Abstract
Bone defects affect many individuals globally and can result in significant suffering and impairment, particularly among the elderly population. In addition, current treatment options for critical-size bone defects, such as autologous or allogeneic bone graft transplantation, present significant challenges. Within this clinical scenario the identification of novel and effective approaches for bone regeneration is urgently needed, and options derived from tissue engineering may be particularly appealing. Bone tissue engineering for bone regeneration involves the application of seed cells, growth factors, and biomaterials to create bioactive substitutes for repairing bone defects. In recent decades, advancements in stem cell research and biological biomaterials have led to remarkable breakthroughs in the field of bone regeneration. In particular, various categories of stem cells have been isolated, characterized, and employed in tissue engineering approaches. This review summarizes the applications of the main types of stem cells currently used for bone regeneration through tissue engineering approaches, and it also pays attention to the most appealing materials for it.
1 Introduction
Approximately 15% of the whole-body weight consists of hard bone. Of this, cortical bone (the outer layer) accounts for about 80% of total adult bone mass. It has a relatively low porosity of 3%–5%, it is highly resistant to mechanical loads (bending and torsion) and it can sustain weight along with providing structural integrity and physical support (1). The remaining ∼ 20% of adult bone mass is made up of the cancellous bone (the inner layer), which is characterized by a honeycomb-like trabecular connection and a porosity of about 80%–90% (1). Besides, the bone is composed of organic components, that include type I collagen, non-collagenous proteins and inorganic components (i.e., hydroxyapatite crystals formed from calcium and phosphate ions). The interaction of these elements determines bone’s mechanical properties, including compressive strength and fracture toughness (2–4). Furthermore, along with its supporting function, bone plays a role in muscular function, hematopoiesis, and protection of internal organs including that of the nervous system (1). Considering the fundamental role of bone within the body, this tissue is extensively studied and bone defects, resulting from several conditions (trauma, cancer, infection, surgical complication, osteoporosis), are a global concern significantly compromising life quality (5–8). While bone distinguishes for ability to self-regenerate in case of minor injuries, defects that are 1.5 times the diameter of the bone are considered as critical-sized defects, requiring surgery to avoid non-union, malunion, or pathological fractures (1, 9).
The autologous or allogeneic bone graft transplantation (known as bone grafting) is currently considered the gold standard approach for treating bone defects (10). Autologous bone grafting involves using bone tissue from the patient’s own distal donor site. This approach does not induce immune rejection and the implants possess osteoinductive capacity. However, obtaining bone grafts is often faced with the challenges of limited tissue availability or various postoperative consequences, including enduring pain with possible sensory loss, infection, bleeding at the donor site, prolonged wound drainage, need for second surgery; consequently, this strategy is not adequate for children or aging patients (1, 11). Allografts are decellularized matrices taken from donor patients, displaying a bone structure and extracellular matrix (ECM) comparable to the original bone. Differently from autografts, allografts undergo lower integration and vascularization in the implant site. Additionally, they present the risk of spreading infections or triggering an immunological rejection; despite cryogenic treatments that may reduce immune rejection, mechanical strength may be correspondingly affected. Compared to autografts, allografts also display a reduced osteoinductive behavior and no cellular component, due to irradiation or freeze-drying processing they are exposed to (12). It is rare for either of these methods to fully restore the full function of injured bone tissue (13–15); however, autologous bone grafts are still considered the gold standard for bone defects management (11). Thus, considering these significant limits, researchers dedicated toward the identification of alternative on-the-bench smart substitutes. Since 1987, merging materials science and cell biology skills, a new field called “Tissue Engineering” stood out (16). The aim of this field is to address the scarcity of tissues/organs accessible for transplantation. In the case of bone, the goal is to mitigate the drawbacks associated with the aforementioned treatment options, including donor site injury, infection transmission, immunological rejection, and limited availability (12), while enhancing patient prognosis by substituting damaged tissue with a comparable one in terms of structure and function (2). This process involves utilizing scaffolds, seed cells, and biologically active growth factors to create bioactive substitutes that can restore normal bone function and repair areas of bone defects.
Together with an increasing understanding of material science, advancements in molecular biology, cell biology, and biochemistry have played a significant role in enhancing our comprehension of cell differentiation mechanisms and tissue growth, along with the cell-ECM interactions. Consequently, the use of stem cells has gained significance in the process of bone regeneration via tissue engineering: together with a great ability to proliferate, they also show a reduced inclination toward senescence versus differentiated cells. Additionally, several studies have demonstrated that stem cells can induce osteogenesis, thus resulting beneficial in the treatment of profound bone defects as consequence of trauma or inadequate blood supply (17, 18).
This narrative review focuses on the major categories of stem cells in bone tissue engineering and considers the main advancements on their use in this field. In addition, biomaterials employed in bone tissue engineering are presented as an extensive representation of this appealing and promising method of regeneration.
2 Different sources of stem cells for bone regeneration
Stem cells, that can be recognized both in embryos and in adult tissues, are defined as unspecialized cells endowed with the ability to self-renew and to differentiate into more than one cell lineage. Typically, there are different steps of specialization that include (19–21): totipotent stem cells, pluripotent stem cells, multipotent stem cells, oligopotent stem cells and unipotent stem cells. Totipotent stem cells (e.g., zygote, up to 4 days from egg fertilization) have the highest differentiation potential; they can form both embryo and extra-embryonic structures and can divide and differentiate into cells of the whole organism. Pluripotent stem cells (e.g., embryonic stem cells, inner cell mass of pre-implantation embryos) form cells of all germ layers except for extraembryonic structures. Multipotent stem cells (e.g., hematopoietic stem cells) can specialize in discrete cells of specific cell lineages; oligopotent stem cells (e.g., myeloid stem cells) can differentiate into several cell types. Unipotent stem cells have the narrowest differentiation capabilities and can divide repeatedly (19). Generally, these cells can differentiate into specialized tissue when exposed to a suitable environment (22).
The identification of suitable cells for bone regeneration encompasses many factors. Together with the ability to differentiate into osteoblasts, it is important to assess whether the cells can be easily accessed, the quantity that can be obtained and whether the source of the cells may raise ethical concerns (23). This section will specifically discuss three types of stem cells: mesenchymal stromal cells (MSCs), embryonic stem cells, and induced pluripotent stem cells. Currently, MSCs are the most studied type, embryonic stem cells and induced pluripotent stem cells are gaining popularity among researchers despite issues (mainly related to safeness and ethics) for future translation in clinical practice. Figure 1 is a schematic diagram of the acquisition methods along with osteogenic differentiation markers of these three types of cells.
FIGURE 1
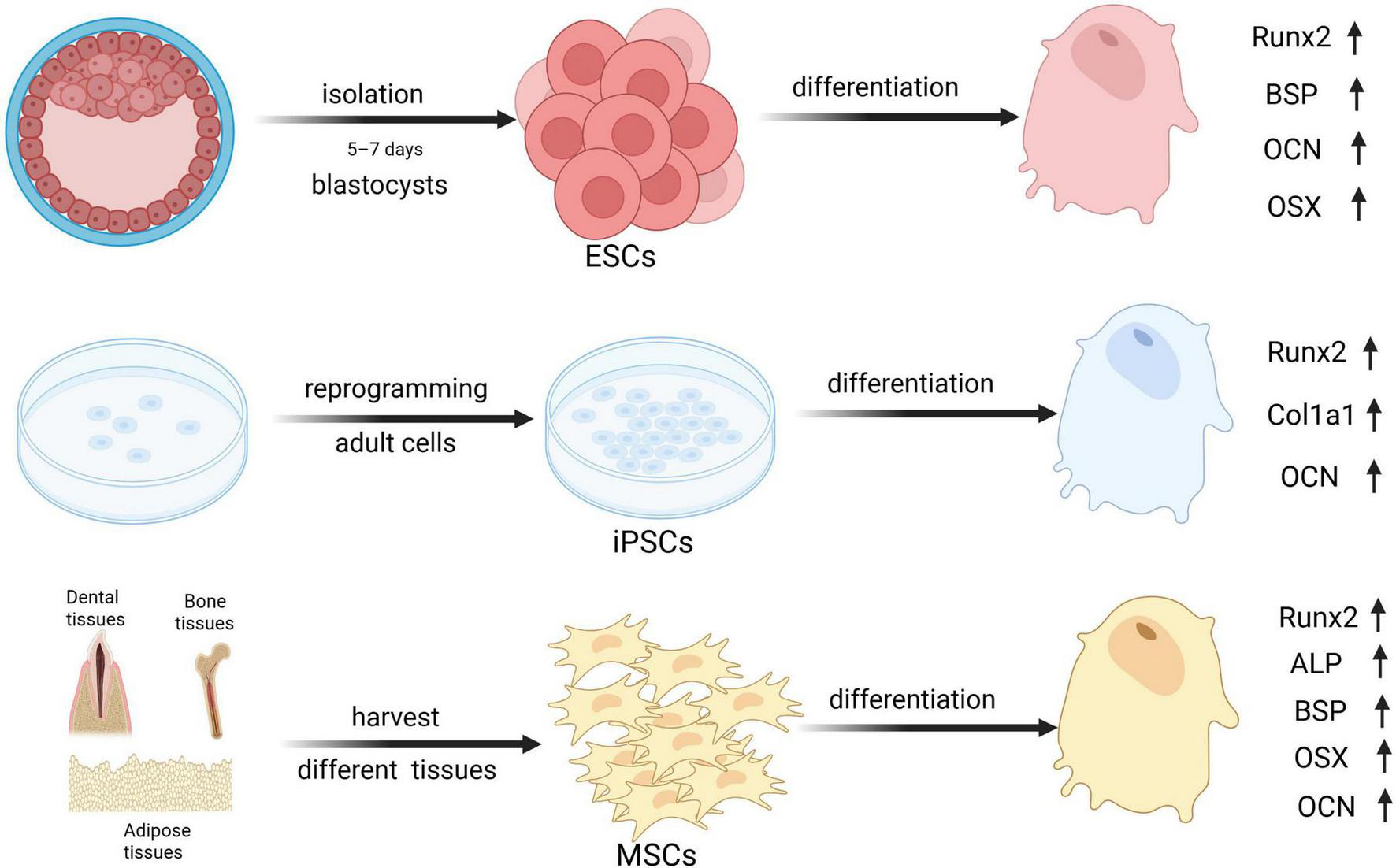
The graphs of osteogenic markers across An error in the conversion from LaTeX to XML has occurred here. 18 different stem cell types. Runx2, Runt-related transcription factor 2; BSP, Bone sialoprotein; OCN, Osteocalcin; OSX, Osterix; Col1a1, Collagen Type I Alpha 1 Chain; ALP, Alkaline phosphatase. Created with BioRender.com.
2.1 Mesenchymal stromal cells: therapeutic promise and the challenge of senescence
Mesenchymal stromal cells are a multipotent and heterogeneous cell population with the ability to differentiate into numerous types including chondrocytes, myoblasts, osteoblasts, and adipocytes (24). According to the International Society for Cellular Therapy (ISCT), there are three widely accepted criteria to define MSCS; they must: (i) be plastic-adherent in standard culture conditions; (ii) show a multipotent phenotype (i.e., ability to differentiate into adipocytes, osteoblasts and chondrocytes); (iii) exhibit the presence of CD73, CD90, and CD105 surface markers but not CD11b, CD14, CD19, CD34, CD45, CD79α, and the HLA-DR (25). There are several MSCs sources within the body (bone marrow, adipose tissue, periosteum, vessels wall, peripheral circulation, muscle, tendon, umbilical cord blood, skin, dental tissues). For clinical applications such as fracture repair, an ideal MSC source should be easily accessible, harvestable through non-invasive procedures, capable of rapid in vitro expansion, and able to survive and integrate effectively at the implantation site without promoting tumorigenesis (26).
However, a major limitation in using autologous MSCs for therapeutic purposes relies on the phenomenon of cellular senescence, which significantly compromises the cells regenerative potential. Typically, senescent MSCs distinguish for an enlarged and flattened aspect, presence of cytoplasmatic granuli and increased lysosomal mass (27). All these characteristics are well identifiable during long-term culture in vitro and are associated with reduced proliferation, lower adhesion to plastic surface, impaired colony-forming ability, and a marked decline in osteogenic differentiation potential (28, 29). Moreover, senescent MSCs often tend to differentiate toward adipose tissue (30, 31). While the MSCs characterizing surface markers (CD73, CD90, CD105) remain stable with senescence, others are downregulated (CD106 and CD146) or upregulated (CD264 and CD295) (31–33). Biomarkers like senescence-associated β-galactosidase (SA-β-gal) and α-L-fucosidase (SA-α-Fuc) are commonly employed to identify senescent cells (27, 34). At the molecular level, senescence is associated with irreversible cell cycle arrest mediated by the upregulation of p53/p21WAF1/CIP1 and p16INK4A (35). Additionally, senescent MSCs develop a senescence-associated secretory phenotype (SASP) with secretion of pro-inflammatory cytokines (e.g., IL-6, IL-8), growth factors, and proteases. The SASP factors together with perpetuating senescence also induce premature senescence in neighboring cells via paracrine signaling, ultimately disrupting tissue regeneration and immune modulation (36, 37).
Though MSCs are widely recognized for their immunosuppressive and anti-inflammatory roles, senescent MSCs can exhibit pro-inflammatory behavior, contributing to tissue dysfunction and impaired healing (38, 39). Considering that MSCs potential for proliferation/differentiation decreases with patient’s age and age-associated comorbidities, allogeneic MSCs can be used instead of autologous MSCs, preserving regenerative efficacy (19, 40).
Senescence in MSCs can arise from several mechanisms, including replicative senescence (due to telomere shortening), oncogene-induced senescence, stress-induced senescence, and developmental senescence. Interestingly, it seems that MSCs senescence is a modifiable risk factor, and intense research efforts are dedicated toward the identification of effective strategies to reverse senescence-associated changes allowing to enhance MSCs use as therapy as well as application for regenerative medicine purposes (34, 41).
To date, bone marrow and adipose-derived MSCs are the two most investigated types of stem cells. Furtherly, dental-derived types are becoming a desirable option for bone tissue engineering too (Table 1).
TABLE 1
| Stem cell type | Source | Advantages | Disadvantages |
| BMMSCs | Bone marrow derived from long bone or jawbone during transplants or orthopedic surgeries | 1. Accessible source for cell harvesting 2. Easy to cultivate in vitro 3. Ease of preparation 4. Can be easily induced to differentiate into osteoblasts |
1. Painful acquisition process 2. Potential for more serious complications 3. Low content in bone marrow tissue 4. Risk of infection in bone tissue 5. Difficulties in obtaining sufficient quantity |
| ADSCs | Collection of superficial subcutaneous adipose tissue during liposuction or reconstructive surgery | 1. Easy to obtain a sufficient quantity 2. Can be painless for patients to provide enough quantity 3. Can be cultured for a long time in vitro with a low apoptosis rate 4. Lower risk of infection 5. The osteogenic capacity does not decrease with cell proliferation |
1. The freezing and subsequent thawing can reduce their ability 2. Cell contamination can reduce the proliferation and differentiation ability 3. Lower osteogenic capacity than BMMSCs 4. High tendency to differentiate into adipocytes |
| DMSCs | 1. Dental pulp from exfoliated deciduous teeth or extracted permanent tooth 2. Periodontal or gingival tissues 3. Dental follicle tissues. |
1. Can be easily harvested from medical waste 2. Can be relatively easy to access and less invasive 3. Higher proliferation rate |
Lower osteogenic capacity |
| ESCs | Pre-implantation stage blastocyst’s inner cell mass after fertilization for 5–7 days | 1. Can differentiate into any type of cell in the body 2. Have unlimited self-renewal capacity 3. Use as disease model |
1. Destruction of an embryo at the blastocyst stage with consequent ethical issues 2. Are available only in limited quantities 3. Limited by the ethics and laws of many countries 4. High rate of mutation 5. Risk of causing teratoma |
| IPSCs | Several differentiated mature somatic cells | 1. No ethical issues compared to embryonic stem cells 2. Easy to obtain 3. HLA histocompatibility 4. Use as disease model 5. Have the ability to be reprogrammed 6. Have a wider range of applications |
1. Risk of tumor formation 2. Risk of genomic instability 3. Risk of immune rejection 4. Cost |
Source, advantages, and disadvantages of different types of stem cells for bone regeneration.
2.1.1 Bone marrow mesenchymal stromal cells
Bone marrow mesenchymal stromal cells (BMMSCs) were the earliest MSCs successfully separated and recognized as effective for bone tissue engineering. Together with the capacity for self-renewal, they also show multidirectional differentiation toward the osteoblast and chondrogenic lineages upon exposure to specific stimuli. Due to their advantageous properties, including relatively easy access, high proliferative capacity, and the ability to readily differentiate into osteoblasts, BMMSCs are now regarded as the most superior type of MSCs for bone regeneration (20).
Several studies have been conducted to assess the function of BMMSCs in bone tissue engineering. In an in vitro study by Zhang et al., BMMSCs were grown in an osteo-induced media after being seeded onto a three-dimensional polycaprolactone/tricalcium phosphate (TCP) scaffold. As indicated by the increased expression of osteogenic genes (RUNX-2, ALP, ON, collagen type I) and calcium deposition, BMMSCs demonstrated an effective osteogenic capacity (21). Hayashi et al., implanted rat BMMSCs/hydroxyapatite composites into a subcutaneous ectopic ossification model, in an in vivo study. Six weeks post-implantation, micro-CT imaging and histologic analysis revealed enhanced new bone formation at the surgery site (22). Recent studies have highlighted the significance of biomaterials in modulating the expression of osteogenic genes in BMMSCs and promoting their proliferation and differentiation into osteoblasts. For instance, porous calcium silicate ceramic materials containing silicon and strontium can significantly boost the expression of osteogenic genes in BMMSCs (23). Additionally, the surface characteristics of the magnesium alloy scaffold are essential in boosting the adherence of BMMSCs, along with stimulating their development into bone cells. These attributes also enhance the expression of genes associated with bone formation (24). Furthermore, it has been observed that the osteogenic capacity of BMMSCs varies depending on their origin. Aghaloo et al. (25) found that jawbone-derived MSCs exhibited more osteogenic gene expression and had a higher ability for mineralization compared to long bone-derived MSCs. Similarly, Zhou et al. (26) found that jawbone-derived MSCs exhibited better osteogenic potential and higher levels of Vascular Endothelial Growth Factor (VEGF) secretion compared to femoral BMMSCs under similar culture conditions. According to the in vitro investigations by Akintoye et al. (27), iliac crest-derived BMMSCs produced denser bone tissue, while the jawbone-derived MSCs were able to generate more bone tissue under the same induction culture conditions. Collectively, jawbone-derived MSCs can be considered an effective stem cell source for maxillofacial bone regeneration (28).
Bone marrow mesenchymal stromal cells are usually isolated from anatomical sites as the sternum or pelvic region during bone marrow transplants or orthopedic surgeries and are associated with patient suffering together with potential serious complications (29). Additionally, the available amounts are limited and their senescence increases with patients’ age (Figure 2) (19). These are the main challenges for their application in bone regeneration (Table 1).
FIGURE 2
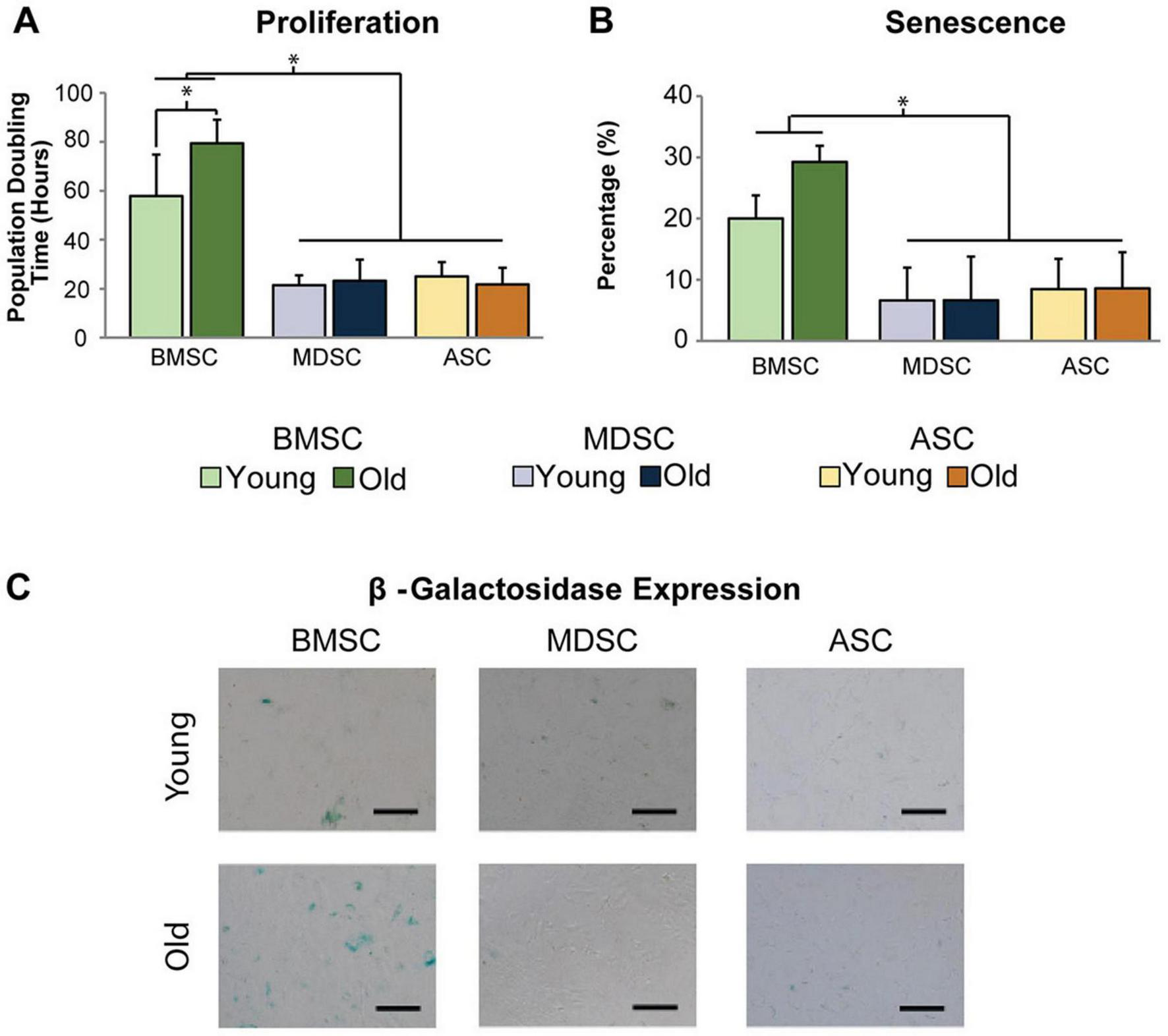
Difference of expansion capacity and senescence of MSCs in age-related changes. (A) The population doubling hours (represents the proliferation capacity) of old BMMSCs is obviously longer than those of young BMMSCs. (B,C) The quantitative and qualitative research of β-galactosidase staining (represents the senescence) exhibits that the senescence of old BMMSCs is more serious than young BMMSCs, however, the age-related changes of adipose-derived stem cells and muscle derived stem cells are not obvious. [Adapted with permission from Ref. (19)] p < 0.05.
2.1.2 Adipose-derived mesenchymal stromal cells
Adipose-derived MSCs (ADSCs) were initially recognized and described as BMMSCs substitutes for bone tissue engineering in 2001 (30). ADSCs can be obtained by liposuction under local anesthesia, commonly from superficial subcutaneous adipose tissue (31). Compared to BMMSCs isolation, their extraction can be painless for patients while providing enough autologous cells for tissue engineering purposes (32). Besides, ADSCs exhibit similar multilineage differentiation capabilities to BMMSCs, including differentiation into skeletal muscle, adipose tissue, tendon, bone, and cartilage. Additionally, the quantity of ADSCs obtainable from adipose tissue and their proliferation capacity are both greater than those of BMMSCs. Furthermore, they can be cultured in vitro for extended periods with a low apoptosis rate (33). In vitro studies have demonstrated that the osteogenic potential of ADSCs is not diminished with cell proliferation, in contrast to BMMSCs. Moreover, unlike BMMSCs, the osteogenic potential and the expression of osteogenic genes of ADSCs from elderly patients was similar to that of ADSCs isolated from younger patients (34–36).
The clinical use of ADSCs includes implantation into bone defects using undifferentiated ADSCs, ADSC-derived extracellular vesicles (EVs), or ADSC-derived osteoblasts following in vitro differentiation. Longaker’s group conducted initial in vivo study where they seeded murine ADSCs on apatite-coated poly-(lactic-co-glycolic acid) (PLGA) scaffolds to evaluate their ability to regenerate bone tissue (36). Lendeckel et al. used a combination of autologous ADSCs with fibrin glue to address cranial defects (37). According to experimental evidences, EVs produced by human ADSCs can be progressively and consistently released when immobilized under mild chemical conditions on polydopamine-coated PLGA scaffolds. EVs secretion can enhance the proliferation, osteogenic differentiation, and migration of human MSCs. Furthermore, the results obtained from experiments conducted on living organisms demonstrated that this approach significantly enhanced the process of bone regeneration in a model with a critical-size bone defect (38). Mesimäki et al. presented an innovative approach for addressing maxillary defects in an adult by using the patient’s own ADSCs along with recombinant human bone morphogenetic protein-2 (BMP-2) and β-tricalcium phosphate (β-TCP) granules. After eight months from surgery, the patient showed the growth of new, fully developed, healthy, and well-supplied bone, which successfully fused with the surrounding tissue and remained stable (39). Thesleff et al. employed ADSCs as alternative approach for calvarial reconstruction with successful results in adult patients (40). Several in vivo studies showed that ADSCs have a greater ability to promote angiogenesis compared to BMMSCs, especially when cultured under hypoxic conditions, showing a 5-fold increase in VEGF secretion. BMMSCs and ADSCs were placed in PLGA scaffolds and then implanted into a subcutaneous pouch in nude mice. The results highlighted that the number of newly formed blood vessels and bone tissue characteristics were significantly higher/better than that obtained using scaffolds alone or cells alone. This synergistic effect can reduce the number of implanted cells and thus reduce the costs associated with bone regeneration (41–43). Overall, ADSCs can be employed in combination with various scaffolds and growth factors to facilitate bone regeneration in several conditions.
Certainly, heterogenicity of the stromal vascular fraction cells obstacles the effective separation of ADSCs (44). In addition, the process of ADSCs freezing and thawing diminishes their capacity to induce bone regeneration with a significant negative effect on the growth and proliferation of cells in vitro (45). Furthermore, ADSCs obtained from patients with systemic diseases like osteoporosis may have a diminished osteogenic potential (46). Several studies have confirmed that the osteogenic capacity of ADSCs is lower than that of BMMSCs. Although both of them showed good proliferation and differentiation when cultured on chitosan/β-1,3-glucan/hydroxyapatite scaffolds, BMMSCs still showed greater adherence, and proliferative capacity (41, 47).
A further source of ADSCs, with potential applications in bone tissue engineering, has been recently identified in infrapatellar fat pad (IFP) adipose tissue. In fact, according to in vitro-characterization studies, IFP-derived stem cells show a differentiation potential toward chondrogenic, and osteogenic lineages producing mRNAs of COL1A1, SPARC, and GLUT1; moreover, the significant expression of cortactin gene suggests that their differentiation might be regulated by mechano-transduction. However, despite being an alternative to subcutaneous ADSCs, it must be considered that they might be reprogramed by the inflammatory environment. More investigations are needed for a broad understanding of IFP stem cells’ regenerative potential (Table 1).
2.1.3 Dental-derived mesenchymal stromal cells
Dental-derived mesenchymal stromal cells (DMSCs) are stem cells obtained from several oral tissues including periodontal ligament, dental pulp, apical papilla, dental follicle and also gingiva (48). Laino et al. were the first to successfully isolate stem cells from human oral tissues and demonstrate their capacity for self-renewal and differentiation into various cell types. Following the implantation into mice with weakened immune systems, researchers observed the formation of a lamellar bone structure consisting of osteocytes at the implant site (49). Many in vivo and in vitro studies have demonstrated the osteogenic capacity of various DMSCs. In contrast to BMMSCs and ADSCs, DMSCs are more readily accessible and their isolation is less invasive; particularly, they can be easily harvested from medical debris, making them a desirable potential source of MSCs for bone tissue engineering purposes (50).
Many researches also highlighted other favorable properties of DMSCs over BMMSCs. For example, the proliferation rate of DMSCs such as dental pulp stem cells, dental follicle stem cells, and the proliferation rate of periodontal stem cells was higher than that of both BMMSCs and ADSCs (51, 52). Although different DMSCs all originate from neural crest cells that arise from the embryonic ectoderm germ layer, there are differences in their phenotypes and osteogenic potential (53, 54). The osteogenic capacity of periodontal stem cells and dental follicle stem cells is only slightly lower than that of BMMSCs and ADSCs, and are both higher than that of dental pulp stem cells and stem cells from human exfoliated deciduous teeth (SHEDs). In a comparative study focusing on SHEDs and dental pulp stem cells, it was demonstrated that SHEDs possess a greater osteogenic potential, while dental pulp stem cells are more likely to produce osseous dentin than bone tissue. This may be attributed to a higher degree of “stemness” and pluripotency in SHEDs (55, 56). Nakajima et al. (57) conducted a comparative study between dental pulp stem cells, SHEDs, and BMMSCs for bone tissue engineering. A scaffold made of PLGA was employed for calvaria defects repair in immunodeficient mice. The results showed that SHEDs are associated with the greatest amount of osteoid and collagen fibers which were also spread extensively. Additionally, the study determined that SHEDs have sufficient bone regeneration capability to effectively restore bone defects. A comparative study was conducted by Vater et al. to assess the bone regeneration capacity of DPSCs and BMMSCs in presence of mineralized collagen matrix (MCM) scaffolds in a critical-size calvarial defect. The results indicated that dental pulp stem cells had a significantly inferior capacity to repair the lesion than BMMSCs (58). Moreover, as showed by Alge et al., the dental pulp stem cells had a greater degree of efficiency in undergoing osteogenic differentiation compared to the BMMSCs (59). A study used hydrogen peroxide and serum deprivation to mimic a potentially harsh microenvironment after transplantation to induce stem cell apoptosis. It was demonstrated that dental pulp stem cells and periodontal stem cells were more resistant to apoptosis than dental follicle stem cells and umbilical cord MSCs which is similar to BMMSCs and ADSCs (29) Taken collectively, periodontal stem cells provide the best alternative to BMMSCs in DMSCs, and SHEDs are also an appealing option for MSCs (Table 1).
2.2 Embryonic stem cells
Embryonic stem cells (ESCs) are the cells obtained from the inner cell mass of blastocysts at the pre-implantation stage up to 4 days after fertilization. These cells have a high degree of pluripotency, enabling them to differentiate into any cell type found in the body. Additionally, they have an unlimited capacity for self-renewal and can be guided to transform into osteoblasts when exposed to specific conditions. This renders them highly promising for bone tissue engineering applications (60, 61). In an in vivo study, researchers combined human ESCs, stimulated by dexamethasone for 24 h, with sterile poly-D, L-lactide foams and implanted them into Severe Combined Immunodeficient (SCID) mice. After 35 days, mineralized tissue formation was observed at the implantation site without evidence of teratoma. (62). However, ESCs are available only in limited quantities, and their application is ruled by the ethics and laws of many countries (63). Besides, the direct use of ESCs in treating bone defects carries a non-negligible risk of teratoma formation (64). Therefore, exerting a precise control over the differentiation toward osteoblasts and maintaining optimal osteogenic culture conditions in vitro are very important (65). Besides, to ensure safety in tissue engineering, ESCs must be correctly committed to the desired lineage at the time of implantation (66). However, if ESCs are differentiated into desired cell types in vitro to avoid the risk for teratomas, they can cause an immunological response when transplanted, thus the safety of the treatment cannot be guaranteed (67). These challenges have restricted the utilization of ESCs in bone regeneration (Table 1).
2.3 Induced pluripotent stem cells
Induced pluripotent stem cells (IPSCs) are mature differentiated cells that have been reprogrammed to a pluripotent state. This reprogramming is achieved by inducing the expression of four specific transcription factors known as a reprogramming cocktail, which typically includes Oct4/Sox2/c-Myc/KLF4 or Oct4/Sox2/NANOG/LIN28 (68–70). Therefore, IPSCs can be developed as cell lines, as they are programmable to generate multiple cell types from a single cell (71). In contrast to MSCs, which are limited to differentiating into mesodermal tissues, IPSCs can differentiate into ectodermal, mesodermal, and endodermal tissues. This enables a broader spectrum of applications (72).
Presently, recurring to IPSCs is becoming a desirable option for bone tissue engineering overcoming the potential risk of teratoma associated with ESCs (64). Prior research has demonstrated that the ability of IPSCs to form bone tissue is comparable to that of ESCs (73). However, this does not mean that IPSCs will be safer to use than ESCs, some other researches have demonstrated that IPSCs are at a higher risk of tumor development. This risk may be attributed to the re-reprogramming process, which frequently employs genes that have high expression in a variety of cancers (64, 74). In recent years, new induction techniques have been developed to reduce the tumorigenic risk. These protocols avoid the use of oncogenic transcription factors, like c-Myc (70). There are research that also suggest to differentiate IPSCs before implantation. It is crucial to ensure that no undifferentiated cells are introduced during the implantation process to prevent contamination (75). Despite promising, IPSCs suffer from genomic instability and immune rejection, so their application is at a preliminary stage (76).
3 MSCs derivatives and bone regeneration
Mesenchymal stromal cells derivatives are divided into two categories: extracellular vesicles (EVs) and bioactive factors. Evs have emerged as pivotal mediators within tissues; certain proteins, lipids, and nucleic acids found in Evs can be transferred and operate as signaling molecules to change cellular behavior (77). Thus, Evs have a key role not only in guaranteeing normal physiological processes but also in regulating several disease-related mechanisms (78–80). Based on their biogenesis, Evs have been classified into three main subgroups: exosomes, microvesicles, and apoptotic bodies (80–82). However, in accordance with the latest consensus guidelines “Minimal Information for Studies of Extracellular Vesicles” (MISEV2023) from the International Society for Extracellular Vesicles (ISEV), this nomenclature is “discouraged” unless the subcellular origin can be proved; conversely, the term Evs is “recommended” (83). As for the “operational terms” like small Evs (diameter < 200 nm) and large Evs (diameter > 200 nm), although their use is allowed, caution is required, as this classification is possibly influenced by the method used for characterization (84).
Bioactive factors encompass a range of interleukins, cytokines, chemokines, proteins, and growth factors, as well as cell-free nucleic acids (such as miRNA, mRNA, and lncRNA), and lipids (such as sphingolipids, cholesterol, and ceramides) (85).
3.1 Extracellular vesicles
Extracellular vesicles are membrane vesicles released by most cultured cells that facilitate cell-cell communication through transferring bioactive substances like proteins or nucleic acid to the recipient cells (86). MSCs also release Evs, and studies show that MSC-derived Evs serve a similar function in tissue repair as MSCs (86, 87). The present mainstream view is that the benefits of MSCs in tissue regeneration are attributed to secreted nutritional factors, among which Evs may play a key role (87). Due to the above characteristics, Evs are recognized as appealing molecules in bone tissue engineering, with a significant position in cellular regeneration therapy of bones. The majority of research has demonstrated that Evs regulate bone regeneration-related pathways (e.g., immunomodulatory effects during bone regeneration (87), enhance local angiogenesis (88), microRNA helps regulate the process of bone regeneration (89), and many other aspects). Researchers have proved that Evs produced from MSCs can regulate osteogenic-related pathways, including the Smad pathway activated by Bmpr2/Acvr2b competitive receptors (90), adjusting the TAF15/RUNX2 to transmit SNHG7 (91), blocking excessive activation of the canonical Wnt signaling pathway (82), activating the AKT/mTOR pathway and other pathways involved in bone regeneration (92). The properties of Evs that promote bone regeneration depend critically on the host’s immunological response to them (93). According to the findings, dental pulp stem cell-derived Evs (DPSC-Evs) help switch macrophage phenotype from M1 to M2. Additionally, in rats with experimental periodontitis, Qiao et al. found that DPSC-Evs were able to stimulate periodontal epithelium healing and prevent alveolar bone loss in vivo (88). A study has proved that MSC-Evs stimulate early angiogenesis and support bone regeneration (94). The Evs of human umbilical cord MSCs facilitate healing of the bone fracture by promoting angiogenesis with HIF-1α (Figure 3) (95). The miR-21/notch1/dll4 signal pathway may be involved in this process, which enhances angiogenesis to mend massive bone defects (96), the Evs secreted by human deciduous tooth stem cells regulate angiogenesis and osteogenesis through the AMPK signaling pathway, promoting alveolar bone regeneration and facilitating periodontal bone regeneration (97). Endogenous non-coding ribonucleic acid microRNA binds to the 3′ untranslated region (UTR) (or 5′ seed region) of the target messenger ribonucleic acid, hence acting as a negative regulatory factor for post-transcriptional gene expression (98). By transferring miRNA to recipient cells, Evs can control epigenetic processes and the biological function of those cells in bone remodeling. The MSC-Evs are now implicated in the bone regeneration process in a variety of ways. These include miR-1260a (99), miR-31 (100), miR-375 (101), miR-23a, miR-17 (101), miR-182 (101), and many others. Apart from the previously discussed potential processes, researchers have suggested that PLIN5-driven regulation of lipid metabolism is the means by which Evs produced from MSCs regulate bone remodeling (102). It was discovered that BMSC Evs mediate the autophagy level of MC3T3-E1 cells, which stimulates osteogenic differentiation (103). Although the majority of recent research has demonstrated that Evs are essential for bone repair, efforts are still needed to truly apply them in clinical practice, as there are limitations such as insufficient availability of extracellular vesicles and the influence of donor age (104).
FIGURE 3
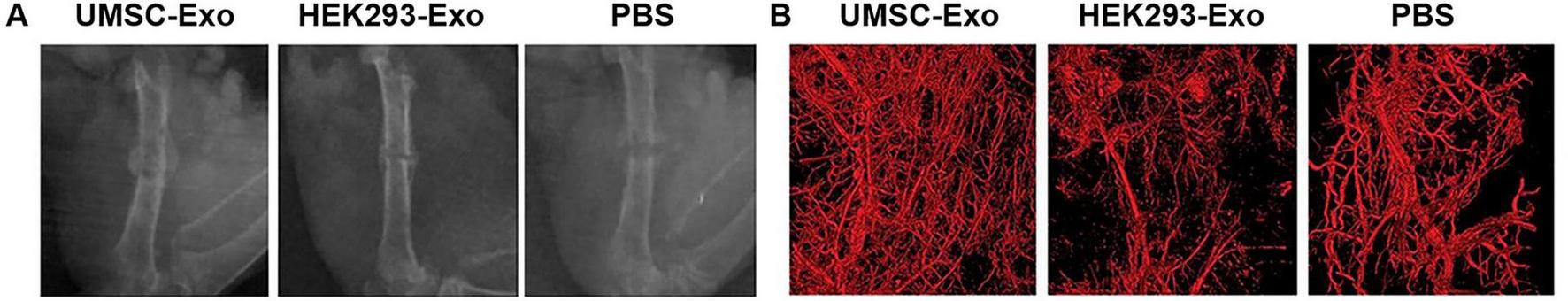
Radiographic analysis of the fracture healing in different conditions. (A) Representative X-ray images of the fractures on post-operative day 14, the uMSC-Evs group had larger callus volumes than those of the HEK293-Evs and PBS groups (served as control groups) (B) Callus and vessel volumes were reconstructed and qualitatively evaluated by high-resolution micro-CT, the employment of uMSC-Evs led to obviously increased vessel volumes. [Adapted with permission from Ref. (95)].
3.2 Bioactive factors
Bioactive factors play important roles in cell proliferation, differentiation, and immune regulation. Growth factors, chemokines, inflammatory cytokines, and other bioactive substances are associated with bone regeneration (105). Similarly, MSCs can secrete bone-regeneration-related bioactive factors, which may be another pathway for MSCs to exert bone regeneration effects. Yamada, et al. used cytokine antibody array and Enzyme-Linked Immunosorbent Assay (ELISA) to detect the cytokine secreted by BMMSCs, DPSCs, and deciduous tooth stem cells. The results highlighted that 11 cytokines were associated with tissue regeneration, including growth factors (e.g., Angiogenin, HGF, TGF-β1, EGFR), chemokines (e.g., MCP-1, MCP-2, GRO), and inflammatory cytokines (e.g., Osteoprotegerin, TIMP-2, IL-6, LAP) and were secreted by the three sources of MSCs (105). Mesenchymal stromal cells conditioned medium (MSCs-CM) contains several bioactive factors. Katagiri et al. (84) conducted the first clinical study on alveolar bone restoration by using human MSCs secretome, in which he found that MSCs-CM is safe to use, is responsible of fewer inflammatory symptoms, and has enormous osteogenic potential in bone tissue engineering (Figure 4) (84). In recent years, an increasing number of scholars have used freeze-dried MSCs-CM for research on bone tissue engineering and found that freeze-dried MSCs-CM and its byproducts can enhance bone cells’ osteogenic potential and the production of new bone (106–108). Some scholars have also begun to study the possible pathways for MSCs-CM to promote bone regeneration. Freeze-dried MSCs-CM contains bioactive factors (chemokines, cytokines, etc.) that stimulate macrophage polarization and adjust the surrounding microenvironment to promote osteogenesis (109). The bioactive components interact to form a network that has osteogenic effects. Consequently, freeze-dried MSCs-CM is a promising derivative of MSCs. Bioactive factors share with EXOs the same critical issues associated with storage.
FIGURE 4
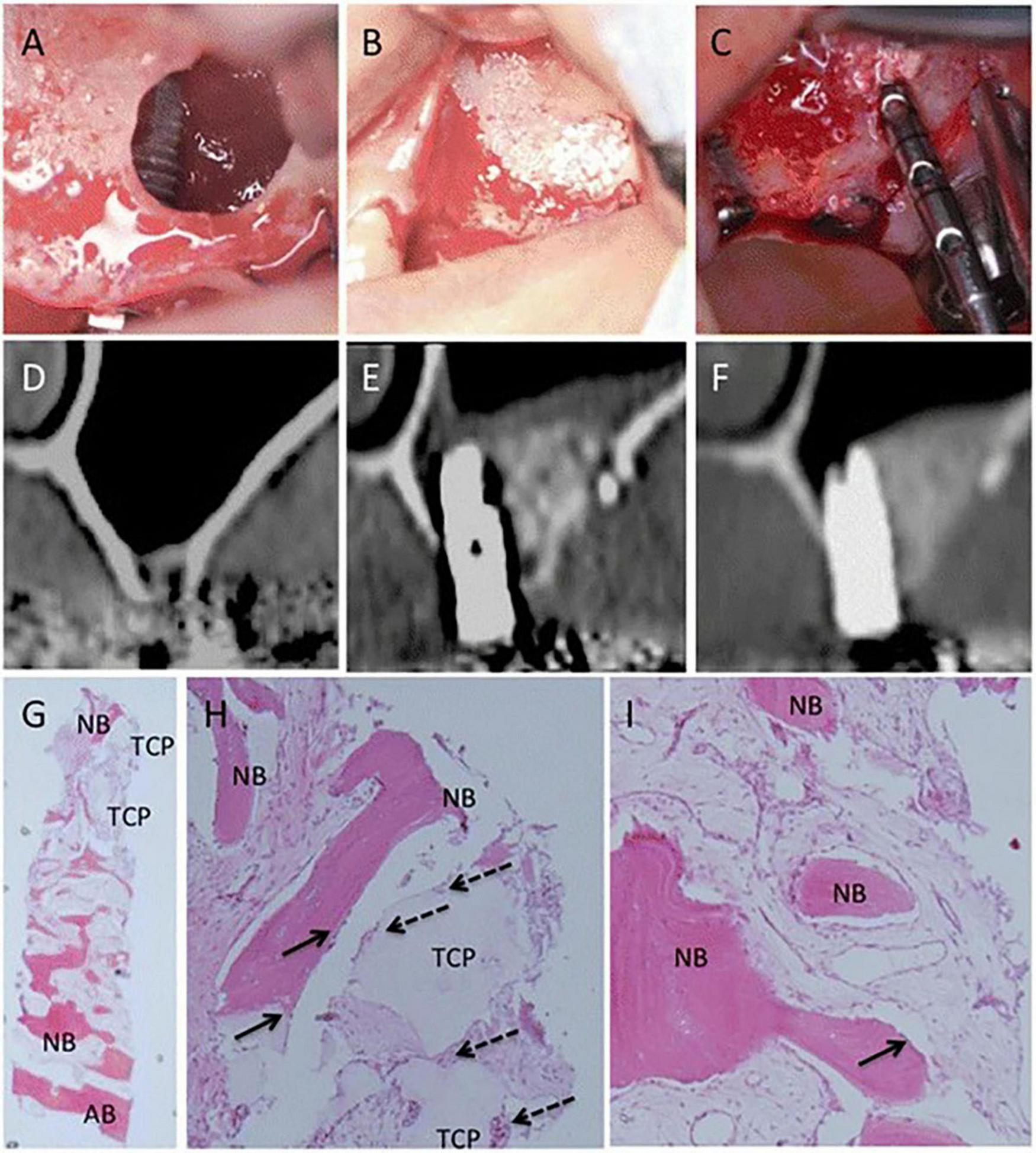
Clinical, radiographic, and histological observations of a woman in maxillary sinus floor elevation (SFE) and simultaneous MSC-CM/β-TCP implantation. (A,B) The implant and MSC-CM/β-TCP is implanted into the position of SFE surgery (C) The implant position was almost covered with newly formed bone and residue of β-TCP after 6 months, and the osseointegration of the implants is satisfactory. (D) CT images before SFE procedure I (E,F) 3 months and 6 months after SFE and MSC-CM/β-TCP implantation. (G–I) Histologic findings of the newly formed bone 6 months after the operation. The residual β-TCP is replaced from the edge by the new bone (NB) throughout the biopsy sample and infiltration of inflammatory cells was not severe. (G, ×12.5) (H, ×100) (I, ×100) [Adapted with permission from Ref. (84)].
Both Evs and secreted bioactive factors have important advantages in bone regeneration and thus, potentially also in bone tissue engineering. Additionally, more studies in these fields will help to develop promising tissue restoration options for patients. If future breakthroughs are made in the production and storage of stem cell derivatives, it is these novel resources may be used also in clinical practice.
4 Biomaterials for scaffolds in bone tissue engineering
Scaffolds are the third essential component in tissue engineering, together with cells and biological active growth factors. Therefore, the selection of materials for scaffold construction and their specific structural design is of paramount importance (110). Scaffolds provide structural support by forming a three-dimensional (3D) framework that facilitates cell adhesion, development, and proliferation, while also promoting the deposition of ECM. Certainly, in addition to biocompatibility and an appropriate biodegradation rate, which ensures the scaffold is gradually replaced by host tissue, bone scaffolds are expected to show adequate stiffness, pore sizes (> 100 μm), surface topology, load-bearing capacity (111). The exceptional strength of bone, with a tensile strength ranging from 700 to 1400 kg/cm2 and compressive strength from 1400 to 2100 kg/cm2, is primarily due to the alignment of collagen fibers and mineral crystals parallel to the bone’s long axis. Additionally, the elasticity of bone tissue, with a modulus of elasticity estimated between 420 and 700 kg/cm2, is essential for proper bone function (110).
Moreover, bone scaffolds must be manufactured in a specific shape in order to adapt to complex bone defects and trigger osteoinduction, osteoconduction, and osteogenesis. Osteoinduction induces the differentiation of cells by activating external growth factors, which in turn initiates bone formation; osteoconduction, on the other hand, creates the matrix facilitating bone cell adhesion. Besides, the effective bone generation by cells can be induced by osteogenesis (112, 113).
Different biomaterials are used in bone-related applications; briefly, these include polymers, ceramics, biodegradable metals, and composite materials from a combination of polymer and ceramics (114).
4.1 Polymers
Polymers for scaffolds fabrication are generally classified into natural polymers and synthetic polymers. Natural polymers can be either protein or polysaccharide based. Proteins have amino acid sequences that can promote cell adhesion by integrin-binding domains; scaffolds based on polysaccharides need to be improved by chemical surface modifications, combination with osteoconductive materials, integrin-binding sequences, or cell adhesion proteins. Unfortunately, they are susceptible to contamination and batch-to-batch variability, as well as inadequate mechanical properties, requiring the adoption of strategies (e.g., modulation of concentration, porosity, polymerization conditions, and addition of inorganic compounds) to improve their strength. Collagen, silk fibroin, alginate, chitosan and hyaluronic acid are the most thoroughly researched natural polymers for bone tissue engineering. (115–117).
Collagen is the predominant protein in mammals. There are about 28 different forms of collagen and types I, II, III, and V are essential components of bone, cartilage, muscle, skin, and tendon. Furthermore, they can be found in fibrillar form that exhibit intricate 3D arrangements in the ECM. Bone tissue mainly consists of type I with a little presence of type V collagen. Collagen structure serves as an anchorage for nanosized hydroxyapatite crystals (118). It descends that collagen is an appealing biomaterial for bone regeneration, especially type I; in fact, it distinguishes for biocompatibility, high porosity, hydrophilicity, low antigenicity, and good resorption (3).
However, as collagen displays low mechanical strength and lacks mineralization, embedding additive organic and inorganic materials is a beneficial solution to overcome this limit also enhancing properties like mineralization capability, cell adhesion, and stem cell differentiation (119). These two major categories can be recognized: collagen/inorganic bioactive materials (bioceramics, β-TCP, hydroxyapatite, and graphene oxide), and collagen/polymers (natural polymers including chitosan, alginate, and silk fibroin; synthetic polymers including PCL, PLGA, and PVA) (120). Modifying or combining collagen with other materials it is possible to obtain many different bone substitutes such as sponges, microfibers or spheres, and hydrogel (121).
Salgado et al. (122) studied the behavior of human bone marrow stromal cells (HBMSC) on collagen/nanohydroxyapatite particles (nanoHA) cryogel where, to enhance the osteogenic induction of the scaffold, the nanoHA were homogeneously dispersed over the pore’s walls of the type I collagen matrix. The presence of nanoHA enhanced cellular attachment and spreading in vitro; in particular, scaffolds with higher nanoHA content (e.g., 70%) supported greater cell proliferation compared to those with lower concentrations (e.g., 30%) and promoted a more pronounced osteogenic differentiation, as indicated by increased alkaline phosphatase (ALP) activity and osteocalcin (OCN) expression. In vivo studies involving subcutaneous and bone implantation in rodent models demonstrated that collagen-nanoHA cryogels facilitated tissue ingrowth and bone formation.
Annamalai et al. (123) showed the ability of injectable MSC-laden chitosan-collagen microtissues in bridging the cavity of the critical-sized calvarial defect in an animal model of disease. Specifically, these chitosan–collagen biocomposites distinguished for osteoconductivity and also showed to enhance MSCs differentiation as providing a suitable microenvironment for cells in bone repair/integration.
Toosi et al. (124) demonstrated that collagen sponges + PGA fibers characterized by interconnected porous structures show both good biocompatibility and degradability, also improving the initial adhesion, proliferation and osteogenic differentiation activities of BMSCs.
Graphene oxide (GO) can enhance the osteogenic differentiation of MSCs. Liu et al. (125) developed a highly porous aerogel made of GO and type I collagen. Different concentrations of GO were adopted: 0%, 0.05%, 0.1%, and 0.2% w/v). In vitro studies related results proved that 0.1% GO-collagen aerogel exhibited the better biomineralization rate and cell compatibility within the cohort. Specifically, rat bone marrow mesenchymal stem cells were used. Moreover, the better outcomes were observed for this group also in vivo, in rat cranial defect models. Study evidences suggested 0.1% GO-collagen aerogel as promising biocompatible scaffold for bone regeneration and tissue engineering.
Regarding collagen isolation for fabrication of medical devices, the most frequently used sources are porcine skin and bovine skin/tendons. The extraction process involves the use of chemical solutions such as neutral saline solutions or acidic solutions, along with enzymatic methods by employing pepsin, papain, and collagenase (126).
Silk fibroin originates from silkworms, including the Bombyx mori (B. mori) species, which is the dominant source of silk worldwide. Raw silk consists of two fibroin fibers that run parallel to one another and are bound together by a coating of sericin on the outside. After the process of degumming is completed to remove sericin from the raw silk, the resultant fibroin fibers have a shiny and smooth appearance. Fibroin fibers have a combination of desirable physiochemical properties along with exceptionally remarkable mechanical characteristics (such as fracture strain, strength, and toughness), making them exhibit qualities that are beyond those of several synthetic or natural fibers, thus undoubtedly becoming one of the most remarkable natural protein fibers (127). It can be integrated with calcium phosphate bioceramics such as hydroxyapatite (Hap), β-TCP, or calcium sulfate, which are commonly used as grafting materials in clinical applications (128). Additionally, the combination leads to the formation of silk fibroin-based composite scaffolds, which enhance the adhesion, proliferation, and differentiation of cells. Silk fibroin-based biomaterials could also be developed into several material forms, such as films, hydrogels, sponges, 3D structures, and nanoparticles (127).
Wang et al. (129) showed that BMSCs harvested from rabbits subjected to ovariectomy and transplanted in alginate gels back into the same rabbits lead to enhanced bone formation and stiffness.
Sartika et al. (130) demonstrated the ability of pure 3D silk fibroin scaffolds, seeded with human adipose-derived mesenchymal stem cells (hASCs), in promoting osteogenic differentiation of the cells in vitro. Moreover, at six and twelve weeks postimplantation in rat calvarial defect model, histological staining results revealed that the SF-hASCs scaffold was associated with bone extracellular matrix deposition in the defect regions. Immunohistochemical staining confirmed this evidence; osteoblast-related genes (BMP-2, COL1a1, and OCN) were also expressed, suggesting scaffold ability to achieve effective bone remodeling. Silk fibroin proved to be an effective carrier for stem cells, to be used as an osteoconductive bioscaffold for bone tissue engineering.
Alginate is a biopolymer derived from a variety of brown algae. Firstly, the aqueous alkali solution, generally including NaOH, is employed to initially treat the brown algae. Then, the extract undergoes filtration, and the filtrate is combined with calcium chloride to cause the precipitation of alginate. Subsequently, the conversion of the salt into alginic acid can be achieved by subjecting the alginate salt to diluted hydrochloric acid (HCl) treatment. The sodium alginate soluble in water could be obtained after the purifying process (131). Alginate is a linear polysaccharide that includes homopolymeric units of 1,4-linked (-D-mannuronic acid) and (-L-guluronic acid) in its chemical structure. The mannuronic acid block segments exhibit a straight and flexible structure, but the (1→4) connections to guluronic acid form an obstruction around the carboxyl groups. The glucuronic acid block segments contribute to the folded and rigid structural conformation, which is the main cause of the significant stiffness of the molecular chains. Typically, alginates that have a high concentration of mannuronic acid are often immunogenic. Moreover, in comparison to alginates with a high glucuronic acid content, these alginates are also more effective in stimulating the production of cytokines. Contents in mannuronic and glucuronic acid depend on the source of extraction that, in turn, also influences specific block lengths and thus the properties of the material (126, 132). Alginate is extensively utilized in the biomedical field because of its inherent properties such as simple manipulation and affordability, compatibility with living tissues, exceptional mucosal adhesion, ability to form a gel in situ, and resistance to degrade. Alginate can be used to create various 3D scaffold materials and the properties of these materials, including osteogenic differentiation, biocompatibility, mechanical strength, cell adhesion, and proliferation, can be influenced by factors including the composition, molecular weight (generally ranging from 32 to 400 kDa), purity, and concentration. High molecular weight alginate is preferable for hard tissues tissue engineering (131, 133).
Salem et al. (127) developed a composite scaffold integrating calcium titanate nanoparticles into a 3D-printed alginate scaffold seeded with MSCs. The support showed to significantly enhanced bone regeneration in critical-sized calvarial defects; moreover, it was detected an improved bone mineral density, nearly complete defect closure in micro-CT imaging, and enhanced histological outcomes with new bone and marrow cavity. According to gene expression analysis, it was observed an upregulation of osteogenic and angiogenic markers, together with downregulation of osteoclast-related genes.
Alginate/gelatin (Alg-Gel) hydrogels were prepared by Ferjaoui et al. to be associated with MSCs and guide bone tissue formation (128, 134). Specifically, the behavior (attachment, morphology, proliferation, and osteogenic differentiation) of dental pulp stem cell (DPSC) was assessed. Briefly, the cells showed good adhesion to the hydrogels and proliferation. A good osteogenic activity was obtained with hydrogels made of 8% alginate and 12% gelatin. The study showed that stiffness of Alg-Gel gel can guide osteogenesis in vitro in absence of DPSCs properties alteration.
Chitosan is a deacetylated form of the natural polymer chitin, which is present in the exoskeletons of crustaceans such as shrimp, crabs, and the walls of fungi. Chitin can be converted to chitosan by partial enzymatic or chemical deacetylation; generally, it is regarded as chitosan, chitin with a deacetylation degree that can vary from 30% to 95%. Both chitin and chitosan belong to a family of linear polysaccharides consisting in glucosamine and N-acetyl-D-glucosamine repeated units linked by covalent b-1-4-glucosidic bonds that are disrupted, especially by enzymatic reactions, under biodegradation (135–137). Chitosan is an alkalescent compound that is not soluble in water or organic solvents. However, it can dissolve in acidic solutions with a pH lower than 6.5 by undergoing protonation of its primary amine groups (138, 139). With a molecular weight (MW) of 1.2 × 105 g/mol, it shares a structural similarity to glycosaminoglycan, an essential element of bone matrix and cell surface (140). This similarity allows it to regulate the availability and function of many osteoclastic and osteogenic factors (141, 142). Chitosan displays keynote characteristics including biodegradability, biocompatibility, anti-infection, antioxidant, and hemostatic properties. Moreover, it can deliver medications or antibiotics in the peri-implant site as a drug carrier (143, 144). Both MW and deacetylation degree modulate chitosan physicochemical properties. Typically, the level of deacetylation is directly related to the solubility, viscosity, biocompatibility, mucosal adhesion, as well as antibacterial and hemostatic activities. Simultaneously, the crystallinity and biodegradability of chitosan diminish when the degree of deacetylation is reduced. Furthermore, the biodegradability and antioxidant activity correspond to the molar mass and tridimensional shape (137). Interestingly, chitosan has been regarded as a superior polysaccharide for fabricating bone scaffolds. However, its mechanical strength is reduced due to its linear form, which hinders its effectiveness in bone tissue synthesis. In consideration of this, recurring to crosslinkers (e.g., dextrins, genipins, and purines) is mandatory (142). Additionally, to overcome this limit, chitosan combination with other materials including natural polymers (collagen, alginate, gelatin, silk fibroin) and synthetic polymers (polylactic acid – PLA, polycaprolactone – PCL, poly-(lactic-co-glycolic) acid – PLGA, poly – l – lactic acid – PLLA), ceramics (calcium phosphate ceramic, bioglass ceramic) has been reported (145).
Zang et al. (146) reported about the development of chitosan-based scaffolds combined with bovine-derived xenografts (BDXs). The chitosan/BDX (mass of 40:60) scaffolds showed significantly enhanced compressive strength than the chitosan scaffold; moreover, they also induced better cell attachment and promoted more osteogenic differentiation of human jawbone marrow-derived mesenchymal stem cells than the CS scaffold. Moreover, for repairing calvarial bone defects. Showed enhanced compressive strength, (hJBMMSCs). The chitosan/BDX composite scaffold with a mass ratio of 40:60 demonstrated superior bone regeneration capacity in critical-size rat calvarial defects, supporting new bone formation and mature lamellar bone formation 8 weeks postimplantation.
Georgopoulou et al. (147) developed crosslinked chitosan/gelatin (CS:Gel, 40:60%) scaffolds with a gel-like, porous structure. These scaffolds supported strong cell adhesion, infiltration, and proliferation of MC3T3-E1 pre-osteoblasts (after 7 days) and human bone marrow-derived mesenchymal stem cells (BM-MSCs) (after 14 days). Compared to standard tissue culture surfaces, the scaffolds significantly increased collagen secretion and enhanced osteogenic gene expression (RUNX2, ALP, OSC) in BM-MSCs. In vivo implantation in mouse femurs showed extracellular matrix formation and collagen production by fibroblasts with minimal inflammation, indicating good biocompatibility and osteogenic potential.
Hyaluronic acid (HA) is a simple, anionic, and non-sulfated glycosaminoglycan (GAG) made of repeating D-glucuronic acid and N-acetylglucosamine disaccharide units, linked together by β-1,4 and β-1,3 glycosidic bonds (115, 148). Its molecular weight can vary widely, from a few hundred up to 4 million Da, allowing it to retain large amounts of water due to its hydroxyl groups that are negatively charged (115, 149). Using HA with a molecular weight of 100 kDa falls within an acceptable range in the field of biomedical science (150). Differently from other GAGs, HA is synthesized at the cell membrane by hyaluronan synthases (HAS) enzymes and does not attach to a core protein (151).
Whether diluted in a physiological solution, HA has a gel-like, viscoelastic structure; moreover, it can form a complex macromolecular network displaying a viscoelastic behavior in case the high molar mass of HA and high concentrations are used. However, such viscoelastic material suffer from poor mechanical integrity over the long term. To overcome this limit, HA-based hydrogels characterized by tailored mechanical properties, can be prepared recurring to covalent cross-linking (152). HA is rapidly degraded in tissues by hyaluronidase enzymes, which breaks it into smaller fragments. Due to its biophysical and biochemical properties HA is a key component in biological systems with broad biomedical applications (115).
Chiang et al. (153) demonstrated that intra-articular injection of allogenic MSCs with HA gels in rabbits can prevent osteoarthritis progression better than HA alone. It was observed a reduced formation of osteocyte as well as less subchondral bone exposure and cartilage wearing.
Li et al. (154) showed that BM-MSCs and HA together are effective in improving the femoral trochlear and condyle defects as compared to HA alone in a beagle canine model.
Boekel et al. (155) evaluated the potential of ADSCs combined with HA for bone tissue engineering in rats with critical femoral bone defects. Five treatment groups were compared: control (no graft), HA alone, ADSCs alone, ADSCs + HA, and osteoinduced ADSCs + HA. After 23 days, the ADSCs + HA group showed significantly higher bone contact surface and bone surface density than control and HA-only groups; these results were confirmed by histological analyses. As for gene expression study by RT-PCR, no significant differences were observed in collagen type I or osteopontin levels, but osteonectin expression was elevated in the HA and osteoinduced ADSCs + HA groups. The combination of ADSCs with HA (without prior osteoinduction) improves bone regeneration revelaing effective for bone tissue engineering.
Synthetic polymers, compared to natural polymers, may show lower cell attachment, bioactivity, and osteoconductivity but take advantage of several properties that include tunability, design flexibility, and processability (156). These characteristics allow the fabrication of scaffolds whose characteristics can be modulated according to the specific applications. Additionally, they could be mass-produced and have an extended shelf life compared to natural alternatives (2, 156). Coatings such as bioceramic particles may improve surface performances toward bone regeneration, and aliphatic polyesters, including PCL, PDLA, and PLGA, are the most commonly utilized synthetic polymers (116).
PCL is a biodegradable aliphatic semi-crystalline polymer with a melting temperature above body temperature (about 59°C∼64°C). PCL exhibits a rubbery state characterized by exceptional mechanical qualities at physiological temperature, including high toughness, strength, and elasticity, which vary according to its molecular weight. Along with being non-toxic and biocompatible, PCL also has a longer degradation time (2–3 years) in comparison to other polyester materials. Under physiological circumstances, degradation occurs by microorganisms or by hydrolysis of its aliphatic ester linkage under physiological conditions (2). More specifically, whether used as a biomedical device, PCL experiences a degradation in two stages: first, water is responsible for ester linkages rupture by hydrolytic degradation; then, enzymes perform an intracellular degradation (157). The MW of PCL directly affects the properties of derived scaffolds. Specifically, scaffolds composed of low MW PCL have more hydrophilic and harder surfaces, as well as better mechanical properties in comparison with scaffolds composed of higher MW PCL. These characteristics also contribute to enhanced proliferation and osteogenic differentiation of cells (158). PCL has the potential for load-bearing applications (159).
Xue et al. (160) explored the potential of PCL nanofiber scaffolds in supporting stem cell-based bone regeneration. To this purpose, human MSCs of different origins (umbilical cord, bone marrow and adipose tissue) were cultured on PCL scaffolds. The PCL nanofibers were effective in supporting MSC adhesion, proliferation, and long-term viability. Interestingly, PCL scaffolds significantly enhanced osteogenic differentiation in all MSC types, with bone marrow-derived MSCs showing the strongest effect.
Xu et al. (161) combined 3D-printed polycaprolactone (PCL) scaffolds with BMSCs and self-assembling peptides (SAPs), aiming to enhance both bone regeneration and vascularization. In accordance with in vitro evidences, the PCL/SAP scaffolds improved BMSC proliferation and osteogenesis compared to PCL alone. In vivo (8-weeks implantation), the PCL/BMSC/SAP scaffolds led to significantly greater bone regeneration and neovascularization than PCL or PCL/BMSC controls.
Polylactic acid is thermoplastic aliphatic polyester that is biodegradable and hydrophobic. Its precursors, which means the lactic acid monomers or lactides, are commonly generated through fermenting renewable agricultural supplies (162). In consideration of the chiral nature of lactic acid, showing two asymmetric centers, it can form three different conformations of isomers (L-PLA, D-PLA, D, L-PLA); thus, different structures with different properties can be prepared. PLA and its isomers have attracted considerable interest in the manufacture of medical implants due to their favorable biological compatibility and mechanical properties. Furthermore, due to gradual degradation and high strength they are suitable for supportive structures and load-bearing constructs since they gradually transfer the load to the adjacent tissue while the damaged part is healing (163).
Bahraminasab et al. (164) considered the in vivo healing of critical-sized bone defects in rat calvaria by means of 3D-printed PLA scaffolds, both cell-free and seeded with BMSCs. Histological analysis developed at 8 and 12 weeks post-implantation showed that the two scaffold types were able to significantly enhance healing versus empty controls. Specifically, it was observed the presence of new bone and connective tissue at the defect sites, with the most substantial bone formation and maturation in the stem cell-seeded group at 12 weeks.
Poly-(lactic-co-glycolic) acid is a synthetic linear copolymer and it is possible to obtain various types of PLGA by changing the proportion of lactide acid (LA) to glycolide acid (GA) during the polymerization process (134). As a substitute material for bone, PLGA biodegradability is a fundamental characteristic to consider; in fact, it directly correlates to bone regeneration. Specifically, the LA: GA ratio, monomer order, but also end groups have a role in PLGA devices’ degradation rate, while the MW and the transition temperature value affect its degradation rate (165). The clinical usage of pure PLGA for fabricating bone scaffolds is hindered by its lack of osteoconductivity and inadequate mechanical characteristics in bearing loads. Thus, PLGA is frequently employed with other substances like ceramics or bioactive glass, or it can be properly modified to be more biomimetic and capable of bone tissue engineering (134).
Bhuiyan et al. (166) reported about a novel nano-hydroxyapatite-poly(D,L-lactide-co-glycolide)-collagen biomaterial (nHAP-PLGA-collagen) with mechanical properties similar to that of human cancellous bone. To assess nHAP-PLGA-collagen bone-forming potential, hMSCs were seeded on 2D films and 3D porous scaffolds. Experimental evidence in 2D showed that hMSCs proliferated, formed mineralized nodules, and displayed high ALP activity, suggesting osteogenic differentiation. In 3D scaffolds, hMSCs migrated, filled the porous network, and over 35 days expressed ALP, osteocalcin, and deposited bone-like minerals, without any adipogenic/chondrogenic differentiation. These results highlighted the scaffold selectivity in supporting osteogenesis, revealing it as a promising candidate for bone regeneration.
4.2 Bioactive ceramics
Ceramics are formed by applying heat or heat with pressure to a mixture of at least one metal and a non-metallic solid or a non-metal, or a combination of at least two non-metallic solids. They are characterized by great mechanical strength, strong biological compatibility, and minimal biodegradability, which typically makes them unsuitable for tissue engineering applications. However, ceramic or ceramic derivatives are widely utilized in bone regeneration because of their osteoconductive capacity. To address the issue of low biodegradability, introducing porosity with interconnected pores into the derived scaffolds is a key strategy. This porosity not only enhances biodegradability but also stimulates tissue ingrowth. (167). Due to the absence of protein content, there have been no reports of immunological responses, foreign body reactions, or systemic toxicities associated with their use (168).
The calcium salts of orthophosphoric acid, named calcium phosphates, are able to generate compounds that are composed of H2PO4–, HPO32–, or PO43–. Tricalcium phosphate (Ca3(PO4)2 and hydroxyapatite Ca10(PO4)6(OH)2 are the two main biologically relevant calcium phosphate salts for bone (169). The properties of calcium phosphates have a significant impact on bioactivity, particularly in terms of their ability to promote adhesion, proliferation, and osteogenesis in osteoblasts. To exhibit these bioactive features, degradation and ion release in calcium phosphates are important. These events increase the local concentration of calcium and phosphate ions and stimulate bone minerals formation on calcium phosphates surface (170). Derived biomaterials have gained wide attention by virtue of their excellent biocompatibility, bioactivity, and similarity to bone mineral components (171).
Interestingly, ceramics can be combined with PLA-based polymers to form composites that attract extensive attention for their potential to link the customizable degradability and efficient release properties of polymers with the osteoconductivity and sustained release features of ceramics (167). Certainly, the ideal properties of a ceramic composite are: (i) a certain biodegradation rate to assure bone remodeling; (ii) microporosity to support cell ingrowth; (iii) mechanical stability/ease of handling; (iv) osteoconductivity; (v) growth factors/cells delivery.
Gendviliene et al. (172) evaluated 3D-printed PLA/HA-based scaffolds for bone regeneration using a critical-size calvarial defect model in Wistar rats. The authors compared three groups consisting in: negative and Bio-Oss® controls, PLA and PLA/HA scaffolds, and PLA/HA scaffolds improved with dental pulp stem cells or ECM. After 8 weeks, analyses based on micro-CT and histology highlighted that PLA/HA ECM scaffolds were able to guarantee outcomes in terms of bone regeneration comparable to that of Bio-Oss®. Notably, the PLA-only group was associated with marked inflammatory reactions during degradation. Overall, study results suggest PLA/HA ECM scaffolds as promising bone graft alternatives, suggesting further research on ECM effects and material ratios.
4.3 Biodegradable metals
During the last 20 years, biodegradable metallic materials have been broadly investigated as promising candidates for the repair of bone tissue because of their ability to degrade naturally over time. Biodegradable metals are materials designed to corrode slowly inside the organism. This corrosion releases products that can be absorbed or processed by cells and tissues. Eventually, the metal completely dissolves, leaving no residue behind (173).
The development of biodegradable metals has focused on iron (Fe), magnesium (Mg), and zinc (Zn), as well as their alloys or composites. Their use in orthopedic surgeries can reduce the issues related to second surgeries for non-degradable metallic implant removal (174, 175).
Fe-based biodegradable materials are highly regarded due to their inner porous structure, exceptional mechanical properties, adaptable shape, biocompatibility, and ability to degrade without releasing hydrogen. In addition, iron and its alloys demonstrate considerable mechanical strength. However, the rate at which they degrade is inadequate to keep up with the bones growth speed (176, 177).
Yang et al. (178) reported about 3D-printed Fe scaffolds with HA nanocoating. The supports showed a precise macropore architecture and a compressive strength within the natural bone range. Overall, scaffold characteristics showed to significantly enhance cell viability, ALP activity, and osteogenic differentiation of rabbit bone marrow stem cells, suggesting 3D-printed, HA-coated Fe scaffolds as promising supports for bone tissue engineering.
Magnesium and its alloys are recognized for their exceptional mechanical characteristics, ability to degrade naturally, and compatibility with living organisms; besides, magnesium alloys have an elastic behavior comparable to that of human bone, making them capable of temporary implants. Within the biological environment, magnesium alloys used for bone replacement could be completely degraded and they can be gradually substituted by newly regenerated tissue without second-surgery requirements. This feature makes them suitable for metallic implants utilized in bone regeneration treatment when the therapy needs transient reinforcement. Unfortunately, devices utilizing magnesium alloys suffer from rapid degradation in vivo, resulting in a progressive decline in mechanical capabilities. Additionally, these devices emit harmful by-products because of side reactions and accumulation of corrosion (177).
Lumbikananda et al. (179) investigated the effects of magnesium chloride (MgCl2) on the proliferation and osteogenic differentiation of human periodontal ligament stem cells highlighting that low concentrations of MgCl2 (from 0.1 to 1 mM) significantly enhanced cell proliferation, colony formation as well as osteogenic differentiation; conversely, higher concentrations (> 10 mM) were cytotoxic. Osteogenic stimulation led to mineralized nodule formation, increased ALP activity, and osteogenic genes upregulation. Overall, 0.1 mM MgCl2 was identified as the optimal concentration to support human periodontal ligament stem cells SC function with potential for periodontal and alveolar bone regeneration.
Zinc is an essential microelement of human bodies with the properties of tolerable rates of corrosion and biocompatibility, which makes it appropriate for orthopedic applications. It has been reported to enhance bone repair by promoting cell proliferation, osteogenic differentiation of BMMSCs, formation of vessels, and inhibition of osteoclast differentiation. Moreover, materials with zinc can also play anti-bacterial activity due to bacteria wall damage following reactive oxygen species (ROS) production (180–182). Nevertheless, an excessive amount of zinc in the body can have adverse consequences, such as impairing normal growth and leading to anemia by disrupting iron absorption. Pure zinc has enough mechanical strength in scaffolds. However, pure zinc has some limitations, including lower corrosion rates in vivo and relatively inferior mechanical characteristics. Although there are zinc-based biomaterials specifically designed for extended durability to employ in orthopedic surgery, the excessive release of Zn2+ during breakdown in the body might have cytotoxic effects and hamper bone integration (177).
Yusa et al. (183) showed that zinc-modified titanium (Zn-Ti) surfaces significantly enhance osteogenic differentiation of human dental pulp stem cells (DPSCs) which showed increased expression of osteoblast-related genes (COL I, BMP2, ALP, Runx2, OPN, and VEGF A), along with higher ALP activity and protein expression. Furtherly, Alizarin Red S staining confirmed enhanced ECM mineralization on Zn-Ti versus controls. Zn-Ti surfaces distinguished for their ability to promote osteogenesis in DPSCs, representing an appealing strategy to promote bone regeneration.
5 Bone regeneration by MSCs: an overview on Human Clinical Trials
Current clinical studies recurring to MSCs embedded in scaffolds for bone regeneration were reported in a systematic review by Theodosaki et al., (184) (Table 2). The authors analyzed 14 clinical trials which involved 138 patients suffering from various bone defects which were treated by stem cells combined with scaffolds materials. Different MSCs sources were considered and selected because of their differentiation potential into osteoblasts, ease of harvest, and proximity to the defect site (in accordance with the principle of site-specific tissue repair). These included: bone marrow-derived MSCs (BMMSCs) (from the iliac crest and alveolar bone); dental-origin MSCs such as periodontal ligament stem cells (PDLSCs) and dental pulp stem cells (DPMSCs and DDPSCs); and adipose-derived MSCs, from buccal fat pads (BFSCs) or abdominal tissue (ADMSCs). Preliminarily, all MSCs used were cultured ex vivo, expanded to 105–107 cells per graft, and characterized for specific surface markers, in accordance with the International Society for Cellular Therapy (ISCT) guidelines.
TABLE 2
| Study | Study design | Type of defect | Number of patients | Gender male/female | Age | Type of mesen- chymal stem cell | Type of scaffold | Control group | Inter- vention group | Follow up | Adverse events | Outcomes | Conclusion |
| Apatzidou et al. (194) NCT02449005 Greece |
RCT | Intrabony periodontal defect | 27 | 9/18 | 20–68 | a-BMMSCs | Collagen fleece (Parasorb®) | GB: collagen fleece + aFPL GC: MAF |
GA: Collagen fleece + aFPL + 5 × 106a-BMMSCs/0.5 cm3 | 6 weeks 3,6,9,12 months 3 years |
Not observed | EHI, safety, CAL, PD, recession, radiographic bone fill (BF) | All variables howed significant clinical improvement with no statistical difference between the groups. Greater radiographic improvement in GA-GC/GB |
| Chen et al. (195) NCT01357785 China |
RCT | Intrabony periodontal defect | 30 (48intra-bony defects) | 18–65 | PDLSCs | Bone xenograft BioOss® |
Graft + GBR | Graft + PDLSCs + GBR | 2 weeks, 3,6,12 months | Moderate swelling and pain in some patients | Safety, blood tests, BF CAL, PD, GR | X-ray filling of bone lesions was observed in both groups, with no sta-tistically significant difference between the groups. The increase was pro- portional to time. | |
| Hernandez-Mondaraz et al. (196) ISRCTN12831118 Mexico |
RCT | Intrabony periodontal defect | 22 | 14/7 | 59.4 ± 5.19 (55–64) years |
DPSCs | Lyophilized PVP Sponge (clg-PVP®) |
Collagen sponge + colla- gen membrane (biomed extend) | Collagen sponge + 5 × 106 DPMSCs + collagen membrane (biomed extend) | 6 months | Pain con-trolled with painkillers | PD, tooth mobility, bone density (HU), antioxidant and interleukin levels (TAS, SOD, LPO, IL) | The increase in bone density was almost twice as high in the intervention group, with no statistically significant differ- ence between the groups. |
| Khojasteh et al. (197) NCT02859025 Iran |
RCT | Alveolar cleft | 10 | 3 adults, 7 children (8–14 years old) | BFSCs (buccal fat pad MSCs) | NBBM (natural bovine bone mineral) Cerabone® | Iliac crest bone graft + collagen membrane | 10>6 BFSCs + 2 ml NBBM + LRCP/Iliac crest bone + collagen membrane | Every 2 weeks, 6 months | There was a partial dehiscence in one patient and partial exposure of the graft site | Soft tissue healing, volume of bone filling radio- graphically | An increase in newly formed bone was observed in all 3 groups, with the BFSCs + iliac bone group showing the largest increase, with no statistically significant difference | |
| Sanchez et al. (198) EudraCT 2013-00435-77 Japan | Quasi RCT intrabony | Intrabony periodontal defect | 20 | 14/16 | 25–70 years | PDLSCs | Bone xenograft with collagen BioOSS-Collagen | XBS | 1 × 107 PDLSCs + 100 mg XBS | 6,12 months | Mild, moderate pain and swelling. Physiological closure of the lesion | PD, CAL, REC, FMPS, FMBS intrasurgically measured size of the lesion, quality of life questionnaire, aesthetic result assessment | An improvement in periodontal markers was observed in all 2 groups, with no statistically significant differ-ence between the groups. |
| Akhlaghi et al. (199) Iran | CCT | Alveolar bone defect | 9 | 3/6 | 25,87 years (19,53) |
BFSCs | HAM (human amniotic membrane) | Iliac crest bone graft + NBBM + HAM | Iliac crest bone graft + NBBM + HAM + BFSCs | 5 months | Not observed | Clinical healing, radiographic deficit filling, the feasibility of placing implants | Greater bone healing was observed vertically and horizontally in the intervention group without statistical significance |
| Ismail et al. (200) NCT01626625 Indonesia |
CCT | Non-union of long bone fractures | 10 | 8/2 | 7–72 years | BMMSCs | HA (hydroxya- patite) | Iliac crest bone graft | 14–18 × 106 BMMSCs + HA | 1–12 months | Not observed | Assessment of pain, LEFS + DASH to assess functionality, radiographic healing of fracture with Lane-Sandee, Tiedelman | Faster healing b3 months was observed in the intervention group, at 1 year the differences were assimilated between the groups. |
| Khojasteh et al. (201) Iran |
CCT | Alveolar bone defect | 8 | 5/3 | 38,91 years | BFPSCs (buccal fat pad MSCs) | FDBA (freeze-dried bone allograft SureOss) | Autologous iliac crest bone graft + FDBA | Autologous iliac crest bone graft + FDBA + 1 × 105 BFPSCs | Every 2 weeks, 5 months | No inflammation of a foreign body was observed | Soft tissue healing, X-ray change in bone width, histological % of new bone | A greater increase in bone thickness was observed in the intervention group radiographically, as well as a greater percentage of new bone histologically. |
| Šponer et al. (202) EudraCT2012-005599-33 Czech Republic |
CCT | Femoral bone defect (hiparthroplasty) | 37 | 15/22 | 44–76 years | BMMSCs | Tricalcium phosphate (β-TCP Vitoss®) | (B9) B -TCP (C9) sponge allograft | (A19) 15 + −4.5 × 106 MSC + β-TCP | 6 weeks, 3,6,12 months |
Not observed | Hip Harris score to assess pain and function, bone healing according to Gie guidelines | Integration of the graft into the intervention group was observed at 6 months and trabecular bone formation at 12 months. There was a significant statistical difference only between group C to B. |
| Gjerde et al. (203) NCT02751125 EudraCT2012-003139-50 Norway |
Single arm | Alveolar bone defect | 11(13), 14 sides | 4/7 | 52–75 years average age is 65 | BMMSCs | Calcium phosphate biomaterial BCP (HA 20%, β-TCP 80%)-MBCP + ® | 20 × 106 MSCs/1 cm3 + BCP + regenerative membrane (PTFE) |
6 months | Not observed | Radiological bone deficit filling, histomor- phometric factors of bone filling, feasibility of placement and osseointegration of implants | An increase in keratinized tissues was observed. An increase in bone was observed both in thickness and volume. Histologi- cally, integration of BCP granules and formation of new bone were observed. Finally, the stability of the implants (ostell values) was increased. | |
| Gomez-Barrena et al. (204) ORTHO-1 NCT01842477 Spain France Germany Italy | Single arm | Non-union of long bone fractures | 28(30) | 15/13 | 39 ± 13 years | BMMSCs | Calcium phosphate biomaterial BCP (HA 20%, β-TCP 80%)-MBCP + ® | 3,6,12 months, with reporting of adverse reactions | 19 mild to moderate adverse reactions not related to the interven- tion were observed | X-ray evaluation of fracture healing, Pain reduction (VAS scale) | Gradual healing of fractures was observed, where in 1 year there was complete healing in 92.8% of patients. Healing was delayed in smokers at 6 and 12 months, and to a small extent in tibia fractures. The sex and time since the initial fracture did not affect healing. | ||
| Relondo et al. (205) NCT01389661 EudraCT2010-024246-30 Spain | Single arm | Maxillary cyst | 9(11) | 2/7 | 36 ± 14 years | a-BMMSCs | 3D BioMax serum autologous cross-linked serum- scaffold matrix | 2 weeks, 3–4, 6–8 months | Not observed | Clinical assessment of healing, radiographic increase in bone density (HU) | An increase in bone density was observed in all lesions. | ||
| Takedashi et al. (206) UMIN000007698 Japan | Single arm | Intrabony periodontal defect | 12 | 2/10 | 53,25 ± 9,15 ετών(43–72) | ADSCs | Fibrin gel Beriplast ® | 3,6,9 months | Transient pain, poolitis and dental sensitivity, delayed wound healing | PD, CAL, BOP, GI alveolar bone growth rate, bone filling rate | There was an improvement in periodontal markers as well as the creation of a new alveolar bone, proportionally increasing over time. | ||
| Tanikawa et al. (207) NCT01932164 Brazil | Single arm with historical control | Alveolar cleft | 6 | 3/3 | 10,16 ετών (8–12) | DPSCs | Hydroxy- apatite + collagen sponge (Bio-Oss collagen®) | G1: sponge + rhBMP2 G2: iliac crest bone graft |
1 × 106 DPSCs + HA and collagen sponge | 1,2,3 weeks, 6,12 months | Not observed | Clinical side effects, duration of hospitalization, radiographic deficit filling, tooth eruption. | Satisfactory bone regeneration and tooth eruption (66.7%) and reduced morbidity compared to the control groups. |
Human clinical trials.
RCT, Randomized clinical trial; CCT, Controlled clinical trial (non-randomized clinical trial); 3aFPL, autologous fibrin/platelet lysate; MAF, Minimally invasive flap; EHI, Early healing index; CAL, Clinical attachment level; PD, pocket depth; BF, bone fill; REC, recession; GBR, Guided bone regeneration; FMPS, full-mouth plaque score; FMBS, full-mouth bleeding score; OHIP-14, Oral Health Impact Profile-14; VAS, Visual analog scale; LEFS, Lower extremity functionality scale; DASH, Disabilities of the arms, shoulder and hand score; LRCP, Lateral ramus cortical bone; rhBMP2, recombinant human bone morphogenetic protein-2. [Adapted with permission from Theodosaki et al. (184)].
Regarding the clinical conditions addressed, these included: infrabony periodontal defects, alveolar ridge atrophy, alveolar clefts, cystic bone defects of the maxilla, and non-unions of long bones. Different scaffolds were focused, with different compositions which included both natural materials (i.e., collagen sponges and human amniotic membranes) synthetic biomaterials (β-TCP and HA), and hybrid constructs, some of which incorporated platelet lysates or were pre-treated in osteogenic media before implantation.
In terms of trial design, are reported: randomized controlled trials (RCTs), controlled clinical trials (CCTs) and single-arm studies; the follow-up varies from 3 months to 3 years. Briefly, all the studies lead to successful bone regeneration with significant clinical and radiographic improvements; incidence of adverse events was low, often mild and procedure-related (e.g., transient swelling or pain). Interestingly, the combination of MSCs with scaffolds is associated with outcomes that are comparable or superior to that of traditional bone grafting. Moreover, improved bone density, better soft tissue healing, and eventual improved quality of life or implant integration were observed. However, the authors recognize the early phase of many of these studies, where results suffer from a limited sample sizes and a high methodological heterogeneity, limiting the generalizability of the results. Certainly, there is need for more standardized, large-scale, multicenter clinical trials characterized by rigorous study designs in order to validate the efficacy/safety/cost-effectiveness of MSC-based therapies addressing bone regeneration.
6 Conclusions and Perspectives
To date, considering the great impact of bone defects management in clinical practice and the shortcomings of autologous and allogeneic bone grafting therapies, such as such as donor site morbidity, high costs, and limited graft size, there is an urgent need for innovative approaches in bone regeneration (87). In recent years, together with advances in bone biology research and growing knowledge of biomaterials, stem cells have proved their attractive potential in bone tissue engineering. This is owing to their capacity to rapidly proliferate, differentiate into multiple lineages, and their low tendency toward senescence. According to tissue engineering principles, the ideal bone scaffold should possess features comparable to those of autograft bone, such as biological safety, long-term viability, and osteogenic and angiogenic properties, while avoiding the constraints inherent to autografts, such as donor site morbidity, high costs, and size limitations (65). Current research has made considerable progress in exploring the types of seed cells and the therapeutic ideas based on stem cell derivatives. However, challenges persist in achieving consistent vascularization in large bone defects as well as in ensuring adequate mechanical properties that match those of native bone, particularly in load-bearing applications (185). In this context, induced tissue-specific stem cells (iTSCs) have emerged as a promising, safer alternative to iPSCs. These cells, generated via transient expression of OSKM factors (OSKM refers to: Oct4, Sox2, Klf4, and c-Myc) from somatic sources such as ADSCs, exist in an intermediate reprogramming state and, interestingly, they do not form teratomas once transplanted, unlike fully reprogrammed pluripotent stem cells. Notably, human iTSCs (hiTSCs) generated from aged ADSCs by a synthetic self-replicating RNA vector retain the ability to proliferate for multiple passages and differentiate into osteoblasts, while expressing markers consistent with their tissue of origin. Their stable expansion, tissue-specific memory, and multipotent differentiation capacity lead the researchers to recognize the iTSCs as a valuable and practical tool for cell-based bone regeneration therapies (186).
Future research is needed to better understand the processes behind stem cell-mediated bone regeneration and fracture repair, as well as the interactive mechanisms between scaffolding materials and seed cells. At present, there is still a lack of reports about the therapeutic use of stem cells and their derivatives in bone tissue engineering (182). Additionally, to date there is increasing evidence reporting about the risk of immune response following allogeneic MSCs implantation, with consequent reduction of their associated therapeutic potential (187). Despite the initial belief that MSCs are immune privileged, several evidence highlight that allogeneic MSC (allo-MSC) therapies are prone to immune rejection. Once transplanted, allo-MSCs often exhibit poor survival and limited engraftment because of recognition and removal by the host immune system. Moreover, experimental evidence have highlighted that allo-MSCs can shift from an immunosuppressive to an immunogenic state in response to environmental cues including inflammation, with consequent activation of both innate and adaptive immune responses. This includes the involvement of CD8+ and CD4+ cytotoxic T cells, B cells, natural killer (NK) cells, and particularly macrophages, which are involved into rejection via antibody-dependent mechanisms. Because of the formation of donor-specific antibodies and anti-donor memory responses repeat administrations are difficult, in turn increasing the risk of rapid rejection. Moreover, the tissue from MSCs are isolated can influence their immunogenic profile, with adipose-derived MSCs showing higher likelihood of inducing HLA class I-specific antibodies versus bone marrow-derived cells. Because of these challenges associated with the immune response, the therapeutic effectiveness of allo-MSCs is limited in turn highlighting the need for strategies to mitigate immune rejection in MSC-based therapies (188). Acellular strategies, including EVs derived from stem cells, offer promising cell-free alternatives with lower immunogenic risks and improved storage and handling potential (189, 190). In addition, as carriers of stem cells and growth factors, as well as providers of 3D structure for implants, the material selection and structural design of the scaffolds have been extensively studied. Unfortunately, this process still primarily employs the trial-and-error method to obtain ideal scaffolds, which is time-consuming and costly (182). This highlights the need for standardized and predictive scaffold design strategies that can accelerate development while reducing variability. Ideally, next-generation scaffolds may incorporate responsive or “smart” biomaterials capable of sensing and reacting to mechanical, biochemical, or thermal stimuli, thereby adapting to the in vivo environment and improving regenerative efficacy. Advances in material sciences, such as hybrid composites with tunable degradation profiles are also contributing to progress in the field (191). In the future, along with the design and manufacture of more desirable bone tissue engineering implants, research methodologies are expected to make significant advances. One of the notable developments will be the application of artificial intelligence in bone tissue engineering. For instance, researchers can use machine learning to model the optimal morphology of scaffolds and the bioactivity of materials to facilitate scaffold design and optimization (192); computational modeling can also be used to predict the response of cells and tissues to a variety of stimuli, helping researchers to better understand the underlying mechanisms of stem cells in bone regeneration (193). These approaches, combined with bioreactor-based tissue conditioning and immune-informed scaffold design, may significantly improve integration and performance of engineered constructs. Greater attention will be devoted to the practical application of stem cells and their derivatives in the medical treatment of bone defects. To ensure successful clinical translation, future efforts must also address methods standardization, large-animal preclinical validation, and long-term safety. Ultimately, integrating stem cell therapy within the broader context of precision medicine - taking advantage from immune modulation, gene editing technologies, and converging advances in bioengineering - will pave the way for more personalized and effective regenerative therapies (185). Certainly, the ideal solution to bone defects appears to be a combination of a biomaterial scaffold, cell biology approaches, and growth factors, within an optimized mechanical environment (190).
Statements
Author contributions
MJ: Methodology, Writing – original draft, Investigation, Writing – review and editing, Conceptualization. TS: Investigation, Conceptualization, Writing – review and editing, Writing – original draft. ZZ: Writing – review and editing, Writing – original draft. YW: Writing – review and editing, Conceptualization, Investigation, Supervision. LY: Conceptualization, Writing – original draft, Investigation. JS: Writing – review and editing. RC: Writing – review and editing. VM: Writing – review and editing. AP: Writing – review and editing. ES: Conceptualization, Writing – original draft, Writing – review and editing. CJ: Writing – review and editing, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for publication of this article. Open Access funding provided by University of Padua, Open Science Committee.
Acknowledgments
Figure 1 is drawn using biorender software, license number is TL28JZGQVS.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ECM, Extracellular matrix; MSCs, Mesenchymal stromal cells; ISCT, International Society for Cellular Therapy; BMMSCs, Bone marrow mesenchymal stromal cells; TCP, Tricalcium phosphate; VEGF, Vascular Endothelial Growth Factor; ADSCs, Adipose-derived mesenchymal stromal cells; PLGA, Poly-(lactic-co-glycolic acid); BMP-2, Bone morphogenetic protein-2; IFP, Infrapatellar fat pad; DMSCs, Dental-derived mesenchymal stromal cells; SHEDs, Stromal cells from human exfoliated deciduous teeth; ESCs, Embryonic stem cells; MCM, Mineralized collagen matrix; SCID, Severe Combined Immunodeficient; IPSCs, Induced pluripotent stem cells; EVs, Extracellular vesicles; SFE, Maxillary sinus floor elevation; MW, Molecular weight; Runx2, Runt-related transcription factor 2; BSP, Bone sialoprotein; OCN, Osteocalcin; OSX, Osterix; Col1a1, Collagen Type I Alpha 1 Chain; ALP, Alkaline phosphatase.
References
1.
Xue N Ding X Huang R Jiang R Huang H Pan X et al Bone tissue engineering in the treatment of bone defects. Pharmaceuticals. (2022) 15:879. 10.3390/ph15070879
2.
Dwivedi R Kumar S Pandey R Mahajan A Nandana D Katti D et al Polycaprolactone as biomaterial for bone scaffolds: review of literature. J Oral Biol Craniofac Res. (2020) 10:381–8. 10.1016/j.jobcr.2019.10.003
3.
Ferreira A Gentile P Chiono V Ciardelli G . Collagen for bone tissue regeneration.Acta Biomater. (2012) 8:3191–200. 10.1016/j.actbio.2012.06.014
4.
Tavoni M Dapporto M Tampieri A Sprio S . Bioactive calcium phosphate-based composites for bone regeneration.J Composites Sci. (2021) 5:227. 10.3390/jcs5090227
5.
Moradi S Golchin A Hajishafieeha Z Khani M Ardeshirylajimi A . Bone tissue engineering: adult stem cells in combination with electrospun nanofibrous scaffolds.J Cell Physiol. (2018) 233:6509–22. 10.1002/jcp.26606
6.
Atala A . Tissue engineering, stem cells and cloning: current concepts and changing trends.Expert Opin Biol Ther. (2005) 5:879–92. 10.1517/14712598.5.7.879
7.
Johnell O Kanis J . An estimate of the worldwide prevalence and disability associated with osteoporotic fractures.Osteoporos Int. (2006) 17:1726–33. 10.1007/s00198-006-0172-4
8.
Yousefi A James P Akbarzadeh R Subramanian A Flavin C Oudadesse H . Prospect of stem cells in bone tissue engineering: a review.Stem Cells Int. (2016) 2016:6180487. 10.1155/2016/6180487
9.
Wang W Yeung K . Bone grafts and biomaterials substitutes for bone defect repair: a review.Bioact Mater. (2017) 2:224–47. 10.1016/j.bioactmat.2017.05.007
10.
Storti G Scioli M Kim B Orlandi A Cervelli V . Adipose-derived stem cells in bone tissue engineering: useful tools with new applications.Stem Cells Int. (2019) 2019:3673857. 10.1155/2019/3673857
11.
Schmidt A . Autologous bone graft: is it still the gold standard?Injury. (2021) 52:S18–22. 10.1016/j.injury.2021.01.043
12.
Amini A Laurencin C Nukavarapu S . Bone tissue engineering: recent advances and challenges.Crit Rev Biomed Eng. (2012) 40:363–408. 10.1615/critrevbiomedeng.v40.i5.10
13.
Calori G Mazza E Colombo M Ripamonti C . The use of bone-graft substitutes in large bone defects: any specific needs?Injury. (2011) 42:S56–63. 10.1016/j.injury.2011.06.011
14.
Codrea C Croitoru A Baciu C Melinescu A Ficai D Fruth V et al Advances in Osteoporotic Bone Tissue Engineering. J Clin Med. (2021) 10:253. 10.3390/jcm10020253
15.
Pereira H Cengiz I Silva F Reis R Oliveira J . Scaffolds and coatings for bone regeneration.J Mater Sci Mater Med. (2020) 31:1–16. 10.1007/s10856-020-06364-y
16.
Berthiaume F Maguire T Yarmush M . Tissue engineering and regenerative medicine: history, progress, and challenges.Annu Rev Chem Biomol Eng. (2011) 2:403–30. 10.1146/annurev-chembioeng-061010-114257
17.
Grayson W Bunnell B Martin E Frazier T Hung B Gimble J . Stromal cells and stem cells in clinical bone regeneration.Nat Rev Endocrinol. (2015) 11:140–50. 10.1038/nrendo.2014.234
18.
McGue C Mañón V Viet C . Advances in tissue engineering and implications for oral and maxillofacial reconstruction.J Calif Dent Assoc. (2021) 49:685–94.
19.
Beane O Fonseca V Cooper L Koren G Darling E . Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells.PLoS One. (2014) 9:e115963. 10.1371/journal.pone.0115963
20.
Winning L El Karim I Lundy F . A comparative analysis of the osteogenic potential of dental mesenchymal stem cells.Stem Cells Dev. (2019) 28:1050–8. 10.1089/scd.2019.0023
21.
Zhang Z Teoh S Chong M Schantz J Fisk N Choolani M et al Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells. (2009) 27:126–37. 10.1634/stemcells.2008-0456
22.
Hayashi O Katsube Y Hirose M Ohgushi H Ito H . Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue.Calcif Tissue Int. (2008) 82:238–47. 10.1007/s00223-008-9112-y
23.
Saranya K Raja S Subhasree R Gnanamani A Das S Rajendran N . Fabrication of nanoporous sodium niobate coating on 316L SS for orthopaedics.Ceramics Int. (2017) 43:11569–79. 10.1016/j.ceramint.2017.05.104
24.
Lee J Jung J Lee H Jeong S Cho K Hwang S et al Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. (2012) 13:219–24. 10.1016/j.intimp.2012.03.024
25.
Aghaloo T Chaichanasakul T Bezouglaia O Kang B Franco R Dry S et al Osteogenic potential of mandibular vs. long-bone marrow stromal cells. J Dent Res. (2010) 89:1293–8. 10.1177/0022034510378427
26.
Zhou W Zhang J Lin K Chen F . Comparison between mandibular and femur derived bone marrow stromal cells: osteogenic and angiogenic potentials in vitro and bone repairing ability in vivo.RSC Adv. (2017) 7:56220–8. 10.1039/C7RA07139F
27.
Akintoye S Lam T Shi S Brahim J Collins M Robey P . Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals.Bone. (2006) 38:758–68. 10.1016/j.bone.2005.10.027
28.
Arinzeh T . Mesenchymal stem cells for bone repair: preclinical studies and potential orthopedic applications.Foot Ankle Clin. (2005) 10:651–65. 10.1016/j.fcl.2005.06.004
29.
Zhang Y Xing Y Jia L Ji Y Zhao B Wen Y et al An in vitro comparative study of multisource derived human mesenchymal stem cells for bone tissue engineering. Stem Cells Dev. (2018) 27:1634–45. 10.1089/scd.2018.0119
30.
Zuk P Zhu M Mizuno H Huang J Futrell J Katz A et al Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. (2001) 7:211–28. 10.1089/107632701300062859
31.
Macchi V Tiengo C Porzionato A Stecco C Vigato E Parenti A et al Histotopographic study of the fibroadipose connective cheek system. Cells Tissues Organs. (2009) 191:47–56. 10.1159/000226276
32.
Shafaei H Kalarestaghi H . Adipose-derived stem cells: an appropriate selection for osteogenic differentiation.J Cell Physiol. (2020) 235:8371–86. 10.1002/jcp.29681
33.
Dmitrieva R Minullina I Bilibina A Tarasova O Anisimov S Zaritskey A . Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: differences and similarities.Cell Cycle. (2012) 11:377–83. 10.4161/cc.11.2.18858
34.
Schäffler A Büchler C . Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies.Stem Cells. (2007) 25:818–27. 10.1634/stemcells.2006-0589
35.
Chen H Lee M Chen C Chuang S Chang L Ho M et al Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. (2012) 16:582–92. 10.1111/j.1582-4934.2011.01335.x
36.
Shi Y Nacamuli R Salim A Longaker M . The osteogenic potential of adipose-derived mesenchymal cells is maintained with aging.Plast Reconstr Surg. (2005) 116:1686–96. 10.1097/01.prs.0000185606.03222.a9
37.
Lendeckel S Jödicke A Christophis P Heidinger K Wolff J Fraser J et al Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. (2004) 32:370–3. 10.1016/j.jcms.2004.06.002
38.
Li W Liu Y Zhang P Tang Y Zhou M Jiang W et al Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl Mater Interfaces. (2018) 10:5240–54. 10.1021/acsami.7b17620
39.
Mesimäki K Lindroos B Törnwall J Mauno J Lindqvist C Kontio R et al Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. (2009) 38:201–9. 10.1016/j.ijom.2009.01.001
40.
Thesleff T Lehtimäki K Niskakangas T Mannerström B Miettinen S Suuronen R et al Cranioplasty with adipose-derived stem cells and biomaterial: a novel method for cranial reconstruction. Neurosurgery. (2011) 68:1535–40. 10.1227/NEU.0b013e31820ee24e
41.
Im G . Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: their comparative efficacies and synergistic effects.J Biomed Mater Res A. (2017) 105:2640–8. 10.1002/jbm.a.36089
42.
Rehman J Traktuev D Li J Merfeld-Clauss S Temm-Grove C Bovenkerk J et al Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. (2004) 109:1292–8. 10.1161/01.CIR.0000121425.42966.F1
43.
Kim Y Kim H Cho H Bae Y Suh K Jung J . Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia.Cell Physiol Biochem. (2007) 20:867–76. 10.1159/000110447
44.
Baer P Geiger H . Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity.Stem Cells Int. (2012) 2012:812693. 10.1155/2012/812693
45.
James A Levi B Nelson E Peng M Commons G Lee M et al Deleterious effects of freezing on osteogenic differentiation of human adipose-derived stromal cells in vitro and in vivo. Stem Cells Dev. (2011) 20:427–39. 10.1089/scd.2010.0082
46.
Patrikoski M Mannerström B Miettinen S . Perspectives for clinical translation of adipose stromal/stem cells.Stem Cells Int. (2019) 2019:5858247. 10.1155/2019/5858247
47.
Przekora A Vandrovcova M Travnickova M Pajorova J Molitor M Ginalska G et al Evaluation of the potential of chitosan/β-1,3-glucan/hydroxyapatite material as a scaffold for living bone graft production in vitro by comparison of ADSC and BMDSC behaviour on its surface. Biomed Mater. (2017) 12:015030. 10.1088/1748-605X/aa56f9
48.
Li B Ouchi T Cao Y Zhao Z Men Y . Dental-derived mesenchymal stem cells: state of the art.Front Cell Dev Biol. (2021) 9:654559. 10.3389/fcell.2021.654559
49.
Laino G d’Aquino R Graziano A Lanza V Carinci F Naro F et al A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. (2005) 20:1394–402. 10.1359/JBMR.050325
50.
Morsczeck C Schmalz G Reichert T Völlner F Galler K Driemel O . Somatic stem cells for regenerative dentistry.Clin Oral Investig. (2008) 12:113–8. 10.1007/s00784-007-0170-8
51.
Kern S Eichler H Stoeve J Klüter H Bieback K . Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue.Stem Cells. (2006) 24:1294–301. 10.1634/stemcells.2005-0342
52.
Li X Bai J Ji X Li R Xuan Y Wang Y . Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation.Int J Mol Med. (2014) 34:695–704. 10.3892/ijmm.2014.1821
53.
Huang G Gronthos S Shi S . Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine.J Dent Res. (2009) 88:792–806. 10.1177/0022034509340867
54.
Mantesso A Sharpe P . Dental stem cells for tooth regeneration and repair.Expert Opin Biol Ther. (2009) 9:1143–54. 10.1517/14712590903103795
55.
Wang X Sha X Li G Yang F Ji K Wen L et al Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol. (2012) 57:1231–40. 10.1016/j.archoralbio.2012.02.014
56.
Tatullo M Falisi G Amantea M Rastelli C Paduano F Marrelli M . Dental pulp stem cells and human periapical cyst mesenchymal stem cells in bone tissue regeneration: comparison of basal and osteogenic differentiated gene expression of a newly discovered mesenchymal stem cell lineage.J Biol Regul Homeost Agents. (2015) 29:713–8.
57.
Nakajima K Kunimatsu R Ando K Ando T Hayashi Y Kihara T et al Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. (2018) 497:876–82. 10.1016/j.bbrc.2018.02.156
58.
Vater C Männel C Bolte J Tian X Goodman S Zwingenberger S . Effectiveness of dental pulp-derived stem cells and bone marrowderived mesenchymal stromal cells implanted into a murine critical bone defect.Curr Stem Cell Res Ther. (2022) 17:480–91. 10.2174/1574888X17666220215100732
59.
Alge D Zhou D Adams L Wyss B Shadday M Woods E et al Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. (2010) 4:73–81. 10.1002/term.220
60.
Zakrzewski W Dobrzyñski M Szymonowicz M Rybak Z . Stem cells: past, present, and future.Stem Cell Res Ther. (2019) 10:1–22. 10.1186/s13287-019-1165-5
61.
Nishikawa S Jakt L Era T . Embryonic stem-cell culture as a tool for developmental cell biology.Nat Rev Mol Cell Biol. (2007) 8:502–7. 10.1038/nrm2189
62.
Bielby R Boccaccini A Polak J Buttery L . In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells.Tissue Eng. (2004) 10:1518–25. 10.1089/ten.2004.10.1518
63.
Trávníčková M Bačáková L . Application of adult mesenchymal stem cells in bone and vascular tissue engineering.Physiol Res. (2018) 67:831–50. 10.33549/physiolres.933820
64.
Ben-David U Benvenisty N . The tumorigenicity of human embryonic and induced pluripotent stem cells.Nat Rev Cancer. (2011) 11:268–77. 10.1038/nrc3034
65.
Shi R Huang Y Ma C Wu C Tian W . Current advances for bone regeneration based on tissue engineering strategies.Front Med. (2019) 13:160–88. 10.1007/s11684-018-0629-9
66.
Asatrian G Pham D Hardy W James A Peault B . Stem cell technology for bone regeneration: current status and potential applications.Stem Cells Cloning. (2015) 8:39–48. 10.2147/SCCAA.S48423
67.
English K Wood K . Immunogenicity of embryonic stem cell-derived progenitors after transplantation.Curr Opin Organ Transplant. (2011) 16:90–5. 10.1097/MOT.0b013e3283424faa
68.
Takahashi K Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors.Cell. (2006) 126:663–76. 10.1016/j.cell.2006.07.024
69.
Takahashi K Tanabe K Ohnuki M Narita M Ichisaka T Tomoda K et al Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. (2007) 131:861–72. 10.1016/j.cell.2007.11.019
70.
Yu J Vodyanik M Smuga-Otto K Antosiewicz-Bourget J Frane J Tian S et al Induced pluripotent stem cell lines derived from human somatic cells. Science. (2007) 318:1917–20. 10.1126/science.1151526
71.
Zhang J Liu Y Chen Y Yuan L Liu H Wang J et al Adipose-derived stem cells: current applications and future directions in the regeneration of multiple tissues. Stem Cells Int. (2020) 2020:8810813. 10.1155/2020/8810813
72.
Medvedev S Shevchenko A Zakian S . Induced pluripotent stem cells: problems and advantages when applying them in regenerative medicine.Acta Naturae. (2010) 2:18–27.
73.
Cowan C Shi Y Aalami O Chou Y Mari C Thomas R et al Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. (2004) 22:560–7. 10.1038/nbt958
74.
Hadzimustafic N D’Elia A Shamoun V Haykal S . Human-Induced Pluripotent Stem Cells in Plastic and Reconstructive Surgery.Int J Mol Sci. (2024) 25:1863. 10.3390/ijms25031863
75.
Peterson S Garitaonandia I Loring J . The tumorigenic potential of pluripotent stem cells: what can we do to minimize it?Bioessays. (2016) 38:S86–95. 10.1002/bies.201670915
76.
Si J Wang X Shen S . Perinatal stem cells: a promising cell resource for tissue engineering of craniofacial bone.World J Stem Cells. (2015) 7:149–59. 10.4252/wjsc.v7.i1.149
77.
Gugliandolo A Fonticoli L Trubiani O Rajan T Marconi G Bramanti P et al Oral bone tissue regeneration: mesenchymal stem cells, secretome, and biomaterials. Int J Mol Sci. (2021) 22:5236. 10.3390/ijms22105236
78.
Verweij F Balaj L Boulanger C Carter D Compeer E D’Angelo G et al The power of imaging to understand extracellular vesicle biology in vivo. Nat Methods. (2021) 18:1013–26. 10.1038/s41592-021-01206-3
79.
Gupta D Liang X Pavlova S Wiklander O Corso G Zhao Y et al Quantification of extracellular vesicles in vitro and in vivo using sensitive bioluminescence imaging. J Extracell Vesicles. (2020) 9:1800222. 10.1080/20013078.2020.1800222
80.
El Andaloussi S Mäger I Breakefield X Wood M . Extracellular vesicles: biology and emerging therapeutic opportunities.Nat Rev Drug Discov. (2013) 12:347–57. 10.1038/nrd3978
81.
Khalyfa A Sanz-Rubio D . The mystery of red blood cells extracellular vesicles in sleep apnea with metabolic dysfunction.Int J Mol Sci. (2021) 22:4301. 10.3390/ijms22094301
82.
Lei F Li M Lin T Zhou H Wang F Su X . Treatment of inflammatory bone loss in periodontitis by stem cell-derived exosomes.Acta Biomater. (2022) 141:333–43. 10.1016/j.actbio.2021.12.035
83.
Welsh J Goberdhan D O’Driscoll L Buzas E Blenkiron C Bussolati B et al Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. (2024) 13:e12404. 10.1002/jev2.12404
84.
Katagiri W Osugi M Kawai T Hibi H . First-in-human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchymal stem cells.Head Face Med. (2016) 12:1–10. 10.1186/s13005-016-0101-5
85.
Bar J Lis-Nawara A Grelewski P . Dental pulp stem cell-derived secretome and its regenerative potential.Int J Mol Sci. (2021) 22:12018. 10.3390/ijms222112018
86.
Luo Z Li F Liu Y Rao S Yin H Huang J et al Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. (2019) 11:20884–92. 10.1039/c9nr02791b
87.
Yang H Cong M Huang W Chen J Zhang M Gu X et al The effect of human bone marrow mesenchymal stem cell-derived exosomes on cartilage repair in rabbits. Stem Cells Int. (2022) 2022:5760107. 10.1155/2022/5760107
88.
Qiao X Tang J Dou L Yang S Sun Y Mao H et al Dental pulp stem cell-derived exosomes regulate anti-inflammatory and osteogenesis in periodontal ligament stem cells and promote the repair of experimental periodontitis in rats. Int J Nanomedicine. (2023) 18:4683–703. 10.2147/IJN.S420967
89.
Zhu Q Tang Y Zhou T Yang L Zhang G Meng Y et al Exosomes derived from mesenchymal stromal cells promote bone regeneration by delivering miR-182-5p-inhibitor. Pharmacol Res. (2023) 192:106798. 10.1016/j.phrs.2023.106798
90.
Liu A Lin D Zhao H Chen L Cai B Lin K et al Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials. (2021) 272:120718. 10.1016/j.biomaterials.2021.120718
91.
Huang M Chen H Peng G Sun H Peng H Li H et al Exosomes from artesunate-treated bone marrow-derived mesenchymal stem cells transferring SNHG7 to promote osteogenesis via TAF15-RUNX2 pathway. Regen Med. (2022) 17:819–33. 10.2217/rme-2022-0065
92.
Liang B Liang J Ding J Xu J Xu J Chai Y . Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway.Stem Cell Res Ther. (2019) 10:1–11. 10.1186/s13287-019-1410-y
93.
Fan L Guan P Xiao C Wen H Wang Q Liu C et al Exosome-functionalized polyetheretherketone-based implant with immunomodulatory property for enhancing osseointegration. Bioact Mater. (2021) 6:2754–66. 10.1016/j.bioactmat.2021.02.005
94.
Takeuchi R Katagiri W Endo S Kobayashi T . Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis.PLoS One. (2019) 14:e0225472. 10.1371/journal.pone.0225472
95.
Zhang Y Hao Z Wang P Xia Y Wu J Xia D et al Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. (2019) 52:e12570. 10.1111/cpr.12570
96.
Zhang Y Xie Y Hao Z Zhou P Wang P Fang S et al Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl Mater Interfaces. (2021) 13:18472–87. 10.1021/acsami.0c22671
97.
Wu J Chen L Wang R Song Z Shen Z Zhao Y et al Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. ACS Biomater Sci Eng. (2019) 5:3561–71. 10.1021/acsbiomaterials.9b00607
98.
Liu T Hu W Zou X Xu J He S Chang L et al Human periodontal ligament stem cell-derived exosomes promote bone regeneration by altering MicroRNA profiles. Stem Cells Int. (2020) 2020:8852307. 10.1155/2020/8852307
99.
Wu D Chang X Tian J Kang L Wu Y Liu J et al Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J Nanobiotechnology. (2021) 19:1–19. 10.1186/s12951-021-00958-6
100.
Lu J Yu N Liu Q Xie Y Zhen L . Periodontal ligament stem cell exosomes key to regulate periodontal regeneration by miR-31-5p in mice model.Int J Nanomedicine. (2023) 18:5327–42. 10.2147/IJN.S409664
101.
Chen S Tang Y Liu Y Zhang P Lv L Zhang X et al Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. (2019) 52:e12669. 10.1111/cpr.12669
102.
Kong L Zhao H Wang F Zhang R Yao X Zuo R et al Endocrine modulation of brain-skeleton axis driven by neural stem cell-derived perilipin 5 in the lipid metabolism homeostasis for bone regeneration. Mol Ther. (2023) 31:1293–312. 10.1016/j.ymthe.2023.02.004
103.
Ma S Li S Zhang Y Nie J Cao J Li A et al BMSC-derived exosomal CircHIPK3 promotes osteogenic differentiation of MC3T3-E1 cells via mitophagy. Int J Mol Sci. (2023) 24:2785. 10.3390/ijms24032785
104.
Brunello G Zanotti F Trentini M Zanolla I Pishavar E Favero V et al Exosomes derived from dental pulp stem cells show different angiogenic and osteogenic properties in relation to the age of the donor. Pharmaceutics. (2022) 14:908. 10.3390/pharmaceutics14050908
105.
Yamada Y Nakamura-Yamada S Umemura-Kubota E Baba S . Diagnostic cytokines and comparative analysis secreted from exfoliated deciduous teeth, dental pulp, and bone marrow derived mesenchymal stem cells for functional cell-based therapy.Int J Mol Sci. (2019) 20:5900. 10.3390/ijms20235900
106.
Shanbhag S Kampleitner C Al-Sharabi N Mohamed-Ahmed S Apaza Alccayhuaman K Heimel P et al Functionalizing collagen membranes with MSC-conditioned media promotes guided bone regeneration in rat calvarial defects. Cells. (2023) 12:767. 10.3390/cells12050767
107.
Shanbhag S Al-Sharabi N Kampleitner C Mohamed-Ahmed S Kristoffersen E Tangl S et al The use of mesenchymal stromal cell secretome to enhance guided bone regeneration in comparison with leukocyte and platelet-rich fibrin. Clin Oral Implants Res. (2024) 35:141–54. 10.1111/clr.14205
108.
Novello S Tricot-Doleux S Novella A Pellen-Mussi P Jeanne S . Influence of periodontal ligament stem cell-derived conditioned medium on osteoblasts.Pharmaceutics. (2022) 14:729. 10.3390/pharmaceutics14040729
109.
Katagiri W Takeuchi R Saito N Suda D Kobayashi T . Migration and phenotype switching of macrophages at early-phase of bone-formation by secretomes from bone marrow derived mesenchymal stem cells using rat calvaria bone defect model.J Dent Sci. (2022) 17:421–9. 10.1016/j.jds.2021.08.012
110.
Yu L Wei M . Biomineralization of collagen-based materials for hard tissue repair.Int J Mol Sci. (2021) 22:944. 10.3390/ijms22020944
111.
Lee S Du X Kim I Ferguson S . Scaffolds for bone-tissue engineering.Matter. (2022) 5:2722–59. 10.1016/j.matt.2022.06.003
112.
Grémare A Guduric V Bareille R Heroguez V Latour S L’heureux N et al Characterization of printed PLA scaffolds for bone tissue engineering. J Biomed Mater Res A. (2018) 106:887–94. 10.1002/jbm.a.36289
113.
Ferraz M . An overview on the big players in bone tissue engineering: biomaterials, scaffolds and cells.Int J Mol Sci. (2024) 25:3836. 10.3390/ijms25073836
114.
Iaquinta M Mazzoni E Bononi I Rotondo J Mazziotta C Montesi M et al Adult stem cells for bone regeneration and repair. Front Cell Dev Biol. (2019) 7:268. 10.3389/fcell.2019.00268
115.
Xing F Zhou C Hui D Du C Wu L Wang L et al Hyaluronic acid as a bioactive component for bone tissue regeneration: fabrication, modification, properties, and biological functions. Nanotechnol Rev. (2020) 9:1059–79. 10.1515/ntrev-2020-0084
116.
Filippi M Born G Chaaban M Scherberich A . Natural polymeric scaffolds in bone regeneration.Front Bioeng Biotechnol. (2020) 8:474. 10.3389/fbioe.2020.00474
117.
Hao Z Song Z Huang J Huang K Panetta A Gu Z et al The scaffold microenvironment for stem cell based bone tissue engineering. Biomater Sci. (2017) 5:1382–92. 10.1039/c7bm00146k
118.
Cen L Liu W Cui L Zhang W Cao Y . Collagen tissue engineering: development of novel biomaterials and applications.Pediatr Res. (2008) 63:492–6. 10.1203/PDR.0b013e31816c5bc3
119.
Zhang D Wu X Chen J Lin K . The development of collagen based composite scaffolds for bone regeneration.Bioact Mater. (2018) 3:129–38. 10.1016/j.bioactmat.2017.08.004
120.
Qin D Wang N You X Zhang A Chen X Liu Y . Collagen-based biocomposites inspired by bone hierarchical structures for advanced bone regeneration: ongoing research and perspectives.Biomater Sci. (2022) 10:318–53. 10.1039/d1bm01294k
121.
Fan L Ren Y Emmert S Vučkoviæ I Stojanovic S Najman S et al The use of collagen-based materials in bone tissue engineering. Int J Mol Sci. (2023) 24:3744. 10.3390/ijms24043744
122.
Salgado C Grenho L Fernandes M Colaço B Monteiro F . Biodegradation, biocompatibility, and osteoconduction evaluation of collagen-nanohydroxyapatite cryogels for bone tissue regeneration.J Biomed Mater Res A. (2016) 104:57–70. 10.1002/jbm.a.35540
123.
Annamalai R Hong X Schott N Tiruchinapally G Levi B Stegemann J . Injectable osteogenic microtissues containing mesenchymal stromal cells conformally fill and repair critical-size defects.Biomaterials. (2019) 208:32–44. 10.1016/j.biomaterials.2019.04.001
124.
Toosi S Naderi-Meshkin H Kalalinia F Peivandi M HosseinKhani H Bahrami A et al PGA-incorporated collagen: toward a biodegradable composite scaffold for bone-tissue engineering. J Biomed Mater Res A. (2016) 104:2020–8. 10.1002/jbm.a.35736
125.
Liu S Zhou C Mou S Li J Zhou M Zeng Y et al Biocompatible graphene oxide-collagen composite aerogel for enhanced stiffness and in situ bone regeneration. Mater Sci Eng C Mater Biol Appl. (2019) 105:110137. 10.1016/j.msec.2019.110137
126.
Hernández-González A Téllez-Jurado L Rodríguez-Lorenzo L . Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: a review.Carbohydr Polym. (2020) 229:115514. 10.1016/j.carbpol.2019.115514
127.
Salem N ElShebiney S Mabrouk M Kishta M Galal A Osama L et al Enhanced bone regeneration using mesenchymal stem cell-loaded 3D-printed alginate-calcium Titanate scaffolds: a Calvarial defect model study. Int J Biol Macromol. (2025) 302:140516. 10.1016/j.ijbiomac.2025.140516
128.
Ferjaoui Z López-Muñoz R Akbari S Chandad F Mantovani D Rouabhia M et al Design of alginate/gelatin hydrogels for biomedical applications: fine-tuning osteogenesis in dental pulp stem cells while preserving other cell behaviors. Biomedicines. (2024) 12:1510. 10.3390/biomedicines12071510
129.
Wang Z Goh J Das De S Ge Z Ouyang H Chong J et al Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. (2006) 12:1753–61. 10.1089/ten.2006.12.1753
130.
Sartika D Wang C Wang D Cherng J Chang S Fan G et al Human adipose-derived mesenchymal stem cells-incorporated silk fibroin as a potential bio-scaffold in guiding bone regeneration. Polymers. (2020) 12:853. 10.3390/polym12040853
131.
Venkatesan J Bhatnagar I Manivasagan P Kang K Kim S . Alginate composites for bone tissue engineering: a review.Int J Biol Macromol. (2015) 72:269–81. 10.1016/j.ijbiomac.2014.07.008
132.
Pawar S Edgar K . Alginate derivatization: a review of chemistry, properties and applications.Biomaterials. (2012) 33:3279–305. 10.1016/j.biomaterials.2012.01.007
133.
Li Y Xu Z Wang J Pei X Chen J Wan Q . Alginate-based biomaterial-mediated regulation of macrophages in bone tissue engineering.Int J Biol Macromol. (2023) 230:123246. 10.1016/j.ijbiomac.2023.123246
134.
Gentile P Chiono V Carmagnola I Hatton P . An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering.Int J Mol Sci. (2014) 15:3640–59. 10.3390/ijms15033640
135.
Wang W Meng Q Li Q Liu J Zhou M Jin Z et al Chitosan derivatives and their application in biomedicine. Int J Mol Sci. (2020) 21:487. 10.3390/ijms21020487
136.
Mawazi S Kumar M Ahmad N Ge Y Mahmood S . Recent applications of chitosan and its derivatives in antibacterial, anticancer, wound healing, and tissue engineering fields.Polymers. (2024) 16:1351. 10.3390/polym16101351
137.
Guillén-Carvajal K Valdez-Salas B Beltrán-Partida E Salomón-Carlos J Cheng N . Chitosan, gelatin, and collagen hydrogels for bone regeneration.Polymers. (2023) 15:2762. 10.3390/polym15132762
138.
Aranaz I Alcántara A Civera M Arias C Elorza B Heras Caballero A et al Chitosan: an overview of its properties and applications. Polymers. (2021) 13:3256. 10.3390/polym13193256
139.
Melro E Antunes F da Silva G Cruz I Ramos P Carvalho F et al Chitosan films in food applications. tuning film properties by changing acidic dissolution conditions. Polymers. (2020) 13:1. 10.3390/polym13010001
140.
Saurav S Sharma P Kumar A Tabassum Z Girdhar M Mamidi N et al Harnessing natural polymers for nano-scaffolds in bone tissue engineering: a comprehensive overview of bone disease treatment. Curr Issues Mol Biol. (2024) 46:585–611. 10.3390/cimb46010038
141.
Ressler A . Chitosan-based biomaterials for bone tissue engineering applications: a short review.Polymers. (2022) 14:3430. 10.3390/polym14163430
142.
Wong S Yee M Chin K Ima-Nirwana SA . Review of the application of natural and synthetic scaffolds in bone regeneration.J Funct Biomater. (2023) 14:286. 10.3390/jfb14050286
143.
Babu S Shanmugavadivu A Selvamurugan N . Tunable mechanical properties of chitosan-based biocomposite scaffolds for bone tissue engineering applications: a review.Int J Biol Macromol. (2024) 272:132820. 10.1016/j.ijbiomac.2024.132820
144.
Mahmood A Maher N Amin F Alqutaibi A Kumar N Zafar M . Chitosan-based materials for dental implantology: a comprehensive review.Int J Biol Macromol. (2024) 268:131823. 10.1016/j.ijbiomac.2024.131823
145.
Kudiyarasu S Karuppan Perumal M Rajan Renuka R Manickam Natrajan P . Chitosan composite with mesenchymal stem cells: properties, mechanism, and its application in bone regeneration.Int J Biol Macromol. (2024) 275:133502. 10.1016/j.ijbiomac.2024.133502
146.
Zang S Zhu L Luo K Mu R Chen F Wei X et al Chitosan composite scaffold combined with bone marrow-derived mesenchymal stem cells for bone regeneration: in vitro and in vivo evaluation. Oncotarget. (2017) 8:110890–903. 10.18632/oncotarget.22917
147.
Georgopoulou A Papadogiannis F Batsali A Marakis J Alpantaki K Eliopoulos A et al Chitosan/gelatin scaffolds support bone regeneration. J Mater Sci Mater Med. (2018) 29:1–13. 10.1007/s10856-018-6064-2
148.
Zhai P Peng X Li B Liu Y Sun H Li X . The application of hyaluronic acid in bone regeneration.Int J Biol Macromol. (2020) 151:1224–39. 10.1016/j.ijbiomac.2019.10.169
149.
Hwang H Lee C . Recent progress in hyaluronic-acid-based hydrogels for bone tissue engineering.Gels. (2023) 9:588. 10.3390/gels9070588
150.
Li X Lin H Yu Y Lu Y He B Liu M et al In situ rapid-formation sprayable hydrogels for challenging tissue injury management. Adv Mater. (2024) 36:e2400310. 10.1002/adma.202400310
151.
Ricard-Blum S Vivčs R Schaefer L Götte M Merline R Passi A et al A biological guide to glycosaminoglycans: current perspectives and pending questions. FEBS J. (2024) 291:3331–66. 10.1111/febs.17107
152.
Szarpak A Auzély-Velty R . Hyaluronic acid single-network hydrogel with high stretchable and elastic properties.Carbohydr Polym. (2023) 320:121212. 10.1016/j.carbpol.2023.121212
153.
Chiang E Ma H Wang J Liu C Chen T Hung S . Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits.PLoS One. (2016) 11:e0149835. 10.1371/journal.pone.0149835
154.
Li L Duan X Fan Z Chen L Xing F Xu Z et al Mesenchymal stem cells in combination with hyaluronic acid for articular cartilage defects. Sci Rep. (2018) 8:9900. 10.1038/s41598-018-27737-y
155.
Boeckel D Sesterheim P Peres T Augustin A Wartchow K Machado D et al Adipogenic mesenchymal stem cells and hyaluronic acid as a cellular compound for bone tissue engineering. J Craniofac Surg. (2019) 30:777–83. 10.1097/SCS.0000000000005392
156.
Alavi M Memarpour S Pazhohan-Nezhad H Salimi Asl A Moghbeli M Shadmanfar S et al Applications of poly(lactic acid) in bone tissue engineering: a review article. Artif Organs. (2023) 47:1423–30. 10.1111/aor.14612
157.
Woodward S Brewer P Moatamed F Schindler A Pitt C . The intracellular degradation of poly(epsilon-caprolactone).J Biomed Mater Res. (1985) 19:437–44. 10.1002/jbm.820190408
158.
Hou Y Wang W Bartolo P . Investigation of polycaprolactone for bone tissue engineering scaffolds: in vitro degradation and biological studies.Materials Design. (2022) 216:110582. 10.1016/j.matdes.2022.110582
159.
Yang X Wang Y Zhou Y Chen J Wan Q . The application of polycaprolactone in three-dimensional printing scaffolds for bone tissue engineering.Polymers. (2021) 13:2754. 10.3390/polym13162754
160.
Xue R Qian Y Li L Yao G Yang L Sun Y . Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue-derived mesenchymal stem cells.Stem Cell Res Ther. (2017) 8:1–9. 10.1186/s13287-017-0588-0
161.
Xu H Wang C Liu C Li J Peng Z Guo J et al Stem cell-seeded 3d-printed scaffolds combined with self-assembling peptides for bone defect repair. Tissue Eng Part A. (2022) 28:111–124. 10.1089/ten.TEA.2021.0055
162.
Gritsch L Conoscenti G La Carrubba V Nooeaid P Boccaccini A . Polylactide-based materials science strategies to improve tissue-material interface without the use of growth factors or other biological molecules.Mater Sci Eng C Mater Biol Appl. (2019) 94:1083–101. 10.1016/j.msec.2018.09.038
163.
Maadani A Salahinejad E . Performance comparison of PLA- and PLGA-coated porous bioceramic scaffolds: mechanical, biodegradability, bioactivity, delivery and biocompatibility assessments.J Control Release. (2022) 351:1–7. 10.1016/j.jconrel.2022.09.022
164.
Bahraminasab M Talebi A Doostmohammadi N Arab S Ghanbari A Zarbakhsh S . The healing of bone defects by cell-free and stem cell-seeded 3D-printed PLA tissue-engineered scaffolds.J Orthop Surg Res. (2022) 17:320. 10.1186/s13018-022-03213-2
165.
Jin S Xia X Huang J Yuan C Zuo Y Li Y et al Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. (2021) 127:56–79. 10.1016/j.actbio.2021.03.067
166.
Bhuiyan D Middleton J Tannenbaum R Wick T . Bone regeneration from human mesenchymal stem cells on porous hydroxyapatite-PLGA-collagen bioactive polymer scaffolds.Biomed Mater Eng. (2017) 28:671–85. 10.3233/BME-171703
167.
Habraken W Wolke J Jansen J . Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering.Adv Drug Deliv Rev. (2007) 59:234–48. 10.1016/j.addr.2007.03.011
168.
Burg K Porter S Kellam J . Biomaterial developments for bone tissue engineering.Biomaterials. (2000) 21:2347–59. 10.1016/s0142-9612(00)00102-2
169.
Barrére F van Blitterswijk C de Groot K . Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics.Int J Nanomedicine. (2006) 1:317–32.
170.
Jeong J Kim J Shim J Hwang N Heo C . Bioactive calcium phosphate materials and applications in bone regeneration.Biomater Res. (2019) 23:4. 10.1186/s40824-018-0149-3
171.
Xu H Wang P Wang L Bao C Chen Q Weir M et al Calcium phosphate cements for bone engineering and their biological properties. Bone Res. (2017) 5:17056. 10.1038/boneres.2017.56
172.
Gendviliene I Simoliunas E Alksne M Dibart S Jasiuniene E Cicenas V et al Effect of extracellular matrix and dental pulp stem cells on bone regeneration with 3D printed PLA/HA composite scaffolds. Eur Cell Mater. (2021) 41:204–15. 10.22203/eCM.v041a15
173.
Liu Y Zheng Y Chen X Yang J Pan H Chen D et al Fundamental theory of biodegradable metals—definition, criteria, and design. Adv Funct Mater. (2019) 29:1805402. 10.1002/adfm.201805402
174.
Fan L Chen S Yang M Liu Y Liu J . Metallic materials for bone repair.Adv Healthc Mater. (2024) 13:e2302132. 10.1002/adhm.202302132
175.
Li P Dai J Li Y Alexander D Čapek J Geis-Gerstorfer J et al Zinc based biodegradable metals for bone repair and regeneration: bioactivity and molecular mechanisms. Mater Today Bio. (2024) 25:100932. 10.1016/j.mtbio.2023.100932
176.
Wegener B Sichler A Milz S Sprecher C Pieper K Hermanns W et al Development of a novel biodegradable porous iron-based implant for bone replacement. Sci Rep. (2020) 10:9141. 10.1038/s41598-020-66289-y
177.
Al-Shalawi F Mohamed Ariff A Jung D Mohd Ariffin M Seng Kim C Brabazon D et al Biomaterials as implants in the orthopedic field for regenerative medicine: metal versus synthetic polymers. Polymers. (2023) 15:2601. 10.3390/polym15122601
178.
Yang C Huan Z Wang X Wu C Chang J . 3D Printed Fe scaffolds with HA nanocoating for bone regeneration.ACS Biomater Sci Eng. (2018) 4:608–16. 10.1021/acsbiomaterials.7b00885
179.
Lumbikananda S Tikkhanarak K Pongjantarasatian S Trachoo V Namangkalakul W Osathanon T . Osteogenic induction activity of magnesium chloride on human periodontal ligament stem cells.Int Dent J. (2025) 75:1431–40. 10.1016/j.identj.2024.11.013
180.
Li H Li M Ran X Cui J Wei F Yi G et al The role of zinc in bone mesenchymal stem cell differentiation. Cell Reprogram. (2022) 24:80–94. 10.1089/cell.2021.0137
181.
Wen X Wang J Pei X Zhang X . Zinc-based biomaterials for bone repair and regeneration: mechanism and applications.J Mater Chem B. (2023) 11:11405–25. 10.1039/d3tb01874a
182.
Kolomenskaya E Butova V Poltavskiy A Soldatov A Butakova M . Application of artificial intelligence at all stages of bone tissue engineering.Biomedicines. (2023) 12:76. 10.3390/biomedicines12010076
183.
Yusa K Yamamoto O Takano H Fukuda M Iino M . Zinc-modified titanium surface enhances osteoblast differentiation of dental pulp stem cells in vitro.Sci Rep. (2016) 6:29462. 10.1038/srep29462
184.
Theodosaki A Tzemi M Galanis N Bakopoulou A Kotsiomiti E Aggelidou E et al Bone regeneration with mesenchymal stem cells in scaffolds: systematic review of human clinical trials. Stem Cell Rev Rep. (2024) 20:938–66. 10.1007/s12015-024-10696-5
185.
Hussen B Taheri M Yashooa R Abdullah G Abdullah S Kheder R et al Revolutionizing medicine: recent developments and future prospects in stem-cell therapy. Int J Surg. (2024) 110:8002–24. 10.1097/JS9.0000000000002109
186.
Saitoh I Sato M Kiyokawa Y Inada E Iwase Y Ibano N et al Induced tissue-specific stem cells (iTSCs): their generation and possible use in regenerative medicine. Pharmaceutics. (2021) 13:780. 10.3390/pharmaceutics13060780
187.
Schu S Nosov M O’Flynn L Shaw G Treacy O Barry F et al Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. (2012) 16:2094–103. 10.1111/j.1582-4934.2011.01509.x
188.
Li Y Jin M Guo D Shen S Lu K Pan R et al Unveiling the immunogenicity of allogeneic mesenchymal stromal cells: challenges and strategies for enhanced therapeutic efficacy. Biomed Pharmacother. (2024) 180:117537.
189.
Jia Z Zhang S Li W . Harnessing stem cell-derived extracellular vesicles for the regeneration of degenerative bone conditions.Int J Nanomedicine. (2023) 18:5561–78.
190.
Smolinska V Csobonyeiova M Zamborsky R Danisovic L . Stem cells and their derivatives: an implication for the regeneration of nonunion fractures.Cell Transplant. (2023) 32:09636897231183530. 10.1177/09636897231183530
191.
Wang Z Sun Y Li C . Advances in 3D printing technology for preparing bone tissue engineering scaffolds from biodegradable materials.Front Bioeng Biotechnol. (2024) 12:1483547. 10.3389/fbioe.2024.1483547
192.
Bagherpour R Bagherpour G Mohammadi P . Application of artificial intelligence in tissue engineering.Tissue Eng Part B Rev. (2025) 31:31–43. 10.1089/ten.TEB.2024.0022
193.
Azuaje F . Computational discrete models of tissue growth and regeneration.Brief Bioinform. (2011) 12:64–77. 10.1093/bib/bbq017
194.
Apatzidou DA Bakopoulou AA Kouzi-Koliakou K Karagiannis V Konstantinidis A . A tissue-engineered biocomplex for periodontal reconstruction. A proof-of-principle randomized clinical study.J Clin Periodontol (2021) 48:1111–25. 10.1111/jcpe.13474
195.
Chen FM Gao LN Tian BM Zhang XY Zhang YJ Dong GY et al Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther (2016) 7:1–11. 10.1186/s13287-016-0288-1
196.
Hernandez-Monjaraz B Santiago-Osorio E Ledesma-Martinez E Aguiniga-Sanchez I Sosa-Hernandez NA Mendoza-Nunez VM . Dental Pulp Mesenchymal stem cells as a treatment for periodontal disease in older adults. Stem Cells Int. (2020) 2020:8890873. 10.1155/2020/8890873
197.
Khojasteh A Kheiri L Behnia H Tehranchi A Nazeman P Nadjmi N et al Lateral Ramus cortical bone plate in alveolar cleft osteoplasty with concomitant use of buccal fat pad derived cells and autogenous bone: phase I clinical trial. BioMed Res Int . (2017) 2017:1. 10.1155/2017/6560234
198.
Sanchez N Fierravanti L Nunez J Vignoletti F Gonzalez-Zamora M Santamaria S et al Periodontal regeneration using a xenogeneic bone substitute seeded with autologous periodontal ligament-derived mesenchymal stem cells: a 12-month quasi-randomized controlled pilot clinical trial. J Clin Periodontol (2020) 47:1391–402. 10.1111/jcpe.13368
199.
Akhlaghi F Hesami N Rad MR Nazeman P Fahimipour F Khojasteh A . Improved bone regeneration through amniotic membrane loaded with buccal fat pad-derived MSCs as an adjuvant in maxillomandibular reconstruction.J Cranio-Maxillofacial Surg (2019) 47:1266–73. 10.1016/j.jcms.2019.03.030
200.
Ismail HD Phedy P Kholinne E Djaja YP Kusnadi Y Merlina M et al Mesenchymal stem cell implantation in atrophic nonunion of the long bones: a translational study. Bone Joint Res (2016) 5:287–93. 10.1302/2046-3758.57.2000587
201.
Khojasteh A Sadeghi N . Application of buccal fat pad-derived stem cells in combination with autogenous iliac bone graft in the treatment of maxillomandibular atrophy: a preliminary human study.Int J Oral Maxillofacial Surg (2016) 45:864–71. 10.1016/j.ijom.2016.01.003
202.
Šponer P Kučera T Brtkova J Urban K Koči Z Měřička P et al Comparative study on the application of mesenchymal stromal cells combined with tricalcium phosphate Scaffold into femoral bone defects. Cell Transpl (2018) 27:1459–68. 10.1177/0963689718794918
203.
Gjerde C Mustafa K Hellem S Rojewski M Gjengedal H Yassin MA et al Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res Ther (2018) 9:1–15. 10.1186/s13287-018-0951-9
204.
Gomez-Barrena E Padilla-Eguiluz N Rosset P Gebhard F Hernigou P Baldini N et al Early efficacy evaluation of mesenchymal stromal cells (MSC) combined to biomaterials to treat long bone non-unions. Injury. (2020) 51:S63–73. 10.1016/j.injury.2020.02.070
205.
Relondo ML Redondo LM Garcia V Peral B Verrier A Becerra J et al Repair of maxillary cystic bone defects with mesenchymal stem cells seeded on a cross-linked serum scaffold. J Cranio-Maxillofacial Surg (2018) 46:222–9. 10.1016/j.jcms.2017.11.004
206.
Takedachi M Sawada K Sakura K Morimoto C Hirai A Iwayama T et al Periodontal tissue regeneration by transplantation of autologous adipose tissue derived multi-lineage progenitor cells. Sci Rep (2022) 12:1–11. 10.1038/s41598-022-11986-z
207.
Tanikawa DYS Pinheiro CCG Almeida MCA Oliveira CRGCM Coudry RA Rocha DL et al Deciduous dental pulp stem cells for maxillary alveolar reconstruction in cleft lip and palate patients. Stem Cells Int . (2020) 2020:6234167. 10.1155/2020/6234167
Summary
Keywords
stem cells, bone tissue engineering, biomaterials, bone regeneration, regenerative medicine, osteogenic differentiation
Citation
Jiao M, Shuai T, Zhao Z, Wu Y, Yu L, Sun J, De Caro R, Macchi V, Porzionato A, Stocco E and Jin C (2025) Categories, applications, and potential of stem cells in bone regeneration: an overview. Front. Med. 12:1606100. doi: 10.3389/fmed.2025.1606100
Received
04 April 2025
Accepted
31 July 2025
Published
20 August 2025
Volume
12 - 2025
Edited by
Hongju Wu, Tulane University, United States
Reviewed by
Xiaolei Li, University of Pennsylvania, United States
Jose Gregorio Marchan Alvarez, Karolinska Institutet (KI), Sweden
Updates
Copyright
© 2025 Jiao, Shuai, Zhao, Wu, Yu, Sun, De Caro, Macchi, Porzionato, Stocco and Jin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chanyuan Jin, jinchanyuanjcy@163.comElena Stocco, elena.stocco@unipd.it
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.