- 1Division of Nuclear Medicine, Imaging Institute of Southern Switzerland, Ente Ospedaliero Cantonale, Bellinzona and Lugano, Switzerland
- 2Division of Nuclear Medicine, Candiolo Cancer Institute, Turin, Italy
- 3IRCCS Ospedale Policlinico San Martino, Genoa, Italy
- 4Department of Health Sciences (DISSAL), University of Genoa, Italy
- 5Department of Diagnostic Imaging (Radiology) and Nuclear Medicine, University Hospital San Pedro and Centre for Biomedical Research of La Rioja (CIBIR), Logroño, La Rioja, Spain
- 6Servicio Cántabro de Salud, Santander, España
- 7Faculty of Biomedical Sciences, Università della Svizzera italiana, Lugano, Switzerland
- 8Faculty of Biology and Medicine, University of Lausanne, Lausanne, Switzerland
Background: Prostate-specific membrane antigen (PSMA) ligand PET/CT has significantly improved prostate cancer (PCa) imaging. However, in patients with poorly differentiated PCa or neuroendocrine transdifferentiation, [18F]fluorodeoxyglucose ([18F]FDG) PET/CT may provide additional diagnostic information. This systematic review evaluates the diagnostic value of combining [18F]FDG PET/CT with PSMA ligand PET/CT in PCa patients.
Methods: A systematic literature search of studies assessing the added diagnostic value of dual-tracer [18F]FDG and PSMA ligands PET/CT in PCa patients was conducted using PubMed/MEDLINE and Cochrane Library databases and available information was summarized.
Results: Fourteen studies (n = 901 patients) met the inclusion criteria. The dual-tracer approach identified [18F]FDG-positive/PSMA-negative (FDG+/PSMA−) lesions in a subset of patients, particularly those with Gleason Score (GS) ≥ 9. However, in patients with GS < 8, [18F]FDG PET/CT did not significantly improve lesion detection over PSMA ligand PET/CT alone.
The presence of FDG+/PSMA− lesions correlated with aggressive tumor biology, increased risk of metastases, and worse prognosis.
Conclusion: Literature data showed that [18F]FDG PET/CT may serve as a valuable complementary imaging modality for high risk PCa patients potentially influencing staging and treatment decisions. Future prospective studies are warranted to further elucidate the prognostic significance and cost-effectiveness of combining [18F]FDG PET/CT with PSMA ligand PET/CT in PCa patients.
1 Introduction
Prostate cancer (PCa) represents the third most frequently diagnosed malignancy worldwide (1, 2). This high prevalence highlights the importance of improving diagnostic and therapeutic approaches to manage the disease effectively.
Metastatic PCa is initially hormone-sensitive; however, over time, it can acquire resistance to therapy, leading to the development of metastatic castration-resistant prostate cancer (mCRPC) (3).
This progression represents a significant clinical challenge, making it crucial to accurately identify the locations and extent of the disease to guide appropriate therapeutic strategies.
Since their introduction, prostate-specific membrane antigen (PSMA) ligand positron emission tomography/computed tomography (PET/CT) has markedly enhanced imaging sensitivity in PCa (4–6).
Although PSMA ligand PET/CT is generally considered superior to fluorine-18 fluorodeoxyglucose ([18F]FDG) PET/CT for detecting PCa lesions (7, 8), evidence from some studies suggests that [18F]FDG PET/CT may have utility or even demonstrates higher diagnostic sensitivity in patients with poorly differentiated adenocarcinoma or neuroendocrine differentiation compared to those with well-differentiated adenocarcinoma (9–11).
This review aims to summarize the existing evidence on the use of dual-tracer PET, combining [18F]FDG PET/CT and PSMA ligand PET/CT, for staging PCa at initial diagnosis and in a recurrence/progression setting.
2 Materials and methods
2.1 Protocol
The present systematic review was conducted following a predefined protocol (12), and the “Preferred Reporting Items for a Systematic Review and Meta-Analysis” (PRISMA 2020 statement) was used as a benchmark in its production (13).
The protocol for this systematic review was not pre-registered (as permitted by item 24 of the PRISMA checklist).
The first step in this systematic review was to define the research question using the PICO framework (Population, Intervention, Comparator, Outcomes). The focus was on patients diagnosed with PCa (Population) who underwent PSMA ligand PET/CT imaging (Intervention), with the addition of [18F]FDG PET/CT (Comparator). The main outcomes assessed were the potential changes in diagnostic accuracy, staging, and management of PCa when [18F]FDG PET/CT is included alongside PSMA ligand PET/CT imaging. Two independent reviewers (G.T. and C.M.I.) conducted the literature search, study selection, and quality assessment. Any discrepancies between reviewers were resolved through a consensus meeting with a third reviewer (A.R.).
2.2 Literature search strategy, information sources, and eligibility criteria
After formulating the review question, two independent reviewers (G.T. and C.M.I.) conducted a systematic search of PubMed/MEDLINE and the Cochrane Library to identify studies evaluating the diagnostic value of dual-tracer [18F]FDG and PSMA ligand PET/CT imaging in PCa. The search strategy incorporated terms such as “PSMA” and “FDG”, tailored to the context of dual-tracer imaging approaches. Boolean operators (AND, OR) were employed to refine the search and ensure relevant studies were included. No restrictions were applied regarding the publication year. Additionally, the reference lists of all included studies were screened for potentially relevant articles. The search was last updated on October 8, 2024.
Reviews, letters, comments, editorials and case reports were excluded from the systematic review to ensure methodological rigor. Only studies published in English were considered eligible for inclusion in the review. We aimed to summarize the available evidence for dual-tracer PET with [18F]FDG and PSMA ligands for PCa staging and restaging. Studies using dual-tracer PET for the purpose of radioligand therapy were excluded because they were outside the scope of this systematic review.
2.3 Study selection process and data collection
The titles and abstracts of all retrieved articles were independently screened by two reviewers (G.T. and C.M.I.) to assess eligibility based on predefined inclusion and exclusion criteria. Discrepancies were resolved through discussion. Relevant studies were selected systematically, and data extraction was performed using a standardized form to ensure consistency and accuracy. Information was collected from full texts, tables, figures, and supplementary materials.
For each included study, the extracted data encompassed: general study details (authors, publication year, country, study design, and funding sources); patient characteristics (sample size, enrollment date, age, clinical setting, Gleason score [GS], prostate-specific antigen [PSA] level and PSA doubling-time and previous treatment); key characteristics of the index test (type of PET tracers, two-scan interval, administered activity of each radiopharmaceutical, uptake times, scan time per bed, image analysis, image evaluators and other PET tracers, when reported); and outcomes of the included studies (validation of PET findings, detection rate of [18F]FDG PET/CT compared to PSMA ligand PET/CT, correlation of [18F]FDG PET/CT findings with GS and PSA levels, additional diagnostic value by using [18F]FDG PET/CT and main findings).
2.4 Quality assessment (risk of bias assessment)
The National Institutes of Health (NIH) quality assessment tool was used to evaluate the quality of the included studies and the risk of bias. Two reviewers independently evaluated the methodological quality of the studies (G.T. and A.R.). Reviewer disagreements regarding the quality assessment were resolved through a consensus meeting.
3 Results
3.1 Literature search and study selection
The literature search resulted in the identification of 382 records, which were assessed for eligibility according to the criteria described in the Materials and methods section. Based on predefined inclusion and exclusion criteria, 332 records were excluded because they were either unrelated to the field of interest or classified as case reports/case series or reviews/editorials/letters, even if relevant to the field. Of the remaining 50 studies, 36 were excluded after an in-depth evaluation of the full text, leaving 14 studies deemed eligible for inclusion in this systematic review. The selection process is summarized in Figure 1.
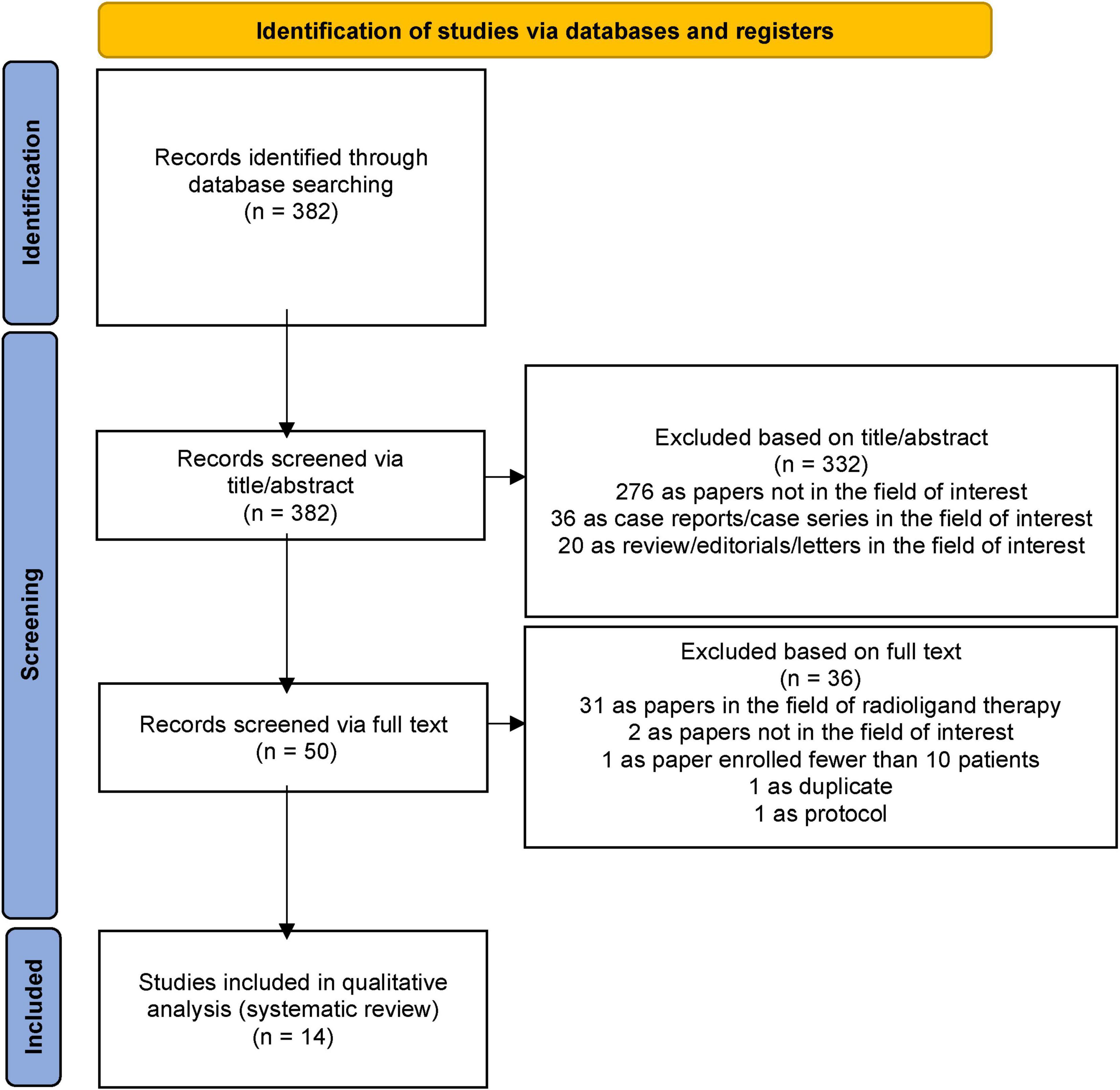
Figure 1. PRISMA flowchart summarizing the study selection process. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Research question: What is the diagnostic value of adding [18F]FDG PET/CT to PSMA ligand PET/CT in the diagnostic pathway of patients with prostate cancer.
Research string: ((FDG) OR (fluorodeoxyglucose)) AND ((PSMA) OR (prostate specific membrane antigen)) AND (prostat*).
Database screened: PubMed/MEDLINE and the Cochrane Library.
3.2 Study characteristics
The 14 studies selected based on predefined inclusion and exclusion criteria comprise a total of 901 prostate cancer patients who underwent both PSMA ligand and [18F]FDG PET/CT imaging. Their characteristics are analyzed in detail in Tables 1–3. The studies were published between 2020 and 2024 across various countries [China (9/14), Canada (1/14), Finland (1/14), Germany (1/14), South Korea (1/14), and the USA (1/14)]. Regarding study design, 13 out of 14 were single-center studies, while one was multicenter (14). Furthermore, seven studies were prospective, and the remaining seven were retrospective. All studies provide information on funding within the text.
The number of patients per study ranges from a minimum of 10 to a maximum of 145, as shown in Table 2. Patients were enrolled in the studies between June 2017 and November 2021, aged 50 to 86 years. At the time of diagnostic imaging, patients were in the following clinical settings: primary PCa staging (3/14), mCRPC (4/14), biochemical recurrence (BCR) (3/14), primary staging + BCR (1/14), hormone-sensitive prostate cancer (HSPC) + CRPC (1/14), early PSA progression on androgen-deprivation therapy (ADT) (1/14), and PCa (1/14). In 13 out of 14 publications, information on Gleason score (GS), PSA levels, and prior treatments is provided, as detailed in Table 2.
As shown in the index test key characteristics in Table 3, in addition to [18F]FDG used in all studies, the PSMA ligands employed were [68Ga]Ga-PSMA-11 in 7/14 studies, [18F]F-PSMA-1007 in 3/14, [18F]DCFPyL in 1/14, [68Ga]Ga-PSMA-617 in 1/14, [68Ga]Ga-PSMA-11 + [18F]F-PSMA-1007 in 1/14, and [68Ga]Ga-PSMA in 1/14. The interval between the two acquisitions, PET/CT with [18F]FDG and PSMA ligand, was reported in 13 of the 14 studies, ranging from 1 to 48 days. The administered activity for each tracer was detailed in 12 of the 14 studies. Comprehensive data, including the time between radiotracer administration and acquisition, as well as additional acquisition and image analysis parameters, are presented in Table 3. Notably, 4 out of 14 studies also employed a third radiotracer in addition to [18F]FDG and PSMA ligand. However, the results of these additional tracers were excluded from this review as they fall outside the scope of its focus.
3.3 Risk of bias and applicability
According to the NIH quality assessment tool, none of the included studies had critical low quality.
3.4 Results of individual studies
3.4.1 Lesions identified exclusively with [18F]FDG (FDG+/PSMA−)
A comprehensive analysis of the studies under review and data extraction from each revealed that, in most studies, certain lesions were positive only on [18F]FDG PET/CT and negative on PSMA ligand PET/CT. Further details regarding the added diagnostic value of [18F]FDG PET/CT in patients with prostate cancer are presented in Table 2.
3.4.2 Correlation between GS, PSA levels, and [18F]FDG PET/CT positivity
An evaluation of the included studies demonstrated a significant correlation between GS, PSA levels, and [18F]FDG PET/CT-positive lesions in patients with PCa across different disease stages. Specifically, this association was evident for GS ≥ 9 according to Xu et al. (17), for GS ≥ 8 according to the first study by Chen et al. (26), and for GS ≥ 8 and PSA ≥ 7.9 ng/mL in the second study by Chen et al. (21). The clinical characteristics of patients in different studies and additional reports supporting this correlation are detailed in Tables 2, 4.
3.4.3 Prognostic significance
The prognostic role of integrating [18F]FDG PET/CT into the diagnostic pathway for patients with PCa was thoroughly evaluated by Kim et al. (18), who concluded that the addition of [18F]FDG PET/CT to PSMA ligand PET/CT can help identify tumors with aggressive biological behavior.
4 Discussion
PSMA-ligand PET/CT is widely used for staging and biochemical recurrence of PCa. However, PCa may appear negative on PSMA-ligand PET/CT (28, 29), and, as observed in the studies analyzed in this review, low PSMA expression could be associated with [18F]FDG positivity.
An analysis of the available data confirms that FDG+/PSMA− lesions are not uncommon in PCa and can be observed in a significant proportion of patients in different settings (14, 20, 21). Additionally, a subgroup of mCRPCa patients, accounting for 6% in the study by Pouliot et al., exhibited exclusively FDG+/PSMA− disease (i.e., absence of PSMA + disease), with a similar prevalence to the 10% reported in the TheraP trial (30).
For these patients, [18F]FDG PET/CT could serve as a useful complementary diagnostic tool at various stages of prostate cancer management, given that, until now, [18F]FDG positivity has primarily been considered at a late stage of the disease as a selection criterion before radioligand therapy or as a poor prognostic factor following such treatments (31).
However, the utility of [18F]FDG PET/CT may extend to the staging phase, as demonstrated in the study by Kim et al., which also evaluated its prognostic impact (18). The collected data indicated that PCa exhibiting [18F]FDG uptake were significantly larger, associated with ductal-dominant histology, and characterized by higher GS and initial PSA levels compared to those [18F]FDG negative (18). Similarly, Chen et al. found that patients with FDG+/PSMA− lesions had higher GS and PSA levels than those without such lesions (21).
Of particular relevance is the observation that patients with [18F]FDG-avid tumors had a higher incidence of lymph node and/or distant metastases at initial staging, as well as an increased biochemical recurrence rate postoperatively, compared to those with non-[18F]FDG-avid tumors (18).
Using a dual-tracer approach could enable more accurate diagnostic assessment in a specific subgroup of patients. Furthermore, these data suggest that identifying this patient subgroup could have significant prognostic implications. However, as reported in the study by Chen et al. (21), only 23.2% of the analyzed patients had at least one FDG+/PSMA− lesion, underscoring the need for careful patient selection for [18F]FDG PET/CT. The objective is to optimize its use, reserving it for cases where it provides a clear diagnostic benefit, particularly in high-risk PCa patients.
An analysis of the studies suggests that the GS may serve as a valuable criterion for predicting the presence of FDG+/PSMA− lesions. A recurring finding across the reviewed studies was that patients with a high GS were more likely to present FDG+/PSMA− lesions than those with a lower score. Specifically, Xu et al. highlighted this correlation for a GS ≥ 9 (17), while both studies by Chen et al. found an association starting from a GS of 8 (21, 26).
Previous studies have also confirmed the diagnostic value of [18F]FDG PET/CT in patients with PCa and high GS (9, 32). In line with this evidence, our analysis suggests that the GS is a key predictor for identifying patients with FDG+/PSMA− lesions. Supporting this finding, Chen et al. demonstrated that the probability of detecting FDG+/PSMA− lesions was 0% in patients with a GS < 8, while it reached 61.5% in those with a GS ≥ 8. Adding [18F]FDG PET/CT in the low-score group did not improve the detection rate compared to PSMA-ligand PET/CT alone. Conversely, in patients with high GS, integrating [18F]FDG PET/CT increased the detection rates of local recurrence, lymph node metastases, and distant metastases from 0 to 7.7%, 30.8 to 61.5%, and 53.8 to 61.5%, respectively, compared to PSMA-ligand PET/CT alone (21). These findings suggest that [18F]FDG PET/CT may not be indicated in patients with a low probability of FDG+/PSMA− lesions but could be more beneficial in those with a high likelihood of such lesions.
While the correlation between a GS ≥ 9 and the presence of FDG+/PSMA− lesions appears well established (17), the association with a GS of 8 requires further investigation. Moreover, the implications of these findings will help in selecting patients for radionuclide therapy, as a prerequisite for this is that PCa lesions are PSMA + (33).
Although studies with therapeutic intent were excluded from the present systematic review, several pivotal clinical trials that employed dual-tracer PET imaging for patient selection in [177Lu]Lu—PSMA radioligand therapy deserve detailed discussion. In the TheraP trial, involving patients with mCRPC who had previously received docetaxel, [18F]FDG PET/CT was systematically used during screening to identify and exclude individuals with discordant disease, and approximately 18% of screened patients were excluded due to this finding (30). Similarly, the ENZA-p trial, which randomized patients with treatment-naïve mCRPC to enzalutamide ± [177Lu]Lu-PSMA-617, incorporated [18F]FDG PET/CT at baseline to refine patient selection. While the exclusion criteria in this study were less stringent than in TheraP, the use of [18F]FDG PET/CT remained a central component in identifying those with low or heterogeneous PSMA expression, who may be less likely to benefit from PSMA-targeted therapy (34). Moreover, the UpFrontPSMA trial extended this approach into an earlier disease setting, evaluating [177Lu]Lu-PSMA-617 followed by docetaxel versus docetaxel alone in patients with de novo high-volume metastatic hormone-sensitive prostate cancer (35). [18F]FDG PET/CT was used at baseline to exclude patients with extensive FDG+/PSMA− disease, highlighting its growing role in the late-stage setting and in therapeutic stratification during initial management. These studies underscore a paradigm shift whereby [18F]FDG PET/CT is no longer viewed solely as a diagnostic adjunct but increasingly as a biological stratification tool. However, as confirmed by our systematic review, identifying patients with FDG+/PSMA− mismatch disease remains a substantial unmet clinical need, and more studies are needed to establish predictive factors for this condition. While guidelines and expert consensus frequently recommend [18F]FDG PET/CT “only in selected cases”, precise criteria, whether clinical, biochemical, or imaging-based, are largely undefined. Recent efforts have been made to address this gap. For example, Pan et al. developed a prediction nomogram based on clinical and imaging features (including SUVmax, PSA levels, bone metastases, prior docetaxel treatment, and alkaline phosphatase), achieving an AUC of 0.83 for the model and demonstrating its potential to guide the use of [18F]FDG PET/CT more selectively in mCRPC (36). Furthermore, a study by Telli et al. has shown that the [18F]FDG-derived tumor burden is an independent prognostic factor for overall survival, even among patients eligible for PSMA-based radioligand therapy, regardless of treatment received. This finding highlights the prognostic value of [18F]FDG PET/CT beyond simple eligibility determination and supports its role in treatment planning (37). Notably, predictive factors for FDG+/PSMA− disease may vary depending on the phase of PCa’s natural history. GS (particularly ≥ 8) appears to be a significant predictor in the primary staging setting. In contrast, in advanced mCRPC, disease biologic behavior may be influenced by prior systemic therapies and treatment-induced neuroendocrine differentiation, potentially diminishing the relevance of the original histological grading.
However, it is noteworthy that studies employing dual-tracer PET imaging for the purpose of selecting patients for [177Lu]-PSMA radioligand therapy were excluded from this systematic review. These studies were not considered eligible (as stated in the methods section) as the primary aim of our review was to explore the diagnostic potential of [18F]FDG PET/CT in PCa patients across different clinical settings, regardless of pre-therapeutic stratification. Therefore, in order to maintain methodological consistency and ensure alignment with the objectives of this review, such studies were excluded from the analysis.
Finally, it should be underlined that our systematic review has several limitations. The most important are the limited number of available studies on the topic of interest and the significant heterogeneity of the clinical characteristics of patients enrolled (including different PCa settings) and methodological aspects of eligible studies. Due to this heterogeneity a meta-analysis is not appropriate (12). Another consideration emerging from the literature is the non-negligible incidence of false-positive bone findings on PSMA-ligand PET/CT scans. The occurrence of unspecific bone uptakes varies considerably depending on the PSMA tracer used, with [18F]PSMA-1007 being associated with the highest prevalence (38). Consequently, the actual number of PSMA-positive bone lesions that truly represent metastatic deposits may be overestimated, particularly in the studies employing [18F]PSMA-1007 (15, 18, 19, 25).
Notably, we have not considered the specific diagnostic accuracy values of [18F]FDG PET/CT and PSMA—ligand PET/CT scans in PCa. The current evidences already showed that PSMA—ligand PET/CT had higher diagnostic accuracy in detecting PCa lesions compared with [18F]FDG PET/CT both in terms of sensitivity and specificity (39). Results from a previous meta-analysis already showed that the pooled sensitivities of PSMA—ligand PET/CT ranged from 91 to 92% compared to 75% for [18F]FDG PET/CT; the pooled specificity ranged from 73 to 88% for PSMA—ligand PET/CT compared to 64% for [18F]FDG PET/CT (39).
As dosimetric aspects are relevant for nuclear medicine imaging and therapy of PCa (40), we would like to underline that performing dual-tracer PET/CT will increase the radiation exposure for the patients. Future studies should better clarify the advantages of performing dual-tracer PET/CT compared to the potential risks related to an increased radiation exposure. Several methods could be used to reduce the radiation exposure related to the PET and CT component of hybrid imaging (41, 42).
Even taking into account these limitations, we believe that our evidence-based manuscript has provided significant information about dual-tracer PET/CT in PCa. In particular, compared to a previous systematic review on the same topic (43), we have included more studies excluding case reports (affected by significant biases) providing an update of the literature through a systematic summary and suggesting further studies to solve the current knowledge gaps. As practical application, we can suggest the use of dual-tracer PET/CT in selected cases of PCa, in particular in aggressive variants, but this approach should be confirmed by further well-designed studies.
5 Conclusion
In conclusion, our systematic review demonstrated that dual-tracer PET/CT approach improved PCa lesion detection due to [18F]FDG-positive/PSMA-negative (FDG+/PSMA−) lesions in a subset of PCa patients, particularly those with GS ≥ 9. However, in patients with GS < 8, [18F]FDG PET/CT did not significantly improve lesion detection over PSMA ligand PET/CT alone. The presence of FDG+/PSMA− lesions correlated with aggressive tumor biology, increased risk of metastases, and worse prognosis.
Available literature data suggest that adding [18F]FDG PET/CT to PSMA-ligand PET/CT may have a role in PCa in selected patients, particularly those with aggressive variants of PCa. Although the potential added value of dual-tracer PET/CT for PCa lesion detection in this specific patient group has been established, the prognostic impact and cost-effectiveness of dual-tracer PET/CT in PCa remain to be determined through further studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CI: Writing – review and editing, Writing – original draft. MC: Writing – review and editing, Writing – original draft. AR: Writing – original draft, Writing – review and editing. MB: Writing – original draft, Writing – review and editing. RD: Writing – review and editing, Writing – original draft. GP: Writing – review and editing, Writing – original draft. GT: Writing – review and editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
3. Ryan C, Smith M, Fizazi K, Saad F, Mulders P, Sternberg C, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. (2015) 16:152–60. doi: 10.1016/S1470-2045(14)71205-7
4. Oprea-Lager D, MacLennan S, Bjartell A, Briganti A, Burger I, de Jong I, et al. European association of nuclear medicine focus 5: Consensus on molecular imaging and theranostics in prostate cancer. Eur Urol. (2024) 85:49–60. doi: 10.1016/j.eururo.2023.09.003
5. Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: A systematic review and meta-analysis. Eur Urol. (2020) 77:403–17. doi: 10.1016/j.eururo.2019.01.049
6. Annunziata S, Pizzuto D, Treglia G. Diagnostic performance of PET imaging using different radiopharmaceuticals in prostate cancer according to published meta-analyses. Cancers (Basel). (2020) 12:2153. doi: 10.3390/cancers12082153
7. Crocerossa F, Marchioni M, Novara G, Carbonara U, Ferro M, Russo G, et al. Detection rate of prostate specific membrane antigen tracers for positron emission tomography/computerized tomography in prostate cancer biochemical recurrence: A systematic review and network meta-analysis. J Urol. (2021) 205:356–69. doi: 10.1097/JU.0000000000001369
8. Fendler W, Calais J, Eiber M, Flavell R, Mishoe A, Feng F, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localising recurrent prostate cancer: A prospective single-arm clinical trial. JAMA Oncol. (2019) 5:856–63. doi: 10.1001/jamaoncol.2019.0096
9. Jadvar H. Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: Utility and limitations. Eur J Nucl Med Mol Imaging. (2013) 40:S5–10. doi: 10.1007/s00259-013-2361-7
10. Richter J, Rodríguez M, Rioja J, Peñuelas I, Martí-Climent J, Garrastachu P, et al. Dual tracer 11C-choline and FDG-PET in the diagnosis of biochemical prostate cancer relapse after radical treatment. Mol Imaging Biol. (2010) 12:210–7. doi: 10.1007/s11307-009-0243-y
11. Dondi F, Antonelli A, Suardi N, Guerini A, Albano D, Lucchini S, et al. /CT and conventional imaging for the assessment of neuroendocrine prostate cancer: A systematic review. Cancers (Basel). (2023) 15:4404. doi: 10.3390/cancers15174404
12. Sadeghi R, Treglia G. Systematic reviews and meta-analyses of diagnostic studies: A practical guideline. Clin. Transl. Imaging. (2017) 5:83–7. doi: 10.1007/s40336-016-0219-2
13. Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
14. Pouliot F, Saad F, Rousseau E, Richard P, Zamanian A, Probst S, et al. Intrapatient intermetastatic heterogeneity determined by triple-tracer PET imaging in mCRPC patients and correlation to survival: The 3TMPO cohort study. J Nucl Med. (2024) 65:1710–7. doi: 10.2967/jnumed.124.268020
15. Pabst K, Mei R, Lückerath K, Hadaschik B, Kesch C, Rawitzer J, et al. Detection of tumour heterogeneity in patients with advanced, metastatic castration-resistant prostate cancer on [68Ga]Ga-/[18F]F-PSMA-11/-1007, [68Ga]Ga-FAPI-46 and 2-[18F]FDG PET/CT: A pilot study. Eur J Nucl Med Mol Imaging. (2024) 52:342–53. doi: 10.1007/s00259-024-06891-8
16. Pan J, Wu J, Wang B, Zhu B, Liu X, Gan H, et al. Interlesional response heterogeneity is associated with the prognosis of abiraterone treatment in metastatic castration-resistant prostate cancer. Med. (2024) 5:1475–84.e3. doi: 10.1016/j.medj.2024.07.020
17. Xu L, Chen R, Yu X, Liu J, Wang Y. 18F-FDG PET is not inferior to 68Ga-PSMA PET for detecting biochemical recurrent prostate cancer with a high gleason score: A head-to-head comparison study. Diagnostics (Basel). (2023) 14:7. doi: 10.3390/diagnostics14010007
18. Kim J, Lee S, Kim D, Kim H, Oh K, Kim S, et al. Combination of [18F]FDG and [18F]PSMA-1007 PET/CT predicts tumour aggressiveness at staging and biochemical failure postoperatively in patients with prostate cancer. Eur J Nucl Med Mol Imaging. (2024) 51:1763–72. doi: 10.1007/s00259-023-06585-7
19. Malaspina S, Ettala O, Tolvanen T, Rajander J, Eskola O, Boström P, et al. Flare on [18F]PSMA-1007 PET/CT after short-term androgen deprivation therapy and its correlation to FDG uptake: Possible marker of tumor aggressiveness in treatment-naïve metastatic prostate cancer patients. Eur J Nucl Med Mol Imaging. (2023) 50:613–21. doi: 10.1007/s00259-022-05970-y
20. Pan J, Wei Y, Zhang T, Liu C, Hu X, Zhao J, et al. Stereotactic radiotherapy for lesions detected via 68Ga-prostate-specific membrane antigen and 18F-fluorodexyglucose positron emission tomography/computed tomography in patients with non-metastatic prostate cancer with early prostate-specific antigen progression on androgen deprivation therapy: A prospective single-center study. Eur Urol Oncol. (2022) 5:420–7. doi: 10.1016/j.euo.2022.02.002
21. Chen R, Wang Y, Zhu Y, Shi Y, Xu L, Huang G, et al. The added value of 18F-FDG PET/CT compared with 68Ga-PSMA PET/CT in patients with castration-resistant prostate cancer. J Nucl Med. (2022) 63:69–75. doi: 10.2967/jnumed.120.262250
22. Fourquet A, Rosenberg A, Mena E, Shih J, Turkbey B, Blain M, et al. A Comparison of 18F-DCFPyL, 18F-NaF, and 18F-FDG PET/CT in a prospective cohort of men with metastatic prostate cancer. J Nucl Med. (2022) 63:735–41. doi: 10.2967/jnumed.121.262371
23. Shi Y, Wu J, Xu L, Zhu Y, Wang Y, Huang G, et al. The heterogeneous metabolic patterns of ganglia in 68Ga-PSMA, 11C-choline, and 18F-FDG PET/CT in prostate cancer patients. Front Oncol. (2021) 11:666308. doi: 10.3389/fonc.2021.666308
24. Shi Y, Xu L, Zhu Y, Wang Y, Chen R, Liu J. Use of 68Ga-PSMA-11 and 18F-FDG PET-CT dual-tracer to differentiate between lymph node metastases and ganglia. Front Oncol. (2021) 11:646110. doi: 10.3389/fonc.2021.646110
25. Zhou X, Li Y, Jiang X, Wang X, Chen S, Shen T, et al. Intra-individual comparison of 18F-PSMA-1007 and 18F-FDG PET/CT in the evaluation of patients with prostate cancer. Front Oncol. (2021) 10:585213. doi: 10.3389/fonc.2020.585213
26. Chen R, Wang Y, Shi Y, Zhu Y, Xu L, Huang G, et al. Diagnostic value of 18F-FDG PET/CT in patients with biochemical recurrent prostate cancer and negative 68Ga-PSMA PET/CT. Eur J Nucl Med Mol Imaging. (2021) 48:2970–7. doi: 10.1007/s00259-021-05221-6
27. Wang B, Liu C, Wei Y, Meng J, Zhang Y, Gan H, et al. A prospective trial of 68Ga-PSMA and 18F-FDG PET/CT in non-metastatic prostate cancer patients with an early PSA progression during castration. Clin Cancer Res. (2020) 26:4551–8. doi: 10.1158/1078-0432.CCR-20-0587
28. Weber M, Kurek C, Barbato F, Eiber M, Maurer T, Nader M, et al. PSMA-ligand PET for early castration-resistant prostate cancer: A retrospective single-center study. J Nucl Med. (2021) 62:88–91. doi: 10.2967/jnumed.120.245456
29. Weber M, Hadaschik B, Ferdinandus J, Rahbar K, Bögemann M, Herrmann K, et al. Prostate-specific membrane antigen-based imaging of castration-resistant prostate cancer. Eur Urol Focus. (2021) 7:279–87. doi: 10.1016/j.euf.2021.01.002
30. Hofman M, Emmett L, Sandhu S, Iravani A, Joshua A, Goh J, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet. (2021) 397:797–804. doi: 10.1016/S0140-6736(21)00237-3
31. Michalski K, Ruf J, Goetz C, Seitz A, Buck A, Lapa C, et al. Prognostic implications of dual tracer PET/CT: PSMA ligand and [18F]FDG PET/CT in patients undergoing [177Lu]PSMA radioligand therapy. Eur J Nucl Med Mol Imaging. (2021) 48:2024–30. doi: 10.1007/s00259-020-05160-8
32. Öztürk H, Karapolat I. 18F-fluorodeoxyglucose PET/CT for detection of disease in patients with prostate-specific antigen relapse following radical treatment of a local-stage prostate cancer. Oncol Lett. (2016) 11:316–22. doi: 10.3892/ol.2015.3903
33. Almeida L, Etchebehere E, García Megías I, Calapaquí Terán A, Hadaschik B, Colletti P, et al. Radioligand Therapy in Prostate Cancer: Where Are We and Where Are We Heading? Clin Nucl Med. (2024) 49:45–55. doi: 10.1097/RLU.0000000000004919
34. Emmett L, Subramaniam S, Crumbaker M, Nguyen A, Joshua A, Weickhardt A, et al. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. (2024) 25:563–71. doi: 10.1016/S1470-2045(24)00135-9
35. Azad A, Bressel M, Tan H, Voskoboynik M, Suder A, Weickhardt A, et al. Sequential [177Lu]Lu-PSMA-617 and docetaxel versus docetaxel in patients with metastatic hormone-sensitive prostate cancer (UpFrontPSMA): A multicentre, open-label, randomised, phase 2 study. Lancet Oncol. (2024) 25:1267–76. doi: 10.1016/S1470-2045(24)00440-6
36. Pan J, Zhang T, Chen S, Bu T, Zhao J, Ni X, et al. Nomogram to predict the presence of PSMA-negative but FDG-positive lesion in castration-resistant prostate cancer: A multicenter cohort study. Ther Adv Med Oncol. (2024) 16:17588359231220506. doi: 10.1177/17588359231220506
37. Telli T, Lopes L, Karpinski M, Pabst K, Grünwald V, Shi K, et al. Prognostic value of [18F]FDG- and PSMA-PET in patients evaluated for [177Lu]Lu-PSMA therapy of mCRPC. Eur J Nucl Med Mol Imaging. (2025): doi: 10.1007/s00259-025-07198-y Epub ahead of print.
38. Rizzo A, Morbelli S, Albano D, Fornarini G, Cioffi M, Laudicella R, et al. The Homunculus of unspecific bone uptakes associated with PSMA-targeted tracers: A systematic review-based definition. Eur J Nucl Med Mol Imaging. (2024) 51:3753–64. doi: 10.1007/s00259-024-06797-5
39. Yu W, Zhao M, Deng Y, Liu S, Du G, Yan B, et al. Meta-analysis of 18 F-PSMA-1007 PET/CT, 18 F-FDG PET/CT, and 68Ga-PSMA PET/CT in diagnostic efficacy of prostate cancer. Cancer Imaging. (2023) 23:77. doi: 10.1186/s40644-023-00599-y
40. Dadgar H, Pashazadeh A, Norouzbeigi N, Assadi M, Al-Balooshi B, Baum R, et al. Targeted radioligand therapy: Physics and biology, internal dosimetry and other practical aspects during 177Lu/225Ac treatment in neuroendocrine tumors and metastatic prostate cancer. Theranostics. (2025) 15:4368–97. doi: 10.7150/thno.107963
41. Singh P, Diwakar M, Gupta R, Kumar S, Chakraborty A, Bajal E, et al. A method noise-based convolutional neural network technique for CT image denoising. Electronics. (2022) 11:3535. doi: 10.3390/electronics11213535
42. Diwakar M, Singh P. CT image denoising using multivariate model and its method noise thresholding in non-subsampled shearlet domain. Biomed Signal Process Control. (2020) 57:101754. doi: 10.1016/j.bspc.2019.101754
Keywords: prostate cancer, PSMA, [18F]FDG, PET/CT, dual-tracer, hybrid imaging, diagnostic, Gleason score
Citation: Iacovitti CM, Cuzzocrea M, Rizzo A, Bauckneht M, Delgado Bolton RC, Paone G and Treglia G (2025) Diagnostic value of dual-tracer PET/CT with [18F]FDG and PSMA ligands in prostate cancer: an updated systematic review. Front. Med. 12:1607227. doi: 10.3389/fmed.2025.1607227
Received: 07 April 2025; Accepted: 12 June 2025;
Published: 09 July 2025.
Edited by:
Taja Lozar, Institute of Oncology Ljubljana, SloveniaReviewed by:
Prabhishek Singh, Bennett University, IndiaAditi Mulgaonkar, University of Texas Southwestern Medical Center, United States
Copyright © 2025 Iacovitti, Cuzzocrea, Rizzo, Bauckneht, Delgado Bolton, Paone and Treglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giorgio Treglia, Z2lvcmdpb21lZG51Y0BsaWJlcm8uaXQ=
 Cesare Michele Iacovitti1
Cesare Michele Iacovitti1 Marco Cuzzocrea
Marco Cuzzocrea Alessio Rizzo
Alessio Rizzo Matteo Bauckneht
Matteo Bauckneht Roberto C. Delgado Bolton
Roberto C. Delgado Bolton Gaetano Paone
Gaetano Paone Giorgio Treglia
Giorgio Treglia