- Department of Geriatrics, Hangzhou Third People’s Hospital, Hangzhou, China
Background: Sarcopenia and osteoporosis, as two prevalent geriatric syndromes, synergistically elevate risks of falls, fractures, and disability in older adults. Despite shared pathophysiological mechanisms–including hormonal dysregulation, chronic inflammation, and attenuated mechanical loading. Existing studies have yet to establish consensus regarding the epidemiological association strength and interaction dynamics between sarcopenia and osteoporosis, particularly as heterogeneous characteristics–including sex, geographic region, and population subgroups–remain insufficiently characterized. This study aimed to quantitatively evaluate the sarcopenia-osteoporosis association in older adults through systematic review and meta-analysis of global observational studies, while analyzing the moderating effects of geographic location, sex, population characteristics, and diagnostic criteria on outcomes.
Methods: We searched PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI) databases until September 2024. Fourteen observational studies quantifying muscle mass/function and bone mineral density were included. Two investigators independently performed literature screening and data extraction. Study quality was assessed using the Newcastle-Ottawa Scale (NOS). Studies were meta-analyzed by Review Manager 5.4 and Stata 17.0.
Results: A total of 14 studies (n = 182307) were included, and the meta-analysis showed that patients with sarcopenia had a significantly higher risk of osteoporosis (OR = 3.16, 95% CI: 2.47 to 4.02, p < 0.001). Subgroup analyses demonstrated an OR of 4.74 [3.19, 7.06] for osteoporosis in the male sarcopenia group compared to females (OR = 3.46; 95% CI, 2.50–4.78). Geographically, European populations exhibited the highest risk (OR = 4.37; 95% CI, 3.72–5.13), surpassing Asian (OR = 2.66; 95% CI, 1.74–4.07) and American cohorts (OR = 2.32; 95% CI, 1.54–3.49). Community-dwelling individuals showed greater susceptibility (OR = 3.70; 95% CI, 3.24–4.23) compared to inpatient and outpatient populations.
Conclusion: Our meta-analysis demonstrates that sarcopenia significantly elevates osteoporosis susceptibility, with heterogeneous risk profiles across geographic regions and population subgroups. However, limitations inherent to the methodological quality and sample size of included studies necessitate validation through large-scale prospective cohort investigations.
1 Introduction
The global demographic shift toward aging populations has brought increasing attention to geriatric health challenges. Sarcopenia and osteoporosis, two prevalent geriatric syndromes, manifest as progressive declines in muscle mass and bone mineral density respectively. Epidemiological data indicate a sarcopenia prevalence of 10%–27% among individuals aged ≥60 years (1), while osteoporosis affects 30%–50% of this population (2). Emerging evidence reveals significant pathophysiological intersections and clinical synergies between these conditions, warranting systematic investigation.
From an etiological perspective, shared risk mediators include senescence-associated biological processes, hormonal alterations (particularly in estrogen, androgen, and growth hormone levels), nutritional insufficiencies (notably protein, vitamin D, and calcium deficiencies), chronic low-grade inflammation, and reduced physical activity (3). Clinically, these conditions exhibit bidirectional progression: sarcopenia-induced muscle weakness impairs osteogenic mechanical loading, accelerating bone density loss, while osteoporosis-related pain and fracture risk exacerbate mobility limitations, thereby perpetuating musculoskeletal deterioration.
Despite growing research interest, current understanding remains constrained by methodological heterogeneity across studies. Discrepancies in diagnostic criteria, population characteristics, and assessment protocols have yielded inconsistent findings regarding the magnitude and mechanisms of sarcopenia-osteoporosis associations. This knowledge gap underscores the imperative for rigorous evidence synthesis through systematic review and meta-analysis. Such methodology enables quantification of correlation strength, identification of heterogeneity sources, and elucidation of potential pathophysiological convergences.
This study intends to comprehensively collect studies on the correlation between sarcopenia and osteoporosis in older adults through systematic evaluation and meta-analysis. Literature was strictly screened, data were extracted and quality evaluated from studies that met the inclusion criteria, and meta-analysis was performed using appropriate statistical models, aiming at clarifying the degree of correlation between the two in the elderly population, further exploring their potential common pathogenic mechanisms and influencing factors, and providing a more reliable basis for early clinical diagnosis, prevention and comprehensive treatment, so as to improve the health status and quality of life of the elderly.
2 Data and methods
2.1 Literature search strategy
Two researchers independently searched PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI), spanning from their respective inceptions to September 1, 2024. The search terms were “sarcopenia,” “osteoporosis,” and “elderly.”
2.2 Inclusion and exclusion criteria
Studies were included if they fulfilled the following conditions: ① the type of study was observational design (covering cross-sectional, case-control or cohort studies); ② Sarcopenia was defined as the primary exposure variable, and the control group consisted of individuals with normal skeletal muscle function. ③ Osteoporosis was clearly defined as the primary outcome measure. ④ Extractable effect size data (e.g., OR value and 95% CI) or dichotomous outcome indicators were provided. The definition of sarcopenia should be based on established criteria, such as those proposed by the Asian Working Group for Sarcopenia (AWGS), the European Working Group on Sarcopenia in Older People (EWGSOP), or the skeletal muscle mass index (SMI). Osteoporosis diagnosis should be based on well - recognized methods, preferably dual - energy X - ray absorptiometry (DXA) according to the World Health Organization (WHO) criteria.
Exclusion criteria comprised: ① repetitive literature; ② non-clinical studies (e.g., animal experiments, reviews, case reports and Meta-analysis); ③ disease-specific subgroup studies (e.g., patients with renal failure); ④ literature with missing data or unable to obtain complete information.
2.3 Literature extraction and quality evaluation
Two researchers executed the search process separately, independently screened the literature, extracted data, and cross-validated the data based on the pre-set inclusion and exclusion criteria. In case of disagreement, a group discussion or a third-party expert (Liu Luping) was asked to intervene for a decision. The extracted information covered the following key parameters: first author, year of publication, sample size, gender distribution, geographical area of study, population type (e.g., community-dwelling, inpatients, outpatients), diagnostic criteria for sarcopenia, odds ratios (ORs) for osteoporosis.
We assessed the methodological quality using the Newcastle-Ottawa Scale (NOS) (4), which scores three dimensions (total score of 9): study population selection, between-group comparability, and assessment of outcomes. The assessment was done independently by two researchers, Jin Sha and Zheng Fengming, and reviewed by a third person (Liu Luping). The risk of bias was categorized into three levels based on the total score: high risk (<5 points), medium risk (6–7 points), and low risk (8–9 points).
2.4 Statistical analysis
All statistical analyses were done by Review manager 5.4 and Stata 17.0 software. Inter-study heterogeneity was assessed by the Q-test I2 statistic. If I2 was ≥50% or P < 0.05, a random-effects model was selected; conversely, a fixed-effects model was used. Subgroup analyses were performed to explore the causes of heterogeneity. In addition, publication bias was assessed using funnel plots. If asymmetry was found, Egger’s test was performed (significance level set at p less than 0.05).
3 Results
3.1 Literature search process and results
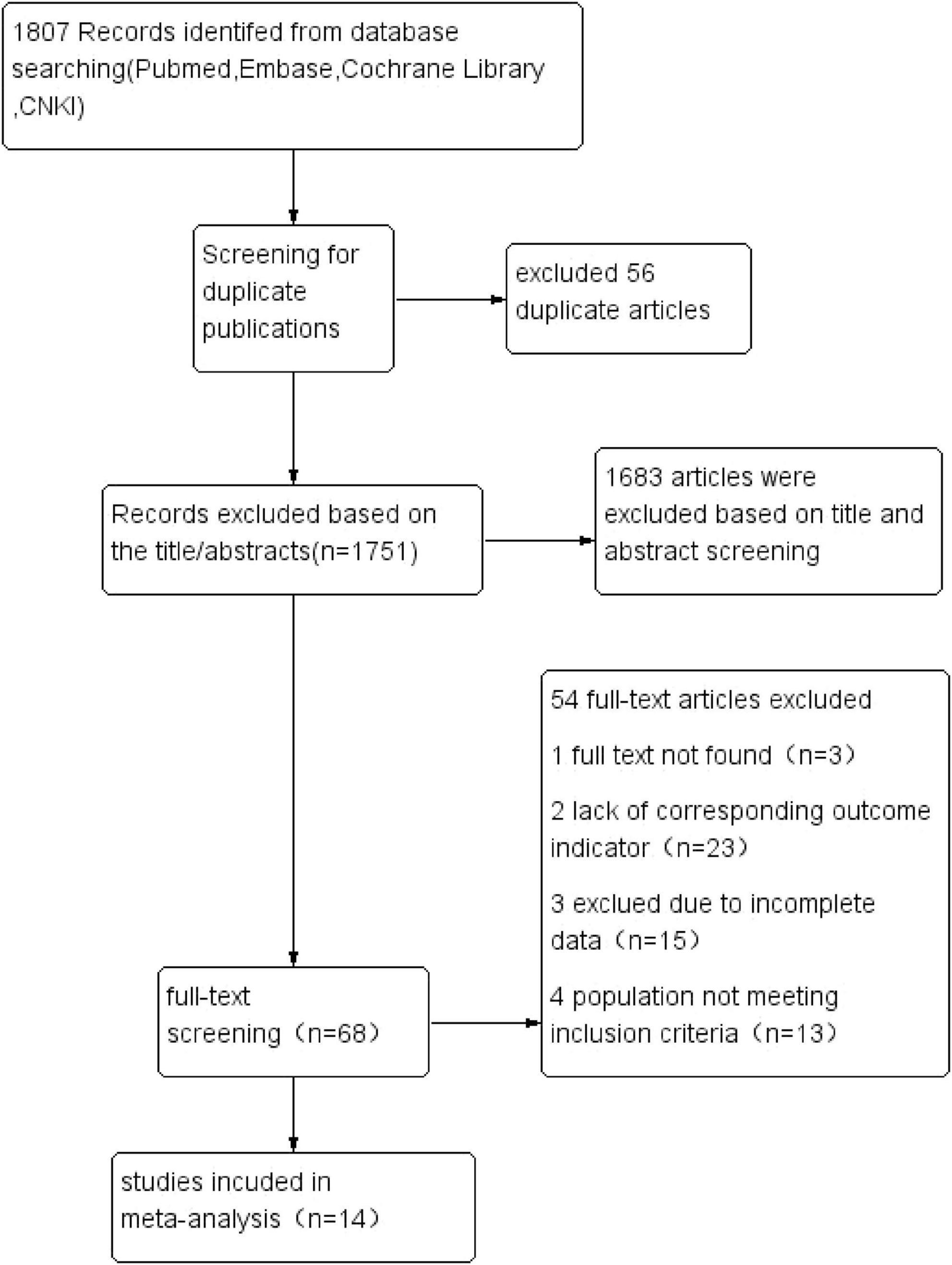
The systematic search identified 1,807 articles across PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI). After removal of 56 duplicates, 1,751 records underwent title/abstract screening, yielding 68 potentially eligible studies. Following full-text review and application of inclusion criteria, 14 observational studies (5–18) were ultimately included for meta-analysis. The final cohort comprised 10 cross-sectional studies and 4 prospective cohort studies, encompassing 182,307 individuals with sarcopenia. Figure 1 illustrates the PRISMA-compliant selection flowchart.
3.2 Basic characteristics of the included literature
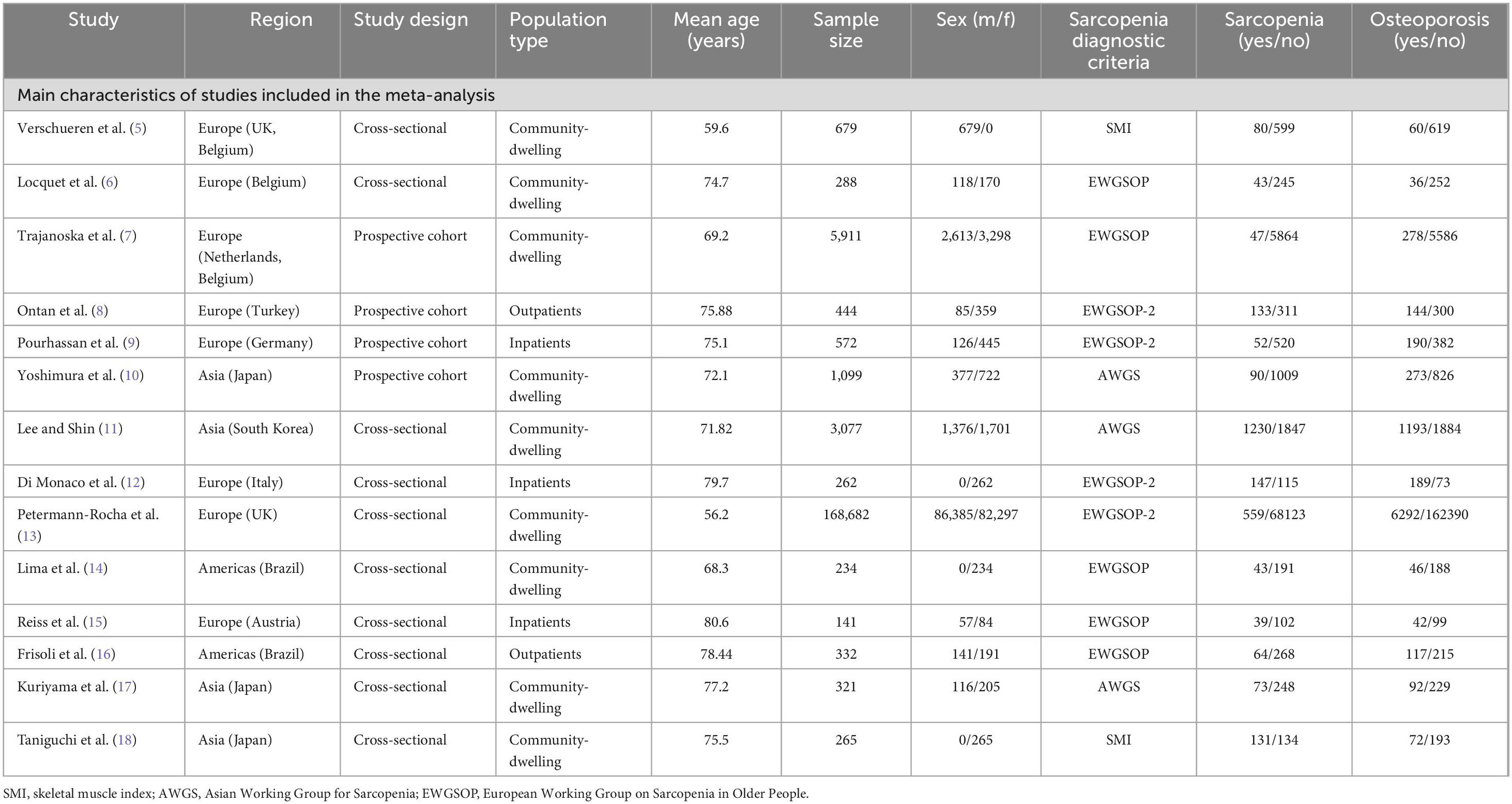
The baseline characteristics of the included studies are summarized in Table 1. This meta-analysis pooled data from 182,307 participants (92,073 males; 90,234 females), comprising 2,641 sarcopenic cases and 179,666 non-sarcopenic controls. The studies were conducted across diverse geographical regions spanning Asia, Europe, and the Americas. With respect to study populations, 9 investigations focused on community-dwelling individuals (5–7, 10, 11, 13, 14, 17, 18), 3 studies involved hospitalized patients (9, 12, 15), and 2 reports examined outpatient cohorts (8, 16).
3 studies employed the Asian Working Group for Sarcopenia (AWGS) diagnostic criteria (10, 11, 17), while 5 investigations applied the European Working Group on Sarcopenia in Older People (EWGSOP) operational definitions (6, 7, 14–16). 2 studies adopted skeletal muscle mass index (SMI) thresholds (5, 18), and 4 implemented the updated EWGSOP-2 consensus criteria (8, 9, 12, 13).
Methodological quality assessment using the Newcastle-Ottawa Scale (NOS) revealed robust study design across all included publications. Each study achieved a NOS score ≥7, indicating high methodological quality in terms of participant selection, comparability assessment, and outcome ascertainment.
3.3 Meta-analysis results
The systematic review incorporated 14 observational studies evaluating the sarcopenia-osteoporosis association. Seven investigations (5, 6, 8, 11, 12, 14, 18) reported odds ratios (ORs) with 95% confidence intervals (CIs) for the sarcopenia-osteoporosis association, while seven studies (7, 9, 10, 13, 15–17) provided dichotomous outcome data. Given substantial heterogeneity among studies (I2 = 67%, P < 0.05), a random-effects model was employed. Pooled analysis revealed a 3.16-fold (OR = 3.16, 95% CI: 2.47–4.02; P < 0.001) increased osteoporosis risk in sarcopenic individuals (n = 2641) compared to non-sarcopenic controls (n = 179,666) (Figure 2). Subgroup analyses were also performed to explore sources of heterogeneity.
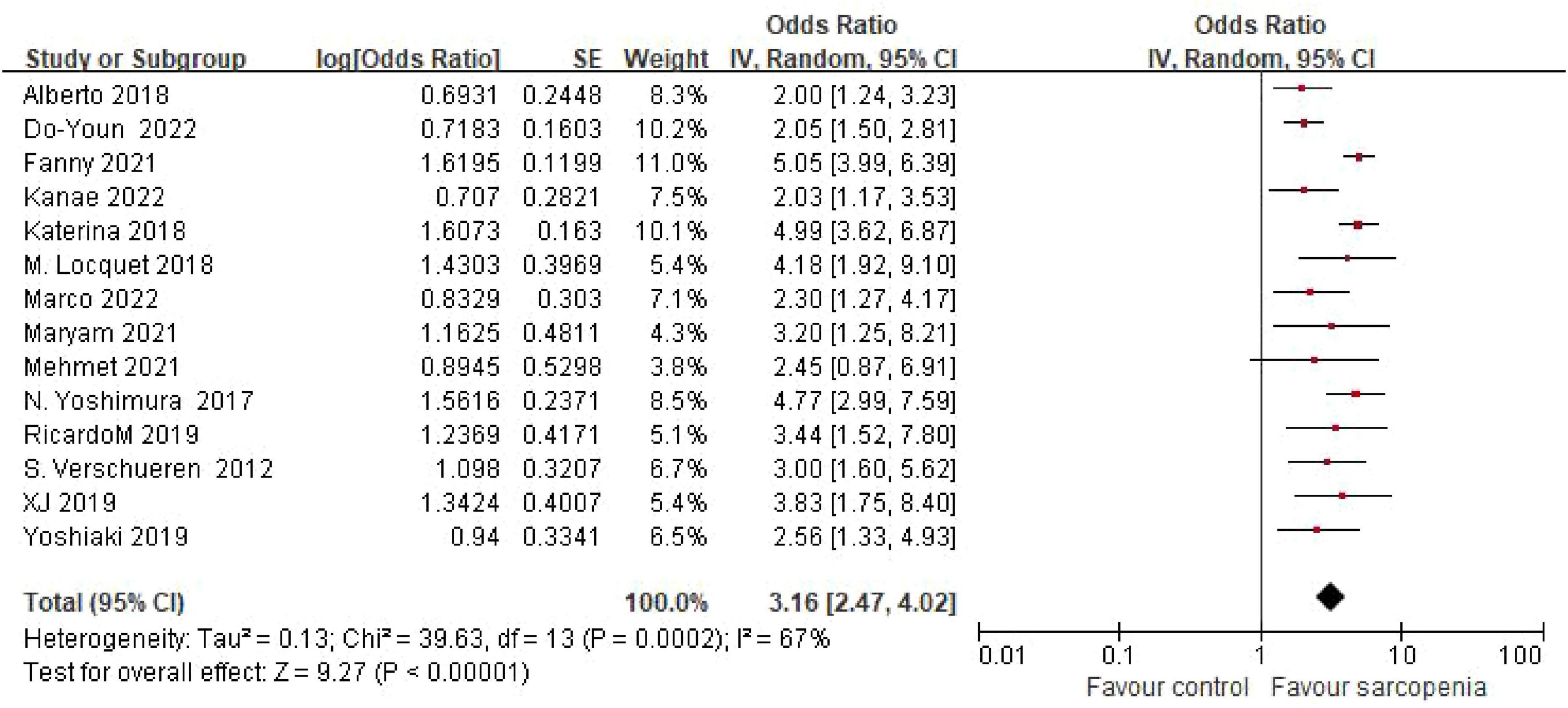
Figure 2. Forest plot of odds ratio (OR) for osteoporosis risk in sarcopenic vs. non - sarcopenic based on Meta – analysis.
3.4 Subgroup analyses
3.4.1 Sex-specific risk gradients
Sex-stratified meta-analysis revealed pronounced sex-specific risk gradients (Figure 3). Pooled estimates from 7 male-only cohorts demonstrated a 4.74-fold increased osteoporosis risk in sarcopenic males (OR = 4.74, 95% CI: 3.19–7.06). Conversely, female participants (8 studies) exhibited a 3.46-fold risk elevation (OR = 3.46, 95% CI: 2.50–4.78). No statistically significant sex-based interaction was detected (P = 0.23), though male effect magnitudes were numerically greater.
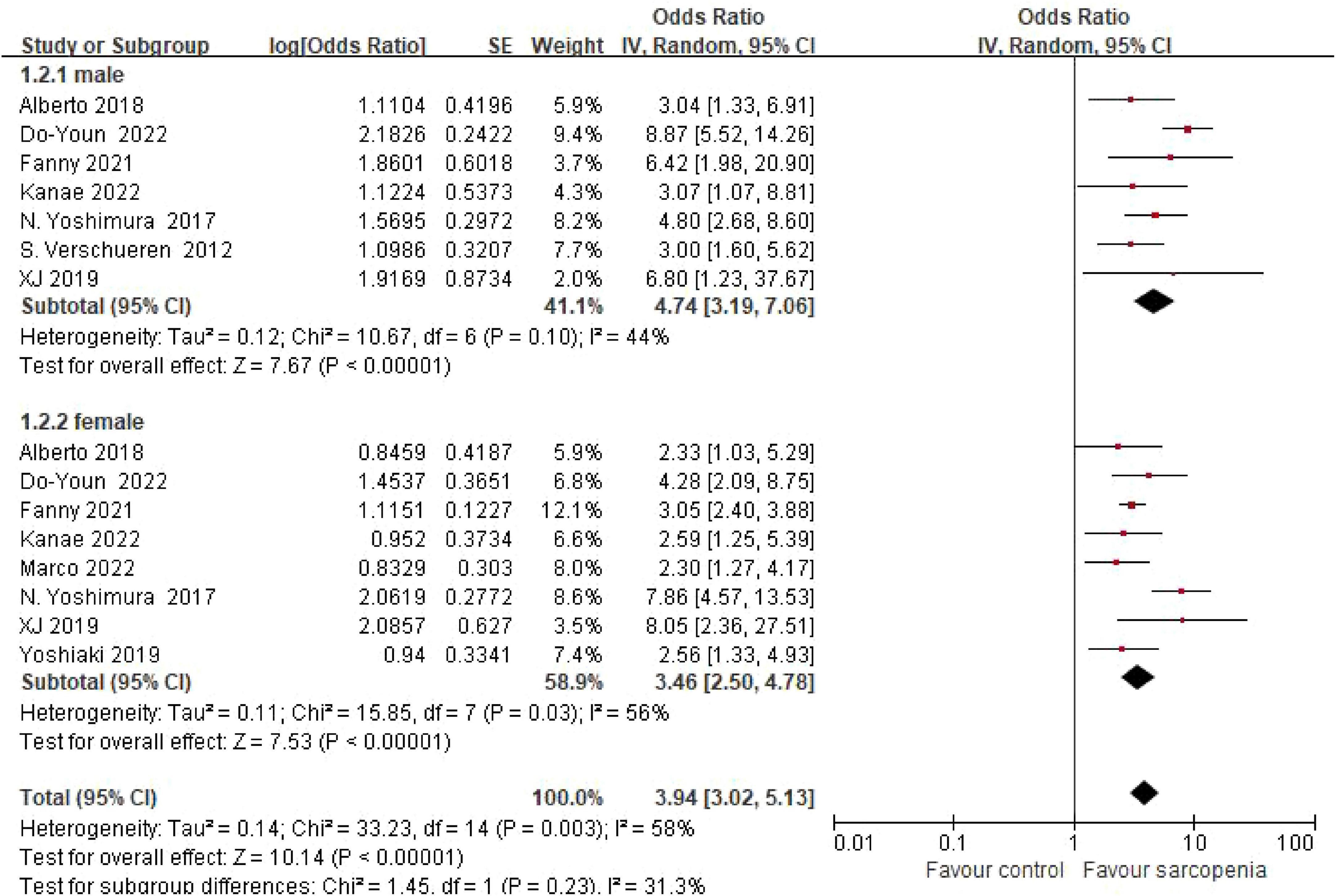
Figure 3. Meta-analysis forest plot of osteoporosis risk ratio (OR) in sarcopenic vs. non-sarcopenic subjects across gender subgroups.
3.4.2 Geographic disparities in risk estimates
Eight studies originated from Europe, two from North America, and four from Asia. Pooled estimates revealed odds ratios (ORs) of 4.74 (95% CI: 3.19–7.06) for European cohorts, 2.66 (95% CI: 1.74–4.07) for Asian populations, and 2.32 (95% CI: 1.54–3.49) for North American groups. No statistically significant differences were observed between geographic subgroups (P = 0.07) (Figure 4).
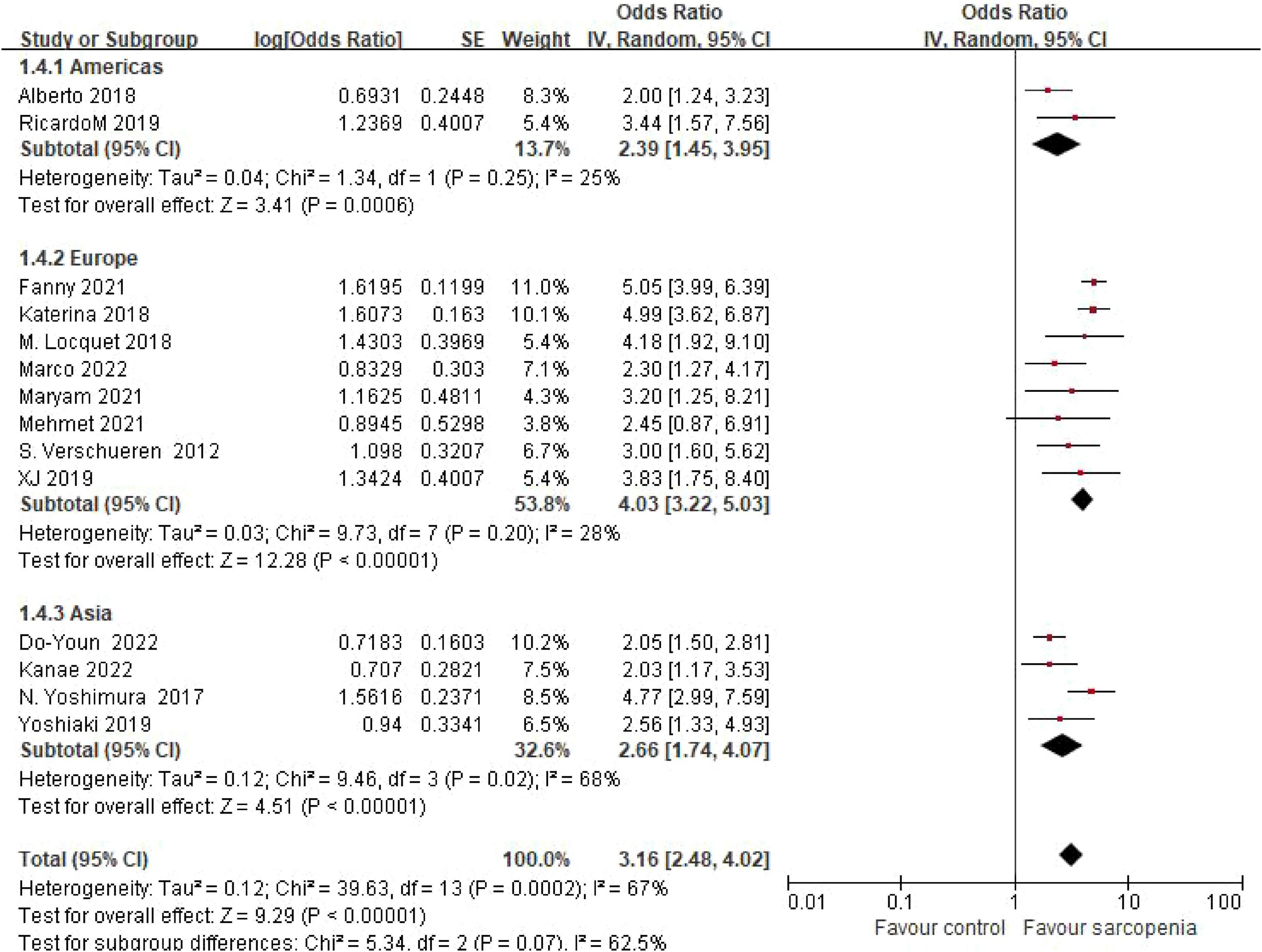
Figure 4. Forest plot of osteoporosis risk ratio (OR) in sarcopenia vs. non - sarcopenia patients by geographic regions.
3.4.3 Healthcare setting-specific associations
Subgroup analysis by participant setting showed marked differences in risk estimates. Community-dwelling populations (9 studies) had the highest osteoporosis risk (OR = 3.41, 95% CI: 2.55–4.57), followed by inpatients (2 studies, OR = 2.85, 95% CI: 1.87–4.35) and outpatients (3 studies, OR = 2.07, 95% CI: 1.34–3.20). No statistically significant differences were observed between healthcare settings (P = 0.17) (Figure 5).
![Forest plot showing odds ratios for three subgroups: community residents, outpatients, and hospitalized patients. Each subgroup has its studies with odds ratios, confidence intervals, and weights. Overall effect favors sarcopenia with a total odds ratio of 3.15 [2.49, 4.00]. Heterogeneity and test effects are indicated, with a diamond representing each subgroup’s summary effect and the total effect on the right.](https://www.frontiersin.org/files/Articles/1603879/fmed-12-1603879-HTML/image_m/fmed-12-1603879-g005.jpg)
Figure 5. Forest plot of osteoporosis risk ratio (OR) in sarcopenia vs. non - sarcopenia patients by healthcare settings.
3.4.4 Grouping according to diagnostic criteria
Three studies using Asian Working Group for Sarcopenia (AWGS) criteria demonstrated an OR of 2.69 (95% CI: 1.55–4.67), while five studies applying European Working Group on Sarcopenia in Older People (EWGSOP) criteria yielded an OR of 3.53 (95% CI: 2.34–5.32). Four studies implementing updated EWGSOP-2 criteria reported an OR of 3.38 (95% CI: 2.06–5.55), and two studies using skeletal muscle mass index (SMI)-based thresholds showed an OR of 2.78 (95% CI: 1.77–4.37). Subgroup analysis by diagnostic criteria revealed consistent risk estimates across definitions. No statistically significant differences were observed between diagnostic subgroups (P = 0.81) (Figure 6).
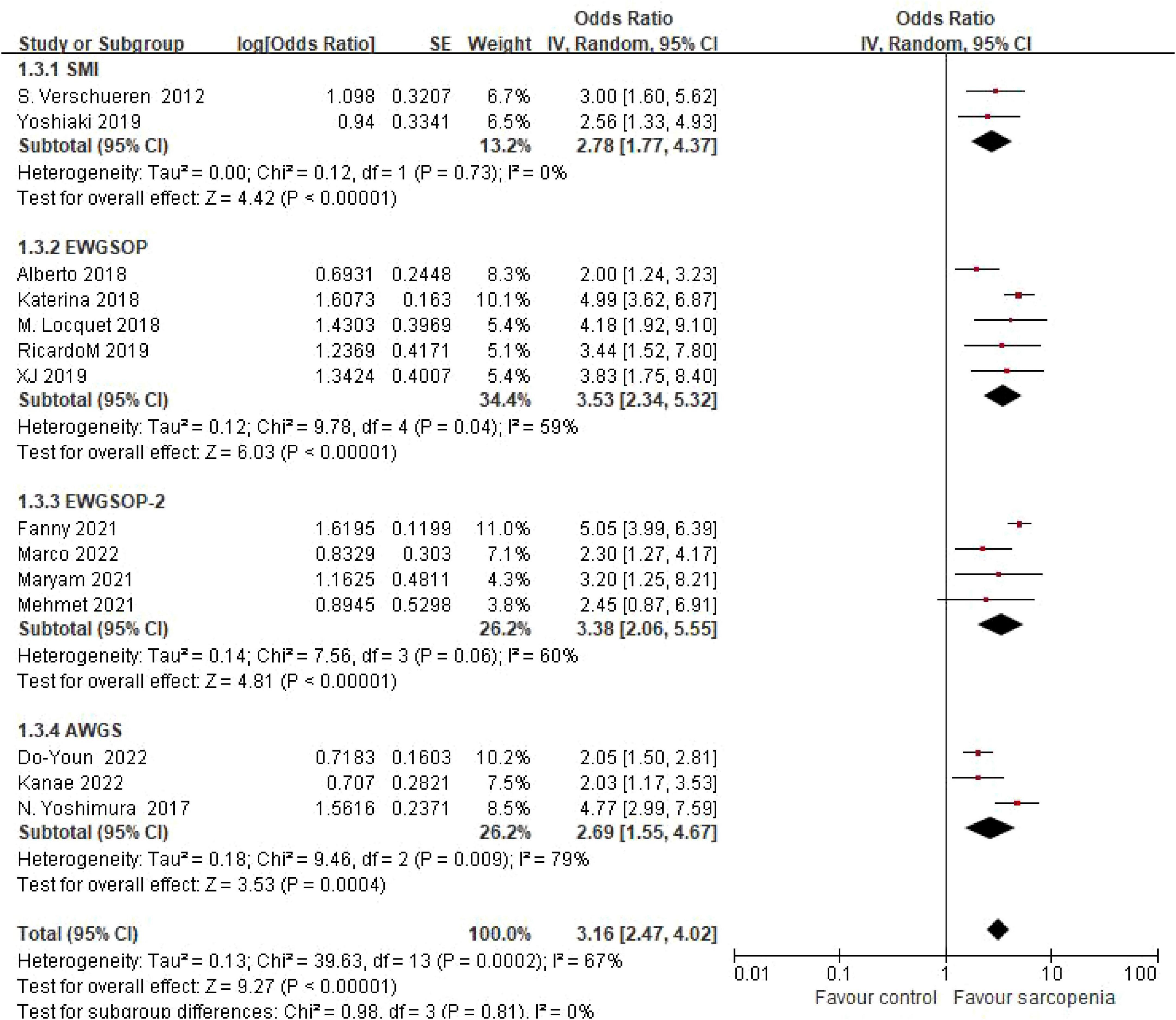
Figure 6. Forest plot comparing osteoporosis OR between sarcopenic and non - sarcopenic groups by diagnostic criteria.
3.4.5 Grouping according to study design
Four cohort studies showed an OR of 4.59 (95% confidence interval: 3.59–45.87), while 10 cross-sectional studies indicated an OR of 2.85 (95% confidence interval: 2.05–3.95). Subgroup analysis grouped by study design revealed consistent risk estimates across different study types. There was a statistically significant difference between subgroups (P = 0.02) (Figure 7).
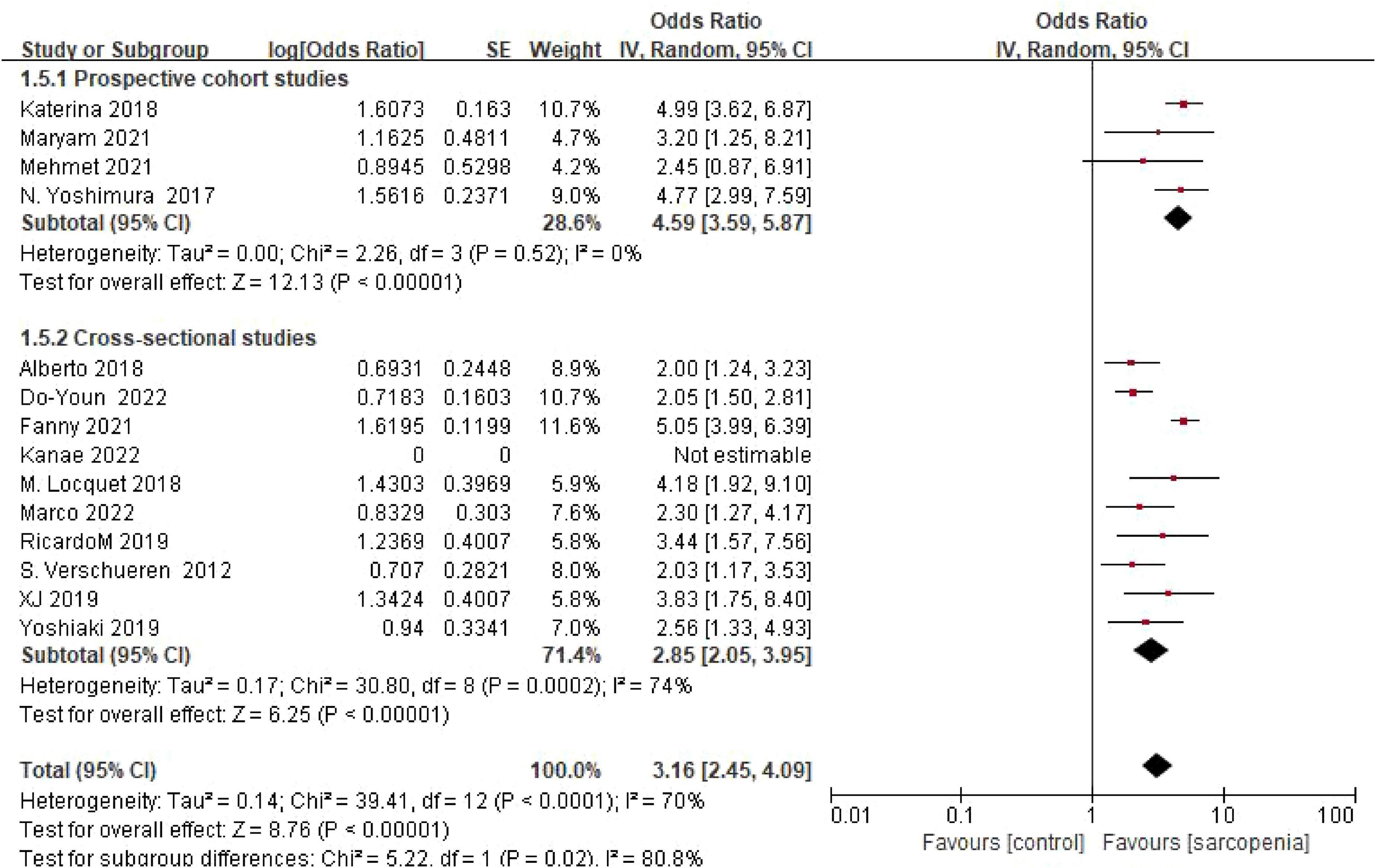
Figure 7. Forest plot comparing osteoporosis OR between prospective cohort studies and cross-sectional studies.
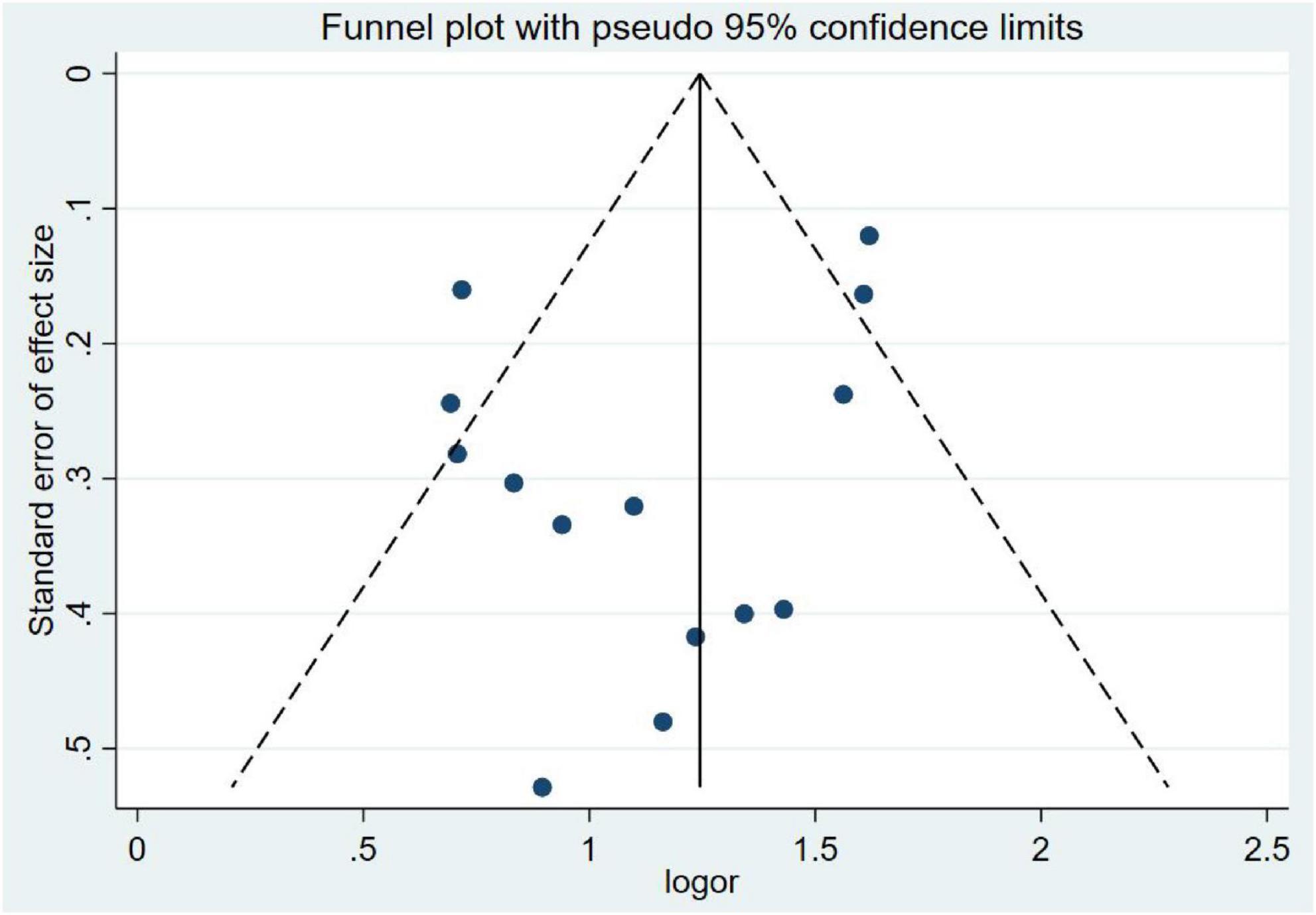
3.5 Publication bias
Publication bias was evaluated using funnel plot symmetry and Egger’s regression test. Visual inspection of the funnel plot (Figure 8) revealed approximate symmetry, with no obvious asymmetry suggesting small-study effects. Egger’s regression analysis demonstrated no statistically significant publication bias (P = 0.20), confirming the absence of systematic bias in the included studies.
4 Discussion
This meta-analysis of 14 studies (n = 182,307) provides the first systematic quantification of the sarcopenia-osteoporosis association, demonstrating a 3.16-fold increased osteoporosis risk in sarcopenic individuals compared to controls (OR = 3.16, 95% CI: 2.47–4.02). These findings underscore the pathophysiological synergy between these geriatric syndromes, likely mediated by shared mechanisms including hormonal dysregulation (e.g., IGF-1 deficiency, vitamin D insufficiency) and chronic inflammation (19).
Notably, subgroup analyses revealed sex-specific trends: males showed numerically higher risk (OR = 4.74) compared to females (OR = 3.46), though interaction testing was non-significant (P = 0.23), potentially reflecting estrogen’s protective effects on female bone health. Geographic disparities emerged, with European populations demonstrating numerically higher risk (OR = 4.74) than other subgroups (P = 0.07), possibly due to genetic predispositions and lifestyle factors (e.g., protein intake patterns). Community-dwelling individuals also exhibited greater osteoporosis risk (OR = 3.41) compared to inpatients (OR = 2.85) and outpatients (OR = 2.07), which may reflect earlier-stage sarcopenia where compensatory mechanisms preserve muscle function but fail to maintain bone integrity.
Based on our findings, we recommend that sarcopenia screening should be integrated into osteoporosis risk assessments. Since sarcopenia is significantly associated with an increased risk of osteoporosis, early identification of sarcopenia can help clinicians identify patients at high risk of osteoporosis. This can enable timely interventions such as exercise programs, nutritional supplements, and lifestyle modifications to prevent or delay the development of osteoporosis. Clinicians should also be aware of the differences in risk among different populations (such as gender, geographical regions) when assessing osteoporosis risk.
Importantly, consistent risk estimates across diagnostic criteria (e.g., EWGSOP vs. AWGS) highlight the cross-cultural stability of the muscle-bone density relationship. These findings reinforce the integrative bone-muscle axis, where mechanical loading, endocrine signaling, and paracrine networks coordinate mass homeostasis, and suggest that early sarcopenia intervention may mitigate osteoporosis risk in aging populations.
Sarcopenia and osteoporosis exhibit significant pathophysiological overlap, with accumulating evidence indicating shared regulatory pathways and bidirectional interactions (20). As integral components of the locomotor system, bone and muscle demonstrate coordinated mass changes across the lifespan, orchestrated by a triad of mechanical loading, endocrine regulation, and paracrine signaling networks (21). Notably, exercise deprivation, disuse atrophy, and aging-induced catabolism trigger synchronous bone-muscle degeneration, as posited by the Mechanical Homeostasis Theory, where reduced mechanical stimuli disrupt bone anabolic processes, leading to microarchitectural deterioration (22, 23). Within this framework, skeletal muscle serves as the primary transducer of mechanical loading, providing critical anabolic signals for bone maintenance.
While mechanical coupling is well-established, the systemic mechanisms governing bone-muscle mass equilibrium remain incompletely understood. Emerging evidence highlights secreted factors as key mediators: signaling molecules including myostatin, activin, and pro-inflammatory cytokines reciprocally regulate bone and muscle metabolism (24, 25). Skeletal muscle has recently been redefined as an endocrine organ, secreting myokines that not only modulate glucose metabolism but also form regulatory networks with osteokines, providing novel insights into integrated bone-muscle physiology.
At the molecular level, the Wnt/β-catenin signaling pathway emerges as a dual regulator, governing both bone mass homeostasis and skeletal muscle development (25). Osteocyte-derived sclerostin (encoded by SOST), a potent Wnt antagonist, acts as a pivotal node in this cross-talk, suggesting osteocytes establish biochemical dialogues with muscle tissues through secreted factors.
In summary, sarcopenia confers a significant osteoporosis risk, emphasizing the need for integrated screening strategies. Future longitudinal studies are required to clarify causal relationships and identify novel therapeutic targets within the bone-muscle metabolic axis.
In conclusion, sarcopenia is associated with an increased risk of osteoporosis, and early prevention of osteoporosis may focus on better identification and prevention of sarcopenia. In the future, more high-quality longitudinal studies are needed to provide insight into the correlation and potential mechanisms between sarcopenia and osteoporosis.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SJ: Writing – original draft, Data curation, Formal analysis, Software, Methodology. FZ: Writing – review & editing, Software, Data curation. HL: Writing – review & editing, Visualization. LL: Writing – review & editing, Data curation. JY: Writing – review & editing, Methodology.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. (2017) 16:21. doi: 10.1186/s40200-017-0302-x
2. Compston J, McClung M, Leslie W. Osteoporosis. Lancet. (2019) 393:364–76. doi: 10.1016/S0140-6736(18)32112-3
3. Cruz-Jentoft A, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
4. Lo C, Mertz D, Loeb M. Newcastle-ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
5. Verschueren S, Gielen E, O’Neill T, Pye S, Adams J, Ward K, et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos Int. (2013) 24:87–98. doi: 10.1007/s00198-012-2057-z
6. Locquet M, Beaudart C, Bruyère O, Kanis J, Delandsheere L, Reginster J. Bone health assessment in older people with or without muscle health impairment. Osteoporos Int. (2018) 29:1057–67. doi: 10.1007/s00198-018-4384-1
7. Trajanoska K, Schoufour J, Darweesh S, Benz E, Medina-Gomez C, Alferink L, et al. Sarcopenia and its clinical correlates in the general population: the rotterdam study. J Bone Miner Res. (2018) 33:1209–18. doi: 10.1002/jbmr.3416
8. Ontan M, Dokuzlar O, Ates Bulut E, Soysal P, Isik A. The relationship between osteoporosis and sarcopenia, according to EWGSOP-2 criteria, in outpatient elderly. J Bone Miner Metab. (2021) 39:684–92. doi: 10.1007/s00774-021-01213-6
9. Pourhassan M, Buehring B, Stervbo U, Rahmann S, Mölder F, Rütten S, et al. Osteosarcopenia, an asymmetrical overlap of two connected syndromes: data from the OsteoSys study. Nutrients. (2021) 13:3786. doi: 10.3390/nu13113786
10. Yoshimura N, Muraki S, Oka H, Iidaka T, Kodama R, Kawaguchi H, et al. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int. (2017) 28:189–99. doi: 10.1007/s00198-016-3823-0
11. Lee D-Y, Shin S. Association of Sarcopenia with osteopenia and osteoporosis in community-dwelling older korean adults: a cross-sectional study. J Clin Med. (2021) 11:129. doi: 10.3390/jcm11010129
12. Di Monaco M, Castiglioni C, Bardesono F, Freiburger M, Milano E, Massazza G. Is sarcopenia associated with osteoporosis? A cross-sectional study of 262 women with hip fracture. Eur J Phys Rehabil Med. (2022) 58:638–45. doi: 10.23736/S1973-9087.22.07215-X
13. Petermann-Rocha F, Ferguson L, Gray S, Rodríguez-Gómez I, Sattar N, Siebert S, et al. Association of sarcopenia with incident osteoporosis: a prospective study of 168,682 UK biobank participants. J Cachexia Sarcopenia Muscle. (2021) 12:1179–88. doi: 10.1002/jcsm.12757
14. Lima R, de Oliveira R, Raposo R, Neri S, Gadelha A. Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women. Arch Osteoporos. (2019) 14:38. doi: 10.1007/s11657-019-0591-4
15. Reiss J, Iglseder B, Alzner R, Mayr-Pirker B, Pirich C, Kässmann H, et al. Sarcopenia and osteoporosis are interrelated in geriatric inpatients. Z Gerontol Geriatr. (2019) 52:688–93. doi: 10.1007/s00391-019-01553-z
16. Frisoli A, Martin F, Carvalho A, Borges J, Paes A, Ingham S. Sex effects on the association between sarcopenia EWGSOP and osteoporosis in outpatient older adults: data from the SARCOS study. Arch Endocrinol Metab. (2018) 62:615–22. doi: 10.20945/2359-3997000000087
17. Kuriyama K, Matsui Y, Suzuki Y, Mizuno T, Watanabe T, Takemura M, et al. Relationship between sarcopenia classification and thigh muscle mass, fat area, muscle CT value and osteoporosis in middle-aged and older Japanese adults. Bone. (2022) 163:116487. doi: 10.1016/j.bone.2022.116487
18. Taniguchi Y, Makizako H, Kiyama R, Tomioka K, Nakai Y, Kubozono T, et al. The association between osteoporosis and grip strength and skeletal muscle mass in community-dwelling older women. Int J Environ Res Public Health. (2019) 16:1228. doi: 10.3390/ijerph16071228
19. Polito A, Barnaba L, Ciarapica D, Azzini E. Osteosarcopenia: a narrative review on clinical studies. Int J Mol Sci. (2022) 23:5591. doi: 10.3390/ijms23105591
20. Luisella C, Maria L. Muscle-bone interactions: basic and clinical aspects. Endocrine (2014) 45:165–77. doi: 10.1007/s12020-013-0026-8
21. Sharir A, Stern T, Rot C, Shahar R, Zelzer E. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development. (2011) 138:3247–59. doi: 10.1242/dev.063768
22. Shao M, Wang Q, Lv Q, Zhang Y, Gao G, Lu S. Advances in the research on myokine-driven regulation of bone metabolism. Heliyon. (2024) 10:e22547. doi: 10.1016/j.heliyon.2023.e22547
23. Mao Y, Jin Z, Yang J, Xu D, Zhao L, Kiram A, et al. Muscle-bone cross-talk through the FNIP1-TFEB-IGF2 axis is associated with bone metabolism in human and mouse. Sci Transl Med. (2024) 16:eadk9811. doi: 10.1126/scitranslmed.adk9811
24. Capulli M, Paone R, Rucci N. Osteoblast and osteocyte: games without frontiers. Arch Biochem Biophys. (2014) 561:3–12. doi: 10.1016/j.abb.2014.05.003
Keywords: sarcopenia, osteoporosis, correlation, systematic review, meta-analysis
Citation: Jin S, Zheng F, Liu H, Liu L and Yu J (2025) The correlation between sarcopenia and osteoporosis in the elderly: a systematic review and meta-analysis. Front. Med. 12:1603879. doi: 10.3389/fmed.2025.1603879
Received: 01 April 2025; Accepted: 15 September 2025;
Published: 01 October 2025.
Edited by:
Marios Kyriazis, National Gerontology Center, CyprusReviewed by:
Zitong Zhang, Icahn School of Medicine at Mount Sinai, United StatesZiyue Wang, University of Massachusetts Medical School, United States
Copyright © 2025 Jin, Zheng, Liu, Liu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengming Zheng, MzkwMTgzNTYyQHFxLmNvbQ==
 Sha Jin
Sha Jin Fengming Zheng*
Fengming Zheng*