- 1Department of Hematology and Oncology, University of Arkansas for Medical Sciences, Little Rock, AR, United States
- 2MD Anderson Cancer Center, Houston, TX, United States
- 3Northeast Georgia Medical Center, Ganiesville, GA, United States
- 4Department of Internal Medicine, Dr. NTR University of Health Sciences, Vijayawada, India
- 5Indira Gandhi Medical College, Shimla, India
- 6Genesis Cancer and Blood Institute, Little Rock, AR, United States
Non-small cell lung cancer (NSCLC) remains a leading cause of cancer mortality, with late-stage diagnosis contributing to poor survival. Circulating tumor DNA (ctDNA) has emerged as a non-invasive biomarker for screening, diagnosis, and monitoring, with limitations about sensitivity and specificity challenges. The integration of artificial intelligence (AI) offers a promising avenue to enhance ctDNA applications in NSCLC by improving mutation detection rates and sensitivities, refining minimal residual disease (MRD) predictions, enabling earlier detection of relapse, sometimes earlier than imaging, differentiating tumor vs. non-tumor derived signals to improve specificities. AI achieves 0.002% mutant allelic fraction detection, 94% relapse detection sensitivity, and 5.2-month lead time over imaging. This narrative review explores the role of ctDNA in NSCLC management, highlighting how AI amplifies its utility across screening, diagnosis, treatment evaluation, MRD detection, and disease surveillance while outlining key opportunities, challenges, and future directions.
Introduction
The lung cancer incidence continues to increase with contrary change in survival, especially of stage IV disease (1, 2). Screening with low-dose computed tomography (LDCT) among certain high-risk individuals is the current recommendation, which still is underutilized, partly due to the risk of exposure to radiation and false positive results leading to invasive procedures causing potential harm (3). Tissue biopsies are the gold standard for diagnosis but carries procedural risks in 15%–30% of cases and may yield insufficient samples for molecular profiling (4). Moreover, traditional protein based biomarkers lack sensitivity and specificity for early detection and monitoring. The search for an exceptionally reliable, non-invasive biomarker is underway not only for lung cancer screening but also to guide initial diagnostics, precision treatments, predicting prognosis, and finding early relapse and actionable targets (5).
On the same lines, liquid biopsies broadly refer to the identification of one of the cell components derived from the tumor in the bodily fluids representing a viable surrogate of the tumor tissue (6). These components include circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), cell-free tumor RNA (cfRNA), exosomes and tumor-educated platelets (TEP) (7). ctDNA has been widely studied and increasingly used noninvasive biomarker in addition to invasive tissue biopsy in many solid cancers including non-small cell lung cancer (NSCLC), leading its approval from United States Food and Drug Administration (U.S. FDA) especially in NSCLC (8). The integration of artificial intelligence (AI) and machine learning (ML) with ctDNA analysis further amplified its potential in revolutionizing its capabilities, from improving detection sensitivity to uncovering complex mutational patterns (9). In this review, we summarized the physiology of ctDNA and its application in NSCLC, including its role in screening, early diagnosis, individualized treatment, MRD detection, disease surveillance, treatment resistance and the future perspectives.
Pathophysiology, isolation, and analysis of ctDNA
Mandel and Metais (10) first reported the presence of nucleic acids in blood circulation. Cell free DNA (cfDNA) can be found at low levels in the blood of healthy subjects and can be elevated in inflammatory, ischemic and pregnancy states. cfDNA was also reported to be found in other body fluids namely urine and spinal fluid (11–14). Circulating tumor DNA (ctDNA) is a type of cfDNA released from cancer cells due to a variety of processes. It ranges from 180 to 200 base pairs in length and has mutations pertaining to the tumor (15). The process of ctDNA isolation and analysis is illustrated in Figure 1.
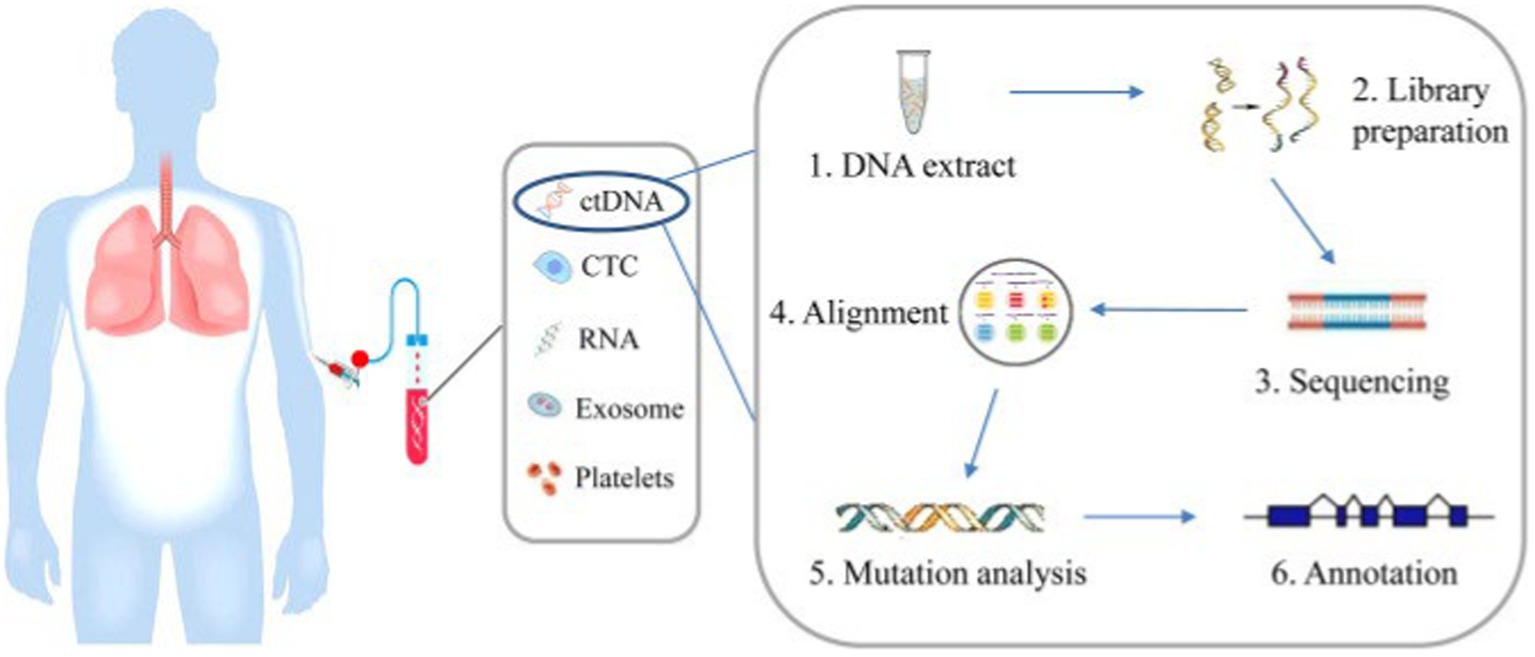
Figure 1. Blood is collected from patients and ctDNA is extracted from blood plasma and mutations can be analyzed by next generation sequencing involving a few steps, including DNA extraction, DNA library preparation, sequencing, sequence alignment, mutation annotation, and so on (58).
Baseline ctDNA levels in NSCLC patients were shown to correlate with disease stage, burden, metabolism and higher cfDNA levels may independently predict poor progression free survival (PFS) and overall survival (OS) (16). Lower ctDNA levels at earlier stages of disease due to smaller disease burden poses a threat to disease detection and some studies recommended combining ctDNA with other cutting edge diagnostics like exosomal RNA to increase the sensitivity of detection (17). However, recent advancements in sequencing like digital polymerase chain reaction (PCR) and next-generation sequencing (NGS) will alleviate this problem with better mutant allelic fraction detections up to 0.05% (11). In addition to the recent technological advances, incorporating artificial intelligence (AI) and machine learning (ML) algorithms with these sequencing technologies to refine diagnostic accuracy by enhancing the detection of low-frequency mutations there by improving the sensitivity and specificity of ctDNA assays, particularly in early-stage NSCLC where tumor burden is minimal (18).
ctDNA role in lung cancer screening
The United States preventive services task force (USPSTF) recommends LDCT for screening lung cancers among high-risk populations. If suspicious lesions were noted, standardized reporting was implemented using Lung reporting and data system (L-RADS) for further follow up and management. Using Lung-RADS criteria, ≥6 mm has been chosen as the lower limit of threshold for solid nodules to minimize false positive rates without affecting false negative rates (3). Lung cancer prognosis did not improve significantly in the last few decades even with advancement in therapeutics which was attributed to delay in diagnosis due to minimal early stage symptoms (19, 20). This is reflected in the tremendous difference of 5 year survival of 2 and 71% among patients with NSCLC diagnosed at stage IV disease and early stages, respectively (19). Therefore, early diagnosis of lung cancer patients might improve patient outcomes, thereby implicating the need for novel markers to aid in achieving the goal.
One of the major goals in oncology is detecting cancers at an early stage thereby being able to treat them with curative intent leading to better outcomes, which sparked the curiosity in finding a pan-screening test to be used in otherwise healthy subjects. Traditional protein-based biomarkers carry the risk of poor sensitivity and specificity there by limiting its routine use in earlier detection (21). Also, those markers were not available to all the available cancers, especially lung cancer. This led to the idea of using ctDNA as a potential marker for identifying cancers at an earlier stage. Like protein-based markers, cancer cells are thought to secrete actively or passively ctDNA into the circulation which help us not only to identify the tumor type but also targetable mutations to guide better therapeutics.
The Circulating Cell-free Genome Atlas study (CCGA) by GRAIL, used a multi-cancer early detection test utilizing cfDNA and AI and reported an overall sensitivity of 51.5% (Range: 14.5–92.2%) in detecting cancers with sensitivity directly proportional to the disease stage (22). The study used AI and ML by employing targeted methylation-based sequencing of cfDNA, using a ML classifier to differentiate tumor derived signals from non-tumor derived ones which improved specificity (reported at 99%) to enable cancer type identification. Similarly, Cohen et al. (23) reported sensitivities ranging from 69 to 98% for the detection of five cancer types (ovary, liver, stomach, pancreas, and esophagus) with changes depending on cancer stage and type. Sensitivities were highest in higher stages and solid tumors of ovarian or liver origin and least in earlier stages and breast cancer and also increased when combined with imaging modalities like PET-CT scan and standard protein biomarkers for some of these cancers (23–25). For lung cancer, the probability of detection was reported as 75% and both studies reported an overall specificity of 99%. The sensitivity, which is one of the crucial aspects of a screening test, is lower especially in earlier stages, which might be a drawback for some cancers but useful in other cancers like lung or liver where the earlier stages have better sensitivities (26).
Recent advancements in ctDNA-based screening have significantly improved early detection of non-small cell lung cancer (NSCLC). Mathios et al. (1) utilized cfDNA fragmentome analysis with ML to achieve 75% sensitivity for Stage I–II NSCLC and 95% specificity, offering a novel approach to distinguish tumor-derived signals from non-tumor cfDNA. Similarly, Yin et al. (27) demonstrated that combining ctDNA with protein biomarkers (CEA, SqCC, CYFRA21-1) increased sensitivity to 86.4% for early-stage NSCLC, highlighting the potential of multi-analyte approaches. Additionally, Tan et al. (28) reported a 65% sensitivity for Stage I–II NSCLC using ultradeep sequencing, with a specificity of 98.5%, addressing challenges like low mutant allele fractions and clonal hematopoiesis of indeterminate potential (CHIP) through tumor-informed sequencing. These studies collectively underscore the evolving role of advanced sequencing, bioinformatics, and multi-biomarker strategies in enhancing lung cancer screening accuracy for high-risk populations.
Although a direct comparison with standard protein biomarkers was not performed, the available results enlighten us for a possible new horizon in the future with better emerging technologies for improving the sensitivities (Table 1). On the contrary, Pons-Belda et al. extrapolated using the current available data from Ct-DNA diagnostic methods and reported that the current detection methods will identify tumors of size 10-15 mm in diameter. Tumors below this size lead to incredibly low mutant allelic fraction about 0.01% which will reduce the sensitivity rendering the use of this technology implausible for screening methods (29).
Another important setback with using ctDNA as a screening test is the presence of clonal hematopoiesis of indeterminate potential (CHIP), a benign condition usually noted in healthy people of older age that causes the release of mutant DNA into the circulation thereby causing false positive rates. Although CHIP-based mutations can be differentiated from the real mutations from malignancy using advanced sequencing steps, this poses a time consuming and costly process, limiting its role as a lung cancer screening tool (30).
ctDNA role in primary diagnosis and treatment evaluation
In addition to traditional TNM staging, NSCLC classification evolved to other subtypes based on the detection of various genetic mutations and subsequent treatment with concomitant targeted therapies, leading to better outcomes (31). Tissue biopsy remains to be the gold standard in diagnosing lung cancer. More commonly tested genetic mutations in NSCLC include EGFR mutations, ALK rearrangements, and ROS1 fusions in addition to MET, RET, BRAF, HER2, and NTRK1. Plasma based conventional tumor markers like carcinoembryonic antigen (CEA), neuron specific enolase (NSE), etc., were of limited utility in aiding primary diagnosis (32). The intrinsic features of ctDNA seems to be an attractive non-invasive method to help in primary diagnosis. It emerged as an equally effective alternative noninvasive detection method for aiding in primary diagnosis and detecting resistance mutations compared to more invasive biopsy testing and may be used with other available biomarkers to enhance the quality of primary diagnosis results (33).
The quest to replace high risk, invasive tissue biopsy to low risk, least invasive, patient tolerated liquid biopsy is ongoing. Even though earlier small scale studies showed discordance between the somatic variations among tissue and plasma samples, recently performed large scale and appropriately designed studies reported better concordance between the samples, thereby encouraging the use of ctDNA in primary diagnostics (34, 35). It was suggested by international association for the study of lung cancer (IASLC) that ctDNA implementation might improve the patient outcome and it should be routinely implemented in clinical practices (36). On the same lines, data from the ENSURE, AURA phase 2 extension cohort and AURA 2 studies lead to approval of Cobas EGFR Mutation Test v2 (Roche Molecular Diagnostics, Pleasanton, CA) by U.S. FDA to detect specific mutations (exon 19 deletion or exon 21 [L858R] substitution) in patients’ blood with NSCLC to determine candidates for treatment with erlotinib as well as in patients with T790M mutations who would benefit from Osimertinib (37–39). European agency also approved another ctDNA test (Thera screen EGFR RGQ PCR Kit, Qiagen, Valencia, CA) to detect EGFR mutations when tumor tissue is insufficient (40).
All these studies supporting the use of ctDNA in aiding primary diagnosis had an unquestionable specificity of around 99%. However, the negative predictive value is low, which will lead to false negative results and should always be followed by gold standard tissue-based testing. Patients who progress on Osimertinib should always checked for EGFR-C797S, and other rare genetic alterations (BRAF-V600, KRAS, HER2 and MET) and ctDNA will be an extremely useful least invasive intervention with a quick turnaround time, allowing targeting additional alterations (41–43).
In addition to EGFR related mutations, ctDNA can be used to detect other genetic changes too. In the largest prospective cfDNA study by Leighl et al. (33), among previously non treated metastatic NSCLC, liquid biopsies using cfDNA identified FDA approved markers (i.e., ALK, BRAF, EGFR, and ROS1) in addition to other alterations (ERBB2, RET, MET amplifications and exon 14 skipping) at a high concordance rate with tissue biopsies reaching up to 98% among FDA approved ones signifying its role in effective genotyping thereby precisely designing therapies for patients.
Dong et al. (44) demonstrated that ctDNA-guided de-escalation of tyrosine kinase inhibitors in advanced NSCLC achieved complete remission in 60% of patients, with a 95% concordance rate for actionable mutations (EGFR, ALK, ROS1). Provencio et al. (45) showed that ctDNA clearance post-neoadjuvant nivolumab plus chemotherapy in Stage IIIA NSCLC predicted improved 5-year overall survival (HR 0.35, p = 0.002), highlighting its utility in immunotherapy settings. Longitudinal ctDNA monitoring is increasingly vital for detecting treatment resistance. Ding et al. found that an early ctDNA nadir within 6 weeks of chemoimmunotherapy predicted better progression-free survival and overall survival in metastatic NSCLC (HR 2.8, p < 0.001), with emergent mutations (e.g., KRAS, MET) indicating resistance. These advancements underscore ctDNA’s potential to guide adaptive therapy (46).
ctDNA in detecting MRD
The pursuit to identify early relapse post curative treatment in any malignancy is a matter of high regard. Current practices involve relying on clinical, radiological, and plasma-based tumor markers to identify early relapse in NSCLC patients treated with curative intent. The use of ctDNA in detecting minimal residual disease (MRD) among NSCLC patients treated with curative intent at their various post-treatment time points was forthcoming. It has been already shown to identify early relapse in the aforementioned group in various small retrospective studies, and their clinical validation through large prospective trials is ongoing.
Chaudhuri et al. first observed the ctDNA levels in a group of unresectable NSCLC patients varying from Stages I–III. They reported that 17 out of 32 patients, with detectable ctDNA within 4 months of completed treatment had lower freedom from progression (FFP) and disease specific survival than those with undetectable ctDNA in the same time point. On the same lines, all 17 with MRD +ve but only 1 out of 15 MRD −ve of them relapsed in the same follow-up period. The study utilized Cancer Personalized Profiling by deep sequencing (CAPP-Seq), a targeted Next generation sequencing (NGS) approach focusing on recurrent NSCLC mutations to achieve high sensitivity (down to 0.002% mutant allele fraction) but potentially missing rare mutations (47).
Similarly, Modling et al., conducted a retrospective study on unresectable stage IIB-IIIB NSCLC patients who received chemoradiotherapy (CRT) initially and further stratified into consolidation with immune checkpoint inhibitor (ICI) and no consolidation group. Plasma samples for ctDNA were checked pre-CRT, post-CRT and median of 11 weeks into ICI therapy. Among no consolidation cohorts, 1 out of 12 post CRT MRD −ve and all 17 of post CRT MRD +ve relapsed in 12 months of follow up. Among the consolidation group, increased freedom from progression (median 22 months vs. 5 months) was observed among MRD +ve post CRT with decreasing ctDNA levels pre-ICI to early on ICI than increasing ct DNA levels during the same time (48).
Chen et al., conducted a prospective study in November 2016 among NSCLC patients with stages I-III who underwent surgical resection for curative intent and ctDNA measured at various time points including (1) immediately before surgery, (2) 5 min, 30 min, and 2 h after surgery, and (3) 1, 3, and 30 days after surgery. Based on the study results, they concluded that the median half-life of ctDNA was 35 min and its longer in patients with positive ctDNA 1–30 days post-surgery than those with undetectable levels in the same period. The authors also suggested measuring ctDNA post operatively as early as 3 days can accurately prognosticate survival by predicting the relapse risk (49).
Another prospective study by Abbosh et al. reported the detection of ctDNA levels at or before clinical relapse among 82% of Stage IA-IIIB NSCLC patients who underwent surgical resection. However, in patients who remained relapse-free during a median follow up of 1,184 days, ctDNA was detected at only one of the 199 timepoints (50).
Recent studies have strengthened ctDNA’s role in detecting minimal residual disease (MRD) in NSCLC. Gale et al. (51) validated ctDNA in 88 early-stage NSCLC patients post-treatment, achieving 90% sensitivity and 95% specificity for MRD detection, predicting relapse 4.8 months before radiographic recurrence. Similarly, Isbell et al. (52) used ultrasensitive sequencing to detect ctDNA in early-stage NSCLC, reporting 92% sensitivity for MRD post-surgery and a 5–7-month lead time, with tumor-informed panels reducing CHIP-related false positives. The LUNGCA-1 study further confirmed ctDNA’s utility, finding that persistent ctDNA at 3–7 days post-surgery predicted relapse with 92% sensitivity and a 6.1-month lead time in 330 patients (53).
The aforementioned studies in surgical resection patients used targeted deep sequencing with limited panels to detect ctDNA. This is evident in a study by Abbosh et al., where 10 patients during clinical follow up were diagnosed with non-lung primary malignancy but their ctDNA did not identify them. Zviran et al., used tumor-based whole genome sequencing (WGS) to detect MRD in the plasma samples at 2.5 weeks before and after surgery among NSCLC patients. On a median follow-up of 18 months, 50% of post-surgery MRD positive patients relapsed while 100% of MRD negative group non-relapsed (54).
All the above studies emphasize the role of ctDNA in MRD detection among early-stage NSCLC patients treated with curative intent by providing insights into the long-term prognosis (Table 2). There is potential for AI models to refine the sensitivity of targeted sequencing panels and integrate longitudinal ctDNA data with clinical variables to predict relapse earlier and with greater accuracy. However, challenges include optimizing AI algorithms to account for tumor heterogeneity and reducing false negatives, particularly when using limited gene panels (55). In the study by Chaudhuri et al., ctDNA predicted relapse in about three-fourths of patients a median of 5.2 months earlier than radiological relapse, thereby providing more time to change therapies for better outcomes. In addition, they reported 100% sensitivity and specificity of ctDNA in detecting recurrences using an ever-positive vs. never-positive approach. However, the sensitivity of MRD detection in post-surgical patients is not optimal, except in Zviran et al., where tumor-based WGS was used. Based on the above evidence, multiple prospective clinical trials are ongoing with the possible outcome of routinely using ctDNA for MRD and disease surveillance, thereby improving outcomes and alleviating toxicities through precision treatment (56).
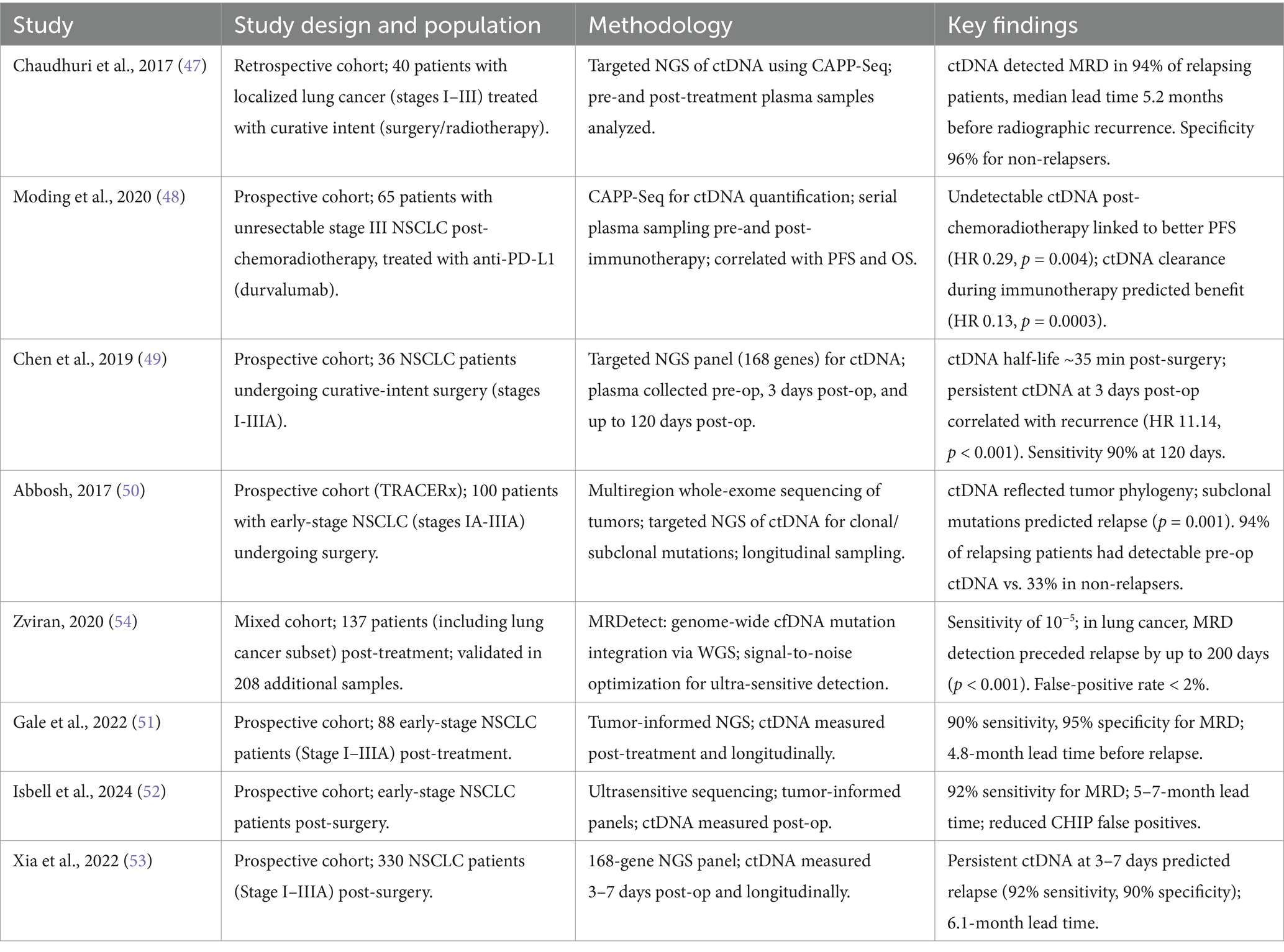
Table 2. Summary of studies evaluating circulating tumor DNA (ctDNA) as a biomarker for minimal residual disease (MRD) detection in lung cancer.
Future perspectives
The field of ctDNA research is rapidly evolving, with ongoing advancements in sequencing technologies, bioinformatics, and multi-analyte approaches. The integration of AI and ML into ctDNA analysis holds promise for improving the accuracy and efficiency of mutation detection. Additionally, the development of multi-cancer early detection (MCED) tests that combine ctDNA with other biomarkers, such as exosomal RNA and protein markers, could enhance the sensitivity and specificity of cancer screening.
AI is significantly transforming ctDNA analysis for NSCLC. For instance, Gale et al. employed AI bioinformatics to achieve 90% sensitivity in detecting MRD and 95% specificity in identifying resistance mutations like EGFR T790M. Similarly, Mathios et al. used ML on cfDNA fragmentomes, reaching 75% sensitivity for early-stage NSCLC. Furthermore, Kris et al. (57) utilized AI to analyze ctDNA changes over time in neoadjuvant atezolizumab patients, predicting relapse with 85% accuracy. These studies demonstrate AI’s capability to combine various data types and account for the diverse nature of tumors. However, challenges such as validating AI models across diverse cohorts, managing computational complexity, and ensuring cost-effectiveness must be addressed to realize these future directions fully.
Conclusion
Circulating tumor DNA (ctDNA) has emerged as a transformative tool in the management of non-small cell lung cancer (NSCLC), offering a non-invasive, dynamic, and comprehensive approach to cancer detection and monitoring. From screening and diagnosis to treatment selection and MRD detection, ctDNA has demonstrated significant potential to improve patient outcomes and redefine the standard of care in NSCLC. AI integration enhances its utility by achieving ultra-low detection limits and improved specificity.
While challenges remain, including the need for improved sensitivity in early-stage disease, clonal hematopoiesis interference and the integration of ctDNA into clinical workflows, ongoing research and technological advancements are expected to address these limitations. Clinicians should integrate ctDNA testing into routine practice, particularly for most common actionable mutations like EGFR, ALK and for MRD monitoring post-curative treatment. The continued evolution of ctDNA-based technologies, coupled with the integration of multi-analyte approaches and AI-driven analysis, holds promise for revolutionizing cancer care and achieving the ultimate goal of precision oncology.
Author contributions
NT: Writing – original draft, Writing – review & editing. MB: Writing – review & editing. BS: Writing – review & editing. PS: Writing – review & editing. KM: Writing – review & editing. KV: Writing – review & editing. SK: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mathios, D, Johansen, JS, Cristiano, S, Medina, JE, Phallen, J, Larsen, KR, et al. Detection and characterization of lung cancer using cell-free DNA fragmentomes. Nat Commun. (2021) 12:5060. doi: 10.1038/s41467-021-24994-w
2. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Jonas, DE, Reuland, DS, Reddy, SM, Nagle, M, Clark, SD, Weber, RP, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US preventive services task force. JAMA. (2021) 325:971–87. doi: 10.1001/jama.2021.0377
4. Corcoran, RB, and Chabner, BA. Application of cell-free DNA analysis to Cancer treatment. N Engl J Med. (2018) 379:1754–65. doi: 10.1056/NEJMra1706174
5. Kan, CFK, Unis, GD, Li, LZ, Gunn, S, Li, L, Soyer, HP, et al. Circulating biomarkers for early stage non-small cell lung carcinoma detection: supplementation to low-dose computed tomography. Front Oncol. (2021) 11:555331. doi: 10.3389/fonc.2021.555331
6. Lianidou, E, and Pantel, K. Liquid biopsies. Genes Chromosomes Cancer. (2019) 58:219–32. doi: 10.1002/gcc.22695
7. Lone, SN, Nisar, S, Masoodi, T, Singh, M, Rizwan, A, Hashem, S, et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. (2022) 21:79. doi: 10.1186/s12943-022-01543-7
8. Jenkins, S, Cross, D, and Scudder, SA. Osimertinib (TAGRISSO™) and the cobas® EGFR mutation test v2. Companion and complementary diagnostics. Academic Press (2019) 429–43.
9. Wang, HY, Lin, WY, Zhou, C, Yang, ZA, Kalpana, S, and Lebowitz, MS. Integrating artificial intelligence for advancing multiple-cancer early detection via serum biomarkers: a narrative review. Cancers (Basel). (2024) 16:862. doi: 10.3390/cancers16050862
10. Mandel, P, and Metais, P. Nuclear acids in human blood plasma. CR Seances Soc Biol Fil. (1948) 142:241–3.
11. Newman, AM, Bratman, SV, To, J, Wynne, JF, Eclov, NCW, Modlin, LA, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. (2014) 20:548–54. doi: 10.1038/nm.3519
12. Reckamp, KL, Melnikova, VO, Karlovich, C, Sequist, LV, Ross Camidge, D, Wakelee, H, et al. A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J Thorac Oncol. (2016) 11:1690–700. doi: 10.1016/j.jtho.2016.05.035
13. De Mattos-Arruda, L, Mayor, R, Ng, CKY, Weigelt, B, Martínez-Ricarte, F, Torrejon, D, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. (2015) 6:8839. doi: 10.1038/ncomms9839
14. Heitzer, E, Ulz, P, and Geigl, JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. (2015) 61:112–23. doi: 10.1373/clinchem.2014.222679
15. Stroun, M, Anker, P, Lyautey, J, Lederrey, C, and Maurice, PA. Isolation and characterization of DNA from the plasma of cancer patients. Eur J Cancer Clin Oncol. (1987) 23:707–12. doi: 10.1016/0277-5379(87)90266-5
16. Lee, Y, Park, S, Kim, WS, Lee, JC, Jang, SJ, Choi, J, et al. Correlation between progression-free survival, tumor burden, and circulating tumor DNA in the initial diagnosis of advanced-stage EGFR-mutated non-small cell lung cancer. Thorac Cancer. (2018) 9:1104–10. doi: 10.1111/1759-7714.12793
17. Krug, AK, Enderle, D, Karlovich, C, Priewasser, T, Bentink, S, Spiel, A, et al. Improved EGFR mutation detection using combined exosomal RNA and circulating tumor DNA in NSCLC patient plasma. Ann Oncol. (2018) 29:2143. doi: 10.1093/annonc/mdy261
18. Ginghina, O, Hudita, A, Zamfir, M, Spanu, A, Mardare, M, Bondoc, I, et al. Liquid biopsy and artificial intelligence as tools to detect signatures of colorectal malignancies: a modern approach in patient’s stratification. Front Oncol. (2022) 12:856575. doi: 10.3389/fonc.2022.856575
19. Goldstraw, P, Chansky, K, Crowley, J, Rami-Porta, R, Asamura, H, and Eberhardt, WEE. The IASLC lung Cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung Cancer. J Thorac Oncol. (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
20. Gridelli, C, Rossi, A, Carbone, DP, Guarize, J, Karachaliou, N, Mok, T, et al. Non-small-cell lung cancer. Nat Rev Dis Primers. (2015) 1:9. doi: 10.1038/nrdp.2015.9
21. Orive, D, Echepare, M, Bernasconi-Bisio, F, Sanmamed, MF, Pineda-Lucena, A, de la Calle-Arroyo, C, et al. Protein biomarkers in lung Cancer screening: technical considerations and feasibility assessment. Arch Bronconeumol. (2024) 60:S67–76. doi: 10.1016/j.arbres.2024.07.007
22. Klein, EA, Richards, D, Cohn, A, Tummala, M, Lapham, R, Cosgrove, D, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. (2021) 32:1167–77. doi: 10.1016/j.annonc.2021.05.806
23. Cohen, JD, Li, L, Wang, Y, Thoburn, C, Afsari, B, Danilova, L, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. (2018) 359:926–30. doi: 10.1126/science.aar3247
24. Douville, C, Cohen, JD, Ptak, J, Popoli, M, Schaefer, J, Silliman, N, et al. Assessing aneuploidy with repetitive element sequencing. Proc Natl Acad Sci USA. (2020) 117:4858–63. doi: 10.1073/pnas.1910041117
25. Lennon, AM, Buchanan, AH, Kinde, I, Warren, A, Honushefsky, A, Cohain, AT, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science. (2020) 369:9601. doi: 10.1126/science.abb9601
26. Zill, OA, Banks, KC, Fairclough, SR, Mortimer, SA, Vowles, JV, Mokhtari, R, et al. The landscape of actionable genomic alterations in cell-free circulating tumor DNA from 21,807 advanced Cancer patients. Clin Cancer Res. (2018) 24:3528–38. doi: 10.1158/1078-0432.CCR-17-3837
27. Yin, JX, Hu, WW, Gu, H, and Fang, JM. Combined assay of circulating tumor DNA and protein biomarkers for early noninvasive detection and prognosis of non-small cell lung Cancer. J Cancer. (2021) 12:1258–69. doi: 10.7150/jca.49647
28. Tan, AC, Lai, GGY, Saw, SPL, Chua, KLM, Takano, A, Ong, BH, et al. Detection of circulating tumor DNA with ultradeep sequencing of plasma cell-free DNA for monitoring minimal residual disease and early detection of recurrence in early-stage lung cancer. Cancer. (2024) 130:1758–65. doi: 10.1002/cncr.35263
29. Pons-Belda, OD, Fernandez-Uriarte, A, and Diamandis, EP. Can circulating tumor DNA support a successful screening test for early cancer detection? The grail paradigm. Diagnostics. (2021) 11:2171. doi: 10.3390/diagnostics11122171
30. Keller, L, Belloum, Y, Wikman, H, and Pantel, K. Clinical relevance of blood-based ctDNA analysis: mutation detection and beyond. Br J Cancer. (2021) 124:345–58. doi: 10.1038/s41416-020-01047-5
31. Nooreldeen, R, and Bach, H. Current and future development in lung cancer diagnosis. Int J Mol Sci. (2021) 22:8661. doi: 10.3390/ijms22168661
32. Howlader, N, Forjaz, G, Mooradian, MJ, Meza, R, Kong, CY, Cronin, KA, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. (2020) 383:640–9. doi: 10.1056/nejmoa1916623
33. Leighl, NB, Page, RD, Raymond, VM, Daniel, DB, Divers, SG, Reckamp, KL, et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non–small cell lung cancer. Clin Cancer Res. (2019) 25:4691–700. doi: 10.1158/1078-0432.ccr-19-0624
34. Merker, JD, Oxnard, GR, Compton, C, Diehn, M, Hurley, P, Lazar, AJ, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. J Clin Oncol. (2018) 36:1631–41. doi: 10.1200/jco.2017.76.8671
35. Park, S, Olsen, S, Ku, BM, Lee, MS, Jung, HA, Sun, JM, et al. High concordance of actionable genomic alterations identified between circulating tumor DNA-based and tissue-based next-generation sequencing testing in advanced non-small cell lung cancer: the Korean lung liquid versus invasive biopsy program. Cancer. (2021) 127:3019–28. doi: 10.1002/cncr.33571
36. Rolfo, C, Mack, PC, Scagliotti, GV, Baas, P, Barlesi, F, Bivona, TG, et al. Liquid biopsy for advanced non-small cell lung cancer (NSCLC): a statement paper from the IASLC. J Thorac Oncol. (2018) 13:1248–68. doi: 10.1016/j.jtho.2018.05.030
37. Wu, YL, Zhou, C, Liam, CK, Wu, G, Liu, X, Zhong, Z, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. (2015) 26:1883–9. doi: 10.1093/annonc/mdv270
38. Yang, JCH, Ahn, MJ, Kim, DW, Ramalingam, SS, Sequist, LV, Su, WC, et al. Osimertinib in pretreated T790M-positive advanced non–small-cell lung cancer: aura study phase II extension component. J Clin Oncol. (2017) 35:1288–96. doi: 10.1200/jco.2016.70.3223
39. Goss, G, Tsai, CM, Shepherd, FA, Bazhenova, L, Lee, JS, Chang, GC, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. (2016) 17:1643–52. doi: 10.1016/s1470-2045(16)30508-3
40. Douillard, JY, Ostoros, G, Cobo, M, Ciuleanu, T, McCormack, R, Webster, A, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer. (2014) 110:55–62. doi: 10.1038/bjc.2013.721
41. Thress, KS, Paweletz, CP, Felip, E, Cho, BC, Stetson, D, Dougherty, B, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non–small cell lung cancer harboring EGFR T790M. Nat Med. (2015) 21:560–2. doi: 10.1038/nm.3854
42. Ortiz-Cuaran, S, Scheffler, M, Plenker, D, Dahmen, L, Scheel, AH, Fernandez-Cuesta, L, et al. Heterogeneous mechanisms of primary and acquired resistance to third-generation EGFR inhibitors. Clin Cancer Res. (2016) 22:4837–47. doi: 10.1158/1078-0432.ccr-15-1915
43. Guibert, N, Hu, Y, Feeney, N, Kuang, Y, Plagnol, V, Jones, G, et al. Amplicon-based next-generation sequencing of plasma cell-free DNA for detection of driver and resistance mutations in advanced non-small cell lung cancer. Ann Oncol. (2018) 29:1049–55. doi: 10.1093/annonc/mdy005
44. Dong, S, Wang, Z, Zhang, JT, Yan, B, Zhang, C, Gao, X, et al. Circulating tumor DNA-guided De-escalation targeted therapy for advanced non-small cell lung Cancer: a nonrandomized controlled trial. JAMA Oncol. (2024) 10:932–40. doi: 10.1001/jamaoncol.2024.1779
45. Provencio, M, Nadal, E, Insa, A, García Campelo, R, Casal, J, Dómine, M, et al. Perioperative chemotherapy and nivolumab in non-small-cell lung cancer (NADIM): 5-year clinical outcomes from a multicentre, single-arm, phase 2 trial. Lancet Oncol. (2024) 25:1453–64. doi: 10.1016/S1470-2045(24)00498-4
46. Ding, H, Yuan, M, Yang, Y, and Xu, XS. Identifying key circulating tumor DNA parameters for predicting clinical outcomes in metastatic non-squamous non-small cell lung cancer after first-line chemoimmunotherapy. Nat Commun. (2024) 15:6862. doi: 10.1038/s41467-024-51316-7
47. Chaudhuri, AA, Chabon, JJ, Lovejoy, AF, Newman, AM, Stehr, H, Azad, TD, et al. Early detection of molecular residual disease in localized lung Cancer by circulating tumor DNA profiling. Cancer Discov. (2017) 7:1394–403. doi: 10.1158/2159-8290.CD-17-0716
48. Moding, EJ, Liu, Y, Nabet, BY, Chabon, JJ, Chaudhuri, AA, Hui, AB, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small cell lung Cancer. Nat Cancer. (2020) 1:176–83. doi: 10.1038/s43018-019-0011-0
49. Chen, K, Zhao, H, Shi, Y, Yang, F, Wang, LT, Kang, G, et al. Perioperative dynamic changes in circulating tumor DNA in patients with lung Cancer (DYNAMIC). Clin Cancer Res. (2019) 25:7058–67. doi: 10.1158/1078-0432.CCR-19-1213
50. Abbosh, C, Birkbak, NJ, Wilson, GA, Jamal-Hanjani, M, Constantin, T, and Salari, R. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. (2017) 545:446–51. doi: 10.1038/nature22364
51. Gale, D, Heider, K, Ruiz-Valdepenas, A, Hackinger, S, Perry, M, Marsico, G, et al. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann Oncol. (2022) 33:500–10. doi: 10.1016/j.annonc.2022.02.007
52. Isbell, JM, Goldstein, JS, Hamilton, EG, Liu, SY, Eichholz, J, Buonocore, DJ, et al. Ultrasensitive circulating tumor DNA (ctDNA) minimal residual disease (MRD) detection in early stage non-small cell lung cancer (NSCLC). J Clin Oncol. (2024) 42:8078. doi: 10.1200/JCO.2024.42.16_suppl.8078
53. Xia, L, Mei, J, Kang, R, Deng, S, Chen, Y, Yang, Y, et al. Perioperative ctDNA-based molecular residual disease detection for non-small cell lung Cancer: a prospective multicenter cohort study (LUNGCA-1). Clin Cancer Res. (2022) 28:3308–17. doi: 10.1158/1078-0432.CCR-21-3044
54. Zviran, A, Schulman, RC, Shah, M, Hill, STK, Deochand, S, Khamnei, CC, et al. Genome-wide cell-free DNA mutational integration enables ultra-sensitive cancer monitoring. Nat Med. (2020) 26:1114–24. doi: 10.1038/s41591-020-0915-3
55. Bamodu, OA, Chung, CC, and Pisanic, TR 2nd. Harnessing liquid biopsies: exosomes and ctDNA as minimally invasive biomarkers for precision cancer medicine. J Liq Biopsy. (2023) 2:100126. doi: 10.1016/j.jlb.2023.100126
56. Pellini, B, and Chaudhuri, AA. Circulating tumor DNA minimal residual disease detection of non–small-cell lung cancer treated with curative intent. J Clin Oncol. (2022) 40:567–75. doi: 10.1200/jco.21.01929
57. Kris, MG, Grindheim, JM, Chaft, JE, Lee, JM, Johnson, BE, Rusch, VW, et al. 1O dynamic circulating tumour DNA (ctDNA) response to neoadjuvant (NA) atezolizumab (atezo) and surgery (surg) and association with outcomes in patients (pts) with NSCLC. Ann Oncol. (2021) 32:S1373. doi: 10.1016/j.annonc.2021.10.017
Keywords: lung cancer, screening, minimal residual disease, circulating tumor DNA, artificial intelligence
Citation: Thalambedu N, Balla M, Sivasubramanian BP, Sadaram P, Malla KP, Vasipalli KP and Kakadia S (2025) Integrating artificial intelligence with circulating tumor DNA for non-small cell lung cancer: opportunities, challenges, and future directions. Front. Med. 12:1612376. doi: 10.3389/fmed.2025.1612376
Edited by:
Udhaya Kumar, Baylor College of Medicine, United StatesReviewed by:
Lei Cheng, Tongji University, ChinaSuleiman Zakari, Federal University of Health Sciences Otukpo, Nigeria
Copyright © 2025 Thalambedu, Balla, Sivasubramanian, Sadaram, Malla, Vasipalli and Kakadia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nishanth Thalambedu, bmlzaGFudGgubWVkY0BnbWFpbC5jb20=
 Nishanth Thalambedu
Nishanth Thalambedu Mamtha Balla
Mamtha Balla Barath Prashanth Sivasubramanian
Barath Prashanth Sivasubramanian Prasanth Sadaram1
Prasanth Sadaram1