- 1Shenzhen Key Laboratory of Spine Surgery, Department of Spine Surgery, Peking University Shenzhen Hospital, Shenzhen, China
- 2Department of Spine Surgery, Shenzhen Xinhua Hospital, Shenzhen, China
- 3Department of Pathology, Peking University Shenzhen Hospital, Shenzhen, China
Malignant melanoma bone marrow infiltration induced coagulation dysfunction and spinal epidural haematoma with paraplegia is extremely rare. It typically presents as anemia, coagulation dysfunction or disseminated intravascular coagulation, immune thrombocytopenia. In severe cases, it can lead to spinal epidural hematoma, compressing the spinal cord and nerve roots, resulting in motor and sensory dysfunction, and even paraplegia. Due to the rarity and complexity of this condition, it is prone to misdiagnosis or delayed diagnosis, ultimately leading to a poor prognosis. This paper reports the case of a 14-year-old female who was urgently admitted to the emergency department with low back pain for 1 month, accompanied by systemic mucous membranes bleeding and ecchymosis for half a month, numbness and incomplete paralysis in both lower limbs for 3 days. Laboratory tests indicated pancytopenia and abnormal coagulation function. Magnetic resonance imaging: T11-L1 epidural irregular abnormal signals in the spinal canal, with a range of about 16 mm × 17 mm × 65 mm. Further physical examination revealed that a large, cauliflower-like black mole on the right scalp, which had been present since childhood and had recurrently ulcerated without healing. Bone marrow aspiration biopsy confirmed the diagnosis of malignant melanoma with bone marrow infiltration, leading to coagulation dysfunction and SEH with paraplegia. However, despite 2 months of aggressive symptomatic and supportive treatment, the child ultimately succumbed to malignant melanoma forever. This report shares our experience with the diagnosis and treatment of this case, highlighting the necessity of thoroughly reassess the medical history and conduct a detailed physical examination.
Introduction
Malignant melanoma (MM) is a highly malignant melanocytic tumor, accounting for 1–3% of all malignant tumors (1). Its main primary site is the skin tissue, and it can be widely metastasised throughlymph and blood circulation, and common metastatic sites include the lung, brain, liver, bone marrow or intestines (2, 3). Patients with malignant melanoma bone marrow infiltration usually present with anemia, coagulopathy or disseminated intravascular coagulation (DIC) and immune thrombocytopenia (ITP) (4–6). When coagulation dysfunction occurs, it may lead to bleeding in multiple parts of the body. Among them, spinal epidural hematoma (SEH) is a rare complication, that can cause compression of the spinal cord and nerve roots, resulting in motor and sensory dysfunction, and even paraplegia (7, 8). As far as we know, cases of coagulopathy secondary to MM bone marrow metastasis resulting in SEH have rarely been reported in the literature. The pathogenesis of SEH may be associated with local vascular damage and systemic coagulopathy secondary to MM bone marrow infiltration, however, the precise pathological mechanisms remain incompletely understood. The etiology of SEH is predominantly attributed to trauma, surgery, anesthesia, and other invasive procedures. Here, we report a case of a rare MM bone marrow infiltration induced coagulation dysfunction and SEH, which eventually developed into paraplegia. By detailed analysis of the diagnosis and treatment process, we aim to further explore the diagnosis and treatment strategies of complications related to MM bone marrow infiltration and provide a reference for clinical practice.
Case report
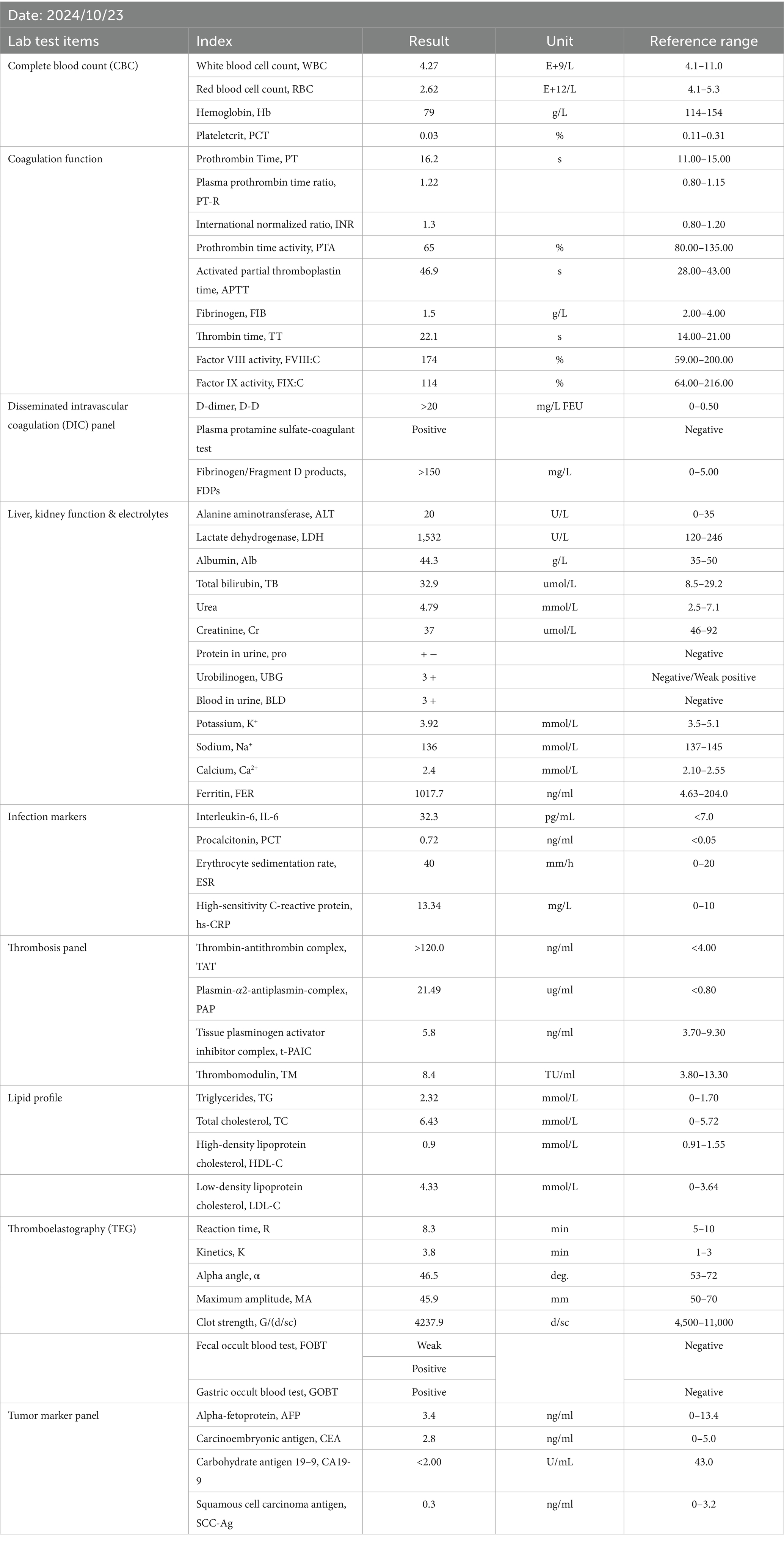
On Oct. 23, 2024, a 14-year-old female was urgently admitted to the emergency department (ED) with low back pain for 1 month, accompanied by systemic mucous membranes bleeding and ecchymosis for half a month, numbness and incomplete paralysis in both lower limbs for 3 days. One month ago, the patient suffered from lumbar pain due to an accidental sprain while running. Half a month ago, the back pain was further worsened after acupuncture on the latissimus dorsi muscles on both sides of the spine, accompained by swelling of the lumbar and buttock muscles, ecchymosis, and active bleeding gums. Fever appeared 4 days ago, with a maximum temperature of 38.5 °C, accompanied by acute urinary retention, the back pain worsened, and progressive decline in muscle strength of both lower limbs. After symptomatic conservative treatment such as antibiotics, analgesics drugs, and blood product transfusion, there was failure improvement in symptoms. No other special treatment. Parents are healthy, and there is no family history of genetic disease or similar disease. Physical examination: Forced high fowler’s position (60°–90°), severe malnutrition, anemia, pale systemic skin and mucous membranes, scattered in large areas of bruising and ecchymosis, especially in the lower limbs, slightly convex skin surface (Figure 1C). Both frontal nasal cavities have been tamponade to stop bleeding, the lumbar and buttock muscles are slightly swollen, and subcutaneous hematoma has formed. The movement of thoracolumbar and both lower limbs was significantly limited, the thoracolumbar and back tenderness and percussion pain. The grade muscle strength of both upper limbs, iliopsoas muscle, quadriceps femoris, tibialis anterior and extensor hallucis longus were 5-/5, 3-/5, 3-/5, 0/5, and 0/5, respectively. Sensation in the perineum and below the plane of the inguinal region was significantly reduced. Active range of motion (AROM) in bilateral lower extremities: hip flexion 0°–45°, knee flexion 0°-90°, ankle dorsiflexion 0°. Brudzinski sign (−), Kernig sign (−), and Babinski sign (−), absent tendon reflexes, ankle and patellar clonus (The child was unable to tolerate physical examination due to severe pain.). Laboratory data: white blood cell (WBC) 4.27 × 109/L, red blood cell (RBC) 2.62 × 1012/L, hemoglobin (HGB) 79 g/L, platelet (PLT) 24 × 109/L, prothrombin time (PT) 16.20s, activated partial thromboplastin time (APTT) 46.90s, D-dimer >20 mg/L FEU (Table 1). Radiographic data: MRI (magnetic resonance imaging): T11-L1 epidural irregular abnormal signals in the spinal canal, with a range of about 16 mm × 17 mm × 65 mm (Figures 2A–E). Based on the above clinical and imaging presentations, the diagnoses of Thoracolumbar spinal epidural hematoma with bilateral lower limb incomplete paraplegia (ASIA-C, T10-L1), DIC (ISTH overt DIC score: 5 points), Febrile fever of unknown origin (FUO), and Traumatic iliopsoas hematoma formation were made. Hematonosis, infection, autoimmune, syndrome of cauda equina (SCE), or malignancy, such as primary or metastatic epidural tumor, were included in the differential diagnosis. At the time of examination (3 days after onset), reflexes were absent, possibly due to acute cord compression; however, as evolution over time was not documented, further histochemical and histopathological examinations were necessary for differential diagnosis. Given that the patient did not exhibit the typical signs of cauda equina syndrome—which is characterized by a flaccid bladder and overflow incontinence—this diagnosis was excluded. The evacuation of T10-L1 epidural hematoma and expanded decompression of spinal canal were performed. The surgery procedure involved resection of the entire lamina at T10 and the right lamina at T11-L1, revealing, as anticipated from the pre-operative MRI, a hematoma situated on the right side of the spinal canal, which substantially compressed the spinal cord, resulting in minimal dural pulsation (Figure 1B). Then, the evacuated hematoma was sent for routine pathology (Figure 1D). Post-operatively, there was no significant improvement in systemic symptoms. The post-op bone marrow aspiration biopsy showed active proliferation of bone marrow, with more basophils and phagocytes. Unknown cells accounted for 77.00%, and myeloid sarcoma or mast cell leukemia was suspected, while neuroblastoma cell nature could not be excluded. There was a positive immunohistochemical reaction of CD117 and Ki-67, with negative results for the other markers tested in the pathological tissue from the post-op epidural hematoma and bone marrow aspiration biopsy. Combined with flow cytometry, histomorphology, and immunohistochemistry, leukemia was highly suspected. However, as the patient’s condition showed no significant improvement, it was deemed necessary to thoroughly reassess the medical history and conduct a detailed physical examination. It was noted that the child had a large cauliflower-like black mole on the right scalp since childhood, which repeatedly ulcerated and failed to heal (Figure 1A). Subsequently, immunohistochemical staining for MM- related markers on bone marrow and hematoma specimens was conducted, and the results showed positive staining for HMB45, Melan-A, SOX10, S100, and PRAME, with negative results for the other markers. Based on the patient’s scalp lesion, combined with positive immunohistochemical (IHC) staining for tumor markers in both the intraoperative hematoma specimen and the post-op iliac bone marrow biopsy, the results confirmed the diagnosis of MM. However, despite 2 months of aggressive symptomatic and supportive treatment, the child ultimately succumbed to MM forever.
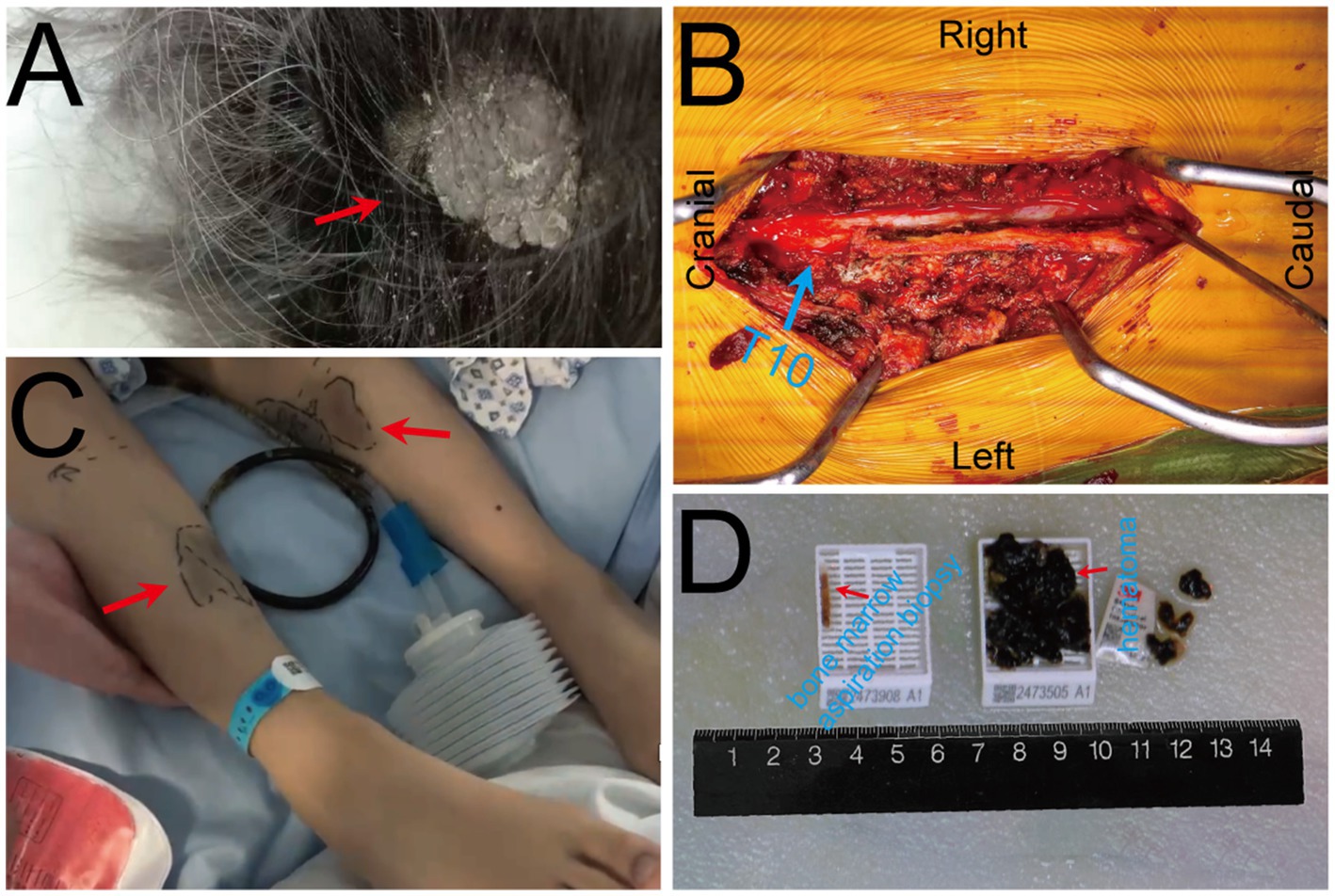
Figure 1. Pre-operative clinical and intra-operative images. (A) A large cauliflower-like black mole on the right scalp (arrow); (B) Intra-operative view of the surgical field with T10 vertebral body marked by blue. (C) Scattered bruising and ecchymosis on bilateral lower limbs (arrows); (D) Bone marrow aspiration biopsy specimen (left) and intra-operative evacuated hematoma specimen (right).
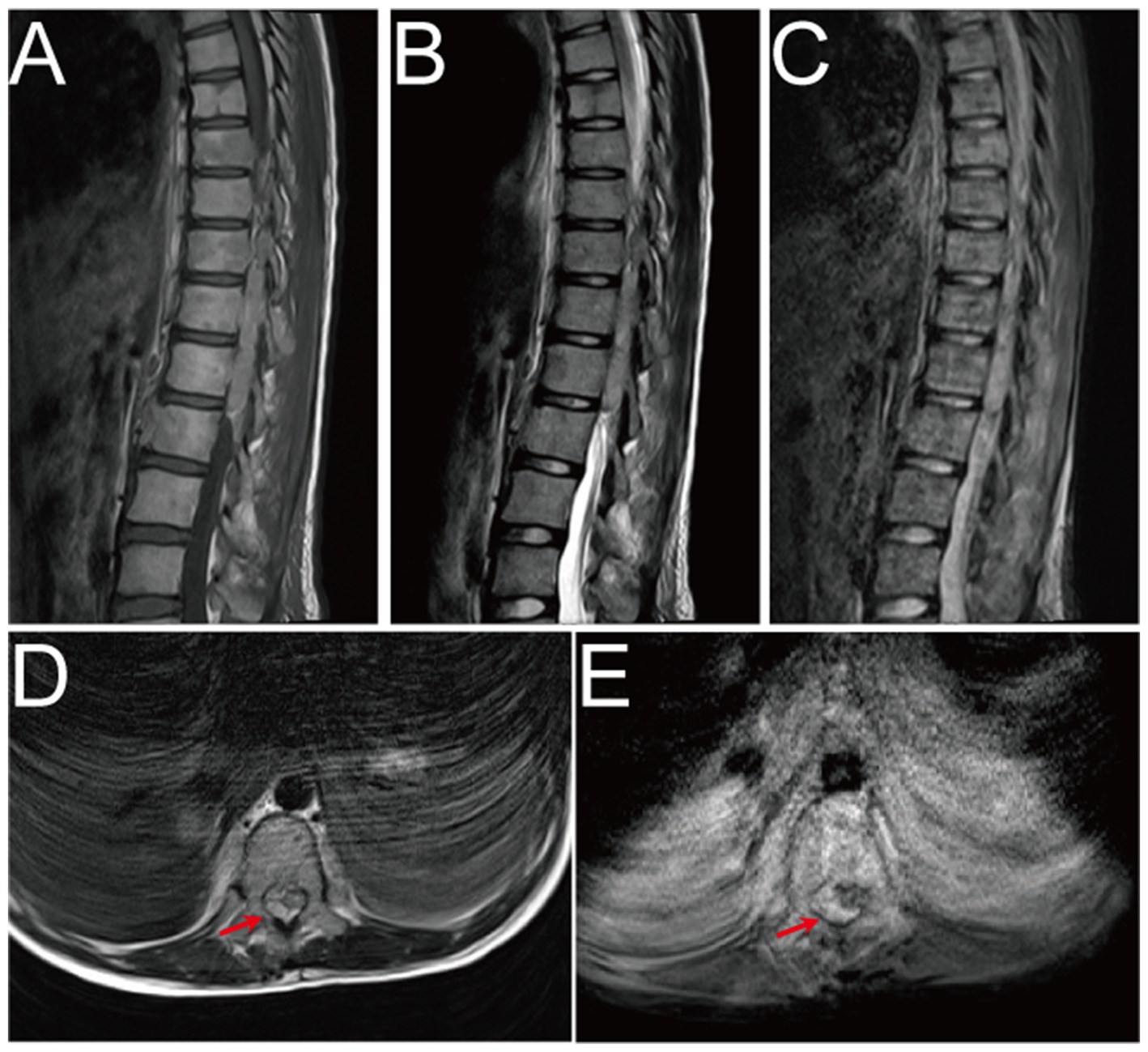
Figure 2. Pre-operative MRI image. (A–E) Pre-operative MRI showed patchy heterogeneous signals in the vertebral body and appendages, and epidural hematoma at the T10-L1 level. The hematoma was crescent-shaped, measuring approximately 16 mm × 17 mm × 65 mm. Both T1WI and T2WI showed hyperintensity, while fat-saturation proton density-weighted sequences showed hyperintensity.
Discussion
This study presents a rare pediatric case of malignant melanoma (MM) bone marrow infiltration, accompanied by spinal cord compression leading to paraplegia. To date, no previously reported cases have documented pediatric paraplegia secondary to MM bone marrow infiltration. Following the PRISMA guidelines for systematic reviews, a comprehensive and systematic literature review was conducted until March 28, 2025, across multiple databases—including ClinicalKey, Embase, Google Scholar, Medline, Ovid, PubMed, Scopus, and Web of Science—using the search terms “malignant melanoma bone marrow infiltration” OR “malignant melanoma bone marrow metastasis” across all fields. A total of 336,935 publications were identified. After removing duplicate records, 336,718 articles remained. Screening for the publication type “Case Report” filtered the results to 36 articles. Following a full-text review, 3 articles were excluded due to irrelevance to melanoma metastasis. Consequently, 33 studies were included for final analysis (Supplementary Figure 2). A systematic literature review and analysis of 36 cases of MM bone marrow infiltration revealed the following: male 63.89% (23/36) over female 36.11% (13/36). The primary sites of melanoma were distributed as follows: Skin (52.78%, 19/36), Ocular (13.89%, 5/36), Nasal (8.33%, 3/36), Occult (16.67%, 6/36), Retroperitoneal (2.78%, 1/36), Rectal and Vaginal mucosa (2.78%, 1/36), and Not reported (2.78%, 1/36). Hematologic abnormalities were common among these cases. Disseminated intravascular coagulation (DIC) was observed in 11.11% (4/36) of patients. Additional hematologic complications included anemia (19.44%, 7/36), leukocytosis (11.11%, 4/36), thrombocytopenia (27.78%, 10/36), pancytopenia (22.22%, 8/36), coagulopathy (2.78%, 1/36), carcinocythemia (2.78%, 1/36), microangiopathic hemolytic anemia (2.78%, 1/36), leucoerythroblastic picture (8.33%, 3/36), and hypercalcemia (2.78%, 1/36). The prognosis involvement remains extremely poor, with 58.33% (21/36) of patients succumbing to the disease within days to months following diagnosis. Notably, only three patients demonstrated favorable therapeutic responses, with two patients achieving complete remission or significant clinical improvement following nivolumab/ipilimumab therapy and one patient responding positively to pembrolizumab. Additionally, the overall prognosis is extremely poor, with the majority of patients (58.33%, 21/36) succumbing to the disease within days to months following diagnosis, despite undergoing chemotherapy or supportive treatment. Notably, two patients achieved complete relief after treatment with Nivolumab/Ipilimumab, while one patient exhibited good response to Pembrolizumab (Table 2). Unfortunately, this patient did not receive post-discharge immunotherapy with either nivolumab/ipilimumab combination or pembrolizumab monotherapy. In addition, Shimizu et al. (9). conducted a systematic review of 29 studies and record that bone metastatic melanoma accounted for 13% (3,130/23,998) with a male-to-female distribution of 56 to 44%. Further analysis of 21 studies revealed the prevalence of bone pain in 3.48% (180/5167), total skeletal-related events (SREs) in 26.88% (1,389/5167). Among the SREs events, pathological fracture occurred in 6.93% (358/5167), spinal cord compression in 2.88% (149/5167), hypercalcemia in 0.74% (38/5167), radiotherapy in 11.83% (611/5167), and surgery to the bone in 9.72% (502/5167).
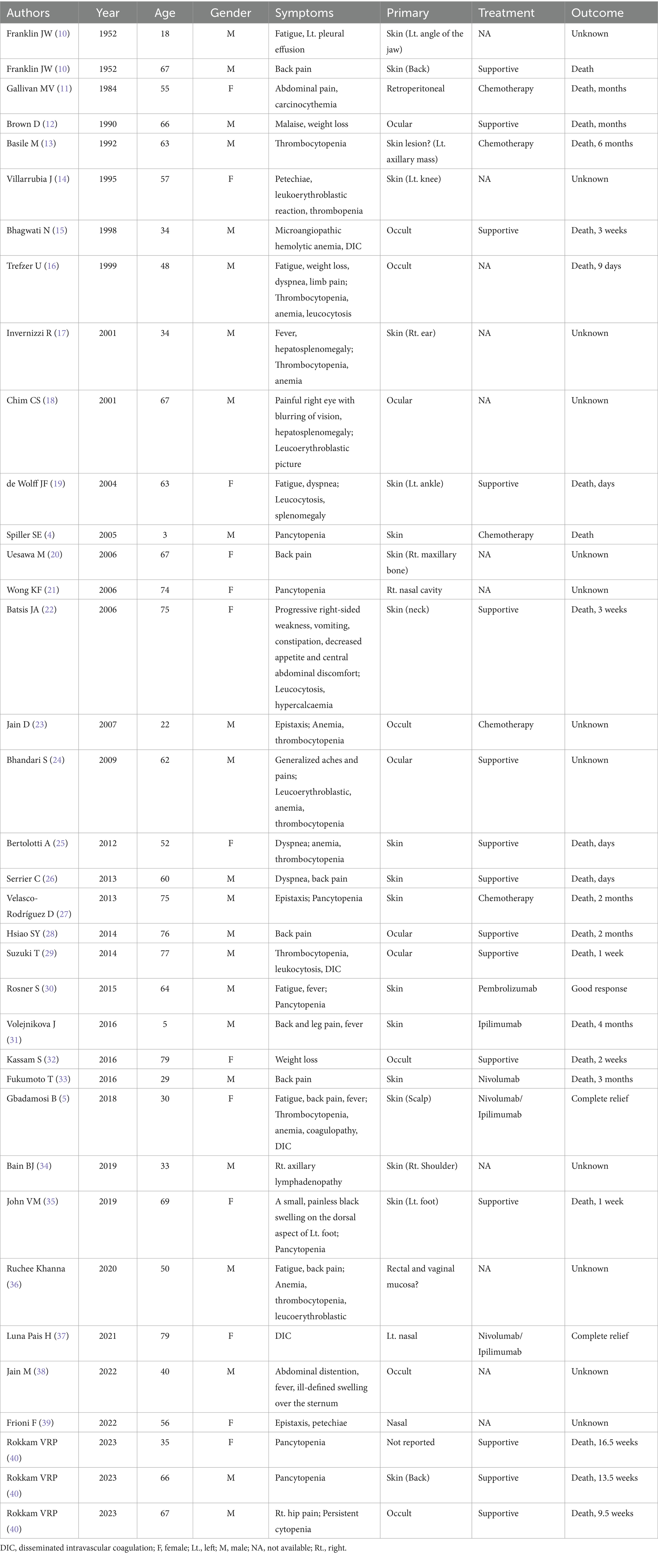
Table 2. Systematic review and comprehensive analysis of bone marrow infiltration in malignant melanoma.
Melanoma is the third most prevalent skin cancer globally, accounting for 1–3% of all malignant tumors. In recent years, the incidence has been steadily rising, with a higher prevalence in males than females. It is projected that by 2040, the number of cases will reach 510,000, with an estimated 96,000 deaths (41–43). Among pediatric melanoma patients, the majority are diagnosed post-puberty (44). MM arised from neuroectoderm-derived pigment-producing cell, which is initiated and driven to migrate to other sites by environmental, genetic, constitutional, and epigenetic factors, as well as acquired mutations, during embryonic development, involves skin (13–38%), distant lymph nodes (5–34%), lung (18–36%), liver (14–20%), central nervous system (2–20%), bones (4–17%), gastrointestinal tract (1–8%), adrenal glands (1–11%), pancreas (3%), pleura (3%), heart (<1%), kidneys (<1%), and thyroid (<1%) (45, 46).
There are four major clinical-pathological subtypes of melanoma: superficial spreading melanoma (SSM), nodular melanoma (NM), acral lentiginous melanoma (ALM), and lentigo malignan melanoma (LMM). In early stages, melanoma exhibits a highly metastatic potential, capable of spreading via lymphatic, hematogenous (3, 47). In the head and neck, it can manifest as recurrent epistaxis, anosmia, and headache. Spinal cord metastasis typically manifests as back pain and symptoms of spinal cord compression. Bone marrow infiltration may present with symptoms such as pain, anemia, thrombocytopenia, bone marrow failure, leukoerythroblastosis, pancytopenia, and leukopenia, with DIC in 11% of cases (4, 48). In the case we reported, the children had a pigmented mass on the right scalp since childhood. However, due to inadequate attention, the lesion repeatedly ulcerated. The children later experienced spontaneous epistaxis accompanied by extensive ecchymosis after physical exercise. Multiple blood tests consistently revealed DIC, and both bone marrow aspiration and intra-operative hematoma pathological biopsy showed that it was consistent with the diagnosis of MM bone marrow infiltration. Regrettably, as documented in the literature, the prognosis of bone marrow metastasis is extremely poor, with a median survival time of only 2 months (48).
To our knowledge, this case is exceptionally rare, as DIC was triggered by MM bone marrow infiltration, and even more unusual is the presence of SEH within the thoracolumbar spinal canal on MRI. SEH is a rare yet potentially fatal disease, with an incidence <1/1000000, accounting for about 1% of spinal epidural space-occupying lesions (49). Over the past 3 years, around 1,000 cases have been documented in the literature worldwide. Common etiologies include trauma, coagulopathy, drugs, hypertension, arteriovenous malformations or spinal puncture. Hematoma typically occur in the dorsal cervicothoracic and thoracolumbar segments, with typically manifests as patients severe knife pain at the site of hemorrhage, and some may experience painless intervals, followed by progressive paralysis below the affected spinal cord level (7, 8, 50). MRI is the preferred imaging modality when SEH is suspected. In acute phase (<24 h), T1-weighted images showed isointensity, while T2-weighted images demonstrate hyperintensity. In subacute phase (>24 h), both T1 and T2-weighted images showed hyperintensity. In chronic phase, both T1 and T2-weighted images showed hypointensity. Fat-saturation proton density-weighted images were used to distinguish hematoma from epidural fat (51). A consensus from most reported cases suggests that once SEH was diagnosed, emergent or at least urgent surgical intervention is necessary, with earlier intervention leading to a better prognosis. Besides, pre-operative neurologic status is also a critically important prognostic indicator (50, 52–54). Therefore, once MM bone marrow infiltration with SEH was diagnosed, surgery (hemilaminectomy or laminectomy with irrigation and debridement) or conservative management based on the imaging data and neurologic status should be selected as soon as possible (52). In this case, despite urgent surgical intervention and subsequent systemic treatment for MM, the outcome was unfortunately unsatisfactory, and succumbed to the disease, after 2 months.
Conclusion
In conclusion, MM bone marrow infiltration induced coagulation dysfunction and SEH with paraplegia is a rare and complex clinical disease, that can be highly challenging to diagnose by pre-operative image. Therefore, we recommend repeated systematic and comprehensive medical history and physical examination, especially neurologic assessment, which is necessary, and urgent MRI is essential for accurate differential diagnosis, and multidisciplinary consultations should be considered when necessary. Once diagnosed, prompt surgical intervention is critical, as early intervention is associated with a significantly favorable prognosis. Besides, given the aggressive nature of MM bone marrow infiltration, early recognition and prompt intervention are paramount. Further research is warranted to better understand the pathophysiology, optimize therapeutic strategies, and improve patient outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
SL: Formal analysis, Visualization, Writing – original draft, Writing – review & editing, Software, Investigation. BZ: Conceptualization, Methodology, Data curation, Investigation, Writing – original draft, Writing – review & editing, Software, Visualization, Formal analysis. YS: Formal analysis, Data curation, Writing – review & editing, Investigation. JG: Writing – review & editing, Formal analysis. WY: Formal analysis, Supervision, Writing – review & editing, Investigation, Conceptualization. QX: Writing – review & editing, Investigation, Supervision, Formal analysis, Visualization. JL: Visualization, Writing – review & editing, Investigation. SM: Data curation, Supervision, Writing – review & editing, Investigation, Resources, Conceptualization, Validation, Project administration, Visualization, Formal analysis, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The financial support was received for “Supported by Shenzhen High-level Hospital Construction Fund”, “Supported by Peking University Shenzhen Hospital Scientific Research Fund” and “Guangdong Provincial Basic and Applied Basic Research Fund Enterprise Joint Fund (Public Health and Medical Health Field) in 2024”. Project Number: 2024A1515220105.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1601774/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | Diagnosis and treatment schedule.
SUPPLEMENTARY FIGURE 2 | Literature search and screening flow chart (PRISMA).
References
1. de la Monte, SM, Moore, GW, and Hutchins, GM. Patterned distribution of metastases from malignant melanoma in humans. Cancer Res. (1983) 43:3427–33.
2. Rigel, DS, Friedman, RJ, and Kopf, AW. The incidence of malignant melanoma in the United States: issues as we approach the 21st century. J Am Acad Dermatol. (1996) 34:839–47. doi: 10.1016/S0190-9622(96)90041-9
3. Adler, NR, Wolfe, R, Kelly, JW, Haydon, A, McArthur, GA, McLean, CA, et al. Tumour mutation status and sites of metastasis in patients with cutaneous melanoma. Br J Cancer. (2017) 117:1026–35. doi: 10.1038/bjc.2017.254
4. Spiller, SE, Hawkins, DS, Finn, LS, Sze, RW, and Sybert, V. Metastatic malignant melanoma presenting as pancytopenia in a three-year-old boy. Pediatr Blood Cancer. (2005) 45:60–3. doi: 10.1002/pbc.20200
5. Gbadamosi, B, Ezekwudo, D, Nayak, B, Yu, Z, Gjorgova-Gjeorgjievski, S, Xie, M, et al. Effective immunotherapy in bone marrow metastatic melanoma presenting with disseminated intravascular coagulopathy. Case Reports Immunol. (2018) 2018:1–8. doi: 10.1155/2018/4520294
6. Evangelidis, P, Tragiannidis, K, Gavriilaki, E, and Tragiannidis, A. Impact of Thrombopoietin receptor agonists on pathophysiology of pediatric ImmuneThrombocytopenia. Curr Issues Mol Biol. (2025) 47:65. doi: 10.3390/cimb47010065
7. Domenicucci, M, Mancarella, C, Santoro, G, Dugoni, DE, Ramieri, A, Arezzo, MF, et al. Spinal epidural hematomas: personal experience and literature review of more than 1000 cases. J Neurosurg Spine. (2017) 27:198–208. doi: 10.3171/2016.12.SPINE15475
8. Dildar, N, Ayaz, SB, Aamir, MO, and Ahmad, N. Spontaneous spinal epidural hemorrhage following disseminated intravascular coagulation resulting in paraplegia: a case report. J Spinal Cord Med. (2019) 42:265–9. doi: 10.1080/10790268.2017.1387717
9. Shimizu, MR, van de Langerijt, ON, Torres, D, de Groot, T, and Groot, OQ. Incidence, risk factors, and survival of bone metastases and skeletal-related events in melanoma patients: a systematic review and quality assessment of 29 studies. J Bone Oncol. (2024) 46:100603. doi: 10.1016/j.jbo.2024.100603
10. Franklin, JW, Zavala, DC, and Radcliffe, CE. The detection of malignant melanoma by bone marrow aspiration; a report of two cases. Blood. (1952) 7:934–41.
11. Gallivan, MV, and Lokich, JJ. Carcinocythemia (carcinoma cell leukemia). Report of two cases with English literature review. Cancer. (1984) 53:1100–2. doi: 10.1002/1097-0142(19840301)53:5<1100::aid-cncr2820530514>3.0.co;2-k
12. Brown, D, Boniuk, M, and Font, RL. Diffuse malignant melanoma of iris with metastases. Surv Ophthalmol. (1990) 34:357–64. doi: 10.1016/0039-6257(90)90111-8
13. Basile, M, Moskowitz, B, Harris, J, Blumberg, N, and Bennett, JM. Malignant melanoma: primary presentation in bone marrow and lymph node. Med Pediatr Oncol. (1992) 20:75–7. doi: 10.1002/mpo.2950200117
14. Villarrubia, J, de Misa, RF, Escribano, L, Bellas, C, and Velasco, JL. Amelanotic bone marrow infiltration secondary to pigmented malignant melanoma. J Dermatol. (1995) 22:620–2. doi: 10.1111/j.1346-8138.1995.tb03885.x
15. Bhagwati, N, Seno, R, Dutcher, JP, and Oleksowicz, L. Fulminant metastatic melanoma complicated by a microangiopathic hemolytic anemia. Hematopathol Mol Hematol. (1998) 11:101–8.
16. Trefzer, U, Schlegel, C, Sterry, W, Späth-Schwalbe, E, Possinger, K, and Denkert, C. Fulminant intravascular disseminating malignant melanoma mimicking acute leukemia. Blood. (1999) 94:1483–4. doi: 10.1182/blood.V94.4.1483
17. Invernizzi, R, and Pecci, A. The irreplaceable image: a case of metastatic malignant melanoma with bone marrow involvement. Haematologica. (2001) 86:447.
18. Chim, CS, and Trendell Smith, NJ. Primary malignant ocular melanoma: a bone marrow diagnosis. Br J Haematol. (2001) 112:2. doi: 10.1046/j.1365-2141.2001.02651.x
19. de Wolff, JF, Planken, EV, and den Ottolander, GJ. Extreme leucocytosis and splenomegaly in metastasised melanoma. Neth J Med. (2004) 62:164–7.
20. Uesawa, M, Sato, K, Ozaki, K, Nagai, T, Muroi, K, and Ozawa, K. Bone marrow metastasis of malignant melanoma. Intern Med. (2006) 45:341. doi: 10.2169/internalmedicine.45.1616
21. Wong, KF. Metastatic melanoma. Br J Haematol. (2006) 134:554. doi: 10.1111/j.1365-2141.2006.06184.x
22. Batsis, JA, and Barry, MJ. Metastatic malignant melanoma presenting with hypercalcaemia and bone marrow involvement. J Eur Acad Dermatol Venereol. (2006) 20:432–4. doi: 10.1111/j.1468-3083.2006.01435.x
23. Jain, D, Singh, T, Kumar, N, and Daga, MK. Metastatic malignant melanoma in bone marrow with occult primary site--a case report with review of literature. Diagn Pathol. (2007) 2:38. doi: 10.1186/1746-1596-2-38
24. Bhandari, S, Jack, F, Hussain, K, and Bell, A. Metastatic melanoma in the marrow: a black and white diagnosis. Br J Haematol. (2009) 147:1. doi: 10.1111/j.1365-2141.2009.07620.x
25. Bertolotti, A, Conte, H, Amazan, E, Dutriaux, C, Ezzedine, K, Parrens, M, et al. Metastatic melanoma with leukaemia-like evolution. Acta Derm Venereol. (2013) 93:729–30. doi: 10.2340/00015555-1581
26. Serrier, C, and Lesesve, JF. Metastatic malignant melanoma in the bone marrow. Blood. (2013) 121:721. doi: 10.1182/blood-2012-09-454546
27. Velasco-Rodríguez, D, Castellanos-González, M, Alonso-Domínguez, JM, Martín-González, M, and Villarrubia, J. Metastatic malignant melanoma detected on bone marrow aspiration. Br J Haematol. (2013) 162:432. doi: 10.1111/bjh.12437
28. Hsiao, SY, and Chen, TY. Uveal melanoma with diffuse bone marrow involvement. Blood. (2014) 124:1996. doi: 10.1182/blood-2014-05-573816
29. Suzuki, T, Kusumoto, S, Iida, S, Tada, T, and Mori, F. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. (2014) 53:325–8. doi: 10.2169/internalmedicine.53.1412
30. Rosner, S, Sen, F, and Postow, M. Response after treatment with pembrolizumab in a patient with myelophthisis due to melanoma: the role of checkpoint inhibition in the bone. J Immunother Cancer. (2017) 5:34. doi: 10.1186/s40425-017-0236-3
31. Volejnikova, J, Bajciova, V, Sulovska, L, Geierova, M, Buriankova, E, Jarosova, M, et al. Bone marrow metastasis of malignant melanoma in childhood arising within a congenital melanocytic nevus. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2016) 160:456–60. doi: 10.5507/bp.2016.018
32. Kassam, S, and Shah, C. Amelanotic melanoma in the bone marrow. Blood. (2016) 128:313. doi: 10.1182/blood-2016-03-702043
33. Fukumoto, T, Sakaguchi, M, Oka, M, Nishimura, M, Mukohara, T, and Nishigori, C. Malignant melanoma with bone marrow involvement diagnosed from hypercalcemia: development of a neural cell adhesion molecule stain. J Dermatol. (2017) 44:e105–6. doi: 10.1111/1346-8138.13718
34. Bain, BJ, and Luckit, J. It’s a black day-metastatic melanoma in the bone marrow. Am J Hematol. (2019) 94:1288–9. doi: 10.1002/ajh.25598
35. John, VM, Jayakumar, KL, and Jayaraman, MB. Malignant melanoma with metastases to the bone marrow: a case report. J Cancer Res Ther. (2019) 15:S280
36. 61st Annual Conference of Indian Society of Hematology & blood transfusion (ISHBT). Indian J Hematol Blood Transfus. (2020) 36:1–229.
37. Luna Pais, H, Luz, P, Lobo-Martins, S, Mansinho, A, Sousa, R, Luís, R, et al. Teixeira de Sousa R: immunotherapy in metastatic mucosal melanoma with disseminated intravascular coagulation: a case of success. Case Reports Immunol. (2021) 2021:1–5. doi: 10.1155/2021/5516004
38. Jain, M, Seth, S, Agarwal, R, and Verma, SP. The pigmented cells in bone marrow: metastatic malignant melanoma. Indian J Pathol Microbiol. (2022) 65:485–6. doi: 10.4103/IJPM.IJPM_1444_20
39. Frioni, F, Za, T, D’Alò, F, and Zini, G. Case of metastatic melanoma in bone marrow smear. Int J Lab Hematol. (2022) 44:976–7. doi: 10.1111/ijlh.13835
40. Rokkam, VRP, Kutti Sridharan, G, Thai, TN, Amaraneni, A, and Sundararajan, S. Clinical manifestations and treatment outcomes of metastatic melanoma with bone marrow infiltration. Am J Ther. (2023) 30:e298–300. doi: 10.1097/MJT.0000000000001384
41. Arnold, M, Singh, D, Laversanne, M, Vignat, J, Vaccarella, S, Meheus, F, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. (2022) 158:495–503. doi: 10.1001/jamadermatol.2022.0160
42. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
43. Zhou, L, Zhong, Y, Han, L, Xie, Y, and Wan, M. Global, regional, and national trends in the burden of melanoma and non-melanoma skin cancer: insights from the global burden of disease study 1990-2021. Sci Rep. (2025) 15:5996. doi: 10.1038/s41598-025-90485-3
44. Moscarella, E, Piccolo, V, Argenziano, G, Lallas, A, Longo, C, Castagnetti, F, et al. Problematic lesions in children. Dermatol Clin. (2013) 31:535–547, vii. doi: 10.1016/j.det.2013.06.003
45. Slominski, RM, Kim, TK, Janjetovic, Z, Brożyna, AA, Podgorska, E, Dixon, KM, et al. Malignant melanoma: an overview, new perspectives, and vitamin D signaling. Cancers (Basel). (2024) 16:2262. doi: 10.3390/cancers16122262
46. Belhocine, TZ, Scott, AM, Even-Sapir, E, Urbain, JL, and Essner, R. Role of nuclear medicine in the management of cutaneous malignant melanoma. J Nucl Med. (2006) 47:957–67.
47. Shain, AH, and Bastian, BC. From melanocytes to melanomas. Nat Rev Cancer. (2016) 16:345–58. doi: 10.1038/nrc.2016.37
48. Paolino, G, Carugno, A, Rongioletti, F, Ponzoni, M, Russo, V, Sena, P, et al. Bone marrow metastases: a systematic review of a neglected involvement in malignant melanoma. Melanoma Res. (2024) 34:31–7. doi: 10.1097/CMR.0000000000000942
49. Holtås, S, Heiling, M, and Lönntoft, M. Spontaneous spinal epidural hematoma: findings at MR imaging and clinical correlation. Radiology. (1996) 199:409–13. doi: 10.1148/radiology.199.2.8668786
50. Kreppel, D, Antoniadis, G, and Seeling, W. Spinal hematoma: a literature survey with meta- analysis of 613 patients. Neurosurg Rev. (2003) 26:1–49. doi: 10.1007/s10143-002-0224-y
51. Al-Mutair, A, and Bednar, DA. Spinal epidural hematoma. J Am Acad Orthop Surg. (2010) 18:494–502. doi: 10.5435/00124635-201008000-00006
52. Zhong, W, Chen, H, You, C, Li, J, Liu, Y, and Huang, S. Spontaneous spinal epidural hematoma. J Clin Neurosci. (2011) 18:1490–4. doi: 10.1016/j.jocn.2011.02.039
53. Babayev, R, and Ekşi, M. Spontaneous thoracic epidural hematoma: a case report and literature review. Childs Nerv Syst. (2016) 32:181–7. doi: 10.1007/s00381-015-2768-5
Keywords: bone marrow infiltration, coagulation dysfunction, case report, malignant melanoma, paraplegia, spinal epidural haematoma
Citation: Liu S, Zhang B, Shi Y, Guo J, Yin W, Xu Q, Liu J and Min S (2025) Malignant melanoma bone marrow infiltration induced coagulation dysfunction and spinal epidural haematoma with paraplegia: a case report and literature review. Front. Med. 12:1601774. doi: 10.3389/fmed.2025.1601774
Edited by:
Eleni Gavriilaki, Aristotle University of Thessaloniki, GreeceReviewed by:
Daniel Turudic, University Hospital Center Zagreb, CroatiaPaschalis Evangelidis, Aristotle University of Thessaloniki, Greece
Copyright © 2025 Liu, Zhang, Shi, Guo, Yin, Xu, Liu and Min. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoxiong Min, bXN4MTc3NkAxNjMuY29tBaode Zhang, ZHJiZHpoYW5nQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Songhua Liu
Songhua Liu Baode Zhang
Baode Zhang Yan Shi1
Yan Shi1 Shaoxiong Min
Shaoxiong Min