- 1Department of Infection, Nantong Third People's Hospital, Affiliated Nantong Hospital 3 of Nantong University, Nantong, Jiangsu, China
- 2Department of Emergency, Nantong Third People's Hospital, Affiliated Nantong Hospital 3 of Nantong University, Nantong, Jiangsu, China
- 3Department of Emergency Medicine, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
Rationale: Mycoplasma pneumoniae (MP) is a common pathogen responsible for pediatric community-acquired pneumonia. MP infection can also induce extrapulmonary manifestations, including mucocutaneous eruptions. Mycoplasma pneumoniae-induced rash and mucositis (MIRM) is a relatively newly described entity distinct from erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). However, MIRM remains underrecognized, with limited cases reported in China.
Patient concerns: A previously healthy 10-year-old boy presented with fever, cough, severe mucositis (oral and ocular involvement), and disseminated rash for 5 days.
Diagnoses: The patient was diagnosed with Mycoplasma pneumoniae-induced rash and mucositis (MIRM), confirmed by clinical presentation and laboratory detection of MP nucleic acid.
Interventions: The treatment regimen for the patient included intravenous azithromycin, methylprednisolone at a dosage of 1 mg/kg/day, intravenous immunoglobulin (IVIG), vitamin C, oral antihistamines, topical treatments for mucosal care, ophthalmic ointment, and calamine lotion. After 1 week, due to a persistent cough and a positive Mycoplasma pneumoniae (MP) nucleic acid test, the patient’s treatment was transitioned to oral doxycycline.
Outcomes: The patient experienced significant clinical improvement after 14 days, with resolution of rash, mucosal lesions, and cough. Follow-up tests confirmed clearance of MP nucleic acid and normalization of inflammatory markers.
Lessons: MIRM is a distinct mucocutaneous entity associated with MP infection. Pediatricians should recognize its clinical presentation early to provide prompt targeted therapy and supportive care, thus preventing complications.
Introduction
Mycoplasma pneumoniae (MP) is a frequent cause of respiratory tract infections in children (1). Up to 25% of affected children develop extrapulmonary complications, including mucocutaneous manifestations such as Mycoplasma pneumoniae-induced rash and mucositis (MIRM) (1–4). Recently, MIRM has been recognized as a distinct clinical entity, differentiated from erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), featuring prominent mucosal involvement with limited skin lesions (4, 5). Due to its rarity and recent recognition, MIRM remains underdiagnosed and understudied, especially in China (6). Here, we report a pediatric case of MIRM to enhance clinical awareness and discuss current knowledge on its diagnosis and management.
Case presentation
Patient information
A 10-year-old boy was admitted with a 5-day history of fever and rash. His illness began with a high fever (maximum temperature 39.5°C), cough, and sputum production. Two days later, blistering of the lips developed and gradually eroded. Shortly thereafter, multiple erythematous lesions, some targetoid with small vesicles, appeared on his hands, trunk, and limbs. Initial laboratory tests from a local hospital revealed a white blood cell count of 10.60 × 10^9/L (73.8% neutrophils, 15.4% lymphocytes), and a C-reactive protein level of 27.6 mg/L. Following an inadequate response to empiric antibiotic treatment, he was transferred to our institution.
Clinical findings
On admission, the patient’s temperature was 38.5°C. He appeared lethargic. Physical examination revealed multiple red macules of varying sizes on the trunk and extremities, some with targetoid morphology and small vesicles. Ocular examination revealed significant conjunctival injection with abundant yellowish secretions. The oral mucosa displayed extensive erosions with limited mouth opening, drooling, and marked pain, while pharyngeal erythema was evident. Lungs had coarse breath sounds without rales; cardiovascular and abdominal examinations were unremarkable.
Diagnostic assessment
Laboratory evaluation showed:
• White blood cell count: 9.88 × 10^9/L with 75.4% neutrophils.
• Red blood cell count: 4.89 × 10^12/L, Hemoglobin: 145 g/L.
• Platelet count: 280 × 10^9/L.
• C-reactive protein: 17.02 mg/L.
• Interleukin-6: 9.08 pg./mL; total IgE: 28.7 IU/mL; ESR: 95 mm/h; ferritin: 208.37 ng/mL.
• Coagulation: Prothrombin time 15.9 s; fibrinogen 5.58 g/L; D-dimer 1.38 mg/L.
• Liver and renal functions as well as electrolytes were normal.
M. pneumoniae nucleic acid testing was performed using multiplex PCR, which was positive. This multiplex PCR method was chosen due to its efficacy in detecting multiple pathogens simultaneously, which is critical for accurate diagnosis in complex cases like MIRM. Comprehensive antinuclear antibody spectrum tests were conducted to rule out autoimmune diseases, including tests for ANA indirect immunofluorescence typing and specific antibodies such as anti-nRNP, anti-SM, anti-SSA, and others, all of which were negative. Additionally, given the patient’s age and lack of relevant history, syphilis was considered highly unlikely, and thus, tests for syphilis were not performed. Our institution does not offer Mycoplasma serology testing; therefore, diagnosis relied solely on nucleic acid detection methods. Serologic assays for herpes simplex virus type I (IgG positive, IgM negative) and type II (both negative), echovirus, coxsackie virus, and enterovirus markers were unremarkable. Blood and throat swab cultures were negative. To further clarify the differential diagnosis, a comparative table is provided below, distinguishing Mycoplasma Induced Rash and Mucositis (MIRM) from Erythema Multiforme (EM), Stevens-Johnson Syndrome (SJS), and Toxic Epidermal Necrolysis (TEN) based on mucocutaneous involvement, triggering pathogens, and severity (Table 1). Electrocardiography indicated sinus rhythm and the chest radiograph did not reveal active pulmonary lesions.
Diagnosis
Based on the clinical presentation—limited cutaneous involvement (<10% body surface area) (Figure 1) with prominent mucosal lesions of the eyes and oral cavity—and the positive laboratory evidence for M. pneumoniae, a diagnosis of MIRM was established.
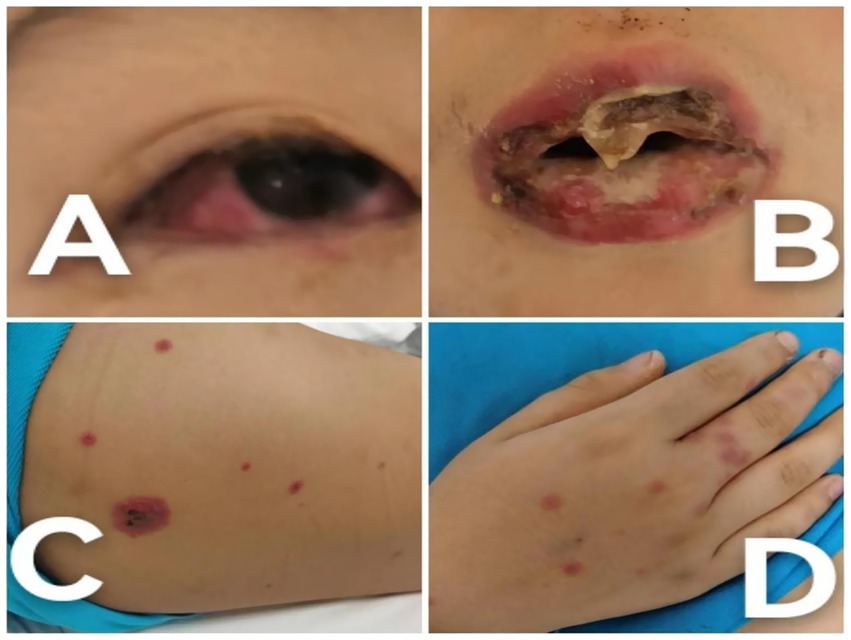
Figure 1. Clinical photographs of the patient at the time of admission. (A) Conjunctival hyperemia accompanied by secretions, illustrating the initial severe inflammatory response. (B) Severe oral mucosal ulceration with mucosal sloughing, highlighting the acute stage of mucocutaneous involvement. (C) Truncal targetoid lesions, depicting the characteristic rash associated with MIRM. (D) Hand lesions with a targetoid appearance, demonstrating the extent of skin involvement.
Treatment and outcome
Treatment initially included azithromycin 0.44 g once daily for 5 days, accompanied by methylprednisolone at a dosage of 1 mg/kg/day, and intravenous immunoglobulin (IVIG) administered as 10 g daily for 2 days, followed by 5 g daily for 4 days. Topical management consisted of vitamin C and compound chlorhexidine solution application to the lips and oral mucosa, erythromycin ophthalmic ointment for the eyes, and external agents on the skin lesions. Oral antihistamines (cetirizine and desloratadine) were also prescribed to manage symptoms.
Three days post-treatment, the fever subsided and skin lesions began to resolve, prompting a gradual taper of methylprednisolone. Initially administered at 40 mg daily for 3 days, the dose was then reduced to 30 mg daily for 5 days, and further tapered to 20 mg daily for 7 days. However, after 1 week, despite the initial treatment, the patient’s persistent cough and positive M. pneumoniae nucleic acid test indicated an incomplete response to azithromycin. This necessitated an adjustment in the treatment regimen, leading to a switch to oral doxycycline 0.1 g every 12 h for 7 days. The choice of doxycycline over other macrolides or alternatives was based on its proven efficacy in treating M. pneumoniae infections, particularly in cases showing resistance to first-line treatments like azithromycin. Additionally, doxycycline is known for its good penetration into respiratory tissues, which is crucial for treating persistent pulmonary symptoms. This decision also considered the growing concern over antimicrobial resistance; doxycycline offers a broader spectrum against potential co-infections and is generally well-tolerated in pediatric patients, making it an appropriate choice in this context. The therapeutic regimen was aimed at preventing long-term sequelae such as persistent mucosal scarring and ocular complications, which are common in untreated or improperly managed cases of MIRM.
After 14 days of hospitalization, the skin lesions had markedly improved, conjunctival signs resolved, and oral mucosal erosions exhibited crusting and healing (Figure 2). Repeat testing confirmed pathogen clearance and normalization of inflammatory markers, allowing for discharge.
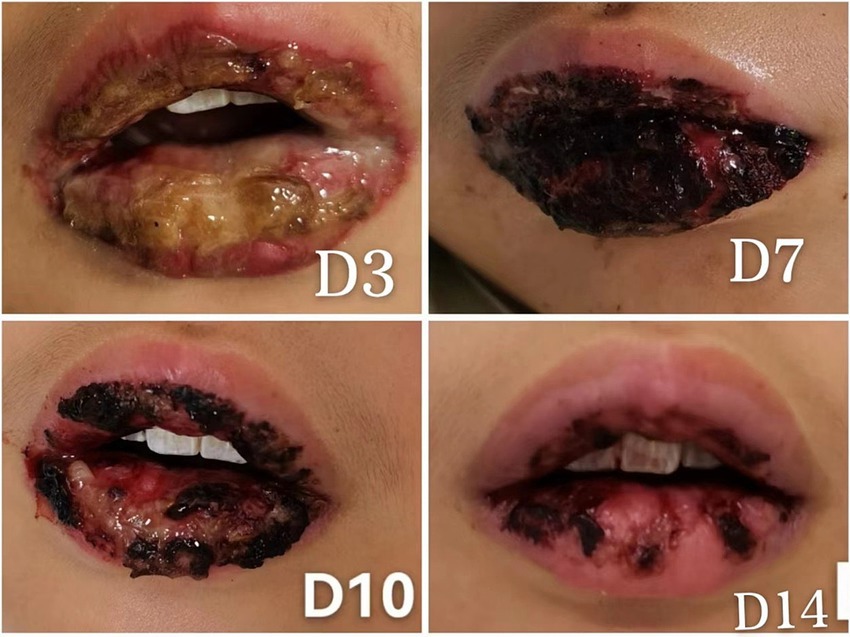
Figure 2. Sequential images showing the healing progression of oral mucosal lesions post-treatment, emphasizing the effectiveness of early intervention. (D3) Day 3 post-admission, expansion of oral mucosal ulcerations with ongoing mucosal repair, indicating the initial response to treatment. (D7) Day 7 post-admission, oral lesions covered with blood crusts, showing signs of healing beneath the crusts. (D10) Day 10 post-admission, partial shedding of the blood crusts revealing the healing mucosa underneath. (D14) Day 14 post-admission, most of the oral mucosal has healed with residual crusting, demonstrating significant recovery.
Discussion
The recognition of Mycoplasma pneumoniae-induced rash and mucositis (MIRM) as a distinct clinical entity, separate from other severe mucocutaneous syndromes such as erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), highlights significant advances in our understanding of infectious immunopathology (4, 7). MIRM is primarily characterized by significant mucosal involvement with limited skin manifestations, often preceded by respiratory symptoms that serve as crucial diagnostic clues (8).
Recent studies elucidate the pathogenesis of MIRM as involving complex immune-mediated mechanisms rather than simple antibody-mediated reactions (9). It is hypothesized that cytokine dysregulation and T-cell mediated cytotoxicity play pivotal roles in causing the characteristic mucosal damage observed in MIRM (9, 10). Specific studies identify cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) as key mediators in this pathogenic process. These cytokines contribute to the T-cell mediated cytotoxicity, further exacerbating the mucosal damage seen in MIRM. The unique cytokine profile, which is distinct from those observed in SJS/TEN, not only aids in distinguishing MIRM clinically but also provides targets for therapeutic intervention, suggesting potential diagnostic markers and therapeutic targets (10).
Reviewing global cases of pediatric MIRM helps in contextualizing the current case within broader epidemiological and clinical patterns. As summarized in the Literature Summary Table, treatments vary widely, with macrolides like azithromycin being common, but outcomes like those reported by Li et al. (11) in Canada and Chowdhury et al. (12) in Singapore show that additional treatments such as immunoglobulins and doxycycline can influence recovery significantly. This variability underscores the importance of tailored treatment approaches based on regional medical practices and antimicrobial resistance patterns (Table 2).
The treatment strategies for MIRM remain largely empirical; however, emerging data suggests that early intervention with targeted therapies such as antibiotics against Mycoplasma pneumoniae, corticosteroids, and intravenous immunoglobulin can significantly modify the disease course (2, 13). These treatments not only reduce the duration of mucosal involvement but also minimize the risk of long-term sequelae. Additionally, the use of cyclosporine A has shown promise in rapidly alleviating symptoms and reducing hospital stays in severe cases. Cyclosporin A, a potent immunosuppressant, inhibits calcineurin, thereby reducing T-cell activation and cytokine release. This mechanism is particularly beneficial in controlling the inflammatory responses observed in severe cases of MIRM, where traditional treatments might be insufficient. This suggests new avenues for therapeutic approaches that could be explored in future clinical trials (11).
The prognosis of MIRM is generally favorable compared to the often grave outcomes associated with SJS and TEN. However, the risk of recurrence and long-term complications such as ocular complications or oral scarring remains a concern (14). Chronic complications like conjunctival scarring and dry eye syndrome may develop, underscoring the importance of vigilant ophthalmologic evaluation and a multidisciplinary approach to care, which should integrate dermatologic, ophthalmologic, and infectious disease expertise (14, 15).
Additionally, recent findings suggest that genetic predispositions, including specific HLA haplotypes, may influence individual immune responses to M. pneumoniae infection, potentially accounting for the variability in clinical presentation and severity observed in MIRM (16–20). This insight not only deepens our understanding of the disease mechanism but may also pave the way for more personalized therapeutic strategies.
In summary, this case report contributes novel insights into the management of Mycoplasma pneumoniae-induced rash and mucositis (MIRM), highlighting a unique clinical presentation and therapeutic response. Notably, this case from China illustrates a robust response to doxycycline, diverging from typical treatment approaches and suggesting regional variations in medical practice that might influence treatment efficacy. This supports the need for a dynamic model of MIRM, recognizing it as distinct from other severe mucocutaneous syndromes such as EM, SJS, and TEN. Early recognition and aggressive management tailored to specific clinical contexts are crucial to prevent complications and improve outcomes. The integration of emerging immunomodulatory therapies, including the successful use of doxycycline in this instance, into standard care protocols, underscores the potential to significantly enhance patient care and adapt treatment strategies to regional medical practices.
Strengths and limitations
This report has several strengths that contribute to the understanding of Mycoplasma pneumoniae-induced rash and mucositis (MIRM). Firstly, it draws from a comprehensive review of literature, incorporating findings from recent systematic studies, which enhances the depth and relevance of the discussion. Secondly, the inclusion of a pediatric case provides practical insights into the clinical presentation and management of MIRM, adding to the real-world applicability of the information. Thirdly, the discussion integrates emerging research on the pathogenesis and potential genetic predispositions involved in MIRM, offering a forward-looking perspective that could influence future therapeutic approaches.
However, there are several limitations to this study. The report is based on a single case study, which may not fully represent the broader spectrum of clinical presentations and outcomes associated with MIRM. This limits the generalizability of the findings. Additionally, potential diagnostic misclassification must be acknowledged, as similar symptoms can occur in other mucocutaneous diseases such as EM, SJS, and TEN, which might lead to diagnostic uncertainty. The absence of comprehensive serologic testing (e.g., lack of IgM/IgG trending) further complicates the accuracy of pathogen identification, possibly affecting the diagnosis and understanding of the immune response. Moreover, while the report discusses potential new treatments such as the use of cyclosporine A, these are based on limited case reports and lack robust evidence from controlled clinical trials. Thus, the efficacy and safety of these treatments remain to be validated in larger, more diverse populations. Furthermore, the discussion on genetic factors influencing MIRM is based on preliminary data, which requires further investigation to confirm these associations and understand their clinical implications. Lastly, as MIRM is a relatively newly recognized condition, long-term follow-up data on patients is scarce, which constrains our understanding of the chronic sequelae and long-term prognosis of the disease. Additionally, there is variability in therapeutic response among individuals, which could influence the efficacy and outcomes of the treatments administered. This factor should be considered when interpreting the results and applying them to broader clinical practice.
Take-away lessons
Mycoplasma pneumoniae-induced rash and mucositis (MIRM) stands as a distinct clinical entity that necessitates early recognition due to its unique presentation, which can often be mistaken for other mucocutaneous syndromes. This differentiation is crucial as it informs the tailored clinical approach required for MIRM, which includes the use of specific antibiotics, corticosteroids, and possibly immunoglobulin therapies aimed at reducing symptom severity and duration. Emerging therapeutic strategies, such as the use of cyclosporine A, highlight the evolving nature of treatment protocols, though these require further validation through robust clinical trials. Additionally, preliminary findings suggest genetic factors may influence individual responses to Mycoplasma pneumoniae, pointing towards the potential for personalized medical approaches in the future. Ongoing research is essential to solidify these findings and to explore the long-term outcomes of MIRM, ensuring that treatment strategies continue to improve and are based on strong scientific evidence.
Patient perspective
Navigating Mycoplasma pneumoniae-induced rash and mucositis (MIRM) poses both physical and emotional challenges for patients and their families. Initially, the onset of respiratory symptoms followed by severe and painful mucosal lesions can be deeply distressing, often leading to significant discomfort and anxiety. The rarity and complexity of MIRM can also cause delays in diagnosis, exacerbating the uncertainty and stress associated with managing this condition.
From a patient’s viewpoint, understanding the illness is crucial. Effective communication with healthcare providers ensures that patients and their families are well-informed about the illness, treatment options, and prognosis. This knowledge is vital for managing symptoms at home and preparing for potential hospital visits or treatments. Additionally, access to psychological support and connecting with others who have faced similar health challenges can provide much-needed emotional support and reduce feelings of isolation.
Conclusion
A timely and accurate diagnosis of MIRM is crucial for instituting appropriate treatment and minimizing morbidity. A multidisciplinary approach, combining antimicrobial therapy with judicious use of corticosteroids, IVIG, and possibly other immunomodulatory agents, has the potential to improve outcomes (18–20). Ongoing research into the immunogenetic underpinnings of MIRM may ultimately enable more individualized treatment strategies and further reduce the risk of long-term sequelae (16–20).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Nantong Third People’s Hospital. The approval number for this study is EL2024043. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XM: Conceptualization, Formal analysis, Methodology, Resources, Supervision, Writing – original draft. MW: Conceptualization, Methodology, Writing – review & editing. XJ: Investigation, Writing – review & editing. LJ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the grants from the project of Nantong Health Commission (MSZ2024063), Nantong Infectious Disease Alliance (NTCRB2025001), the project of Nantong City Natural Science Foundation (JCZ2024008), and Peking Union Medical Foundation — Ruiyi Emergency Medical Research Fund (PUMF01010010-2025-02).
Acknowledgments
We express our sincere gratitude to the patient and his family for their willingness to share this case, enhancing medical education and awareness. Special thanks to our dedicated medical, nursing, and support teams for their expertise and care, which were crucial in managing this complex case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Waites, KB, and Talkington, DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. (2004) 17:697–728. doi: 10.1128/CMR.17.4.697-728.2004
2. Meyer Sauteur, PM, Theiler, M, Buettcher, M, Seiler, M, Weibel, L, and Berger, C. Frequency and clinical presentation of mucocutaneous disease due to Mycoplasma pneumoniae infection in children with community-acquired pneumoniae. JAMA Dermatol. (2020) 156:144–50. doi: 10.1001/jamadermatol.2019.3602
3. Narita, M. Classification of extrapulmonary manifestations due to Mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front Microbiol. (2016) 7:23. doi: 10.3389/fmicb.2016.00023
4. Canavan, TN, Mathes, EF, Frieden, I, and Shinkai, K. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. (2015) 72:239–245.e4. doi: 10.1016/j.jaad.2014.06.026
5. Liakos, W, Xu, A, and Finelt, N. Clinical features of recurrent Mycoplasma pneumoniae-induced rash and mucositis. Pediatr Dermatol. (2021) 38:154–8. doi: 10.1111/pde.14472
6. Jia, Y, Guo, F, Wu, X, and Wenhui, L. Clinical characteristics of rash and mucositis induced by Mycoplasma pneumoniae in children. J Capital Med Univ. (2023) 44:131–6. doi: 10.3969/j.issn.1006-7795.2023.01.019
7. Li, K, and Haber, RM. Stevens-Johnson syndrome without skin lesions (Fuchs syndrome): a literature review of adult cases with Mycoplasma cause. Arch Dermatol. (2012) 148:963–4. doi: 10.1001/archdermatol.2012.681
8. Liu, L, Wang, Y, Sun, J, Wang, W, Hou, J, and Wang, X. Case report: clinical and immunological features of a Chinese cohort with Mycoplasma-induced rash and mucositis. Front Pediatr. (2020) 8:402. doi: 10.3389/fped.2020.00402
9. Chaudhry, R, Ghosh, A, and Chandolia, A. Pathogenesis of Mycoplasma pneumoniae: an update. Indian J Med Microbiol. (2016) 34:7–16. doi: 10.4103/0255-0857.174112
10. Gandelman, JS, Kim, EY, Grzegorczyk, AM, Zejnullahu, K, and Edson, RS. Mycoplasma pneumoniae-induced rash and mucositis in the previously healthy man: a case report and brief review of the literature. Open Forum Infect Dis. (2020) 7:ofaa437. doi: 10.1093/ofid/ofaa437
11. Li, HO, Colantonio, S, and Ramien, ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. (2019) 23:608–12. doi: 10.1177/1203475419874444
12. Roy Chowdhury, S. Mycoplasma pneumoniae-induced rash and mucositis is a distinct entity that needs more recognition. J Paediatr Child Health. (2020) 56:645–6. doi: 10.1111/jpc.14625
13. Lofgren, DH, Lenkeit, C, Palanisamy, J, and Brown, J. Mycoplasma pneumoniae induced rash and mucositis with bilateral otitis media and sinusitis. Cureus. (2020) 12:e7449. doi: 10.7759/cureus.7449
14. Gise, R, Elhusseiny, AM, Scelfo, C, and Mantagos, IS. Mycoplasma pneumonia-induced rash and mucositis: a longitudinal perspective and proposed management criteria. Am J Ophthalmol. (2020) 11:351–6. doi: 10.1016/j.ajo.2020.06.010
15. Valle, J, Nasrollahi, F, and Eilbert, W. Mycoplasma pneumoniae-induced rash and mucositis. Am J Emerg Med. (2022) 54:e5-e7. doi: 10.1016/j.ajem.2021.09.080
16. Mayor-Ibarguren, A, Feito-Rodriguez, M, González-Ramos, J, Del Rosal-Rabes, T, González-Sainz, FJ, Sánchez-Orta, A, et al. Mucositis secondary to Chlamydia pneumoniae infection: expanding the Mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol. (2017) 34:465–72. doi: 10.1111/pde.13140
17. Goyal, A, and Hook, K. Two pediatric cases of influenza B-induced rash and mucositis: Stevens-Johnson syndrome or expansion of the Mycoplasma pneumoniae-induced rash with mucositis (MIRM) spectrum? Pediatr Dermatol. (2019) 36:929–31. doi: 10.1111/pde.13921
18. Ramien, ML. Reactive infectious mucocutaneous eruption: Mycoplasma pneumoniae-induced rash and mucositis and other parainfectious eruptions. Clin Exp Dermatol. (2021) 46:420–9. doi: 10.1111/ced.14404
19. Gamez-Gonzalez, LB, Pena-Varela, C, Ramirez-Lopez, JM, and Yamazaki‐Nakashimada, MA. Adenoviral-induced rash and mucositis: expanding the spectrum of reactive infectious mucocutaneous eruption. Pediatr Dermatol. (2021) 38:306–8. doi: 10.1111/pde.14419
20. Holcomb, ZE, Hussain, S, Huang, JT, and Delano, S. Reactive infectious cutaneous eruption associated with SARS-CoV-2 infection. JAMA Dermatol. (2021) 157:603–5. doi: 10.1001/jamadermatol.2021.0385
21. Santos, RP, Silva, M, Vieira, AP, and Brito, C. Mycoplasma pneumoniae-induced rash and mucositis: a recently described entity. BMJ Case Rep. (2017) 2017:bcr2017220768. doi: 10.1136/bcr-2017-220768
22. Mazori, DR, Nagarajan, S, and Glick, SA. Recurrent reactive infectious mucocutaneous eruption (RIME): insights from a child with three episodes. Pediatr Dermatol. (2020) 37:545–7. doi: 10.1111/pde.14142
23. Lu, H, and Zhang, B. Mycoplasma-induced rash and Mucositis. N Engl J Med. (2023) 389:1601. doi: 10.1056/NEJMicm2305301
24. Poddighe, D, and Bruni, P. Mycoplasma pneumoniae-induced rash and mucositis (MIRM): an unusual mild skin rash associated with severe mucosal involvement. BMJ Case Rep. (2017) 2017:bcr2017220749. doi: 10.1136/bcr-2017-220749
25. Bowling, M, Schmutzler, T, and Glick, S. Mycoplasma pneumoniae-induced mucositis without rash in an 11-year-old boy. Clin Case Rep. (2018) 6:551–2. doi: 10.1002/ccr3.1400
26. Brazel, D, Kulp, B, Bautista, G, and Bonwit, A. Rash and mucositis associated with Mycoplasma pneumoniae and Chlamydophila pneumoniae: a recurrence of MIRM? J Pediatr Infect Dis Soc. (2021) 10:220–4. doi: 10.1093/jpids/piaa028
27. Chao, K, Balin, S, Sorenson, E, and Worswick, S. Mycoplasma-induced pustulosis with perifollicular involvement. Dermatol Online J. (2018) 24:13030/qt5584k6b1
28. Curtiss, P, Melnick, L, Sicco, KL, and Liebman, TN. Mycoplasma pneumoniae, more than a lung disease. Dermatol Online J. (2018) 24:13030/qt8w993185. doi: 10.5070/D3246040695
29. Song, H, Huang, JT, and Tan, JK. Mycoplasma-induced rash and Mucositis in a father and son: a case report. Pediatr Infect Dis J. (2018) 37:e205–6. doi: 10.1097/INF.0000000000001881
30. Sandhu, R, Mareddy, C, Itskowitz, M, Mount, CE, Bhanot, N, and Min, Z. Mycoplasma-induced rash and mucositis in a young patient with red eyes, oral mucositis, and targetoid cutaneous vesicles. Lancet Infect Dis. (2017) 17:562. doi: 10.1016/S1473-3099(16)30515-1
31. Kheiri, B, Alhesan, NA, Madala, S, Assasa, O, Shen, M, and Dawood, T. Mycoplasma pneumoniae-associated Fuchs syndrome. Clin Case Rep. (2017) 6:434–5. doi: 10.1002/ccr3.1350
Keywords: Mycoplasma pneumoniae, rash and mucositis, MIRM, children, case report
Citation: Mo X, Wang M, Jiang X and Jin L (2025) Case Report: Mycoplasma pneumoniae-induced rash and mucositis in a child. Front. Med. 12:1607970. doi: 10.3389/fmed.2025.1607970
Edited by:
Chhavi Gupta, Yashoda Super Speciality Hospital, IndiaReviewed by:
Netto George Mundadan, Government Medical College, Kottayam, IndiaAparna Chakravarty, Amrita Institute of Medical Sciences and Research, Faridabad, India
Copyright © 2025 Mo, Wang, Jiang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Jin, bGppbjEwMjkxMDIwQHN0dS5zdWRhLmVkdS5jbg==
†These authors have contributed equally to this work
 Xiaoying Mo1†
Xiaoying Mo1† Li Jin
Li Jin