- 1Department of Obstetrics and Gynecology, Soroka University Medical Center, Beersheba, Israel
- 2Faculty of Health Science, Ben-Gurion University of the Negev, Beersheba, Israel
- 3Pediatric Dermatology Service, Soroka University Medical Center, Beersheba, Israel
Background: This study investigated the prevalence of acanthosis nigricans and acrochordons as potential markers of diabetes or insulin resistance in diabetic and non-diabetic pregnant women, and examined their potential association with pregnancy complications.
Methods: This prospective cohort study was conducted at a tertiary university medical center and included 62 pregnant women with diabetes during pregnancy and 58 non-diabetic controls. Maternal demographics, obstetrical history, and neonatal outcomes were collected. The presence of acanthosis nigricans and acrochordons was assessed at multiple body sites. Multivariable logistic regression analysis was performed to identify obstetrical complications and perinatal outcomes that are independently associated with acrochordons.
Results: Acanthosis nigricans was more prevalent among women with diabetes, particularly in the axilla (59.7% vs. 32.8%, p = 0.004), under the breast (46.8% vs. 19%, p = 0.002) and in the lower abdomen (35.5% vs. 25.5%, p = 0.021). Similarly, acrochordons were significantly more common in diabetic patients, with a higher prevalence at various sites (p < 0.001). In multivariable regression analysis, diabetes during pregnancy and maternal age were independently associated with a high prevalence of acrochordons.
Conclusion: Pregnant women with gestational diabetes showed a significantly higher prevalence of acanthosis nigricans and acrochordons compared to those without. This association suggests that acanthosis nigricans and acrochordons may be potential indicators of underlying insulin resistance during pregnancy.
1 Introduction
Acrochordons (skin tags), are benign, small, soft, and often pedunculated skin tumors frequently found on the neck, axilla, and groin. They represent protrusions of loose fibrous tissue and typically range in size from 2 mm to 6 mm in diameter. While they are usually skin-colored, larger and hyperpigmented lesions may also be observed during dermatological examinations. Although acrochordons are benign and of little clinical significance, studies have reported an increased prevalence in individuals with systemic conditions such as acromegaly and colonic polyps (1, 2). In 1951, Touraine proposed a possible association between acrochordons and diabetes mellitus (DM) (3). Since then, several clinical studies have been conducted to investigate this hypothesis, yielding conflicting results (4–7). Additionally, acrochordons have been observed with increased frequency during the second trimester of pregnancy, often regressing postpartum (8).
Acanthosis nigricans (AN) is a well-recognized cutaneous marker of insulin resistance (9). It has been linked to severe insulin resistance, including in obese women with gestational diabetes mellitus (GDM) (9). Daitchman et al. (10) found that women with AN had higher body weight and were nearly twice as likely to require insulin therapy, with a higher daily dose.
Diabetes during pregnancy is classified into two main categories: pregestational diabetes mellitus (including type 1 and type 2 diabetes) and GDM. GDM is further divided into GDMA1, which is well-controlled without medication, and GDMA2, which requires pharmacological treatment to achieve euglycemia (11). Women with GDM are at increased risk of pregnancy complications such as preeclampsia and cesarean delivery. Moreover, their offspring are at a higher risk of macrosomia, neonatal hypoglycemia, and birth trauma (11). Additional factors associated with diabetes during pregnancy or impaired glucose metabolism postpartum include fasting plasma glucose (FPG), 1-h plasma glucose, hemoglobin A1C (HbA1c) at GDM diagnosis, maternal age, pregestational weight and maximum weight, pregestational body mass index, maternal birth weight, family history of diabetes in first-degree relatives, and prenatal weight (12).
A previous study demonstrated that pregnant women presenting with both acrochordons and acanthosis nigricans had a 4.8-fold higher likelihood (95% CI: 1.9–11.7) of developing GDM compared to those without these dermatological findings (13). However, there is a lack of data regarding the potential benefits of early screening and diagnosis of diabetes among pregnant women with acrochordons and AN to prevent pregnancy-related complications.
In the present study, we aim to evaluate the potential relationship between acrochordons and AN with diabetes during pregnancy, the need for insulin treatment, pregnancy outcomes, and the risk of impaired glucose metabolism in the postpartum period.
2 Materials and methods
2.1 Study design
A prospective cohort study was conducted at Soroka University Medical Center (SUMC) to evaluate the potential association between acrochordons and GDM. SUMC is a tertiary medical center and the sole facility providing obstetric admission and delivery services for the entire Negev sub-district, where approximately 98% of all deliveries take place, with an annual average of around 17,000 births. The study was conducted in accordance with the declaration of Helsinki and all appropriate amendments. The study was reviewed and approved by the local Ethics Committee of SUMC, Israel (Approval Number 0016-22-SOR). Written informed consent was obtained from each patient before entering the study.
2.2 Study population
The study population included pregnant women diagnosed with diabetes during pregnancy, who were recruited from the day-hospitalization clinic and the obstetric emergency room (ER). The control group consisted of pregnant women, without diabetes, randomly recruited from the obstetric and gynecological ER, high-risk pregnancy departments, and the day-hospitalization clinic. All participants provided informed consent before undergoing an examination for the presence of acrochordons and acanthosis nigricans (AN). Data regarding diabetes status and pregnancy parameters were collected and recorded.
To assess the number of acrochordons, a physician conducted a physical examination at four anatomical locations: the neck, inframammary region, axillae, and groin. The total number of acrochordons s was documented, along with the presence of AN. The diagnosis of acrochordons and acanthosis nigricans was performed by trained physician under the direct supervision and instruction of a board-certified dermatologist, including standardized criteria for identification and grading. In relevant cases, such as those first examined during the first trimester, follow-up assessments were conducted. At the time of delivery, pregnancy outcomes were retrieved from the computerized medical records at SUMC. Once all participants had given birth, the collected data were analyzed.
2.3 Statistical analysis
The initial analysis included descriptive statistics, such as means, standard deviations (SD), and graphical representations. Advanced statistical analyses were performed using parametric tests where applicable. Continuous variables with a normal distribution were presented as mean ± SD and compared between study groups using the independent t-test. Non-normally distributed continuous variables were expressed as medians with interquartile ranges (IQR) and analyzed using the Mann–Whitney test. Categorical variables were presented as counts and percentages and analyzed using the chi-square test or Fisher’s exact test, as appropriate. A two-sided p-value of <0.05 was considered statistically significant.
3 Results
3.1 Diabetic vs. non-diabetic
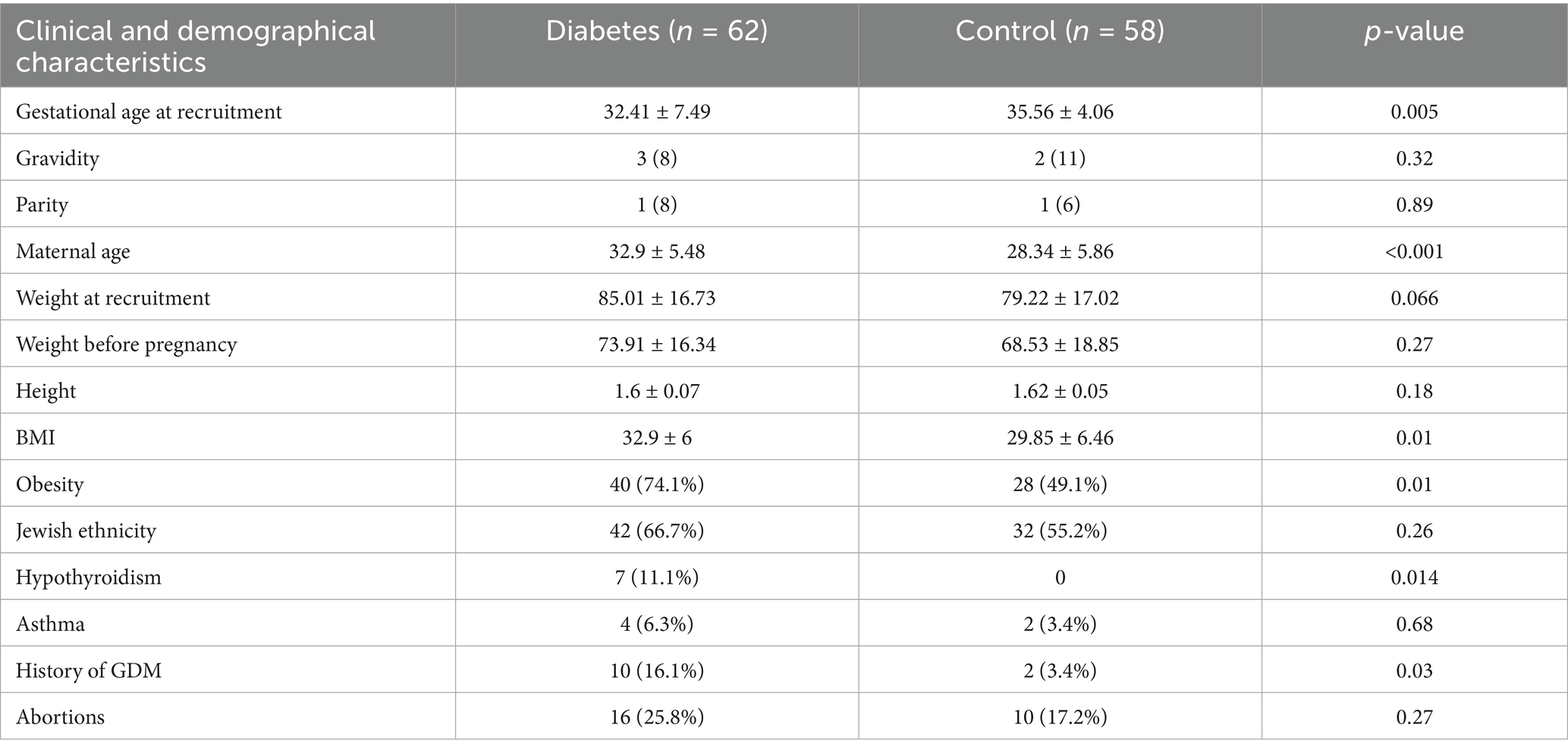
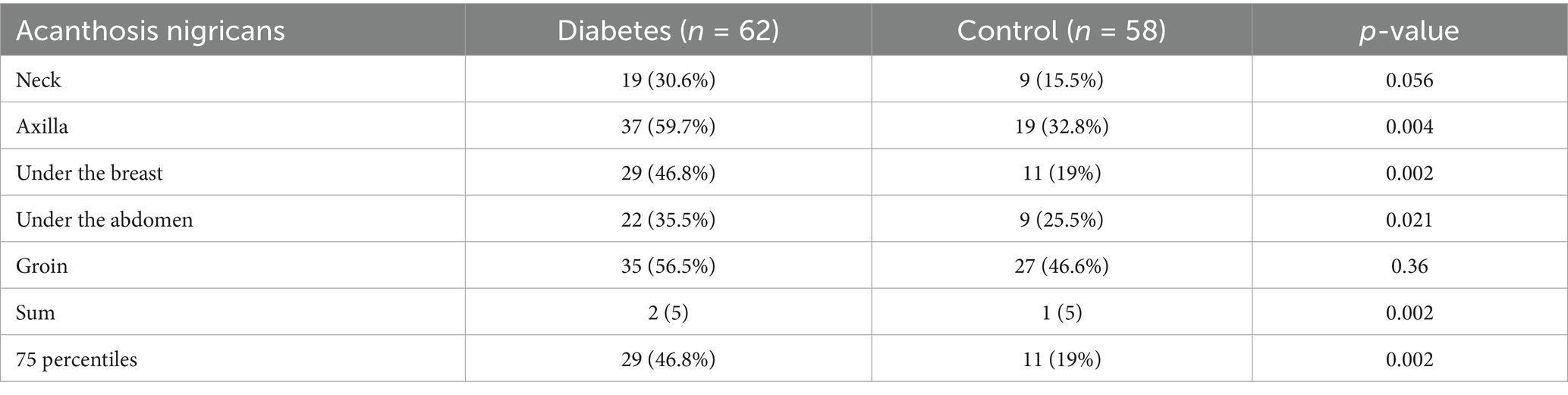
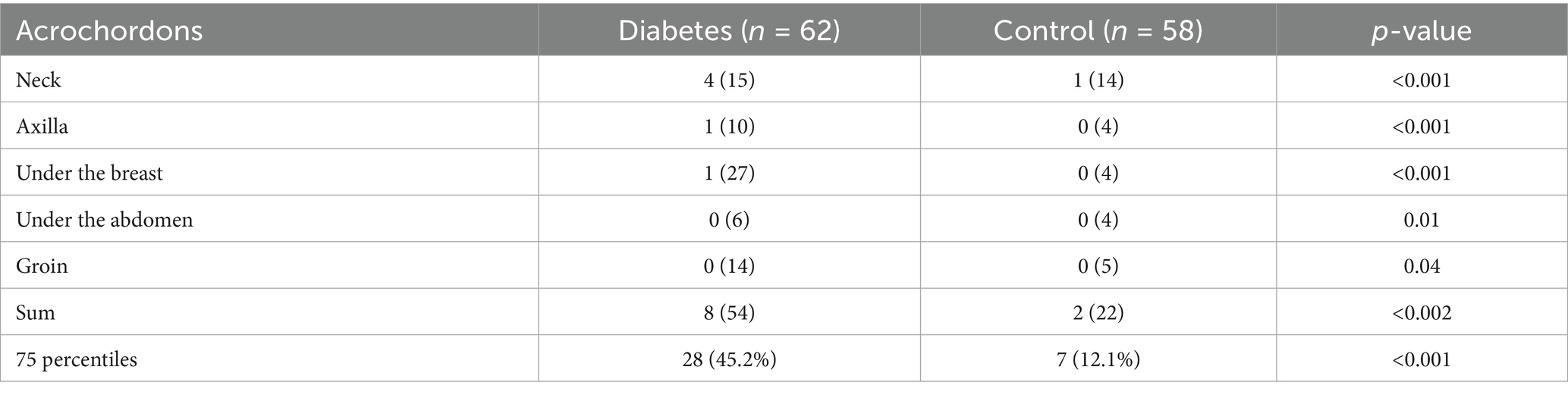
During the study period, 119 women met the inclusion criteria, of whom 62 were diagnosed with diabetes during pregnancy, while 58 served as non-diabetic controls. Women with diabetes during pregnancy were significantly older than those in the control group, with a mean age of 32.9 ± 5.48 years compared to 28.3 ± 5.86 years, respectively (p < 0.001) (Table 1). Additionally, the prevalence of obesity was significantly higher among diabetic women, observed in 74.1% of cases compared to 49.1% in the control group (p = 0.01). Hypothyroidism was significantly more common in the diabetic group, affecting 11.1% of women, whereas no cases were reported in the control group (p = 0.014). A history of GDM in previous pregnancies was significantly more frequent in the diabetic group, with 16.1% of women reporting a prior diagnosis compared to only 3.4% in the control group (p = 0.03) (Table 1). Dermatologic findings were notably more prevalent among women with diabetes. The prevalence of acanthosis nigricans was significantly higher in the diabetic group, particularly in specific body areas. The axilla was the most affected site, with 59.7% of diabetic women exhibiting acanthosis nigricans compared to 32.8% in the control group (p = 0.004) (Table 2). The condition was also more common under the breast, where it was observed in 46.8% of diabetic women compared to 19% of controls (p = 0.002), and in the lower abdomen, where it was present in 35.5% of diabetic women versus 25.5% in the control group (p = 0.021). Similarly, acrochordons were significantly more prevalent among diabetic women, with a broader distribution across multiple body sites, a difference that reached statistical significance (p < 0.001) (Table 3).
3.2 Pregnancy complications and neonatal outcomes
Pregnancy complications and neonatal outcomes among patients with diabetes during pregnancy and the control group are presented in Table 2. Labor induction was performed more frequently among diabetic women, with a rate of 39.7% compared to 22.4% in the control group (p = 0.05). Despite the maternal differences, neonatal outcomes showed no significant variations between the two groups. APGAR scores at both 1 min and 5 min were comparable, and neonatal birth weight did not differ significantly, with a mean weight of 3,123 ± 634 g in the diabetic group compared to 3,040 ± 786 g in controls (p = 0.52) (Table 4).
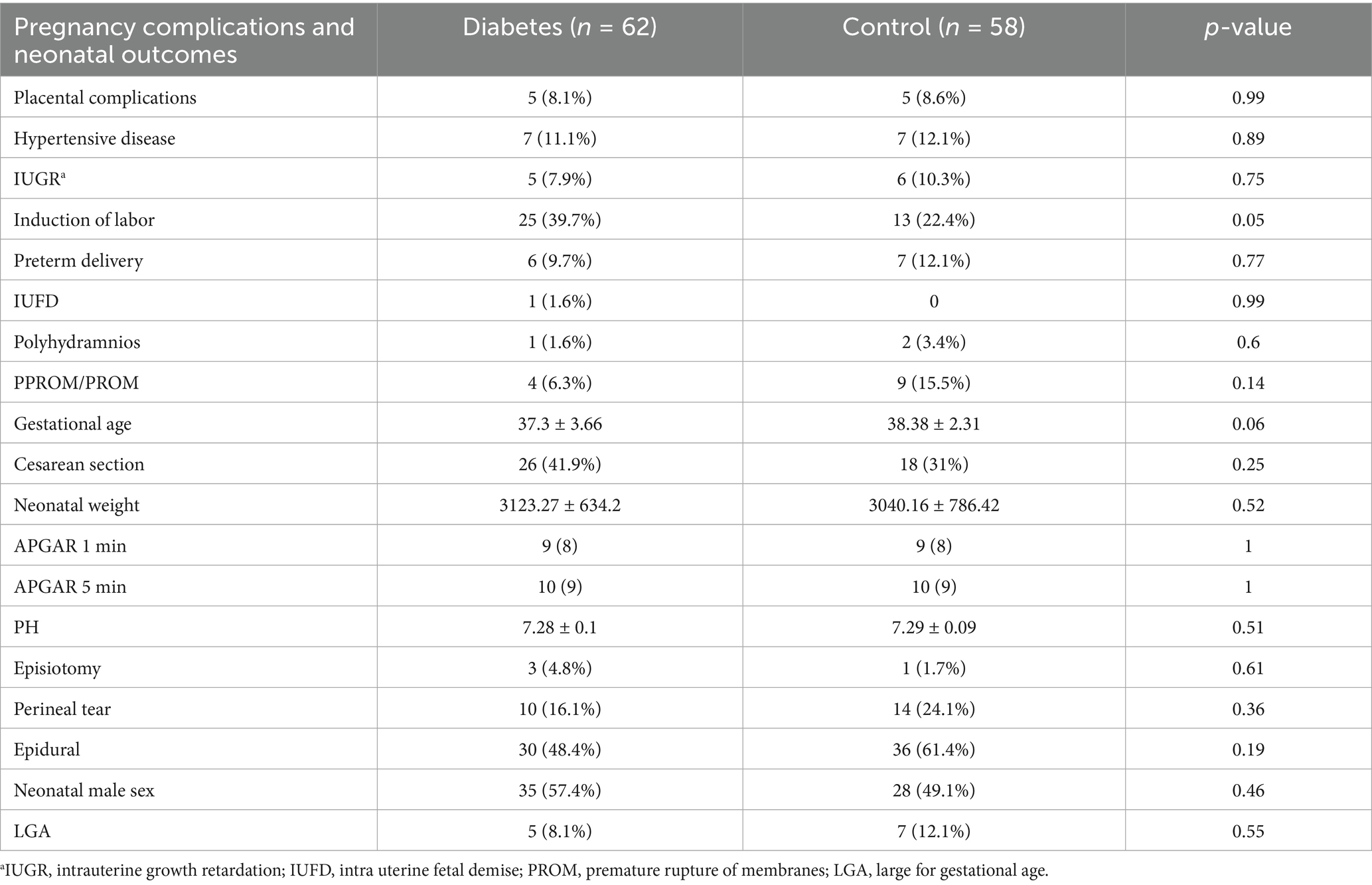
Table 4. Pregnancy complications and neonatal outcomes among patients with diabetes during pregnancy and the control group.
3.3 Multivariable logistic regression analysis
Multivariable regression analysis identified both diabetes during pregnancy and advanced maternal age as independent predictors of a higher prevalence of acrochordons (Table 5). Diabetes in pregnancy remained a significant risk factor even after adjusting for age and other confounders, with an adjusted odds ratio of 5 (95% confidence interval: 1.68–14.88, p = 0.004).
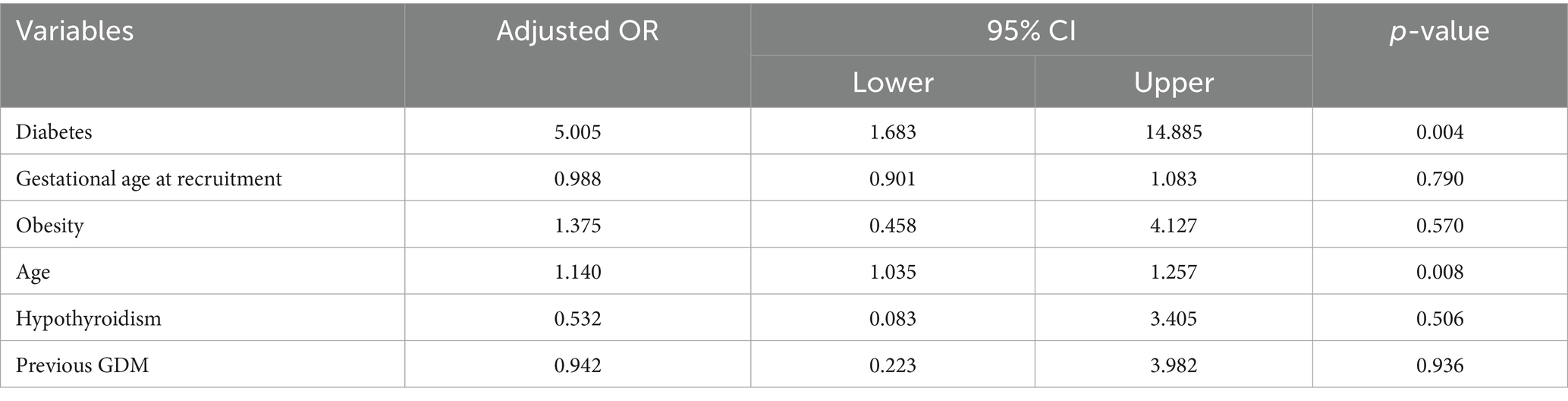
Table 5. Results of multivariable logistic regression analysis with 75 percentiles of acrochordons as the outcome.
4 Discussion
The results of this study confirm that pregnant women with diabetes exhibit a higher prevalence of both acrochordons and acanthosis nigricans, particularly in areas such as the axilla, inframammary region, and lower abdomen. Multivariable regression analysis further identified diabetes during pregnancy and advanced maternal age as independent factors associated with an increased frequency of acrochordons. These findings suggest that acrochordons and acanthosis nigricans may serve as early, visible indicators of metabolic disturbances in pregnant women, facilitating the early detection and monitoring of insulin resistance and related complications.
Our findings align with previous research that demonstrated an association between acanthosis nigricans and insulin resistance, particularly in individuals with obesity and GDM (10). Similarly, prior studies have suggested that acrochordons may be linked to metabolic disturbances, although data on pregnant populations remain limited (3–7). The increased prevalence of these dermatologic conditions among women with diabetes during pregnancy supports their role as clinical markers of insulin resistance. Moreover, previous research has indicated that the presence of both acrochordons and acanthosis nigricans in pregnant women is associated with a significantly higher risk of developing GDM (13), reinforcing the potential clinical relevance of these findings. The current study adds to the growing body of evidence on the topic.
While our findings support the association between dermatologic markers and insulin resistance, we did not find notable differences in neonatal birth weight or APGAR scores between diabetic and non-diabetic groups. Previous studies have explored the relationship between insulin resistance severity and dermatologic markers, such as acanthosis nigricans (10), our study did not directly quantify insulin resistance levels. Further research is needed to determine whether these dermatologic markers correlate with neonatal outcomes or serve only as characteristics of metabolic disturbance.
The identification of acrochordons and acanthosis nigricans as potential markers of metabolic disturbances during pregnancy provides clinicians with a valuable, non-invasive screening tool. Integrating dermatologic assessments into routine prenatal care may enhance early detection and intervention strategies for women at risk of diabetes-related complications. Early identification of metabolic disturbances can contribute to improved pregnancy outcomes by allowing for timely interventions such as dietary modifications, glucose monitoring, and pharmacological management (11, 14). Previous studies have emphasized that proactive management of insulin resistance during pregnancy may reduce the risk of adverse maternal and neonatal outcomes (11, 14), reinforcing the importance of dermatologic markers as potential early warning signs.
The strengths of this study include its prospective cohort design and the use of standardized clinical assessments for dermatological findings. Additionally, the study was conducted at a tertiary medical center, ensuring a well--diverse patient population. However, certain limitations should be acknowledged. The study was conducted at a single medical center, which may limit the generalizability of the findings. While confounders such as maternal age and obesity were accounted for, other potential influences, including genetic predisposition and additional metabolic disorders, were not comprehensively assessed. Additionally, Data on polycystic ovarian syndrome (PCOS) were incompletely documented. As it is a diagnosis of exclusion and several women had menstrual irregularities together with hyperandrogenism without a formal diagnosis, its prevalence in our study population was likely substantially underestimated, precluding reliable subgroup analysis. Further multicenter studies with larger sample sizes are needed to validate these findings and elucidate underlying mechanisms.
5 Conclusion
Pregnant women with gestational diabetes showed a significantly higher prevalence of acanthosis nigricans and acrochordons compared to those without. This association suggests that acanthosis nigricans and acrochordons may be potential indicators of underlying insulin resistance during pregnancy. Early recognition of these skin changes may provide opportunities for preventing or mitigating pregnancy complications associated with gestational diabetes. Ongoing research should further explore the predictive value of these dermatologic markers and their role in refining screening and management protocols for gestational diabetes and related metabolic disorders.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the local Ethics Committee of Soroka University Medical Center (No. 0016-22-SOR). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
EI: Data curation, Investigation, Writing – review & editing, Writing – original draft. TE: Conceptualization, Writing – review & editing, Resources, Investigation, Supervision, Software, Writing – original draft, Project administration. AM: Writing – original draft, Writing – review & editing, Investigation. LY: Writing – original draft, Data curation, Resources, Formal analysis, Validation, Software, Writing – review & editing. AB: Writing – original draft, Writing – review & editing. NB: Writing – original draft, Writing – review & editing. AW: Visualization, Validation, Supervision, Conceptualization, Writing – review & editing, Writing – original draft. AH: Supervision, Writing – review & editing, Writing – original draft, Project administration, Resources, Conceptualization, Methodology, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. The author(s) acknowledge the use of OpenAI’s ChatGPT for assistance in refining the phrasing, grammar, and spelling throughout the manuscript. All final content decisions were made by the author(s).
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nabarro, JDN. Acromegaly. Clin Endocrinol. (1987) 26:481–512. doi: 10.1111/j.1365-2265.1987.tb00805.x
2. Chobanian, SJ, Van Ness, MM, Winters, C, and Cattau, EL. Skin tags as a marker for adenomatous polyps of the colon. Ann Intern Med. (1985) 103:892–3. doi: 10.7326/0003-4819-103-6-892
3. Touraine, A. A new hereditary chain; cutaneous fibromas, diabetes, obesity. Ann Dermatol Syphiligr. (1951) 78:409–16.
4. Shah, R, Jindal, A, and Patel, N. Acrochordons as a cutaneous sign of metabolic syndrome: a case-control study. Ann Med Health Sci Res. (2014) 4:202–5. doi: 10.4103/2141-9248.129040
5. Demir, S, and Demir, Y. Acrochordon and impaired carbohydrate metabolism. Acta Diabetol. (2002) 39:57–9. doi: 10.1007/s005920200014
6. Bustan, RS, Wasim, D, Yderstræde, KB, and Bygum, A. Specific skin signs as a cutaneous marker of diabetes mellitus and the prediabetic state—a systematic review. Dan Med J. (2017) 64:A5316.
7. Rasi, A, Soltani-Arabshahi, R, and Shahbazi, N. Skin tag as a cutaneous marker for impaired carbohydrate metabolism: a case-control study. Int J Dermatol. (2007) 46:1155–9. doi: 10.1111/j.1365-4632.2007.03287.x
8. Winton, GB, Lewis, CW, Winton, GB, and Lewis, CW. Dermatoses of pregnancy. J Am Acad Dermatol. (1982) 6:977–98. doi: 10.1016/s0190-9622(82)70083-0
9. Lopez-Alvarenga, JC, García-Hidalgo, L, Landa-Anell, MV, Santos-Gómez, R, González-Barranco, J, and Comuzzie, A. Influence of skin color on the diagnostic utility of clinical acanthosis nigricans to predict insulin resistance in obese patients. Arch Med Res. (2006) 37:744–8. doi: 10.1016/j.arcmed.2005.12.007
10. Daitchman, J, Vermeulen, MJ, and Ray, JG. Acanthosis nigricans among women with gestational diabetes mellitus and risk of adverse pregnancy outcomes. Diabetes Care. (2008) 31:e30. doi: 10.2337/dc07-2108
11. Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. (2018) 131:e49–64. doi: 10.1097/AOG.0000000000002501
12. Tang, L, Xu, S, Li, P, and Li, L. Predictors of insulin treatment during pregnancy and abnormal postpartum glucose metabolism in patients with gestational diabetes mellitus. Diabetes Metab Syndr Obes. (2019) 12:2655–65. doi: 10.2147/DMSO.S233554
13. Ylmaz, E, Kelekci, KH, and Kelekci, S. Skin tag and acanthosis nigricans: do they have a predictive value for gestational diabetes mellitus? Exp Clin Endocrinol Diabetes. (2011) 119:419–22. doi: 10.1055/s-0030-1270478
Keywords: acanthosis nigricans, acrochordon, pregnancy outcome, metabolic disturbance, dermatological manifestations
Citation: Ilatov E, Eshkoli T, Mashal A, Yahav L, Binyaminov A, Borochov N, Weintraub AY and Horev A (2025) Acanthosis nigricans and acrochordons as clinical markers of metabolic disturbance during pregnancy: a prospective cohort study. Front. Med. 12:1607429. doi: 10.3389/fmed.2025.1607429
Edited by:
Simcha Yagel, Hadassah Medical Center, IsraelReviewed by:
Indu Saxena, All India Institute of Medical Sciences Jodhpur, IndiaAssi Levi, Tel Aviv University, Israel
Copyright © 2025 Ilatov, Eshkoli, Mashal, Yahav, Binyaminov, Borochov, Weintraub and Horev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amir Horev, YW1pcmhyQGNsYWxpdC5vcmcuaWw=
†These authors share first authorship
‡ORCID: Amir Horev, orcid.org/0000-0001-6646-9061
 Erika Ilatov1†
Erika Ilatov1† Tamar Eshkoli
Tamar Eshkoli Adi Y. Weintraub
Adi Y. Weintraub Amir Horev
Amir Horev