- 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2Eye College of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
Foreign body ingestion (FBI) is considered a widespread global health concern, with fishbone ingestion (FI) occurring frequently. However, fishbone-induced intestinal perforation (FIIP) remains rare and is frequently overlooked in the initial differential diagnosis. A case involving a 39-year-old patient presenting with acute abdominal pain was diagnosed as FIIP. Initial laparoscopic surgery was followed by a laparotomy for fishbone removal, resulting in a favorable patient recovery. The existing literature on FIIP is reviewed in this article. Reported cases underscore the necessity of prompt identification of the perforation’s cause and the critical role of thorough medical history-taking. Computed tomography (CT) and ultrasonography are considered essential diagnostic tools in confirming the condition. While ultrasonography serves as a rapid, non-invasive preliminary examination, CT is regarded as more accurate and comprehensive. In regions with high fish consumption, FIIP should be considered in adult patients, especially the elderly. Retained fishbones may result in serious complications and should be removed whenever feasible. Clinical education is considered vital in minimizing delays in diagnosis and treatment. The least invasive treatment strategy should be selected according to the patient’s clinical status.
1 Introduction
Currently, over 4,000 peer-reviewed publications worldwide have reported clinical case reports and case series related to foreign body ingestion (FBI). Most FBIs are expelled spontaneously or result in minor mucosal injuries. However, in rare cases, sharp foreign bodies may lead to gastrointestinal perforation, severe gastrointestinal hemorrhage, sepsis, hepatic abscess, or even death (1–3). It is estimated that approximately 1% of all ingested foreign bodies result in complications, such as mucosal injuries, impaction, or perforation (4). Among these complications, up to 63% are attributed to fish bones, highlighting the clinical relevance of fishbone-related injuries. While intestinal perforation is one of the rarest outcomes, fishbone-induced intestinal perforation (FIIP) remains a distinct and potentially life-threatening condition that warrants heightened clinical awareness (5). Gastrointestinal perforations caused by the FBI have not received sufficient attention. In numerous low- and middle-income countries globally, fish has emerged as a principal source of animal protein and essential nutrients (6, 7). A case of terminal ileal perforation resulting from accidental fishbone ingestion (FI) is presented. Given the absence of prior comprehensive reviews on FIIP, a summary of all relevant studies is warranted. A comprehensive literature review was conducted, focusing on clinical characteristics, patient demographics, diagnostic imaging, surgical and conservative management strategies, and geographical patterns, to enhance diagnostic accuracy and therapeutic outcomes.
2 Case presentation
A 39-year-old male patient was admitted with a 3-day history of abdominal distension. He had no significant medical history and no prior abdominal surgeries. During this time, he reported decreased appetite, absence of bowel movements for 2 days, fever peaking at 38.5°C, and chills. He attributed all symptoms to an upper respiratory tract infection. Upon admission, nausea, vomiting, and diarrhea were denied. Physical examination revealed abdominal distension, fever (38.6°C), diffuse tenderness in the mid-to-upper abdomen, tympany on percussion, abdominal guarding, and absence of rebound tenderness. Laboratory tests demonstrated leukocytosis (WBC count: 11.78 × 109/L), neutrophilia (NEUT count: 8.06 × 109/L), and elevated C-reactive protein (CRP) levels (244.75 mg/L). Computed tomography (CT) scan revealed scattered gas and fluid in parts of the mid-to-upper small intestine without notable dilation, areas of increased and hazy fat density with patchy shadows in the abdomen, adjacent peritoneal thickening, and an irregular arc-shaped hyperdense structure partially penetrating the intestinal wall was identified within a segment of the left mid-abdominal small intestine (Figure 1), measuring approximately 3 cm in length (Figure 1). The patient subsequently recalled ingesting a fishbone of similar length 3 days prior. Emergency laparoscopic exploration was initiated and subsequently converted to open laparotomy. Intraoperatively, marked dilatation of the small intestine was observed, without bile leakage or purulent fluid in the peritoneal cavity. An embedded fishbone was found lodged in the terminal ileum (2 cm from the ileocecal valve), with its tip (approximately 0.5 cm long) protruding through the intestinal wall, while the rest remained in the intestinal lumen. Mild adhesions, localized inflammatory reaction, and minimal purulent exudate were noted at the site of perforation, with no evidence of abscess formation nearby. Through limited enlargement of the perforation site, a 3-cm-long fishbone was completely extracted (Figure 2). The perforation site was closed with four 4-0 absorbable sutures (Figure 2). A peritoneal drain was placed adjacent to the perforation site in the peritoneal cavity for drainage. An additional drain was positioned in the pelvic cavity to prevent postoperative inflammatory exudate accumulation, given that the pelvis is the lowest point of the peritoneal cavity when upright. No evidence of ischemic necrosis in the small intestine was observed, hence bowel resection was unnecessary. The procedure was uneventful. The fishbone was successfully removed, and the intestinal defect was repaired. The patient passed flatus on postoperative day 2, resumed oral intake thereafter, had a bowel movement on postoperative day 5, and was discharged on day 7.
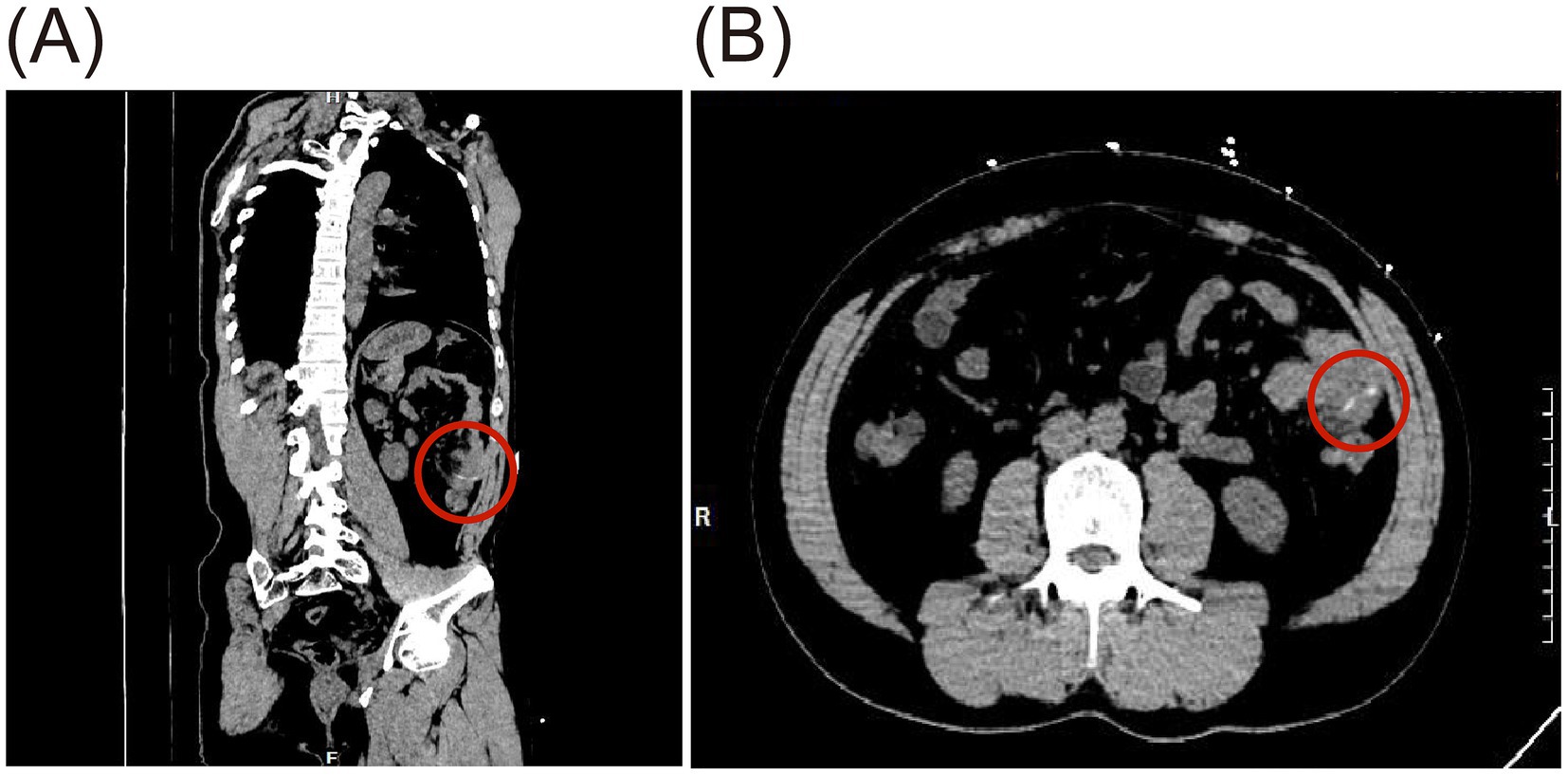
Figure 1. Preoperative CT imaging of small bowel perforation caused by FI, showing a linear hyperdense foreign body about 3 cm long in the small intestine (red circle). (A) Oblique sagittal plane. (B) Coronal plane.

Figure 2. The fishbone that had perforated the small intestine was removed during surgery. (A) Intraoperative findings revealed that the fishbone had perforated the small intestine, and the wound was closed using 4-0 absorbable sutured. (B) The fishbone was successfully removed and displayed.
3 Discussion
FBI may result in clinical outcomes ranging from mild symptoms to life-threatening complications (8), most frequently observed in children between 6 months and 6 years of age (9). Among adults, aside from food bolus impaction secondary to underlying gastrointestinal pathology, FBI is more frequently reported in elderly individuals, patients with intellectual disabilities, individuals with alcohol use disorder, incarcerated populations, and those with psychiatric conditions (10). The majority of FBI cases are asymptomatic, with gastrointestinal perforation constituting a rare complication, occurring in less than 1% of cases (11, 12). Once perforation occurs, the risk of localized infection, gastrointestinal hemorrhage, abscess formation, peritonitis, and sepsis increases significantly. Approximately 1 to 14% of patients may require surgical intervention (11, 13–15). Ingestion of sharp objects—such as animal bones, sewing needles, and toothpicks—represents the major risk factor for gastrointestinal perforation caused by FBI (12).
The reported frequency of FBI varies considerably across published studies. In adults, fishbones constitute the most frequently ingested food-related foreign bodies, accounting for 9 to 45% of cases (16). This variability is likely attributable to regional dietary practices, increased consumption of fish as a protein source, and the recognized health benefits associated with fish consumption (17, 18). Most fishbones are sharp, small, and resistant to gastric acid, making them particularly prone to causing intestinal perforation following ingestion. FIIP typically occurs unintentionally and without the patient’s awareness. When symptoms related to gastrointestinal perforation emerge, patients often have difficulty recalling any history of FBI. In the case presented, the patient initially sought treatment at a local clinic for fever, abdominal distension, and anorexia over a 3-day period, during which an upper respiratory tract infection was diagnosed. The accidental ingestion of a fishbone was recalled only after the CT findings were disclosed. Based on both the literature review and the current case, a multidimensional analysis of FIIP was conducted (Table 1).
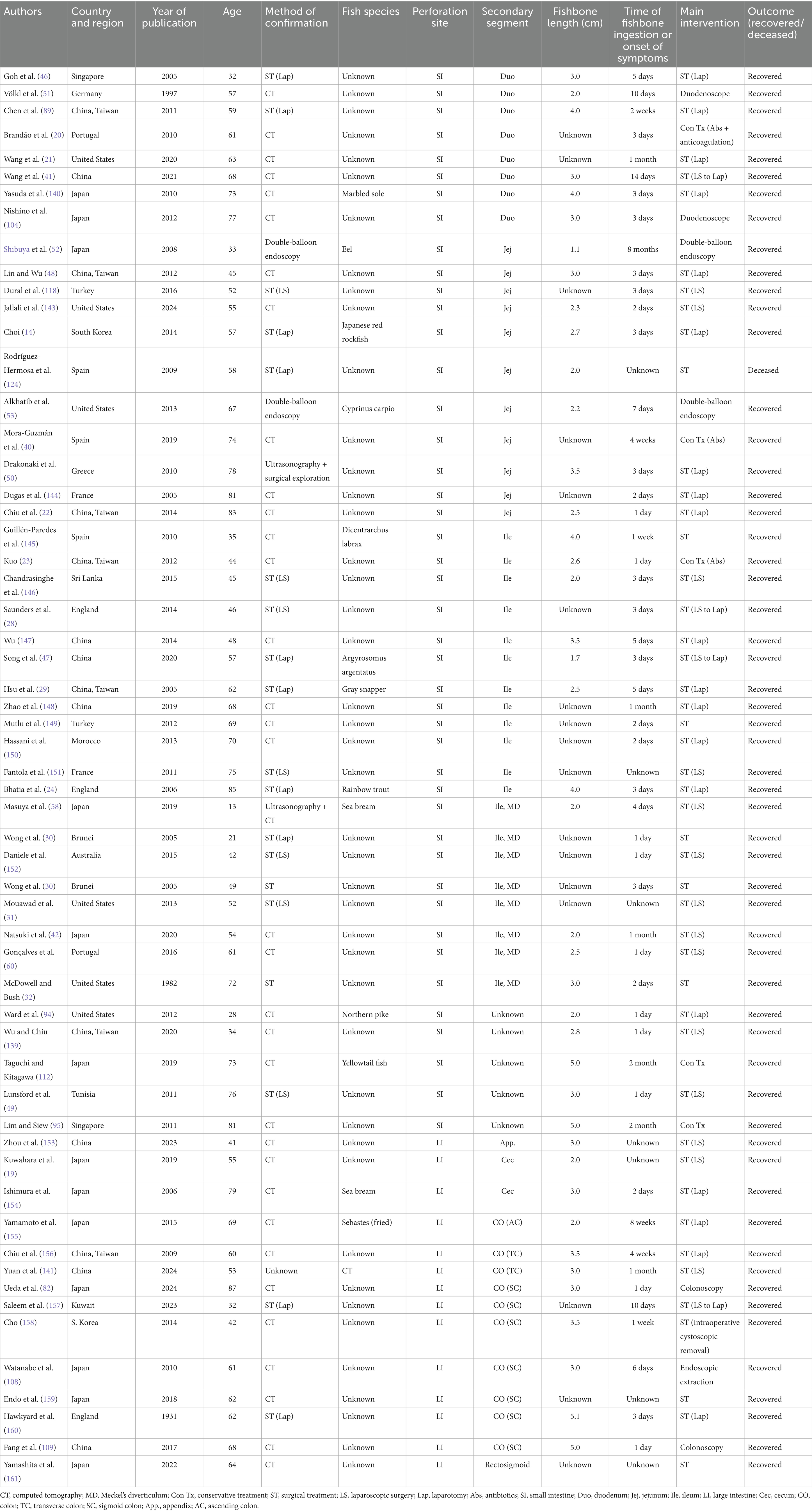
Table 1. Previously published case reports and case series of fishbone-induced intestinal perforation, categorized by country, patient age, diagnostic method, type of fish, location, onset of symptoms or time of ingestion, prognosis, and therapeutic interventions (N = 58).
From 1949 to 2025, a literature search was conducted using search engines including PubMed, Embase, MEDLINE, CINAHL, Ovid, and Cochrane databases. The search terms included “fish bone,” “fishbone,” “fish*,” “seafood,” “intestinal perforation,” “bowel perforation,” “foreign body,” “ingestion,” “peritoneal abscess,” “intestinal injury,” “perforation*,” and “intestin*.” Only articles specifically diagnosing intestinal perforation caused by fishbones were included in the study. Patient characteristics were evaluated. Clinical and imaging findings, subsequent management, and prognosis were determined. Full-text articles of all articles meeting the inclusion criteria were obtained and cross-checked for their references. A summary and analysis of all available data were performed.
3.1 Diagnostic methods of FIIP
3.1.1 Medical history, physical examination, and laboratory tests
With regard to medical history, most patients—particularly elderly individuals—are unable to recall the unintentional ingestion of fishbones, significantly complicating the diagnostic process (19). FIIP commonly presents with nonspecific symptoms, including abdominal pain, distension, fever, nausea, anorexia, rebound tenderness, and abdominal rigidity. Laboratory tests typically reveal elevated inflammatory markers in most FIIP patients (20–22), such as increased WBC counts and CRP levels (23, 24). In the absence of a clear ingestion history, acute abdominal symptoms are frequently attributed to alternative conditions such as cholecystitis, appendicitis, peptic ulcer disease, or intra-abdominal malignancies (25–27). In our FIIP case series, four patients were initially misdiagnosed with acute appendicitis (28–31). While others were preoperatively misdiagnosed with colonic diverticulitis (24) or ruptured abdominal aortic aneurysm (32). Despite limitations arising from patient recall bias and the nonspecific clinical presentation, identifying high-risk dietary behaviors and early subtle manifestations through meticulous history-taking remains essential, particularly in regions where fish constitutes a dietary staple. A systematic approach to clinical history, combined with appropriate diagnostic imaging, is crucial for reducing delays in diagnosis.
3.1.2 Imaging examinations of FIIP
3.1.2.1 X-ray
X-ray can be utilized for the detection of ingested foreign bodies and is particularly useful for identifying metallic objects (33–35), bone tissue (36), and pneumoperitoneum (37). Relative to chicken bones, which are almost always radiopaque (38), the radiolucency of fishbones varies by species (39). Fishbones are typically small and slender, and the perforations they cause are often minute. Even when radiopaque, fishbones may become obscured by surrounding fibrin or omental tissue following intestinal perforation. Additionally, perforation sites are often associated with soft tissue swelling, inflammatory changes, abscess formation, and fluid extravasation (29, 40), further masking the subtle osseous signal of the fishbone on radiographs (Figure 3). Consequently, abdominal radiographic findings are frequently unremarkable (41, 42). In a prospective study involving 358 patients with suspected FI, plain radiography demonstrated a sensitivity of only 32% (43). Therefore, the diagnostic utility of plain abdominal X-rays in detecting ingested fishbones is considered limited and unreliable.
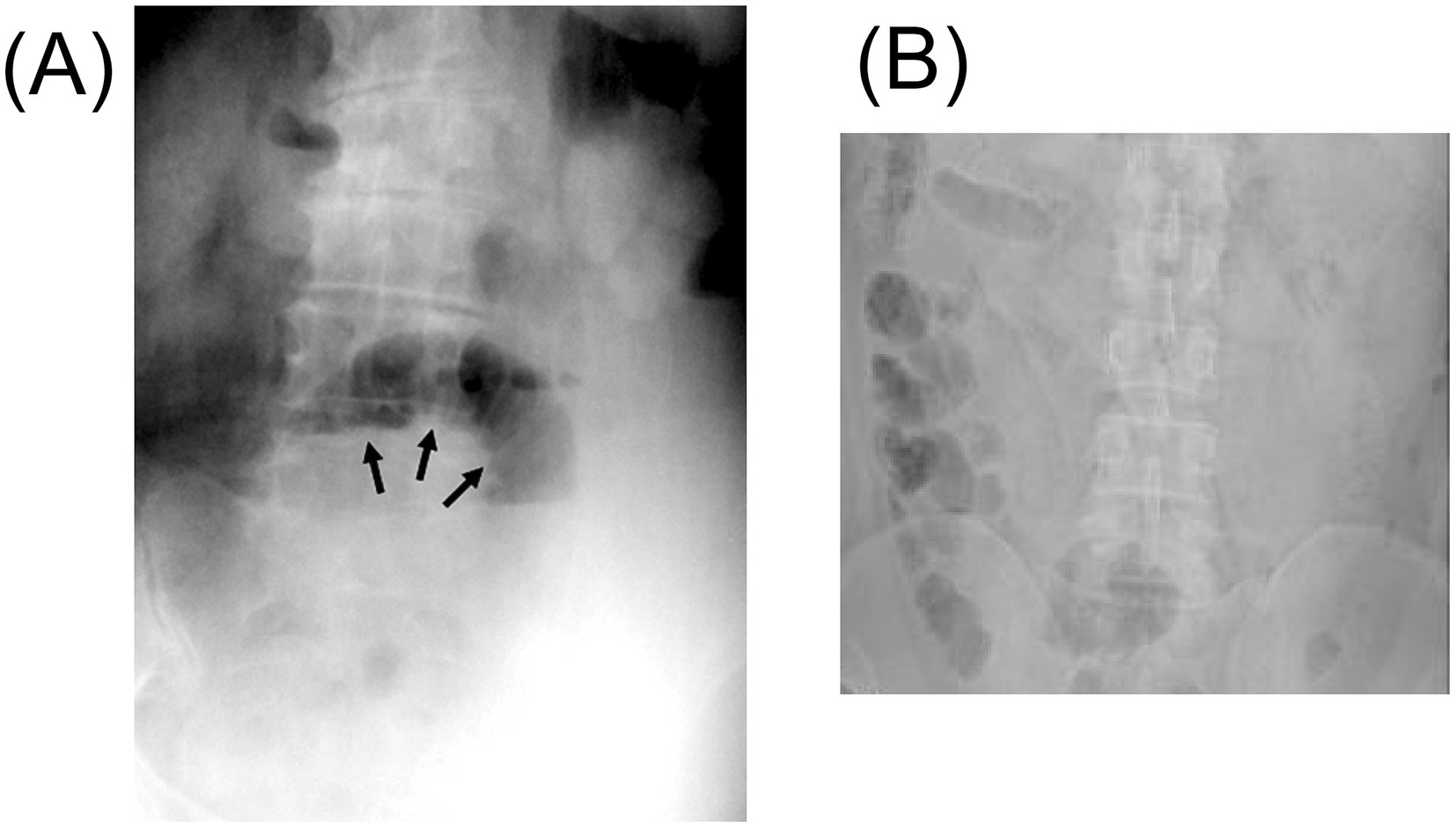
Figure 3. The figure shows the X-ray after intestinal perforation caused by a fishbone. (A) The supine abdominal plain film shows dilated small bowel loops, suggesting the possible presence of small bowel obstruction. There is no obvious radiopaque foreign body (black arrow). Adapted from reference (50) with permission from Galenos. (B) The abdominal X-ray shows the formation of a localized central intestinal obstruction. Adapted from reference (139) with permission from Springer Nature.
3.1.2.2 CT scan
CT examination has played a significant role in the diagnosis of FIIP. Among the 58 cases we collected, over half of the patients were diagnosed with FIIP through CT (n = 58, 35/58, 60.3%). Most patients underwent an abdominal CT scan without oral contrast administration. This approach is also supported by previous studies, as the use of contrast agents may delay diagnosis and subsequent intervention (44). Additionally, intravenous contrast may lead to misinterpretation of fishbones as vascular structures, while oral contrast can obscure radiopaque fishbones within the gastrointestinal lumen (45). On CT imaging, perforation caused by foreign body impaction is typically a gradual process; therefore, the presence of free intraperitoneal air is uncommon. Instead, a localized inflammatory response with fibrin deposition frequently occurs at the perforation site. As a result, free air is often confined and manifests as small gas bubbles or pockets in the mesentery near the site of perforation, forming small gas pockets. As observed in the present case, localized accumulation of small bowel gas and fluid was evident. On non-contrast CT scans, fishbones often appear as linear hyperdense structures (46). Whereas on contrast-enhanced CT, they are visualized as more distinct high-density linear shadows (47). Additionally, CT may also show increased density of peritoneal fat, thickening of the peritoneum and bowel wall (48), inflammatory changes, edema (40, 49), abscess formation (14), and even obstruction (50) (Figure 4). CT scans can generally estimate the approximate length and anatomical location of the fishbone. However, definitive identification requires surgical retrieval.
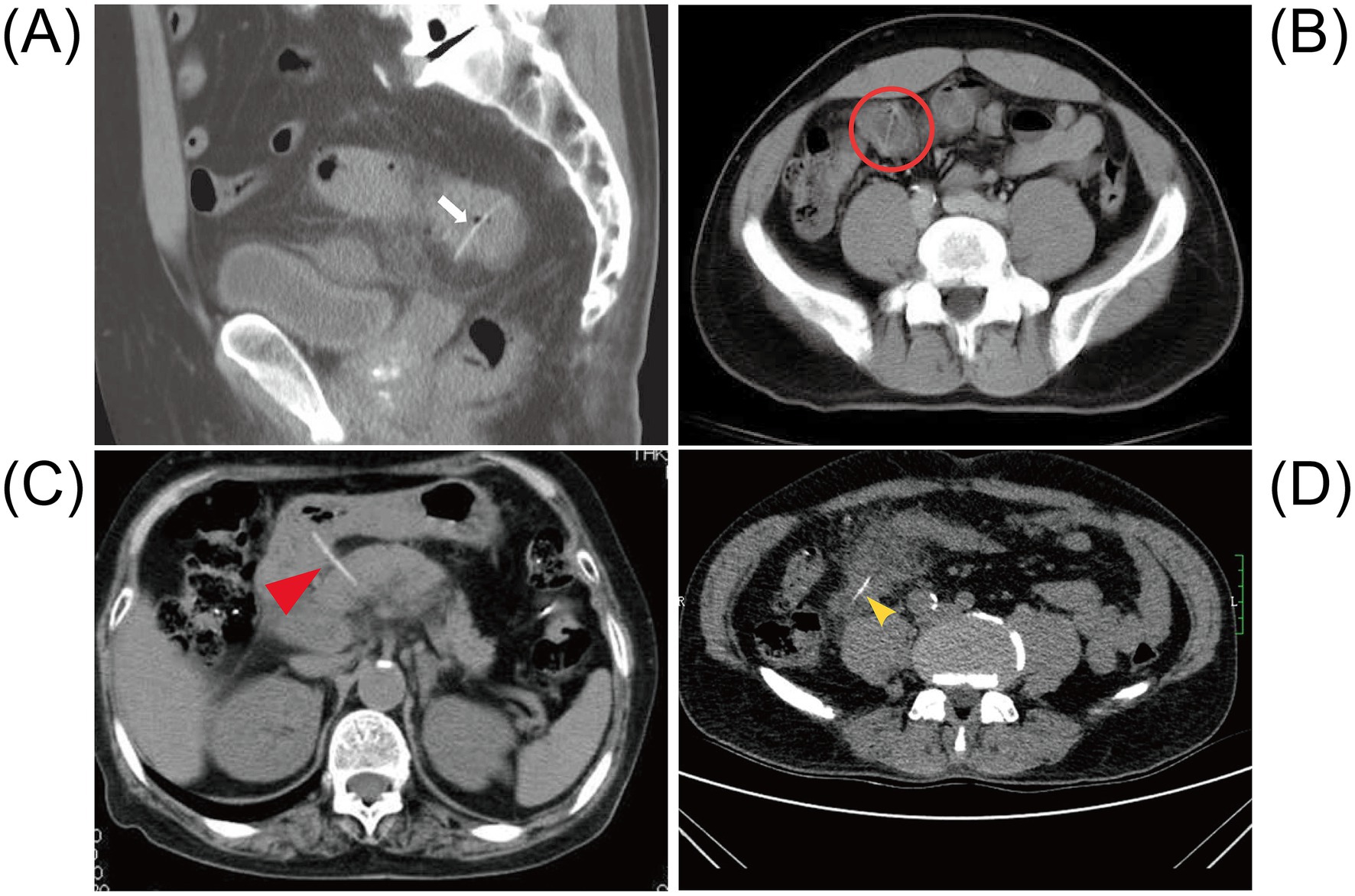
Figure 4. Abdominal CT after intestinal perforation caused by a fishbone. (A) The abdominal CT shows a radiopaque linear foreign body penetrating through the ileocecal wall (white arrow). Adapted from reference (108) with permission from J-STAGE. (B) The non-contrast abdominal CT shows a 26-mm long radiolucent linear shadow located within thickened bowel walls at both ends in the distal ileum (red circle). Adapted from reference (23) with permission from Baishideng. (C) The CT scan reveals a linear calcified body that appears to penetrate the posterior wall of the duodenal bulb and extends into the head of the pancreas. Adapted from reference (140) with permission from Springer Nature. (D) The abdominal CT shows patchy exudation and high-density shadow stripes in the right lower abdomen (yellow arrow). Adapted from reference (141) with permission from Springer Nature.
3.1.2.3 Endoscopy
Endoscopy is frequently utilized to evaluate symptoms resulting from FBI, retrieval is generally successful when ingestion is confirmed, particularly in cases where computed tomography fails to identify the fishbone. When the foreign body is retained within the proximal gastrointestinal tract, endoscopic diagnosis and intervention are more feasible. However, endoscopic management of intestinal perforation secondary to FBI has rarely been reported (51–53) (Table 1). This approach is typically reserved for patients who are asymptomatic at the time of presentation.
3.1.2.4 Ultrasound
Foreign bodies, including radiolucent materials such as fishbones and toothpicks, can be identified using ultrasonography due to their high echogenicity and characteristic posterior acoustic shadowing (54). Nevertheless, the widespread adoption of multidetector CT in emergency departments has restricted ultrasound’s role in assessing acute abdominal pain patients. Ultrasonography offers several advantages over CT, including real-time imaging capability, repeatability, portability, cost-effectiveness, absence of ionizing radiation, and the ability to target symptomatic abdominal regions through palpation-guided scanning (55). Ultrasound is generally effective in assessing gastrointestinal perforations, perilesional tissue changes, and luminal contents in superficial intestinal loops or colonic segments. However, evaluation of deeper anatomical structures may be limited (56). Advances in ultrasonographic technology have significantly enhanced diagnostic capabilities, improving image resolution in intestinal evaluations—even in obese patients or when imaging deeper abdominal regions (57) (Figure 5). FBI is particularly common among pediatric populations. As a radiation-free modality, ultrasound holds unique diagnostic value in children, enabling bedside localization of ingested foreign bodies and facilitating prompt surgical removal. Previous studies have demonstrated the diagnostic accuracy of ultrasound in detecting fishbones, typically visualized as linear hyperechoic structures or calcifications, and in identifying associated findings such as intra-abdominal fluid or masses (29, 50, 58, 59). In clinical and emergency settings, the speed, accessibility, radiation-free nature, and diagnostic precision of ultrasound make it an effective first-line imaging modality, particularly in urgent presentations of acute abdominal pain. However, when patient history is unclear, physical findings are nonspecific, and imaging modalities fail to identify the fishbone, laparoscopic or open surgical exploration is required to confirm the diagnosis of FIIP (49, 60).
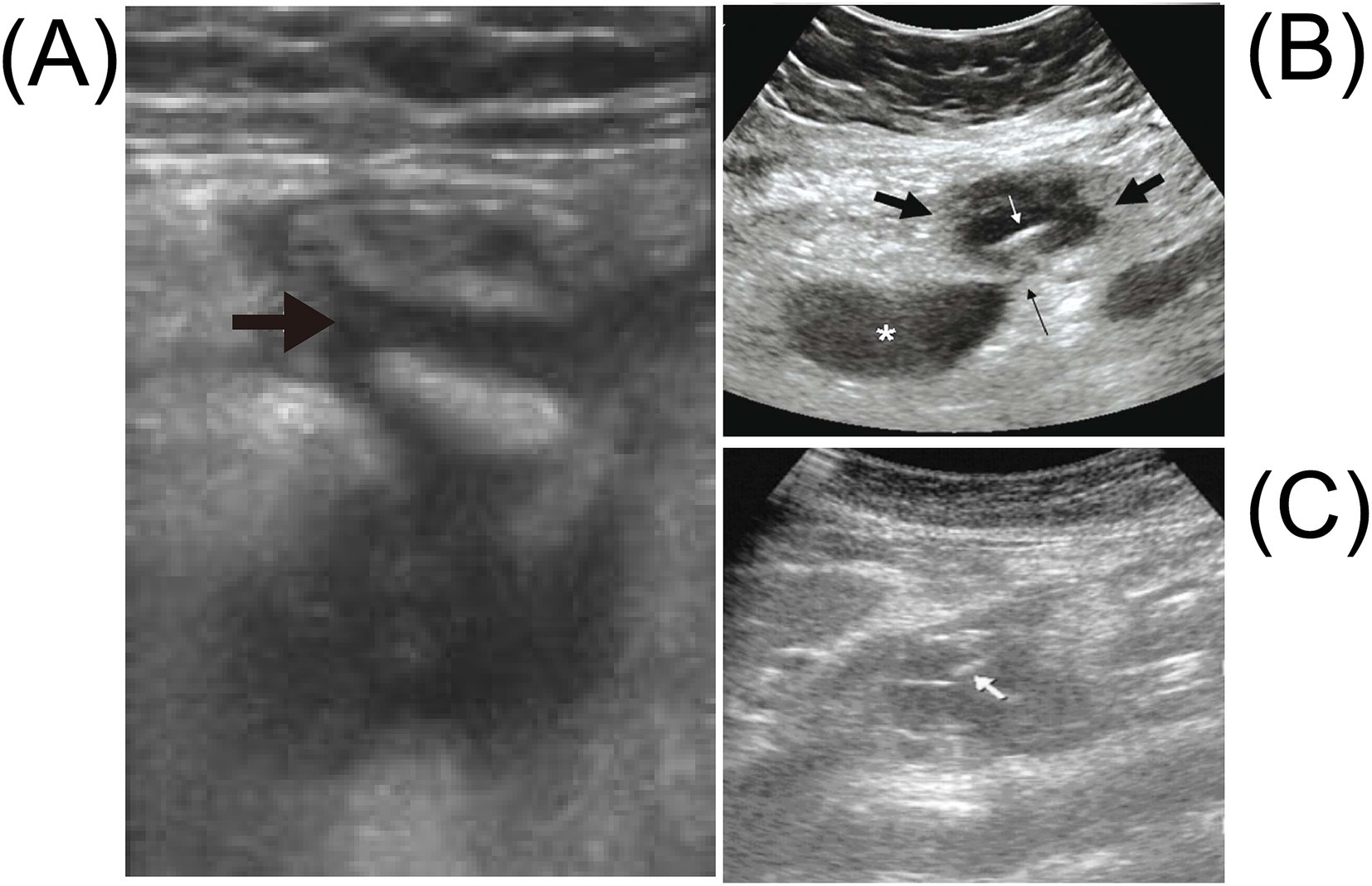
Figure 5. Abdominal ultrasound for intestinal perforation caused by a fishbone shows the following. (A) The abdominal ultrasound examination reveals fluid accumulation in the right lower abdomen (black arrow). Adapted from reference (29) with permission from Elsevier. (B) The transverse sonographic image of the right periumbilical region shows a hypoechoic mass with a maximum diameter of 4.5 cm (thick black arrow), containing a thin linear echogenic structure measuring 3.7 cm in length (white arrow), representing the fishbone. There is also a hypoechoic sentinel loop of small intestine (asterisk) adjacent to the mass without peristalsis, connected to it via a 2-mm-wide linear hypoechoic sinus tract (thin black arrow). Note the intense echogenicity of the omental fat surrounding the lesion. Adapted from reference (50) with permission from Galenos. (C) The abdominal ultrasound shows a hypoechoic mass around the pancreas, with linear echogenic structures around the pancreas (white arrow). Adapted from reference (142) with permission from Oxford Academic.
In conclusion, the timely selection of appropriate diagnostic modalities is essential for the effective management of acute abdominal conditions. In emergency settings, ultrasound serves as an excellent first-line imaging modality, particularly when readily available in the emergency department (61). When performed by experienced operators, ultrasound can effectively identify linear hyperechoic foreign bodies, such as fishbones, and is highly sensitive in detecting abscesses and free intra-abdominal fluid. However, its diagnostic accuracy may be compromised by the presence of intestinal gas, potentially reducing its sensitivity for detecting foreign bodies. Despite these limitations, ultrasonography remains a valuable, non-invasive, radiation-free, and rapid screening tool, especially during the initial evaluation of suspected gastrointestinal perforation.
However, CT remains the most effective imaging modality for diagnosing gastrointestinal foreign bodies. It offers superior spatial resolution and is particularly advantageous for detecting high-density foreign bodies, as well as assessing associated inflammatory changes, abscess formation, and perforation. Its diagnostic sensitivity far exceeds that of X-rays. CT also plays a critical role in determining the necessity of surgical intervention (62). Compared to X-ray, which is typically used for initial evaluation, CT provides more detailed and accurate information regarding the location and impact of foreign bodies.
In certain cases, plain radiography may be performed for basic assessment—particularly to evaluate the presence of free intraperitoneal air or gastrointestinal perforation. However, its sensitivity for detecting fishbones is relatively low, and it is limited in visualizing foreign bodies, especially in the absence of free gas.
When retained fishbones are suspected in the upper gastrointestinal tract (e.g., the esophagus or duodenum), or in the colon and rectum, endoscopy serves as an effective diagnostic and therapeutic modality for localization and retrieval. In situations where imaging and endoscopic findings are inconclusive, or the patient’s clinical condition is unstable and the diagnosis remains uncertain, exploratory laparotomy should be considered as the definitive diagnostic and therapeutic approach.
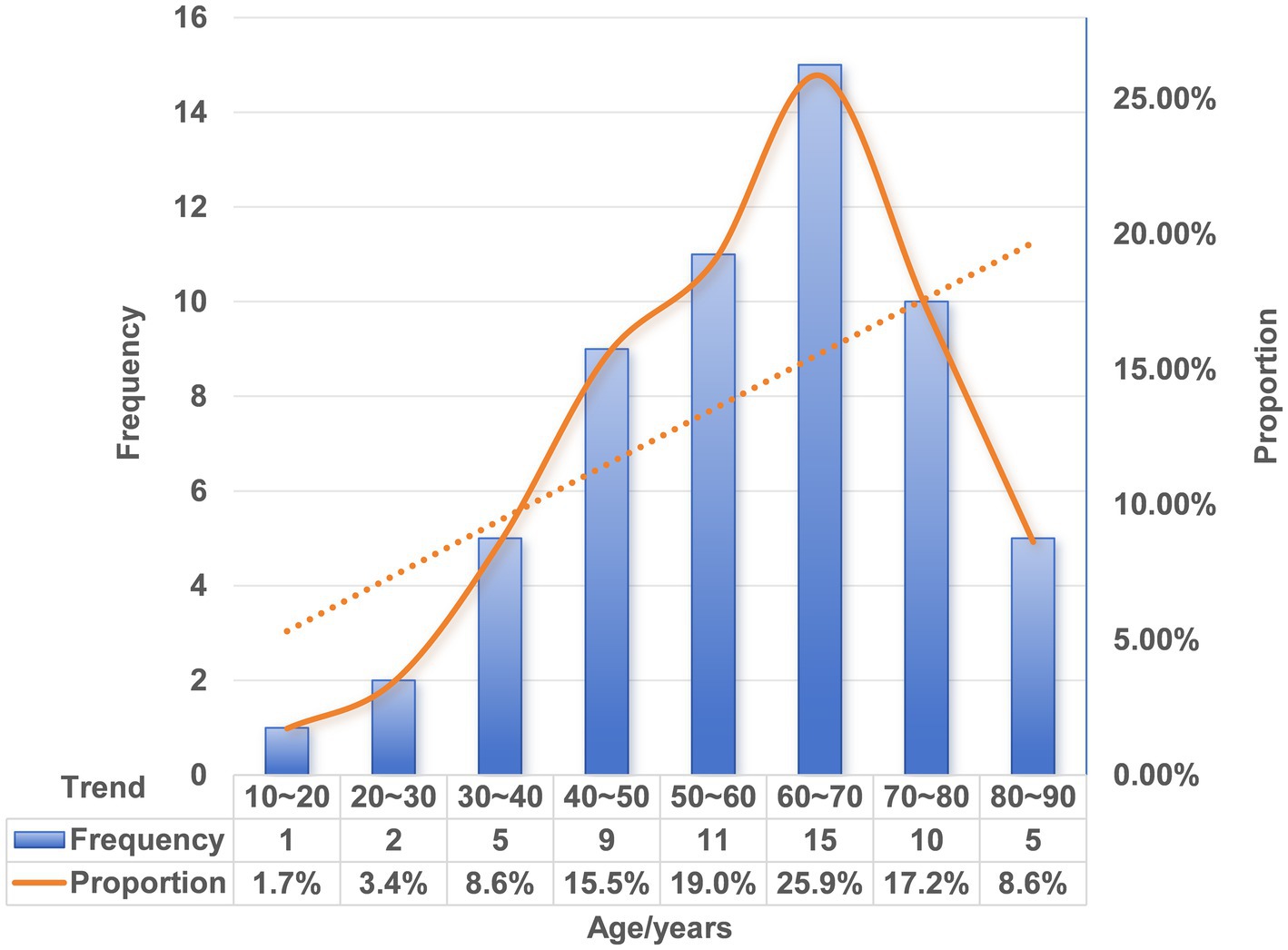
3.2 Age distribution and time to presentation of FIIP
Extensive epidemiological data have shown that FBI is most prevalent among children aged 5 years or younger, accounting for approximately 75% of documented cases (63). In contrast, the incidence of FIIP is higher among older adults than in pediatric populations (64–66). We conducted a statistical review of patient age in published cases of FIIP. Notably, no cases were reported in infants or toddlers. The youngest affected patient was 13 years old, and the oldest was 87. The age distribution demonstrated right skewness, with a marked predominance in older adults (Figure 6 and Table 1). The absence of FIIP in very young children may be attributed to close adult supervision during fish consumption, including removal of fishbones or provision of boneless fish. Moreover, children possess relatively larger tonsils and smaller oral cavities compared to adults, anatomical features that may predispose them to oropharyngeal foreign body impaction rather than gastrointestinal perforation. Existing evidence suggests that younger children are more likely to seek medical attention promptly, whereas elderly individuals often delay seeking care (67). Our case analysis corroborated previous findings, indicating that adults typically postpone medical evaluation until approximately 3 days after FI or symptom onset (n = 51, 35/51, 68.6%). The longest reported interval from ingestion to perforation extended up to 8 months (Table 1). This delay may reflect an underestimation of the clinical risks associated with FI or a failure to recall the ingestion event. Furthermore, elderly patients, who commonly experience tooth loss or use dentures, exhibit reduced tactile sensitivity and increased intestinal fragility with age. Consequently, foreign body ingestion in adults is more likely to remain undetected compared to children, resulting in subtler clinical manifestations. Therefore, beyond established risk factors such as dentures and chicken bone ingestion, increased clinical vigilance regarding FI is warranted in middle-aged and elderly populations.
3.3 Anatomic distribution of FIIP
Prior research has demonstrated that the anatomical distribution of foreign bodies varies according to object type. Fishbones are predominantly located in the tonsils (48.5%) and the base of the tongue (25.0%) (68). In these regions, symptoms typically manifest quickly and are often pronounced, prompting most patients to seek medical attention within 2 h (69). In our analysis of FIIP cases, the mean interval from ingestion or symptom onset to clinical presentation was 14.6 days. After excluding two statistical outliers (240 and 60 days), the adjusted mean presentation time was 8 days (Table 1). These observations indicate that the anatomical site of foreign body impaction correlates with the timing of presentation, the nature of the foreign body, and patient age. For fishbones, even when ingested intentionally, as in the case of our patient, the absence of a foreign body sensation in the oropharynx, tonsils, and esophagus is often perceived as safety. This perception significantly delays FI presentation, thereby increasing the incidence of FIIP.
3.3.1 Small intestine
Anatomical configuration, luminal dimensions, and motility characteristics of the gastrointestinal tract influence the localization of FIIP, with the small intestine being the most frequently affected segment, accounting for 75.9% of cases (Table 2 and Figure 7). The small intestine is a long, tubular structure approximately 6–7 meters in length with an internal diameter of 3–4 cm (70). Its narrow, tortuous lumen, high mobility, and variable loop positioning increase the likelihood of foreign body impaction. Acute angulations, a common occurrence (71), may alter fishbone orientation and increase stress on the intestinal wall, promoting perforation. Among small intestinal segments, the ileum is most frequently involved, accounting for 45.4% of fishbone-related perforations (Table 2 and Figure 7). Compared to the jejunum, the ileum has a smaller lumen, greater length, more densely packed loops, and less vigorous peristalsis, especially in the terminal ileum and at the ileocecal valve (72). These anatomical and functional characteristics contribute to an elevated risk of fishbone retention and subsequent perforation. Multiple studies have identified the distal gastrointestinal tract, especially the ileum, as a common site of perforation, with 182 patients (58.5%) exhibiting involvement at this location (73).
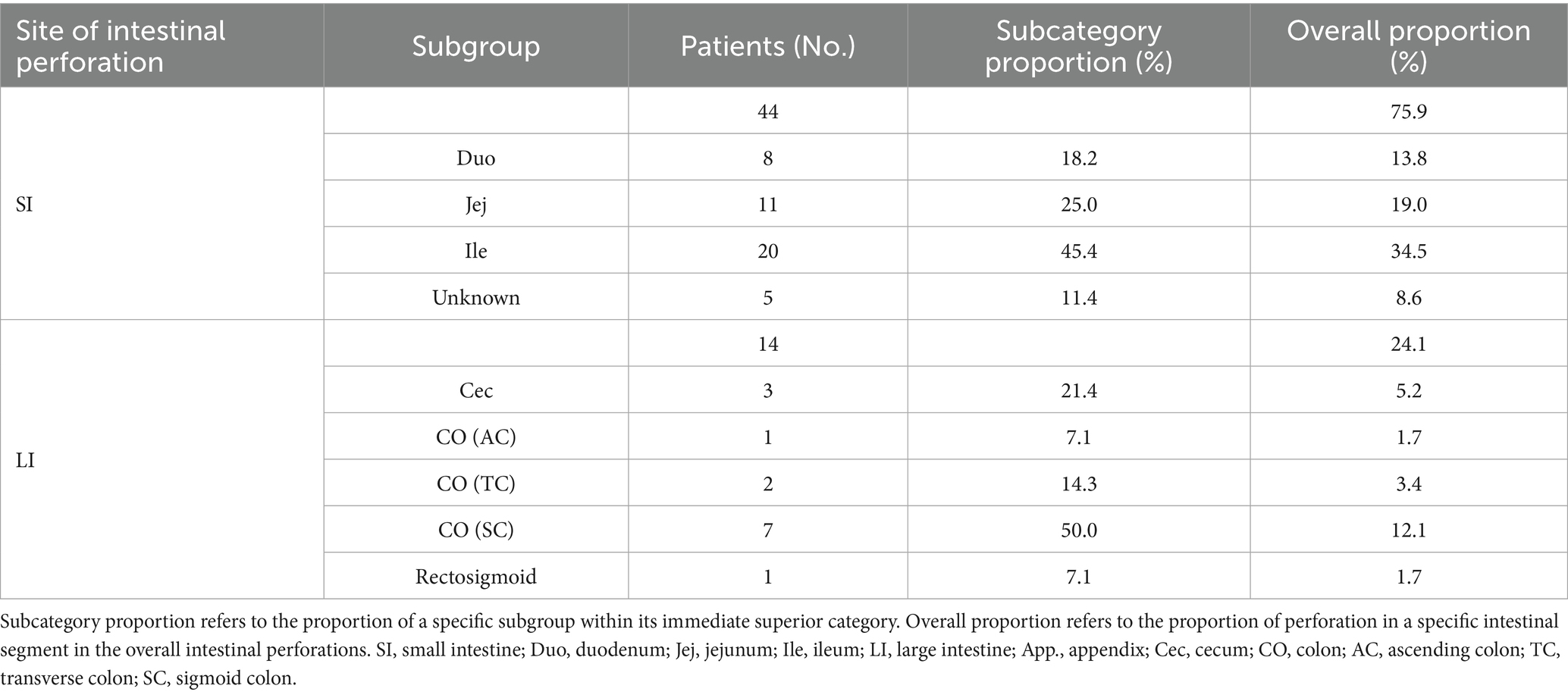
Table 2. Distribution characteristics of intestinal segment perforation caused by fishbones (N = 58).
Figure 7. Location-based heatmap of FIIP frequency. Created with BioRender.com.
In cases of FIIP, a distinct feature is the involvement of Meckel’s diverticulum (MD) of the ileum, reported in eight patients (18.2%, Table 1). MD is a congenital gastrointestinal anomaly characterized by a small sac that protrudes from the small intestine wall. It is usually asymptomatic but may occasionally result in clinical complications (74). The prevalence of MD is estimated at approximately 2.2%, with a higher occurrence in males than females (75). The primary complications associated with MD include gastrointestinal hemorrhage, intestinal obstruction, diverticulitis, intussusception, and ulceration, while perforation and volvulus are considered rare (76). Foreign body-induced perforation of MD is exceedingly uncommon. Anatomically, MD averages 2.9 cm in length and 1.9 cm in width and features a significantly narrower lumen compared to the adjacent small intestine. The impaction of a fishbone within MD increases the likelihood of localized perforation (77). While most ingested foreign bodies pass through the gastrointestinal tract without incident, the fishbones associated with MD perforation in our series measured approximately 2 cm in length (Table 1). It is hypothesized that, even when the length of a fishbone or other foreign body is sufficient to traverse the normal gastrointestinal tract, the presence of MD may increase the risk of perforation. Surgical resection is generally recommended for symptomatic MD (78). However, the management of asymptomatic MD remains controversial. The estimated lifetime risk of complications in individuals with MD is approximately 5–6%. Accumulating evidence supports prophylactic resection of incidentally discovered MD in the absence of complicating factors such as peritonitis, hemodynamic instability, or ascites (79). In clinical practice, clinicians should maintain a high index of suspicion for FBI in patients with asymptomatic diverticula.
3.3.2 Large intestine
Previous studies indicate that the sigmoid colon and cecum in the large intestine are common sites for foreign body perforation (80). The tortuous configuration and angulated structure of the intestinal lumen, coupled with relatively thin intestinal walls (81), predispose sharp or elongated foreign bodies to impaction at anatomical flexures, particularly near the ileocecal valve and rectosigmoid junction. Although fishbone-induced sigmoid colon perforation is rarely reported (82), our analysis indicates that such perforations in the large intestine occur most frequently in the sigmoid colon (50%), followed by the cecum (21.4%) (Table 2 and Figure 7). This distribution is attributed to the sigmoid colon’s anatomical characteristics, including its narrow diameter, increased tortuosity (83), and persistent fecal loading relative to other colonic segments (84). As water is absorbed by the colonic mucosa (85), fecal material becomes firm, and the vigorous peristaltic activity of the sigmoid colon elevates intraluminal pressure, thereby increasing the risk of perforation. Situated in the right iliac fossa, the cecum and ascending colon resemble sac-like structures with a larger diameter than the descending colon, sigmoid colon, and rectum, yet are anatomically constrained by a narrow ileocecal valve (86), limiting the passage of foreign bodies. Once lodged in the cecum, fishbones are more likely to perforate the thinner posterior wall (87).
Penetration of the intestinal wall by fishbones frequently induces localized inflammatory encapsulation and fibrotic barrier formation (88). As a result, fishbones rarely achieve full transmural penetration; when complete penetration occurs, they may migrate to adjacent or distant anatomical sites. One rare but potentially fatal complication is hepatic abscess formation following migration of a fishbone to the liver (59). Multiple case reports have documented instances in which ingested fishbones perforated the duodenum and subsequently migrated to the liver, resulting in hepatic abscesses (46, 89). In rare cases, such migration has been associated with serious vascular complications, including portal vein thrombosis and fistula formation involving the inferior vena cava (20, 21).
3.4 Intervention methods of FIIP
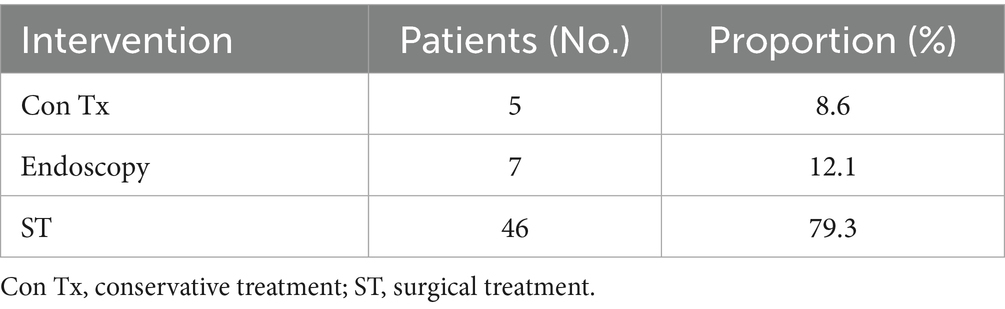
FBI and food bolus impaction are commonly encountered in clinical practice. Over 80% of ingested foreign bodies pass through the gastrointestinal tract spontaneously, without requiring medical intervention. Endoscopic removal is indicated in approximately 20% of cases, whereas surgical intervention is required in fewer than 1% of patients (90). However, intestinal perforation secondary to FBI often necessitates surgical management (91). In our analysis of 58 cases of FIIP, 79.3% of patients underwent surgical treatment, 12.1% received endoscopic extraction, and 8.6% were managed conservatively (Table 3). When fish bone ingestion is suspected as the cause of abdominal symptoms, diagnostic and therapeutic procedures should be conducted in accordance with established clinical protocols (Figure 8).
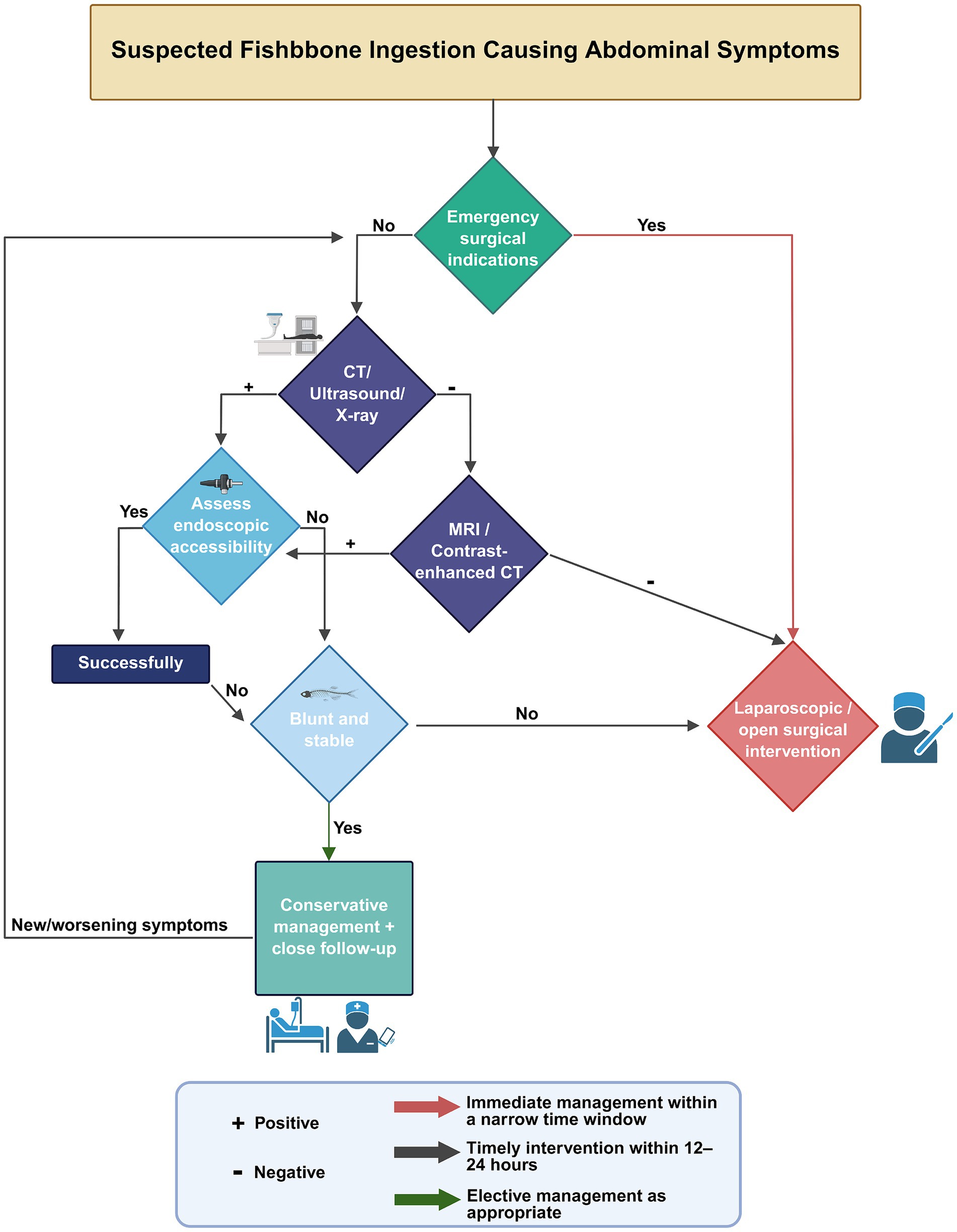
Figure 8. Clinical management algorithm for suspected fishbone ingestion presenting with abdominal symptoms. Created with BioRender.com.
3.4.1 Conservative management
In cases with benign CT findings, such as the absence of peritoneal fluid, abscesses, pneumoperitoneum, fat stranding, or bowel wall thickening, or if these findings remain stable on serial CT scans, conservative management may be appropriate (92). Non-operative treatment of FIIP may also be appropriate for patients with stable vital signs, mild peritoneal signs, or contraindications to surgical intervention (93). For example, in the FIIP case reported by Ward et al. (94), only localized abdominal pain and mild leukocytosis with neutrophilia were observed, without other abnormal findings. The patient was managed with fasting alone, without antibiotics, and remained asymptomatic during 1 year of follow-up after discharge. In other conservatively treated cases, broad-spectrum antibiotics such as piperacillin-tazobactam or amoxicillin-clavulanate were administered in conjunction with bowel rest and supportive care (20, 23, 40). However, effective physician-patient communication is essential to ensure that patients are aware of the potential complications associated with conservative treatment. Close inpatient observation is necessary, and outpatient follow-up should be maintained for at least 1 year after discharge. Notably, there was a rare case of spontaneous expulsion of a 4.5-cm fishbone in an elderly patient with small bowel perforation, which occurred the day before the scheduled surgical intervention, nearly 2 months after ingestion (95). This unusual event may be explained by the thicker portion of the foreign body remaining lodged within the intestinal lumen, which eventually loosened and was expelled naturally.
Conservative management of FBI necessitates careful evaluation of potential complications (96). Foreign bodies lodged at perforation sites may irritate surrounding tissues, leading to chronic inflammation (97). Prolonged retention can result in the development of inflammatory granulomas, enteric fistulas, or intra-abdominal abscesses (98, 99). Elongated foreign bodies may also cause partial or complete intestinal obstruction, particularly in cases involving luminal stenosis, intussusception, or neoplastic lesions (100). Unstable perforation sites may exacerbate symptoms, as intestinal peristalsis can drive the object further into the bowel wall. If dislodged, the foreign body may perforate other intestinal segments, leading to peritonitis. Migration into adjacent organs—including the liver, bladder, uterus, ureter, abdominal wall, or blood vessels—can result in serious complications such as infection, hemorrhage, pseudoaneurysm formation, thrombosis, abscesses, fistulae, or ectopic inflammatory responses (101).
Given these risks, conservative treatment should be pursued cautiously and only under close surveillance. This is particularly important in cases where asymptomatic fish bones are incidentally identified on imaging but remain in situ. For such cases, treatment decisions should be highly individualized. Management should be based on the morphology of the foreign body (e.g., sharpness, length), its anatomical location, and the patient’s comorbidities. Blunt, short, and well-encapsulated fish bones may be managed conservatively, provided that patients undergo structured imaging surveillance, such as repeating CT every 2–3 days initially, then weekly, until the foreign body is expelled or becomes encapsulated and stabilized within the surrounding tissue. In contrast, sharp, long, or poorly fixed fish bones carry a higher risk of delayed perforation or migration. In elderly patients or those with immunosuppression or prior abdominal surgery, clinicians should maintain a lower threshold for early removal, even in the absence of symptoms.
Anatomical location is a crucial factor in guiding management decisions. Foreign bodies located near angulated or narrow segments (e.g., the ileocecal junction or sigmoid colon) present a higher risk of impaction or perforation, and may require earlier intervention. In contrast, asymptomatic fish bones that are stable on imaging and retained in the stomach or rectum are generally accessible and can usually be safely removed via endoscopy. If endoscopic removal is not feasible due to location or technical limitations, surgical consultation should be considered.
For patients with severe comorbidities or poor surgical candidacy, conservative management becomes even more critical. In these cases, sharp foreign bodies retained in situ should be monitored closely with regular imaging. The goal is to prevent delayed perforation or migration, which could worsen the patient’s condition. For patients in whom endoscopic removal is not feasible due to the location of the foreign body, conservative management becomes paramount. Alternative strategies, such as long-term observation and imaging follow-up, should be considered. In the absence of surgical options, the primary focus should be on minimizing further harm, managing symptoms, and preventing complications through vigilant monitoring.
Overall, conservative management of retained fish bones should be carefully selected based on patient factors and the nature of the foreign body, closely monitored, and promptly escalated if any signs of clinical deterioration or complication arise.
3.4.2 Endoscopic treatment
Endoscopic intervention is considered necessary in approximately 10–20% of FBI cases (102). Esophagogastroduodenoscopy is widely employed for the removal of upper gastrointestinal foreign bodies due to its safety, cost-effectiveness, procedural efficiency, and low complication rate (103). Therefore, in cases of duodenal perforation caused by FI, upper gastrointestinal endoscopy may be indicated for patients with mild symptoms and hemodynamic stability (51, 104) (Figure 9). However, reports of endoscopic management for lower gastrointestinal tract perforations remain limited (82). The advent of double-balloon enteroscopy has facilitated complete small intestinal examination and retrieval of retained foreign bodies, although the technique remains technically demanding (105–107). In two documented cases of fishbone-induced jejunal perforation, removal was successfully achieved using double-balloon enteroscopy with a snare, and both patients experienced no postoperative complications with rapid recovery. For sigmoid colon perforations, sigmoidoscopy equipped with a transparent cap enabled direct visualization and successful foreign body extraction (82, 108, 109). Whether suturing is required at the site of perforation remains unclear. In the seven cases of FIIP managed by endoscopic removal collected in this review (51–53, 82, 104, 108, 109), none reported the use of suturing. This may be attributed to the sharp and slender nature of fishbones, which typically result in small perforations that can be sealed by the surrounding inflammatory response, even without sutures. Most endoscopically treated cases did not present with peritonitis, and leakage of intestinal contents was rarely observed (104). In contrast to the others, one case documented the application of a clamp at the perforation site following endoscopic removal. Possibly due to its specific anatomical location. The sigmoid colon contains relatively dry contents compared to other intestinal segments (110), which may reduce the risk of intraperitoneal contamination. When perforation occurs in other segments of the intestine, even if the defect is small or encapsulated and the patient remains clinically stable, the risk of peritonitis due to leakage of liquid intestinal contents after endoscopic removal must still be carefully considered (111). At this stage, the decision to pursue endoscopic management or to suture the perforation site should be evaluated cautiously. Patients presenting with localized abdominal symptoms and stable systemic status may be candidates for conservative treatment using endoscopic techniques. Importantly, conversion from endoscopic removal to surgical intervention necessitates close multidisciplinary coordination and must be performed with heightened vigilance.
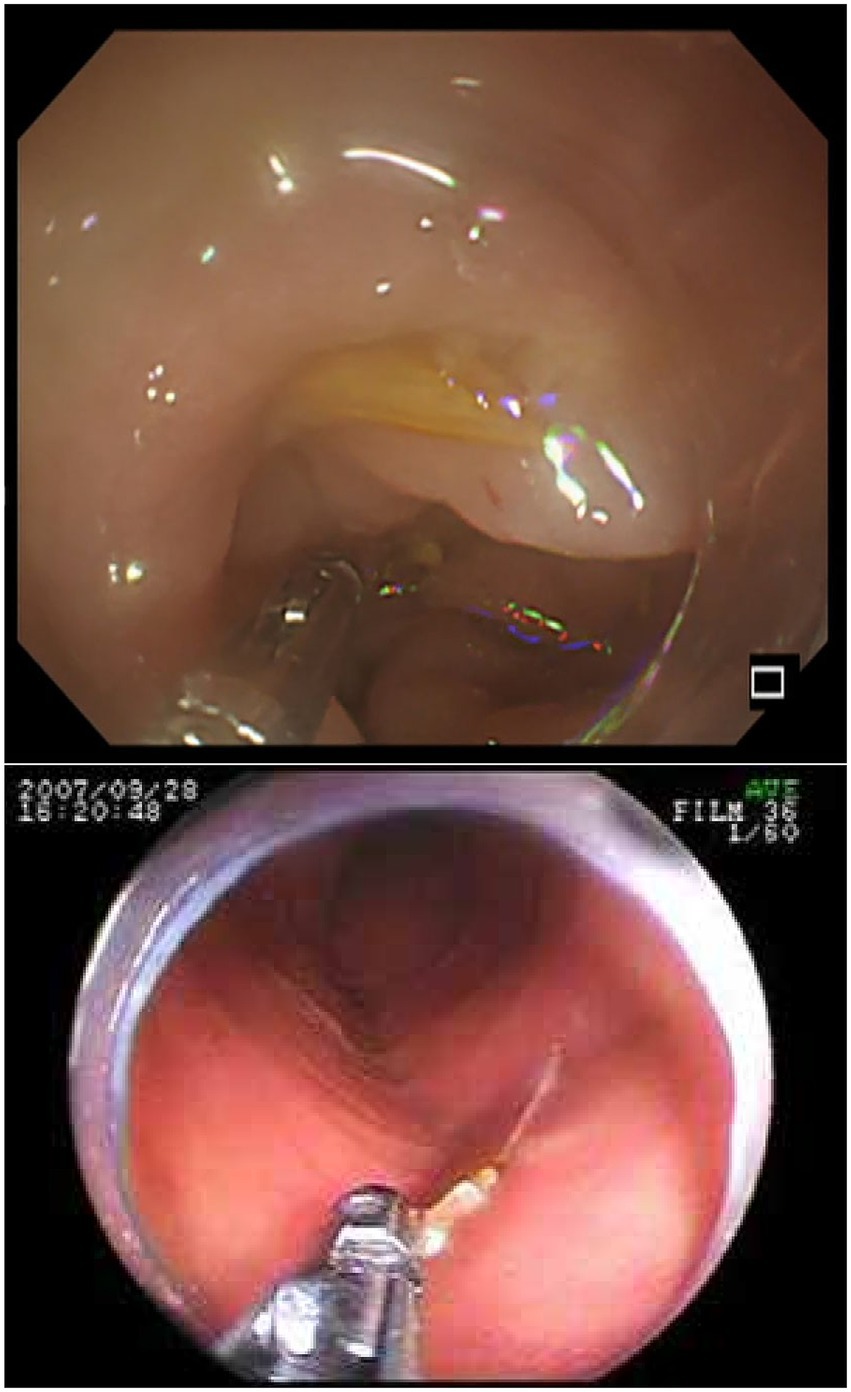
Figure 9. The figure shows the endoscopic findings of intestinal perforation caused by a fishbone. Top image: Endoscopic findings of edematous sigmoid colon mucosa. The fishbone is hidden within the edematous sigmoid colon. Adapted from reference (82) with permission from J-STAGE. Bottom image: The endoscopic image shows a tiny, sharp object lodged in the wall of the distal jejunum. The object was grasped with forceps and pulled out. Adapted from reference (52) with permission from International Scientific Information, Inc.
3.4.3 Surgical treatment
Unlike most other cases of FBI, which seldom necessitate surgical intervention, the majority of FIIP cases in our cohort (78.9%) required operative management. In instances where patients presented with severe acute abdominal pain, emergency surgery was performed without a definitive diagnosis and occasionally even before CT scan (50, 112). Although CT imaging has demonstrated high sensitivity and specificity in detecting perforations caused by ingested foreign bodies, it is often bypassed in life-threatening scenarios. Therefore, in patients presenting with severe abdominal pain and suspected of FBI, an emergency abdominal CT scan should be prioritized to guide management decisions. Exploratory laparotomy is frequently recommended in cases of acute abdominal pain, particularly when signs of peritonitis are present or the diagnosis remains uncertain (113). In conclusion, surgical intervention is typically required for cases where conservative management or endoscopic retrieval has failed, or when the fishbone poses significant risks, such as perforation, obstruction, or infection. Both open and laparoscopic approaches have demonstrated therapeutic efficacy; however, the selection of surgical modality should be based on multiple factors, including the anatomical location and dimensions of the foreign body, the patient’s physiological status, and the extent of associated complications.
Laparotomy has traditionally been considered the gold standard for the management of gastrointestinal foreign body removal. It offers optimal visualization, operative access, and comprehensive exposure of the abdominal cavity. This approach is particularly appropriate in cases where the fishbone has migrated or caused substantial injury to adjacent structures, such as extensive abscess formation, diffuse peritonitis, hemorrhage, or bowel necrosis, all of which demand prompt surgical intervention. Laparotomy is also indicated for patients with a history of multiple laparotomies, dense intra-abdominal adhesions, or in elderly and frail individuals with compromised physiological reserves. Nevertheless, this method is associated with larger incisions, heightened postoperative discomfort, prolonged hospitalization, and an elevated risk of complications, including surgical site infection and adhesion formation.
Conversely, laparoscopic surgery has become increasingly favored in recent years owing to its minimally invasive characteristics. It is associated with smaller incisions, reduced postoperative discomfort, expedited recovery, shorter hospitalizations, and decreased rates of adhesion and surgical site infections (114). This technique is particularly advantageous in younger patients. When the fishbone is situated in an accessible anatomical location and the pathology is limited to a localized perforation, abscess, or solitary lesion, laparoscopy provides a safe and effective therapeutic option. However, this approach may not be appropriate for all patients, especially those with complex or deeply embedded foreign bodies, extensive intra-abdominal adhesions, or disseminated infections. In such scenarios, laparoscopic exploration may result in diagnostic inaccuracy, prolonged operative time, and potential iatrogenic complications (115). For the safety of the patient, laparoscopic exploration may be initially attempted during the early stages of disease or when the diagnosis remains uncertain, with conversion to laparotomy considered if necessary. Alternatively, a combined approach involving laparoscopy and mini-laparotomy may be employed to facilitate direct visualization and manual palpation, enabling thorough assessment of the perforation site (116). In our reported case, initial laparoscopic exploration was performed, but due to significant small bowel dilation and dense adhesions, the fishbone and the site of intestinal wall damage could not be identified, necessitating conversion to open surgery. For patients in whom laparoscopic findings are inconclusive, proceeding to direct laparotomy is recommended (117). During surgery, if intestinal necrosis is identified, resection of the affected bowel segment, primary anastomosis, or stoma creation may be warranted (14, 29, 118).
The increasing adoption of robotic surgery has demonstrated advantages such as enhanced visualization of the surgical field and improved instrument dexterity compared to both open and laparoscopic approaches, while also contributing to shorter hospital stays and a reduced risk of conversion to open surgery (119). Although robotic-assisted procedures have traditionally been reserved for elective surgeries (120), their application in emergency scenarios has been increasingly reported in recent years (121). As costs continue to decline, the integration of robotic surgery into emergency interventions for gastrointestinal perforation caused by foreign body ingestion is expected to expand.
3.5 Prognosis of FIIP
Most ingested foreign bodies do not lead to serious complications, and fatalities remain exceedingly rare (122). Among the reported cases, mortality related to foreign body aspiration is the most common cause of death (123). In the documented literature on fishbone-induced gastrointestinal perforation, only a single fatality has been reported, while the remaining patients experienced favorable clinical outcomes (Table 1). The deceased patient had morbid obesity and more than 16 underlying comorbidities. Additionally, delayed medical intervention—defined as a foreign body ingestion interval exceeding 15 days with the onset of severe symptoms—greatly impeded timely diagnosis and treatment. Despite surgical intervention, the patient ultimately succumbed to multiple organ dysfunction syndrome (124). In contrast, when diagnosis and treatment are performed promptly and comorbidities are minimal, the prognosis for fishbone-induced gastrointestinal perforation is generally favorable.
3.6 Fishbone length of FIIP
Prior studies have mainly emphasized the challenges associated with the size of ingested foreign bodies, particularly regarding their potential to cause gastrointestinal obstruction. Duodenal passage is determined by both the length and diameter of the foreign object, with items exceeding 6 cm in length or 2.5 cm in diameter being significantly less likely to pass through (1). In our cohort of FIIP cases, the fishbone lengths ranged from 1.1 to 5.1 cm, with a mean length of 3.0 cm. The largest dimension recorded was a fish fin measuring 4.0 cm × 3.0 cm (Table 1). These measurements are comparable to the average diameter of the small intestine. Fishbones exceeding the intestinal lumen’s diameter may increase the risk of small bowel perforation. Therefore, in cases of FBI, both the size and physical characteristics—such as sharpness and rigidity—should be carefully evaluated. The mechanism of injury differs markedly between blunt and sharp foreign bodies. Notably, perforation risk is primarily determined by the shape of sharp objects; even a length of 1 cm may be sufficient to penetrate the full thickness of the bowel wall.
3.7 Fish species of FIIP
In most previously reported cases of intestinal perforation caused by fishbones, the specific fish species involved were not identified. Among the documented cases, only 14 mentioned the species. The implicated fishbones were typically slender and pointed (Figure 10). Both the fish species and cooking methods may influence bone morphology, density, and resistance to gastric acid dissolution (125). These factors play a key role in determining the site of impaction and the likelihood of perforation (126). Regional dietary preferences influence the types of fish consumed, potentially explaining geographic variations in incidence (127). Clinically, it is advisable to document the fish species whenever possible, as this may assist in predicting the location, size, and risk associated with the ingested bone, thus improving diagnostic and therapeutic accuracy. Future case reports are encouraged to include such information to enhance clinical understanding and global data sharing.
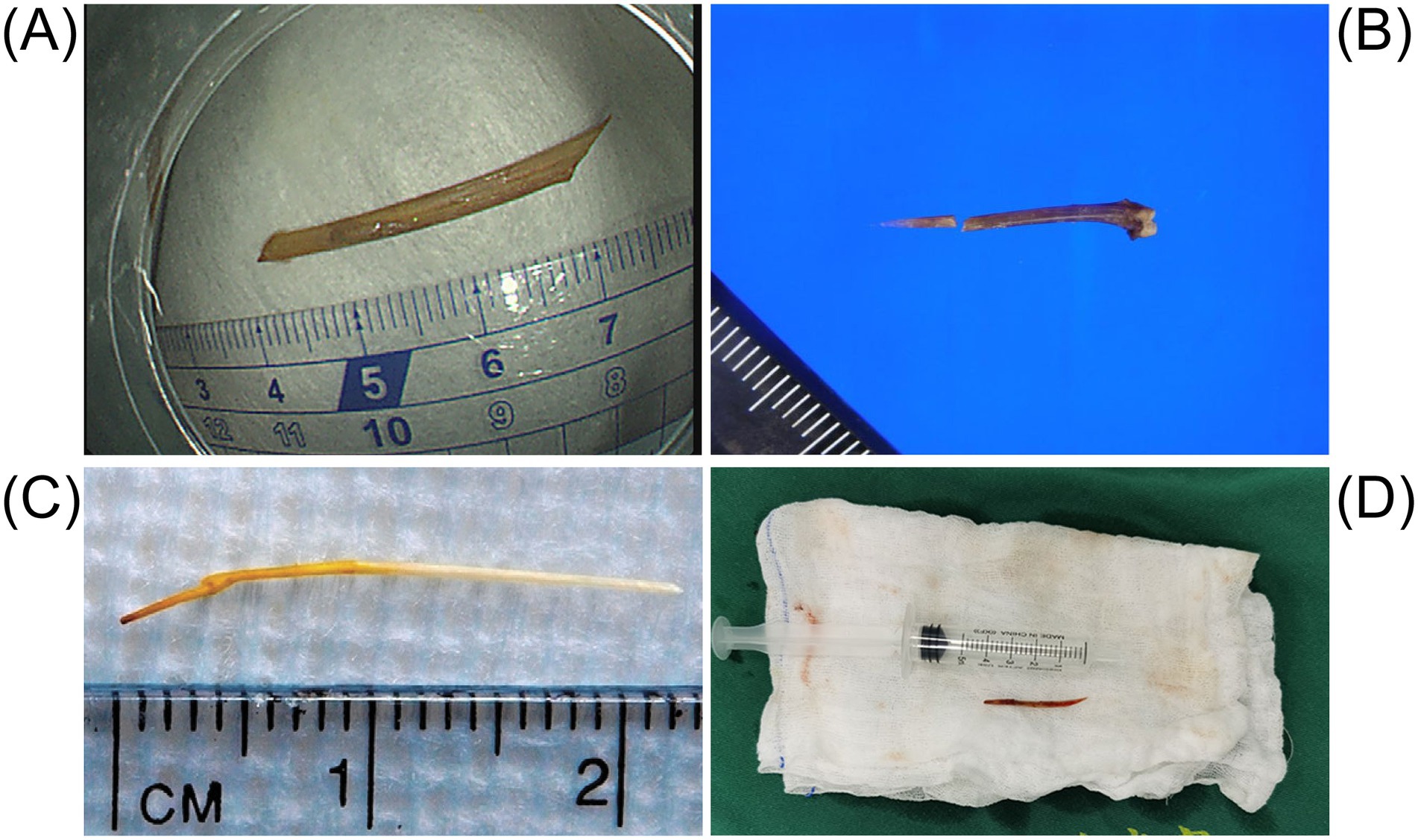
Figure 10. The figure illustrates the shape and length of the fishbone that caused the intestinal perforation. (A) The image shows the fishbone removed by endoscopic examination. Adapted from reference (82) with permission from J-STAGE. (B) The object is a 2-centimeter-long fishbone that was removed from the cecal wall. Adapted from reference (19) with permission from Springer Nature. (C) The object is a carp fishbone that caused the perforation of the jejunum. Adapted from reference (53) with permission from Elsevier. (D) The fishbone retrieved from the abdominal cavity resembles a knife. Adapted from reference (41) with permission from Springer Nature.
3.8 Regional characteristics of FIIP
Fish are abundant in bioactive compounds possessing immunomodulatory, antioxidant, antimicrobial, neuroprotective, and cardioprotective properties (128). Regions with extensive coastlines and rich inland water resources hold substantial potential for aquaculture development (129), in recent years, both global fish consumption and production have steadily increased (130). Asia remains the leading producer in the aquaculture sector, contributing 92% of global output (131), and records the highest per capita fish consumption worldwide (132). This heavy dietary reliance significantly increases the risk of fishbone-related injuries. In our dataset of FIIP cases, 63.8% involved patients from Asia (Figure 11 and Table 1). China stands as the world’s largest producer and consumer of aquatic products (133), supported by its expansive coastline and abundant freshwater resources. In addition to seafood, freshwater fish species such as carp and catfish, which are characterized by numerous sharp bones, are widely consumed, particularly in Asia (134). In our collected cases, 12.1% originated from mainland China and Taiwan, China (Figure 11 and Table 1). Japan accounted for the highest proportion of reported cases globally, at 22.4% (Figure 11 and Table 1). This elevated incidence in Japan may be related to several factors: a traditionally higher consumption of fish compared to meat until 2007 (135), a rapidly aging population with a high dietary intake of fish (136), and cultural preferences for raw fish and fried fishbones (137). Furthermore, underreporting in rural regions—due to limited access to medical facilities—may exacerbate the true burden across Asia (138). Overall, regional dietary customs, high fish consumption driven by aquaculture, and unequal healthcare access collectively contribute to the increased incidence of fishbone-related intestinal perforation across Asia, especially in East Asia.
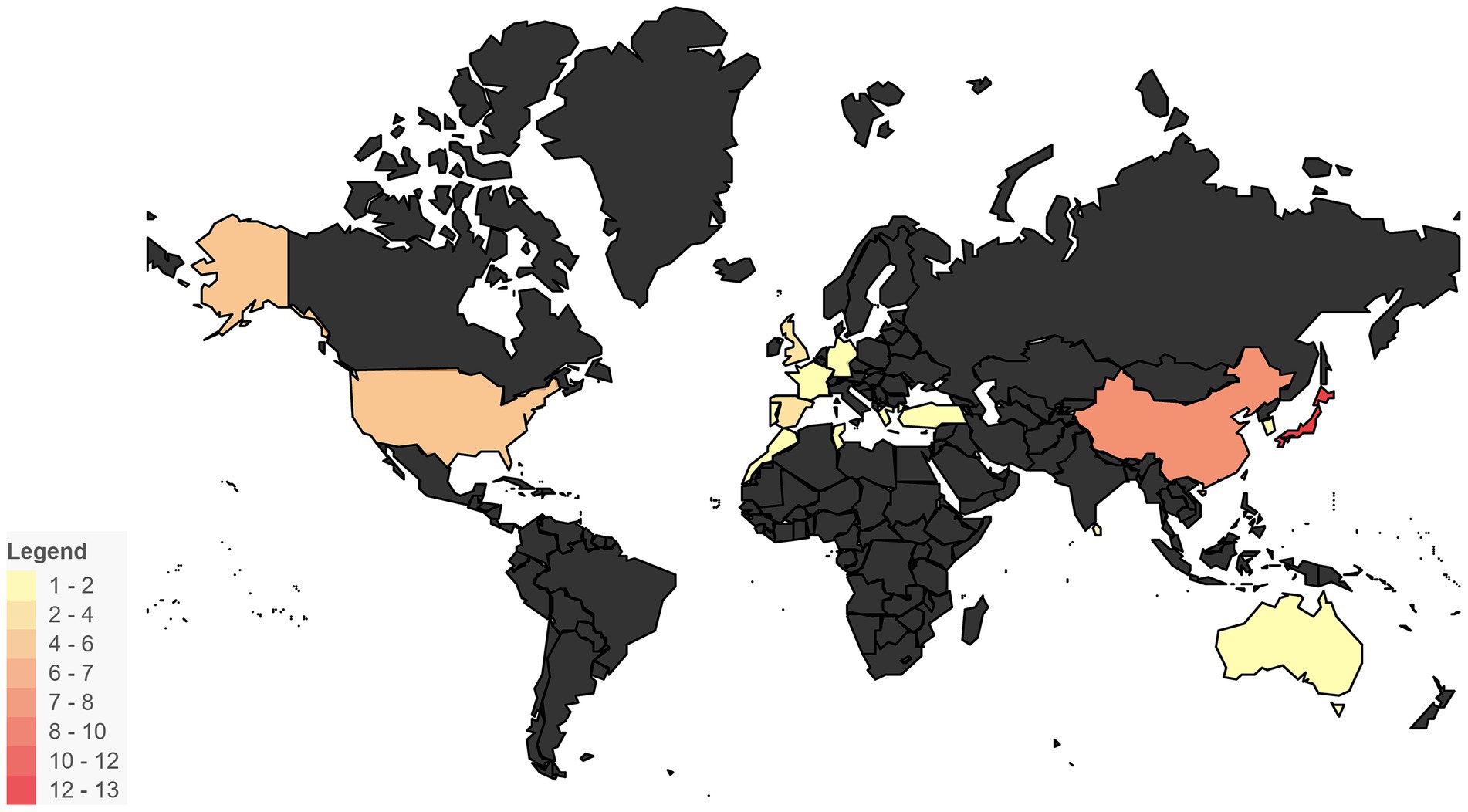
Figure 11. Geographical distribution of FIIP cases. This heatmap illustrates the geographical distribution of FIIP cases, highlighting regions with a higher number of occurrences.
4 Conclusion
FBI remains a prominent clinical concern in emergency medicine. The systematic collection of epidemiological and clinical data on FBI is vital for enhancing diagnostic precision and informing effective treatment strategies. Currently, data on intestinal perforation due to FIIP remain limited. Our study is inherently limited by its retrospective nature and small sample size. However, it provides the first comprehensive analysis of FIIP as a distinct subset of gastrointestinal foreign body injuries. It addresses key aspects, including thorough clinical history-taking, the need for greater awareness of FI risks in elderly populations, identification of high-risk anatomical sites for FIIP, the pivotal role of CT in diagnosis, treatment selection, regional clustering trends, and dietary risk factors linked to fish consumption. We aim for this report to serve as a meaningful reference for advancing both clinical management and future research in this field.
Author contributions
WZ: Supervision, Writing – review & editing, Writing – original draft. JB: Writing – original draft, Visualization, Writing – review & editing. JW: Writing – original draft. CL: Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tseng, HJ, Hanna, TN, Shuaib, W, Aized, M, Khosa, F, and Linnau, KF. Imaging foreign bodies: ingested, aspirated, and inserted. Ann Emerg Med. (2015) 66:570–582e5. doi: 10.1016/j.annemergmed.2015.07.499
2. Hunter, TB, and Taljanovic, MS. Foreign bodies. Radiographics. (2003) 23:731–57. doi: 10.1148/rg.233025137
3. Kehagias, D, Mulita, F, Maroulis, I, and Benetatos, N. Caudate lobe: the last barrier—an unusual place for a foreign body. ANZ J Surg. (2022) 92:1218–20. doi: 10.1111/ans.17226
4. Velitchkov, NG, Grigorov, GI, Losanoff, JE, and Kjossev, KT. Ingested foreign bodies of the gastrointestinal tract: retrospective analysis of 542 cases. World J Surg. (1996) 20:1001–5. doi: 10.1007/s002689900152
5. Pernar, LIM, Mongiu, A, and Sheu, EG. An unusual cause of intestinal perforation. JAMA Surg. (2017) 152:199. doi: 10.1001/jamasurg.2016.4614
6. Du, H, Guo, Y, Bennett, DA, Bragg, F, Bian, Z, Chadni, M, et al. Red meat, poultry and fish consumption and risk of diabetes: a 9 year prospective cohort study of the China Kadoorie Biobank. Diabetologia. (2020) 63:767–79. doi: 10.1007/s00125-020-05091-x
7. Kwasek, K, Thorne-Lyman, AL, and Phillips, M. Can human nutrition be improved through better fish feeding practices? A review paper. Crit Rev Food Sci Nutr. (2020) 60:3822–35. doi: 10.1080/10408398.2019.1708698
8. Sahn, B, Mamula, P, and Ford, CA. Review of foreign body ingestion and esophageal food impaction management in adolescents. J Adolesc Health. (2014) 55:260–6. doi: 10.1016/j.jadohealth.2014.01.022
9. Binder, L, and Anderson, WA. Pediatric gastrointestinal foreign body ingestions. Ann Emerg Med. (1984) 13:112–7. doi: 10.1016/S0196-0644(84)80573-9
10. Vizcarrondo, FJ, Brady, PG, and Juergen, NH. Foreign bodies of the upper gastrointestinal tract. Gastrointest Endosc. (1983) 29:208–10. doi: 10.1016/S0016-5107(83)72586-1
11. Zamary, KR, Davis, JW, Ament, EE, Dirks, RC, and Garry, JE. This too shall pass: a study of ingested sharp foreign bodies. J Trauma Acute Care Surg. (2017) 82:150–5. doi: 10.1097/TA.0000000000001265
12. Selivanov, V, Sheldon, GF, Cello, JP, and Crass, RA. Management of foreign body ingestion. Ann Surg. (1984) 199:187–91. doi: 10.1097/00000658-198402000-00010
13. Schwartz, JT, and Graham, DY. Toothpick perforation of the intestines. Ann Surg. (1977) 185:64–6. doi: 10.1097/00000658-197701000-00010
14. Choi, Y. Peritonitis with small bowel perforation caused by a fish bone in a healthy patient. World J Gastroenterol. (2014) 20:1626. doi: 10.3748/wjg.v20.i6.1626
15. Chen, CK, Su, YJ, Lai, YC, Cheng, HKH, and Chang, WH. Fish bone-related intra-abdominal abscess in an elderly patient. Int J Infect Dis. (2010) 14:e171–2. doi: 10.1016/j.ijid.2009.03.024
16. Ambe, P, Weber, SA, Schauer, M, and Knoefel, WT. Swallowed foreign bodies in adults. Dtsch Arztebl Int. (2012) 109:869–75. doi: 10.3238/arztebl.2012.0869
17. Hoevenaars, FPM, Berendsen, CMM, Pasman, WJ, van den Broek, TJ, Barrat, E, de Hoogh, IM, et al. Evaluation of food-intake behavior in a healthy population: personalized vs. one-size-fits-all. Nutrients. (2020) 12:2819. doi: 10.3390/nu12092819
18. Oken, E, Choi, AL, Karagas, MR, Mariën, K, Rheinberger, CM, Schoeny, R, et al. Which fish should I eat? Perspectives influencing fish consumption choices. Environ Health Perspect. (2012) 120:790–8. doi: 10.1289/ehp.1104500
19. Kuwahara, K, Mokuno, Y, Matsubara, H, Kaneko, H, Shamoto, M, and Iyomasa, S. Development of an abdominal wall abscess caused by fish bone ingestion: a case report. J Med Case Rep. (2019) 13:369. doi: 10.1186/s13256-019-2301-7
20. Brandão, D, Canedo, A, Maia, M, Ferreira, J, and Vaz, G. Duodenocaval fistula as a result of a fish bone perforation. J Vasc Surg. (2010) 51:1276–8. doi: 10.1016/j.jvs.2009.12.049
21. Wang, VY, Wang, VL, Kao, L, and Elwood, DR. A complex game of go fish: a hybrid endoscopic and surgical approach to a fish bone perforation of the portal vein. Am Surg. (2020) 86:153–5. doi: 10.1177/000313482008600317
22. Chiu, WY, Chen, YJ, Cheng, PC, and Shiau, EL. Early presentation of bowel perforation due to fish bone ingestion. QJM. (2014) 107:679–80. doi: 10.1093/qjmed/hcu004
23. Kuo, CC. Medical treatment for a fish bone-induced ileal micro-perforation: a case report. World J Gastroenterol. (2012) 18:5994. doi: 10.3748/wjg.v18.i41.5994
24. Bhatia, R, Deane, AJB, Landham, P, and Schulte, KM. An unusual case of bowel perforation due to fish fin ingestion: bowel perforation due to fish fin ingestion. Int J Clin Pract. (2006) 60:229–31. doi: 10.1111/j.1742-1241.2006.00610.x
25. Bathla, G, Teo, LL, and Dhanda, S. Pictorial essay: complications of a swallowed fish bone. Indian J Radiol Imaging. (2011) 21:63–8. doi: 10.4103/0971-3026.76061
26. Goh, BKP, Jeyaraj, PR, Chan, HS, Ong, HS, Agasthian, T, Chang, KTE, et al. Case report: a case of fish bone perforation of the stomach mimicking a locally advanced pancreatic carcinoma. Dig Dis Sci. (2004) 49:1935–7. doi: 10.1007/s10620-004-9595-y
27. Paixão, TSA, Leão, RV, de Souza Maciel Rocha Horvat, N, Viana, PCC, Da Costa Leite, C, de Azambuja, RL, et al. Abdominal manifestations of fishbone perforation: a pictorial essay. Abdom Radiol. (2017) 42:1087–95. doi: 10.1007/s00261-016-0939-9
28. Saunders, D, Jones, M, Kaushik, M, and Thomas, WM. Fish bone perforation of the terminal ileum presenting as acute appendicitis. BMJ Case Rep. (2014) 2014:bcr2013009533. doi: 10.1136/bcr-2013-009533
29. Hsu, SD, Chan, DC, and Liu, YC. Small-bowel perforation caused by fish bone. World J Gastroenterol. (2005) 11:1884–5. doi: 10.3748/wjg.v11.i12.1884
30. Wong, JH, Suhaili, DN, and Kok, KY. Fish bone perforation of Meckel’s diverticulum: a rare event? Asian J Surg. (2005) 28:295–6. doi: 10.1016/s1015-9584(09)60364-x
31. Mouawad, NJ, Hammond, S, and Kaoutzanis, C. Perforation of Meckel’s diverticulum by an intact fish bone. BMJ Case Rep. (2013) 2013:bcr2012008226. doi: 10.1136/bcr-2012-008226
32. McDowell, DE, and Bush, M. Fish bone perforation of Meckel’s diverticulum simulating a leaking abdominal aortic aneurysm. South Med J. (1982) 75:891–2. doi: 10.1097/00007611-198207000-00037
33. Yogev, D, Mahameed, F, Gileles-Hillel, A, Millman, P, Davidovics, Z, Hashavya, S, et al. Hijab pin ingestions. Pediatrics. (2020) 145:e20193472. doi: 10.1542/peds.2019-3472
34. Alansari, AN, Baykuziyev, T, Soyer, T, Akıncı, SM, Al Ali, KK, Aljneibi, A, et al. Magnet ingestion in growing children: a multi-center observational study on single and multiple magnet incidents. Sci Rep. (2024) 14:4575. doi: 10.1038/s41598-024-55127-0
35. Mubarak, A, Benninga, MA, Broekaert, I, Dolinsek, J, Homan, M, Mas, E, et al. Diagnosis, management, and prevention of button battery ingestion in childhood: a European Society for Paediatric Gastroenterology Hepatology and Nutrition position paper. J Pediatr Gastroenterol Nutr. (2021) 73:129–36. doi: 10.1097/MPG.0000000000003048
36. Kijowski, R, Demehri, S, Roemer, F, and Guermazi, A. Osteoarthritis year in review 2019: imaging. Osteoarthr Cartil. (2020) 28:285–95. doi: 10.1016/j.joca.2019.11.009
37. Hillman, KM. Pneumoperitoneum—a review. Crit Care Med. (1982) 10:476–81. doi: 10.1097/00003246-198207000-00015
38. Berk, RN, and Reit, RJ. Intra-abdominal chicken-bone abscess. Radiology. (1971) 101:311–3. doi: 10.1148/101.2.311
39. Kumar, M, Joseph, G, Kumar, S, and Clayton, M. Fish bone as a foreign body. J Laryngol Otol. (2003) 117:568–9. doi: 10.1258/002221503322113058
40. Mora-Guzmán, I, Valdés De Anca, Á, and Martín-Pérez, E. Intra-abdominal abscess due to fish bone perforation of small bowel. Acta Chir Belg. (2019) 119:66–7. doi: 10.1080/00015458.2018.1493822
41. Wang, R, He, J, Chen, Z, and Wen, K. Migration of fish bones into abdominal para-aortic tissue from the duodenum after leading to duodenal perforation: a case report. BMC Gastroenterol. (2021) 21:82. doi: 10.1186/s12876-021-01662-3
42. Natsuki, S, Iseki, Y, Nagahara, H, Fukuoka, T, Shibutani, M, and Ohira, M. Liver abscess caused by fish bone perforation of Meckel’s diverticulum: a case report. BMC Surg. (2020) 20:121. doi: 10.1186/s12893-020-00783-y
43. Ngan, JH, Fok, PJ, Lai, EC, Branicki, FJ, and Wong, J. A prospective study on fish bone ingestion. Experience of 358 patients. Ann Surg. (1990) 211:459–62. doi: 10.1097/00000658-199004000-00012
44. Kessner, R, Barnes, S, Halpern, P, Makrin, V, and Blachar, A. CT for acute nontraumatic abdominal pain-is oral contrast really required? Acad Radiol. (2017) 24:840–5. doi: 10.1016/j.acra.2017.01.013
45. Goh, BKP, Tan, YM, Lin, SE, Chow, PKH, Cheah, FK, Ooi, LLPJ, et al. CT in the preoperative diagnosis of fish bone perforation of the gastrointestinal tract. AJR Am J Roentgenol. (2006) 187:710–4. doi: 10.2214/AJR.05.0178
46. Goh, BKP, Yong, WS, and Yeo, AWY. Pancreatic and hepatic abscess secondary to fish bone perforation of the duodenum. Dig Dis Sci. (2005) 50:1103–6. doi: 10.1007/s10620-005-2712-8
47. Song, J, Yang, W, Zhu, Y, Fang, Y, Qiu, J, Qiu, J, et al. Ingested a fish bone-induced ileal perforation: a case report. Medicine. (2020) 99:e19508. doi: 10.1097/MD.0000000000019508
48. Lin, CY, and Wu, FZ. Fish bone perforation of small intestine. QJM. (2012) 105:479–80. doi: 10.1093/qjmed/hcr035
49. Lunsford, KE, and Sudan, R. Small bowel perforation by a clinically unsuspected fish bone: laparoscopic treatment and review of literature. J Gastrointest Surg. (2012) 16:218–22. doi: 10.1007/s11605-011-1610-y
50. Drakonaki, E, Chatzioannou, M, Spiridakis, K, and Panagiotakis, G. Acute abdomen caused by a small bowel perforation due to a clinically unsuspected fish bone. Diagn Interv Radiol. (2011) 17:160–2. doi: 10.4261/1305-3825.DIR.3236-09.1
51. Völk, M, Geissler, A, Herold, T, Schäffler, A, Gross, V, and Gmeinwieser, J. The computed tomographic demonstration of duodenal perforation caused by a fish bone. Fortschr Geb Rontgenstr Nuklearmed. (1997) 167:198–200.
52. Shibuya, T, Osada, T, Asaoka, D, Mori, H, Beppu, K, Sakamoto, N, et al. Double-balloon endoscopy for treatment of long-term abdominal discomfort due to small bowel penetration by an eel bone. Med Sci Monit. (2008) 14:CS107-9
53. Alkhatib, AA, Umar, SB, Patel, NC, and Harrison, ME. Balloon-assisted enteroscopy for the treatment of a sealed jejunal perforation: removal of a penetrating fish bone (with video). Gastrointest Endosc. (2013) 77:133–5. doi: 10.1016/j.gie.2012.08.033
54. Matricardi, L, and Lovati, R. Intestinal perforation by a foreign body: diagnostic usefulness of ultrasonography. J Clin Ultrasound. (1992) 20:194–6. doi: 10.1002/jcu.1870200306
55. White, RZ, Rezaian, P, Parasuramar, A, and Sampson, MJ. Ultrasound-assisted foreign body extraction (U-SAFE): review of technique and technical pearls. J Med Imaging Radiat Oncol. (2022) 66:362–9. doi: 10.1111/1754-9485.13313
56. Rooks, VJ, Shiels, WE, and Murakami, JW. Soft tissue foreign bodies: a training manual for sonographic diagnosis and guided removal. J Clin Ultrasound. (2020) 48:330–6. doi: 10.1002/jcu.22856
57. Athreya, S, Radhachandran, A, Ivezić, V, Sant, VR, Arnold, CW, and Speier, W. Enhancing ultrasound image quality across disease domains: application of cycle-consistent generative adversarial network and perceptual loss. JMIR Biomed Eng. (2024) 9:e58911. doi: 10.2196/58911
58. Masuya, R, Okamoto, K, Kidogawa, H, Kamizono, J, and Ieiri, S. Rare pediatric case of Meckel diverticulum penetration caused by a fish bone. Pediatr Int. (2019) 61:731–3. doi: 10.1111/ped.13902
59. Yen, H, and Hsu, Y. Gastrointestinal: pyogenic liver abscess associated with a penetrating fish bone. J Gastroenterol Hepatol. (2010) 25:1900–21. doi: 10.1111/j.1440-1746.2010.06551.x
60. Gonçalves, A, Almeida, M, Malheiro, L, and Costa-Maia, J. Meckel’s diverticulum perforation by a fish bone: a case report. Int J Surg Case Rep. (2016) 28:237–40. doi: 10.1016/j.ijscr.2016.08.026
61. Pinto, A, Pinto, F, Faggian, A, Rubini, G, Caranci, F, Macarini, L, et al. Sources of error in emergency ultrasonography. Crit Ultrasound J. (2013) 5:S1. doi: 10.1186/2036-7902-5-S1-S1
62. Zhong, Z, Li, Z, Xing, Y, and Guo, S. Case report: a large gastric calcifying fibrous tumor treated with endoscopic submucosal excavation. Front Oncol. (2024) 14:1385695. doi: 10.3389/fonc.2024.1385695
63. Mantegazza, C, Destro, F, Ferraro, S, Biganzoli, D, Marano, G, Quitadamo, P, et al. Recent trends in foreign body ingestion (FBI) epidemiology: a national cohort study. Dig Liver Dis. (2025) 57:595–602. doi: 10.1016/j.dld.2024.10.002
64. Oliva, S, Romano, C, de Angelis, P, Isoldi, S, Mantegazza, C, Felici, E, et al. Foreign body and caustic ingestions in children: a clinical practice guideline. Dig Liver Dis. (2020) 52:1266–81. doi: 10.1016/j.dld.2020.07.016
65. van Waas, M, De Bruyne, P, and de Ridder, L. Paediatric multiple magnet ingestion. Lancet Gastroenterol Hepatol. (2021) 6:80. doi: 10.1016/S2468-1253(20)30338-1
66. Orsagh-Yentis, D, McAdams, RJ, Roberts, KJ, and McKenzie, LB. Foreign-body ingestions of young children treated in US emergency departments: 1995–2015. Pediatrics. (2019) 143:e20181988. doi: 10.1542/peds.2018-1988
67. Marquardt, P, Derousseau, T, and Patel, N. Foreign body ingestion: a curious case of the missing denture. Geriatrics. (2020) 5:49. doi: 10.3390/geriatrics5030049
68. Kim, SY, Park, B, Kong, IG, and Choi, HG. Analysis of ingested foreign bodies according to age, type and location: a retrospective observational study. Clin Otolaryngol. (2016) 41:640–5. doi: 10.1111/coa.12576
69. Wu, T, Meng, J, Li, X, and He, S. A rare and peculiar case of fish bone penetration to the submucosa of the posterior pharyngeal wall. Asian J Surg. (2024) 47:1445–6. doi: 10.1016/j.asjsur.2023.11.123
70. Jung, SM, and Kim, S. In vitro models of the small intestine for studying intestinal diseases. Front Microbiol. (2021) 12:767038. doi: 10.3389/fmicb.2021.767038
71. Browning, CM, Cloutier, R, Rich, TC, and Leavesley, SJ. Endoscopy lifetime systems architecture: scoping out the past to diagnose the future technology. Systems. (2022) 10:189. doi: 10.3390/systems10050189
72. Pichardo, J, Zapata, J, Echavarría, R, Ubiñas, R, Báez, P, and Gómez, Á. Gallstone ileus with cholecystoenteric fistula in an elderly female: a case report. Cureus. (2023) 15:e37077. doi: 10.7759/cureus.37077
73. Memon, AA, Siddiqui, FG, Abro, AH, Agha, AH, Lubna, S, and Memon, AS. An audit of secondary peritonitis at a tertiary care university hospital of Sindh, Pakistan. World J Emerg Surg. (2012) 7:6. doi: 10.1186/1749-7922-7-6
74. Ivatury, RR. Meckel’s diverticulum and the eponymous legend. J Trauma Acute Care Surg. (2019) 87:451–5. doi: 10.1097/TA.0000000000002300
75. Levy, AD, and Hobbs, CM. From the archives of the AFIP. Meckel diverticulum: radiologic features with pathologic correlation. Radiographics. (2004) 24:565–87. doi: 10.1148/rg.242035187
76. Sagar, J, Kumar, V, and Shah, DK. Meckel’s diverticulum: a systematic review. J R Soc Med. (2006) 99:501–5. doi: 10.1177/014107680609901011
77. Uppal, K, Tubbs, RS, Matusz, P, Shaffer, K, and Loukas, M. Meckel’s diverticulum: a review. Clin Anat. (2011) 24:416–22. doi: 10.1002/ca.21094
78. Ding, Y, Zhou, Y, Ji, Z, Zhang, J, and Wang, Q. Laparoscopic management of perforated Meckel’s diverticulum in adults. Int J Med Sci. (2012) 9:243–7. doi: 10.7150/ijms.4170
79. Malik, AA, Shams-ul-Bari,, Wani, KA, and Khaja, AR. Meckel’s diverticulum-revisited. Saudi J Gastroenterol. (2010) 16:3–7. doi: 10.4103/1319-3767.58760
80. Devanathan, N, Patel, H, Sargin, P, Zarak, A, and Biglione, A. Sigmoid perforation by an ingested foreign body mimicking acute appendicitis: a case report. Cureus. (2024) 16:e66855. doi: 10.7759/cureus.66855
81. Lu, J, Tan, Y, Liu, D, Li, C, and Zhou, H. Endoscopic submucosal dissection for rectal-sigmoid laterally spreading tumors ≥10 cm: an analysis of 10 cases. Transl Cancer Res. (2021) 10:867–75. doi: 10.21037/tcr-20-2659
82. Ueda, T, Sato, H, Ogimi, T, Deguchi, R, and Suzuki, H. Use of endoscopy to remove fish bone that caused sigmoid colon perforation. Intern Med. (2024) 63:2626–30. doi: 10.2169/internalmedicine.3063-23
83. Manes, G, Andreozzi, P, Omazzi, B, Bezzio, C, Redaelli, D, Devani, M, et al. Efficacy of withdrawal time monitoring in adenoma detection with or without the aid of a full-spectrum scope. Endosc Int Open. (2019) 7:E1135–42. doi: 10.1055/a-0854-3946
84. Paulus, LP, Buehler, A, Wagner, AL, Raming, R, Jüngert, J, Simon, D, et al. Contrast-enhanced multispectral optoacoustic tomography for functional assessment of the gastrointestinal tract. Adv Sci. (2023) 10:e2302562. doi: 10.1002/advs.202302562
85. Guo, L, Zhang, T, Liang, T, Chen, J, and Gao, H. Laparoscopic radical cystectomy with ileal orthotopic neobladder for bladder cancer: current indications and outcomes. Urol Int. (2024) 108:242–53. doi: 10.1159/000535032
86. Hussain, MS, Balagoni, H, Dwivedi, S, and Piper, M. Macroscopic to microscopic—a case of Crohn’s disease progressing to collagenous colitis. Cureus. (2021) 13:e18299. doi: 10.7759/cureus.18299
87. Pérez-Lara, JL, Santana, Y, Hernández-Torres, J, and Díaz-Fuentes, G. Acute colonic pseudo-obstruction caused by dexmedetomidine: a case report and literature review. Am J Case Rep. (2019) 20:278–84. doi: 10.12659/AJCR.913645
88. Zheng, Y, Li, Z, and Zhou, Q. Diagnosis of small intestinal microperforation by cell morphology detection in abdominal puncture fluid: a case report. Int J Surg Case Rep. (2024) 115:109316. doi: 10.1016/j.ijscr.2024.109316
89. Chen, H, Kuo, J, Uen, Y, and Sun, D. Liver abscess secondary to fish bone migration from the duodenum. ANZ J Surg. (2011) 81:206–6. doi: 10.1111/j.1445-2197.2010.05665.x
90. Tepelidis, C, Fotiadis, P, Permekerlis, A, Karastergiou, T, and Kouridakis, P. Descending colon perforation due to ingestion of foreign body. Cureus. (2023) 15:e47479. doi: 10.7759/cureus.47479
91. Ma, T, Zheng, W, An, B, Xia, Y, and Chen, G. Small bowel perforation secondary to foreign body ingestion mimicking acute appendicitis: case report. Medicine. (2019) 98:e16489. doi: 10.1097/MD.0000000000016489
92. Coulier, B, Tancredi, MH, and Ramboux, A. Spiral CT and multidetector-row CT diagnosis of perforation of the small intestine caused by ingested foreign bodies. Eur Radiol. (2004) 14:1918–25. doi: 10.1007/s00330-004-2430-1
93. Chaugale, SB, Singhal, V, Kapoor, D, and Singh, A. Gastrointestinal complications (gangrene or perforation) after corona virus disease 2019—a series of ten patients. Indian J Gastroenterol. (2022) 41:307–12. doi: 10.1007/s12664-021-01218-z
94. Ward, MA, and Tews, MC. Small bowel perforation secondary to fish bone ingestion managed non-operatively. J Emerg Med. (2012) 43:e295–8. doi: 10.1016/j.jemermed.2010.05.039
95. Lim, BK, and Siew, EP. Swallowed fish bone expelled spontaneously after perforating the small bowel. Ann Acad Med Singap. (2011) 40:475–6. doi: 10.47102/annals-acadmedsg.V40N10p475
96. Essrani, R, Hickey, P, and Shah, H. Denture misadventure: the tooth about your colon. Cureus. (2019) 11:e3890. doi: 10.7759/cureus.3890
97. Buck, E, Lee, S, Stone, LS, and Cerruti, M. Protein adsorption on surfaces functionalized with COOH groups promotes anti-inflammatory macrophage responses. ACS Appl Mater Interfaces. (2021) 13:7021–36. doi: 10.1021/acsami.0c16509
98. Kong, W, Du, Q, Xin, Z, Cao, G, Liu, D, Wei, Y, et al. Percutaneous fully endoscopic surgical management of the ruptured epidural catheter: rescue of the novice anesthesiologist from his dilemma. Front Surg. (2022) 9:915133. doi: 10.3389/fsurg.2022.915133
99. Poretti, D, Pescatori, LC, Mauri, G, Sconfienza, LM, and Brambilla, G. Inferior vena cava septic thrombosis due to gut perforation after accidental toothpick ingestion. BJR Case Rep. (2017) 3:20150522. doi: 10.1259/bjrcr.20150522
100. Cull, JN, Jacobson, DL, Lau, GA, Cartwright, PC, Wallis, MC, Skarda, D, et al. Internal hernia with volvulus after major abdominal reconstructions in pediatric urology—an infrequently reported and potentially devastating complication. J Pediatr Urol. (2023) 19:402.e1–7. doi: 10.1016/j.jpurol.2023.04.030
101. Zhang, Z, Yang, M, and Zhang, R. Radiographic grid for locating foreign bodies in maxillofacial emergency trauma. BMC Oral Health. (2024) 24:46. doi: 10.1186/s12903-023-03807-0
102. Magalhães-Costa, P, Carvalho, L, Rodrigues, JP, Túlio, MA, Marques, S, Carmo, J, et al. Endoscopic management of foreign bodies in the upper gastrointestinal tract: an evidence-based review article. GE Port J Gastroenterol. (2016) 23:142–52. doi: 10.1016/j.jpge.2015.09.002
103. Yoo, DR, Im, CB, Jun, BG, Seo, HI, Park, JK, Lee, SJ, et al. Clinical outcomes of endoscopic removal of foreign bodies from the upper gastrointestinal tract. BMC Gastroenterol. (2021) 21:385. doi: 10.1186/s12876-021-01959-3
104. Nishino, T, Shinzato, T, Uramatsu, T, Obata, Y, Arai, H, Hayashida, T, et al. Bacterial peritonitis due to duodenal perforation by a fish bone in an elderly peritoneal dialysis patient. Intern Med. (2012) 51:1715–9. doi: 10.2169/internalmedicine.51.7286
105. Yamamoto, H. Fifteen years since the advent of double-balloon endoscopy. Clin Gastroenterol Hepatol. (2017) 15:1647–50. doi: 10.1016/j.cgh.2017.08.018
106. Ito, T, Shimatani, M, Masuda, M, Nakamaru, K, Mitsuyama, T, Fukata, N, et al. Efficacy and safety of endoscopic stent placement for afferent loop obstruction using a short double-balloon endoscopy. DEN Open. (2023) 3:e154. doi: 10.1002/deo2.154
107. Hendel, JW, Vilmann, P, and Jensen, T. Double-balloon endoscopy: who needs it? Scand J Gastroenterol. (2008) 43:363–7. doi: 10.1080/00365520701799468
108. Watanabe, M, Kou, T, Nishikawa, Y, Sakuma, Y, Kumagai, N, Oda, Y, et al. Perforation of the sigmoid colon by an ingested fish bone. Intern Med. (2010) 49:1041–2. doi: 10.2169/internalmedicine.49.3438
109. Fang, C, Ye, L, Mao, X, and Zhang, J. Endoscopic treatment of a sigmoid perforation caused by an ingested fish bone. Endoscopy. (2017) 49:E82–3. doi: 10.1055/s-0042-124500
110. Garbati, P, Picco, C, Magrassi, R, Signorello, P, Cacopardo, L, Dalla Serra, M, et al. Targeting the gut: a systematic review of specific drug nanocarriers. Pharmaceutics. (2024) 16:431. doi: 10.3390/pharmaceutics16030431
111. Dev, S, Pokhrel, KM, Mulmi, U, Devkota, S, Dev, B, and Bhattarai, A. Chicken bone-induced ileal perforation peritonitis mimicking duodenal perforation peritonitis: a case report. Ann Med Surg. (2012) 85:6202–5. doi: 10.1097/MS9.0000000000001404
112. Taguchi, T, and Kitagawa, H. Fish bone perforation. N Engl J Med. (2019) 381:762–2. doi: 10.1056/NEJMicm1900442
113. Nagy, AG, and James, D. Diagnostic laparoscopy. Am J Surg. (1989) 157:490–3. doi: 10.1016/0002-9610(89)90642-9
114. Dellino, M, Cerbone, M, Laganà, AS, Vitagliano, A, Vimercati, A, Marinaccio, M, et al. Upgrading treatment and molecular diagnosis in endometrial cancer-driving new tools for endometrial preservation? Int J Mol Sci. (2023) 24:9780. doi: 10.3390/ijms24119780
115. Sermonesi, G, Tian, BWCA, Vallicelli, C, Abu-Zidan, FM, Damaskos, D, Kelly, MD, et al. Cesena guidelines: WSES consensus statement on laparoscopic-first approach to general surgery emergencies and abdominal trauma. World J Emerg Surg. (2023) 18:57. doi: 10.1186/s13017-023-00520-9
116. Zhang, S, Wang, Q, Feng, Y, Zhang, G, Chen, Y, Zheng, W, et al. Clip or tattooing: a comparative study for preoperative colon cancer endoscopic localization. Front Oncol. (2022) 12:846900. doi: 10.3389/fonc.2022.846900
117. Alemanno, G, Prosperi, P, Di Bella, A, Socci, F, Batacchi, S, Peris, A, et al. Bedside diagnostic laparoscopy for critically ill patients in the intensive care unit: retrospective study and review of literature. J Minimal Access Surg. (2019) 15:56–62. doi: 10.4103/jmas.JMAS_232_17
118. Dural, AC, Çelik, MF, Yiğitbaş, H, Akarsu, C, Doğan, M, and Alış, H. Laparoscopic resection and intracorporeal anastomosis of perforated small bowel caused by fish bone ingestion. Turk J Trauma Emerg Surg. (2016) 22:572–4. doi: 10.5505/tjtes.2016.88137
119. Li, M, Liu, Q, Zhang, T, Gao, Y, Tan, X, Yin, Z, et al. Evaluating the learning curve of robotic radical antegrade modular pancreatosplenectomy: a retrospective cohort study. Int J Surg. (2022) 101:106612. doi: 10.1016/j.ijsu.2022.106612
120. Gage, D, Neilson, T, Pino, MG, Eiferman, D, and Knight-Davis, J. Establishment of a 24/7 robotic acute care surgery program at a large academic medical center. Surg Endosc. (2024) 38:4663–9. doi: 10.1007/s00464-024-11036-x
121. Ceccarelli, G, Catena, F, Avella, P, Tian, BW, Rondelli, F, Guerra, G, et al. Emergency robotic surgery: the experience of a single center and review of the literature. World J Emerg Surg. (2024) 19:28. doi: 10.1186/s13017-024-00555-6
122. Yu, B, Yeo, IH, and Park, JY. Life-threatening esophageal perforation due to blister pack ingestion. Am J Emerg Med. (2023) 65:219.e1–3. doi: 10.1016/j.ajem.2022.12.031
123. Irwin, RS, Ashba, JK, Braman, SS, Lee, HY, and Corrao, WM. Food asphyxiation in hospitalized patients. JAMA. (1977) 237:2744–5. doi: 10.1001/jama.1977.03270520054024
124. Rodríguez-Hermosa, JI, Ruiz-Feliú, B, Roig-García, J, Albiol-Quer, M, Planellas-Giné, P, and Codina-Cazador, A. Lethal intestinal perforation after foreign body ingestion in a superobese patient. Obes Surg. (2009) 19:1183–5. doi: 10.1007/s11695-008-9667-8
125. Cho, SH, Lee, S, Park, JI, La Yang, Y, Kim, SR, Ahn, J, et al. Age-associated spinal stenosis in the turquoise killifish. iScience. (2023) 26:107877. doi: 10.1016/j.isci.2023.107877
126. Shishido, T, Suzuki, J, Ikeda, R, Kobayashi, Y, and Katori, Y. Characteristics of fish-bone foreign bodies in the upper aero-digestive tract: the importance of identifying the species of fish. PLoS One. (2021) 16:e0255947. doi: 10.1371/journal.pone.0255947
127. Lu, Y, Shi, C, Jin, X, He, J, and Yin, Z. Domestication of farmed fish via the attenuation of stress responses mediated by the hypothalamus-pituitary-inter-renal endocrine axis. Front Endocrinol. (2022) 13:923475. doi: 10.3389/fendo.2022.923475
128. Chen, J, Jayachandran, M, Bai, W, and Xu, B. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem. (2022) 369:130874. doi: 10.1016/j.foodchem.2021.130874
129. Maulu, S, Musuka, CG, Molefe, M, Ngoepe, TK, Gabriel, NN, Mphande, J, et al. Contribution of fish to food and nutrition security in Southern Africa: challenges and opportunities in fish production. Front Nutr. (2024) 11:1424740. doi: 10.3389/fnut.2024.1424740
130. Fiorella, KJ, Okronipa, H, Baker, K, and Heilpern, S. Contemporary aquaculture: implications for human nutrition. Curr Opin Biotechnol. (2021) 70:83–90. doi: 10.1016/j.copbio.2020.11.014
131. Naylor, RL, Hardy, RW, Buschmann, AH, Bush, SR, Cao, L, Klinger, DH, et al. A 20-year retrospective review of global aquaculture. Nature. (2021) 591:551–63. doi: 10.1038/s41586-021-03308-6
132. Bennett, G, Bardon, LA, and Gibney, ER. A comparison of dietary patterns and factors influencing food choice among ethnic groups living in one locality: a systematic review. Nutrients. (2022) 14:941. doi: 10.3390/nu14050941
133. Asche, F, Yang, B, Gephart, JA, Smith, MD, Anderson, JL, Camp, EV, et al. China’s seafood imports-not for domestic consumption? Science. (2022) 375:386–8. doi: 10.1126/science.abl4756
134. Xu, H, Wu, T, Budhathoki, M, Fang, DS, Zhang, W, and Wang, X. Consumption patterns and willingness to pay for sustainable aquatic food in China. Foods. (2024) 13:2435. doi: 10.3390/foods13152435
135. Sasaki, K, Motoyama, M, Watanabe, G, and Nakajima, I. Meat consumption and consumer attitudes in Japan: an overview. Meat Sci. (2022) 192:108879. doi: 10.1016/j.meatsci.2022.108879
136. Nomura, M, Yamaguchi, M, Inada, Y, and Nishi, N. Current dietary intake of the Japanese population in reference to the planetary health diet-preliminary assessment. Front Nutr. (2023) 10:1116105. doi: 10.3389/fnut.2023.1116105
137. Yoshikawa, E, Nishi, D, and Matsuoka, YJ. Association between frequency of fried food consumption and resilience to depression in Japanese company workers: a cross-sectional study. Lipids Health Dis. (2016) 15:156. doi: 10.1186/s12944-016-0331-3
138. Aljassim, N, and Ostini, R. Health literacy in rural and urban populations: a systematic review. Patient Educ Couns. (2020) 103:2142–54. doi: 10.1016/j.pec.2020.06.007
139. Wu, CW, and Chiu, YW. Unintentional fish bone ingestion causing perforation of small intestine. Intern Emerg Med. (2021) 16:1371–2. doi: 10.1007/s11739-020-02519-5
140. Yasuda, T, Kawamura, S, Shimada, E, and Okumura, S. Fish bone penetration of the duodenum extending into the pancreas: report of a case. Surg Today. (2010) 40:676–8. doi: 10.1007/s00595-009-4110-x
141. Yuan, F, Liu, Y, and Wen, M. Fish bone perforation of the transverse colon. Intern Emerg Med. (2024) 19:1771–2. doi: 10.1007/s11739-024-03591-x
142. Huang, YH, Siao, FY, and Yen, HH. Pre-operative diagnosis of pancreatic abscess from a penetrating fish bone. QJM. (2013) 106:955–6. doi: 10.1093/qjmed/hcs166
143. Jallali, M, Zenati, H, Korbi, A, Chaouch, MA, Jabra, SB, Korbi, I, et al. Small bowel perforation with ingestion of a fish bone: case report. Pan Afr Med J. (2024) 48:94. doi: 10.11604/pamj.2024.48.94.42796
144. Dugas, B, Bernard, B, Odet, E, Naouri, A, Bernard, P, and Valette, P. Bad dentition and risk of jejunal perforation by fish bone. J Am Geriatr Soc. (2005) 53:1632–3. doi: 10.1111/j.1532-5415.2005.53487_2.x
145. Guillén-Paredes, MP, Lirón-Ruiz, R, Torralba-Martínez, JA, Martín-Lorenzo, JG, and Aguayo-Albasini, JL. Intestinal perforation caused by incidental ingestion of a fish bone: value of CT in the diagnosis. Rev Esp Enferm Dig. (2010) 102:569–70. doi: 10.4321/S1130-01082010000900016
146. Chandrasinghe, PC, and Pathirana, CK. Laparoscopically detected and nonsurgically managed ileal perforation by an ingested fish bone: a case report. J Med Case Rep. (2015) 9:43. doi: 10.1186/s13256-015-0526-7
147. Wu, CX. Rare case of omentum-wrapped abscess caused by a fish bone penetrating the terminal ileum. World J Gastroenterol. (2014) 20:11456. doi: 10.3748/wjg.v20.i32.11456
148. Zhao, SG, Xu, JJ, Xu, L, Zheng, JF, Zhou, ZC, and Jiang, LQ. Ileal perforation caused by a fish bone shortly after drug-eluting stent implantation for acute myocardial infarction. J Int Med Res. (2019) 47:2709–15. doi: 10.1177/0300060519842778
149. Mutlu, A, Uysal, E, Ulusoy, L, Duran, C, and Selamoglu, D. A fish bone causing ileal perforation in the terminal ileum. Turk J Trauma Emerg Surg. (2012) 18:89–91. doi: 10.5505/tjtes.2012.90912
150. Hassani, KIM, and Toughrai, I. Péritonite par perforation grêlique secondaire à une arête de poisson. Pan Afr Med J. (2013) 15:107. doi: 10.11604/pamj.2013.15.107.3025
151. Fantola, G, Cenedese, A, Barth, X, and Monneuse, O. Laparoscopic diagnosis and treatment of small bowel perforation by fish bone. ANZ J Surg. (2011) 81:205–6. doi: 10.1111/j.1445-2197.2010.05664.x
152. Daniele, L, Elliott, D, Wong, MS, and Free, J. Perforation of Meckel’s diverticulum by an intact fish bone: a case report and literature review. ANZ J Surg. (2017) 87:E206–7. doi: 10.1111/ans.13125
153. Zhou, W, Ding, L, Dong, T, and Liu, X. Unusual case of acute appendicitis with perforation caused by an ingested fish bone. Asian J Surg. (2024) 47:1421–2. doi: 10.1016/j.asjsur.2023.11.111
154. Ishimura, T, Takenaka, A, Sakai, Y, Fujii, T, Jo, Y, and Fujisawa, M. Hydronephrosis caused by intra-abdominal abscess from cecal perforation by an ingested fish bone. Int J Urol. (2006) 13:1350–1. doi: 10.1111/j.1442-2042.2006.01552.x
155. Yamamoto, M, Yamamoto, K, Sasaki, T, Fukumori, D, Yamamoto, F, Igimi, H, et al. Successfully treated intra-abdominal abscess caused by fish bone with perforation of ascending colon: a case report. Int Surg. (2015) 100:428–30. doi: 10.9738/INTSURG-D-14-00163.1
156. Chiu, JJ, Chen, TL, and Zhan, YL. Perforation of the transverse colon by a fish bone: a case report. J Emerg Med. (2009) 36:345–7. doi: 10.1016/j.jemermed.2007.11.007
157. Saleem, A, Alenezi, S, Abdulbaqi, S, Saud, A, and Al-Shadidi, N. Multiple abdominopelvic abscesses caused by fishbone: a case report of rare etiology and literature review. Int J Surg Case Rep. (2023) 110:108608. doi: 10.1016/j.ijscr.2023.108608
158. Cho, MK. Fish bone migration to the urinary bladder after rectosigmoid colon perforation. World J Gastroenterol. (2014) 20:7075. doi: 10.3748/wjg.v20.i22.7075
159. Endo, S, Minakuchi, H, and Yoshida, T. Peritoneal dialysis patient having fish bone-induced colon perforation. Clin Exp Nephrol. (2019) 23:717–8. doi: 10.1007/s10157-018-1670-3
160. Hawkyard, A, and Armitage, G. Peritonitis due to perforation of the bowel by foreign bodies. BMJ. (1931) 1:577–8. doi: 10.1136/bmj.1.3665.577
161. Yamashita, K, Komohara, Y, Uchihara, T, Arima, K, Uemura, S, Hanada, N, et al. A rare case of perforation of a colorectal tumor by a fish bone. Clin J Gastroenterol. (2022) 15:598–602. doi: 10.1007/s12328-022-01622-8
Glossary
FBI - Foreign body ingestion
FI - Fishbone ingestion
CT - Computed tomography
FIIP - Fishbone-induced intestinal perforation
WBC - White blood cell
NEUT - Neutrophil
CRP - C-reactive protein
MD - Meckel’s diverticulum
Con Tx - Conservative treatment
ST - Surgical treatment
LS - Laparoscopic surgery
Lap - Laparotomy
Abs - Antibiotics
SI - Small intestine
Duo - Duodenum
Jej - Jejunum
Ile - Ileum
LI - Large intestine
Cec - Cecum
CO - Colon
TC - Transverse colon
SC - Sigmoid colon
App. - Appendix
AC - Ascending colon
Keywords: foreign bodies, intestinal perforation, diagnosis, abdomen, acute
Citation: Zuo W, Bai J, Wei J and Long C (2025) Fishbone ingestion is a non-negligible cause of intestinal perforation. Front. Med. 12:1607262. doi: 10.3389/fmed.2025.1607262
Edited by:
Angel Lanas, University of Zaragoza, SpainReviewed by:
Andee Dzulkarnaen Zakaria, Universiti Sains Malaysia, MalaysiaPavel Skok, University of Maribor, Slovenia
Copyright © 2025 Zuo, Bai, Wei and Long. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiexin Bai, YmFpamlleGluQHN0dS5jZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work
 Wenwei Zuo1†
Wenwei Zuo1† Jiexin Bai
Jiexin Bai Jing Wei
Jing Wei