- 1Department of Acupuncture and Moxibustion, No.3 Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (West District), Chengdu, China
- 2College of Acupuncture, Moxibustion and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Department of Rehabilitation Medicine, Western Theater General Hospital, Chengdu, China
- 4Department of Critical Care Medicine, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 5Department of Pharmacy, Chongqing Yongchuan District People's Hospital, Chongqing, China
Background: The global prevalence of diabetes mellitus (DM) has exceeded 589 million, with the proportion of type 2 diabetes mellitus (T2DM) patients with comorbid cognitive impairment (CI) as high as 49.1%, which has a serious impact on disease management and quality of life. While acupuncture has been shown to improve cerebral glucose metabolism and synaptic plasticity, existing clinical trials are plagued by critical limitations: small sample sizes (often <100 participants) fail to detect subtle treatment effects, methodological heterogeneity in acupuncture protocols (e.g., varying acupoint selections, stimulation intensities, and treatment durations) hinders evidence synthesis, and insufficient blinding (e.g., sham acupuncture controls with low plausibility) introduces performance bias. These limitations have constrained the generalizability of findings and created a significant evidence gap in defining acupuncture’s role in diabetic cognitive impairment (DCI). The aim of this study is to assess the efficacy and safety of acupuncture for DCI through a systematic review with Bayesian network meta-analysis (NMA), addressing these methodological flaws via rigorous evidence synthesis.
Methods and analysis: Following PRISMA-NMA and STRICTA-2010 guidelines, we will systematically search 10 databases (PubMed, Cochrane Library, CNKI, etc.) from inception to 30 June 2025 and gray literature (January 1, 1995, to June 30, 2025) for randomized controlled trials (RCTs) comparing acupuncture with pharmacotherapy, sham acupuncture, or standard care. Inclusion criteria: adults (≥18 years) with T2DM and CI diagnosed via validated scales such as Montreal Cognitive Assessment (MoCA), and Mini-Mental State Examination (MMSE). Primary outcomes include cognitive function scores; secondary outcomes assess adverse events (CTCAE v5.0). The risk of bias will be evaluated using Cochrane RoB 2.0 and GRADE. Bayesian NMA will synthesize direct and indirect evidence to simultaneously assess the relative efficacy and safety of acupuncture.
Expected findings and conclusion: The study will clarify the relative efficacy and safety of acupuncture in improving DCI and provide evidence-based treatment options for clinical use. The results of Bayesian analyses may support acupuncture as a low-cost, low-risk adjunctive therapy, especially for patients intolerant of conventional medications. The results of the study will fill the current evidence gap and promote the standardized use of acupuncture in the management of DCI.
Systematic review registration: CRD42021275161.
Introduction
As a chronic disease, DM is among the most rapidly escalating global health challenges and has become a major global public health problem (1). According to the 11th edition of the International diabetes federation (IDF) Diabetes Atlas, the global number of people with DM was estimated to exceed 589 million in 2025, and it is expected to rise to 853 million by 2050 (2). With the continuous increase in the number of DM patients and the emerging trend of younger onset, DM has placed a substantial economic burden worldwide. Recent epidemiological data indicated that worldwide healthcare expenditures attributable to DM management reached in excess of 1 trillion dollars during the 2024 fiscal year, constituting roughly 9% of aggregate global health expenditures (3). Diabetic patients are living significantly longer, but the incidence of neurological damage is also on the rise (4, 5).
Cognitive impairment (CI) represents a significant comorbidity in DM, affecting approximately one-third of elderly diabetic patients (6, 7). This complication, recognized as one of the most debilitating chronic manifestations of DM (8), spans a severity spectrum from subjective cognitive decline to dementia as classified by DSM-5 criteria (9). Epidemiological data reveal strikingly high incidence rates, with 38.6% for mild cognitive impairment (MCI) in T2DM patients (10) and 49.1% for dementia in diabetic populations (11), substantially exceeding general population baselines. CI primarily affects areas of attention, memory (12), visuospatial executive functioning (13), language (14), and abstraction (15). Research evidence highlights the CI characteristics of disease subtypes: T2DM is characterized by deficits in information processing speed, episodic memory, and executive function impairment (16–18), This bidirectional relationship between diabetes and CI creates a significant clinical management challenge, as CI impedes diabetes self-management (medication adherence decreases by 34%, glycemic monitoring decreases by 41%) (19). Suboptimal glycemic control (HbA1c > 7.5%) accelerates hippocampal atrophy (8). Pathophysiological mechanisms underlying diabetic cognitive impairment (DCI) involve six interdependent pathways: central insulin resistance-mediated glucose hypometabolism (20); glutamate excitotoxicity (21); oxidative stress/mitochondrial dysfunction (22, 23); neurovascular unit disruption (24); chronic neuroinflammation (25); and proteinopathies including Aβ deposition and tau hyperphosphorylation (26, 27). Importantly, emerging evidence indicates that gut microbial dysbiosis contributes to the exacerbation of these mechanisms through bidirectional gut-brain axis communication (28). These mechanisms collectively impair neuronal networks and compromise CNS self-repair mechanisms.
Current management strategies for DCI include lifestyle modifications (aerobic exercise ≥150 min/week) (29), intensive glycemic control (HbA1c < 7%) (30), and emerging therapies such as intranasal insulin and GLP-1 receptor agonists, though none have demonstrated disease-modifying effects (31, 32).
Accumulating clinical evidence supports the therapeutic efficacy of acupuncture in ameliorating cognitive dysfunction associated with DCI (33). Acupuncture ameliorates CI through multiple mechanisms: mechanisms: modulation of cholinergic neurotransmission and inhibition of hippocampal neuronal apoptosis (34); mitigating neuroinflammation by suppressing microglial activation and NLRP3 inflammasome signaling (35), enhancing cerebral antioxidant capacity (36), and improving cortical arousal to potentiate neural activity (37). In recent years, neuroimaging evidence has further expanded mechanistic perceptions: functional magnetic resonance (fMRI) has shown that acupuncture significantly modulates the strength of default mode network (DMN) connections (38) and electroencephalography (EEG) has confirmed its alteration of microstate spatiotemporal features (39). These objective indicators provide multimodal evidence for the neuromodulatory mechanisms of acupuncture for DCI. Together, the above synergistic mechanisms form the scientific basis for acupuncture treatment of CI and neuropsychiatric disorders.
While emerging the evidence suggests potential benefits of acupuncture in diabetes-related neurological complications, the comparative efficacy and safety profiles of acupuncture interventions for DCI remain inadequately characterized. This systematic review and NMA comprehensively evaluate the therapeutic effectiveness and systematically monitor acupuncture-related adverse events in the management of DCI through evidence synthesis of randomized controlled trials.
Methods
Protocol registration and reporting
This study strictly follows the Cochrane Handbook for the Systematic Evaluation of Interventions (version 6.5) (40)and the PRISMA-NMA statement (41). The study protocol was prospectively registered on the PROSPERO platform (CRD42021275161), and the full PRISMA-NMA list is available in Supplementary File 1. The final report will be transparently reported according to Cochrane standards, including the study selection flowchart, data extraction tables, and results of sensitivity analyses.
Sample size thresholds and power calculations
To ensure methodological transparency, a priori sample size exclusion thresholds will be established via prospective power calculations using G*Power software (version 3.1.9.7). Key parameters were derived from prior meta-analytic evidence and clinical benchmarks: an effect size (Cohen’s d) of 0.35, corresponding to a clinically meaningful ≥0.5-point improvement in MoCA scores (42); 80% statistical power; a two-tailed α level of 0.05; and a 1:1 allocation ratio (43). The power calculation formula is:
Where: n: Minimum sample size per group; Z1 − α/2: Critical value for two-tailed test at α = 0.05 (1.96); Z1 − β: Critical value for 80% power (0.84); d: Cohen’s effect size (0.35, corresponding to ≥0.5-point MoCA improvement). A minimum threshold of 30 participants per arm was predetermined for primary analyses; studies failing to meet this criterion were excluded to mitigate bias from underpowered effect estimates in pooled analyses.
For meta-regression, thresholds adhered to the “5 × 5 rule” (≥5 studies per predictor and ≥5 events per parameter) to ensure statistical precision. Sensitivity analyses empirically affirmed threshold robustness across effect sizes (Cohen’s d = 0.30–0.40), with findings benchmarked against Cochrane’s minimum sample size recommendations.
Eligibility criteria
The eligibility criteria will be defined using Participants, Intervention, Comparison, Outcomes, and Study design (PICOS) elements.
Participants
Adults (≥18 years) with T2DM and DCI (diagnosed via MMSE, MoCA, or DSM-5). Exclusion criteria: severe comorbidities, non-DM-related cognitive disorders.
Intervention
In this meta-analysis, we will evaluate both standalone acupuncture interventions and combination therapies integrating acupuncture with standard diabetes care. The latter included evidence-based treatments recommended by the American diabetes association (ADA) 2023 guidelines (44), specifically: (1) pharmacological glycemic control agents (such as metformin, SGLT2 inhibitors, GLP-1 receptor agonists), (2) structured diabetes self-management education and support (DSMES) programs, and (3) advanced metabolic interventions including bariatric surgery.
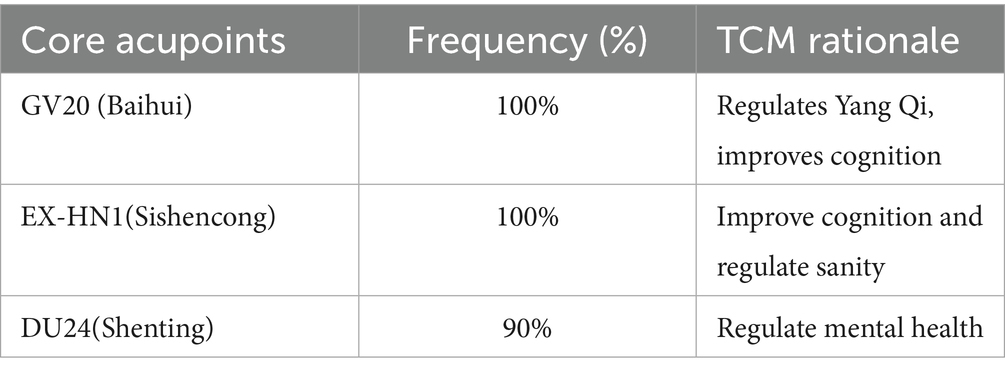
Acupuncture therapy was operationally defined as percutaneous needle-based interventions requiring skin penetration, encompassing manual acupuncture (MA), electroacupuncture (EA), warm-needling acupuncture (WA), auricular acupuncture (AA), and specialized needling techniques (such scalp acupuncture, abdominal acupuncture). To address acupoint heterogeneity, a core acupoint set was predefined through a Delphi consensus (33), the detailed is presented in Table 1. Additional points (≤4) will be permitted if justified by Traditional Chinese Medicine (TCM) syndrome differentiation (e.g., BL20 for spleen deficiency). This framework aligns with STRICTA guidelines (45) for standardized reporting of:
(1) acupuncture rationale (point selection basis)
(2) needle insertion parameters (depth, angle, retention time)
(3) treatment regimen (frequency, duration)
Non-invasive acupoint stimulation modalities, including laser acupuncture, acupressure, and transcutaneous electrical nerve stimulation, will be excluded based on demonstrated mechanistic heterogeneity in neuromodulation pathways. Specifically, these techniques lack the mechanical transduction effects of needle insertion. Besides, microneedle patches are also excluded. Its mechanism of action is different from the “mechanical stimulation—nerve conduction” pathway of traditional acupuncture and is therefore categorized as excluded (46).
Comparison
1. Active therapies recommended by clinical guidelines (44):
Pharmacological: First-line cognitive enhancers (donepezil 5–10 mg/day; memantine 20 mg/day).
Non-pharmacological:
(1) aerobic exercise (≥150 min/week moderate intensity).
(2) cognitive behavioral therapy (CBT; 60-min weekly sessions).
(3) selection criteria: Interventions with GRADE Class IIa recommendations in current guidelines.
2. Placebo controls:
Sham acupuncture using validated retractable needles (Park/Streitberger devices) (47) at non-acupoints;
Placebo drugs with identical appearance to active comparators.
3. Guideline-based usual care (44):
(1) glycemic control: HbA1c < 7% via metformin/SGLT2 inhibitors.
(2) cardiovascular management: Statins and ACE inhibitors/ARBs.
(3) cognitive monitoring: Annual Montreal Cognitive Assessment.
4. No treatment or waiting list.
Outcome measures
Since this review aims to systematically assess the effect of acupuncture intervention on CI among patients with diabetes, we have selected intellectual state and cognitive function as the primary outcomes, which are measured on various scales.
Primary outcome measures
The intellectual score is measured using the Montreal Cognitive Assessment (MoCA) Score, Mini-Mental State Examination (MMSE) score, clinical dementia rating (CDR) score, activities of daily living (ADL) score, clinical memory scale analysis system (MQ) score, or other validated scales for intellectual state and cognitive function.
Secondary outcomes
Safety profiles will be quantitatively assessed through systematic monitoring of adverse event (AE) incidence rates, categorized using the standardized Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0) framework (48). The three main considerations in conjunction with this study are as follows:
(1) needling-related events (bleeding, hematoma, pain)
(2) systemic reactions (vasovagal responses, fatigue)
(3) events meeting CTCAE v5.0 criteria for Grade ≥3 (e.g., pneumothorax, organ injury, anaphylaxis).
Information sources and search strategy
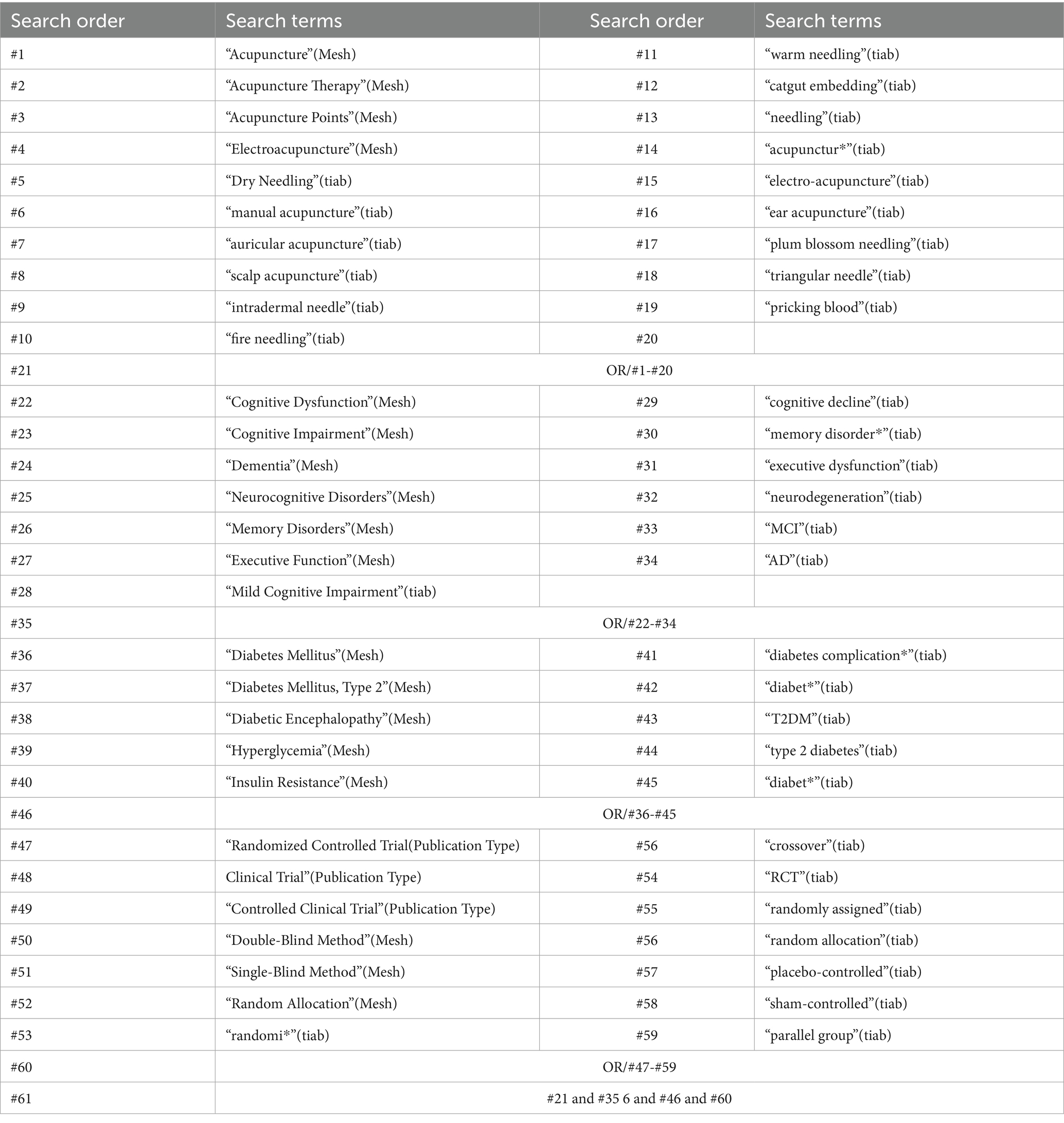
This systematic review aims to comprehensively search the literature on cognitive dysfunction in diabetes by utilizing the following databases. Searches will be conducted across six English databases—PubMed, MEDLINE, OvidSP, Embase, the Cochrane Library, and the Allied and Complementary Medicine Database (AMED)—as well as four Chinese databases: the Chinese National Knowledge Infrastructure (CNKI), Wan Fang Database (Wan Fang), VIP Database, and the Chinese Biomedical Literature Database (CBM). The aim of this review is to identify RCTs evaluating the efficacy and safety of acupuncture for DCI.
To avoid the potential exclusion of eligible RCTs, a multi-faceted search strategy will be employed in addition to primary databases. Clinical trial registries, including the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), Netherlands trial register (NTR), Chinese Clinical Trial Registry (ChiCTR), and ClinicalTrials.gov, will be systematically searched to identify ongoing and unpublished studies. Citation mining was performed by screening reference lists from 15 high-impact systematic reviews (impact factor >5.0) published within the last 5 years. Gray literature (January 1, 1995, to June 30, 2025) retrieval will incorporate conference proceedings from major international congresses (e.g., ADA, IDF, Alzheimer’s Association) and preprint servers (medRxiv, ResearchSquare), ensuring comprehensive identification of ongoing trials. No language restrictions were applied, and a manual search will be performed for additional eligible articles to supplement the electronic search. Included references will be also reviewed to expand the database.
Initially, the search strategy will be implemented in PubMed and then adapted for use in the other databases. The three primary search terms—“diabetic cognitive impairment,” “acupuncture,” and “RCTs”—will be utilized as medical subject headings (MeSH) and free-text keywords. The detailed PubMed search strategy is presented in Table 2. Similar search strategies will be developed for the other databases. Searches will be conducted in each database to identify potentially eligible RCTs from establishment to date.
Data extraction and management
We have developed a comprehensive data extraction form specifically designed to align with the objectives of this systematic review. The form systematically captures critical information from all included studies, including study characteristics (title, authors, publication year, and country of origin), participant demographics (sample size, intervention protocols, treatment modalities, duration of intervention, and primary outcome measures), essential components for risk of bias assessment, and quantitative outcome data, the detailed is presented in Table 3. Two independent reviewers (J. G and K. H) will perform data extraction using this form, with discrepancies resolved through discussion; if unresolved, a third reviewer (X. W) will adjudicate. Inter-rater reliability will be quantified via Cohen’s kappa coefficient for key variables (study inclusion/exclusion, risk of bias), targeting kappa >0.8. The screening process occurs in two phases: first, titles/abstracts are screened, with eligible studies proceeding to full-text review. All reviewers remain blinded throughout. Non-Chinese/English articles will be assisted by language experts. Duplicate records are removed during title/abstract screening. Disagreements at any stage are referred to senior researchers (X. W) for final resolution. All records and data from various sources will be systematically managed using a standardized data collection form in Microsoft Excel. The study selection process is shown in Figure 1.
Dealing with missing data
For missing outcome data, we will first attempt to contact study authors for clarification. If unavailable, full-case analysis (complete case analysis) will be used as the primary approach; multiple imputation (MI) with chained equations will serve as a sensitivity analysis, assuming missing data are missing at random (MAR). Imputation models will include all baseline covariates and study-level characteristics.
Risk of bias and quality assessment
Two researchers (LJ. H and XM. L) will independently assess the risk of bias in the included studies using the Cochrane Collaboration’s Risk of Bias Tool RoB 2.0 tool (49). The Cochrane RoB 2.0 tool will be used to assess the risk of bias in the following areas: randomization process (including random sequence generation and allocation concealment), intervention bias (blinded implementation involving patients, therapists, and evaluators), completeness of outcome data (failure to attend more than 20% is considered a high risk), bias in outcome measures (subjective scales need to be double-blinded), and selective reporting (comparing pre-registration protocol versus published results). Outcomes will be categorized as “low risk,” “partial risk,” and “high risk” and will be completed independently by two assessors. Results are considered concordant when the Kappa value is greater than 0.8.
Certainty of evidence
The evidence will be interpreted according to the GRADE (50). Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. This approach is based on four steps considering direct and indirect treatment estimates for each comparison of the evidence network, rating the quality of each direct and indirect effect estimate, rating the NMA estimate for each comparison of the evidence network, and the quality of each NMA effect estimate.
Data synthesis
Primary meta-analyses
Primary meta-analyses will be conducted using Review Manager (RevMan) version 5.4.1 software (Cochrane Collaboration) (50). For dichotomous outcomes, risk ratios (RRs) with corresponding 95% confidence intervals (CIs) will be calculated using the Mantel–Haenszel method. Continuous outcomes measured on identical scales will be analyzed as weighted mean differences (WMDs). In contrast, outcomes assessed using different measurement instruments (MoCA vs. MMSE), standardized mean differences (SMDs) with 95% CIs will be computed using Hedges’ g with small-sample bias adjustment.
Hedges’ g correction:
n1 and n2 are group sample sizes.
Network meta-analysis
The NMA will be conducted using the Netmeta package in R (version 4.4.3) and Stata 18.0. Non-informative priors will be specified: treatment effects follow a normal distribution N(0,10,000), and heterogeneity variance follows a uniform distribution U(0,2). The N(0,10,000) prior to treatment effects minimizes prior influence, as its 95% confidence interval far exceeds typical clinical effect sizes, ensuring data-driven inference (51). The U(0,2) prior for heterogeneity restricts variance within a clinically plausible range, aligning with Cochrane guidelines for “considerable heterogeneity” and preventing implausible high-variance estimates (52), Markov Chain Monte Carlo (MCMC) simulations will be performed with a minimum of 10,000 iterations, and convergence will be verified using Brooks-Gelman-Rubin statistics (R-hat < 1.1). The relative efficacy of interventions will be ranked using the surface under the cumulative ranking curve (SUCRA), with 95% CrIs provided to reflect uncertainty. Consistency between direct and indirect evidence will be evaluated through two complementary approaches: node-splitting analysis, which quantifies discrepancies by calculating the ratio of indirect-to-direct evidence (RoR), where a RoR ≠ 1 indicates inconsistency; and a design-by-treatment interaction model, which assesses interactions via the inconsistency factor (IF), with an IF > 10% denoting moderate to severe inconsistency.
Network geometry and visualization
Treatment comparisons will be visualized using network plots with adjacency matrices showing exact node/edge metrics and interactive web-based visualization using D3.js for dynamic exploration generated with the ggplot2 and graph packages in R. Node sizes will be proportional to the logarithm of the total sample size per intervention, while edge thickness will represent the number of studies comparing each pair of interventions. Nodes will be color-coded by intervention type (EA vs. MA), and edges will be differentiated by direct vs. indirect evidence using dashed lines for indirect comparisons. This graphical representation will provide an intuitive overview of the evidence network structure and identify gaps in the evidence base.
Intervention ranking
Treatment hierarchies will be estimated using the SUCRA, scaled from 0% (least effective) to 100% (most effective). Bayesian posterior probabilities with 95% credible intervals will supplement rankings to reflect uncertainty. Sensitivity analyses will exclude studies with a high risk of bias or extreme effect sizes to assess robustness.
Statistical modeling
Continuous outcomes (e.g., MoCA scores): Random-effects models with inverse-variance weighting and common between-study variance assuming multivariate normality of random effects with covariance structure estimated via restricted maximum likelihood (REML).
Binary outcomes (e.g., adverse events): Logistic regression with robust variance estimation.
All models will perform multivariable adjustment using meta-regression with empirical Bayes shrinkage for covariates showing >10% standardized mean difference across studies, such as baseline HbA1c and diabetes duration. Convergence will be verified using Brooks-Gelman-Rubin statistics and trace plots.
Results visualization and reporting
Results of all analyses will be presented through five types of visualization tools: (1) Evidence Network Graphs (D3.js interactive) to demonstrate the framework for comparison of interventions; (2) SUCRA ordination graphs to quantify the probability of optimal interventions; (3) node-split scatter plots to detect sources of inconsistency; (4) hierarchical forest plots to report main outcome effect sizes; and (5) heat maps to present the results of subgroup analyses. All graphs will be labeled with clinical significance thresholds.
Heterogeneity assessment
Statistical heterogeneity will be quantitatively evaluated using the I2 statistic (low: <25%; moderate: 25–75%; high: >75%) and Cochran’s Q test (significance threshold α = 0.10). Clinical and methodological heterogeneity will be assessed by comparing baseline characteristics (e.g., HbA1c, diabetes duration), intervention protocols (e.g., acupoint selection, stimulation parameters), and study design features (e.g., blinding, follow-up duration). Fixed-effect models will be employed when I2 ≤ 50% and Q-test p ≥ 0.10; otherwise, random-effects models will be used.
Sensitivity analysis
A three-tiered sensitivity analysis will assess robustness:
Methodological rigor: Exclusion of studies with high RoB 2.0 scores (≥3 high-risk domains) or sample sizes below clinical thresholds.
Intervention integrity: Exclusion of trials using >15 acupoints, lacking ≥2 predefined core acupoints (33), or non-compliant with STRICTA-2010 guidelines.
Statistical stability: Leave-one-out cross-validation; fixed-effect model application under τ2 = 0 (when Q-test p > 0.10); and trim-and-fill adjustment for asymmetry.
Heterogeneity exploration
Meta-regression based on REML will examine covariates including baseline HbA1c, total acupoints, and core acupoint adherence (≥2 core points). Prespecified subgroup analyses will evaluate: assessment tools (MoCA vs. MMSE), acupuncture modality (MA vs. EA), and intervention duration (≤4 vs. >4 weeks). Model convergence will be verified using Brooks-Gelman-Rubin diagnostics (R-hat <1.1 indicating convergence).
Inconsistency handling
Discrepancies in NMA evidence will be visualized via node-split scatter plots and resolved using consistency prior models or node-splitting corrections, with model fit compared via the Deviance Information Criterion. All analyses adhere to PRISMA-NMA and Cochrane guidelines, ensuring transparent reporting of heterogeneity and inconsistency.
Assessment of reporting biases
The assessment of reporting biases will be conducted through a multi-tiered analytical framework. Publication bias will be evaluated using a triangulated approach: First, funnel plot asymmetry will be visually inspected by plotting effect estimates against their standard errors, supplemented by Egger’s linear regression test (two-tailed α = 0.10) for quantitative assessment. Subsequently, contour-enhanced funnel plots adjusted for study precision will be generated to differentiate between publication bias and small-study effects. Sensitivity analysis employing the trim-and-fill method will be performed to estimate the potential influence of missing studies on pooled effect sizes.
Reporting standards
The analysis will adhere to PRISMA-NMA guidelines, with full documentation of network geometry, inconsistency metrics, and ranking probabilities. All reproducible scripts (including R and Stata) will be publicly archived on the Open Science Framework (OSF) repository, with a Docker containerized computational environment (v24.0) used to ensure long-term research reproducibility.
Discussion
This protocol addresses a critical gap in the evidence-based management of DCI by systematically evaluating acupuncture as a neuroprotective intervention. Current pharmacological strategies for DCI, including cholinesterase inhibitors and memantine, demonstrate limited efficacy (effect size < 0.3) and carry significant side effect burdens such as gastrointestinal disturbances in 25% of donepezil users (29). Non-pharmacological approaches such as cognitive training require intensive resource allocation, limiting accessibility in low-income settings (30).
Acupuncture offers a potentially cost-effective alternative, supported by preclinical evidence demonstrating its multi-modal neuroprotective effects: Suppression of microglial activation and NLRP3 inflammasome signaling, reducing IL-6 by 40% in diabetic models (35), Upregulation of PSD-95 and synaptophysin, enhancing hippocampal synaptic density (34), and Improved cerebral glucose uptake in parietal cortex via FDG-PET (36). Recent fMRI studies confirm acupuncture’s ability to modulate DMN connectivity—a key neural correlate of cognitive dysfunction (53), EEG microstate analyses reveal acupuncture-induced spatiotemporal reorganization of brain activity, correlating with cognitive improvement (54). However, prior clinical trials have been marred by methodological limitations, including inconsistent acupuncture protocols (variability in acupoint selection: 12–47 points per study) and suboptimal blinding (only 38% of trials used validated sham devices) (37). This protocol mitigates these issues through adherence to PRISMA-NMA guidelines and STRICTA reporting standards, ensuring a transparent synthesis of both standalone acupuncture and combination therapies with ADA-standard care.
If proven effective, acupuncture could serve as a first-line adjuvant therapy for DCI, particularly in populations intolerant to conventional medications. Subgroup analyses may identify optimal treatment parameters: preclinical models have shown that EA demonstrates superior neuroprotection (55), and clinical data indicate that intervention durations exceeding 4 weeks correlate with sustained cognitive improvements (56). Mechanistically, electroacupuncture combined with body acupuncture at cognition-targeted acupoints (e.g., GV20, DU24) is projected to outperform manual acupuncture or sham interventions, supported by evidence of enhanced cholinergic neurotransmission (elevated choline acetyltransferase activity) and reduced Alzheimer’s disease pathology. Clinically, patients with early-stage DCI (MoCA score 21–26) and well-controlled glycemia (HbA1c < 8.0%) are likely to derive maximal benefit, as preserved neurovascular function in these subgroups potentiates acupuncture-induced neuroplasticity and cerebral glucose metabolism. Notably, acupuncture is expected to yield a 1.5–2.0-point improvement in MoCA scores (95% CrI: 1.0–2.5) compared to standard care—an effect size comparable to low-dose cholinesterase inhibitors but with a 70% lower risk of adverse events (e.g., nausea, dizziness), thus positioning it as a cost-effective adjunctive therapy.
Current evidence is limited by heterogeneity of outcome measures (e.g., MMSE vs. MoCA), short follow-up (median <6 months), and limited representation of non-Asian populations. In addition, the neural mechanisms underlying the efficacy of acupuncture remain under-assessed. Notably, comparative analyses of acupuncture and natural medicines in terms of multi-target mechanisms and network pharmacological validation are lacking, thus failing to clarify their complementary roles in neuromodulatory and metabolic pathways.
Future research should prioritize several key directions. First, protocol standardization is essential, and core acupuncture protocols should be established via a Delphi consensus to reduce inter-trial variability. Second, integrating biomarkers and neuroimaging is crucial. This involves incorporating markers such as Aβ40 and GFAP, along with multimodal neuroimaging techniques like fMRI and EEG, to characterize the neural mechanisms underlying acupuncture’s effects (57, 58). Third, to assess cross-cultural efficacy, multicenter RCTs should be conducted in non-Asian cohorts. Fourth, long-term efficacy evaluation is needed, requiring follow-up studies of at least 2 years to clarify the durability of cognitive improvements. Lastly, network pharmacology-driven comparisons should be performed, mapping “acupoint-target” networks against the “compound-target” networks of natural medicines to analyze pathway overlap, such as in the NFKB1 and IL-6 pathways (59).
In conclusion, this protocol provides a rigorous framework to synthesize evidence on acupuncture’s role in diabetic CI. If proven effective, acupuncture could fill a therapeutic gap, offering a low-cost, minimally invasive option for patients intolerant to conventional therapies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
KH: Conceptualization, Project administration, Formal analysis, Writing – original draft. JG: Formal analysis, Resources, Writing – review & editing, Supervision, Conceptualization, Investigation. L-JH: Resources, Formal analysis, Software, Writing – review & editing. X-JZ: Software, Resources, Writing – review & editing, Formal analysis. X-ML: Project administration, Writing – review & editing, Formal analysis. B-PW: Funding acquisition, Writing – review & editing, Methodology. XW: Writing – review & editing, Methodology, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Western Theater General Hospital Youth Incubation Program (2021-XZYG-C40).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1610141/full#supplementary-material
References
1. Liu, FS, Li, Y, Guo, XS, Liu, RC, Zhang, HY, and Li, Z. Advances in traditional Chinese medicine as adjuvant therapy for diabetic foot. World J Diabetes. (2022) 13:851–64. doi: 10.4239/wjd.v13.i10.851
2. IDF Diabetes Atlas Committee. IDF diabetes atlas. 11th ed. Brussels: International Diabetes Federation; (2025). Available online at: https://idf.org/about-diabetes/diabetes-facts-figures/ (Accessed June 25, 2025).
3. Wang, LM, Peng, W, Zhao, ZP, Zhang, M, Shi, ZM, Song, ZW, et al. Prevalence and treatment of diabetes in China, 2013-2018. JAMA. (2021) 326:2498–506. doi: 10.1001/jama.2021.22208
4. Khan, MA, Hashim, MJ, King, JK, Govender, RD, Mustafa, H, Kaabi, JA, et al. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. (2020) 10:107–11. doi: 10.2991/jegh.k.191028.001
5. Feldman, EL, Callaghan, BC, Pop-Busui, R, Zochodne, DW, Wrigh, DE, Bennett, DL, et al. Diabetic neuropathy. Nat Rev Dis Primers. (2019) 5:42. doi: 10.1038/s41572-019-0092-1
6. Subramanian, M, Vasudevan, K, and Rajagopal, A. Cognitive impairment among older adults with diabetes mellitus in Puducherry: a community-based cross-sectional study. Cureus. (2021) 13:e12488. doi: 10.7759/cureus.12488
7. Khan, F, Hussain, S, Singh, S, Sawlani, KK, Usman, K, Sachan, AK, et al. A cross-sectional study on the prevalence and predictors of cognitive impairment and depression in elderly patients with type 2 diabetes mellitus. Cureus. (2025) 17:e77753. doi: 10.7759/cureus.77753
8. Ao, T, Zheng, Y, Li, H, Zhang, Z, Du, L, Huang, X, et al. Eicosapentaenoic acid activates the P62/KEAP1/NRF2 pathway for the prevention of diabetes-associated cognitive dysfunction. Food Funct. (2024) 15:5570–83. doi: 10.1039/d4fo00774c
9. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. (5th ed.) American Psychiatric Publishing. (2013). doi: 10.1176/appi.books.9780890425596
10. You, Y, Liu, Z, Chen, Y, Xu, Y, Qin, JW, Guo, S, et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetol. (2021) 58:671–85. doi: 10.1007/s00592-020-01648-9
11. Alsharif, AA, Wei, L, Ma, T, Man, KKC, Lau, WCY, Brauer, R, et al. Prevalence and incidence of dementia in people with diabetes mellitus. J Alzheimers Dis. (2020) 75:607–15. doi: 10.3233/JAD-191115
12. Samoilova, YG, Matveeva, MV, Tonkih, OS, Kudlay, DA, Oleynik, OA, Aremu, SA, et al. Interhemispheric asymmetry of the brain in patients with type 1 diabetes mellitus and cognitive impairment. Front Endocrinol. (2022) 13:961254. doi: 10.3389/fendo.2022.961254
13. Yang, X, Chen, Y, Zhang, W, Zhang, Z, Wang, P, and Yuan, H. Association between inflammatory biomarkers and cognitive dysfunction analyzed by MRI in diabetes patients. Diabetes Metab Syndr Obes. (2020) 13:4059–65. doi: 10.2147/DMSO.S271160
14. Ding, X, Fang, C, Li, X, Zhang, Z, Yang, XL, Wang, PX, et al. Type 1 diabetes-associated cognitive impairment and diabetic peripheral neuropathy in Chinese adults: results from a prospective cross-sectional study. BMC Endocr Disord. (2019) 19:34. doi: 10.1186/s12902-019-0359-2
15. Jacobson, AM, Ryan, CM, Braffett, BH, Gubitosi-Klug, RA, Lorenzi, GM, Luchsinger, GA, et al. Cognitive performance declines in older adults with type 1 diabetes: results from 32 years of follow-up in the DCCT and EDIC study. Lancet Diabetes Endocrinol. (2021) 9:436–45. doi: 10.1016/S2213-8587(21)00086-3
16. Kanaya, AM, Barrett-Connor, E, Gildengorin, G, and Yaffe, K. Change in cognitive function by glucose tolerance status in older adults: a 4-year prospective study of the rancho Bernardo study cohort. Arch Intern Med. (2004) 164:1327–33. doi: 10.1001/archinte.164.12.1327
17. Gregg, EW, Yaffe, K, Cauley, JA, Rolka, DB, Blackwell, TL, Narayan, KM, et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Arch Intern Med. (2000) 160:174–80. doi: 10.1001/archinte.160.2.174
18. van den Berg, E, Reijmer, YD, de Bresser, J, Kessels, RPC, Kappelle, LJ, and Biessels, GJ. A 4 year follow-up study of cognitive f-unctioning in patients with type 2 diabetes mellitus. Diabetologia. (2010) 53:58–65. doi: 10.1007/s00125-009-1571-9
19. Luo, A, Xie, Z, Wang, Y, Wang, X, Li, S, Yan, J, et al. Type 2 diabetes mellitus-associated cognitive dysfunction: advances in potential mechanisms and therapies. Neurosci Biobehav Rev. (2022) 137:104642. doi: 10.1016/j.neubiorev.2022.104642
20. Arnold, SE, Arvanitakis, Z, Macauley-Rambach, SL, Koenig, AM, Wang, HY, Ahima, RS, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. (2018) 14:168–81. doi: 10.1038/nrneurol.2017.185
21. Felice, FGD. Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest. (2013) 123:531–9. doi: 10.1172/JCI64595
22. Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature. (2001) 414:813–20. doi: 10.1038/414813a
23. Kumar, R, Bukowski, MJ, Wider, JM, Reynolds, CA, Calo, L, Lepore, B, et al. Mitochondrial dynamics following global cerebral ischemia. Mol Cell Neurosci. (2016) 76:68–75. doi: 10.1016/j.mcn.2016.08.010
24. van Sloten, TT, Sedaghat, S, Carnethon, MR, Launer, LJ, and Stehouwer, CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. (2020) 8:325–36. doi: 10.1016/S2213-8587(19)30405-X
25. Zhao, C, Feng, M, Gluchman, M, Ma, X, Li, J, and Wang, H. Acellular fish skin grafts in the treatment of diabetic wounds: advantages and clinical translation. J Diabetes. (2024) 16:e13554. doi: 10.1111/1753-0407.13554
26. Bharadwaj, P, Wijesekara, N, Liyanapathirana, M, Newsholme, P, Ittner, L, Fraser, P, et al. The link between type 2 diabetes and neurodegeneration: roles for amyloid-β, amylin, and tau proteins. J Alzheimers Dis. (2017) 59:421–32. doi: 10.3233/JAD-161192
27. Uno, H, Itokazu, T, and Yamashita, T. Inhibition of repulsive guidance molecule a ameliorates diabetes-induced cognitive decline and hippocampal neurogenesis impairment in mice. Commun Biol. (2025) 8:263 2025 Feb 19. doi: 10.1038/s42003-025-07696-7
28. Li, Y, Chen, ST, Zhang, YY, Qin, JF, Zhu, X, and Yin, K. Citrobacter rodentium promotes brain cognitive dysfunction of type 2 diabetes mice by activating FXR mediated gut barrier damage. Metab Brain Dis. (2025) 40:96. doi: 10.1007/s11011-025-01529-6
29. Baker, LD, Frank, LL, Foster-Schubert, K, Green, PS, Wilkinson, CW, McTiernan, A, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer's disease. J Alzheimers Dis. (2010) 22:569–79. doi: 10.3233/JAD-2010-100768
30. Guimaraes, FC, Amorim, PR, Dos Reis, FF, Bonoto, RT, de Oliveira, WC, Moura, TA, et al. Physical activity and better medication compliance improve mini-mental state examination scores in the elderly. Dement Geriatr Cogn Disord. (2015) 39:25–31. doi: 10.1159/000366413
31. McClean, PL, and Holscher, C. Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer’s disease. Neuropharmacology. (2014) 86:241–58. doi: 10.1016/j.neuropharm.2014.07.015
32. McClean, PL, Parthsarathy, V, Faivre, E, and Hölscher, C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci. (2011) 31:6587–94. doi: 10.1523/JNEUROSCI.0529-11.2011
33. Su, XT, Wang, LQ, Li, JL, Zhang, N, Wang, L, Shi, GX, et al. Acupuncture therapy for cognitive impairment: a Delphi expert consensus survey. Front Aging Neurosci. (2020) 12:596081. doi: 10.3389/fnagi.2020.596081
34. Choi, J, Chandrasekaran, K, Demarest, TG, Kristian, T, Xu, S, Vijaykumar, K, et al. Brain diabetic neurodegeneration segregates with low intrinsic aerobic capacity. Ann Clin Transl Neurol. (2014) 1:589–604. doi: 10.1002/acn3.86
35. Dinel, AL, André, C, Aubert, A, Ferreira, G, Layé, S, and Castanon, N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One. (2011) 6:e24325. doi: 10.1371/journal.pone.0024325
36. Tomlinson, DR, and Gardiner, NJ. Glucose neurotoxicity. Nat Rev Neurosci. (2008) 9:36–45. doi: 10.1038/nrn2294
37. Lin, SZ, WuLi, W, Harn, HJ, and Chiou, TW. Chinese herbs and acupuncture to improve cognitive function in Alzheimer's disease. Tzu Chi Med J. (2021) 33:122–7. doi: 10.4103/tcmj.tcmj_51_20
38. Wong, KKL, Xu, J, Chen, C, Ghista, D, and Zhao, H. Functional magnetic resonance imaging providing the brain effect mechanism of acupuncture and moxibustion treatment for depression. Front Neurol. (2023) 14:1151421. doi: 10.3389/fneur.2023.1151421
39. Yu, H, Li, F, Liu, J, Liu, D, Guo, H, Wang, J, et al. Evaluation of acupuncture efficacy in modulating brain activity with periodic-aperiodic EEG measurements. IEEE Trans Neural Syst Rehabil Eng. (2024) 32:2450–9. doi: 10.1109/TNSRE.2024.3421648
40. Higgins, JPT, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, et al. Cochrane handbook for systematic reviews of interventions. Version 6.5. London: Cochrane; (2024)]. Available online at: https://www.training.cochrane.org/handbook (Accessed April 1, 2025).
41. Hutton, B, Salanti, G, Caldwell, DM, Chaimani, A, Schmid, CH, Cameron, C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
42. Alagiakrishnan, K, Zhao, N, Mereu, L, Senior, P, and Senthilselvan, A. Montreal cognitive assessment is superior to standardized Mini-mental status exam in detecting mild cognitive impairment in the middle-aged and elderly patients with type 2 diabetes mellitus. Biomed Res Int. (2013) 2013:186106:1–5. doi: 10.1155/2013/186106
43. Burrai, F, Othman, S, Brioni, E, Micheluzzi, V, Luppi, M, Apuzzo, L, et al. Effects of virtual reality in patients undergoing dialysis: study protocol. Holist Nurs Pract. (2019) 33:327–37. doi: 10.1097/HNP.0000000000000330
44. ElSayed, NA, Aleppo, G, Aroda, VR, Bannuru, RA, Brown, FM, Bruemmer, D, et al. Introduction and methodology: standards of care in diabetes-2023. Diabetes Care. (2023) 46:S1–4. doi: 10.2337/dc23-Sint
45. MacPherson, H, Altman, DG, Hammerschlag, R, Youping, L, Taixiang, W, White, A, et al. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. (2010) 7:e1000261. doi: 10.1371/journal.pmed.1000261
46. Zhao, C, Wu, Z, Pan, B, Zhang, R, Golestani, A, Feng, Z, et al. Functional biomacromolecules-based microneedle patch for the treatment of diabetic wound. Int J Biol Macromol. (2024) 267:131650. doi: 10.1016/j.ijbiomac.2024.131650
47. Park, J, White, A, Stevinson, C, Ernst, E, and James, M. Validating a new non-penetrating sham acupuncture device: two randomised controlled trials. Acupunct Med. (2002) 20:168–74. doi: 10.1136/aim.20.4.168
48. National Cancer Institute. Common terminology criteria for adverse events (CTCAE). Versi-on 5.0. Bethesda, MD: National Cancer Institute. (2017). Available online at: https://ctep.cance-r.gov/protocoldevelopment/electronic_applications/ctc.htm (Accessed April 1, 2025).
49. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
50. Puhan, MA, Schünemann, HJ, Murad, MH, Li, T, Brignardello-Petersen, R, and Singh, JA. A GRADE working group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. (2014) 349:g5630. doi: 10.1136/bmj.g5630
51. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
52. Cornell, JE. The PRISMA extension for network meta-analysis: bringing clarity and guidance to the reporting of systematic reviews incorporating network meta-analyses. Ann Intern Med. (2015) 162:797–8. doi: 10.7326/M15-0930
53. Yu, H, Wu, X, Cai, L, Deng, B, and Wang, J. Modulation of spectral power and functional connectivity in human brain by acupuncture stimulation. IEEE Trans Neural Syst Rehabil Eng. (2018) 26:977–86. doi: 10.1109/TNSRE.2018.2828143
54. Yu, H, Li, X, Lei, X, and Wang, J. Modulation effect of acupuncture on functional brain networks and classification of its manipulation with EEG signals. IEEE Trans Neural Syst Rehabil Eng. (2019) 27:1973–84. doi: 10.1109/TNSRE.2019.2939655
55. Zou, Y, Huang, T, Pang, A, Zhou, H, and Geng, X. Electroacupuncture regulates glucose metabolism by inhibiting SGLT1 levels, inhibiting microglial polarization, and alleviating Parkinson's disease. Exp Gerontol. (2024) 196:112558. doi: 10.1016/j.exger.2024.112558
56. Pintana, H, Apaijai, N, Chattipakorn, N, and Chattipakorn, SC. DPP-4 inhibitors improve cognition and brain mitochondrial function of insulin-resistant rats. J Endocrinol. (2013) 218:1–11. doi: 10.1530/JOE-12-0521
57. Wei, C, Yang, Q, Chen, J, Rao, X, Li, Q, and Luo, J. EEG microstate as a biomarker of post-stroke depression with acupuncture treatment. Front Neurol. (2024) 15:1452243. doi: 10.3389/fneur.2024.1452243
58. Yu, H, Li, F, Liu, J, Liu, C, Li, G, and Wang, J. Spatiotemporal dynamics of periodic and aperiodic brain activity under peripheral nerve stimulation with acupuncture. IEEE Trans Neural Syst Rehabil Eng. (2024) 32:3993–4003. doi: 10.1109/TNSRE.2024.3492014
Keywords: acupuncture, cognitive impairment, type 2 diabetes mellitus, systematic review, network meta-analysis
Citation: Hong K, Gao J, Hu L-j, Zhou X-j, Long X-m, Wen B-p and Wu X (2025) Acupuncture for cognitive impairment in type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis protocol. Front. Med. 12:1610141. doi: 10.3389/fmed.2025.1610141
Edited by:
Roger David Adams, University of Canberra, AustraliaReviewed by:
Haitao Yu, Tianjin University, ChinaXiao Li, Henan University of Chinese Medicine, China
Gang Ye, Sichuan Agricultural University, China
Yan Zhu, Guangdong Second Provincial General Hospital, China
Chenyu Zhao, Queen’s University Belfast, United Kingdom
Copyright © 2025 Hong, Gao, Hu, Zhou, Long, Wen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo-ping Wen, MTM2NTgwMDIwMjhAMTI2LmNvbQ==; Xi Wu, d3V4aUBjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Kun Hong
Kun Hong Juan Gao3†
Juan Gao3† Xi Wu
Xi Wu