Abstract
Background:
Propofol combined with sufentanil is the most commonly used anesthesia regimen for painless gastroscopy in China. However, this combination carries a higher risk of circulatory and respiratory depression. Esketamine, with its strong analgesic and sympathetic excitatory effects, may be a safer alternative. This study aimed to evaluate the safety and efficacy of propofol-sufentanil versus propofol-esketamine for painless gastroscopy in adults.
Methods:
120 participants were randomly assigned to four groups: PS (propofol 2 mg/kg + sufentanil 0.1 μg/kg), PE1 (propofol 2 mg/kg + esketamine 0.05 mg/kg), PE2 (propofol 2 mg/kg + esketamine 0.1 mg/kg), and PE3 (propofol 2 mg/kg + esketamine 0.2 mg/kg). The primary outcome was the incidence of reflex cough during gastroscopy insertion. Secondary outcomes included hemodynamic changes, pulse oxygen saturation, induction time, recovery time, discharge time, propofol consumption, and the occurrence of adverse events.
Results:
There was no significant difference in reflex cough, body movement response, or propofol injection pain between the PS, PE2, and PE3 groups, but these incidences were significantly lower than in the PE1 group (p < 0.05). Hypotension occurred less frequently in PE2 and PE3 compared to PS and PE1 (p = 0.001), with more stable hemodynamics observed in PE2 and PE3. However, the incidence of tachycardia was significantly higher in the PE3 group than in the others (p < 0.05). Fewer participants in PE3 and PS required additional propofol compared to PE1 (p < 0.05), with no significant difference between PS, PE2, and PE3 (p > 0.05). Induction time was significantly shorter in PE3 compared to PS and PE1, with no difference between PE3 and PE2 (p > 0.05). However, recovery time was longest in PE3 (p = 0.002). No significant differences were found in other outcomes (p > 0.05).
Conclusion:
Considering the superior safety and efficacy observed in the PE2 group, we recommend the combination of 2 mg/kg propofol and 0.1 mg/kg esketamine as the optimal anesthesia for painless gastroscopy. This combination provides several benefits, including reduced reflex cough, stable hemodynamics, and faster recovery, making it a valuable clinical practice.
Introduction
Gastroscopy is widely regarded as the gold standard for diagnosing upper gastrointestinal diseases (1). However, as an invasive procedure, it can provoke adverse reactions such as reflexive coughing and vomiting during endoscope insertion, which in turn may increase the risk of respiratory and cardiovascular complications. To reduce these risks and improve patient comfort, many hospitals have adopted the practice of performing gastroscopies under sedation, commonly known as “painless gastroscopy.” This approach aims to alleviate procedural discomfort and minimize the likelihood of adverse effects.
The choice of sedative agents is critical to the success of painless gastroscopy, as the balance between efficacy and safety must be carefully considered. Traditionally, the most commonly used sedative combination for this purpose has been propofol in conjunction with opioids, particularly sufentanil. Propofol is widely utilized due to its rapid onset, quick recovery, and favorable pharmacokinetic profile, which allows for swift induction and recovery from sedation (2). These characteristics make it an attractive option for both anesthesiologists and patients. However, despite its advantages, propofol is not without its complications. Common side effects include pain at the injection site, respiratory depression, and circulatory instability, all of which pose significant risks, especially for patients with underlying health conditions such as cardiovascular or respiratory diseases (3).
To enhance sedation quality and minimize the required dosage of propofol, opioids like sufentanil are often administered concurrently. Sufentanil, a potent opioid, provides effective analgesia and has been shown to reduce the necessary dose of propofol, thus decreasing the incidence of certain side effects. However, opioids, including sufentanil, carry inherent risks such as respiratory depression, apnea, bradycardia, chest wall rigidity, and other gastrointestinal symptoms such as nausea and vomiting. These side effects, particularly respiratory depression, are of significant concern, as they can lead to life-threatening complications during the procedure (4). The safety concerns surrounding the use of propofol and sufentanil in combination have spurred interest in identifying alternative sedative combinations that may offer better safety profiles without compromising the effectiveness of sedation and analgesia.
Esketamine, a dextral isomer of ketamine, has emerged as a promising alternative for sedation and analgesia. Esketamine primarily acts on NMDA (N-methyl-D-aspartate) receptors and provides both sedative and analgesic effects. Research has shown that esketamine’s analgesic potency is twice that of ketamine and four times that of its R-isomer, making it a potent agent for pain management (5). Additionally, esketamine is associated with a lower incidence of adverse effects, particularly those related to psychiatric side effects such as hallucinations and confusion, which are commonly associated with ketamine use. This reduction in psychiatric side effects is due to the absence of R-ketamine in esketamine, as R-ketamine is believed to be responsible for many of the negative mental health effects seen with ketamine (6). However, some studies suggest that high doses of esketamine may still increase the risk of psychiatric side effects (7). Nonetheless, when used in low doses, esketamine has demonstrated a strong analgesic effect with a relatively low risk of these adverse effects (8). In addition to its analgesic properties, esketamine also possesses sympathomimetic effects, meaning it can help stabilize the circulatory system during anesthesia. This is particularly beneficial when used in combination with propofol, as propofol is known to cause respiratory and circulatory suppression. Esketamine’s ability to maintain circulatory stability can counterbalance these effects, providing a more balanced and safer sedation profile during procedures like gastroscopy.
Despite the pharmacological advantages of esketamine, there are still limited clinical reports on its use in combination with propofol for painless gastroscopy. Most of the existing literature focuses on other combinations, such as propofol and sufentanil (9). This gap in the literature underscores the need for further investigation into the potential benefits of combining propofol with esketamine, particularly in the context of painless gastroscopy. Therefore, this study aims to evaluate the safety and efficacy of two sedative combinations—propofol-sufentanil and propofol-esketamine—in adults undergoing painless gastroscopy. Through a comparative analysis, the study seeks to identify a safer and more effective anesthetic regimen that enhances patient comfort while minimizing the risk of adverse effects during the procedure.
Materials and methods
A total of 120 patients scheduled for painless gastroduodenoscopy at the Endoscopy Center of the Second Affiliated Hospital of Shantou University Medical College between August and December 2022 were included in this study. This was a single-center, prospective, randomized, double-blind trial, approved by the hospital’s Ethics Review Committee (Protocol No. 2022–165) and registered with the Chinese Clinical Trial Center (No. chiCTR 2,300,069,846). All participants provided written informed consent.
Inclusion and exclusion criteria
The study included 120 patients who met the following criteria: (1) aged 18–64 years; (2) ASA I-II; (3) BMI 18.5–23.9; (4) fully understood the study’s purpose and signed informed consent. Exclusion criteria were: (1) ASA III or higher, or myocardial infarction within the past 6 months; (2) poorly controlled or untreated hypertension; (3) chronic lung diseases or recent acute upper respiratory tract infection or pneumonia; (4) liver or kidney decompensation; (5) neuropsychiatric disorders or epilepsy; (6) anticipated difficult airway; (7) breastfeeding or allergies to eggs, seafood, soy, or medications; (8) history of psychotropic drug or narcotic abuse.
Randomization and blindness
Patients meeting the inclusion criteria were randomly assigned into four groups using computer-generated randomization. An independent researcher, who was not involved in the sedation process, was responsible for executing the randomization procedure. The anesthesia regimens for each group were as follows: the control group (PS) received 2 mg/kg propofol combined with 0.1 μg/kg sufentanil; trial group 1 (PE1) received 2 mg/kg propofol with 0.05 mg/kg esketamine; trial group 2 (PE2) received 2 mg/kg propofol with 0.1 mg/kg esketamine; and trial group 3 (PE3) received 2 mg/kg propofol with 0.2 mg/kg esketamine. The drugs were prepared by a researcher not involved in the sedation process and administered by an anesthesiologist who was blinded to the group assignments. Data collection was carried out by a separate researcher who was also blinded to the group allocations. To ensure blinding, both the anesthesiologists and data collectors, as well as the endoscopists and patients, remained unaware of the group assignments throughout the study.
Sample size estimation
The sample size for this study was determined through a preliminary pre-test, which indicated the following efficacy in preventing reflex cough during gastroscopy insertion: 80, 50, 80, and 90% for the PS, PE1, PE2, and PE3 groups, respectively. A power analysis was performed with a significance level (α) of 0.05 and a desired power (β) of 0.80, considering a 15% anticipated loss to follow-up. Based on these parameters, the calculated sample size was 120 participants, with 30 participants per group.
Anesthesia sedation process
The day before the gastroscopy, the anesthesiologist should conduct a routine visit, recording the patient’s basic information such as age, gender, height, weight, body mass index (BMI), medical history, anesthesia contraindications, and ASA classification. For any potential conditions that may affect anesthesia safety, relevant tests should be arranged. For patients meeting the trial inclusion criteria, the anesthesia and endoscopy process, along with the potential benefits and risks of participating in the trial, should be explained in detail to the patient or their immediate family. After obtaining informed consent from the patient or family member, both the anesthesia consent and the clinical trial consent should be signed, and the patient can then be included in the trial.
A researcher not involved in anesthesia or data collection diluted the required drugs to a final volume of 10 mL using normal saline, resulting in sufentanil at 1 μg/mL and esketamine at concentrations of 0.5 mg/mL, 1 mg/mL, and 2 mg/mL. The propofol stock solution had a concentration of 10 mg/mL and was used directly without dilution.
All patients fasted for at least 8 h and refrained from drinking for at least 2 h before the gastroscopy. Fifteen minutes before the procedure, they consumed about 100 mL of a vesicle removal solution (streptomycin granules, simethicone emulsion, sodium bicarbonate, and water) prepared by the endoscopic nurse. Upon entering the room, patients were positioned on their left side, and intravenous access was established in the right upper limb. Sodium potassium magnesium calcium and glucose injection were administered, and low-flow oxygen (2 L/min) was delivered via a nasal catheter. Routine monitoring included ECG, pulse oximetry, and left upper limb arterial blood pressure.
Patients received intravenous injections of specific doses of sufentanil or esketamine, followed by a 2 mg/kg dose of propofol. Anesthesia induction was monitored by recording the time from the end of drug administration to the disappearance of the eyelash reflex. Sedation depth was assessed using the Ramsay Sedation Scale (RSS), with a target RSS score of ≥5 indicating adequate sedation. If the RSS score was <5, an additional 0.5 mg/kg of propofol was administered. Endoscopy was initiated once the RSS reached ≥5. During the procedure, the anesthesiologist continuously monitored the patient, administering 0.5 mg/kg doses of propofol as needed to maintain an RSS score of ≥5, ensuring proper sedation and patient safety. The doses of propofol, sufentanil, and esketamine used during anesthesia were also recorded. Blood pressure (BP), heart rate (HR), and pulse oxygen saturation (SpO2) were measured at five time points: T0 (upon entering the room), T1 (when eyelash reflex ceased), T2 (when the gastroscope was inserted), T3 (at the end of the procedure), and T4 (5 min after the procedure).
Hypotension was defined as a systolic blood pressure below 90 mmHg or a decrease of more than 30% from baseline; hypertension as an increase of more than 30% from baseline; and hypoxemia as an SpO2 below 94%. Bradycardia was defined as a heart rate below 60 beats per minute, and tachycardia as a heart rate above 100 beats per minute.
If the patient experiences hypotension during the examination, fluid rehydration should be increased, and 10 mg of intravenous ephedrine should be administered. If bradycardia occurs, 0.5 mg of atropine should be injected intravenously. In the case of hypertension, sedation depth should be adjusted accordingly, and the procedure should be paused if necessary. If hypoxemia occurs, increase oxygen flow and support the jaw to open the airway. If blood oxygen levels cannot be restored, insert a nasopharyngeal airway and suspend the endoscopy. A simple balloon mask can be used to administer oxygen if needed. If the patient coughs during endoscope insertion, additional anesthesia should be given. In the event of serious adverse effects, such as severe coughing that interferes with the procedure or anaphylactic shock, the trial should be immediately suspended, and blinding measures should be implemented to ensure the patient’s safety.
Primary and secondary outcome
The primary outcome of this study is the incidence of reflex cough following gastroscopy insertion. Secondary outcomes include hemodynamic indices (T0-T4), induction time, recovery time (from the end of examination to when the patient’s Ramsay score reaches 2), discharge time (from recovery time to when the PADSS score is ≥9), propofol consumption, patient and endoscopist satisfaction, and adverse events. Adverse events include pain from propofol injection, hypoxemia, hypotension, hypertension, arrhythmia, delirium, lethargy, nausea and vomiting, body movement, apnea, excessive oral secretions, and psychotomimetic effects.
Statistical analysis
Data collection adhered to the protocols of the prospective intervention study. Statistical analysis was conducted using SPSS 25.0. Continuous variables were presented as mean ± SD, and categorical variables as N (%). Before analysis, the Kolmogorov–Smirnov test and histograms were used to assess data normality. For continuous variables, analysis of variance (ANOVA) was performed, with post hoc comparisons conducted using the Student–Newman–Keuls (SNK) test. Data collected through repeated measures were analyzed using a repeated-measures ANOVA. Categorical variables were assessed using either the chi-square test or Fisher’s exact test. A p-value less than 0.05 was considered statistically significant.
Results
A total of 120 participants who met the trial inclusion criteria were enrolled. No participants were withdrawn due to adverse events during the examination. Follow-up was conducted by telephone 1 day after gastroscopy, with no participants lost to follow-up. Therefore, all 120 patients successfully completed the trial and were included in the data analysis.
General information
No significant differences were observed in age, sex, BMI, body weight, ASA grade, or other variables among the four groups (p > 0.05) (Table 1).
Table 1
| General information | Group PS | Group PE1 | Group PE2 | Group PE3 | p value |
|---|---|---|---|---|---|
| Gender (male/female) | 13/17 | 13/17 | 10/20 | 13/17 | 0.818 |
| ASA (I/II) | 21/9 | 22/8 | 23/7 | 22/8 | 0.952 |
| Age (years) | 40.7 ± 11.5 | 41.6 ± 10.0 | 36.8 ± 8.1 | 37.5 ± 8.2 | 0.054 |
| Height (cm) | 163 [157.7, 171] | 160 [158, 171.25] | 161.5 [158.75, 169.25] | 162 [158.75, 170] | 0.997 |
| Weight (kg) | 58.5 [50, 64.3] | 57.5 [49.8, 66.5] | 55 [50, 63.5] | 57.3 [50, 61.3] | 0.186 |
| BMI (kg/m2) | 21.77 [19.02, 23.28] | 21.06 [19.61, 23.67] | 21.05 [19.64, 23.4] | 20.80 [19.4, 22.79] | 0.960 |
| Smoking (yes/no) | 4/26 | 2/28 | 2/28 | 3/27 | 0.900 |
| Drink (yes/no) | 2/28 | 0/30 | 2/28 | 1/29 | 0.753 |
| Hypertension (yes/no) | 4/26 | 1/29 | 2/28 | 2/28 | 0.625 |
| Diabetes (yes/no) | 0/30 | 1/29 | 0/30 | 0/30 | 1.000 |
| COPD (yes/no) | 0/30 | 0/30 | 0/30 | 0/30 | 1.000 |
| Asthma (yes/no) | 0/30 | 0/30 | 0/30 | 0/30 | 1.000 |
General information of individuals.
Incidence of adverse effects
The incidence of adverse effects in the four groups during and within 24 h after the examination was presented (Table 2). Significant differences were observed among the groups for reflex cough (p = 0.029) (Figure 1A), body movement (p = 0.001) (Figure 1B), propofol injection pain (p = 0.002) (Figure 1C), tachycardia (p = 0.048), hypotension (p = 0.001), and drowsiness (p = 0.001). The incidence of drowsiness in group PS was significantly higher than in groups PE1 and PE2 within 24 h after the examination, but there was no significant difference compared with group PE3.
Table 2
| Adverse effects | Group PS | Group PE1 | Group PE2 | Group PE3 | p value |
|---|---|---|---|---|---|
| Cough | 4 (13.3%)a | 11 (36.7%)b | 3 (10%)a | 3 (10%)a | 0.029 |
| Body movement | 6 (20%)a | 18 (60%)b | 7 (23.3%)a | 5 (16.7%)a | 0.001 |
| Injection pain | 8 (26.7%)a | 20 (66.7%)b | 9 (30%)a | 8 (26.7%)a | 0.002 |
| Hypoxemia | 1 (3.3%) | 1 (3.3%) | 0 (0) | 1 (3.3%) | 1.000 |
| Tachycardia | 1 (3.3%)a | 5 (16.7%)a,b | 4 (13.3%)a,b | 9 (30%)b | 0.048 |
| Bradycardia | 9 (30%) | 2 (6.7%) | 4 (13.3%) | 3 (10%) | 0.095 |
| Hypotension | 9 (30%) a | 6 (20%)a,b | 1 (3.3%)b | 0 (0)b | 0.001 |
| Hypertension | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Delirium | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Drowsiness | 12 (40%)a | 2 (6.7%)b | 2 (6.7%)b | 5 (16.7%)a,b | 0.001 |
| Dizzy | 9 (30%) | 12 (40%) | 11 (36.6%) | 12 (40%) | 0.835 |
| Nausea | 1 (3.3%) | 0 (0) | 1 (3.3%) | 0 (0) | 1.000 |
| Emesis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Visual disturbance | 0 (0) | 0 (0) | 1 (3.3%) | 1 (3.3%) | 1.000 |
| Psychoactive effects | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
Incidence of adverse effects.
“ab” indicates the difference in diastolic blood pressure between the four groups at the same time. Groups sharing the same letter have no statistically significant difference (p > 0.05), while groups with different letters show a statistically significant difference (p < 0.05).
Figure 1

Comparison of the incidence of reflex cough, body movement, and propofol injection pain among the groups. (A) The incidence of reflex cough was significantly higher in the PE1 group compared to the PS, PE2, and PE3 groups (p < 0.05). There were no significant differences in reflex cough incidence among the other three groups (p > 0.05). (B) The incidence of body movement during examination differed significantly among the four groups (p = 0.001). The PE1 group had a significantly higher incidence than the PS, PE2, and PE3 groups (p < 0.05). There were no significant differences among the other three groups. (C) The incidence of propofol injection pain differed significantly among the four groups (p = 0.002), with the PE1 group showing a significantly higher incidence. No significant differences were found between the PS, PE2, and PE3 groups (p > 0.05).
Vital signs
The vital parameters included systolic blood pressure (SBP), diastolic blood pressure (DBP), HR, and SpO2, measured at five time points: T0, T1, T2, T3, and T4 (Supplementary Tables S1–S4). Changes in these parameters for the four groups at each time point were shown (Figures 2A–C). There were no significant differences in vital signs among the four groups at T0 (p > 0.05). However, significant differences in HR, SBP, and DBP were observed at T1, T2, T3, and T4 (p < 0.05). No significant differences were found in SpO2 across the groups at the five time points (p > 0.05). The incidence of hypotension was significantly lower in groups PE2 and PE3 compared to group PS, with no significant difference between group PS and group PE1. The incidence of hypotension was 20, 3.3, and 0% in groups PE1, PE2, and PE3, respectively. While no statistical difference was observed among the three groups, a dose-dependent decreasing trend was noted.
Figure 2
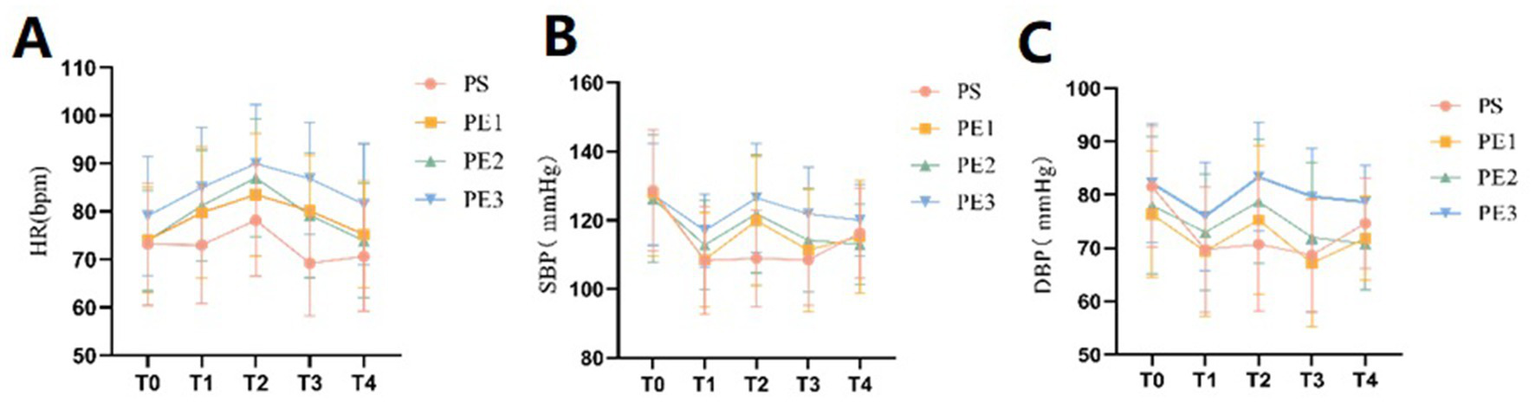
Comparison of vital sign parameters of the four groups at different time points. (A–C) The incidence of hypotension differed significantly among the four groups (p = 0.001). The PE2 and PE3 groups had a significantly lower incidence than the PS and PE1 groups. Systolic and diastolic blood pressure, as well as heart rate, remained stable in the PE2 and PE3 groups during T1, T2, and T3 compared to the PS and PE1 groups. However, the tachycardia rate was significantly higher in the PE3 group than in the other groups (p < 0.05).
Time index
The statistics of induction, procedure, recovery, and discharge times among the four groups were presented (Table 3). The induction time in group PE3 was significantly shorter than in groups PS and PE1, but no significant difference was found between group PE2 and group PS (Figure 3A). The recovery time in group PE3 was significantly longer than in the other three groups, with no significant differences among the other three groups (Figure 3B). There were no significant differences in procedure time and discharge time among the four groups.
Table 3
| Time index | Group PS | Group PE1 | Group PE2 | Group PE3 | p value |
|---|---|---|---|---|---|
| Induction time | 60 [53,66.75]a | 60 [48,70.75] a | 50 [40.75,66.25] a,b | 45 [40,50.25]b | <0.001 |
| Procedure time | 5.78 ± 0.59 | 5.67 ± 0.60 | 5.44 ± 0.81 | 5.50 ± 0.73 | 0.256 |
| Recovery time | 5.52 ± 1.76a | 5.16 ± 1.66a | 5.40 ± 2.23a | 6.89 ± 1.75b | 0.002 |
| Discharge time | 12.63 ± 2.96 | 11.41 ± 2.86 | 12.71 ± 2.28 | 12.83 ± 2.11 | 0.121 |
Time index.
“ab” indicates the difference in diastolic blood pressure between the four groups at the same time. Groups sharing the same letter have no statistically significant difference (p > 0.05), while groups with different letters show a statistically significant difference (p < 0.05).
Figure 3
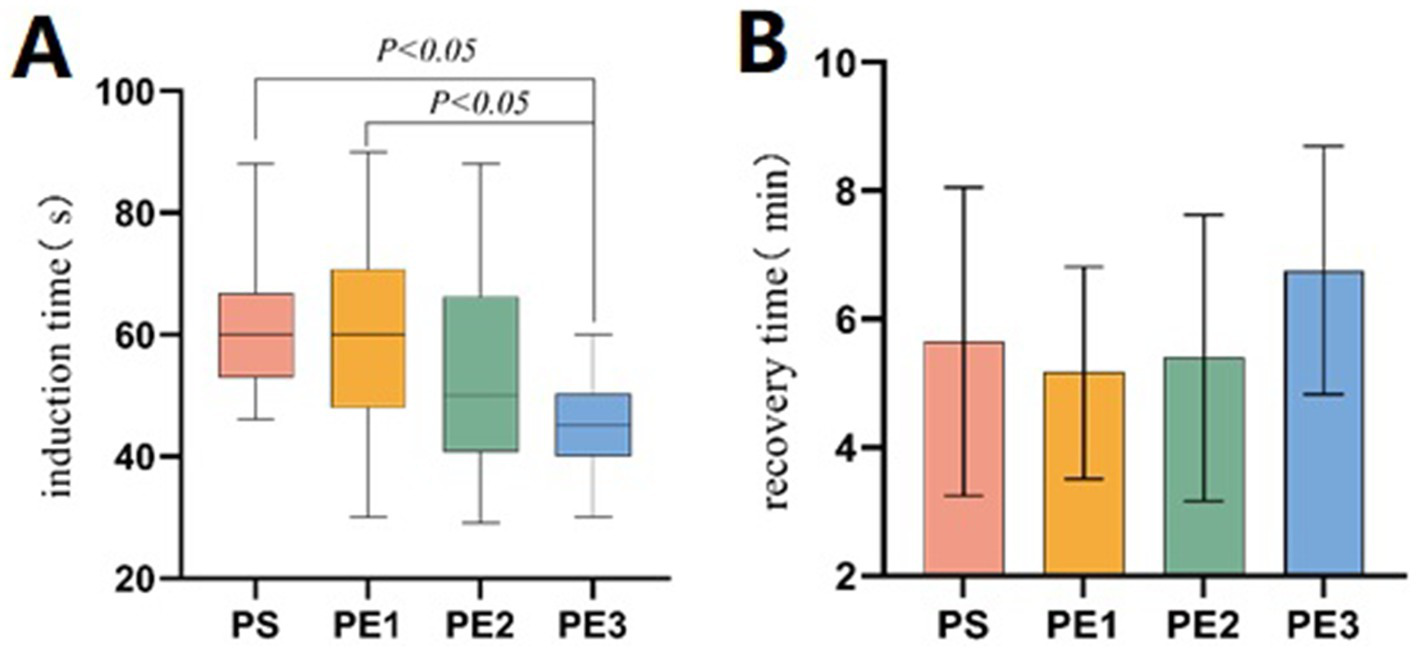
Comparison of induction and recovery times among the groups. (A) The induction time differed significantly among the four groups (p < 0.001). The induction time in the PE3 group was significantly shorter than in the PS and PE1 groups (p < 0.05), but there was no significant difference compared to the PE2 group (p > 0.05). (B) The recovery time differed significantly among the four groups (p = 0.002). The recovery time in the PE3 group was significantly longer than in the other three groups (p < 0.05). No significant difference was found between the PS, PE1, and PE2 groups (p > 0.05).
Propofol consumption
Patients in groups PS, PE1, PE2, and PE3 who required additional propofol only needed one dose to meet the examination needs, with numbers of 6, 18, 13, and 5, respectively (p = 0.001). The need for additional propofol was significantly lower in groups PE3 and PS compared to group PE1 (p < 0.05). However, there was no statistically significant difference in the need for additional propofol between the PS, PE2, and PE3 groups (Figure 4).
Figure 4
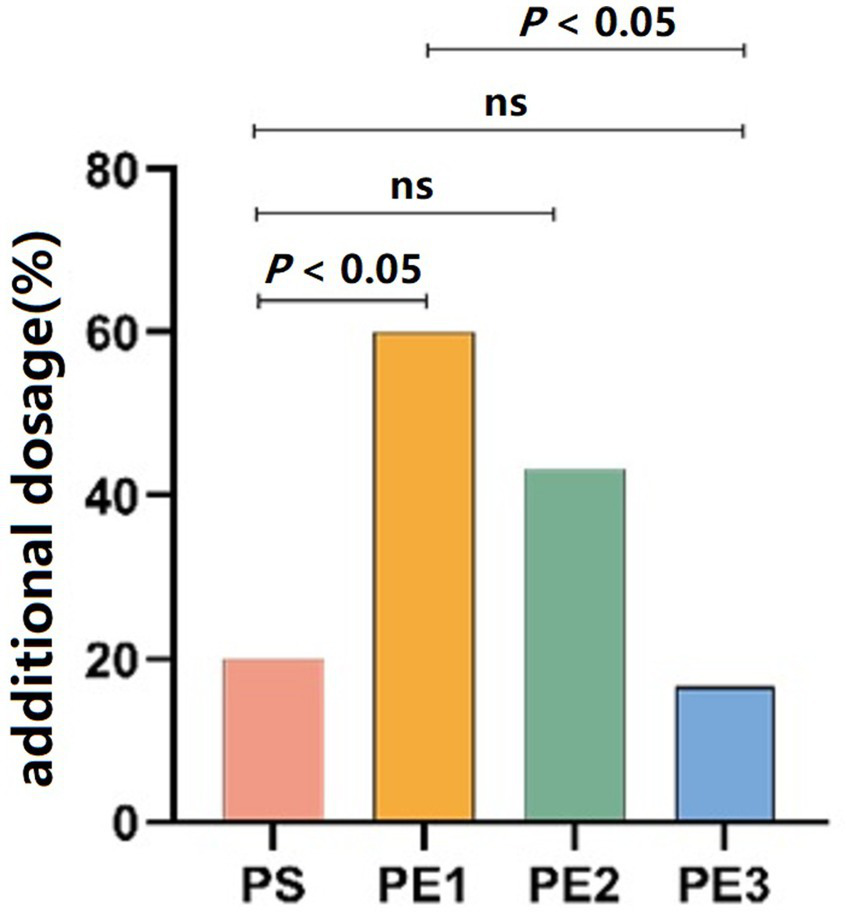
Comparison of propofol consumption across the groups. The number of patients requiring additional propofol was significantly lower in the PE3 and PS groups compared to the PE1 group (p < 0.05), but there was no significant difference between the PS, PE2, and PE3 groups.
Discussion
Reflex cough is a common complication of gastroscopy that causes discomfort for patients, affects their physiology, and interferes with the procedure and observation for endoscopists. Evaluating whether a painless anesthesia or sedation regimen offers advantages in effectively suppressing reflex cough is crucial. Coughing is a protective airway reflex to expel foreign objects from the respiratory tract, and it can be classified into voluntary, induced, and reflex coughs (10). The main difference between induced and reflex coughs lies in the intensity of the stimulation. When the stimulation reaches a certain threshold, induced cough can turn into reflex cough. Reflex cough can be triggered by irritation during gastroscopy placement or from oropharyngeal secretions. While propofol can inhibit upper respiratory responses and raise the threshold for reflex cough, the strong pharyngeal stimulation caused by gastroscopy can still provoke reflex coughing (11). Severe coughing increases abdominal pressure and activates the sympathetic nervous system, leading to significant hemodynamic fluctuations in patients (11). To minimize these adverse effects, propofol is often combined with other anesthetics such as sufentanil, midazolam, etomidate, or ketamine. However, no sedation regimen has yet been developed that fully satisfies patients, endoscopists, and anesthesiologists.
Esketamine is an NMDA receptor antagonist with stronger sedative effects. Due to the dose-dependent side effects of ketamine, low-dose esketamine can reduce the incidence of anesthetic side effects. Studies have shown that low-dose esketamine helps reduce adverse events, such as reflex cough and hypotension, by stimulating the sympathetic nervous system, providing analgesia, and antagonizing NMDA receptors (12). In a clinical trial comparing the incidence of reflex cough during painless gastroscopy, propofol combined with saline, sufentanil, or ketamine resulted in reflex cough rates of 40, 17, and 3%, respectively (13). The ketamine dose in this study was 0.4 mg/kg, which is equivalent to 0.2 mg/kg of esketamine based on titer calculations. Our study found that doses of esketamine at 0.1 mg/kg or higher effectively inhibited reflex cough. However, 0.05 mg/kg of esketamine did not significantly reduce the occurrence of reflex cough, likely due to insufficient analgesia at this lower dose.
Propofol combined with sufentanil can easily cause hypotension after anesthesia induction (14). Some studies suggest that this is due to arterial dilation caused by propofol, leading to reduced systemic vascular resistance, rather than venous dilation or changes in myocardial contractility (15). Esketamine has a sympathetic-like effect, which constricts resistant blood vessels and partially alleviates the cardiovascular depression caused by propofol. Zhou et al. (16) reported that 0.5 mg/kg of esketamine could effectively improve peripheral perfusion and mean arterial pressure after anesthesia induction. In our study, we also found that the sufentanil group experienced a more significant decrease in blood pressure compared to the esketamine groups, with the incidence of hypotension being dose-dependent on esketamine. Therefore, we speculate that esketamine’s protective effect against hypotension may be dose-dependent. However, further clinical studies with larger sample sizes are needed.
Literature indicates that esketamine doses below 0.25 mg/kg primarily produce an analgesic effect, with 0.125–0.25 mg/kg having additional sedative effects (17, 18). Studies have also shown that combining 1.5 mg/kg propofol with 0.2 mg/kg esketamine reduces induction time in painless gastroenteroscopy, suggesting that 0.2 mg/kg esketamine may enhance the sedative effect of propofol (19). In our study, we found that 0.2 mg/kg or higher doses of esketamine shortened the induction time, consistent with previous research. However, there are some discrepancies when comparing our findings to those of Zhang et al. (20), who reported that 0.2 mg/kg esketamine prolonged recovery time after general anesthesia. Similarly, Feng et al. (21) found that 0.5 mg/kg esketamine significantly prolonged recovery time in a dose-dependent manner compared to 0.15 mg/kg and 0.25 mg/kg doses during painless gastroenteroscopy. In contrast, our study did not observe a prolongation of recovery time with doses below 0.2 mg/kg, while doses of 0.2 mg/kg or higher appeared to extend recovery time. The differences in our findings may result from variations in study design, patient populations, and procedures. The combination of esketamine with other sedatives, along with the specific clinical context, could also influence recovery outcomes. Further research is needed to clarify how esketamine dosage affects both induction and recovery phases.
The optimal dosage of esketamine for painless gastroenteroscopy remains unclear. It has been reported that 0.5 mg/kg esketamine alone is safe and effective for gastroscopy in Chinese patients (12). However, most clinical anesthesiologists believe this dose may be linked to a higher risk of side effects, such as increased secretions and adverse mental effects. Low-dose esketamine primarily serves an analgesic role and is often combined with propofol for anesthesia, reducing the need for higher doses of propofol (22). In our study, we found that as the combined esketamine dose increased, the number of patients requiring additional propofol decreased. This suggests that esketamine may reduce propofol use in painless gastroenteroscopy in a dose-dependent manner.
Visual disturbances are a common adverse effect of esketamine. Previous studies have shown that esketamine may increase the incidence of visual impairment in a dose-dependent manner, with high-dose administration being a primary cause of prolonged recovery times and poorer recovery quality in patients (23, 24). Notably, in our study, only one patient in the PE3 group experienced transient visual impairment, characterized by blurred vision that resolved within 1 h, while no visual impairment was observed in the other groups. Based on these findings, we hypothesize that low-dose esketamine, as used in painless gastroscopy, does not significantly increase the risk of visual impairment. However, we emphasize the importance of remaining vigilant for this potential adverse effect in clinical practice.
Drowsiness is a common adverse effect associated with opioids (25). In this study, we found that low doses of esketamine did not significantly increase the incidence of drowsiness after the procedure, whereas high doses of esketamine and sufentanil exhibited similar potential to induce drowsiness in patients. Importantly, no other adverse mental effects were observed in our investigation. Several factors may explain these findings: First, esketamine lacks R-ketamine, which is believed to cause adverse mental effects (26). Second, mental side effects are mainly dose-dependent, and the low dose of esketamine used in this study likely contributed to the absence of such effects. Third, propofol can mitigate the psychogenic side effects of esketamine by inhibiting c-fos expression in the cingulate cortex, a mechanism linked to ketamine-induced mental side effects (27). Finally, esketamine can help alleviate cognitive dysfunction and brain damage caused by propofol (28). Based on these factors and our results, we conclude that low-dose esketamine combined with propofol for painless gastroscopy may not increase the incidence of psychiatric side effects.
Opioids and propofol can both cause respiratory depression (29). Ketamine can reduce ventilation insufficiency by stimulating the sympathetic nervous system, which helps maintain spontaneous breathing and airway reflexes (30). In our study, hypoxemia occurred infrequently in both the sufentanil and esketamine groups. We attribute this to two factors. First, the patients in our study were classified as ASA I or II, excluding those who are overweight or obese, as these individuals are more susceptible to respiratory insufficiency during induction. Second, all patients received a low-flow (2 L/min) nasal oxygen catheter, which effectively mitigated respiratory depression caused by propofol and sufentanil. Consequently, none of the four anesthesia regimens used in this study resulted in significant respiratory depression during painless gastroscopy.
This study compared the safety and efficacy of different doses of esketamine combined with propofol for painless gastroscopy, aiming to provide new insights into esketamine’s application. However, several limitations should be addressed in future research. First, the age range of 18 to 64 years may not fully represent older populations, and future studies should expand the sample size and consider age stratification. Second, manual intravenous administration could lead to dosing inaccuracies compared to more precise methods like target-controlled infusion (TCI). Incorporating TCI with real-time bispectral index (BIS) monitoring would improve dosing accuracy and sedation control. Lastly, variability in technique among gastroscopic operators could have contributed to inconsistent propofol administration. Future studies should standardize operator training or use a consistent group of operators to minimize this bias and improve procedural uniformity.
Conclusion
The combination of 0.1 mg/kg esketamine and 2 mg/kg propofol demonstrates a favorable balance of safety and efficacy for painless gastroscopy. This regimen is associated with a low incidence of reflex cough, stable hemodynamics, and no significant effect on recovery time or major adverse events, making it a promising anesthetic option for such procedures. Future studies should examine the effects of different esketamine doses to optimize safety and effectiveness. Long-term research is also necessary to evaluate the sustained safety and efficacy of this anesthetic combination across diverse clinical settings.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of The Second Affiliated Hospital of Shantou University Medical College (Protocol no. 2022–165). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
R-sG: Data curation, Writing – review & editing, Investigation, Formal analysis, Writing – original draft. X-YZ: Writing – review & editing, Investigation, Writing – original draft, Formal analysis, Methodology. S-PW: Writing – review & editing, Investigation, Data curation. X-YH: Investigation, Data curation, Writing – review & editing. Z-yL: Writing – review & editing, Validation, Supervision. Y-FZ: Project administration, Writing – review & editing, Funding acquisition, Conceptualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Shantou Medical Health Science and Technology Plan ([2021] No. 68–25).
Acknowledgments
We express our sincere gratitude to all the patients, nurses, and doctors who are participating in or contributing to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1606134/full#supplementary-material
Abbreviations
ASA, American Society of Anesthesiogists; SBP, Systolic Blood Pressure; DBP, Diastolic blood pressure; HR, Heart Rate; SpO2, Saturation of Pulse Oxygen; BMI, Body Mass Index; PACU, Post Anesthesia Care Unit; ERCP, Endoscopic RetrogradeCholangiopancreatography; RSS, Ramsay Sedation Scale; MAP, Mean Arterial Pressure; SPSS, Statistical Product and Service Solutions; PADSS, Post-Anesthetic Discharge Scoring System; RCT, Randomized Clinical Trial.
References
1.
Nguyen L Räsänen N Berggren F van Nieuwenhoven MA . Assessing age-related risks of gastrointestinal pathologies: a comparative study of gastroscopy outcomes across decades. Ther Adv Gastroenterol. (2024) 17:17562848241290446. doi: 10.1177/17562848241290446
2.
Li J Wang X Liu J Wang X Li X Wang Y et al . Comparison of ciprofol (HSK3486) versus propofol for the induction of deep sedation during gastroscopy and colonoscopy procedures: a multi-Centre, non-inferiority, randomized, controlled phase 3 clinical trial. Basic Clin Pharmacol Toxicol. (2022) 131:138–48. doi: 10.1111/bcpt.13761
3.
Kotani Y Pruna A Turi S Borghi G Lee TC Zangrillo A et al . Propofol and survival: an updated meta-analysis of randomized clinical trials. Crit Care. (2023) 27:139. doi: 10.1186/s13054-023-04431-8
4.
Gui YK Zeng XH Xiao R Xi WF Zhang D Liu Y et al . The effect of dezocine on the median effective dose of Sufentanil-induced respiratory depression in patients undergoing spinal Anesthesia combined with low-dose dexmedetomidine. Drug Des Devel Ther. (2023) 17:3687–96. doi: 10.2147/DDDT.S429752
5.
Mion G Himmelseher S . Esketamine: less drowsiness, more analgesia. Anesth Analg. (2024) 139:78–91. doi: 10.1213/ANE.0000000000006851
6.
Kawczak P Feszak I Bączek T . Ketamine, Esketamine, and Arketamine: their mechanisms of action and applications in the treatment of depression and alleviation of depressive symptoms. Biomedicine. (2024) 12:2283. doi: 10.3390/biomedicines12102283
7.
Nogo D Nazal H Song Y Teopiz KM Ho R McIntyre RS et al . A review of potential neuropathological changes associated with ketamine. Expert Opin Drug Saf. (2022) 21:813–31. doi: 10.1080/14740338.2022.2071867
8.
Ma J Wang F Wang J Wang P Dou X Yao S et al . The effect of low-dose Esketamine on postoperative neurocognitive dysfunction in elderly patients undergoing general Anesthesia for gastrointestinal Tumors: a randomized controlled trial. Drug Des Devel Ther. (2023) 17:1945–57. doi: 10.2147/DDDT.S406568
9.
Xiao X Xiao N Zeng F Chen H Zhang L He X . Gastroscopy sedation: clinical trial comparing propofol and sufentanil with or without remimazolam. Minerva Anestesiol. (2022) 88:223–9. doi: 10.23736/S0375-9393.21.15917-6
10.
Jakusova J Brozmanova M . Methods of cough assessment and objectivization. Physiol Res. (2023) 72:687–700. doi: 10.33549/physiolres.935062
11.
Yin N Xia J Cao YZ Lu X Yuan J Xie J . Effect of propofol combined with opioids on cough reflex suppression in gastroscopy: study protocol for a double-blind randomized controlled trial. BMJ Open. (2017) 7:e014881. doi: 10.1136/bmjopen-2016-014881
12.
Wang J Huang J Yang S Cui C Ye L Wang SY et al . Pharmacokinetics and safety of Esketamine in Chinese patients undergoing painless gastroscopy in comparison with ketamine: a randomized, open-label clinical study. Drug Des Devel Ther. (2019) 13:4135–44. doi: 10.2147/DDDT.S224553
13.
Yin S Hong J Sha T Chen Z Guo Y Li C et al . Efficacy and tolerability of sufentanil, dexmedetomidine, or ketamine added to propofol-based sedation for gastrointestinal endoscopy in elderly patients: a prospective, randomized, controlled trial. Clin Ther. (2019) 41:1864–1877.e0. doi: 10.1016/j.clinthera.2019.06.011
14.
Miao Y Zheng M Li Q Xiong L Feng J Liu X et al . Comparison of propofol-esketamine versus propofol-sufentanil for deep sedation and analgesia in children with autism: a randomized double-blind clinical trial. Autism Res. (2024) 17:1356–64. doi: 10.1002/aur.3172
15.
Saugel B Bebert EJ Briesenick L Hoppe P Greiwe G Yang D et al . Mechanisms contributing to hypotension after anesthetic induction with sufentanil, propofol, and rocuronium: a prospective observational study. J Clin Monit Comput. (2022) 36:341–7. doi: 10.1007/s10877-021-00653-9
16.
Zhou N Liang X Gong J Li H Liu W Zhou S et al . S-ketamine used during anesthesia induction increases the perfusion index and mean arterial pressure after induction: a randomized, double-blind, placebo-controlled trial. Eur J Pharm Sci. (2022) 179:106312. doi: 10.1016/j.ejps.2022.106312
17.
Sinner B Graf BM . Ketamine. Handb Exp Pharmacol. (2008) 182:313–33. doi: 10.1007/978-3-540-74806-9_15
18.
Zhou JS Chen Z Liu YY Zhong M-L Zhong Q Wei J et al . Observation on the analgesic effect of different doses of a combination of Esketamine and dexmedetomidine administered for percutaneous endoscopic transforaminal discectomy: a randomized, double-blind controlled trial. CNS Drugs. (2024) 38:547–58. doi: 10.1007/s40263-024-01083-2
19.
Zhan Y Liang S Yang Z Luo Q Li S Li J et al . Efficacy and safety of subanesthetic doses of esketamine combined with propofol in painless gastrointestinal endoscopy: a prospective, double-blind, randomized controlled trial. BMC Gastroenterol. (2022) 22:391. doi: 10.1186/s12876-022-02467-8
20.
Zhang C He J Shi Q Bao F Xu J . Subanaesthetic dose of esketamine during induction delays anaesthesia recovery a randomized, double-blind clinical trial. BMC Anesthesiol. (2022) 22:138. doi: 10.1186/s12871-022-01662-0
21.
Feng M Shi G Cui W Zhang N Xie Q Zhang W . The median effective concentration of propofol in combination with different doses of esketamine during gastrointestinal endoscopy in adults. Front Pharmacol. (2022) 13:1034236. doi: 10.3389/fphar.2022.1034236
22.
Huang X Lin F Chen Q Hu X . Safety and efficacy of the combination of esketamine and propofol in procedural sedation/analgesia: a systematic review and meta-analysis. Minerva Anestesiol. (2023) 89:680–9. doi: 10.23736/S0375-9393.23.17100-8
23.
Kan Z Min W Dai Y Zhang P . Intravenous esketamine as an adjuvant for sedation/analgesia outside the operating room: a systematic review and meta-analysis. Front Pharmacol. (2024) 15:1287761. doi: 10.3389/fphar.2024.1287761
24.
Zheng XS Shen Y Yang YY Zheng X‐S Yang Y‐Y He P et al . ED50 and ED95 of propofol combined with different doses of esketamine for children undergoing upper gastrointestinal endoscopy: a prospective dose-finding study using up-and-down sequential allocation method. J Clin Pharm Ther. (2022) 47:1002–9. doi: 10.1111/jcpt.13635
25.
Smallwood NE Pascoe A Wijsenbeek M Russell AM Holland AE Romero L et al . Opioids for the palliation of symptoms in people with serious respiratory illness: a systematic review and meta-analysis. Eur Respir Rev. (2024) 33:230265. doi: 10.1183/16000617.0265-2023
26.
Wei Y Chang L Hashimoto K . A historical review of antidepressant effects of ketamine and its enantiomers. Pharmacol Biochem Behav. (2020) 190:172870. doi: 10.1016/j.pbb.2020.172870
27.
Nakao S Nagata A Miyamoto E Masuzawa M Murayama T Shingu K . Inhibitory effect of propofol on ketamine-induced c-Fos expression in the rat posterior cingulate and retrosplenial cortices is mediated by GABAA receptor activation. Acta Anaesthesiol Scand. (2003) 47:284–90. doi: 10.1034/j.1399-6576.2003.00040.x
28.
Xu G Wang Y Chen Z Zhang Y Zhang X Zhang G . Esketamine improves propofol-induced brain injury and cognitive impairment in rats. Transl Neurosci. (2022) 13:430–9. doi: 10.1515/tnsci-2022-0251
29.
Oğuz AK Soyalp C Tunçdemir YE Tekeli AE Yüzkat N . Sedoanalgesia with dexmedetomidine in daily anesthesia practices: a prospective randomized controlled trial. BMC Anesthesiol. (2025) 25:45. doi: 10.1186/s12871-025-02918-1
30.
Suleiman A Wongtangman K Eikermann M Stucke AG . Neuroanatomical and pharmaco-physiological effects of hypoxia and esketamine on breathing, the sympathetic nerve system, and cortical function. Br J Anaesth. (2025) 134:277–80. doi: 10.1016/j.bja.2024.11.011
Summary
Keywords
esketamine, propofol, painless gastroscopy, reflex cough, safety
Citation
Gu R-s, Zhuang X-Y, Wu S-P, Huang X-Y, Lin Z-y and Zhang Y-F (2025) Safety and efficacy evaluation of low-dose of esketamine combined with propofol for painless gastroscopy: a single-center, randomized, double-blind, parallel controlled clinical trial. Front. Med. 12:1606134. doi: 10.3389/fmed.2025.1606134
Received
08 April 2025
Accepted
26 August 2025
Published
10 September 2025
Volume
12 - 2025
Edited by
Somchai Amornyotin, Mahidol University, Thailand
Reviewed by
Joao Massud, Independent Researcher, São Paulo, Brazil
Takehito Sato, Nagoya University Hospital, Japan
Sandeep Bhushan, Chengdu Second People's Hospital, China
Updates
Copyright
© 2025 Gu, Zhuang, Wu, Huang, Lin and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Fa Zhang, 10yfzhang1@stu.edu.cn
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.