- 1College of Nursing, Shanxi Medical University, Taiyuan, Shanxi, China
- 2Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
- 3Institute of High Energy Physics and National Center for Nanoscience and Technology, CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, Chinese Academy of Sciences, Beijing, China
- 4Guangdong-Hong Kong-Macao Greater Bay Area National Institute of Nanotechnology Innovation and Research, Guangzhou, Guangdong, China
- 5Guangzhou Guangna Huichuan Technology Co., Guangzhou, Guangdong, China
Background and Purpose: Acute radiation dermatitis (ARD) is a prevalent complication among breast cancer patients undergoing radiotherapy. Fullerene possesses broad-spectrum free radical scavenging properties, which may be beneficial in mitigating oxidative stress. This study aimed to evaluate the impact of fullerene on both the incidence and severity of ARD in breast cancer patients.
Methods: This study involved 88 breast cancer patients who met the inclusion criteria. Participants were randomly assigned to either the fullerene group or the control group in a 1:1 ratio. The primary endpoint was the grading of ARD and the cumulative dose of ionizing radiation at the first occurrence of ARD. Secondary endpoints included patient-reported symptoms, such as pain and quality of life (QoL).
Results: The Radiation Therapy Oncology Group (RTOG) score was significantly lower in the fullerene group than in the control group, and the cumulative dose of ionizing radiation at the first occurrence of ARD was higher. There was a statistically significant difference between the two groups (P < 0.05). Regarding the secondary endpoints, the pain scores exhibited a significant reduction in the fullerene group as compared with the control group (P < 0.05). The results of the Skindex-16 scale showed that the quality of life was better in the fullerene group than in the control group (P < 0.01).
Conclusion: This trial indicated that fullerene could reduce the grading of ARD, delay the occurrence of ARD, alleviate patients’ symptoms, and improve the patients’ overall quality of life.
Clinical trial registration: https://www.chictr.org.cn/index.html, identifier CTR2400079800.
1 Introduction
Cancer is a significant public health issue, with female breast cancer remaining the leading cause of both cancer incidence and cancer-related mortality among women worldwide (1). The American Cancer Society (ACS) estimates that in 2024, there were 310720 new cases of breast cancer in the United States, representing 15.5% of all new cancer diagnoses (2). Radiotherapy is used in >50% of patients with cancer, about 40% of cancer can be cured (3–5).
However, according to statistical data, acute radiation dermatitis (ARD) occurs to varying degrees in 95% of cancer patients undergoing radiotherapy and is a common complication among those receiving radiotherapy for breast, head and neck cancers (6, 7). ARD can contribute to a negative patient experience, reduce health-related quality of life, and lead to poor compliance with administered treatments (8).
In the 2023 clinical practice guidelines published by the Multinational Association of Supportive Care in Cancer (MASCC), the most promising interventions for the prevention and management of ARD include photobiomodulation therapy, Mepitel film, Hydrofilm, olive oil, oral enzyme mixtures, mometasone furoate, and betamethasone (9). A gold-standard treatment for the prevention and management of ARD has yet to be established, despite the extensive body of evidence supporting various therapeutic regimens (7).
Fang Fu Gao is a cream containing Trolamine. Trolamine is a compound similar to non-steroidal anti-inflammatory agents with concomitant antioxidant effects and has been considered a safe and tolerable ARD topical intervention (10, 11), which has been supported by multiple randomized trials and meta-analyses (12–14). Furthermore, trolamine-based emulsions have been a standard therapy for radiation dermatitis in Europe and the United States for over three decades (15).
Fullerene, known as a “free radical sponge,” is a great choice for skin radioprotection because of its broad-spectrum free radical scavenging performance, good chemical stability, and excellent biosafety (16). Currently, the research on hydroxy fullerene used in radiotherapy protection mainly focuses on basic research, and the in vitro and in vivo experiments proved that fullerene agents are effective against ARD (17). Therefore, we designed and large-scale synthesized protective ointment with fullerene as the main ingredient (EOSSKY Fullerene moisturizing and Repairing cream). This study aimed to conduct a randomized control trial to investigate the prophylactic effects of EOSSKY Fullerene moisturizing and Repairing cream versus control intervention (Fang Fu Gao, a cream containing Trolamine) for ARD in patients with breast cancer receiving radiotherapy. It also provides a new idea for the prevention of ARD and provides a reference for the application and popularization of fullerene in ARD patients.
2 Methods and materials
2.1 Study design and sample size
The design of this study was a randomized controlled trial comparing EOSSKY Fullerene moisturizing and Repairing cream with a control intervention in 88 breast cancer patients undergoing radiation therapy. The study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (approval number: 2023, ethics number 190) and written informed consent was obtained from all participants. The CONSORT guidelines and checklist, Good Clinical Practice principles, and the Declaration of Helsinki were rigorously followed. Trial registration: Chinese Clinical Trial Registry identifier: CTR2400079800; Date of registration: 12/01/2024.
The results of our pilot study indicated that EOSSKY Fullerene moisturizing and Repairing cream has good therapeutic effects and safety. The sample size was calculated using the formula for comparing two sample rates, with α = 0.05 and β = 0.1. Considering a 15% dropout rate, the final total sample size was determined to be 88 cases, with 44 cases in the EOSSKY Fullerene moisturizing and Repairing cream group and 44 cases in the control group.
2.2 Patient selection
Eligibility criteria for the study participants included the following: patients diagnosed with breast cancer based on pathological examination; female patients aged between 18 and 75; surgery with modified radical or breast-conserving surgery; fully healed surgical incision; planning to receive postoperative radiation therapy; Karnofsky Performance Status (KPS) score ≥70; no severe endocrine or metabolic disease; Exclusion criteria included: receiving concurrent chemotherapy; presence of skin disease; receiving other interventions to prevent or treat ARD during treatment.
2.3 Radiotherapy
Both groups of patients received conformal intensity modulated radiation therapy (IMRT) in a fractionated manner, with a single dose of 2 Gy, five fractions per week up to a total dose of 50–60 Gy.
2.4 Randomization and blinding
Participants were recruited from the department of oncology, The Second Hospital of Shanxi Medical University. Before the study initiation, a computer-generated random allocation sequence (1–88) was produced using SPSS 26.0, which assigned random sequence numbers to either Group A (experimental group) or Group B (control group) in a 1:1 allocation ratio. The allocation results were sequentially sealed in opaque envelopes and distributed sequentially at the time of patient admission. Since the drug colors of the experimental group and the control group were significantly different, the blind method was not adopted.
2.5 Grouping and ARD management
In this study, patients were divided into the Fang Fu Gao group and the fullerene group. Both groups received routine health education and were provided with information about ARD before the start of the treatment. Besides, for the fullerene group and control group, patients were asked to start topical application of EOSSKY Fullerene moisturizing and Repairing cream and control intervention cream, respectively, on the area of skin being irradiated at the onset of radiotherapy, three times a day at the same dose until the treatment was over.
2.6 Study end points
The primary endpoint were the grading of ARD and the cumulative dose of ionizing radiation at the first occurrence of ARD. The secondary study endpoints were patient-reported symptoms, including pain, quality of life, and adverse events. ARD was graded using the North American Radiation Therapy Oncology Group (RTOG) grading criteria, skin pain was assessed using the Visual Analog Scale (VAS) assessment tool, and QoL was measured using the Skindex-16 scale. Adverse events (e.g., edema, pruritus) were assessed by a combination of daily researcher observations and patient-reported symptoms.
2.7 Clinical evaluation
Acute radiation dermatitis was assessed every day by the investigator throughout the treatment period, and the grading of ARD and the cumulative dose of ionizing radiation were recorded. Patients’ skin pain was evaluated weekly during treatment using the VAS assessment tool. The higher the score, the more intense the pain. The patients’ QoL was assessed using the Skindex-16 scale every week. Adverse events were assessed daily by a combination of researcher observation and patient reports.
2.8 Statistical analysis
The IBM SPSS Statistics 26 software was used for statistical analysis. If the continuous variables conform to a normal distribution, the mean ± standard deviation is used to describe, and the two independent sample t-test is used to compare continuous variables between groups. If not, the medians and quartiles would be used to describe, and the Wilcoxon Rank Sum test would be used to compare the differences between the two groups. Categorical variables are described as frequencies and percentages, and Fisher’s exact test or Chi-square test is used to compare variables between groups. P < 0.05 was considered the basis of statistical significance.
3 Results
3.1 Patient characteristics
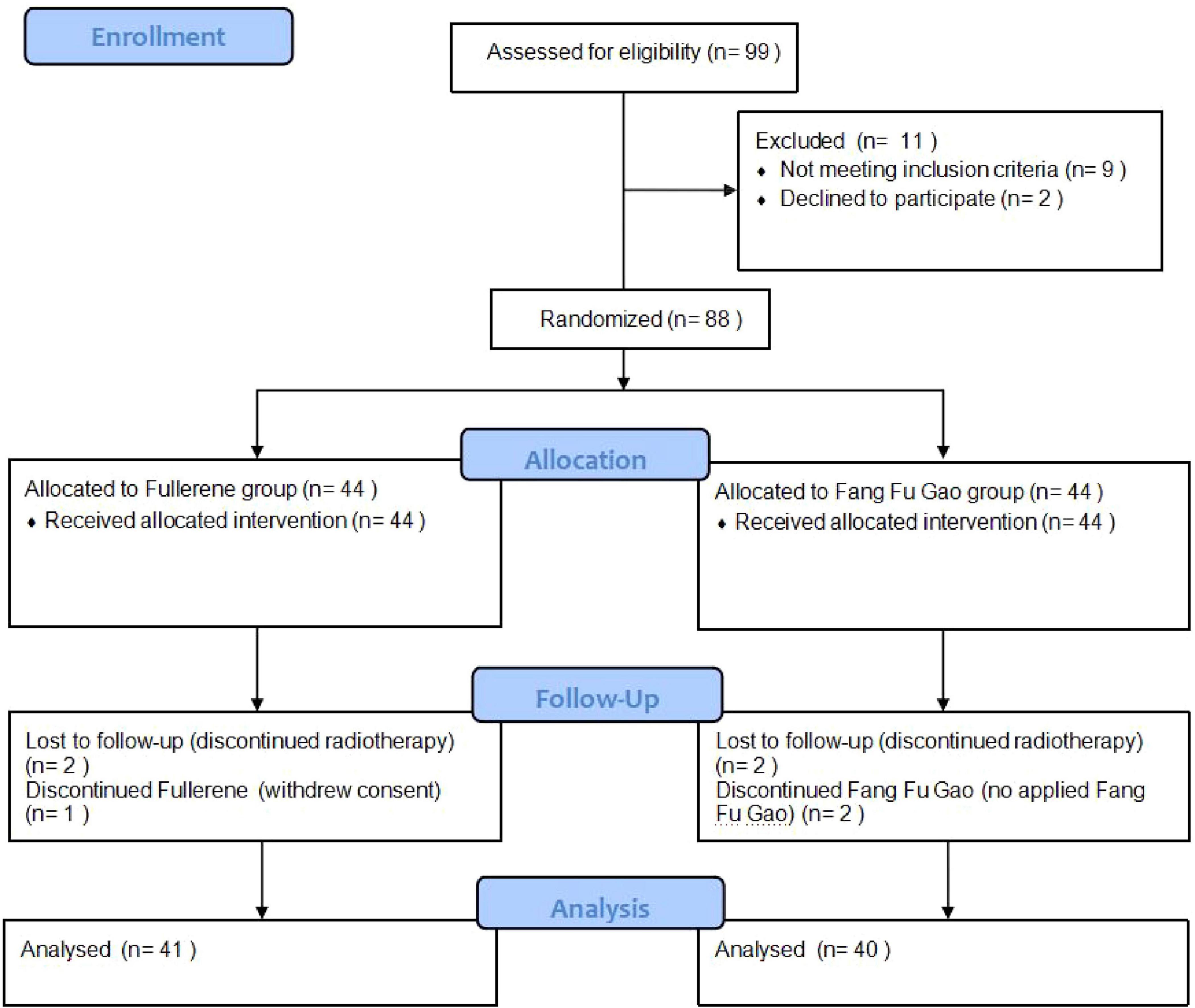
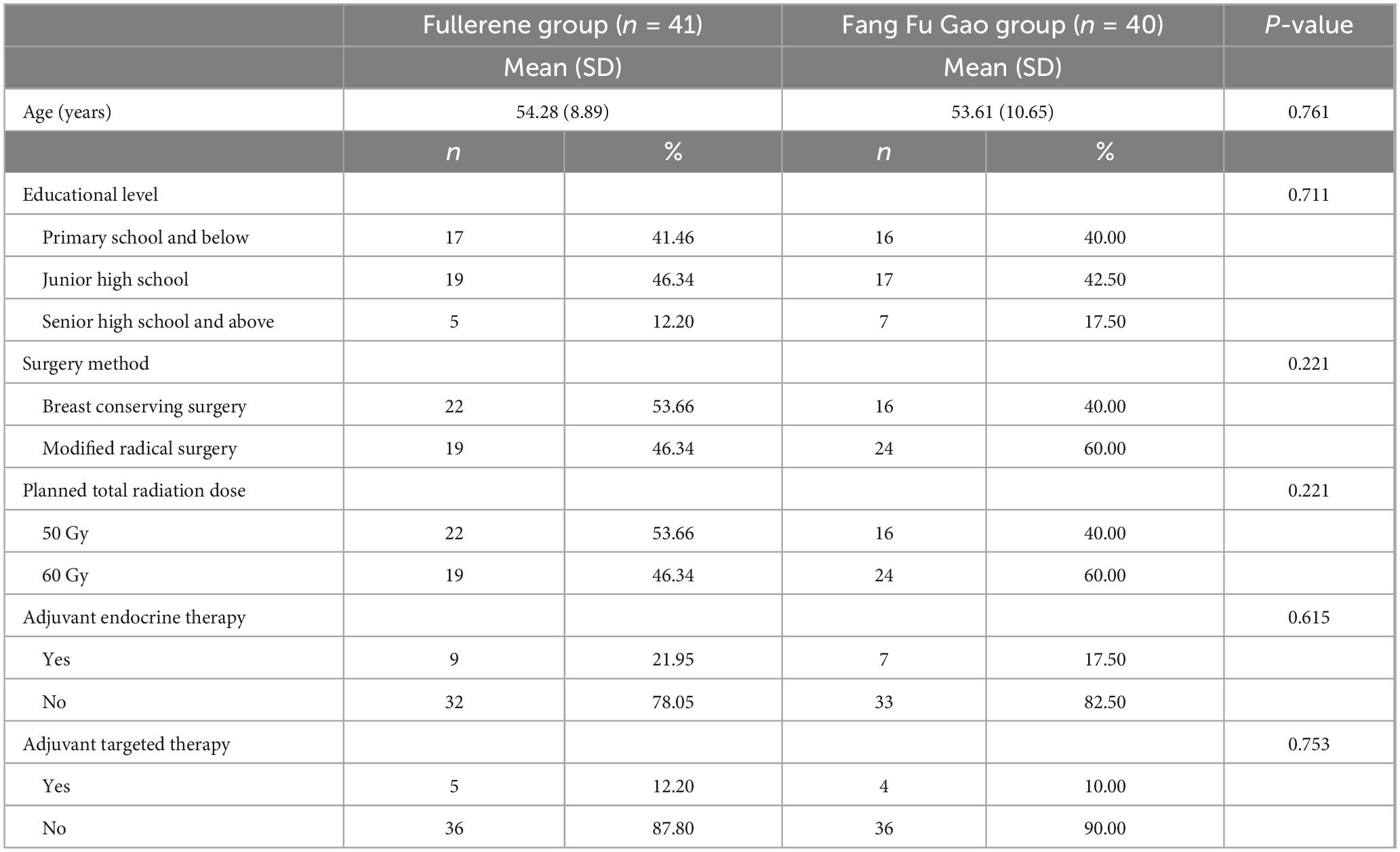
A total of 88 patients were included in this study between May 2023 and January 2025 (Figure 1). Forty-four patients received EOSSKY Fullerene moisturizing and Repairing cream, while the other forty-four patients received Fang Fu Gao as the control group. After randomization, one patient in the fullerene group withdrew consent, and two patients failed to complete radiotherapy. Two patients in the control intervention group were excluded due to incomplete radiotherapy, and two patients did not use Fang Fu Gao. As a result, a total of 81 eligible patients were ultimately included. The baseline characteristics of the two groups had no statistically significant differences (P > 0.05; Table 1).
3.2 Efficacy
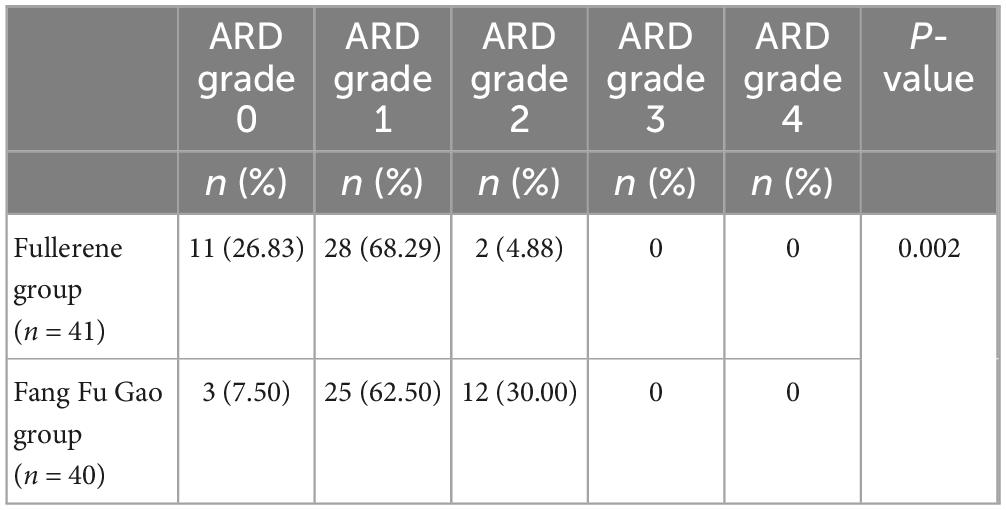
According to the RTOG classification, the ARD grading at the end of radiotherapy was significantly better in the fullerene group compared with the Fang Fu Gao group (Table 2). There was a statistically significant difference between the two groups (P < 0.05).
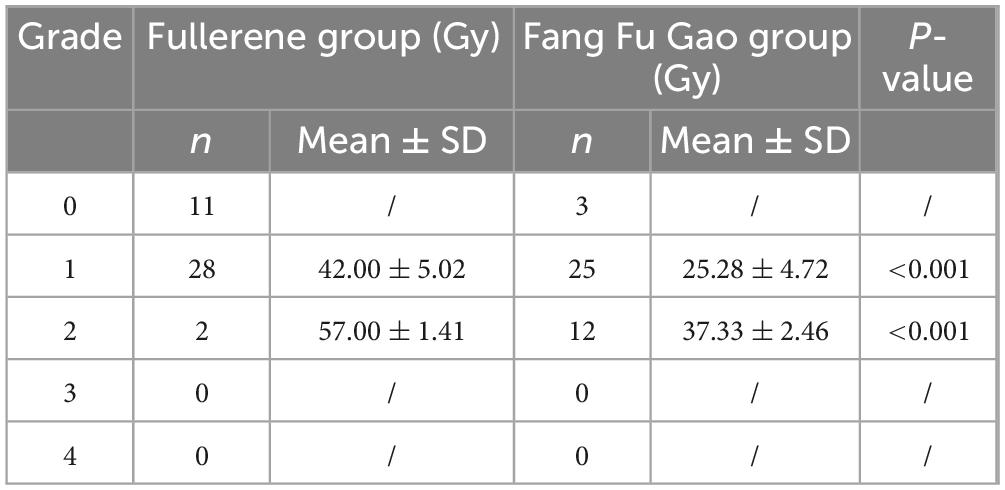
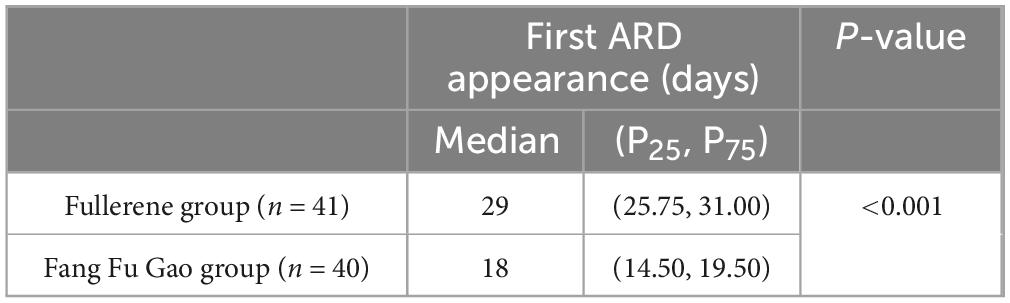
The cumulative dose of ionizing radiation at the first occurrence of ARD was higher in the fullerene group than in the control group (Table 3). In other words, the use of fullerene delayed the incidence of ARD in the intervention group. This was confirmed by comparing the time to the first ARD appearance in the two groups (Table 4). There was a statistically significant difference between the two groups (P < 0.01).
After evaluation of adverse events, no adverse events unrelated to the intended use of the medication were identified.
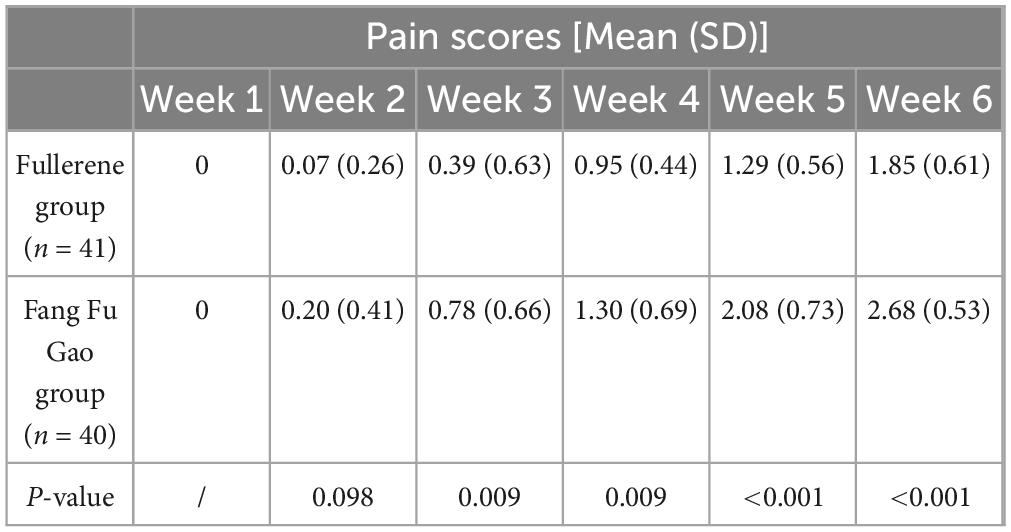
The pain scores in the fullerene group were consistently lower than those in the control group (Table 5). There was a significant difference in pain between the fullerene and control groups starting from the third week (P < 0.05).
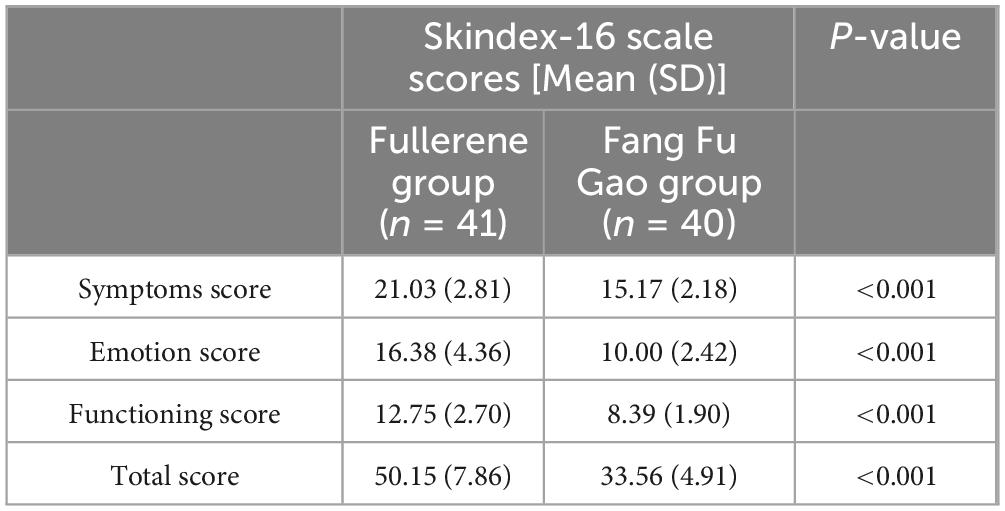
The results of the Skindex-16 scale scores showed that the quality of life was better in the fullerene group than in the control group after radiation therapy (Table 6). There was a statistically significant difference between the two groups (P < 0.001).
4 Discussion
Because the skin is the first organ exposed to radiation, ARD is the most common profound side effect of breast cancer patients undergoing radiotherapy (18). As cumulative radiation doses are received, skin damage becomes increasingly apparent, manifesting as erythema, dryness, peeling, folliculitis, itching, hyperpigmentation, pain, and mucosal ulceration (19). The pathophysiology of ARD involves X-rays causing DNA damage through direct interactions with DNA, as well as producing excessive reactive oxygen species (ROS). Additionally, indirect radioactive reactions with water further damage basal keratinocytes and hair follicle stem cells (16, 20, 21).
Currently, numerous medications are available for preventing ARD, but most cannot be recommended due to a low-quality evidence, a lack of supporting data, or conflicting findings across multiple trials (9). Fullerene is composed entirely of carbon atoms, which form hollow spheres resembling a football. These structures have conjugated double bonds, high electron affinity, and polarity, giving them an excellent ability to scavenge free radicals (22, 23). Research has indicated that fullerene can scavenge ROS and reactive nitrogen species (RNS) due to its special structure (24). Moreover, fullerene is more efficient in scavenging free radicals than conventional antioxidants, such as vitamin E or superoxide dismutase (SOD) (16, 25). The study (17) has demonstrated the radioprotective properties of fullerene as skin radioprotective agent in animal experiments. Thus, fullerene holds potentially radioprotective agent against ionizing radiation.
To the best of our knowledge, this is the first clinical study to investigate the prophylactic effect of fullerene on ARD. Our results demonstrate that EOSSKY Fullerene Moisturizing and Repairing Cream effectively reduces the grading and incidence of ARD in breast cancer patients undergoing radiotherapy. Furthermore, the cumulative dose of ionizing radiation at the first occurrence of ARD was significantly higher in the fullerene group compared to the control group, and the first appearance of ARD did occur later than in the control group. This may be attributed to fullerene’s ability to not only effectively scavenge various ROS, but also to induce endogenous phase II antioxidant enzymes and modulate cell antioxidant status by upregulating the antioxidant pathways in response to oxidative stress (26).
At the secondary endpoint, the fullerene group reported less pain. Additionally, according to the results of the Skindex-16, the fullerene group showed a significantly improvement in the patients’ QoL after radiotherapy. All of these findings suggest that EOSSKY Fullerene Moisturizing and Repairing Cream provides significant benefits in preventing ARD following radiotherapy for breast cancer.
However, this study has some limitations. First, the sample size was relatively small. Second, due to the different properties of EOSSKY Fullerene Moisturizing and Repairing Cream and the control intervention, it was not possible to blind physicians or patients, which may have introduce bias into the results. Third, although preclinical data and our clinical trials have demonstrated its safety and effectiveness, its long-term efficacy still requires further evaluation. Fourth, this study did not have biological evidence to confirm the antioxidant mechanism of fullerenes in patients due to resource constraints and ethical considerations, and future studies are recommended to bridge this translational gap.
5 Conclusion
In summary, this study found that EOSSKY Fullerene Moisturizing and Repairing Cream reduced grade and incidence of ARD, delayed the onset of skin reactions, alleviated pain, and significantly improved the patients’ QoL. These findings have important implications for the prevention of ARD in breast cancer and provides a valuable foundation for future research.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Second Hospital of Shanxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft. XS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft. JG: Conceptualization, Methodology, Project administration, Resources, Writing – review and editing. ZG: Methodology, Resources, Visualization, Writing – review & editing. XD: Methodology, Resources, Visualization, Writing – review and editing. YQ: Methodology, Resources, Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by a grant of the Mechanistic Research and Application Evaluation of Medical Fullerene Products in the Prevention and Treatment of Clinical Radiation Dermatitis.
Acknowledgments
We express our gratitude to the Department of Oncology of the Second Hospital of Shanxi Medical University for supporting this project.
Conflict of interest
YQ was employed by Guangzhou Guangna Huichuan Technology Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel R, Giaquinto A, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Surveillance, Epidemiology, and End Results Program. Female breast cancer — cancer stat facts. (2025). https://seer.cancer.gov/statfacts/ (accessed February 13, 2025).
3. De Ruysscher D, Niedermann G, Burnet N, Siva S, Lee A, Hegi-Johnson F. Radiotherapy toxicity. Nat Rev Dis Prim. (2019) 5:15. doi: 10.1038/s41572-019-0073-4
4. Maier P, Wenz F, Herskind C. Radioprotection of normal tissue cells. Strahlenther Onkol. (2014) 190:745–52. doi: 10.1007/s00066-014-0637-x
5. Citrin D, Cotrim A, Hyodo F, Baum B, Krishna M, Mitchell J. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. (2010) 15:360–71. doi: 10.1634/theoncologist.2009-S104
6. Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: A review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. (2019) 81:558–67. doi: 10.1016/j.jaad.2019.02.047
7. Behroozian T, Bonomo P, Patel P, Kanee L, Finkelstein S, van den Hurk C, et al. Multinational association of supportive care in cancer (MASCC) clinical practice guidelines for the prevention and management of acute radiation dermatitis: International Delphi consensus-based recommendations. Lancet Oncol. (2023) 24:e172–85. doi: 10.1016/S1470-204500067-0
8. Rzepecki A, Birnbaum M, Ohri N, Daily J, Fox J, Bodner W, et al. Characterizing the effects of radiation dermatitis on quality of life: A prospective survey-based study. J Am Acad Dermatol. (2019) 86:161–3. doi: 10.1016/j.jaad.2019.03.011
9. Behroozian T, Goldshtein D, Ryan Wolf J, van den Hurk C, Finkelstein S, Lam H, et al. MASCC clinical practice guidelines for the prevention and management of acute radiation dermatitis: part 1) systematic review. EClinicalMedicine. (2023) 58:101886. doi: 10.1016/j.eclinm.2023.101886
10. Menêses AG, Reis PEDD, Guerra ENS, Canto GL, Ferreira EB. Use of trolamine to prevent and treat acute radiation dermatitis: A systematic review and meta-analysis. Rev Latino Am Enferm. (2018) 26:e2929. doi: 10.1590/1518-8345.2035.2929
11. Boisnic S, Branchet-Gumila MC, Nizri D, Ben Slama L. Histochemical and biochemical modifications induced by experimental irradiation of human skin maintained in survival conditions and modulation by application of an emulsion containing trolamine. Int J Tissue React. (2003) 25:9–18.
12. Abbas H, Bensadoun RJ. Trolamine emulsion for the prevention of radiation dermatitis in patients with squamous cell carcinoma of the head and neck. Support Care Cance.r (2012) 20:185–90. doi: 10.1007/s00520-011-1110-3
13. Ho AY, Olm-Shipman M, Zhang Z, Siu CT, Wilgucki M, Phung A, et al. A randomized trial of mometasone furoate 0.1% to reduce high-grade acute radiation dermatitis in breast cancer patients receiving postmastectomy radiation. Int J Radiat Oncol Biol Phys. (2018) 101:325–33. doi: 10.1016/j.ijrobp.2018.02.006
14. Wang R, Wu F, Wang D, Liu K. Clinical effect of Biafine* in preventing and treating radioactive skin destruction of nasopharyngeal carcinoma patients caused by concurrent intensity-modulated radiotherapy and chemotherapy. Chin J Clin Oncol. (2008) 5:58–63. doi: 10.1007/s11805-008-0058-7
15. Del Rosso JQ, Bikowski J. Trolamine-containing topical emulsion: clinical applications in dermatology. Cutis. (2008) 81:209–14.
16. Zhao M, Wang C, Xie J, Ji C, Gu Z. Eco-Friendly and Scalable Synthesis of Fullerenols with High Free Radical Scavenging Ability for Skin Radioprotection. Small. (2021) 17:e2102035. doi: 10.1002/smll.202102035
17. Yin H, Gao Y, Chen W, Tang C, Zhu Z, Li K, et al. Topically applied fullerenols protect against radiation dermatitis by scavenging reactive oxygen species. Discov Nano. (2023) 18:101. doi: 10.1186/s11671-023-03869-7
18. Guangmei D, Weishan H, Wenya L, Fasheng W, Jibing C. Evolution of radiation-induced dermatitis treatment. Clin Transl Oncol. (2024) 26:2142–55. doi: 10.1007/s12094-024-03460-1
19. Kole A, Kole L, Moran M. Acute radiation dermatitis in breast cancer patients: challenges and solutions. Breast Cancer. (2017) 9:313–23. doi: 10.2147/BCTT.S109763
20. Ward J. The complexity of DNA damage: relevance to biological consequences. Int J Radiat Biol. (1994) 66:427–32. doi: 10.1080/09553009414551401
21. Hymes S, Strom E, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment. J Am Acad Dermatol. (2006) 54:28–46. doi: 10.1016/j.jaad.2005.08.054
22. Zhou Y, Li J, Ma H, Zhen M, Guo J, Wang L, et al. Biocompatible [60]/[70] Fullerenols: Potent defense against oxidative injury induced by reduplicative chemotherapy. ACS Appl Mater Interfaces. (2017) 9:35539–47. doi: 10.1021/acsami.7b08348
23. Xiao L, Aoshima H, Saitoh Y, Miwa N. Highly hydroxylated fullerene localizes at the cytoskeleton and inhibits oxidative stress in adipocytes and a subcutaneous adipose-tissue equivalent. Free Radic Biol Med. (2011) 51:1376–89. doi: 10.1016/j.freeradbiomed.2011.05.026
24. Jacevic V, Djordjevic A, Srdjenovic B, Milic-Tores V, Segrt Z, Dragojevic-Simic V, et al. Fullerenol nanoparticles prevents doxorubicin-induced acute hepatotoxicity in rats. Exp Mol Pathol. (2017) 102:360–9. doi: 10.1016/j.yexmp.2017.03.005
25. Grebowski J, Konopko A, Krokosz A, DiLabio G, Litwinienko G. Antioxidant activity of highly hydroxylated fullerene C and its interactions with the analogue of α-tocopherol. Free Radic Biol Med. (2020) 160:734–44. doi: 10.1016/j.freeradbiomed.2020.08.017
Keywords: fullerene, acute radiation dermatitis, radiotherapy, breast cancer, randomized controlled trial
Citation: Wang Q, Shi X, Guo J, Gu Z, Dong X and Qin Y (2025) Topical EOSSKY fullerene moisturizing and repairing cream for preventing acute radiation dermatitis in breast cancer patients undergoing radiotherapy: a randomized controlled trial. Front. Med. 12:1604012. doi: 10.3389/fmed.2025.1604012
Received: 01 April 2025; Accepted: 29 June 2025;
Published: 22 July 2025.
Edited by:
Sebastian Yu, Kaohsiung Medical University, TaiwanReviewed by:
Yan Liu, Akershus University Hospital, NorwayHuixin Wu, Oregon Health and Science University, United States
Copyright © 2025 Wang, Shi, Guo, Gu, Dong and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinli Guo, Z2psZ2JkQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Qi Wang
Qi Wang Xiaoning Shi
Xiaoning Shi Jinli Guo2*
Jinli Guo2*