- 1Department of Critical Care Medicine, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, Sichuan, China
- 2Department of Cardiovascular Surgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Objective: This study aimed to investigate independent risk factors of severe postoperative hypoxaemia in patients with type A aortic dissection (TAAD), for providing a reference for clinical healthcare professionals to identify high-risk patients early and formulate postoperative severe hypoxaemia prevention and intervention plans.
Methods: This was a retrospective, observational study, patients with Stanford TAAD who underwent aortic surgery (n = 755) between March 2021 and July 2024 were enrolled. Relevant preoperative and intraoperative clinical data were collected. Univariate and logistic regression analyses were used to identify risk factors of severe hypoxaemia after TAAD.
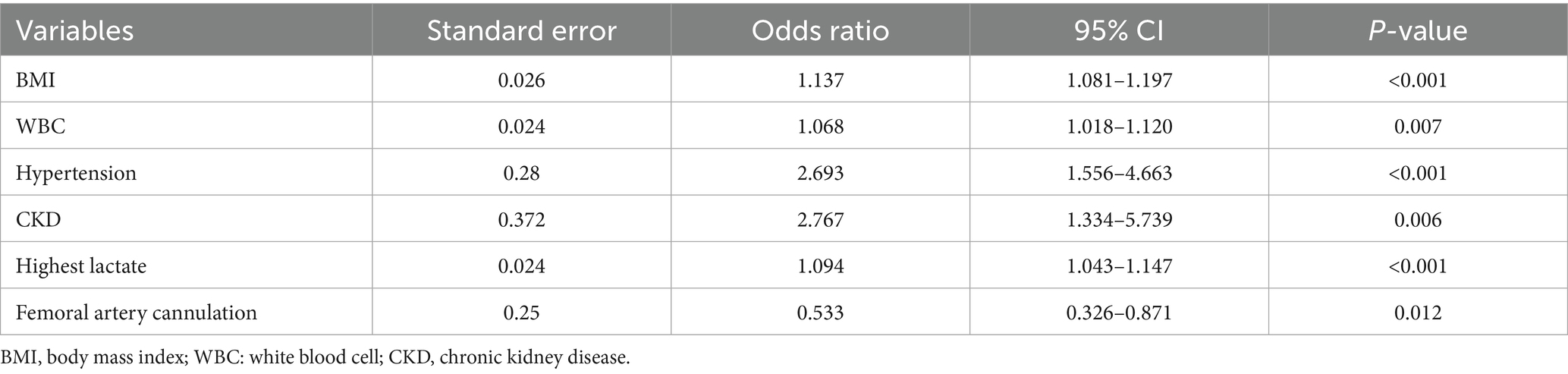
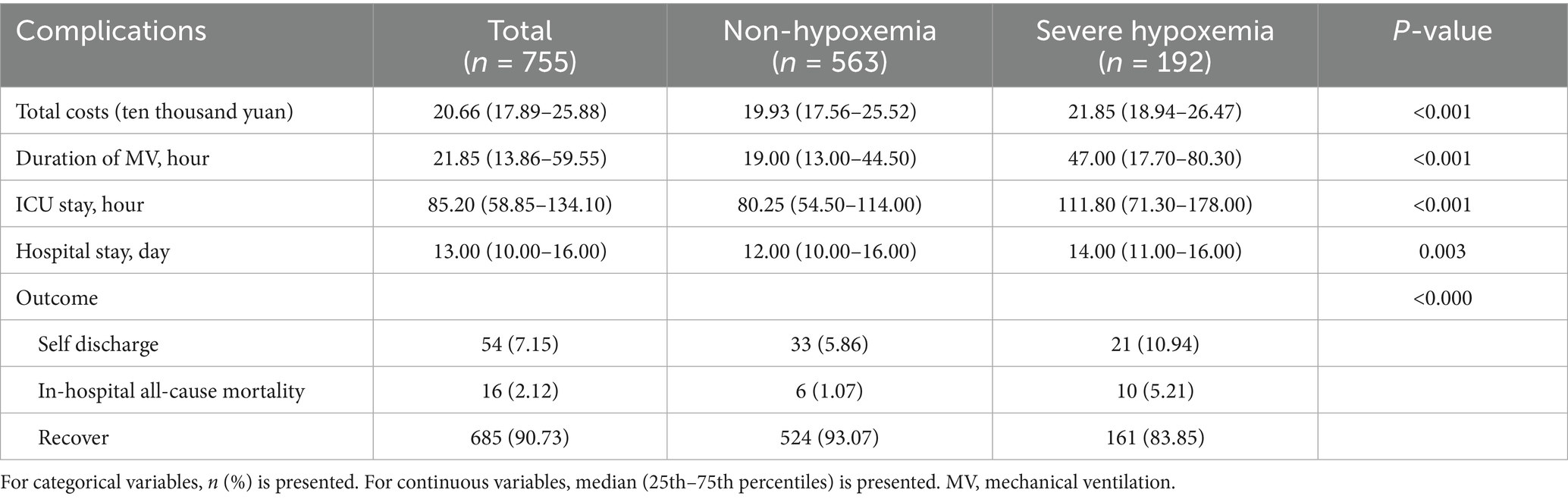
Results: Of the 755 patients with postoperative TAAD, 192 (25.43%) experienced severe postoperative hypoxaemia. Multivariable logistic regression analysis revealed body mass index (BMI) (odds ratio [OR] = 1.137), white blood cell count (WBC) (OR = 1.068), hypertension (OR = 2.693), chronic kidney disease (CKD) (OR = 2.767), highest lactate level (OR = 1.094) as independent predictors of severe postoperative hypoxaemia. Femoral artery cannulation (OR = 0.533) was a protective factor against severe postoperative hypoxaemia. Severe hypoxaemia increased hospitalization costs, prolonged mechanical ventilation duration, and extended intensive care unit and hospital stay durations.
Conclusion: The high severe hypoxaemia prevalence after TAAD is associated with poor clinical outcomes. Preoperative BMI, WBC, hypertension, CKD and intraoperative highest lactate level were risk factors for severe postoperative hypoxaemia. Clinical healthcare professionals should closely monitor high-risk patients, intensify oxygenation index surveillance, and intervene promptly to minimize hypoxaemia and improve patient prognosis.
Introduction
Stanford type A aortic dissection (TAAD), which is defined as a dissection involving the ascending aorta with possible extension to the aortic arch or brachiocephalic artery, is a life-threatening condition requiring immediate open surgical intervention owing to its association with severe morbidity and mortality (1). Recent studies have shown that the incidence of TAAD is approximately 2.6 to 7.2 per 100,000 person-years (2, 3). Without surgical intervention, mortality rates reach 50% within the first 48 h; hence, guidelines recommend urgent surgery in all patients (4–6); data from the International Registry of Aortic Dissection (IRAD) indicates that an overall in-hospital mortality rate of 16.7–31.4% (7). Survival rates following TAAD have significantly increased in the past 20 years owing to improved surgical, technical, and perioperative management; however, the incidence of postoperative complications remains high (6, 8). Postoperative hypoxaemia, characterized by respiratory failure, remains a common TAAD complication after surgery, sample sizes range from 60 to 403, with an incidence ranging from 16.7 to 68.75% (9). Postoperative hypoxaemia is followed by prolonged mechanical ventilation, increased length of stay in the intensive care unit (ICU) and hospital, increased hospitalization costs, damage to the lung and other organ functions, and even increased mortality (10–12). Therefore, preventing postoperative hypoxaemia after TAAD is essential. Several studies have focused on the risk factors for postoperative hypoxaemia (10, 11, 13); however, few have focused on the risk factors for severe hypoxaemia (PaO2/FiO2 ratio < 100 mmHg) after TAAD (14–17); sample sizes ranged from 162 to 492, moreover, the risk factors included were not comprehensive. Our center is the largest aortic dissection surgery center in Southwest China, the cases were well represented, in this study, to investigate risk factors for severe postoperative hypoxaemia (defined as PaO2/FiO2 < 100 mmHg) and to provide guidance for preventing this complication. In addition, the variables were screened based on the results of a meta-analysis conducted by our team and expert consultation, in order to identify high-risk patients early and accurately, our study only included preoperative and intraoperative possible 54 variables. These objectives logically lead into a retrospective study design that would involve collecting relevant patient data, performing statistical analyses (univariate and logistic regression), and identifying independent risk factors.
Methods
Study design and settings
This single-center retrospective observational clinical study enrolled consecutive patients who underwent TAAD repair at the our center between March 2021 and July 2024. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee (Number ID: 2024 Annual Audit 848). The requirement for written informed consent was waived because of the observational and retrospective nature of this study.
Study population
The study comprised 755 consecutive adult patients (age ≥ 18 years) (573 [75.89%] males) who underwent TAAD repair and were hospitalized during this period in the ICU after surgery. Patients who died intraoperatively and those with missing data were excluded. The diagnostic criteria for hypoxaemia according to the Berlin Criteria for acute respiratory distress syndrome (18) were mild (200 mm Hg < PaO2/FiO2 ≤ 300 mm Hg), moderate (100 mm Hg < PaO2/FiO2 ≤ 200 mm Hg), and severe (PaO2/FiO2 ≤ 100 mm Hg) hypoxaemia. In the present study, postoperative severe hypoxaemia was defined as PaO2/FiO2 ≤ 100 mmHg. Collected data on hypoxaemia in the first 72 h after surgery, blood gas analyses 4–6 times daily in patients after TAAD surgery, calculating the lowest oxygen index in 72 h. The 755 patients were divided into two groups, namely severe hypoxaemia (n = 192) and non-hypoxaemia (n = 563) groups.
Data collection
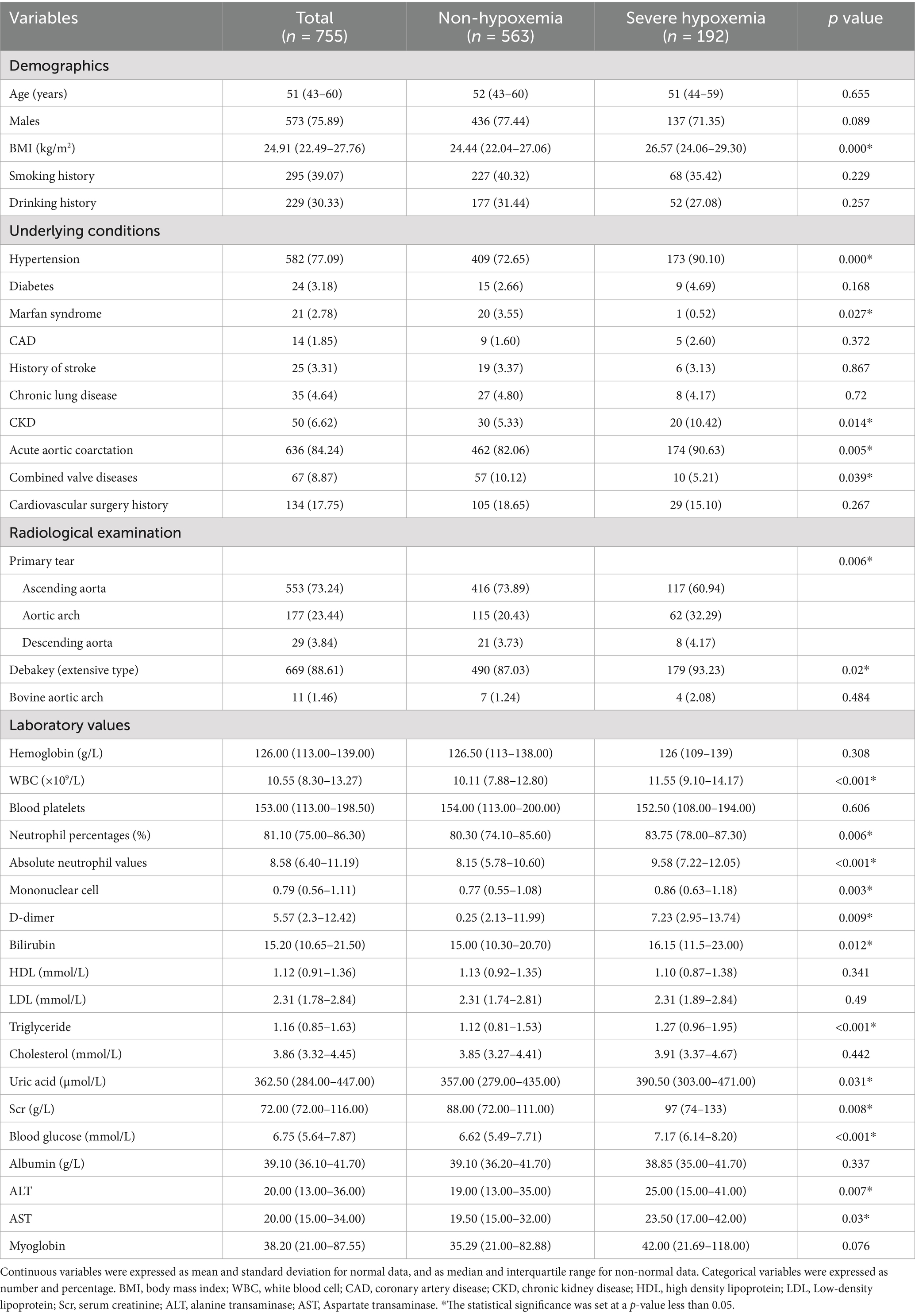
Clinical data were collected using the electronic medical record management system of the hospital. Preoperative variables in this study were as follows: demographic variables such as age, gender, body mass index (BMI), smoking history and drinking history; underlying conditions such as hypertension, diabetes mellitus, coronary heart disease, stroke, chronic kidney disease (CKD), chronic lung disease, combined valve diseases, and history of cardiovascular surgery; radiological examination, including primary tear and deBakey type of aortic dissection; laboratory tests Pre-operative Collection of the results of the most recent tests conducted before operation, including hemoglobin, white blood cell count, neutrophil percentages, d-dimer, bilirubin, high-density lipoprotein, low-density lipoprotein, triglyceride, uric acid, serum creatinine, blood glucose, albumin, alanine transaminase, aspartate transaminase, and myoglobin.
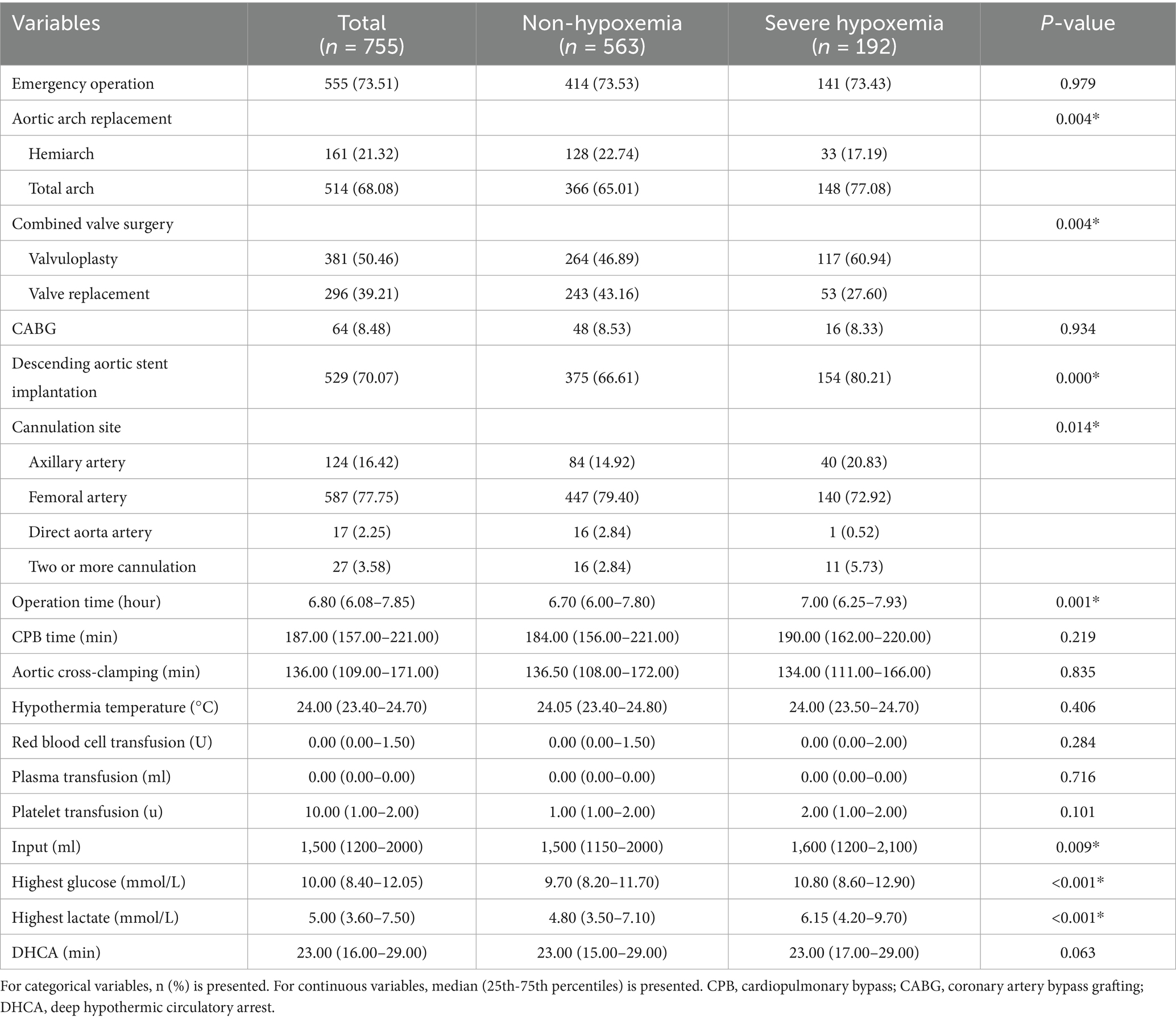
Intraoperative variables were as follows: emergency surgery, aortic arch replacement, combined valve surgery, coronary artery bypass grafting, descending aortic stent implantation, cannulation site (which is used to establish cardiopulmonary bypass, for example, axillary artery, femoral artery, direct aorta artery), cerebral perfusion, operation time, cardiopulmonary bypass (CPB) time, aortic cross-clamping time, hypothermia temperature, blood transfusion volume, highest glucose levels, highest lactic acid levels, and deep hypothermic circulatory arrest time. Highest lactic acid defined as the highest lactate value measured by blood gas analysis after the start of CPB.
Postoperative variables were the total costs, duration of mechanical ventilation, length of ICU stay, length of hospitalization, and self-discharge or mortality. Mortality is defined as postoperative all-cause deaths that occurred in the hospital.
Statistical analysis
Statistical analyses were performed using SPSS version 22.0. The Kolmogorov–Smirnov test was used to evaluate whether continuous variables were normally distributed. Continuous variables were expressed as mean ± standard deviation when normally distributed and as median (25th–75th percentiles) when skewed. Categorical variables are presented as absolute values and relative frequencies. Univariate logistic regression analysis was conducted to screen for potential risk factors of severe postoperative hypoxaemia. Normally distributed continuous variables with homogeneous variance were compared using Student’s t-test; otherwise, the Mann–Whitney U test was used. Categorical variables were compared using chi-squared or Fisher’s exact test. Similarly, a multivariate logistic regression analysis model (forward stepwise) was employed to test for independent predictors of severe hypoxaemia for variables with statistical significance in the univariate analysis (p < 0.05), and the odds ratio (OR) was calculated with a 95% confidence interval (CI). The clinical outcomes were compared between the severe hypoxaemia and non-hypoxaemia groups. p-value <0.05 was considered statistically significant.
Results
General clinical data
In this study, 759 patients underwent TAAD repair with cardiopulmonary bypass at the West China Hospital of Sichuan University between March 2021 and July 2024. Of these, four were excluded; one was <18 years and three died within 24 h after surgery. Therefore, 755 patients were included, with 192 (25.43%) having severe postoperative hypoxaemia.
Preoperative data
Preoperative patient variables are presented in Table 1. These include demographic characteristics such as sex (573 males; 75.89%) and median age (51 years; interquartile range 43–60); underlying conditions such as hypertension (582; 77.09%), chronic lung disease (35; 4.64%), CKD (50; 6.62%), and history of cardiovascular disease (134; 17.75%); and radiological examination such as the location of primary tear in the ascending aorta (553; 73.24%).
Intraoperative data
The operative variables of the patients are listed in Table 2. There were 555 emergency surgeries (73.51%), 514 total arch replacements (68.08%), with a mean operative time of 6.8 h, extracorporeal circulation time of 187 min, highest intraoperative blood glucose level of 10 mmol/L, and highest intraoperative lactate level of 5 mmol/L.
Risk factors for postoperative hypoxaemia
Preoperative and intraoperative variables were included in the logistic regression analysis to evaluate risk factors for postoperative hypoxaemia (Table 3). BMI (OR 1.137; 95% CI [1.081–1.197]; p < 0.001), WBC (OR 1.068; 95% CI [1.018–1.120]; p = 0.007), hypertension (OR 2.693; 95% CI [1.556–4.663]; p < 0.001), CKD (OR 2.767; 95% CI [1.334–5.739]; p = 0.006), highest lactate level (OR 1.094; 95% CI [1.043–1.147]; p < 0.001) were independent risk factors for severe hypoxaemia. However, femoral cannulation (OR 0.533; 95% CI [0.326–0.871]; p = 0.012) was a protective factor against severe postoperative hypoxaemia.
Clinical outcomes
The effect of severe postoperative hypoxaemia on clinical outcomes in TAAD was assessed and analyzed. The results suggest that the proportion of self-discharge or mortality (p < 0.0001) was significantly higher in the hypoxaemia group than in the non-hypoxaemia group. Total costs (21.85 vs. 19.93 ten thousand yuan; p < 0.001), ventilation time (47 vs. 19 h; p < 0.001), length of ICU stay (111.80 h vs. 80.25 h; p <0.001), and length of hospitalization (14.00 vs. 12.00 day; p = 0.003) of patients in the hypoxaemia group was significantly higher than those of patients in the non-hypoxaemia group (Table 4).
Discussion
Hypoxaemia is a common postoperative complication in patients with TAAD. The incidence of severe hypoxaemia was 25.43% (192 out of 755) in our study, which is consistent with the results of a recent study (24.2%) (15); however, it is less than those reported in the study of Guan et al. (13). Previously reported risk factors for hypoxaemia after TAAD surgery include age, smoking history, renal insufficiency, higher BMI, white blood cell count, hematocrit, PaO2/FiO2 ratio, Stanford type, pH, cardiopulmonary bypass time, postoperative lactic acid level, postoperative creatinine level, and intraoperative aortic occlusion time (11, 12, 15, 19). Our study further confirmed that preoperative BMI, WBC, hypertension, CKD, and intraoperative maximum lactate level were risk factors for severe postoperative hypoxaemia.
This study reveals that higher BMI is associated with the development of postoperative and severe hypoxaemia, which is consistent with the results of previous studies (12, 13, 15, 20). In the current study, the average BMI of the included patients was 24.91, which was consistent with previous studies. Gong et al. (21) reported an evident decrease in lung compliance and respiratory resistance in patients with obesity. In addition, the inflammatory response and oxidative stress may be involved in the process of lung injury in aortic dissection caused by obesity (22). Therefore, this study highlights the significance of preventing severe postoperative hypoxaemia in patients with high BMI. Similarly, the WBC, a biomarker that reflects the systemic inflammatory response, representing higher inflammatory responses which may contribute to respiratory dysfunction, was another independent predictor of severe hypoxaemia, it is consistent with the results of previous studies (12, 23). Additionally, 90.11% of patients in the severe hypoxaemia group had a history of hypertension, which was higher than that reported in the study of Sheng et al. (15) (73.1%). Patients need to maintain low blood pressure with post-TAAD. Guo et al.’s (24) study revealed that altered pulmonary circulation and insufficient tissue perfusion related to low blood pressure were responsible for the development of hypoxaemia.
CKD is an additional independent predictor of severe hypoxaemia in our study, which has been reported previously (10, 11, 15, 25). Zhou et al. (10) reported that the APACHE II score was independently associated with the development of severe postoperative hypoxaemia, in which renal function may play a vital role. Sheng et al. (15) reported that the inflammatory response, regulation of erythropoietin production by the kidney, and the resultant oxygen delivery may play an essential role (26).
Highest intraoperative lactate levels and cerebral perfusion were independent risk factors for hypoxaemia, the mean maximum intraoperative lactate level was 6.15 mmol/L in the hypoxaemia group. Lactic acid is an intermediate product of anaerobic glycolysis, which is metabolized in the liver and kidney primarily through glycogenosis or direct oxidative decomposition. In addition, lactic acid is primarily produced under anaerobic conditions, and tissue hypoxia can elevate its levels in the body, is a marker of tissue hypoxia. Anesthesia, cardiopulmonary bypass, and surgical procedures during TAAD can cause tissue hypoxia and increase lactic acid levels (27, 28), patients who have undergone heart surgery usually exhibit elevated lactate levels (29). Wang et al. (19) showed that median lactic acid was 3.6 mmol/L before surgery and 5.0 mmol/L after surgery in patients with acute TAAD. Maintaining sufficient oxygen delivery is the critical determinant to satisfy metabolic needs during CPB, during CPB, maintain adequate tissue perfusion tissue, increasing the pump flow, increasing hemoglobin level and decreasing temperature. Furthermore, it’s worth noting that that patients with femoral artery cannulation had a lower incidence of severe hypoxaemia. Generally speaking, femoral artery cannulation is always the first choice, less time compared with axillary artery cannulation, may quicker establishment of CPB in patients with TAAD surgery (30, 31), Wang et al. (14) showed that CPB ≥ 257.5 min was the risk of postoperative severe acute lung injury after ATAAD. In our research, the femoral artery is the most common site of cannulation (77.75%), CPB time was 187 min.
In this study, patients who experienced severe postoperative hypoxaemia presented with higher rates of self-discharge or mortality and had a longer duration of mechanical ventilation support, length of ICU stay, length of hospitalization, and higher total costs. However, consistent with previous studies (10–12), we did not follow deaths after discharge. Therefore, clinicians should intervene in the early postoperative hypoxemia according to the risk factors.
Limitations
This study had a few limitations. First, this is a single-center retrospective study, making it subject to inherent selection and information biases. Second, preoperative and intraoperative data were collected as much as possible; however, emergency surgery accounted for 73.51% of cases, and preoperative variables, such as PaO2/FiO2 and C-reactive protein data, were missed more than 20%, therefore, inclusion in the analysis was not considered. Third, we only collected data on hypoxaemia in the first 72 h after surgery, and changes in PaO2/FiO2 ratio over time were not analyzed. Finally, outcome measures included only short-term outcomes; long-term outcomes were not followed up.
In conclusion, hypoxaemia is a common postoperative complication of Stanford TAAD. The study indicated that preoperative BMI, hypertension, CKD, white blood cell count and intraoperative maximum lactate level were independent risk factors for severe postoperative hypoxaemia in patients with TAAD. Severe hypoxaemia is closely associated with poor clinical outcomes. These results indicate that early detection of postoperative hypoxaemia may improve prognosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ML: Conceptualization, Data curation, Investigation, Writing – original draft. YX: Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. TL: Formal analysis, Investigation, Software, Writing – review & editing. LZ: Project administration, Supervision, Visualization, Writing – review & editing. JF: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Benedetto, U, Dimagli, A, Kaura, A, Sinha, S, Mariscalco, G, Krasopoulos, G, et al. Determinants of outcomes following surgery for type a acute aortic dissection: the UK national adult cardiac surgical audit. Eur Heart J. (2021) 43:44–52. doi: 10.1093/eurheartj/ehab586
2. Li, R, and Prastein, D. Female patients have higher in-hospital mortality after type a aortic dissection repair: a population study from the national inpatient sample. World J Surg. (2024) 48:1783–90. doi: 10.1002/wjs.12241
3. Yuan, X, Mitsis, A, and Nienaber, CA. Current understanding of aortic dissection. Life (Basel). (2022) 12:1606. doi: 10.3390/life12101606
4. Erbel, R, Aboyans, V, Boileau, C, Bossone, E, Bartolomeo, RD, Eggebrecht, H, et al. 2014 esc guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the european society of cardiology (esc). Eur Heart J. (2014) 35:2873–926. doi: 10.1093/eurheartj/ehu281
5. Zhu, Y, Lingala, B, Baiocchi, M, Tao, JJ, Toro, AV, Khoo, JW, et al. Type a aortic dissection-experience over 5 decades: JACC historical breakthroughs in perspective. J Am Coll Cardiol. (2020) 76:1703–13. doi: 10.1016/j.jacc.2020.07.061
6. Isselbacher, EM, Preventza, O, Hamilton, BIJ, Augoustides, JG, Beck, AW, Bolen, MA, et al. ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 80:e223–393. doi: 10.1016/j.jacc.2022.08.004
7. Tsai, TT, Trimarchi, S, and Nienaber, CA. Acute aortic dissection: perspectives from the international registry of acute aortic dissection (irad). Eur J Vasc Endovasc Surg. (2009) 37:149–59. doi: 10.1016/j.ejvs.2008.11.032
8. Sfeir, PM, Issa, K, and Ayoub, CM. Mesenteric malperfusion syndromes in type a aortic dissection: current management strategies. J Cardiothorac Vasc Anesth. (2021) 35:3711–9. doi: 10.1053/j.jvca.2021.05.056
9. Xiang, Y, Luo, T, Zeng, L, and Dai, X. Risk factors for postoperative hypoxemia after Stanford type a aortic dissection: a systematic review and meta-analysis. Chin J Clin Thorac Cardiov Surg. (2023) 30:1483–9. doi: 10.7507/1007-4848.202112062
10. Zhou, J, Pan, J, Yu, Y, Huang, W, Lai, Y, Liang, W, et al. Independent risk factors of hypoxemia in patients after surgery with acute type a aortic dissection. Ann Palliat Med. (2021) 10:7388–97. doi: 10.21037/apm-21-1428
11. Song, XC, Nie, S, Xiao, JL, Shen, X, Hong, L, Chen, SY, et al. Risk factors and long-term prognosis for postoperative hypoxemia in patients with acute type a aortic dissection: a retrospective observational study. Medicine. (2022) 101:e32337. doi: 10.1097/MD.0000000000032337
12. Ge, H, Jiang, Y, Jin, Q, Wan, L, Qian, X, and Zhang, Z. Nomogram for the prediction of postoperative hypoxemia in patients with acute aortic dissection. BMC Anesthesiol. (2018) 18:146. doi: 10.1186/s12871-018-0612-7
13. Guan, X, Li, L, Li, J, Jiang, W, Li, H, Wang, X, et al. High preoperative bradykinin level is a risk factor for severe postoperative hypoxaemia in acute aortic dissection surgery. Exp Physiol. (2023) 108:683–91. doi: 10.1113/EP091054
14. Wang, Q, Feng, W, Kuang, J, Wu, J, Yang, J, Li, C, et al. Prediction model for postoperative severe acute lung injury in patients undergoing acute type a aortic dissection surgery. J Card Surg. (2022) 37:1602–10. doi: 10.1111/jocs.16447
15. Sheng, W, Le, S, Song, Y, Du, Y, Wu, J, Tang, C, et al. Preoperative nomogram and risk calculator for postoperative hypoxemia and related clinical outcomes following Stanford type a acute aortic dissection surgery. Front Cardiovasc Med. (2022) 9:851447. doi: 10.3389/fcvm.2022.851447
16. Hao, Z, Wang, Y, Zhu, Y, Liu, L, and Xiao, Y. Severe hypoxemia after Stanford a aortic dissection surgery: a clinical analysis of 162 cases. Acta Acad Med Mil Tert. (2020) 42:929–36. doi: 10.16016/j.1000-5404.202001031
17. Ju, F, Liu, N, Pan, X, Qiao, H, Li, L, Rong, T, et al. A prediction model for severe postoperative hypoxemia after surgery for Standford type a aortic dissection. Natl Med J China. (2016) 96:1001–6.
18. Ranieri, VM, Rubenfeld, GD, Thompson, BT, Ferguson, ND, Caldwell, E, Fan, E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
19. Wang, H, Xu, Z, Dai, X, and Chen, L. Predicting postoperative hypoxemia risk factors in the patients after triple-branched stent graft implantation surgery with acute type a aortic dissection: a retrospective study. J Card Surg. (2022) 37:3642–50. doi: 10.1111/jocs.16884
20. Wang, M, Jia, S, Pu, X, Sun, L, Liu, Y, Gong, M, et al. A scoring model based on clinical factors to predict postoperative moderate to severe acute respiratory distress syndrome in Stanford type a aortic dissection. BMC Pulm Med. (2023) 23:515. doi: 10.1186/s12890-023-02736-6
21. Gong, M, Wu, Z, Xu, S, Li, L, Wang, X, Guan, X, et al. Increased risk for the development of postoperative severe hypoxemia in obese women with acute type a aortic dissection. J Cardiothorac Surg. (2019) 14:81. doi: 10.1186/s13019-019-0888-9
22. Kawai, T, Autieri, MV, and Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. (2021) 320:C375–91. doi: 10.1152/ajpcell.00379.2020
23. Zhao, Y, Yue, Y, Wang, Y, Zhao, W, and Feng, G. The risk factors for postoperative acute respiratory distress syndrome in Stanford type a acute aortic dissection patients. Am J Transl Res. (2021) 13:7318–26.
24. Guo, Z, Yang, Y, Zhao, M, Zhang, B, Lu, J, Jin, M, et al. Preoperative hypoxemia in patients with type a acute aortic dissection: a retrospective study on incidence, related factors and clinical significance. J Thorac Dis. (2019) 11:5390–7. doi: 10.21037/jtd.2019.11.68
25. Pan, X, Ju, F, Liu, N, Zheng, J, Sun, L, Zheng, S, et al. The relationship bet ween preoperative renal failure and severe postoperative hypoxemia of patients received surgical procedures for Stanford a aortic dissection. Chin J Surg. (2016) 54:628–31. doi: 10.3760/cma.j.issn.0529-5815.2016.08.016
26. Schödel, J, and Ratcliffe, PJ. Mechanisms of hypoxia signalling: new implications for nephrology. Nat Rev Nephrol. (2019) 15:641–59. doi: 10.1038/s41581-019-0182-z
27. Chiang, BB, Stevens, K, Etoch, SW, Cerrito, P, Gray, LJ, and Dowling, RD. Blood lactic acid levels after artificial heart implantation. ASAIO J. (2001) 47:683–5. doi: 10.1097/00002480-200111000-00022
28. Sun, R, Chao, K, and Yang, H. Effect of lactic acid levels on the prognosis of critically ill patients after cardiac and non-cardiac surgery: an analysis of 549 cases. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2018) 30:123–7. doi: 10.3760/cma.j.issn.2095-4352.2018.02.006
29. Andersen, LW. Lactate elevation during and after major cardiac surgery in adults: a review of etiology, prognostic value, and management. Anesth Analg. (2017) 125:743–52. doi: 10.1213/ANE.0000000000001928
30. Benedetto, U, Mohamed, H, Vitulli, P, and Petrou, M. Axillary versus femoral arterial cannulation in type a acute aortic dissection: evidence from a meta-analysis of comparative studies and adjusted risk estimates. Eur J Cardiothorac Surg. (2015) 48:953–9. doi: 10.1093/ejcts/ezv035
Keywords: TAAD, severe postoperative hypoxaemia, risk factors, retrospective cohort study, clinical outcomes
Citation: Lin M, Xiang Y, Luo T, Zeng L and Fan J (2025) Risk factors and clinical outcomes of severe postoperative hypoxaemia following surgical repair of Stanford type A aortic dissection: a retrospective cohort study. Front. Med. 12:1603262. doi: 10.3389/fmed.2025.1603262
Edited by:
Robert Jeenchen Chen, Stanford University, United StatesReviewed by:
Maria Paparoupa, University Medical Center Hamburg-Eppendorf, GermanyChiyuan Zhang, Central South University, China
Copyright © 2025 Lin, Xiang, Luo, Zeng and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingxiu Fan, ZmFuamluZ3hpdUBzY3UuZWR1LmNu
 Min Lin
Min Lin Yuping Xiang
Yuping Xiang Tianhui Luo1
Tianhui Luo1