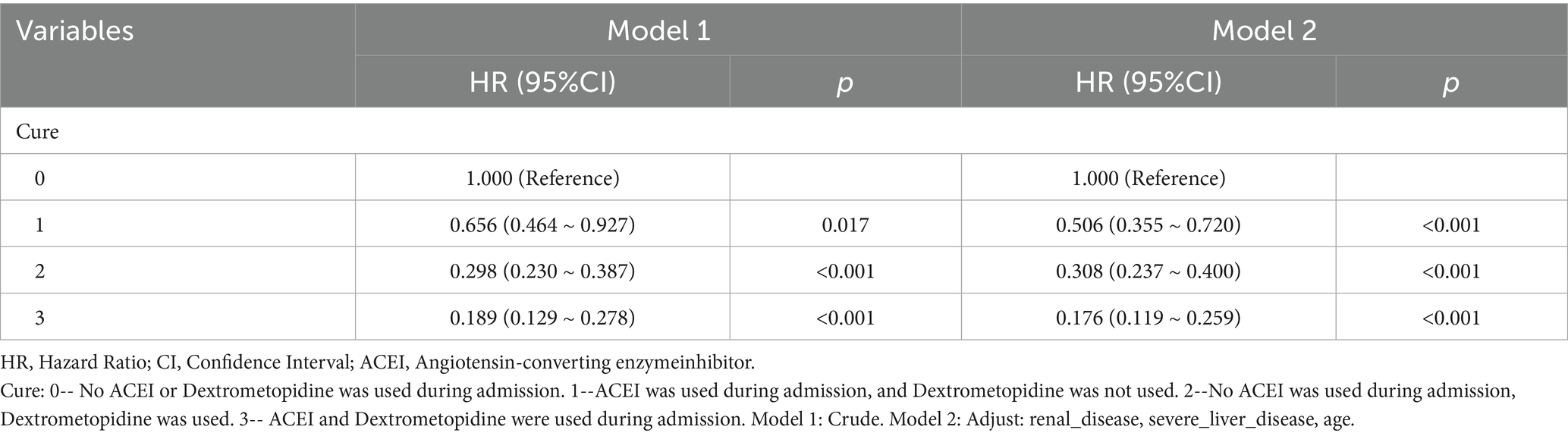
Table 10. Cox regression model and correction model affecting mortality of ARDS patients within 180 days after admission.
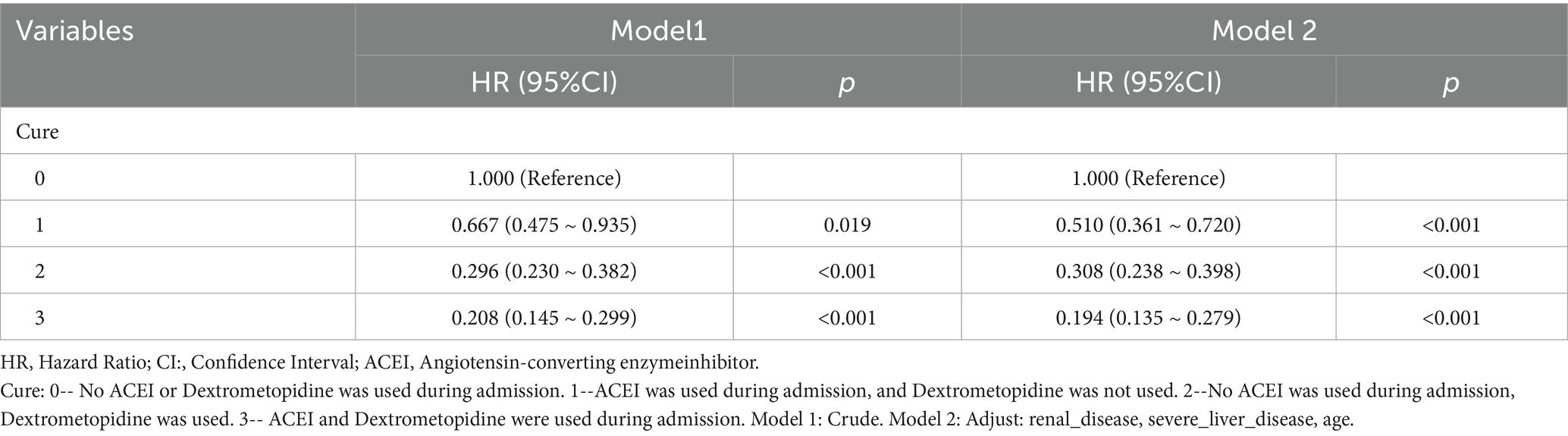
Table 11. Cox regression model and correction model affecting mortality of ARDS patients within 365 days after admission.
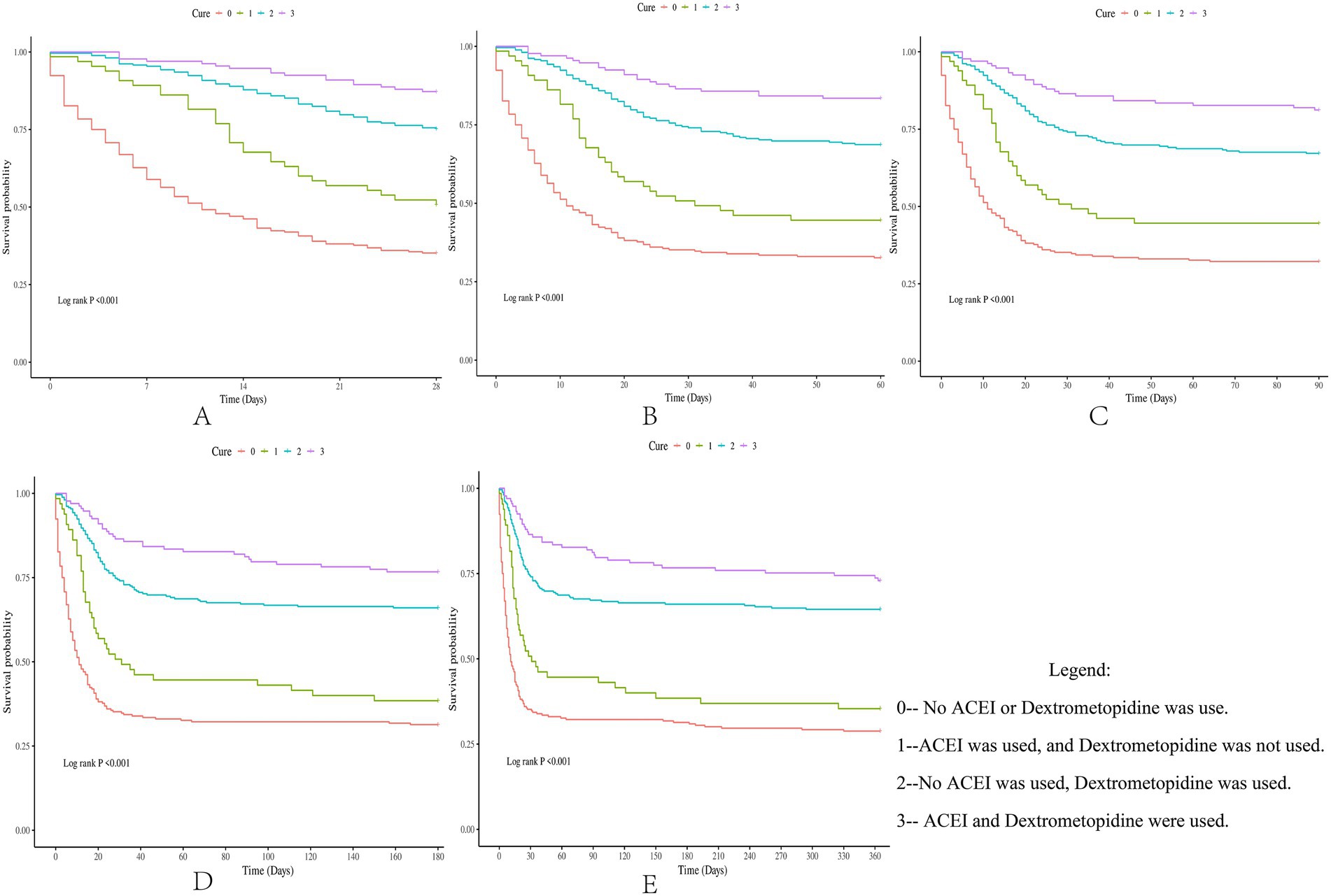
Figure 2. Difference in survival rates between the drug group and the control group (Kaplan-Meier survival curve).
4 Discussion
ARDS is characterized by diffuse pulmonary interstitial and alveolar edema caused by injury to pulmonary capillary endothelial cells and alveolar epithelial cells induced by non-cardiogenic diseases, such as trauma and severe infection, followed by acute hypoxic respiratory insufficiency (22–27). Current research indicates that ARDS can be divided into two distinct subphenotypes. One subphenotype is characterized by more severe inflammation, shock, and metabolic acidosis, and a poorer clinical prognosis. In a randomized trial examining PEEP strategies, these subtypes responded differently to treatment (28). Current treatment methods include improved ventilation (29–32), respiratory support (33, 34), sedation (35), muscle relaxation (36), and early nerve block (37–39).
Previous studies have shown that both ACEI and dexmedetomidine monotherapies benefit patients with ARDS. However, whether the combined application of ACEIs and dexmedetomidine can play a synergistic role in improving the survival rate of patients with ARDS remains to be explored. Four treatment methods were established in this study: neither ACEIs nor dexmedetomidine was used; ACEIs were used instead of dexmedetomidine; dexmedetomidine was used instead of ACEIs; and ACEIs and dexmedetomidine were used. By comparing the survival rate differences of patients with ARDS under four different treatment methods, this study explored whether the combined application of ACEIs and dexmedetomidine could further enhance the improvement in the prognosis of patients with ARDS. By constructing a multi-model Cox regression coarse model, the results showed that compared to the other three treatment methods, the combined application of ACEIs and dexmedetomidine had the strongest ability to reduce the risk of death in patients with ARDS. Subsequently, by adjusting for the interference of covariates, the adjusted model showed that, compared with the other three groups, patients with ARDS who received ACEIs and dexmedetomidine had the highest survival rate, and this conclusion was maintained within 0–365 days after admission. Finally, through the Kaplan–Meier survival curve analysis in this study, it was further verified that the combined application of ACEIs and dexmedetomidine had a synergistic effect on improving the prognosis of patients with ARDS.
As the combined application of dexmedetomidine and ACEIs has not yet been reported, it is unfeasible to draw conclusions from previous studies to explain our results. Therefore, we can only explore why ACEIs combined with dexmedetomidine significantly improve the survival rate of patients with ARDS from the perspective of the mechanism of drug action.
ACEIs are a class of drugs that reduce the production of angiotensin II by inhibiting angiotensin-converting enzymes, which dilate blood vessels and reduce peripheral resistance (40, 41). In addition, ACEIs may have anti-inflammatory effects because angiotensin II is thought to be involved in pro-inflammatory responses (42, 43). In ARDS, an excessive inflammatory response in the lungs may be an important factor leading to lung injury (24). Therefore, inhibiting angiotensin II synthesis may help reduce inflammation and improve lung function (44, 45). Dexmedetomidine is a selective α2 adrenergic receptor agonist that promotes sodium and water excretion. As ARDS is often associated with fluid retention and pulmonary edema, the use of dexmedetomidine may also help improve fluid balance, indirectly reduce pulmonary edema, and improve oxygenation. In addition, dexmedetomidine maintained hemodynamic stability (46). Finally, dexmedetomidine neither leads to deeper sedation levels nor does it lead to respiratory depression, which is conducive to rapid patient recovery (47, 48).
Based on the above drug mechanism analysis, we found that the combination of ACEIs and dexmedetomidine has certain potential advantages. For example, the anti-inflammatory effects of ACEIs and the sedative and protective effects of dexmedetomidine may synergistically improve lung function and oxygenation. The combination of these may intervene in the pathophysiological process of ARDS through multiple targets, reduce the mortality rate, and shorten the duration of mechanical ventilation. In addition, ACEIs can inhibit ROS generation, and dexmedetomidine activates the Nrf2 pathway. This combined application may enhance the antioxidant capacity of alveolar epithelial cells and reduce apoptosis.
Although we tried our best to improve the research plan, there were some limitations. First, this was a single-center, retrospective study. The strength of the conclusions drawn from this retrospective study is not as strong as that of prospective studies. Therefore, in the future, multicenter prospective studies need to be improved for verification. Second, this study preliminarily explored and verified the improvement effect of ACEIs and dexmedetomidine on the prognosis of patients with ARDS based on statistics and made predictions about the conclusion in combination with the mechanisms of the two types of drugs. The basic experiments need to be improved in the future to further verify these speculations. Finally, because of the limitations of the database, other confounding factors, such as smoking history, may not have been included in this study. Moreover, there has been no further study on the correlation between drug exposure time and the prognosis of patients with ARDS. Despite these limitations, this study provides novel insights for future prospective research and basic experiments.
5 Conclusion
ARDS is a complication with acute onset and poor prognosis, and its treatment remains complex. However, significant progress has been made in the treatment of ARDS. For example, dexmedetomidine has been shown to improve patient prognosis. Prone positioning has improved ventilation. Despite these advances, consensus has not yet been reached on some issues. For example, while ACEIs can improve the prognosis of patients with ARDS, it is still unclear whether combining dexmedetomidine with ACEIs is superior to using dexmedetomidine alone.
In this study, the improvement effect of ACEIs on the prognosis of patients with ARDS was confirmed, the effect of dexmedetomidine was verified, and it was further found that the combined application of dexmedetomidine and ACEIs has a certain synergistic effect on the improvement of the prognosis of patients with ARDS. In the future, it will be necessary to conduct prospective studies to verify the conclusions of this study and improve basic research to explore the underlying molecular mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The MIMIC-IV databases have received ethical approval from the Institutional Review Boards at Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology. As the databases do not contain protected health information, a waiver of informed consent was included in the approval from the Institutional Review Boards at Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology. Therefore, this manuscript does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee. All methods in this study were carried out in accordance with relevant guidelines and regulations (declarations of Helsinki).
Author contributions
QH: Conceptualization, Formal analysis, Project administration, Methodology, Funding acquisition, Writing – original draft, Data curation, Investigation, Resources. XY: Data curation, Writing – original draft, Conceptualization. HY: Writing – original draft. XZ: Formal analysis, Methodology, Conceptualization, Writing – original draft. JT: Software, Writing – original draft, Formal analysis, Conceptualization. ZW: Validation, Funding acquisition, Project administration, Writing – review & editing, Supervision, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank all the staff involved in building the MIMIC-IV database for providing this valuable open-access resource. They also express their sincere gratitude to all the staff of Beth Israel Deaconess Medical Center.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Umbrello, M, Fumagalli, J, Pesenti, A, and Chiumello, D. Pathophysiology and Management of Acute Respiratory Distress Syndrome in obese patients. Semin Respir Crit Care Med. (2019) 40:40–56. doi: 10.1055/s-0039-1685179
2. Gattinoni, L, Pelosi, P, Suter, PM, Pedoto, A, Vercesi, P, and Lissoni, A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med. (1998) 158:3–11. doi: 10.1164/ajrccm.158.1.9708031
3. Bellani, G, Laffey, JG, Pham, T, Fan, E, Brochard, L, Esteban, A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. (2016) 315:788–800. doi: 10.1001/jama.2016.0291
4. Acute Respiratory Distress Syndrome NetworkBrower, RG, Matthay, MA, Morris, A, Schoenfeld, D, Thompson, BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. (2000) 342:1301–8. doi: 10.1056/NEJM200005043421801,
5. Zambon, M, and Vincent, JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. (2008) 133:1120–7. doi: 10.1378/chest.07-2134
6. Ohshimo, S. Oxygen administration for patients with ARDS. J Intensive Care. (2021) 9:17. doi: 10.1186/s40560-021-00532-0
7. Kimura, S, Stoicea, N, Rosero Britton, BR, Shabsigh, M, Branstiter, A, and Stahl, DL. Preventing ventilator-associated lung injury: a perioperative perspective. Front Med (Lausanne). (2016) 3:25. doi: 10.3389/fmed.2016.00025
8. Thompson, BT, and Moss, M. A new definition for the acute respiratory distress syndrome. Semin Respir Crit Care Med. (2013) 34:441–7. doi: 10.1055/s-0033-1351162
9. Matthay, MA, Arabi, Y, Arroliga, AC, Bernard, G, Bersten, AD, Brochard, LJ, et al. A new global definition of acute respiratory distress syndrome. Am J Respir Crit Care Med. (2024) 209:37–47. doi: 10.1164/rccm.202303-0558WS
10. Papazian, L, Pauly, V, Hamouda, I, Daviet, F, Orleans, V, Forel, JM, et al. National incidence rate and related mortality for acute respiratory distress syndrome in France. Anaesth Crit Care Pain Med. (2021) 40:100795. doi: 10.1016/j.accpm.2020.100795
11. Jeffery, MM, Cummins, NW, Dempsey, TM, Limper, AH, Shah, ND, and Bellolio, F. Association of outpatient ACE inhibitors and angiotensin receptor blockers and outcomes of acute respiratory illness: a retrospective cohort study. BMJ Open. (2021) 11:e044010. doi: 10.1136/bmjopen-2020-044010
12. Fang, YP, and Zhang, X. A propensity score-matching analysis of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker exposure on in-hospital mortality in patients with acute respiratory failure. Pharmacotherapy. (2022) 42:387–96. doi: 10.1002/phar.2677
13. Akyüz, A, Işık, F, Aslan, B, Çap, M, Kaya, İ, Atlı, Ö, et al. The effect of RAAS inhibitors on acute hypoxemic respiratory failure and in-hospital mortality in the hypertensive Covid-19 patients. Clin Exp Hypertens. (2021) 43:587–96. doi: 10.1080/10641963.2021.1916947
14. Song, Y, Gao, S, Tan, W, Qiu, Z, Zhou, H, and Zhao, Y. Dexmedetomidine versus midazolam and propofol for sedation in critically ill patients: mining the medical information Mart for intensive care data. Ann Transl Med. (2019) 7:197. doi: 10.21037/atm.2019.04.14
15. Boncyk, C, Devlin, JW, Faisal, H, Girard, TD, Hsu, SH, Jabaley, CS, et al. INhaled sedation versus Propofol in REspiratory failure in the intensive care unit (INSPiRE-ICU1): protocol for a randomised, controlled trial. BMJ Open. (2024) 14:e086946. doi: 10.1136/bmjopen-2024-086946
16. Shi, X, Zhang, J, Sun, Y, Chen, M, and Han, F. Effect of different sedatives on the prognosis of patients with mechanical ventilation: a retrospective cohort study based on MIMIC-IV database. Front Pharmacol. (2024) 15:1301451. doi: 10.3389/fphar.2024.1301451
17. Zhang, Z, Goyal, H, Lange, T, and Hong, Y. Healthcare processes of laboratory tests for the prediction of mortality in the intensive care unit: a retrospective study based on electronic healthcare records in the USA. BMJ Open. (2019) 9:e028101. doi: 10.1136/bmjopen-2018-028101
18. Kurniati, AP, Rojas, E, Hogg, D, Hall, G, and Johnson, OA. The assessment of data quality issues for process mining in healthcare using medical information Mart for intensive care III, a freely available e-health record database. Health Informatics J. (2019) 25:1878–93. doi: 10.1177/1460458218810760
19. Johnson, AE, Bulgarelli, L, Shen, L, Gayles, A, Shammout, A, Horng, S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10:1. doi: 10.1038/s41597-022-01899-x
20. Cusack, R, Garduno, A, Elkholy, K, and Martín-Loeches, I. Novel investigational treatments for ventilator-associated pneumonia and critically ill patients in the intensive care unit. Expert Opin Investig Drugs. (2022) 31:173–92. doi: 10.1080/13543784.2022.2030312
21. Gomes, AP, Costa, B, Marques, R, Nunes, V, and Coelho, C. Kaplan-Meier survival analysis: practical insights for clinicians. Acta Medica Port. (2024) 37:280–5. doi: 10.20344/amp.21080
22. Matthay, MA, Zemans, RL, Zimmerman, GA, Arabi, YM, Beitler, JR, Mercat, A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. (2019) 5:18. doi: 10.1038/s41572-019-0069-0
23. Bossardi Ramos, R, and Adam, AP. Molecular mechanisms of vascular damage during lung injury. Adv Exp Med Biol. (2021) 1304:95–107. doi: 10.1007/978-3-030-68748-9_6
24. Bernard, GR, Artigas, A, Brigham, KL, Carlet, J, Falke, K, Hudson, L, et al. The American-European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. (1994) 149:818–24. doi: 10.1164/ajrccm.149.3.7509706
25. Schmidt, M, Hajage, D, Lebreton, G, Dres, M, Guervilly, C, Richard, JC, et al. Prone positioning during extracorporeal membrane oxygenation in patients with severe ARDS: the PRONECMO randomized clinical trial. JAMA. (2024) 331:446. doi: 10.1001/jama.2023.24491
26. Guérin, C, Reignier, J, Richard, JC, Beuret, P, Gacouin, A, Boulain, T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. (2013) 368:2159–68. doi: 10.1056/NEJMoa1214103
27. Scholten, EL, Beitler, JR, Prisk, GK, and Malhotra, A. Treatment of ARDS with prone positioning. Chest. (2017) 151:215–24. doi: 10.1016/j.chest.2016.06.032
28. Calfee, CS, Delucchi, K, Parsons, PE, Thompson, BT, Ware, LB, Matthay, MA, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. (2014) 2:611–20. doi: 10.1016/S2213-2600(14)70097-9
29. Ashra, F, Chen, R, Kang, XL, Chiang, KJ, Pien, LC, Jen, HJ, et al. Effectiveness of prone position in acute respiratory distress syndrome and moderating factors of obesity class and treatment durations for COVID-19 patients: a meta-analysis. Intensive Crit Care Nurs. (2022) 72:103257. doi: 10.1016/j.iccn.2022.103257
30. Subgroup of Critical Respiratory Diseases. Standardized protocol of prone position ventilation in patients with acute respiratory distress syndrome. Zhonghua Nei Ke Za Zhi. (2020) 59:781–7. doi: 10.3760/cma.j.cn112138-20200430-00439
31. Camporota, L, Sanderson, B, Chiumello, D, Terzi, N, Argaud, L, Rimmelé, T, et al. Prone position in COVID-19 and -COVID-19 acute respiratory distress syndrome: an international multicenter observational comparative study. Crit Care Med. (2022) 50:633–43. doi: 10.1097/CCM.0000000000005354
32. Besch, G, Persoz, MO, and Liaudet, L. Décubitus ventral et syndrome de détresse respiratoire aiguë de l'adulte: de la théorie à la pratique [Prone position mechanical ventilation in the acute respiratory distress syndrome: theoretical and practical considerations]. Rev Med Suisse. (2014) 10:2362–7. doi: 10.1056/NEJMoa1901686
33. Umbrello, M, Marino, A, and Chiumello, D. Tidal volume in acute respiratory distress syndrome: how best to select it. Ann Transl Med. (2017) 5:287. doi: 10.21037/atm.2017.06.51
34. Beitler, JR, Sarge, T, Banner-Goodspeed, VM, Gong, MN, Cook, D, Novack, V, et al. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. (2019) 321:846–57. doi: 10.1001/jama.2019.0555
35. Jabaudon, M, Boucher, P, Imhoff, E, Chabanne, R, Faure, JS, Roszyk, L, et al. Sevoflurane for sedation in acute respiratory distress syndrome. A randomized controlled pilot study. Am J Respir Crit Care Med. (2017) 195:792–800. doi: 10.1164/rccm.201604-0686OC
36. Moore, L, Kramer, CJ, Delcoix-Lopes, S, and Modrykamien, AM. Comparison of Cisatracurium versus Atracurium in early ARDS. Respir Care. (2017) 62:947–52. doi: 10.4187/respcare.05102
37. National Heart, Lung, and Blood Institute PETAL Clinical Trials NetworkMoss, M, Huang, DT, Brower, RG, Ferguson, ND, Ginde, AA, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. (2019) 380:1997–2008.
38. Grawe, ES, Bennett, S, and Hurford, WE. Early paralysis for the management of ARDS. Respir Care. (2016) 61:830–8. doi: 10.4187/respcare.04734
39. Bourenne, J, Hraiech, S, Roch, A, Gainnier, M, Papazian, L, and Forel, JM. Sedation and neuromuscular blocking agents in acute respiratory distress syndrome. Ann Transl Med. (2017) 5:291. doi: 10.21037/atm.2017.07.19
40. Kellner, M, Noonepalle, S, Lu, Q, Srivastava, A, Zemskov, E, and Black, SM. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Adv Exp Med Biol. (2017) 967:105–37. doi: 10.1007/978-3-319-63245-2_8
41. Mittal, M, Siddiqui, MR, Tran, K, Reddy, SP, and Malik, AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. (2014) 20:1126–67. doi: 10.1089/ars.2012.5149
42. Nguyen Dinh Cat, A, Montezano, AC, Burger, D, and Touyz, RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. (2013) 19:1110–20. doi: 10.1089/ars.2012.4641
43. Zheng, J, Li, G, Chen, S, Bihl, J, Buck, J, Zhu, Y, et al. Activation of the ACE2/Ang-(1-7)/mas pathway reduces oxygen-glucose deprivation-induced tissue swelling, ROS production, and cell death in mouse brain with angiotensin II overproduction. Neuroscience. (2014) 273:39–51. doi: 10.1016/j.neuroscience.2014.04.060
44. Ekström, M, and Bornefalk-Hermansson, A. Cardiovascular and antacid treatment and mortality in oxygen-dependent pulmonary fibrosis: a population-based longitudinal study. Respirology. (2016) 21:705–11. doi: 10.1111/resp.12781
45. Kriszta, G, Kriszta, Z, Váncsa, S, Hegyi, PJ, Frim, L, Erőss, B, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on angiotensin-converting enzyme 2 levels: a comprehensive analysis based on animal studies. Front Pharmacol. (2021) 12:619524. doi: 10.3389/fphar.2021.619524
46. McKenzie-Brown, AM. Commentary on: total intravenous anesthesia with dexmedetomidine for hemodynamic stability and enhanced recovery in facial aesthetic surgery. Aesthet Surg J. (2022) 42:NP611–2. doi: 10.1093/asj/sjac183
47. Lewis, K, Piticaru, J, Chaudhuri, D, Basmaji, J, Fan, E, Møller, MH, et al. Safety and efficacy of Dexmedetomidine in acutely ill adults requiring noninvasive ventilation: a systematic review and meta-analysis of randomized trials. Chest. (2021) 159:2274–88. doi: 10.1016/j.chest.2020.12.052
Keywords: dexmedetomidine, ARDS, MIMIC IV, prognosis, clinical decision
Citation: Hong Q, Yang X, Yang H, Zhou X, Tang J and Wen Z (2025) Prognostic impact of angiotensin-converting enzyme inhibitors and dexmedetomidine in acute respiratory distress syndrome: a MIMIC-IV-based retrospective cohort analysis. Front. Med. 12:1601565. doi: 10.3389/fmed.2025.1601565
Edited by:
Liang Zhao, Dalian University of Technology, ChinaReviewed by:
Yingjie Zhu, The Second Affiliated Hospital of Fujian Medical University, ChinaKaimeng Liu, The First Affiliated Hospital of Henan University of Science and Technology, China
Copyright © 2025 Hong, Yang, Yang, Zhou, Tang and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongmei Wen, endlbkBqbHUuZWR1LmNu
 Qingli Hong
Qingli Hong Xiaozan Yang1
Xiaozan Yang1 Huahong Yang
Huahong Yang Zhongmei Wen
Zhongmei Wen