- 1Department of Nephrology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 2Department of Nephrology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 3Department of Nephrology, Jiangxi Medical College, The Second Affiliated Hospital, Nanchang University, Nanchang, China
- 4Department of Rheumatology and Immunology, The Second Hospital of Shandong University, Jinan, China
- 5Department of Minimally Invasive Comprehensive Treatment of Cancer, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 6Department of Nephrology, The Affiliated Taian City Central Hospital of Qingdao University, Taian, China
The association between glomerular diseases and malignancies has been recognized since the 1920s. A diverse spectrum of glomerular lesions can occur in various neoplasms, including both hematologic malignancies and solid tumors. This study presents a case series of paraneoplastic nephrotic syndrome (PNS) associated with three solid tumors: ovarian cancer, pancreatic neuroendocrine tumor (NET), and bladder cancer. The occurrence of PNS is rarely reported in association with these malignancies. Notably, all patients achieved complete or partial remission without receiving corticosteroids or immunosuppressant therapy. These observations accentuate the critical role of malignancy in the pathogenesis of glomerulopathy and underscore the therapeutic primacy of oncological control in such patients.
Introduction
The association between glomerular diseases and malignancies was first recognized as early as the 1920s by Galloway in patients with Hodgkin’s disease (1). Subsequent studies have reported that renal involvement may occur in over 7% of cancer patients. (2, 3). A recent Brazilian retrospective cohort analysis found that 1.97% of patients had concurrent glomerulopathy and neoplasms, with hematologic malignancies being the most common (35.8%), followed by colon and gynecologic tumors (4).
In this study, we present a case series of paraneoplastic nephrotic syndrome (PNS) in association with three solid tumors: ovarian cancer, pancreatic neuroendocrine tumor (NET), and bladder cancer. The occurrence of PNS in conjunction with these malignancies is rarely documented. Notably, all patients achieved complete or partial remission without corticosteroid or immunosuppressive therapy. These observations accentuate the critical role of malignancy in the pathogenesis of glomerulopathy and underscore the therapeutic primacy of oncological control in these patients.
Case report
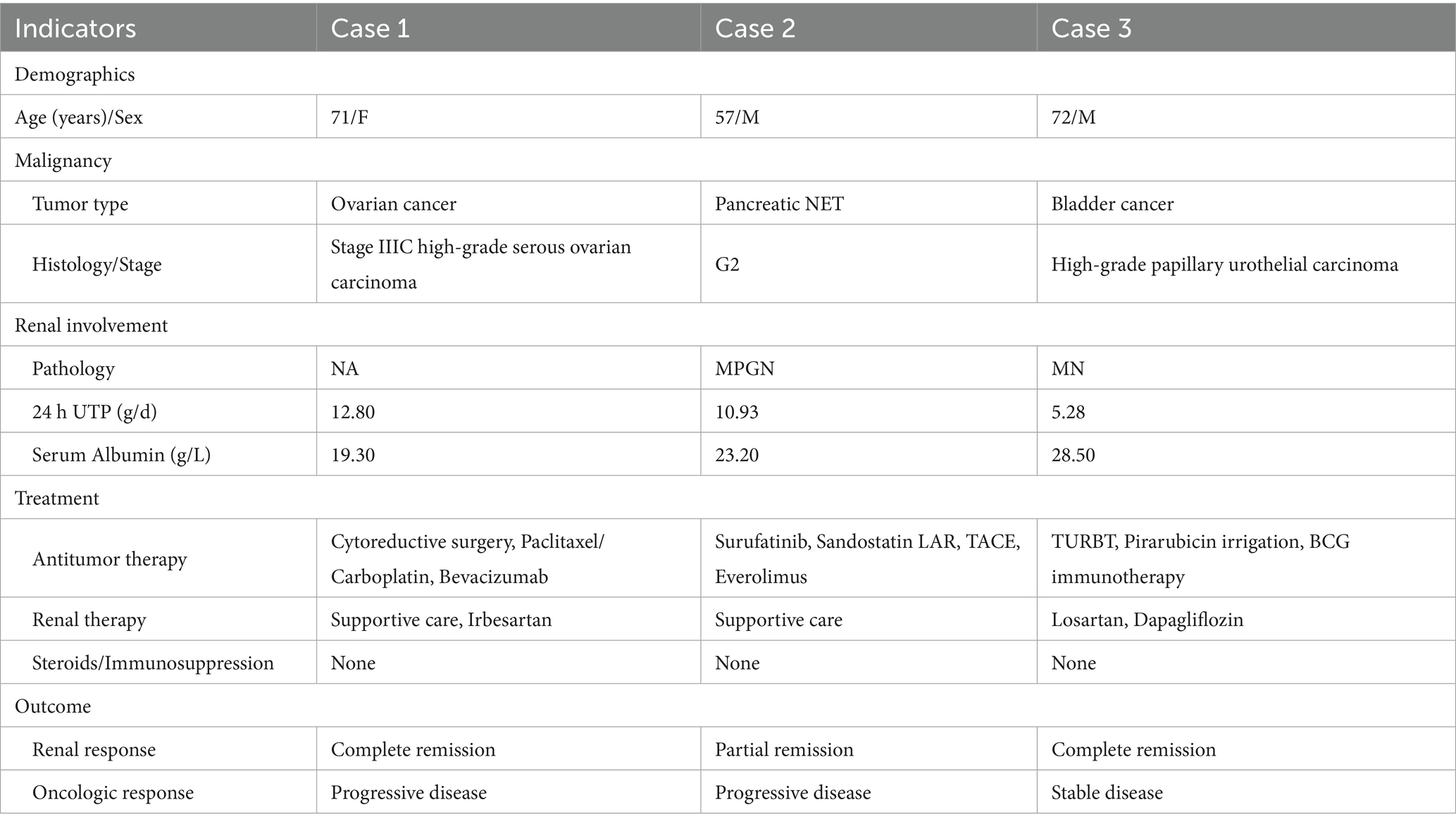
Case 1
A 71-year-old Chinese woman presented with bilateral lower limb edema. Her medical history included well-controlled diabetes mellitus and hypertension, managed with irbesartan. Physical examination revealed periorbital and lower extremity edema with normal blood pressure. Laboratory tests demonstrated nephrotic-range proteinuria (24 h UTP 12.80 g/d), severe hypoalbuminemia (serum albumin 19.30 g/L), and marked hyperlipidemia (triglycerides 4.56 mmol/L, total cholesterol 8.83 mmol/L, and LDL-C 5.73 mmol/L). Renal function remained relatively normal (serum creatinine 0.70 mg/dL, eGFR 87.40 mL/min/1.73m2, CKD-EPI 2009). Serological tests, including phospholipase A2 receptor antibodies (PLA2R-Ab), autoantibodies, viral markers, serum complement, and immunofixation electrophoresis, were all negative. Fundoscopy showed no diabetic retinopathy, consistent with her glycemic control (HbA1c 6.1%).
Gynecological ultrasound and abdominal CT scanning revealed a right adnexal cystic-solid mass (9.1 × 7.4 × 6.2 cm) and an irregular peri-uterine soft tissue lesion with poorly defined boundaries (5.3 × 6.0 cm), respectively. Tumor markers were significantly elevated (CA125 311.00 U/mL [normal<25], HE4 1414.00 pmol/L [normal<140]). The patient declined a renal biopsy and underwent cytoreductive surgery, which confirmed stage IIIC high-grade serous ovarian carcinoma (Figure 1). Post-operative management included intraperitoneal carboplatin and supportive therapy (metformin, insulin, statins, irbesartan, low molecular weight heparin, and diuretics). Tumor markers significantly declined post-operatively (CA125 191 U/mL, HE4 145 pmol/L), while proteinuria persisted (3+), and serum albumin decreased to 16 g/L but increased to 25.9 g/L after albumin infusions. Her renal function remained stable throughout hospitalization, and the patient was discharged with persistent edema.
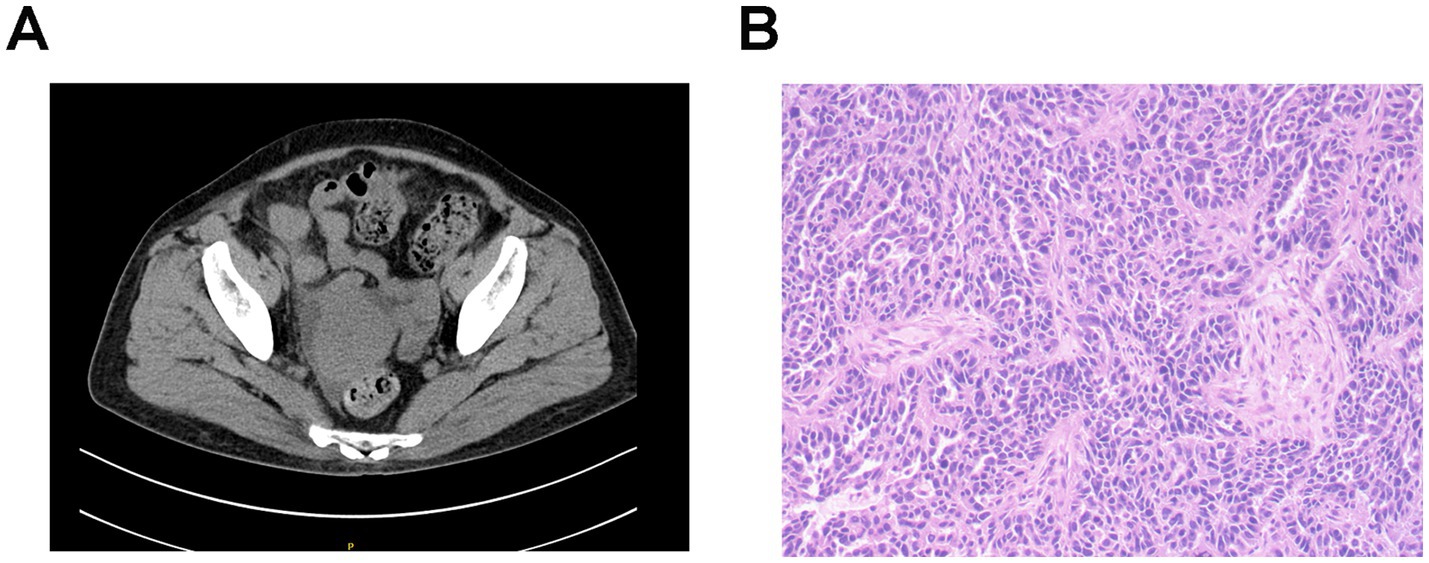
Figure 1. Imaging and pathological characteristics of ovarian cancer in patient 1. (A) Abdominal CT scanning indicates an irregular soft tissue near the uterus without obvious boundaries with the uterus. (B) Histopathology shows right adnexal high-grade serous carcinoma. Tumor dimensions: 6.5 × 5 × 4 cm. Lymphovascular space invasion present (cancer emboli identified). Metastatic lesions identified in: right fallopian tube, right parietal peritoneum, and omentum. Uterus and left adnexa: carcinomatous involvement of the uterine serosal surface, and the left ovary and left fallopian tube show no significant lesions. Immunohistochemistry results: WT-1 (+), Vimentin (−), P16 + (patchy/mottled staining pattern), P53 (−), Ki-67 + (60% proliferation index), ER (−), PR (−), and PAX8 (+).
During the 6-month follow-up, the patient received paclitaxel and carboplatin-based adjuvant chemotherapy. Notably, 1 month after surgery and prior to initiating chemotherapy, laboratory tests showed signs of NS remission, evidenced by reduced proteinuria (2+), improved serum albumin (30 g/L), and resolved edema, without albumin infusion, steroids, or immunosuppressants. Her tumor markers continued to decline at this time point (CA125 45.3 U/mL, HE4 89.0 pmol/L). Complete remission of NS was observed at 4-month post-surgery, with sustained normalization of both CA125 and HE4 levels. At 1-year follow-up, CT scanning showed disease progression with new soft tissue lesions in the left pelvic wall. Consequently, over the next 6 months, the patient received six cycles of combined paclitaxel/carboplatin chemotherapy, with bevacizumab added in the first and the last cycles, and her tumor markers increased (CA125 24.8 U/mL, HE4 299 pmol/L). Nevertheless, despite tumor progression, renal remission persisted throughout the subsequent 1.5 years, with no proteinuria (24 h UTP 0.09 g/d), normal serum albumin (38.2 g/L), and stable renal function (eGFR 90.0 mL/min/1.73m2).
Case 2
A 57-year-old Chinese man was admitted with bilateral lower limb edema for 2 months and elevated serum creatinine for 20 days. His medical history included hypertension, treated with calcium channel blockers, and a pancreatic NET G2 (Figure 2A), for which he had undergone distal pancreatectomy, splenectomy, and metastatic liver resection 18 months prior. Physical examination showed lower extremity edema. Laboratory tests revealed nephrotic-range proteinuria (24 h UTP 4.98 g/d), microscopic hematuria (17.2 RBCs/HPF), hypoalbuminemia (serum albumin 24.9 g/L), hyperlipidemia, and preserved renal function (eGFR 85.0 mL/min·1.73m2, CKD-EPI 2009). All serological tests were negative.
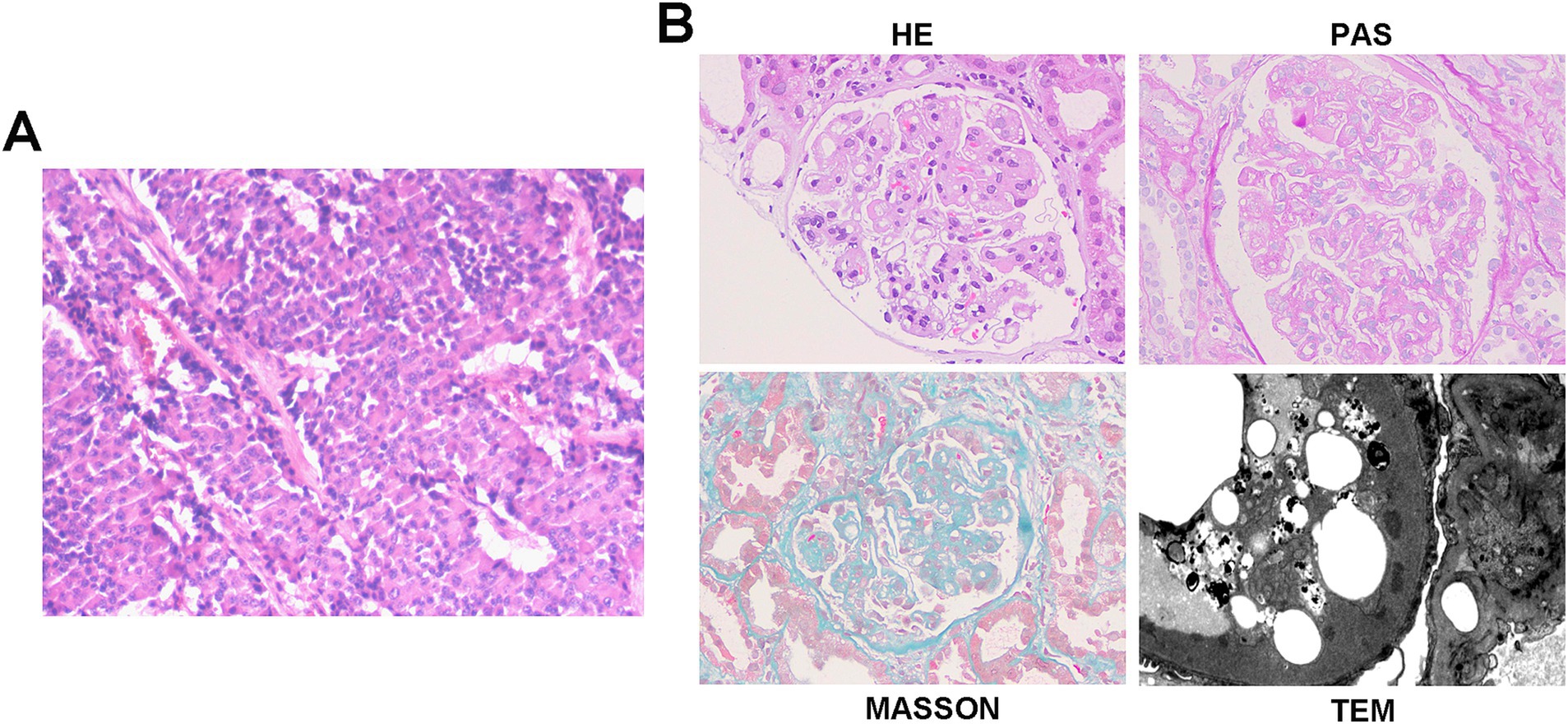
Figure 2. Histopathology of pancreatic NET and kidney in case 2. (A) Histopathology shows pancreatic neuroendocrine tumor, grade 2 (NET G2). Tumor size: 11 × 6 cm. Mitotic rate: 5 per 50 high-power fields (HPFs). Status of resection margins: Negative for tumor involvement. Adjacent structures: pancreatic transection margin: tumor-free; splenic parenchyma: no tumor identified; left adrenal gland: uninvolved. Hepatic metastases: left hepatic lobe: ingle metastatic deposit (10 × 9 cm) confirmed as metastatic NET; resection margin: clear; right hepatic lobe: two metastatic foci (4.5 × 3.5 cm and 2 × 1.8 cm), histologically consistent with metastatic NET; larger lesion abuts the capsular surface; resection margins: free of tumor. Lymph node status: peripancreatic lymph node (1/1): no metastatic involvement. Immunohistochemistry results: CK(AE1/AE3) (+), Vimentin (−), CK7(−), CK8/18(+), CK19 (+), CD56 (+), Syn (+), CgA (+), B-Catenin (+), and Ki-67(20%). (B) Representative of renal pathology of case 2.
Renal biopsy showed 29 glomeruli, eight with segmental sclerosis and adhesions, diffuse capillary wall thickening with double contours, mild-to-moderate mesangial proliferation, and scattered deposits on Masson staining. Interstitial fibrosis (10–20%) and tubular atrophy (20%) with inflammation were present. A diagnosis of mesangial proliferative glomerulonephritis (MPGN) was made (Figure 2B). Retrospective reviews indicated pre-existing proteinuria (2+) with hypoalbuminemia before surgery (38.5 g/L). Postoperatively, the patient received surufatinib for 16 months, with stable disease, and his proteinuria resolved and albumin normalized 3-month post-surgery without any additional interventions.
One month before admission, routine follow-up revealed the onset of NS (4 + proteinuria, 24 h UTP 10.93 g/d, serum albumin 23.2 g/L, LDL-C 8.76 mmol/L) with acute kidney injury (AKI) (serum creatinine 138.2 μmol/L) and radiographic disease progression. Surufatinib was then switched to sandostatin LAR due to suspected nephrotoxicity. Paraneoplastic MPGN was diagnosed based on temporal association, exclusion of other causes, and atypical pathology. Symptomatic treatment was initiated with diuretics, statins, and indobufen due to his intolerance of AECI/ARBs and recent AKI. At discharge, his renal function improved (serum creatinine 86.8 μmol/L) with partial edema resolution.
At the 1-month follow-up, the disease progressed despite sandostatin LAR therapy, prompting transarterial chemoembolization (TACE) and a switch to everolimus. Laboratory results showed decreased proteinuria (2+, 24 h UTP 3.89 g/d) with improved albumin levels (28.5 g/L), lipid profile (LDL-C 2.98 mmol/L), and renal function (serum creatine 71.6 μmol/L, eGFR 107.0 mL/min·1.73m2). At 4 months, disease progression continued, complicated by a liver abscess requiring drainage and antibiotic therapy, and NS was exacerbated (3 + proteinuria, serum albumin 23.3 g/L, LDL-C 7.22 mmol/L), leading to everolimus suspension. Partial remission of NS was observed after 6 months (albumin 30.3 g/L without infusion), allowing everolimus resumption. Sustained partial remission was noted at the 1-year follow-up (2 + proteinuria, albumin 34.0 g/L) with stable renal function (serum creatinine 66.3 μmol/L), which persisted over subsequent local follow-up until the last assessment at 1.5 years.
Case 3
A 72-year-old Chinese man presented with a 5-month history of bilateral lower limb edema. His medical history included atrophic gastritis, treated with rebamipide, and a varicectomy a decade earlier. The patient denied any history of smoking or alcohol consumption. Physical examination revealed mild lower extremity edema. Laboratory findings indicated nephrotic-range proteinuria (24 h UTP 5.28 g/d), microscopic hematuria (4.8 RBCs/HPF), hypoalbuminemia (28.5 g/L), hyperlipidemia (total cholesterol 6.2 mmol/L, LDL-C 4.33 mmol/L), and normal renal function (eGFR 98.0 mL/min·1.73m2, CKD-EPI 2009). All serological tests were negative. Abdominal and urinary ultrasonography showed mild fatty liver, prostatic hyperplasia, and vascular atherosclerosis.
Renal biopsy of 17 glomeruli revealed diffuse capillary wall thickening and podocyte swelling without mesangial proliferation, along with scattered deposits on Masson staining. There was minor tubular atrophy and interstitial fibrosis with mild inflammation. Immunofluorescence showed granular deposits of IgG (IgG1) and light chain deposits along the capillary walls, and PLA2R staining was negative. A diagnosis of atypical membranous nephropathy (MN) or PLA2R-unrelated MN was made (Figure 3A). The patient responded well to diuretics and statins and was discharged on losartan, statins, and indobufen.
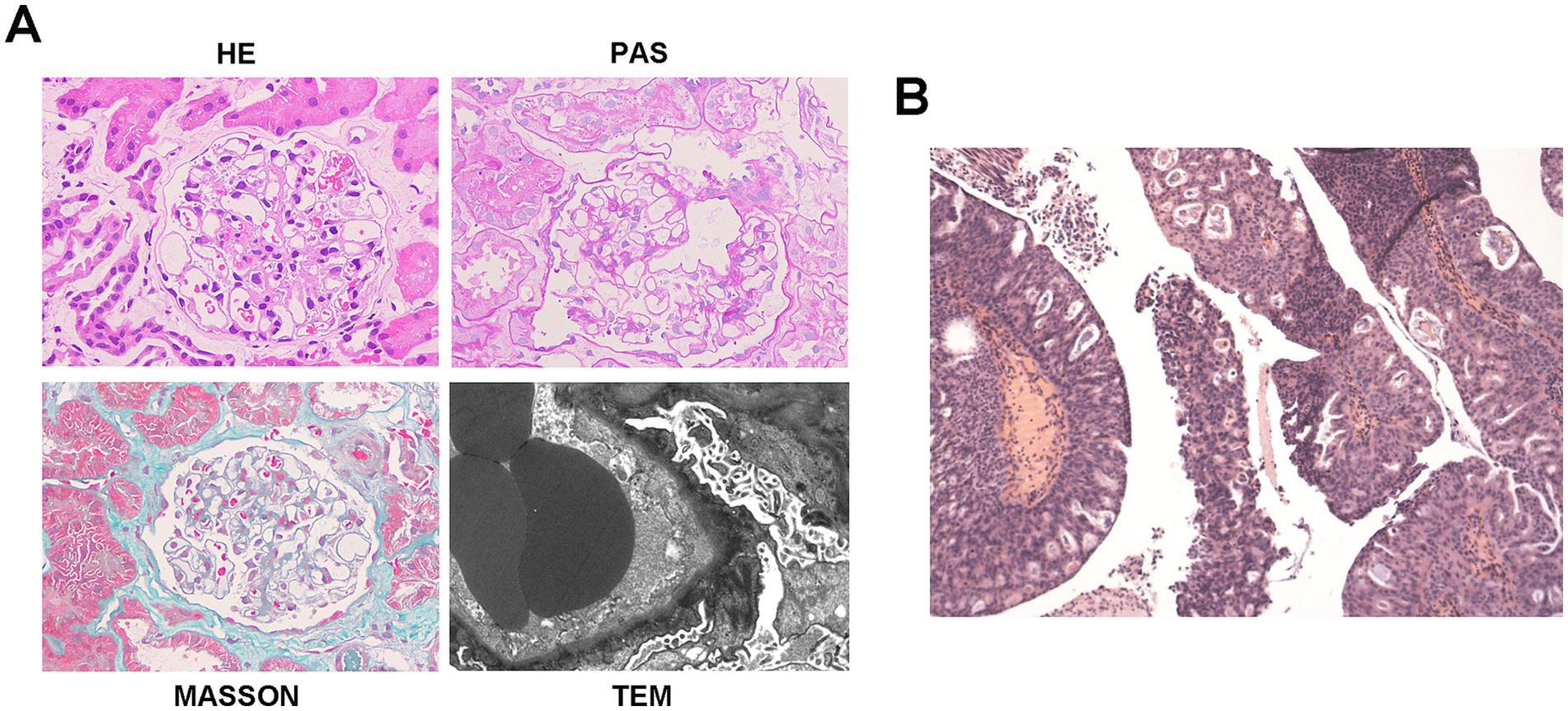
Figure 3. Histopathology of bladder cancer and kidney in case 3. (A) Representative of renal pathology of case 3. (B) Histopathology shows high-grade papillary urothelial carcinoma. Morphological variant: Focal glandular differentiation. Tumor dimensions: 2.0 × 1.5 × 0.5 cm.
At the 4-month follow-up, partial NS remission was observed with reduced proteinuria (24 h UTP 1.56 g/d) and normalized serum albumin (41.6 g/L), though hematuria persisted (40.5 RBCs/HPF). At 5 months, urinary ultrasonography and CT urography showed multiple bladder masses (largest 1.4 × 1.2 cm) and retroperitoneal lymphadenopathy. NS was still in partial remission (24 h UTP 1.44 g/d, serum albumin 39.6 g/L, LDL-C 3.58 mmol/L), with improved urinalysis. The patient underwent transurethral resection of the bladder tumor (TURBT) with postoperative pirarubicin irrigation, which confirmed high-grade papillary urothelial carcinoma with focal adenoid differentiation (Figure 3B). Post-operatively, the patient received Bacillus Calmette–Guerin (BCG) immunotherapy and continued conservative treatment with losartan and dapagliflozin. Three-month post-operatively, near-complete renal remission was achieved, evidenced by minimal proteinuria (24 h UTP 0.62 g/day), normal serum albumin (41.4 g/L), normal lipid levels (LDL-C 1.96 mmol/L), and stable renal function (eGFR 92.49 mL/min/1.73m2). This remission was sustained at the 8-month postoperative follow-up, with repeat urinary ultrasonography showing no evidence of recurrence and a further reduction in proteinuria (24 h UTP 0.10 g/day).
Discussion
Paraneoplastic glomerular diseases refer to glomerular lesions that are indirectly caused by the presence of malignancy through tumor-related mechanisms and which tend to improve following effective treatment of the underlying cancer (5). The clinical diagnosis is often supported by the following characteristics (6, 7): (1) Clinical manifestations of renal injury have a time correlation with tumor (5, 8), especially within 2 years (9); (2) effective treatment of tumor, either surgical or chemical, is the premise for clinical and histologic remission of renal injury (10); (3) deterioration of renal function attributed to tumor relapse (5, 11); and (4) a pathophysiological connection between glomerular lesion and tumorigenesis, namely tumor antigens or antibody components detected by pathological examination (6). Among these, MN represents the most frequently reported histologic pattern, although other pathological alterations may occur (5, 12, 13). Importantly, there are currently no established consensus guidelines for the management of both cancer and paraneoplastic glomerulopathy (14, 15). In this context, we present three rare instances of solid malignancy-associated NS, including ovarian cancer, pancreatic NET, and bladder carcinoma, which are highly suggestive of a paraneoplastic etiology. A paramount and unifying observation across all cases is the achievement of complete or partial NS remission solely through antitumor and supportive therapies, without the use of corticosteroids or immunosuppressive agents. This finding accentuates a critical role of underlying malignancy in the pathogenesis of glomerulopathy in these patients and underscores the therapeutic primacy of oncological control. Clinical characteristics, management, and outcomes of three presenting cases are summarized in Table 1.
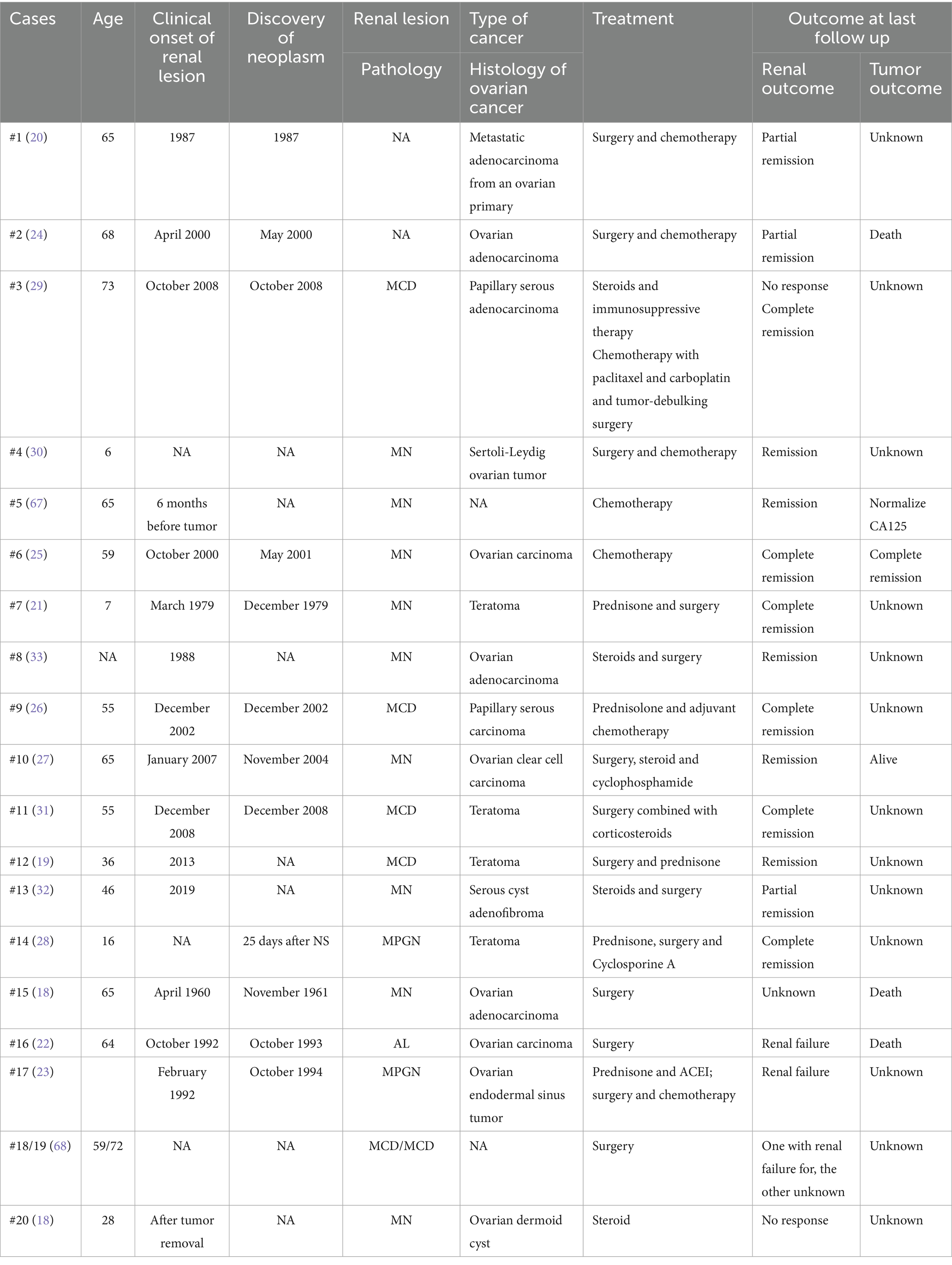
Ovarian cancer, one of the most common gynecologic malignancies, frequently presents with non-specific symptoms in its early stages (16, 17). Its association with paraneoplastic glomerulopathy remains rare, with only 20 documented cases to date (including 5 benign teratomas) (Table 2) (18–33). Notably, 70% (14/20) of these patients achieved renal remission during follow-up, despite approximately half receiving steroids or immunosuppressive therapy, suggesting a favorable renal prognosis in ovarian cancer-associated PNS, although oncologic outcomes were frequently unreported. Recent studies have also indicated associations between gynecological neoplasms and glomerular lesions, although the histological characteristics remain undefined (4). In our first case, the temporal dynamics between tumor activity and renal response were particularly revealing. Although a definitive histological diagnosis is lacking, complete and sustained NS remission was induced following cytoreductive surgery and chemotherapy, providing strong clinical evidence for a paraneoplastic etiology. Although the patient had been on long-term irbesartan therapy prior to the onset of NS, and spontaneous remission could not be entirely ruled out, the immediate response to tumor debulking remains the most plausible explanation and underscores the value of steroid-sparing approaches in such scenarios. Another important point is the potential nephrotoxicity of oncological therapies, such as bevacizumab, used in this case. Bevacizumab is a monoclonal antibody targeting vascular endothelial growth factor (VEGF-A) and is known to be associated with proteinuria and other glomerular lesions, such as glomerular microangiopathy (34, 35). Critically, the temporal sequence of events in this patient, which showed significant renal improvement after tumor-directed therapy but persisted despite the administration of bevacizumab, supports the primacy of a paraneoplastic mechanism–rather than drug-induced nephrotoxicity—is the primary cause. Notably, the renal remission in this case proved remarkably resilient, persisting even upon subsequent oncologic progression, a phenomenon rarely documented in prior ovarian cancer-associated PNS cases. This suggests that initial reduction in tumor burden might disrupt paraneoplastic pathways sufficiently to induce prolonged stabilization of glomerular permeability, independent of later tumor progression, which is consistent with previous findings that glomerular lesions may be indirectly related to tumor burden, invasion, or metastasis (5, 6).
In this case, the initial parallel decline of both CA125 and HE4 with NS remission, followed by their subsequent dissociation (isolated HE4 elevation during cancer progression without NS relapse), revealed a fascinating nuance not previously emphasized in the literature. This dissociation may be clinically significant, since HE4 has been shown to possess higher specificity for ovarian malignancy than CA125 (36, 37), and its elevation has been observed in chronic kidney disease (CKD) independent of renal function (38, 39). Therefore, the isolated HE4 increase likely reflected genuine tumor progression, whereas the sustained NS remission suggested a decoupling of the paraneoplastic mechanism. This observation suggests complex biomarker interactions in PNS that merit further investigation.
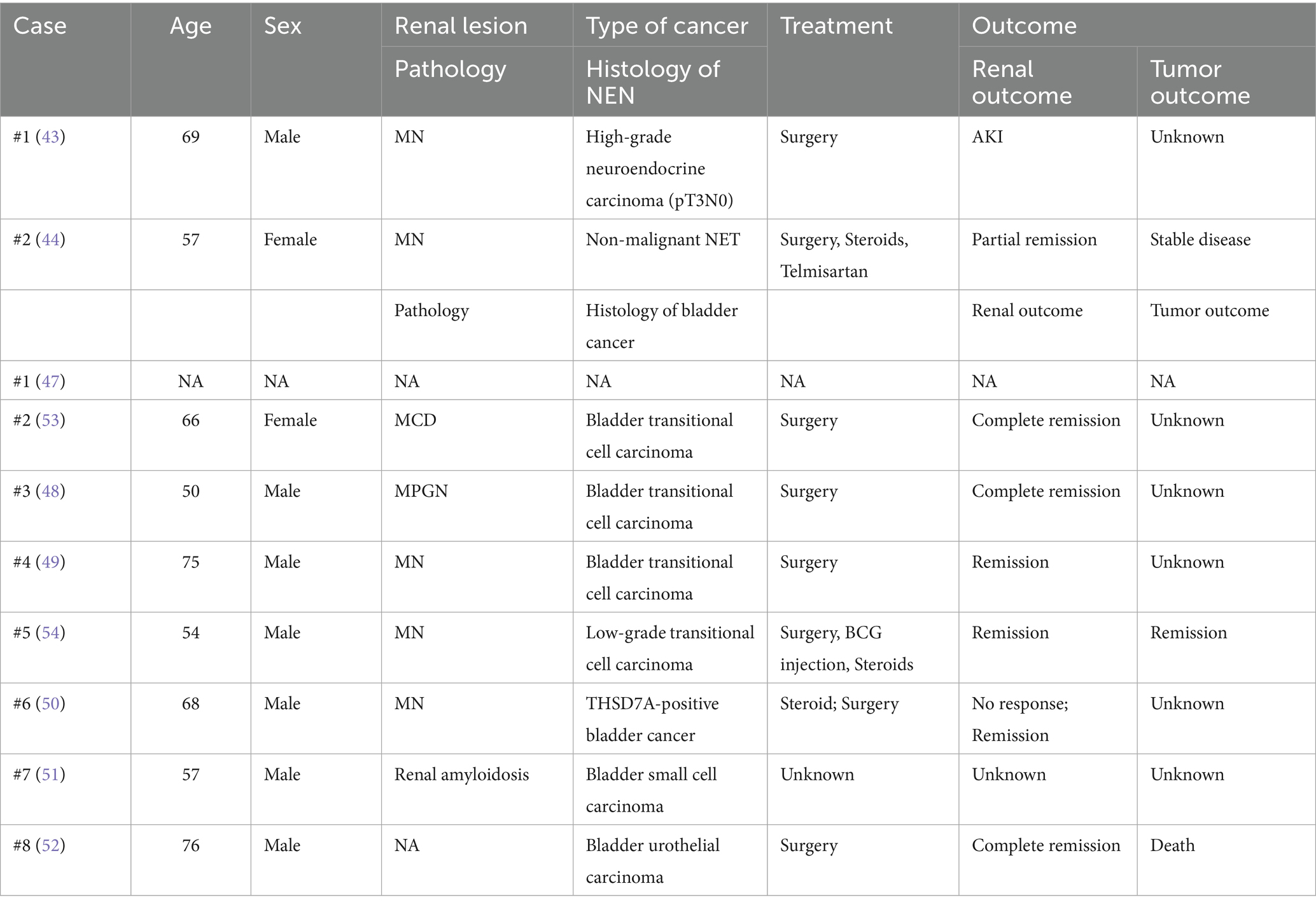
Neuroendocrine neoplasms (NENs) represent a diverse group of tumors predominantly originating from gastroenteropancreatic (GEP) tissues (40, 41). The majority are indolent neuroendocrine tumors (NETs), while approximately 10–20% are neuroendocrine carcinomas (NECs) with rapid disease progression (41, 42). To date, only two cases of NEN-associated PNS have been reported worldwide, one including a case of pancreatic NEC (43), and another a pancreatic NET (Table 3) (44). Our study presents the third global case and the first Asian case of pancreatic NEN-related PNS. Of particular interest is the temporal pattern of the paraneoplastic glomerulopathy, occurring before the administration of sunitinib and remitting during postoperative surufatinib therapy, and recurring with tumor progression. This pattern strongly suggests that tumor burden, rather than drug toxicity (45, 46), serves as the primary driver of renal injury, similar to our first case. More notably, subsequent tumor progression under everolimus therapy was paradoxically associated with partial NS remission, indicating a complex and non-linear relationship between tumor burden and paraneoplastic glomerulopathy. This observation also suggests a potential nephroprotective role for everolimus through mTORC1 inhibition, as demonstrated in previous studies (36, 37), adding a novel therapeutic dimension to the management of such complex cases that has not been adequately described in the existing literature.
In the literature, only eight cases of bladder tumor-related paraneoplastic NS have been reported (47–54), with four cases demonstrating partial or complete NS remission after surgical treatment. Corticosteroid therapy failed to improve proteinuria in two of these cases (49, 50), contrasting with the more favorable renal outcomes observed in ovarian cancer-associated PNS. Our third case of bladder cancer-associated MN provides critical new insights into its diagnostic and therapeutic challenges. Although the pathological pattern itself may not be unusual, the clinical presentation and course were highly instructive, expanding upon the eight previously reported cases of bladder tumor-related PNS (47–54). Different from prior cases (Table 3), in our patient, the manifestation of NS preceded bladder malignancy detection by several months. Significantly, partial remission was achieved with losartan monotherapy even before tumor detection, and this remission was further accelerated following surgical resection. This might be attributed to diagnostic limitations, since initial urological screening with ultrasonography revealed no masses at the time of NS diagnosis, while sensitive methods, including CT urography or contrast-enhanced ultrasound, are not routinely applied for scanning NS patients in the clinical setting, even in the elderly. Furthermore, while malignancy-associated MN is associated with other antigens such as THSD7A (13, 55), these biomarker assays are not yet widely integrated into routine clinical practice in our institution, due to cost constraints. Importantly, the observed treatment response in the third case indicates a dual potential mechanism: first, the reduction in tumor burden removes the antigenic stimulus; second, angiotensin receptor blockers (ARBs) may exert nephroprotective and potential antitumor effects by inhibiting the renin–angiotensin–aldosterone system (RAAS), which has been implicated in tumorigenesis via signaling pathways including Ras/RAF/MAPK/ERK, PI3K/AKT/mTOR, and Wnt/β-catenin (56–61). Therefore, this case highlights the importance of maintaining a high index of suspicion for occult malignancy in patients with NS, even after an initially negative urological evaluation, and the importance of scheduled monitoring, even after treatment initiation.
Current understanding of paraneoplastic glomerulopathy pathogenesis remains incomplete despite several proposed molecular mechanisms (62–66). Our cases contribute to this understanding by demonstrating that: (1) the relationship between tumor burden and glomerulopathy is not always linear and may exhibit complex temporal dynamics; (2) different cancer types may use distinct pathophysiological pathways to induce glomerular injury, which may be reflected in variations of specific biomarkers; (3) non-immunosuppressive treatments, including both RAAS inhibition and targeted antitumor agents, can be effective while potentially minimizing side effects; and (4) certain antineoplastic agents may possess underrecognized nephroprotective effects in PNS. Future research should prioritize the identification of novel biomarkers through the analysis of renal biopsy specimens from cancer patients with glomerular involvement, combined with functional validation in reliable in vitro and in vivo models. Large-scale cohort studies are needed to identify epidemiological characteristics, including the influence of race, ethnicity, age, and gender, as well as temporal relationships between tumor diagnosis and renal manifestations. Such investigations would advance our understanding of disease mechanisms and contribute to the establishment of more precise diagnostic criteria and prognostic indicators for this complex paraneoplastic phenomenon.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Clinical Research Ethics Committee of Shandong Provincial Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MH: Conceptualization, Data curation, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. QM: Data curation, Writing – original draft. LW: Data curation, Writing – original draft. TZ: Writing – original draft. JL: Writing – review & editing. XY: Writing – original draft. ZL: Conceptualization, Data curation, Resources, Writing – original draft, Writing – review & editing. RW: Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China (Grant No. 82100768) and the Natural Science Foundation of Shandong Province (Grant No. ZR2020QH062). All authors approved the final version of the study.
Acknowledgments
We thank all authors for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Puolijoki, H, Mustonen, J, Pettersson, E, Pasternack, A, and Lahdensuo, A. Proteinuria and haematuria are frequently present in patients with lung cancer. Nephrol Dial Transplant. (1989) 4:947–50. doi: 10.1093/ndt/4.11.947
3. Sawyer, N, Wadsworth, J, Wijnen, M, and Gabriel, R. Prevalence, concentration, and prognostic importance of proteinuria in patients with malignancies. Br Med J (Clin Res Ed). (1988) 296:1295–8. doi: 10.1136/bmj.296.6632.1295
4. Laferreira, MS, and Kirsztajn, GM. Potentially paraneoplastic glomerulopathies in a Brazilian cohort: a retrospective analysis. J Bras Nefrol. (2025) 47:e20240131. doi: 10.1590/2175-8239-jbn-2024-0131en
5. Lien, YH, and Lai, LW. Pathogenesis, diagnosis and management of paraneoplastic glomerulonephritis. Nat Rev Nephrol. (2011) 7:85–95. doi: 10.1038/nrneph.2010.171
6. Ronco, PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int. (1999) 56:355–77. doi: 10.1046/j.1523-1755.1999.00548.x
7. Bacchetta, J, Juillard, L, Cochat, P, and Droz, JP. Paraneoplastic glomerular diseases and malignancies. Crit Rev Oncol Hematol. (2009) 70:39–58. doi: 10.1016/j.critrevonc.2008.08.003
8. Pani, A, Porta, C, Cosmai, L, Melis, P, Floris, M, Piras, D, et al. Glomerular diseases and cancer: evaluation of underlying malignancy. J Nephrol. (2016) 29:143–52. doi: 10.1007/s40620-015-0234-9
9. Caza, TN, Hassen, SI, Dvanajscak, Z, Kuperman, M, Edmondson, R, Herzog, C, et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. (2021) 99:967–76. doi: 10.1016/j.kint.2020.07.039
10. Lefaucheur, C, Stengel, B, Nochy, D, Martel, P, Hill, GS, Jacquot, C, et al. Membranous nephropathy and cancer: epidemiologic evidence and determinants of high-risk cancer association. Kidney Int. (2006) 70:1510–7. doi: 10.1038/sj.ki.5001790
11. Cambier, JF, and Ronco, P. Onco-nephrology: glomerular diseases with cancer. Clin J Am Soc Nephrol. (2012) 7:1701–12. doi: 10.2215/CJN.03770412
12. Royal, V, Leung, N, Karam, S, Bridoux, F, and Nasr, SH. Paraneoplastic Glomerulopathies: mechanistic and pathogenic insights. Am J Nephrol. (2025) 23:1–13. doi: 10.1159/000546050
13. Murt, A, Berke, I, Bruchfeld, A, Caravaca-Fontán, F, Floege, J, Frangou, E, et al. Malignancies and glomerulonephritis: when to suspect and when to screen? Clin Kidney J. (2025) 18:sfaf101. doi: 10.1093/ckj/sfaf101
14. Porta, C, Bamias, A, Danesh, FR, Dębska-Ślizień, A, Gallieni, M, Gertz, MA, et al. KDIGO controversies conference on onco-nephrology: understanding kidney impairment and solid-organ malignancies, and managing kidney cancer. Kidney Int. (2020) 98:1108–19. doi: 10.1016/j.kint.2020.06.046
15. Cozzo, D, Orlando, F, Bruno, M, Ogna, A, and Forni Ogna, V. Minimal change glomerular disease associated with solid neoplasms: a systematic review. J Nephrol. (2024) 38:343–52. doi: 10.1007/s40620-024-02084-6
16. Siegel, RL, Miller, KD, and Jemal, A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
17. Beck, LH Jr. Membranous nephropathy and malignancy. Semin Nephrol. (2010) 30:635–44. doi: 10.1016/j.semnephrol.2010.09.011
18. Lee, JC, Yamauchi, H, and Hopper, J Jr. The association of cancer and the nephrotic syndrome. Ann Intern Med. (1966) 64:41–51. doi: 10.7326/0003-4819-64-1-41
19. Jeroudi, A, Kadikoy, H, Gaber, L, Ramanathan, V, Frome, A, Anwar, N, et al. Profound nephrotic syndrome in a patient with ovarian teratoma. Saudi J Kidney Dis Transpl. (2013) 24:777–82. doi: 10.4103/1319-2442.113883
20. Hoyt, RE, and Hamilton, JF. Ovarian cancer associated with the nephrotic syndrome. Obstet Gynecol. (1987) 70:513–4.
21. Beauvais, P, Vaudour, G, Gibod, LB, and Levy, M. Membranous nephropathy associated with ovarian tumour in a young girl: recovery after removal. Eur J Pediatr. (1989) 148:624–5. doi: 10.1007/BF00441515
22. Fernandez-Miranda, C, Mateo, S, Gonzalez-Gomez, C, and Ballestin, C. Systemic amyloidosis and ovarian carcinoma. Postgrad Med J. (1994) 70:505–6. doi: 10.1136/pgmj.70.825.505
23. Salazar-Exaire, D, Rodríguez, A, Galindo-Rujana, ME, Briones, JC, Arenas-Osuna, J, Rocha, LM, et al. Membranoproliferative glomerulonephritis associated with a mixed-cell germinal ovary tumor. Am J Nephrol. (2001) 21:51–4. doi: 10.1159/000046219
24. Forgy, AP, Ewing, TL, and Flaningam, J. Two paraneoplastic syndromes in a patient with ovarian cancer: nephrotic syndrome and paraneoplastic cerebellar degeneration. Gynecol Oncol. (2001) 80:96–8. doi: 10.1006/gyno.2000.6029
25. Kim, YT, Rha, SY, Shim, CY, Sohn, JH, Kim, C, Yu, NC, et al. A case of paraneoplastic nephrotic syndrome in a patient with ovarian carcinoma. Yonsei Med J. (2003) 44:539–43. doi: 10.3349/ymj.2003.44.3.539
26. Ryu, DR, Yoo, TH, Kim, YT, Jeong, HJ, Cho, NH, and Kang, SW. Minimal change disease in a patient with ovarian papillary serous carcinoma. Gynecol Oncol. (2004) 93:554–6. doi: 10.1016/j.ygyno.2004.02.022
27. Ata, A, Gürses, I, Kıykım, A, and Arıcan, A. Nephrotic syndrome associated with gemcitabine use in a patient with ovarian cancer. Am J Case Rep. (2012) 13:268–70. doi: 10.12659/AJCR.883583
28. Kilis-Pstrusinska, K, Szajerka, U, and Zwolinska, D. Unspecific increase of tumor markers in a girl with nephrotic syndrome and ovarian teratoma. Ren Fail. (2013) 35:654–6. doi: 10.3109/0886022X.2013.780614
29. González-Fontal, GR, Restrepo, JG, and Henao-Martínez, AF. Minimal-change disease as a paraneoplastic syndrome in a patient with ovarian carcinoma. NDT Plus. (2011) 4:427–9. doi: 10.1093/ndtplus/sfr106
30. Chen, A, Vander Lugt, M, McAllister-Lucas, LM, Killen, PD, and Blatt, NB. An unusual case of membranous nephropathy associated with an ovarian tumor. Pediatr Nephrol. (2011) 26:2249–51. doi: 10.1007/s00467-011-1994-7
31. Benabdellah, N, Izzedine, H, Bentata, Y, and Haddiya, I. Ovarian tumor and glomerulopathies: case report and review of the literature. Pan Afr Med J. (2019) 34:75. doi: 10.11604/pamj.2019.34.75.6008
32. Ana Stojanoska, NG, Simona, S-G, Svetlana Pavleska-Kuzmanovska, LT, Severova, G, Rambabova-Bushljetic, I, Pushevski, V, et al. Membranous nephropathy as a paraneoplastic syndrome in a patient with ovarian serous Cystadenofibroma - case report. Bantao Journal. (2019) 17:108–10.
33. Torres, VE, and JV, Donadio Jr., Cical aspects of the nephrotic syndrome in systemic diseases. New York, Dekker, (1988). in SI Cameron and RJ Glassock(eds): Nephrotic syndrome, ed 1.: 555–651.
34. Onteddu, NK, Mudupula Vemula, SS, Areddy, VR, Onteddu, J, and Mabbu, T. Bevacizumab-induced nephropathy presenting as crescentic Glomerulopathy. Cureus. (2023) 15:e48787. doi: 10.7759/cureus.48787
35. Jiang, R, Yu, RZ, Yang, HF, Wang, LX, and Lin, JJ. Bevacizumab-associated glomerular microangiopathy: a case report and literature review. BMC Nephrol. (2025) 26:445. doi: 10.1186/s12882-025-04385-9
36. Moore, RG, Brown, AK, Miller, MC, Skates, S, Allard, WJ, Verch, T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. (2008) 108:402–8. doi: 10.1016/j.ygyno.2007.10.017
37. Zhang, M, Zhang, Y, Liu, G, and Ge, L. A new risk algorithm combining D-dimer and HE4 differentiates borderline tumor from patients with ovarian tumor. Transl Cancer Res. (2025) 14:93–101. doi: 10.21037/tcr-24-1276
38. Bolstad, N, Øijordsbakken, M, Nustad, K, and Bjerner, J. Human epididymis protein 4 reference limits and natural variation in a Nordic reference population. Tumour Biol. (2012) 33:141–8. doi: 10.1007/s13277-011-0256-4
39. Yuan, T, and Li, Y. Human epididymis protein 4 as a potential biomarker of chronic kidney disease in female patients with Normal ovarian function. Lab Med. (2017) 48:238–43. doi: 10.1093/labmed/lmx036
40. Dasari, A, Shen, C, Halperin, D, Zhao, B, Zhou, S, Xu, Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. (2017) 3:1335–42. doi: 10.1001/jamaoncol.2017.0589
41. Das, S, and Dasari, A. Epidemiology, incidence, and prevalence of neuroendocrine neoplasms: are there global differences? Curr Oncol Rep. (2021) 23:43. doi: 10.1007/s11912-021-01029-7
42. Sorbye, H, Strosberg, J, Baudin, E, Klimstra, DS, and Yao, JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. (2014) 120:2814–23. doi: 10.1002/cncr.28721
43. Gleeson, FC, Brown, CM, and Herrera Hernandez, LP. A pancreatic mass and bilateral pitting pedal edema: nothing is ever what it seems. Clin Gastroenterol Hepatol. (2012) 10:e3. doi: 10.1016/j.cgh.2011.08.018
44. Forslund, T, Kellokumpu, I, Elomaa, E, Arola, J, and Nuorva, K. Remission of membranous glomerulonephritis after pancreatectomy for pancreatic neuroendocrine neoplasm - a rare coincidence. Clin Nephrol. (2011) 75:42–6. doi: 10.5414/CNP75042
45. Singh, L, Matassa, D, and Li, S. A case of acute kidney injury, proteinuria, and thrombotic Microangiopathy associated with Sunitinib therapy in metastatic pancreatic neuroendocrine tumor. Cureus. (2024) 16:e56660. doi: 10.7759/cureus.56660
46. Khan, A, Consing Gangelhoff, M, Moubarak, S, Herrmann, S, Nooruddin, K, and Alexander, M. Sunitinib induced glomerular thrombotic microangiopathy in a patient with refractory pancreatic neuroendocrine tumour. J Clin Pathol. (2024) 78:357–8. doi: 10.1136/jcp-2024-209851
47. Fukushi, Y, Sugita, A, and Ozu, K. A case of bladder tumor associated with nephrotic syndrome Fukuoka: Nishinihon J. Urol (1978) 40:89–93.
48. Reshi, AR, Mir, SA, Gangoo, AA, Shah, S, and Banday, K. Nephrotic syndrome associated with transitional cell carcinoma of urinary bladder. Scand J Urol Nephrol. (1997) 31:295–6. doi: 10.3109/00365599709070351
49. Fabbian, F, Rizzioli, E, and Catalano, C. Membranous glomerulonephritis and transitional cell carcinoma, improved proteinuria after each tumor resection. G Ital Nefrol. (2003) 20:65–8.
50. Matsumoto, A, Matsui, I, Mano, K, Mizuno, H, Katsuma, Y, Yasuda, S, et al. Recurrent membranous nephropathy with a possible alteration in the etiology: a case report. BMC Nephrol. (2021) 22:253. doi: 10.1186/s12882-021-02457-0
51. Kanat, O, Evrensel, T, Filiz, G, Usta, M, Baskan, E, Dilek, K, et al. Systemic AA amyloidosis and nephrotic syndrome associated with small cell carcinoma of the bladder. Nephrol Dial Transplant. (2003) 18:2453-a–4. doi: 10.1093/ndt/gfg391
52. Mizusawa, H, Mimura, Y, Utazu, H, and Maejima, T. Muscle invasive urinary bladder urothelial carcinoma presenting with secondary nephrotic symptoms. IJU Case Rep. (2021) 4:314–7. doi: 10.1002/iju5.12335
53. Rapoport, J, Kuperman, O, Gopas, Y, Maor, E, Eyal, A, Mostovslavski, M, et al. Nephrotic syndrome associated with transitional cell carcinoma of the bladder. Nephron. (1989) 52:36–9. doi: 10.1159/000185579
54. Singh, NP, Prakash, A, Kubba, S, Ganguli, A, Agarwal, SK, Dinda, AK, et al. Nephrotic syndrome as a complication of intravesical BCG treatment of transitional cell carcinoma of urinary bladder. Ren Fail. (2007) 29:227–9. doi: 10.1080/08860220601098961
55. Hoxha, E, Beck, LH, Wiech, T, Tomas, NM, Probst, C, Mindorf, S, et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy. J Am Soc Nephrol. (2017) 28:520–31. doi: 10.1681/ASN.2016010050
56. Pawlonka, J, Buchalska, B, Buczma, K, Borzuta, H, Kamińska, K, and Cudnoch-Jędrzejewska, A. Targeting the renin-angiotensin-aldosterone system (RAAS) for cardiovascular protection and enhanced oncological outcomes: review. Curr Treat Options in Oncol. (2024) 25:1406–27. doi: 10.1007/s11864-024-01270-9
57. Forrester, SJ, Booz, GW, Sigmund, CD, Coffman, TM, Kawai, T, Rizzo, V, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. (2018) 98:1627–738. doi: 10.1152/physrev.00038.2017
58. Siljee, S, Pilkington, T, Brasch, HD, Bockett, N, Patel, J, Paterson, E, et al. Cancer stem cells in head and neck metastatic malignant melanoma express components of the renin-angiotensin system. Life (Basel). (2020) 10:268. doi: 10.3390/life10110268
59. Kilmister, EJ, and Tan, ST. Cancer stem cells and the renin-angiotensin system in the tumor microenvironment of melanoma: implications on current therapies. Int J Mol Sci. (2025) 26:1389. doi: 10.3390/ijms26031389
60. Wang, J, Nishiyama, A, Matsuyama, M, Wang, Z, and Yuan, Y. The (pro)renin receptor: a novel biomarker and potential therapeutic target for various cancers. Cell Commun Signal. (2020) 18:39. doi: 10.1186/s12964-020-0531-3
61. Sever, N, Yunusov, E, Çelebi, A, Yaşar, A, Majidova, N, Kocaaslan, E, et al. Impact of renin angiotensin system inhibitors on survival of patients with metastatic non-small cell lung cancer. Ann Saudi Med. (2025) 45:18–24. doi: 10.5144/0256-4947.2025.18
62. Bertelli, R, Bonanni, A, Caridi, G, Canepa, A, and Ghiggeri, GM. Molecular and cellular mechanisms for proteinuria in minimal change disease. Front Med (Lausanne). (2018) 5:170. doi: 10.3389/fmed.2018.00170
63. Stuchlova Horynova, M, Raška, M, Clausen, H, and Novak, J. Aberrant O-glycosylation and anti-glycan antibodies in an autoimmune disease IgA nephropathy and breast adenocarcinoma. Cell Mol Life Sci. (2013) 70:829–39. doi: 10.1007/s00018-012-1082-6
64. McAdoo, SP, and Pusey, CD. Antiglomerular Basement Membrane Disease. Semin Respir Crit Care Med. (2018) 39:494–503. doi: 10.1055/s-0038-1669413
65. Li, JN, Cui, Z, Wang, J, Hu, SY, Jia, XY, Guan, Z, et al. Autoantibodies against linear epitopes of myeloperoxidase in anti-glomerular basement membrane disease. Clin J Am Soc Nephrol. (2016) 11:568–75. doi: 10.2215/CJN.05270515
66. Koyama, A, Fujisaki, M, Kobayashi, M, Igarashi, M, and Narita, M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. (1991) 40:453–60. doi: 10.1038/ki.1991.232
67. Topalak, O, Saygili, U, Soyturk, M, Karaca, N, Batur, Y, Uslu, T, et al. Serum, pleural effusion, and ascites CA-125 levels in ovarian cancer and nonovarian benign and malignant diseases: a comparative study. Gynecol Oncol. (2002) 85:108–13. doi: 10.1006/gyno.2001.6575
Keywords: paraneoplastic glomerular disease, ovarian cancer, pancreatic neuroendocrine tumor, bladder cancer, paraneoplastic nephrotic syndrome
Citation: Hu M, Ma Q, Wang L, Zhang T, Lin J, Yang X, Lv Z and Wang R (2025) Glomerular disease associated with cancer: a case series of paraneoplastic nephrotic syndrome. Front. Med. 12:1609192. doi: 10.3389/fmed.2025.1609192
Edited by:
Piergiorgio Messa, University of Milan, ItalyReviewed by:
Martin Windpessl, Clinic Wels-Grieskirchen, AustriaXiangchen Gu, Shanghai University of Traditional Chinese Medicine, China
Copyright © 2025 Hu, Ma, Wang, Zhang, Lin, Yang, Lv and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengsi Hu, ZHJodW1lbmdzaUBzaW5hLmNvbQ==; Zhimei Lv, emhpbWVpbHZAc2luYS5jbg==
 Mengsi Hu
Mengsi Hu Qiqi Ma
Qiqi Ma Liguang Wang
Liguang Wang Tingwei Zhang
Tingwei Zhang Jiangong Lin
Jiangong Lin Xiaowei Yang1
Xiaowei Yang1 Zhimei Lv
Zhimei Lv Rong Wang
Rong Wang